Abstract
BACKGROUND:
Patients with previous coronary artery bypass grafting often require invasive coronary angiography (ICA). However, for these patients, the procedure is technically more challenging and has a higher risk of complications. Observational studies suggest that computed tomography cardiac angiography (CTCA) may facilitate ICA in this group, but this has not been tested in a randomized controlled trial.
METHODS:
This study was a single-center, open-label randomized controlled trial assessing the benefit of adjunctive CTCA in patients with previous coronary artery bypass grafting referred for ICA. Patients were randomized 1:1 to undergo CTCA before ICA or ICA alone. The co–primary end points were procedural duration of the ICA (defined as the interval between local anesthesia administration for obtaining vascular access and removal of the last catheter), patient satisfaction after ICA using a validated questionnaire, and the incidence of contrast-induced nephropathy. Linear regression was used for procedural duration and patient satisfaction score; contrast-induced nephropathy was analyzed using logistic regression. We applied the Bonferroni correction, with P<0.017 considered significant and 98.33% CIs presented. Secondary end points included incidence of procedural complications and 1-year major adverse cardiac events.
RESULTS:
Over 3 years, 688 patients were randomized with a median follow-up of 1.0 years. The mean age was 69.8±10.4 years, 108 (15.7%) were women, 402 (58.4%) were White, and there was a high burden of comorbidity (85.3% hypertension and 53.8% diabetes). The median time from coronary artery bypass grafting to angiography was 12.0 years, and there were a median of 3 (interquartile range, 2 to 3) grafts per participant. Procedure duration of the ICA was significantly shorter in the CTCA+ICA group (CTCA+ICA, 18.6±9.5 minutes versus ICA alone, 39.5±16.9 minutes [98.33% CI, −23.5 to −18.4]; P<0.001), alongside improved mean ICA satisfaction scores (1=very good to 5=very poor; −1.1 difference [98.33% CI, −1.2 to −0.9]; P<0.001), and reduced incidence of contrast-induced nephropathy (3.4% versus 27.9%; odds ratio, 0.09 [98.33% CI, 0.04–0.2]; P<0.001). Procedural complications (2.3% versus 10.8%; odds ratio, 0.2 [95% CI, 0.1–0.4]; P<0.001) and 1-year major adverse cardiac events (16.0% versus 29.4%; hazard ratio, 0.4 [95% CI, 0.3–0.6]; P<0.001) were also lower in the CTCA+ICA group.
CONCLUSIONS:
For patients with previous coronary artery bypass grafting, CTCA before ICA leads to reductions in procedure time and contrast-induced nephropathy, with improved patient satisfaction. CTCA before ICA should be considered in this group of patients.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03736018.
Keywords: coronary angiography, coronary artery bypass, myocardial ischemia
Clinical Perspective.
What Is New?
This study showed for the first time in a randomized clinical trial that in patients with previous coronary artery bypass grafting undergoing invasive coronary angiography, adjunctive computed tomography cardiac angiography improves patient safety, optimizes the angiographic procedure, and increases patient satisfaction.
What Are the Clinical Implications?
Upfront computed tomography cardiac angiography before invasive coronary angiography resulted in reduced procedure times, improved patient satisfaction, and lower incidence of contrast-induced nephropathy.
A lower incidence of procedural complications and clinical events out to 12 months was also seen.
The results suggest that computed tomography cardiac angiography should be considered before invasive coronary angiography in patients with previous coronary artery bypass grafting.
Coronary artery bypass grafting (CABG) is the most common cardiac procedure performed on adults in the developed world, with ≈250 000 patients undergoing the procedure each year in the United States.1 Despite advances in percutaneous coronary intervention, CABG has a major role in the management of patients with coronary artery disease, especially those with multivessel or left main stem disease.2 However, due to accelerated progression of native coronary artery disease after CABG and the high failure rates of saphenous vein grafts, ≈1 in 5 patients will require an invasive angiogram within 3 years of CABG, with up to 15% requiring further revascularization within 5 years.3,4
Invasive coronary angiography (ICA) in patients post-CABG, whilst remaining the gold standard for coronary and graft evaluation, is more challenging than in patients without grafts. An increased number of vessels to engage, variable location of bypass graft ostia, and often incomplete information available regarding the number and type of grafts placed lead to procedures lasting longer, high levels of contrast and radiation exposure, and an increased risk of complications (eg, stroke and contrast-induced nephropathy) compared with patients without previous CABG.5–10 The benefits of procedural developments in ICA have also been questioned, with the possibility of greater contrast use and procedure length with radial access compared with femoral in the post-CABG patient.11 Therefore, the development of techniques to facilitate safer and more efficient ICA is needed.
Computed tomography cardiac angiography (CTCA) is a useful clinical tool in the assessment of patients with previous CABG, providing a noninvasive evaluation of the number and location of bypass grafts, and being highly accurate at detecting graft stenoses, with sensitivity and specificity in excess of 95%.12,13 Previous observational studies have demonstrated the potential benefit of CTCA before ICA in reducing procedural time, contrast administration, and radiation exposure.14,15 BYPASS-CTCA (Randomised Controlled Trial to Assess Whether Computed Tomography Cardiac Angiography Can Improve Invasive Coronary Angiography in Bypass Surgery Patients) was designed to assess, in a randomized controlled trial (RCT), whether CTCA before ICA led to improved procedural metrics, safety, and patient satisfaction.
METHODS
Data Sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request and with approval from the trial steering committee.
Trial Design and Oversight
The trial design has been described previously.16 This study, a single-center RCT performed at St Bartholomew’s Hospital, the largest cardiac center in the United Kingdom, evaluated a strategy of CTCA before ICA in patients with previous CABG. The trial was approved by an independent ethics committee and supported by the Barts cardiovascular clinical trials unit.
Patients
Patients considered eligible were those ≥18 years of age with a history of previous CABG who had been referred for ICA and were able and willing to give written informed consent. Exclusion criteria were cardiac arrest, cardiogenic shock, ST-segment–elevation myocardial infarction (MI), chronic renal failure with an estimated glomerular filtration rate <20 mL/min, pregnancy, intolerance to CTCA (ie, contrast allergy or inability to tolerate beta-blockers), or a current life-threatening condition other than vascular disease that may prevent study completion. Eligible patients were approached either at their pre–angiography assessment visit (for elective patients) or on the ward before invasive angiography (acute patients). They were enrolled after giving written informed consent.
