Abstract
Background
Vitamin D deficiency has been linked with several adverse maternal and fetal outcomes.
Objective
To summarize systematic reviews and meta-analyses evaluating the effects of vitamin D deficiency and of vitamin D supplementation in pregnancy on maternal and offspring health-related outcomes.
Methods
Prior to conducting this umbrella review, we registered the protocol in PROSPERO (CRD42022368003). We conducted searches in PubMed, Embase, and Cochrane Library for systematic reviews and meta-analyses on vitamin D in pregnancy, from database inception to October 2, 2023. All outcomes related to vitamin D in pregnancy obtained from the systematic reviews and meta-analyses were extracted. Data Extraction: Two reviewers independently chose studies and collected information on health outcomes. The quality of the included articles’ methodology was assessed using AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews–2).
Results
We identified 16 eligible systematic reviews and meta-analyses, which included 250,569 women. Our results demonstrated that vitamin D deficiency in pregnancy is associated with increased risk of preterm birth, small-for gestational age/low birth weight infants, recurrent miscarriage, bacterial vaginosis and gestational diabetes mellitus. Vitamin D supplementation in pregnancy increases birth weight, and reduces the risk of maternal pre-eclampsia, miscarriage, and vitamin D deficiency, fetal or neonatal mortality, as well as attention-deficit hyperactivity disorder, and autism spectrum disorder in childhood. In women with gestational diabetes mellitus, vitamin D supplementation in pregnancy can reduce the risk of maternal hyperbilirubinemia, polyhydramnios, macrosomia, fetal distress, and neonatal hospitalization.
Conclusion
Due to the association with adverse maternal and offspring health outcomes, we recommend the vitamin D status in pregnancy should be monitored, particularly in women at high risk of vitamin D deficiency. It is suggested that pregnant women take a dose of >400 IU/day of vitamin D supplementation during pregnancy to prevent certain adverse outcomes.
Subject terms: Diseases, Health care
Background
Vitamin D is a fat soluble steroid hormone important in the homeostasis of multiple organs [1]. In addition to regulating phosphorus and calcium levels and promoting bone mineralization, it has extraskeletal functions in cardiovascular, metabolic, respiratory and immune systems [2–6]. Vitamin D deficiency is reemerging with high-risk groups including people with little sunshine exposure, people with darker skin and pregnant women [7].
During pregnancy, the body undergoes significant physiological change, including requirements and metabolism of vitamin D. Vitamin D deficiency in pregnancy is a common problem worldwide. The prevalence varies between 5% to 90% depending on the country [8–10]. Vitamin D deficiency has been linked with several adverse maternal and fetal outcomes [11–13]. For example, hypertensive disorders of pregnancy, gestational diabetes mellitus (GDM), preterm birth, and low birthweight [14].
To date, several meta-analyses have been conducted to investigate the associations between vitamin D status during pregnancy and maternal and offspring outcomes [15, 16]. Additionally, many meta-analyses have examined the impact of vitamin D supplementation during pregnancy on these outcomes [17, 18]. An umbrella review employs systematic review methods to collect the current high-level evidence from meta-analyses on a specific topic [19]. However, the evidence regarding to vitamin D status during pregnancy associated with maternal and offspring outcomes has not been well-organized using an umbrella review.
To provide clinicians, researchers and policy makers an overview of the complete body of evidence, we synthesized the findings, quality and certainty of systematic reviews and meta-analyses evaluating the effects of vitamin D deficiency and of vitamin D supplementation in pregnancy on maternal and offspring health-related outcomes.
Method
Protocol and registration
Prior to conducting this umbrella review, we registered the protocol in PROSPERO (CRD42022368003) (Tables S1 and S2).
Search methods
Three electronic databases (PubMed, Embase, and Cochrane Library) were searched for relevant systematic reviews and meta-analyses investigating the effects of vitamin D in pregnancy on maternal and infant health from inception to October 2 2023. The complete search strategy is displayed in Table S3. Initial deduplication was performed using EndNote 20, followed by title/abstract screening. Finally, full-text screening was performed to determine whether the remaining meta-analyses met our inclusion criteria. Two authors (C.L.S and P.W.) independently conducted title/abstract screening. Any disagreement was resolved through discussion. In addition, we manually searched the reference lists of the included systematic reviews and meta-analyses or related reviews to identify potential meta-analyses for inclusion.
Eligibility criteria
We used the PECOS (P: population, E: exposure, C: comparison, O: outcomes, and S: study design) framework to determine the inclusion criteria. The criteria were as follows: (1) population: pregnant women; (2) exposure: intake of vitamin D or measured vitamin D levels; (3) comparison: authors defined either a low level of vitamin D supplementation, placebo, no supplementation, or low measured vitamin D levels; (4) outcomes: all health-related outcomes; (5) study design: meta-analyses of randomized controlled or observational studies. If any meta-analyses overlapped with more than one similar outcome, we included the newest meta-analysis and if they were published in the same year, we included the meta-analysis with the largest number of studies in our review. The exclusion criteria were as follows: (1) conference abstract; (2) animal study; (3) meta-analyses not from systematic reviews; and (3) meta-analyses that were not published in English or Chinese.
Data extraction
We extracted the following information from the included meta-analysis: name of first author, publication year, type of clinical outcome, study design, number of studies in each meta-analysis, total sample size, effect sizes (OR: odds ratio, RR: relative risk, MD: mean difference, and SMD: standardized mean difference). One author (C.L.S.) conducted the data extraction, and another author (P.W.) checked the data. Any disagreement was resolved through consensus or by consulting a third reviewer (C.Y.H.).
The pooled estimates with the 95% confidence interval (CI) of clinical outcomes were extracted from each included meta-analysis. The corresponding evidence of heterogeneity (I2 value) was also extracted from the meta-analysis. Moreover, if available, publication bias assessed by Egger’s test or Begg’s test was obtained from the included meta-analysis.
Assessment of quality and evidence
A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR 2) was utilized to assess each meta-analysis’s metrological quality [20]. The instrument has the capability to categorize every meta-analysis’s quality into four groups: high (containing no or one non-critical weakness), moderate (containing multiple non-critical weaknesses), low (containing one critical flaw from protocol registration, adequate literature search, rationale for excluding studies, risk of bias evaluated for every included study, suitable meta-analytical approach, taking into account risk of bias when interpreting review results, and evaluation of publication bias), and critically low (containing multiple critical flaws). Moreover, we used a modified criteria to evaluate the quality of evidence for the meta-analysis [21]. The criteria adopted include the number of included studies, heterogeneity, and differences in results between the two study designs (randomized controlled trials (RCTs) and observational studies) in the same meta-analysis. In general, the quality of evidence is classified into four levels, from the highest (level 1: convincing) to the lowest (level 4: limited/contrasting).