Randomization and Treatment
Patients were randomly assigned in a 1:1 ratio to a strategy of CTCA before ICA or ICA alone. Randomization was performed using an online electronic randomization system and was stratified by acute coronary syndrome (ACS) presentation. Block randomization was used with block size varied randomly. The allocation algorithm was written by the study statistician in Stata (Version 14) using the ralloc command.
Procedures
For patients allocated to CTCA, all CTCAs were performed using a third-generation dual-source computed tomography (CT) scanner (Somatom FORCE; Siemens). In elective ICA cases, CTCA was planned to be performed at least 2 weeks before ICA. For patients presenting with ACS, the CTCA and ICA were performed within 24 to 48 hours on the basis of scanner availability and clinical pathways. Although not mandated by the study protocol, heart rate control was achieved with the use of intravenous beta-blockers at the discretion of the radiographer and supervising cardiologist. Patients with atrial fibrillation were included in the study. All CTCA scans were reported by an independent accredited radiologist/cardiologist detailing the graft anatomy, ostial location, and presence of disease. All coronary angiograms were performed either by, or under the supervision of, an interventional cardiologist. The choice of vascular access and whether to cannulate patent bypass grafts on CTCA were left to the discretion of the operator, but it was recommended not to image grafts found to be occluded on CTCA.
Outcomes
The primary outcome was a coprimary end point consisting of ICA procedure duration (defined as the interval between local anesthesia administration for obtaining vascular access and removal of the last catheter), patient satisfaction scores after ICA (on the basis of a validated questionnaire), and the incidence of contrast-induced nephropathy (CIN; a ≥0.3 or ≥26.5 μmol/L increase in creatinine within 48 hours or ≥1.5× within 1 week as defined by the Kidney Disease Improving Global Outcomes criteria).17,18 Secondary end points included radial access rates, contrast amount (in milliliters) and radiation exposure administered during ICA, the number of catheters used during ICA, the number of grafts not identified during ICA, ICA-related complications (coronary or aortic dissection or periprocedural MI [Society for Cardiovascular Angiography & Interventions definition], stroke, bleeding, or vascular access complications), major adverse cardiac events (MACEs), and major adverse kidney events.19 MACE was defined as all-cause mortality, cardiac mortality, MI (not including periprocedural MI), and unscheduled revascularization. Major adverse kidney event was defined as all-cause mortality, new onset of renal replacement therapy, and persistent renal dysfunction (>50% increase from baseline creatinine).20
Statistical Analysis
The study was sized to ensure that each of the 3 co–primary end points was sufficiently powered. The primary end point requiring the largest sample size was CIN; 510 patients provides 80% power (significance level 0.05) to demonstrate a CIN reduction of 60%, assuming an estimated CIN incidence of 12% in the control arm. We applied the Bonferroni correction and used α=0.017 in the calculations. This gives a total sample size of 618, which was increased to 688 after accounting for dropouts.
The statistical analysis plan (available with the protocol) was finalized before any analysis by trial group assignment. Primary analyses were presented with 98.3% CIs, and P<0.017 was deemed to be statistically significant (to preserve an overall α=5% split over 3 co–primary end points using the Bonferroni method of adjustment). The main analysis of primary end points was conducted on an intention-to-treat population consisting of all those randomized who had available data regardless of which procedures they underwent. The primary outcome of CIN was analyzed using logistic regression; linear regression was used for procedural duration and patient satisfaction score. For all primary end point analyses, estimates were made unadjusted and adjusted for ACS and creatinine level at baseline. Prespecified subgroup analyses were performed on primary outcomes by incorporating and testing interaction terms into the models. In addition, a sensitivity analysis was conducted for the CIN end point excluding participants who did not undergo an ICA. Analyses of secondary outcomes were not adjusted for multiplicity. For the analysis for secondary end points, differences between trial groups were estimated using Cox proportional hazards models for survival outcomes, Poisson regression for count outcomes, linear regression for continuous outcomes, and logistic regression for binary outcomes. Secondary end points are presented with 95% CIs. For the MACE end point, a Kaplan-Meier plot was used to show cumulative incidence in the 2 treatment groups over 1 year of follow-up. All analyses were conducted with the use of Stata software, version 17.0 (StataCorp).
RESULTS
Patients
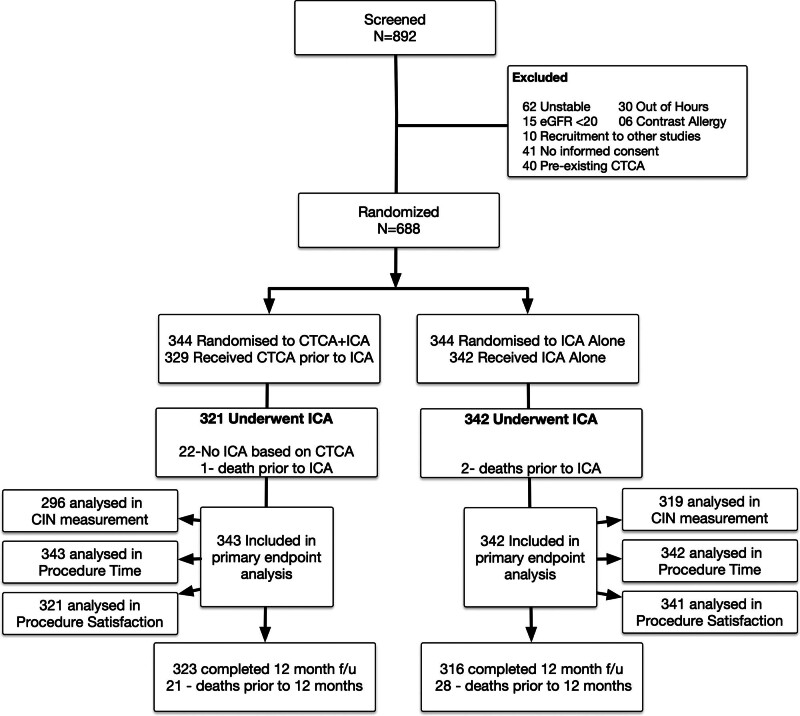
Between November 6, 2018, and August 23, 2021, 688 patients were randomized: 344 in the CTCA+ICA group, and 344 in the ICA-only group (Figure 1). In the CTCA group, 22 patients did not undergo ICA as a result of physician preference on the basis of the CTCA result, and one patient died before ICA. This meant that there were 321 patients in this group who underwent ICA (Figure 1). In the ICA-alone group, 2 patients died after randomization but before ICA, resulting in 342 patients in this group undergoing ICA (Figure 1). Patients were followed up for a median of 1.0 year (377 days).
Figure 1.
Study CONSORT (Consolidated Standards of Reporting Trials) diagram. CIN indicates contrast-induced nephropathy; CTCA, computed tomography cardiac angiography; eGFR, estimated glomerular filtration rate; and ICA, invasive coronary angiography.
Baseline characteristics are shown in Table 1. In the CTCA+ICA group, the median time from CTCA to ICA was 6.9 days (interquartile range [IQR], 0.2–63.0 days). Shorter times were seen in the ACS group (median, 0.3 days [IQR, 0.1–13.7 days]) compared with the elective group (median, 26.9 days [IQR, 3.1–91.8 days]).
Table 1.
Baseline Characteristics.
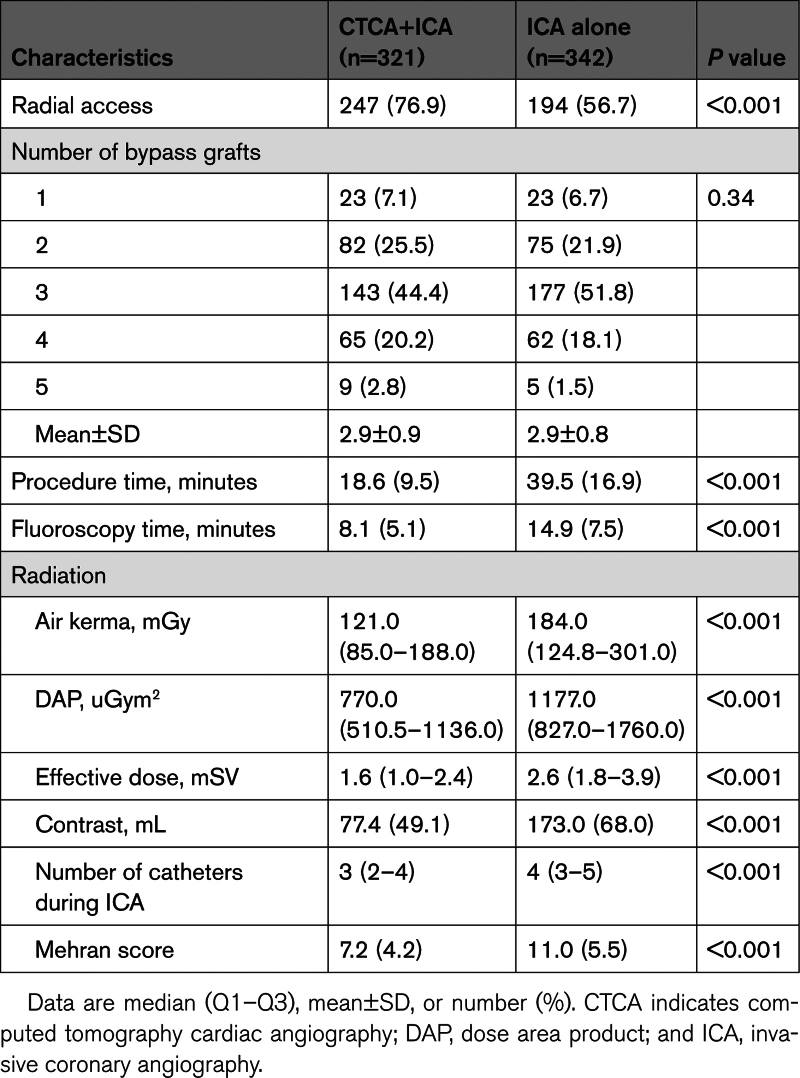
The overall median time from CABG to angiogram was 12.0 years (IQR, 5.7–19.2). In 24% of cases, the graft details were unknown, as the operation note was not available and there had been no subsequent angiogram, and 46.3% of patients had a previous coronary angiogram after CABG. The majority of patients (92.8%) had a left internal mammary artery graft, with arterial grafts comprising 34.2% of grafts overall and the remainder venous. The total number of grafts was similar between the 2 groups (2.9±0.9 in CTCA+ICA and 2.9±0.8 in ICA alone). In the CTCA+ICA group, 36.0% of grafts were patent or occluded on CTCA, so they were not invasively assessed, with one graft not found (0.3%). In the ICA-alone group, 19.1% of all grafts were not imaged either due to being known to be occluded (previous angiography) or being unable to locate at the time of angiography (Table 2). Aortography, performed with a pigtail catheter and 40 mL of contrast, was undertaken as part of invasive angiography in 1.2% of the CTCA+ICA group and 17.3% of the ICA-only group (P<0.001).
Table 2.
Invasive Coronary Angiography Procedural Data
Primary Outcomes
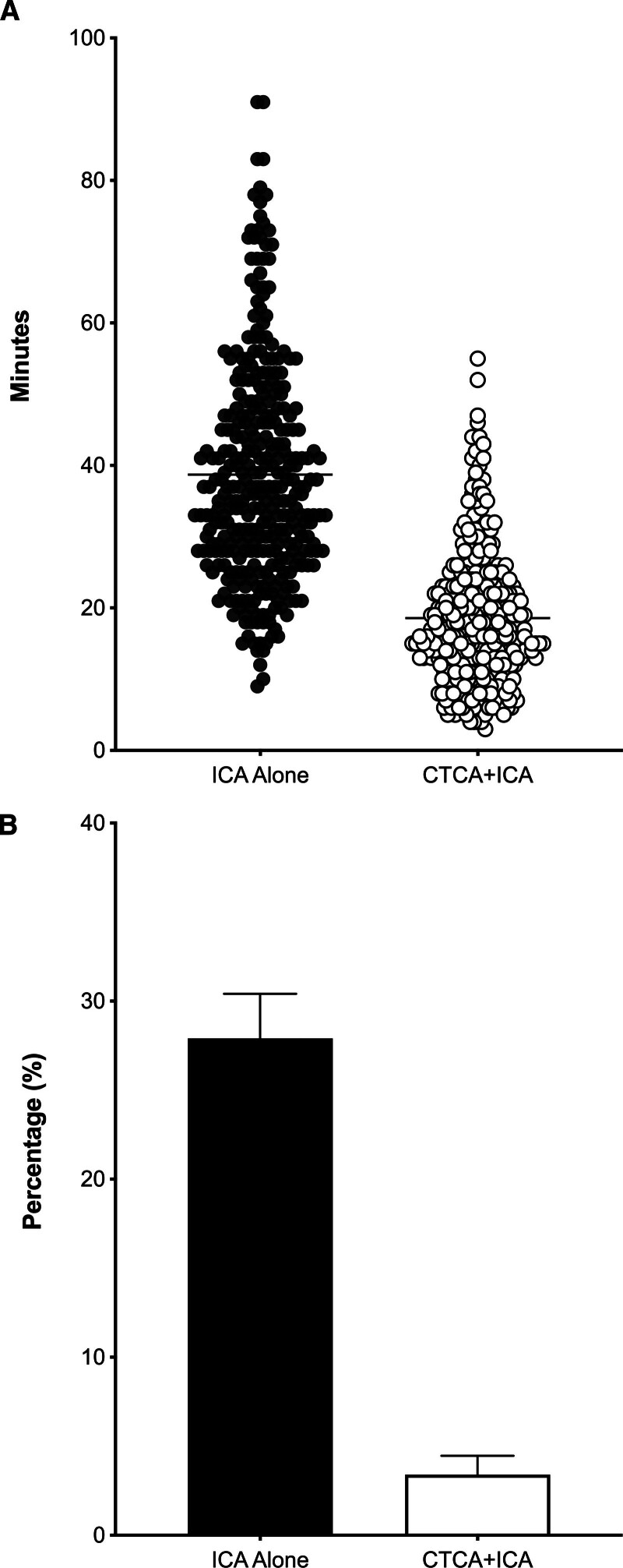
The mean ICA procedure duration was significantly reduced for patients in the CTCA+ICA group compared with patients undergoing ICA alone (CTCA+ICA, 18.6±9.5 minutes versus ICA alone, 39.5±16.9 minutes; P<0.001; Figure 2A). This was an unadjusted difference of −20.9 minutes (98.3% CI, −23.5 to −18.4), with no change seen after adjustment (−20.9 [95% CI, −23.5 to −18.4]; P<0.001). When comparing total procedure time (including percutaneous coronary intervention), procedure time remained significantly reduced in the CTCA+ICA arm, with a mean difference of 10.8 minutes (CTCA+ICA, 70.4±34.7 versus ICA, 81.2±36.4 [95% CI, −19.2 to −2.5]; P=0.01). When combining the CTCA and ICA procedure durations, there remained a significant reduction in the CTCA+ICA group compared with the ICA-alone group (22.1±10.5 minutes versus 39.5±16.9 minutes; P<0.001).
Figure 2.
Procedural duration and incidence of contrast-induced nephropathy. A, Violin plot of the procedural duration of invasive coronary angiography (ICA) for the 2 groups. Mean and SD are shown behind the scatterplots. B, Contrast-induced nephropathy incidence in both the treatment groups. CTCA indicates computed tomography cardiac angiography.