Results
Search results
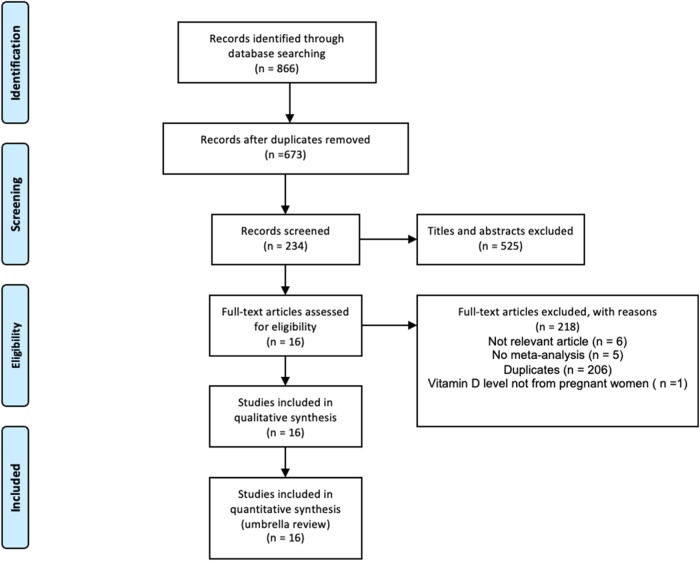
The process of literature search and study selection is shown in Fig. 1. A total of 866 meta-analyses were initially identified from the searches. Following deduplication, 673 meta-analyses remained, which underwent title and abstract screening with 123 meta-analyses selected for full-text screening. Finally, 16 meta-analyses met the inclusion criteria for this umbrella review [15–18, 22–34].
Fig. 1. Selection process of relevant articles.
Three electronic databases were searched for relevant articles. Initial deduplication was performed using EndNote 20, followed by title/abstract screening. Finally, full-text screening was performed to determine whether the remaining meta-analyses met our inclusion criteria.
Study characteristics
Total number of studies included in each included meta-analysis ranged from 4 to 54. The total number of participants for each included meta-analysis ranged from 1,465 to 67,484. Among them, 9 meta-analyses focused on observational studies and reported 16 specific outcomes. The other 7 meta-analyses focused on randomized controlled trials. Of those, 31 specific outcomes were obtained from pregnant women, while 18 specific outcomes were obtained from pregnant women with GDM (Table 1).
Table 1.
Characteristics of the included meta-analyses.
| OBS | First author | Publication year | Study design | No. of studies in MA | No of sample size (individual) | Outcomes | AMSTAR 2 |
|---|---|---|---|---|---|---|---|
| 1 | Tirani [15] | 2023 | Observation | 5 | 9125 | Offspring autism spectrum disorder and offspring attention-deficit hyperactivity disorder | Critically low |
| 2 | Wu [17] | 2023 | RCT | 20 | 1682 | 25(OH)D, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, preterm birth, hyperbilirubinemia, and neonatal hospitalization | Critically low |
| 3 | Rouhani [34] | 2023 | Observation | 32 | 76,394 | Pre-eclampsia | Critically low |
| 4 | Liu [22] | 2022 | RCT | 42 | 11,082 | Fetal or neonatal mortality, preterm birth, birth length, birth head circumference, and birth weight | Critically low |
| 5 | Chen [23] | 2022 | Observation | 14 | 3029 | Recurrent miscarriage | Critically low |
| 6 | Luo [25] | 2022 | RCT | 23 | 5390 | Femur length in the third trimester, humeral length in the third trimester, neonatal WB-BMC, neonatal WB-BMD, and neonatal WB-BA | Critically low |
| 7 | Luo [26] | 2022 | RCT | 7 | 3737 | Asthma or wheeze, eczema, allergic rhinitis, and lower respiratory tract infection for infants | Critically low |
| 8 | Ma [16] | 2022 | Observation | 13 | 4793 | Bacterial vaginosis | Critically low |
| 9 | Motamed [18] | 2022 | RCT | 17 | 1465 | 25(OH)D, hs-CRP, total antioxidant capacity, malondialdehyde, and glutathione | Critically low |
| 10 | Irwinda [27] | 2022 | RCT | 27 | 7567 | Pre-eclampsia, gestational diabetes mellitus, preterm birth, and birth weight | Critically low |
| 11 | Fatima [28] | 2022 | Observation | 44 | 37,838 | Gestational diabetes mellitus | Critically low |
| 12 | Tamblyn [29] | 2022 | Observation | 10 | 7663 | Miscarriage risk | Critically low |
| 13 | Fang [30] | 2021 | Observation | 16 | 8403 | Low birth weight and birth weight | Critically low |
| 14 | Wang [31] | 2021 | RCT | 19 | 1550 | Fasting plasma glucose, fasting insulin level, HOMA-IR, Caesarean section rate, maternal hospitalization rate, postpartum hemorrhage, hyperbilirubinemia, macrosomia, polyhydramnios, fetal distress, preterm birth, and hypoglycemia | Critically low |
| 15 | Tous [32] | 2020 | Observation | 54 | 67,484 | Birth length, birth head circumference, birth weight, preterm birth, and small-for-gestational-age | Critically low |
| 16 | Kang [33] | 2020 | Observation | 4 | 3367 | 25(OH)D for pregnant women | Critically low |
RCT randomized controlled trial, MA meta-analysis, AMSTAR 2 A Measurement Tool to Assess Systematic Reviews 2, 25(OH)D 25-hydroxyvitamin D, WB-BMC whole body bone mineral content, WB-BMD whole body bone mineral density, WB-BA whole body bone area, hs-CRP high sensitivity C-reactive protein, HOMA-IR homeostasis model assessment of insulin resistance.
Assessment of quality and evidence
The metrological quality of each meta-analysis was assessed using AMSTAR 2. All meta-analyses received a grade of “critically low” because more than one critical flaw was detected for each meta-analysis (Table 1). The quality of evidence of the specific outcomes which demonstrated statistically significant difference was assessed. Among the 16 specific outcomes derived from observational studies, most of them (13/16) were positively correlated with vitamin D level during pregnancy (Table S4). Eleven out of these twelve outcomes were graded as “possible” (level 3) due to high heterogeneity (I2 > 50%), while two were graded as “probable” (level 2) due to low heterogeneity. Among the 31 specific outcomes derived from RCTs, only a few outcomes (11/31) were positively correlated with vitamin D supplementation (Table S5). Nine of these eleven outcomes were graded as “convincing” (level 1) but two of them were graded as “limited” (level 4) due to the small number of meta-analyses with these outcomes (n ≤ 3) (Table S6).
Vitamin D in pregnancy related to health outcomes in observational studies
For maternal outcomes, a low level of vitamin D ( < 50 nmol/L), significantly increased the risk of preterm birth (OR = 1.28; 95% CI = 1.08–1.52; number of studies = 21; Table 2A) [32], miscarriage (OR = 1.60; 95% CI = 1.11–2.30; number of studies = 6; Table 2A) [29], and small-for-gestational-age infants (OR = 1.43; 95% CI = 1.08–1.91; number of studies = 19; Table 2B) [32]. Vitamin D deficiency also increased the risk of GDM (OR = 1.38; 95% CI = 1.22 – 1.57; number of studies = 31) [28], recurrent miscarriage (OR = 4.02; 95% CI = 2.23–7.25; number of studies = 12) [23], and bacterial vaginosis (OR = 1.54; 95% CI = 1.25–1.91; number of studies = 14) (Table 2A) [16]. The highest level of vitamin D had a significant lower risk of preeclampsia compared with the lowest level (RR = 0.68; 95% CI = 0.55–0.85; number of studies = 29; Table 2A) [34].
Table 2.