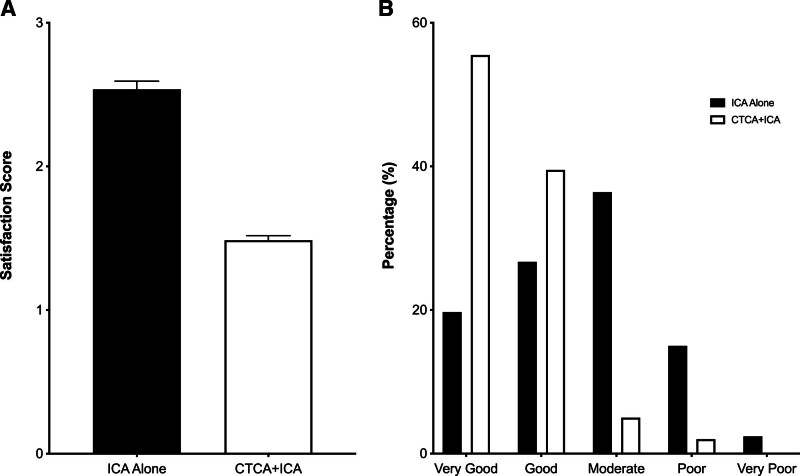
Patient angiography satisfaction questionnaires were completed for 662 patients (99.8%). Patient satisfaction scores (1=very good to 5=very poor) were significantly better in the CTCA+ICA group (1.5±0.6) compared with the ICA-alone group (2.5±1.0; Figure 3A), with a mean difference of −1.1 (98.33% CI, −1.2 to −0.9; P<0.001). In the CTCA+ICA group, 96% of the patients rated their overall satisfaction as very good or good, compared with only 46% of the ICA-alone group (Figure 3B). This benefit was seen consistently across all elements of the questionnaire (Table S3) and across subgroups (Table S4). Across the study, the mean satisfaction score was lower (ie, indicating greater satisfaction) among patients without a complication compared with those with a complication (1.98 versus 2.73; P<0.001). In the CTCA+ICA group, patient satisfaction with the CTCA scan was high, with 98% of patients rating their satisfaction as very good or good (Table S5).
Figure 3.
Patient satisfaction scores. A, Mean overall patient satisfaction score of the invasive coronary angiography (ICA) for both treatment groups. B, Breakdown of overall patient satisfaction for the 2 groups. CTCA indicates computed tomography cardiac angiography.
Postangiography renal function tests were available for 615 patients and demonstrated an overall CIN incidence of 16.1%. Incidence of CIN was significantly reduced in the CTCA+ICA group compared with the ICA-alone group (3.4% versus 27.9%; odds ratio, 0.09 [98.33% CI, 0.04–0.2]; P<0.001; Figure 2B). When patients in the CTCA+ICA group who underwent CTCA only (n=21) were included, the difference persisted, and was consistent across the subgroups (Table S6).
Secondary Outcomes
Regarding secondary outcomes (Table 2), the CTCA+ICA group had significantly higher radial access rates, a lower number of catheters used during ICA, reduction in fluoroscopy time, and a reduction in contrast used during ICA, which persisted even when adding the contrast used during CTCA (CTCA+ICA, 148.9±50.6 versus 173.0±68.0 mL; P<0.001). Percutaneous coronary intervention rates were comparable, with 139 patients (43.3%) in the CTCA+ICA group and 141 patients (41.2%) in the ICA group proceeding to percutaneous coronary intervention (Table S7).
Total effective dose received during ICA was significantly reduced in the CTCA+ICA group (median, 1.6 mSv [IQR, 1.0–2.4]) compared with the ICA-alone group (2.6 mSv [IQR, 1.8–3.9 mSv]; P<0.001). However, the median total effective dose (using a conversion factor of 0.017) for the CTCA was 5.8 mSv (IQR, 3–9.9 mSv), resulting in a combined radiation dose of 7.50 (IQR, 4.5–11.6 mSv) in the CTCA+ICA group, which was significantly greater than in the ICA-alone group (2.6; IQR, 1.8–3.9 mSv; P<0.001).