Summary of meta-analyses of observational studies on vitamin D in pregnancy. (A) Maternal health. (B) Offspring health.
| (A) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | First author | Year | No. of studies in MA | Comparator (vitamin D supplementation or level) | Effects model | Metric of MA | Effect size | 95% CI | I2 (%) | Publication bias |
| Preterm birth | Tous | 2020 | 11 | low level < 30 vs high level ≥ 30 (nmol/L) | random-effect | OR | 1.16 | (0.83 ~ 1.62) | 68 | NA |
| Preterm birth | Tous | 2020 | 21 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | OR | 1.28 | (1.08 ~ 1.52)* | 61 | NA |
| Preterm birth | Tous | 2020 | 10 | low vit D < 75 vs high vit D ≥ 75 (nmol/L) | random-effect | OR | 1.18 | (0.91 ~ 1.54) | 72 | NA |
| Gestational diabetes mellitus | Fatima | 2022 | 31 | vitamin D deficiency vs normal | random-effect | OR | 1.38 | (1.22 ~ 1.57)* | 49 | NA |
| Pre-eclampsia | Rouhani | 2023 | 29 | highest vs lowest circulating 25(OH)D levels | random-effect | RR | 0.68 | (0.55 ~ 0.85)* | 98 | 0.65 |
| Recurrent miscarriage | Chen | 2022 | 12 | vitamin D deficiency vs normal | random-effect | OR | 4.02 | (2.23 ~ 7.25)* | 82 | NA |
| Miscarriage risk | Tamblyn | 2022 | 4 | low level <50 vs high level ≥ 75 (nmol/L) | random-effect | OR | 1.94 | (1.25 ~ 3.02)* | 18 | NA |
| Miscarriage risk | Tamblyn | 2022 | 6 | low level ≤ 75 vs high level > 75 (nmol/L) | random-effect | OR | 1.60 | (1.11 ~ 2.30)* | 35 | NA |
| Bacterial vaginosis | Ma | 2022 | 14 | vitamin D deficiency vs normal | random-effect | OR | 1.54 | (1.25 ~ 1.91)* | 85 | 0.005 |
| 25(OH)D | Kang | 2020 | 8 | type 1 diabetes in childhood vs normal | random-effect | MD | -2.54 | (-4.65 ~ -0.44)* | 52 | 0.04 |
| (B) | ||||||||||
| Birth length | Tous | 2020 | 4 | low level < 30 vs high level ≥ 30 (nmol/L) | random-effect | MD | -0.28 cm | (-0.66 ~ 0.10) | 69 | NA |
| Birth length | Tous | 2020 | 7 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | MD | 0.12 cm | (-0.09 ~ 0.33) | 63 | NA |
| Low birth weight (< 2500 g) | Fang | 2021 | 8 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | OR | 2.39 | (1.25 ~ 4.57)* | 81 | NA |
| Birth weight | Tous | 2020 | 15 | low level < 30 vs high level ≥ 30 (nmol/L) | random-effect | MD | -87.82 g | (-119.73 ~ -55.91)* | 58 | NA |
| Birth weight | Tous | 2020 | 13 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | MD | -19.27 g | (-63.34 ~ 24.80) | 84 | NA |
| Birth weight | Tous | 2020 | 5 | low level < 75 vs high level ≥75 (nmol/L) | random-effect | MD | 15.15 g | (-12.73 ~ 43.04) | 27 | NA |
| Head circumference | Tous | 2020 | 7 | low level < 30 vs high level ≥ 30 (nmol/L) | random-effect | MD | -0.19 cm | (-0.32 ~ -0.06)* | 66 | NA |
| Head circumference | Tous | 2020 | 7 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | MD | -0.47 cm | (-1.11 ~ 0.16) | 98 | NA |
| Small-for-gestational-age | Tous | 2020 | 11 | low level < 30 vs high level ≥ 30 (nmol/L) | random-effect | OR | 1.59 | (1.24 ~ 2.03)* | 71 | NA |
| Small-for-gestational-age | Tous | 2020 | 19 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | OR | 1.43 | (1.08 ~ 1.91)* | 89 | NA |
| Small-for-gestational-age | Tous | 2020 | 7 | low level < 75 vs high level ≥ 75 (nmol/L) | random-effect | OR | 0.98 | (0.81 ~ 1.17) | 39 | NA |
| Attention-deficit hyperactivity disorder | Tirani | 2023 | 5 | highest level vs lowest level | random-effect | OR | 0.59 | (0.44 ~ 0.81)* | 48 | NA |
| Autism spectrum disorder | Tirani | 2023 | 5 | highest level vs lowest level | random-effect | OR | 0.57 | (0.33 ~ 0.99)* | 82 | NA |
| Mental development | Tous | 2022 | 9 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | MD | -1.12 points | (-1.82 ~ -0.42)* | 70 | NA |
| Language development | Tous | 2022 | 7 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | MD | -0.35 points | (-1.00 ~ 0.31) | 78 | NA |
| Motor development | Tous | 2022 | 15 | low level < 50 vs high level ≥ 50 (nmol/L) | random-effect | MD | -0.06 points | (-0.51 ~ 0.40) | 65 | NA |
MA meta-analysis, MD mean difference, OR odds ratio, RR relative risk, NA not available, * significant difference, 25(OH)D 25-hydroxyvitamin D.
For the offspring, a low level of vitamin D ( < 30 nmol/L) significantly reduced birth weight (MD: −87.83; 95% CI: −119.73 ~ −55.91; number of studies = 15; Table 2B) and decreased head circumference compared with a high level of vitamin D (MD = −0.19; 95% CI = −0.32 ~ −0.06; number of studies = 7; Table 2B) [32]. Pregnant women with vitamin D deficiency (<50 nmol/L) had a significantly higher rate of low birth weight (<2500 g) than those without deficiency (OR = 2.39; 95% CI = 1.25 ~ 4.57; number of studies = 8; Table 2B) [30]. A high level of vitamin D ( > 50 nmol/L) significantly decreased the risk of attention-deficit hyperactivity disorder (OR = 0.59; 95% CI = 0.44 ~ 0.81; number of studies = 5; Table 2B) and autism spectrum disorder (OR = 0.57; 95% CI = 0.33 ~ 0.99; number of studies = 5; Table 2B) [15]. Furthermore, a low level of vitamin D ( < 50 nmol/L) significantly decreased the mental development score in the child (MD = −1.12; 95% CI = −1.82 ~ −0.42; number of studies = 9; Table 2B) in comparison to a high level (>50 nmol/L) of vitamin D [32]. Children with type 1 diabetes had significantly lower maternal levels of vitamin D compared to children without type 1 diabetes (MD = −2.54; 95% CI = −4.65 ~ −0.44; number of studies = 8; Table 2A) [33].
Vitamin D supplementation in pregnancy associated with health-related outcomes from RCTs
High dose vitamin D supplementation (>2000 IU/day) reduced the risk of GDM compared with a low dose supplementation (≤2000 IU/day) (OR = 0.7; 95% CI = 0.51–0.95; number of studies = 7; Table 3) [27], while low dose supplementation (≤ 2000 IU/day) reduced the risk of pre-eclampsia compared with placebo (OR = 0.29; 95% CI = 0.09–0.95; number of studies = 3; Table 3). Vitamin D supplementation (>400 IU/day) increased maternal 25-hydroxyvitamin D levels (SMD = 2.07; 95% CI = 1.51 ~ 2.63; number of studies = 15), total antioxidant capacity (SMD = 2.13; 95% CI = 1.04–3.23; number of studies = 9) and glutathione level (SMD = 4.37; 95% CI = 2.90–5.84; number of studies = 9) [18], while decreased maternal the malondialdehyde level (SMD = −0.46; 95% CI = −0.87 ~ −0.05; number of studies = 9) [18], compared with low dose (≤400 IU/day) or placebo (Table 3).
Table 3.
Summary of meta-analyses of randomized controlled trials on vitamin D in pregnancy in relation to maternal health.
| Outcomes | First author | Year | No. of studies in MA | Comparator (vitamin D supplementation or level) | Effects model | Metric of MA | Effect size | 95% CI | I2 (%) | Publication bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Preterm birth | Liu | 2022 | 27 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 0.938 | (0.790 ~ 1.090) | 9 | NA |
| Preterm birth | Wu | 2023 | 10 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | OR | 0.37 | (0.22 ~ 0.62)* | 0 | NA |
| Preterm birth | Irwinda | 2022 | 6 | dose≤ 2000 IU/day vs placebo | random-effect | OR | 0.84 | (0.46 ~ 1.53) | 42 | NA |
| Preterm birth | Irwinda | 2022 | 10 | high dose (> 2000 IU/day) vs low dose (≤ 2000 IU/day) | random-effect | OR | 1.01 | (0.82 ~ 1.26) | 0 | NA |
| Pre-eclampsia | Irwinda | 2022 | 3 | dose ≤ 2000 (IU/day) vs placebo | random-effect | OR | 0.29 | (0.09 ~ 0.95)* | 0 | NA |
| Pre-eclampsia | Irwinda | 2022 | 8 | high dose (> 2000 IU/day) vs low dose (≤2000 IU/day) | random-effect | OR | 0.8 | (0.51 ~ 1.24) | 31 | NA |
| Gestational diabetes mellitus | Irwinda | 2022 | 2 | dose ⩽ 2000 IU/day vs placebo | random-effect | OR | 0.92 | (0.59 ~ 1.42) | 0 | |
| Gestational diabetes mellitus | Irwinda | 2022 | 7 | high dose (> 2000 IU/day) vs low dose (≤2000 IU/day) | random-effect | OR | 0.7 | (0.51 ~ 0.95)* | 0 | NA |
| 25(OH)D | Wu | 2023 | 9 | high dose (≥400 IU/day) vs placebo | random-effect | SMD | 4.07 | (2.73 ~ 5.41)* | 97 | NA |
| 25(OH)D | Motamed | 2022 | 15 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | SMD | 2.07 | (1.51 ~ 2.63)* | 94 | <0.001 |
| 25(OH)D on cord blood | Motamed | 2022 | 3 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | SMD | 1.13 | (-0.28 ~ 2.54) | 96 | 0.77 |
| Total cholesterol | Wu | 2023 | 7 | high dose (≥ 400 IU/day) vs placebo | random-effect | SMD | (-0.67) | (-1.19 ~ -0.14)* | 85 | NA |
| Low-density lipoprotein cholesterol | Wu | 2023 | 7 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | SMD | (-0.49) | (-0.68 ~ -0.29)* | 30 | NA |
| Triglycerides | Wu | 2023 | 6 | high dose (≥ 400 IU/day) vs placebo | random-effect | SMD | (-0.59) | (-1.01 ~ -0.17)* | 77 | NA |
| High-density lipoprotein cholesterol | Wu | 2023 | 8 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | SMD | 0.41 | (0.23 ~ 0.58)* | 0 | NA |
| Caesarean section | Wang | 2021 | 9 | supplement vs placebo or nothing | fixed-effect | RR | 0.75 | (0.63 ~ 0.89)* | 43 | NA |
| Hospitalization | Wang | 2021 | 2 | supplement vs placebo or nothing | fixed-effect | RR | 0.13 | (0.02 ~ 0.98)* | 0 | NA |
| Postpartum hemorrhage | Wang | 2021 | 2 | supplement vs placebo or nothing | fixed-effect | RR | 0.47 | (0.22 ~ 1.00) | 0 | NA |
| Hyperbilirubinemia | Wu | 2023 | 9 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | OR | 0.38 | (0.25 ~ 0.58)* | 0 | NA |
| hs-CRP | Motamed | 2022 | 10 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | SMD | 0.24 | (-0.56 ~ 1.04) | 95 | 0.22 |
| Total antioxidant capacity | Motamed | 2022 | 9 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | SMD | 2.13 | (1.04 ~ 3.23)* | 97 | <0.001 |
| Malondialdehyde | Motamed | 2022 | 6 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | SMD | (-0.46) | (-0.87 ~ -0.05)* | 75 | 0.003 |
| Glutathione | Motamed | 2022 | 9 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | SMD | 4.37 | (2.90 ~ 5.84)* | 98 | <0.001 |
MA meta-analysis, RR relative risk, OR odds ratio, SMD standardized mean difference, NA not available, * significant difference, 25(OH)D 25-hydroxyvitamin D, hs-CRP high sensitivityC-reactive protein.
In terms of the offspring, vitamin D supplementation (>400 IU/day) significantly increased the humeral length in the third trimester (MD = 0.13; 95% CI = 0.06–0.21; number of studies = 2) [25] and birth length (MD = 0.269; 95% CI = 0.024–0.514; number of studies = 20) and significantly decreased the risk of fetal or neonatal mortality (RR = 0.69; 95% CI = 0.482–0.985; number of studies = 13) [22], compared to low dose supplementation (≤400 IU/day) or placebo (Table 4). However, high dose supplementation (>400 IU/day) did not affect other outcomes (femur length in the third trimester, macrosomia, birth weight, rate of low birth weight, or head circumference). Furthermore, vitamin D supplementation in pregnancy improved the neonatal vitamin D level (MD = 27.7; 95% CI = 20.5–34.9; number of studies = 25) and decreased the risk of neonatal vitamin D insufficiency (RR = 0.508; 95% CI = 0.384–0.673; number of studies = 12) (Table 4) [22]. However, it did not improve infant bone health and small-for-gestational-age infants (Table 4).
Table 4.
Summary of meta-analyses of randomized controlled trials on vitamin D in pregnancy in relation to offspring health.