In the CTCA+ICA group, 99.7% of patients had complete diagnostic studies after ICA (one patient did not have a CTCA preprocedure due to logistical reasons), compared with only 75.7% in the ICA-alone group (P<0.001), in which the remaining had bypass grafts that were not evaluated or quantified at the time of ICA.
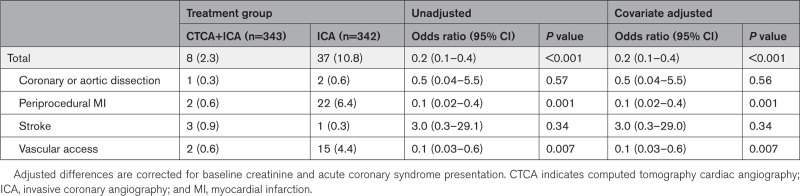
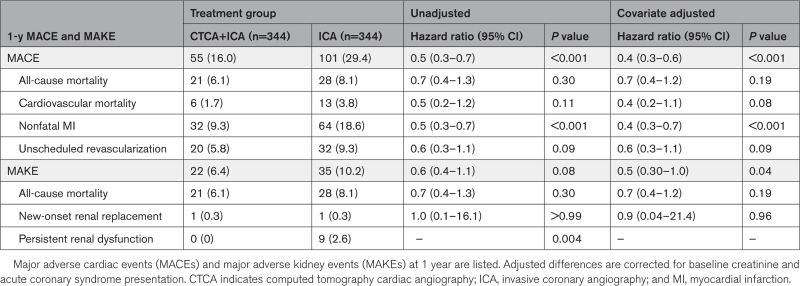
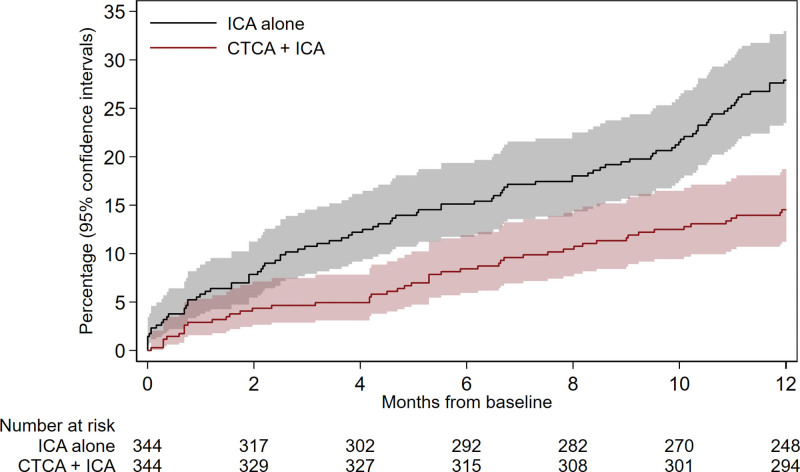
Procedural complication incidence was lower in the CTCA+ICA group (2.3% versus 10.8%; odds ratio, 0.2 [95% CI, 0.1 to 0.4]; P<0.001; Table 3), driven by reduced vascular access complications and periprocedural MI. There was a significant reduction in incidence of 1-year MACE in the CTCA+ICA group compared with the ICA group (16.0% to 29.4%; hazard ratio, 0.4 [95% CI, 0.3–0.6]; P<0.001), driven by reduced rates of spontaneous MI (Table 4; Figure 4). There was a significant reduction in 1-year incidence of major adverse kidney event in the CTCA+ICA group compared with the ICA group (6.4% versus 10.2%; hazard ratio, 0.6 [95% CI 0.3–0.97]; P=0.04), driven by reduced frequency of persistent renal dysfunction (Table 4).
Table 3.
Procedural Complications
Table 4.
Major Adverse Events
Figure 4.
Major adverse cardiac events at 12 months. The cumulative incidence (percentage of population) of major adverse cardiac events during the 12-month follow-up period was estimated by the Kaplan-Meier method; differences were tested using the log-rank test. CTCA indicates computed tomography cardiac angiography; and ICA, invasive coronary angiography.
DISCUSSION
In this RCT of patients with previous CABG undergoing ICA, CTCA before ICA resulted in reduced procedure times, increased patient satisfaction, and lower incidence of CIN compared with ICA alone. Upfront CTCA was also superior for several secondary end points, including reduced contrast dose, reduced ICA radiation exposure, and a lower number of angiography catheters used. Procedural complications were also reduced, with upfront CTCA providing safer procedures with subsequent improved clinical outcomes (MACE) for up to 1 year. This supports the routine use of adjunctive CTCA before ICA to facilitate safe and effective angiography and improve patient outcomes.
The study was designed to assess whether CTCA is a useful adjunct to planned ICA in patients with previous CABG. Previous observational studies have suggested upfront CTCA may reduce the exposure of patients to contrast, radiation, and the clinical risks of invasive procedures.14,15,21 A recently presented RCT (GREECE [Computed Tomography Coronary Angiography in Patients With a Previous Coronary Artery Bypass Graft Surgery Trial]) has provided some preliminary data.22,23 In GREECE, 153 patients with previous CABG and a clinical indication for coronary angiography were randomized to CTCA+ICA (n=84) or ICA alone (n=69). The study reported a primary end point of an increased total contrast volume in the CTCA+ICA arm compared with the ICA-alone arm (209 versus 165 mL; P=0.006), despite a similar incidence of CIN (16% versus 13.8%; P=0.71). Total procedure time (28.5 versus 38.4 minutes; P=0.02) and 30-day MACE (5% versus 16%; P=0.02) were lower in the CTCA arm. These conflicting results likely highlight the underpowered nature of the trial but also uncertainty with respect to full use of the CTCA in this group. Higher volumes of contrast use during ICA with previous CTCA are difficult to explain; however, as the full results are not yet published, it is difficult to draw detailed comparisons to explain the different results seen between GREECE and BYPASS-CTCA. However, the GREECE investigators did conclude that a larger trial with newer CT scanners could lead to a different outcome, which was the case in our study.22
The primary beneficial role of CTCA before ICA in patients with previous CABG is in providing information on the number and location of bypass grafts and, in particular, if they are patent or occluded. This potentially avoids graft-seeking and facilitates selective engagement during ICA. As expected, this allowed for lower volumes of contrast use during ICA, which then expectedly leads to lower CIN incidence.24 The prognostic significance of CIN has been debated in recent years, but there are emerging data that CIN after arterial contrast administration during angiography, especially in patients with preexisting renal dysfunction, is prognostically important.25–27 The CIN incidence of 27.9% in the control group (ICA-only) was higher than estimated in our assumptions, although, to our knowledge, no study has specifically reported CIN incidence after ICA in patients undergoing CABG. The incidence of CIN in the ICA-alone group corresponded to the average Mehran score of 11.0; however, even allowing for the higher volume of contrast, this was higher than in the CTCA group.24 We found that the effect was consistent when using the CIN criteria (≥25% or ≥0.5 mg/dL increase in creatinine at 48 hours) used in the Mehran model, with an incidence of 2.5% in the CTCA+ICA group and 24% in the ICA-alone group. The reduction in CIN remained in the ACS group despite the CTCA (and associated contrast load) often being performed on the same day (median 0.3). Whereas the increased incidence of CIN in the ICA-alone group was associated with persistent renal dysfunction, there was no increase in the incidence of need for renal replacement therapy.