| Outcomes | First author | Year | No. of studies in MA | Comparator (vitamin D supplementation or level) | Effects model | Metric of MA | Effect size | 95% CI | I2 (%) | Publication bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Birth length | Liu | 2022 | 20 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | MD | 0.269 cm | (0.024–0.514)* | 63 | NA |
| Low birth weight rate | Liu | 2022 | 10 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 0.902 | (0.655–1.242) | 32 | NA |
| Birth weight | Liu | 2022 | 33 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | MD | 37.07 g | (-9.669–83.801) | 77 | NA |
| Head circumference | Liu | 2022 | 18 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | MD | 0.149 cm | (-0.016 ~ 0.315) | 68 | NA |
| Small-for-gestational-age | Liu | 2022 | 14 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 0.836 | (0.633 ~ 1.104) | 20 | NA |
| Macrosomia | Liu | 2022 | 3 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 1.097 | (0.600 ~ 2.007) | 0 | NA |
| Femur length in the third trimester | Luoa | 2022 | 2 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | MD | 0.16 | (-0.07 ~ 0.40) | 60 | NA |
| humeral length in the third trimester | Luoa | 2022 | 2 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | MD | 0.13 | (0.06 ~ 0.21)* | 0 | NA |
| Congenital anomalies | Liu | 2022 | 4 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 0.759 | (0.439 ~ 1.314) | 56 | NA |
| 1-min Apgar score | Liu | 2022 | 4 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | MD | 0.093 | (-0.004 ~ 0.190) | 20.5 | NA |
| 5-min Apgar score | Liu | 2022 | 4 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | MD | 0.041 | (-0.051 ~ 0.133) | 43.6 | NA |
| Newborn NICU admission or hospitalization | Liu | 2022 | 5 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 1.00 | (0.830 ~ 1.205) | 0 | NA |
| Asthma or wheeze | Luo | 2022 | 4 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | RR | 1.01 | (0.81 ~ 1.26) | 47 | NA |
| Eczema | Luo | 2022 | 3 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | RR | 0.95 | (0.80 ~ 1.13) | 0 | NA |
| Allergic rhinitis | Luo | 2022 | 3 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | RR | 0.93 | (0.78 ~ 1.11) | 47 | NA |
| Fetal or neonatal mortality | Liu | 2022 | 13 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 0.69 | (0.482 ~ 0.985)* | 0 | NA |
| Lower respiratory tract infection | Luo | 2022 | 3 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | RR | 0.97 | (0.85 ~ 1.11) | 0 | NA |
| Hyperbilirubinemia | Wang | 2021 | 7 | supplement vs placebo or nothing | fixed-effect | RR | 0.47 | (0.33 ~ 0.67)* | 0 | NA |
| Macrosomia | Wang | 2021 | 6 | supplement vs placebo or nothing | fixed-effect | RR | 0.58 | (0.38 ~ 0.89)* | 0 | NA |
| Fetal distress | Wang | 2021 | 2 | supplement vs placebo or nothing | fixed-effect | RR | 0.46 | (0.24 ~ 0.90)* | 0 | NA |
| Polyhydramnios | Wang | 2021 | 4 | supplement vs placebo or nothing | fixed-effect | RR | 0.42 | (0.24 ~ 0.72)* | 0 | NA |
| Hypoglycemia | Wang | 2021 | 4 | supplement vs placebo or nothing | fixed-effect | RR | 0.82 | (0.52 ~ 1.29) | 0 | NA |
| Hospitalization | Wu | 2023 | 4 | high dose (⩾ 400 IU/day) vs placebo | fixed-effect | OR | 0.29 | (0.16 ~ 0.53)* | 0 | NA |
| Vitamin D insufficiency | Liu | 2022 | 12 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | RR | 0.508 | (0.384 ~ 0.673)* | 96 | NA |
| Vitamin D concentration | Liu | 2022 | 25 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | random-effect | MD | 27.7 | (20.5 ~ 34.9)* | 98 | NA |
| WB-BMC | Luoa | 2022 | 2 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | MD | 1.09 | (-0.64 ~ 2.81) | 0 | NA |
| WB-BMD | Luoa | 2022 | 2 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | MD | 0.00 | (0.00 ~ 0.00) | 0 | NA |
| WB-BA | Luoa | 2022 | 2 | high dose (> 400 IU/day) vs low dose (≤ 400 IU/day) or placebo | fixed-effect | MD | 3.71 | (-1.75 ~ 9.18) | 0 | NA |
MA meta-analysis, RR relative risk, MD mean difference, NA not available, *: significant difference, WB-BMC whole body bone mineral content, WB-BMD whole body bone mineral density, WB-BA whole body bone area, NICU neonatal intensive care unit.
Vitamin D supplementation for women with GDM
Two meta-analyses investigated that effects of vitamin D supplementation in pregnancy affected by GDM (Table 5) [17, 31]. It significantly reduced caesarean section rate (RR = 0.75; 95% CI = 0.63–0.89; number of studies = 9), hospitalization rate (RR = 0.13; 95% CI = 0.02–0.98; number of studies = 2), and preterm birth (OR = 0.37; 95% CI = 0.22–0.62; number of studies = 10) [17, 31].
Table 5.
Summary of meta-analyses of randomized controlled trials on vitamin D supplementation for pregnant women with gestational diabetes mellitus and its impact on maternal and offspring health.
| Outcomes | First author | Year | No. of articles in MA | Comparator (vitamin D supplementation or level) | Effects model | Metric of MA | Effect size | 95% CI | I2 (%) | Publication bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Pregnant health | ||||||||||
| Preterm birth | Wu | 2023 | 10 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | OR | 0.37 | (0.22 ~ 0.62)* | 0 | NA |
| Total cholesterol | Wu | 2023 | 7 | high dose (≥ 400 IU/day) vs placebo | random-effect | SMD | (-0.67) | (-1.19 ~ -0.14)* | 85 | NA |
| Low-density lipoprotein cholesterol | Wu | 2023 | 7 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | SMD | (-0.49) | (-0.68 ~ -0.29)* | 30 | NA |
| Triglycerides | Wu | 2023 | 6 | high dose (≥ 400 IU/day) vs placebo | random-effect | SMD | (-0.59) | (-1.01 ~ -0.17)* | 77 | NA |
| High-density lipoprotein cholesterol | Wu | 2023 | 8 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | SMD | 0.41 | (0.23 ~ 0.58)* | 0 | NA |
| 25(OH)D | Wu | 2023 | 9 | high dose (≥ 400 IU/day) vs placebo | random-effect | SMD | 4.07 | (2.73 ~ 5.41)* | 97 | NA |
| HOMA-IR | Wang | 2021 | 8 | supplementation vs placebo or nothing | random-effect | MD | (-1.06 mmol/L) | (-1.40 ~ -0.72)* | 74 | NA |
| Cesarean section | Wang | 2021 | 9 | supplementation vs placebo or nothing | random-effect | RR | 0.75 | (0.63 ~ 0.89)* | 43 | NA |
| Hospitalization | Wang | 2021 | 2 | supplementation vs placebo or nothing | fixed-effect | RR | 0.13 | (0.02 ~ 0.98)* | 0 | NA |
| Postpartum hemorrhage | Wang | 2021 | 2 | supplementation vs placebo or nothing | fixed-effect | RR | 0.47 | (0.22 ~ 1.00) | 0 | NA |
| Fasting plasma glucose | Wang | 2021 | 11 | supplementation vs placebo or nothing | random-effect | MD | (-10.2 mg/dL) | (-13.43 ~ -6.96)* | 80 | NA |
| Fasting insulin level | Wang | 2021 | 8 | supplementation vs placebo or nothing | random-effect | MD | (-5.02 μIU/mL) | (-6.83 ~ -3.20)* | 78 | NA |
| Infant health | ||||||||||
| Hyperbilirubinemia | Wu | 2023 | 9 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | OR | 0.38 | (0.25 ~ 0.58)* | 0 | NA |
| Hospitalization | Wu | 2023 | 4 | high dose (≥ 400 IU/day) vs placebo | fixed-effect | OR | 0.29 | (0.16 ~ 0.53)* | 0 | NA |
| Giant children | Wang | 2021 | 6 | supplementation vs placebo or nothing | fixed-effect | RR | 0.58 | (0.38 ~ 0.89)* | 0 | NA |
| Fetal distress | Wang | 2021 | 2 | supplementation vs placebo or nothing | fixed-effect | RR | 0.46 | (0.24 ~ 0.90)* | 0 | NA |
| Polyhydramnios | Wang | 2021 | 4 | supplementation vs placebo or nothing | fixed-effect | RR | 0.42 | (0.24 ~ 0.72)* | 0 | NA |
| Hypoglycemia | Wang | 2021 | 4 | supplementation vs placebo or nothing | fixed-effect | RR | 0.82 | (0.52 ~ 1.29) | 0 | NA |
MA meta-analysis, OR odds ratio, RR relative risk, MD mean difference, SMD standardized mean difference, NA not available; *significant difference; 25(OH)D 25-hydroxyvitamin D, HOMA-IR homeostasis model assessment of insulin resistance.