Procedure times seen in the ICA-alone group in BYPASS-CTCA were comparable to the ICA-only group of GREECE (38.4 minutes), and with other series of patients after bypass (ranging from 21.9 to 60 minutes).11,22,28,29 The 18.6 minutes of ICA time in the CTCA group is shorter than in all of these aforementioned studies and suggests the use of the information provided specifically by CTCA led to this reduction. This reduction in ICA procedure time was likely because of the need for fewer grafts being invasively imaged at the time of ICA and identifying the location of those grafts that were deemed necessary to cannulate. Although invasive angiography procedure times were reduced, for the elective patients in the CTCA group, this was at the time expense of an extra hospital visit, which may be difficult or not preferable for some patients.
The degree of reduction seen in procedural complications in the CTCA group was surprising and driven by reduced vascular access complications and frequency of periprocedural MI. With knowledge of how many, if any, grafts needed to be engaged, those in the CTCA group had a higher frequency of radial access and therefore a lower frequency of vascular access complications.30 The reassurance of a patent left internal mammary artery during CTCA could mean increasing use of the right radial route in this cohort, avoiding the need for femoral access and avoiding invasive left internal mammary artery cannulation at the time of ICA, which correlates with the findings of L-RECORD (Left Radial Compared to Femoral Approach for Coronary Angiography in Patients With Previous CABG), in which left radial access was noninferior to femoral access for patients undergoing CABG when the anatomy was known.31 Radial access rates are increasing for ICA in patients with CABG, and although our reported rates (67% overall and 57% in ICA alone) are consistent with the available literature, in centers with higher radial access rates, the addition of CTCA may not reduce the incidence of complications to the same extent. The higher incidence of periprocedural MI in the ICA-alone group is likely because of the combination of increased procedure time, higher number of grafts being invasively cannulated, and higher contrast volumes, which may explain the benefits of CTCA in this regard.32
Despite the positive procedural benefits of CTCA, there were significantly higher total doses of radiation received by patients who underwent upfront CTCA. No safety signal was seen in relation to this during the 1-year follow-up; however, this has to be acknowledged as a limitation of the combined approach, with any long-term consequences not known. Radiation doses with newer CT scanners are likely to be reduced and the benefits at ICA may have been underappreciated at our institution on the basis of low frame rates and acquisition doses for invasive angiography; as a consequence, the higher combined metrics of CTCA and ICA may not be reflected at other centers.
The reduction in 1-year MACE with upfront CTCA seen in this study, driven by a reduction in MI, is of interest, although as the trial was not powered for this end point, this should be viewed as hypothesis-generating only and should therefore be the focus of future research. Despite this, there is evidence of improvement in multiple variables and outcomes in the CTCA group that may affect MACE events during follow-up: higher rates of radial access, reduced procedural complications, higher rates of full diagnostic studies, and therefore potentially complete revascularization, reduced incidence of CIN, and improved renal outcomes for up to 1 year.25,33 The synergistic benefits of these factors could explain these findings, and a similar signal was seen in the only other RCT assessing this question (GREECE); however, these findings require prospective validation in an adequately powered multicenter RCT.22
BYPASS-CTCA was a single-center study, and as such, the potential for application across other centers is uncertain. In particular, this approach will not be possible at centers with lower CTCA capacity and could delay angiography and revascularization (although not seen in this study), with a potential negative effect on outcomes. The use of newer CT scanners may have contributed to the positive results demonstrated in BYPASS-CTCA compared with GREECE and make the results less generalizable to centers with older scanners. The interpretation and application of information from CTCA also varies among clinicians. Even at this single center, where many operators perform the studies, differing practice occurs (eg, whether to reimage grafts shown to be patent or occluded on CTCA). The assumption that CTCA findings were accurate and grafts engagement at angiography was not mandated is a limitation of the study, although no safety concern was seen as far as MACEs. As discussed previously, the reduction in procedural complications in the CTCA group may not be found in centers with higher radial access rates. Although planned, the cost-effectiveness evaluation of CTCA in this setting has not been completed so no conclusions around this can currently be made. By its nature, the trial was open-label, which may have affected subjective end points (eg, patient satisfaction), although these subjective end points were assessed by individuals blinded to the patient allocation.
Conclusions
Invasive coronary angiography remains the gold standard for evaluation of both native coronary arteries and grafts; however, in patients with previous CABG, it is technically more challenging and is associated with a higher risk of complications. This study has shown that the use of upfront CTCA before ICA leads to reduced procedure time, improved patient satisfaction, and reduced incidence of CIN. CTCA before ICA, when logistically possible, should be considered for this group of patients.
ARTICLE INFORMATION
Sources of Funding
This project is funded by the National Institute for Health and Care Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-1216-20028). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Disclosures
Dr Pugliese receives institutional research support from Siemens Healthineers. The other authors report no conflicts of interest.
Supplemental Material
BYPASS-CTCA Investigators, Committees, and Collaborators
Tables S1–S7
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACS
- acute coronary syndrome
- BYPASS-CTCA
- Randomised Controlled Trial to Assess Whether Computed Tomography Cardiac Angiography Can Improve Invasive Coronary Angiography in Bypass Surgery Patients
- CABG
- coronary artery bypass grafting
- CIN
- contrast-induced nephropathy
- CT
- computed tomography
- CTCA
- computed tomography cardiac angiography
- GREECE
- Computed Tomography Coronary Angiography in Patients With a Previous Coronary Artery Bypass Graft Surgery Trial
- ICA
- invasive coronary angiography
- IQR
- interquartile range
- L-RECORD
- Left Radial Compared to Femoral Approach for Coronary Angiography in Patients With Previous CABG
- MACE
- major adverse cardiac event
- MI
- myocardial infarction
- RCT
- randomized controlled trial
A complete list of BYPASS-CTCA collaborators is provided in the Supplemental Appendix.
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.064465.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 1379.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Anne-Marie Beirne, Email: anne-marie.beirne@nhs.net.
Matthew Kelham, Email: m.kelham@nhs.net.
Krishnaraj S. Rathod, Email: k.s.rathod@qmul.ac.uk.
Mervyn Andiapen, Email: mervyn.andiapen@nhs.net.
Lucinda Wynne, Email: lucinda.wynne@nhs.net.
Thomas Godec, Email: tom.godec@closedloopmedicine.com.
Nasim Forooghi, Email: nasim.forooghi@nhs.net.
Rohini Ramaseshan, Email: rohini.ramaseshan@nhs.net.
James C. Moon, Email: j.moon@ucl.ac.uk.
Ceri Davies, Email: ceri.davies8@nhs.net.
Christos V. Bourantas, Email: cbourantas@gmail.com.
Andreas Baumbach, Email: a.baumbach@qmul.ac.uk.
Charlotte Manisty, Email: c.manisty@ucl.ac.uk.
Andrew Wragg, Email: andrew.wragg2@nhs.net.
Amrita Ahluwalia, Email: a.ahluwalia@qmul.ac.uk.
Francesca Pugliese, Email: f.pugliese@qmul.ac.uk.
Anthony Mathur, Email: a.mathur@qmul.ac.uk.
REFERENCES
- 1.Alkhouli M, Alqahtani F, Kalra A, Gafoor S, Alhajji M, Alreshidan M, Holmes DR, Lerman A. Trends in characteristics and outcomes of patients undergoing coronary revascularization in the United States, 2003-2016. JAMA Netw Open. 2020;3:e1921326. doi: 10.1001/jamanetworkopen.2019.21326 [DOI] [PubMed] [Google Scholar]
- 2.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. ; Writing Committee Members. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:197–215. doi: 10.1016/j.jacc.2021.09.005 [DOI] [PubMed] [Google Scholar]
- 3.Head SJ, Milojevic M, Taggart DP, Puskas JD. Current practice of state-of-the-art surgical coronary revascularization. Circulation. 2017;136:1331–1345. doi: 10.1161/CIRCULATIONAHA.116.022572 [DOI] [PubMed] [Google Scholar]
- 4.Kwiecinski J, Tzolos E, Fletcher AJ, Nash J, Meah MN, Cadet S, Adamson PD, Grodecki K, Joshi N, Williams MC, et al. Bypass grafting and native coronary artery disease activity. JACC Cardiovasc Imaging. 2022;15:875–887. doi: 10.1016/j.jcmg.2021.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delewi R, Hoebers LP, Råmunddal T, Henriques JP, Angerås O, Stewart J, Robertsson L, Wahlin M, Petursson P, Piek JJ, et al. Clinical and procedural characteristics associated with higher radiation exposure during percutaneous coronary interventions and coronary angiography. Circ Cardiovasc Interv. 2013;6:501–506. doi: 10.1161/CIRCINTERVENTIONS.113.000220 [DOI] [PubMed] [Google Scholar]
- 6.Werner N, Bauer T, Hochadel M, Zahn R, Weidinger F, Marco J, Hamm C, Gitt AK, Zeymer U. Incidence and clinical impact of stroke complicating percutaneous coronary intervention: results of the Euro Heart Survey Percutaneous Coronary Interventions registry. Circ Cardiovasc Interv. 2013;6:362–369. doi: 10.1161/CIRCINTERVENTIONS.112.000170 [DOI] [PubMed] [Google Scholar]
- 7.Sanmartin M, Cuevas D, Moxica J, Valdes M, Esparza J, Baz JA, Mantilla R, Iñiguez A. Transradial cardiac catheterization in patients with coronary bypass grafts: feasibility analysis and comparison with transfemoral approach. Catheter Cardiovasc Interv. 2006;67:580–584. doi: 10.1002/ccd.20633 [DOI] [PubMed] [Google Scholar]
- 8.Nilsson T, Lagerqvist B, Tornvall P. Coronary angiography of patients with a previous coronary artery by-pass operation is associated with a three times increased risk for neurological complications: a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Scand Cardiovasc J. 2009;43:374–379. doi: 10.1080/14017430902842575 [DOI] [PubMed] [Google Scholar]
- 9.Xenogiannis I, Tajti P, Hall AB, Alaswad K, Rinfret S, Nicholson W, Karmpaliotis D, Mashayekhi K, Furkalo S, Cavalcante JL, et al. Update on cardiac catheterization in patients with prior coronary artery bypass graft surgery. JACC Cardiovasc Interv. 2019;12:1635–1649. doi: 10.1016/j.jcin.2019.04.051 [DOI] [PubMed] [Google Scholar]
- 10.Gobel FL, Stewart WJ, Campeau L, Hickey A, Herd JA, Forman S, White CW, Rosenberg Y. Safety of coronary arteriography in clinically stable patients following coronary bypass surgery: post CABG clinical trial investigators. Cathet Cardiovasc Diagn. 1998;45:376–381. doi: 10.1002/(sici)1097-0304(199812)45:4<376::aid-ccd5>3.0.co;2-x [DOI] [PubMed] [Google Scholar]
- 11.Michael TT, Alomar M, Papayannis A, Mogabgab O, Patel VG, Rangan BV, Luna M, Hastings JL, Grodin J, Abdullah S, et al. A randomized comparison of the transradial and transfemoral approaches for coronary artery bypass graft angiography and intervention: the RADIAL-CABG Trial (Radial versus Femoral Access for Coronary Artery Bypass Graft Angiography and Intervention). JACC Cardiovasc Interv. 2013;6:1138–1144. doi: 10.1016/j.jcin.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 12.Barbero U, Iannaccone M, d’Ascenzo F, Barbero C, Mohamed A, Annone U, Benedetto S, Celentani D, Gagliardi M, Moretti C, et al. 64 slice-coronary computed tomography sensitivity and specificity in the evaluation of coronary artery bypass graft stenosis: a meta-analysis. Int J Cardiol. 2016;216:52–57. doi: 10.1016/j.ijcard.2016.04.156 [DOI] [PubMed] [Google Scholar]