In terms of maternal biochemical changes, vitamin D supplementation (≥ 400 IU/day) significantly increased maternal 25-hydroxyvitamin D level (SMD = 4.07; 95% CI = 4.73 ~ 5.41; number of studies = 9) and high-density lipoprotein (SMD = 0.41; 95% CI = 0.23 ~ 0.58; number of studies = 8), and significantly decreased maternal fasting plasma glucose (MD = −0.2; 95% CI = −13.43 ~ −6.96; number of studies = 11), fasting insulin level (MD = −0.2; 95% CI = −6.83 ~ −3.20; number of studies = 8), HOMA-IR (MD = −1.06; 95% CI = −1.40 ~ −0.72; number of studies = 8), total cholesterol (SMD = −0.67; 95% CI = −1.19 ~ −0.14; number of studies = 8), low-density lipoprotein cholesterol (SMD = −0.49; 95% CI = −0.68 ~ −0.29; number of studies = 7), and triglycerides (SMD = −0.59; 95% CI = −1.01 ~ −0.17; number of studies = 18) compared to placebo or no supplementation [17, 31].
Comparison between observational studies and RCTs
A low level of vitamin D during pregnancy significantly increased the risk of GDM and small-for-gestational-age infants, while vitamin D supplementation during pregnancy significantly reduced these risks. On the other hand, a low level of vitamin D during pregnancy significantly increased the risk of preterm birth, but vitamin D supplementation significantly reduced preterm birth risk only for women with GDM and not for those without it. This pattern was also observed in cases of pre-eclampsia. For birth length, a low level of vitamin D during pregnancy did not increase its risk. However, vitamin D supplementation during pregnancy significantly increased birth length.
Discussion
Principal findings
We reviewed 15 systematic reviews and meta-analyses involving 174,175 women. Our findings demonstrate that vitamin D deficiency during pregnancy is associated with adverse outcomes for both the mother and offspring. These outcomes include preterm birth, small-for-gestational-age/low birth weight babies, recurrent miscarriages, bacterial vaginosis, and GDM. Furthermore, vitamin D supplementation during pregnancy increases birth weight, reduces the risk of pre-eclampsia and miscarriage, and improves outcomes in women affected by GDM. However, the methodical quality of all meta-analyses was assessed as critically low.
Comparison with existing literature
We found that having a low level of vitamin D during pregnancy significantly increases the risk of preterm birth and small-for-gestational-age infants, while high-quality evidence suggests vitamin D supplementation during pregnancy did not reduce these risks. As labor has features suggestive of an inflammatory response [35, 36], vitamin D deficiency during pregnancy could lead to increased levels of inflammatory cytokines [37] and potentially lead to preterm birth. Although vitamin D supplementation during pregnancy did not affect the risk of preterm birth, this may be due to the fact that the participants in the RCTs did not all have vitamin D deficiency, and thus the effect of vitamin D supplementation on preterm birth was not significant.
Our umbrella review showed that a low level of vitamin D ( < 50 nmol/L) in pregnancy significantly increased the risk of low birth weight (<2500 g). However, this evidence was considered as low because the result was obtained from observational studies and had high heterogeneity (I2 > 50%). We did not find any association between low level of vitamin D and birth length. On the other hand, a high dose of vitamin D supplementation in pregnancy significantly increased birth length but not increase birth wight. These results were obtained from RCTs, and their evidence was graded as “convincing”. Although heterogeneity was detected in the meta-analyses, the inclusion of a large number of studies (n > 20) strengthens the convincing nature of the results.
The incidence rate of recurrent miscarriage is about 1 ~ 2% [38]. It is critical to investigate the risk factors of recurrent miscarriage in order to determine the optimal treatment and prevent its recurrence. Our review demonstrated that vitamin D deficiency during pregnancy significantly increases the risk of recurrent miscarriage. Although the effect size is large (OR = 4.02) and the number of included articles is substantial (n = 14), there is high heterogeneity in the meta-analysis (I² = 82%) [23]. The assay method, age, and region were considered as sources of heterogeneity [23]. The evidence of this association was assessed as “Possible”. Nevertheless, no meta-analysis has looked into how vitamin D supplementation during pregnancy affects the chance of having recurrent miscarriage. We suggest that RCTs should be conducted to investigate this issue in the future.
Pregnant women with bacterial vaginosis are at risk for adverse outcomes such as low birth weight, preterm birth, fetal death, late miscarriage, and chorioamnionitis [39, 40]. Our review found that vitamin D deficiency during pregnancy is associated with a high risk of bacterial vaginosis. Although the meta-analysis included many studies (n = 14), there was high heterogeneity (I2 = 85%). To date, no meta-analysis has investigated the effect of vitamin D supplementation during pregnancy on the risk of bacterial vaginosis. There is one RCT which showed that vitamin D supplementation for women with vitamin D deficiency may reduce the risk of bacterial vaginosis [41]. Supplementing with vitamin D should reduce the risk of bacterial vaginosis in pregnant women with vitamin D deficiency. Further RCTs should be conducted to confirm this finding.
Pre-eclampsia is a common pregnancy complication affecting approximately 4 million pregnant women every year, and leading to over 70,000 maternal and 500,000 neonatal deaths globally [42, 43]. Our review showed that vitamin D supplementation during pregnancy may lower the risk of pre-eclampsia and, consequently, the risk of neonatal or fetal death. Pro-inflammatory cytokine secretion has been implicated in the pathogenesis of pre-eclampsia, and vitamin D supplementation could reduce the secretion through the inhabitation of Toll-like Receptor 4 monocyte expression [44].
We found low maternal vitamin D levels during pregnancy increased the risk of attention-deficit hyperactivity disorder, autism spectrum disorder, and mental development issues in offspring when compared to high maternal vitamin D levels. Since these results were obtained from observational studies, we deemed the evidence for association as only “possible” or “probable”. However, our finding is supported by the increase in gene expression related to the differentiation of dopaminergic neurons by vitamin D, which affects the neuronal development of the brain during the embryonic period [45]. Moreover, research indicates that children who experience deficiencies in vitamin D are more susceptible to developing attention-deficit hyperactivity disorder or autistic spectrum condition [46, 47], while vitamin D supplementation in children with attention-deficit hyperactivity disorder or autism spectrum disorder had improved cognitive function [48] or reduced severity [49], respectively.
Our comprehensive review found that vitamin D supplementation reduces the risk of GDM. However, vitamin D deficiency during pregnancy significantly increases the risk of GDM. This finding is supported by evidence from both observational studies and RCTs. In addition, a meta-analysis has reported vitamin D plus calcium supplementation can reduce the risk of type 2 diabetes mellitus, but only in people with glucose intolerance [50]. Therefore, vitamin D plays a vital role in preventing GDM. We also demonstrated that vitamin D supplementation for pregnant women with GDM reduced risks of polyhydramnios, preterm birth, Caesarean section, maternal hospitalization, macrosomia, newborn hyperbilirubinemia, and fetal distress, along with biochemical improvements. However, a letter to editor [51] pointed out some concerns with the meta-analysis conducted by Wang et al. [31]. In their update, vitamin D supplementation had no effect on the risk of preterm birth, Caesarean section, maternal hospitalization, macrosomia, or newborn hyperbilirubinemia [51]. However, some outcomes of this updated meta-analysis included only a few studies (n = 2 ~ 3), and was not formally published as an original article.
The RCTs used different doses of vitamin D supplementation to examine its impact on clinical outcomes. To assess the impact of dose on clinical outcomes, we included meta-analyses that compared two significantly different doses (2000 IU/day and 400 IU/day) as the cut-off points. These meta-analyses can help to investigate the effect of dose on clinical outcomes. Additionally, the meta-analyses also compared outcomes between vitamin D supplements (< 2000 IU/day) and placebo. These comparisons helped determine if a specific high dose was necessary to achieve significant improvement. According the current evidence, a very high dose of vitamin D supplement (> 2000 IU/day) only showed a significant improvement in the risk of GDM when compared with a low dose (≤ 2000 IU/day) (Table 3). Otherwise, a low dose of supplements (> 400 IU/day) could be used to improve several clinical outcomes, such as fetal or neonatal mortality, vitamin D insufficiency, and preterm birth (Table 3).
Strengths and limitations
This study well-organized the current evidence of the topic investigating the impact vitamin D status during pregnancy on health-related outcomes. The newest and highest level of evidence of meta-analyses were included. In addition, we assessed the evidence of meta-analyses. However, this comprehensive review does have some limitations. First, some adverse clinical outcomes were reported to be associated with vitamin D deficiency in pregnancy based on the meta-analysis of observational studies. However, vitamin D supplementation in pregnancy could not prevent these adverse clinical outcomes based on the meta-analysis of RCTs. This may be due to the participants in the RCTs did not all have vitamin D deficiency. Second, some meta-analyses only included a few studies (n = 2 to 3), and the results may not be reliable. More primary studies should be conducted to confirm these results. Finally, vitamin D status in pregnancy was assessed at the time of blood sampling and only reflect the vitamin D status over a short period of time period. As daily vitamin D status in blood varies according to diet and sum exposure, long-term measures of vitamin D status would be more reliable, such as measurement from hair samples.
Conclusion
Vitamin D deficiency is commonly found in pregnancy. This review highlights the adverse outcomes associated with low vitamin D levels during pregnancy and that vitamin D supplementation during pregnancy can reduce the risk of some adverse outcomes. Based on our review, it is suggested that pregnant women take a dose of > 400 IU/day of vitamin D supplementation during pregnancy to prevent certain adverse outcomes. However, there is conflicting evidence for the effectiveness of vitamin D supplementation in women with GDM. We recommend monitoring vitamin D levels during pregnancy, and supplementing with vitamin D for pregnant women who are deficient to prevent potential adverse outcomes.
Supplementary information
Author contributions
CLS, PW, MCC, JHW, and CYH conceived the project. CLS and PW searched the articles and extracted the data. CLS and PW revised and edited the manuscript. All authors read and approved the final manuscript.
Data availability
All data used in this study come from the published articles listed in Table 1.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not application.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mei-Chun Chien, Chueh-Yi Huang.
Contributor Information
Jie-Huei Wang, Email: jhwang@ccu.edu.tw.
Chia-Lung Shih, Email: stone770116@gmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41387-024-00296-0.
References
- 1.IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of vitamin D. Recommendations 1981. Eur J Biochem. 1982;124:223–7. [PubMed]
- 2.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolliffe DA, Camargo CA, Jr, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9:276–92. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 4.Demer LL, Hsu JJ, Tintut Y. Steroid Hormone Vitamin D: Implications for Cardiovascular Disease. Circ Res. 2018;122:1576–85. doi: 10.1161/CIRCRESAHA.118.311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganji V, Tangpricha V, Zhang X. Serum Vitamin D Concentration >/=75 nmol/L Is Related to Decreased Cardiometabolic and Inflammatory Biomarkers, Metabolic Syndrome, and Diabetes; and Increased Cardiorespiratory Fitness in US Adults. Nutrients. 2020;12:730. doi: 10.3390/nu12030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudet M, Plesa M, Mogas A, Jalaleddine N, Hamid Q, Al Heialy S. Recent advances in vitamin D implications in chronic respiratory diseases. Respir Res. 2022;23:252. doi: 10.1186/s12931-022-02147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richard A, Rohrmann S, Quack Lotscher KC. Prevalence of Vitamin D Deficiency and Its Associations with Skin Color in Pregnant Women in the First Trimester in a Sample from Switzerland. Nutrients. 2017;9:260. doi: 10.3390/nu9030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karras S, Paschou SA, Kandaraki E, Anagnostis P, Annweiler C, Tarlatzis BC, et al. Hypovitaminosis D in pregnancy in the Mediterranean region: a systematic review. Eur J Clin Nutr. 2016;70:979–86. doi: 10.1038/ejcn.2016.12. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Kumar A, Prasad S, Sharma S. Current Scenario of Vitamin D Status During Pregnancy in North Indian Population. J Obstet Gynaecol India. 2016;66:93–100. doi: 10.1007/s13224-014-0658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–45. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Accortt EE, Lamb A, Mirocha J, Hobel CJ. Vitamin D deficiency and depressive symptoms in pregnancy are associated with adverse perinatal outcomes. J Behav Med. 2018;41:680–9. doi: 10.1007/s10865-018-9924-9. [DOI] [PubMed] [Google Scholar]
- 12.Huang JY, Qiu C, Miller RS, Siscovick DS, Williams MA, Enquobahrie DA. Maternal birthweight is associated with subsequent risk of vitamin D deficiency in early pregnancy. Paediatr Perinat Epidemiol. 2013;27:472–80. doi: 10.1111/ppe.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin LL, Lu FG, Yang SH, Xu HL, Luo BA. Does Maternal Vitamin D Deficiency Increase the Risk of Preterm Birth: A Meta-Analysis of Observational Studies. Nutrients. 2016;8:301. doi: 10.3390/nu8050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palacios C, Kostiuk LK, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi: 10.1002/14651858.CD008873.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirani SA, Balali A, Askari G, Saneei P. Maternal serum 25-hydroxy vitamin D levels and risk of autism spectrum and attention-deficit hyperactivity disorders in offspring: A systematic review and dose-response meta-analysis. Psychiatry Res. 2023;319:114977. doi: 10.1016/j.psychres.2022.114977. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Zhang Z, Li L, Zhang L, Lin Z, Qin H. Vitamin D deficiency increases the risk of bacterial vaginosis during pregnancy: Evidence from a meta-analysis based on observational studies. Front Nutr. 2022;9:1016592. doi: 10.3389/fnut.2022.1016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Song Y, Wang X. Vitamin D Supplementation for the Outcomes of Patients with Gestational Diabetes Mellitus and Neonates: A Meta-Analysis and Systematic Review. Int J Clin Pract. 2023;2023:1907222. doi: 10.1155/2023/1907222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motamed S, Nikooyeh B, Anari R, Motamed S, Mokhtari Z, Neyestani T. The effect of vitamin D supplementation on oxidative stress and inflammatory biomarkers in pregnant women: a systematic review and meta-analysis of clinical trials. BMC Pregnancy and Childbirth. 2022;22:816. doi: 10.1186/s12884-022-05132-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papatheodorou SI, Evangelou E. Umbrella Reviews: What They Are and Why We Need Them. Methods Mol Biol. 2022;2345:135–46. doi: 10.1007/978-1-0716-1566-9_8. [DOI] [PubMed] [Google Scholar]