- 13.Mushtaq S, Conte E, Pontone G, Pompilio G, Guglielmo M, Annoni A, Baggiano A, Formenti A, Mancini ME, Muscogiuri G, et al. Interpretability of coronary CT angiography performed with a novel whole-heart coverage high-definition CT scanner in 300 consecutive patients with coronary artery bypass grafts. J Cardiovasc Comput Tomogr. 2020;14:137–143. doi: 10.1016/j.jcct.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 14.Plessis J, Warin Fresse K, Cahouch Z, Manigold T, Letocart V, Le Gloan L, Guyomarch B, Guerin P. Value of image fusion in coronary angiography for the detection of coronary artery bypass grafts. J Am Heart Assoc. 2016;5:e002233. doi: 10.1161/JAHA.115.002233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones DA, Castle EV, Beirne AM, Rathod KS, Treibel TA, Guttmann OP, Moon JC, Smith EJ, Bourantas CV, Davies LC, et al. Computed tomography cardiac angiography for planning invasive angiographic procedures in patients with previous coronary artery bypass grafting. EuroIntervention. 2020;15:e1351–e1357. doi: 10.4244/EIJ-D-18-01185 [DOI] [PubMed] [Google Scholar]
- 16.Beirne AM, Rathod KS, Castle E, Andiapen M, Richards A, Bellin A, Hammond V, Godec T, Moon JC, Davies C, et al. The BYPASS-CTCA study: the value of computed tomography cardiac angiography (CTCA) in improving patient-related outcomes in patients with previous bypass operation undergoing invasive coronary angiography: study protocol of a randomised controlled trial. Ann Transl Med. 2021;9:1395. doi: 10.21037/atm-21-1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangelsdorff AD. Patient satisfaction questionnaire. Med Care. 1979;17:86–90. doi: 10.1097/00005650-197901000-00008 [DOI] [PubMed] [Google Scholar]
- 18.Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W; Ad-hoc working group of ERBP. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–4272. doi: 10.1093/ndt/gfs375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62:1563–1570. doi: 10.1016/j.jacc.2013.08.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Mor MK, Palevsky PM, Kaufman JS, Thiessen Philbrook H, Weisbord SD, Parikh CR. Postangiography increases in serum creatinine and biomarkers of injury and repair. Clin J Am Soc Nephrol. 2020;15:1240–1250. doi: 10.2215/CJN.15931219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krysztofiak T, Ahmad F, Adams J, Stobo DB, Good R, Byrne J. The value of non-invasive computed tomography coronary angiography in imaging patients with coronary artery bypass grafts. Scott Med J. 2020;65:76–80. doi: 10.1177/0036933020936274 [DOI] [PubMed] [Google Scholar]
- 22.Tsigkas G. Computed Tomography Guided Invasive Coronary Angiography in Patients With a Previous Coronary Artery Bypass Graft Surgery trial (GREECE trial). Presented at EuroPCR 2022; Paris, France; May 17–20, 2022. [DOI] [PubMed] [Google Scholar]
- 23.Tsigkas G, Apostolos A, Synetos A, Latsios G, Toutouzas K, Xenogiannis I, Hamilos M, Sianos G, Ziakas A, Tsiafoutis I, et al. ; GREECE Collaborators. Computed Tomography Guided Invasive Coronary Angiography in Patients With a Previous Coronary Artery Bypass Graft Surgery trial (GREECE trial): rationale and design of a multicenter, randomized control trial. Hellenic J Cardiol. 2021;62:470–472. doi: 10.1016/j.hjc.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068 [DOI] [PubMed] [Google Scholar]
- 25.Mohebi R, Karimi Galougahi K, Garcia JJ, Horst J, Ben-Yehuda O, Radhakrishnan J, Chertow GM, Jeremias A, Cohen DJ, Cohen DJ, et al. Long-term clinical impact of contrast-associated acute kidney injury following PCI: an ADAPT-DES substudy. JACC Cardiovasc Interv. 2022;15:753–766. doi: 10.1016/j.jcin.2021.11.026 [DOI] [PubMed] [Google Scholar]
- 26.Schönenberger E, Martus P, Bosserdt M, Zimmermann E, Tauber R, Laule M, Dewey M. Kidney injury after intravenous versus intra-arterial contrast agent in patients suspected of having coronary artery disease: a randomized trial. Radiology. 2019;292:664–672. doi: 10.1148/radiol.2019182220 [DOI] [PubMed] [Google Scholar]
- 27.Davenport MS, Perazella MA, Nallamothu BK. Contrast-induced acute kidney injury and cardiovascular imaging: danger or distraction? Circulation. 2023;147:847–849. doi: 10.1161/CIRCULATIONAHA.122.062783 [DOI] [PubMed] [Google Scholar]
- 28.Amro A, Mansoor K, Amro M, Hirzallah H, Sobeih A, Kusmic D, Abuhelwa Z, Kanbour M, Elhamdani A, Aqtash O, et al. Transradial versus transfemoral approach for coronary angiography in females with prior bypass surgery. Cureus. 2020;12:e6797. doi: 10.7759/cureus.6797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pingpoh C, Siepe M, Burger K, Zietak T, Valina CM, Ferenc M, Beyersdorf F, Neumann F-J, Hochholzer W. Impact of proximal radiopaque coronary bypass graft markers on postbypass surgery coronary angiography. J Thorac Cardiovasc Surg. 2018;155:1565–1572. doi: 10.1016/j.jtcvs.2017.12.043 [DOI] [PubMed] [Google Scholar]
- 30.Nikolakopoulos I, Vemmou E, Xenogiannis I, Karacsonyi J, Rao SV, Romagnoli E, Tsigkas G, Milkas A, Velagapudi P, Alaswad K, et al. Radial versus femoral access in patients with coronary artery bypass surgery: frequentist and Bayesian meta-analysis. Catheter Cardiovasc Interv. 2022;99:462–471. doi: 10.1002/ccd.30010 [DOI] [PubMed] [Google Scholar]
- 31.Tsigkas G, Makris A, Tsiafoutis I, Koutouzis M, Hamilos M, Katsanos K, Ziakas A, Brilakis ES, Davlouros P, Hahalis G. The L-RECORD study. JACC Cardiovasc Interv. 2020;13:1014–1016. doi: 10.1016/j.jcin.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 32.Abu Arab T, Rafik R, El Etriby A. Efficacy and safety of local intracoronary drug delivery in treatment of no-reflow phenomenon: a pilot study. J Interv Cardiol. 2016;29:496–504. doi: 10.1111/joic.12318 [DOI] [PubMed] [Google Scholar]
- 33.Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, Rubartelli P, Briguori C, Andò G, Repetto A, et al. ; MATRIX Investigators. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–2476. doi: 10.1016/S0140-6736(15)60292-6 [DOI] [PubMed] [Google Scholar]