- 20.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annual Review of Nutrition. 2017;37:131–56. doi: 10.1146/annurev-nutr-071816-064941. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Ding C, Xu R, Wang K, Zhang D, Pang W, et al. Effects of vitamin D supplementation during pregnancy on offspring health at birth: A meta-analysis of randomized controlled trails. Clinical Nutrition. 2022;41:1532–40. doi: 10.1016/j.clnu.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Wang S, Zhang C, Wu X, Zhou L, Zou X, et al. Association between serum vitamin D level during pregnancy and recurrent spontaneous abortion: A systematic review and meta-analysis. American Journal of Reproductive Immunology. 2022;88:e13582. doi: 10.1111/aji.13582. [DOI] [PubMed] [Google Scholar]
- 24.Jasper EA, Nidey NL, Schweizer ML, Ryckman KK. Gestational vitamin D and offspring risk of multiple sclerosis: a systematic review and meta-analysis. Annals of Epidemiology. 2020;43:11–7. doi: 10.1016/j.annepidem.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Luo T, Lin Y, Lu J, Lian X, Guo Y, Han L, et al. Effects of vitamin D supplementation during pregnancy on bone health and offspring growth: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2022;17:e0276016. doi: 10.1371/journal.pone.0276016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo C, Sun Y, Zeng Z, Liu Y, Peng S. Vitamin D supplementation in pregnant women or infants for preventing allergic diseases: a systematic review and meta-analysis of randomized controlled trials. Chin Med J (Engl) 2022;135:276–84. doi: 10.1097/CM9.0000000000001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwinda R, Hiksas R, Lokeswara AW, Wibowo N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal–fetal outcome: Systematic review and meta-analysis. Women’s Health. 2022;18:17455057221111066. doi: 10.1177/17455057221111066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatima K, Asif M, Nihal K, Hussain HU, Hasan AW, Zahid M, et al. Association between vitamin D levels in early pregnancy and gestational diabetes mellitus: A systematic review and meta-analysis. J Family Med Prim Care. 2022;11:5569–80. doi: 10.4103/jfmpc.jfmpc_107_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamblyn JA, Pilarski NSP, Markland AD, Marson EJ, Devall A, Hewison M, et al. Vitamin D and miscarriage: a systematic review and meta-analysis. Fertility and Sterility. 2022;118:111–22. doi: 10.1016/j.fertnstert.2022.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Fang K, He Y, Mu M, Liu K. Maternal vitamin D deficiency during pregnancy and low birth weight: a systematic review and meta-analysis. Journal of Maternal-Fetal and Neonatal Medicine. 2021;34:1167–73. doi: 10.1080/14767058.2019.1623780. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Chen Z, Hu Y, Wang Y, Wu Y, Lian F, et al. The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: A systematic review and meta-analysis. Clinical Nutrition. 2021;40:3148–57. doi: 10.1016/j.clnu.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Tous M, Villalobos M, Iglesias L, Fernández-Barrés S, Arija V. Vitamin D status during pregnancy and offspring outcomes: a systematic review and meta-analysis of observational studies. European Journal of Clinical Nutrition. 2020;74:36–53. doi: 10.1038/s41430-018-0373-x. [DOI] [PubMed] [Google Scholar]
- 33.Kang X, Cui J, Zhang M, Wang Y, Tang W, Chen L. Maternal level of 25-hydroxyvitamin d during pregnancy associated with risk of type 1 diabetes in the offspring, a meta-analysis. Journal of Nutritional Science and Vitaminology. 2020;66:402–8. doi: 10.3177/jnsv.66.402. [DOI] [PubMed] [Google Scholar]
- 34.Rouhani P, Mokhtari E, Lotfi K, Saneei P. The association between circulating 25-hydroxyvitamin D levels and preeclampsia: a systematic review and dose-response meta-analysis of epidemiologic studies with GRADE assessment. Nutr Rev. 2023;81:1267–89. doi: 10.1093/nutrit/nuad006. [DOI] [PubMed] [Google Scholar]
- 35.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104.e1–11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 36.Menon R, Behnia F, Polettini J, Richardson LS. Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes. Semin Immunopathol. 2020;42:431–50. doi: 10.1007/s00281-020-00808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azizieh F, Alyahya KO, Raghupathy R. Association between levels of vitamin D and inflammatory markers in healthy women. J Inflamm Res. 2016;9:51–7. doi: 10.2147/JIR.S103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan Y, Fang L, Li Y, Yu Y, Li Y, Cheng JC, et al. Association of MMP2 and MMP9 gene polymorphisms with the recurrent spontaneous abortion: A meta-analysis. Gene. 2021;767:145173. doi: 10.1016/j.gene.2020.145173. [DOI] [PubMed] [Google Scholar]
- 39.Juliana NCA, Suiters MJM, Al-Nasiry S, Morré SA, Peters RPH, Ambrosino E. The Association Between Vaginal Microbiota Dysbiosis, Bacterial Vaginosis, and Aerobic Vaginitis, and Adverse Pregnancy Outcomes of Women Living in Sub-Saharan Africa: A Systematic Review. Front Public Health. 2020;8:567885. doi: 10.3389/fpubh.2020.567885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abou Chacra L, Fenollar F, Diop K. Bacterial Vaginosis: What Do We Currently Know? Front Cell Infect Microbiol. 2021;11:672429. doi: 10.3389/fcimb.2021.672429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taheri M, Baheiraei A, Foroushani AR, Nikmanesh B, Modarres M. Treatment of vitamin D deficiency is an effective method in the elimination of asymptomatic bacterial vaginosis: A placebo-controlled randomized clinical trial. Indian J Med Res. 2015;141:799–806. doi: 10.4103/0971-5916.160707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. International Journal of Gynecology & Obstetrics. 2019;145:1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022;27:148–69. doi: 10.1016/j.preghy.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Qian L, Wu F, Li M, Wang H. Significance of Toll-like Receptor 4 Signaling in Peripheral Blood Monocytes of Pre-eclamptic Patients. Hypertens Pregnancy. 2015;34:486–94. doi: 10.3109/10641955.2015.1077860. [DOI] [PubMed] [Google Scholar]
- 45.Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193–203. doi: 10.1016/j.neuroscience.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 46.Khoshbakht Y, Bidaki R, Salehi-Abargouei A. Vitamin D Status and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Observational Studies. Adv Nutr. 2018;9:9–20. doi: 10.1093/advances/nmx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Ding R, Wang J. The Association between Vitamin D Status and Autism Spectrum Disorder (ASD): A Systematic Review and Meta-Analysis. Nutrients. 2020;13:86. doi: 10.3390/nu13010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elshorbagy HH, Barseem NF, Abdelghani WE, Suliman HAI, Al-Shokary AH, Abdulsamea SE, et al. Impact of Vitamin D Supplementation on Attention-Deficit Hyperactivity Disorder in Children. Ann Pharmacother. 2018;52:623–31. doi: 10.1177/1060028018759471. [DOI] [PubMed] [Google Scholar]
- 49.Kittana M, Ahmadani A, Stojanovska L, Attlee A. The Role of Vitamin D Supplementation in Children with Autism Spectrum Disorder: A Narrative Review. Nutrients. 2021;14:26. doi: 10.3390/nu14010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo ACQ, Lo CCW. The effect of vitamin D supplementation on glycemic control/glucose metabolism and maternal-neonatal outcomes in women with established gestational diabetes mellitus: An updated meta-analysis. Clin Nutr. 2022;41:2420–3. doi: 10.1016/j.clnu.2022.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study come from the published articles listed in Table 1.