Abstract
A wide variety of gram-negative bacteria utilize a specialized apparatus called the type III secretion system (TTSS) to translocate virulence factors directly into the cytoplasm of eukaryotic cells. These translocated effectors contribute to the pathogen's ability to infect and replicate within plant and animal hosts. The amino terminus of effector proteins contains sequences that are necessary and sufficient for both secretion and translocation by TTSS. Portions of these sequences contain binding sites for type III chaperones, which facilitate efficient secretion and translocation of specific effectors through TTSS. In this study, we have utilized the yeast two-hybrid assay to identify protein-protein interactions between effector and chaperone proteins encoded within Salmonella pathogenicity island 1 (SPI-1). Several interactions were identified including a novel interaction between the effector protein, SspA (SipA), and a putative chaperone, InvB. InvB was demonstrated to bind to the amino terminus of SspA in the bacterial cytoplasm. Furthermore, InvB acts as a type III chaperone for the efficient secretion and translocation of SspA by SPI-1. InvB also permitted translocation of SspA through the SPI-2 TTSS, indicating that it is an important regulator in the recognition of SspA as a target of TTSS. Finally, it was determined that InvB does not alter the transcription of sspA but that its absence results in reduced SspA protein levels in Salmonella enterica serovar Typhimurium.
Gram-negative plant and animal pathogens utilize specialized protein export machines termed type III secretion systems (TTSS) that mediate translocation of effector proteins from the bacterial cytoplasm into the host eukaryotic cell cytosol (25). These TTSS are important for the pathogenesis of Yersinia, Salmonella, Erwinia, and Pseudomonas and many other pathogenic bacteria (18). Most TTSS described exhibit contact-dependent secretion of effector proteins (34, 45, 47). In Salmonella spp., two completely separate TTSS are expressed at different times and are required for different aspects of bacterial colonization and persistence in the host organism (17, 18, 31). One TTSS, which exhibits contact-dependent secretion, is encoded in Salmonella pathogenicity island 1 (SPI-1) and is involved in bacterial interactions with epithelial cells (10), while the second TTSS is encoded in SPI-2 and is expressed in intracellular bacteria and translocates proteins across the phagosomal membrane (17, 31).
All TTSS are comprised of over 20 gene products that can be grouped into one of four classes: bacterial membrane apparatus proteins, translocon proteins, translocated effector proteins, and type III chaperones. Apparatus proteins form the needle structure that spans the inner and outer membranes of the bacteria (23), while the translocon proteins insert themselves into the eukaryotic cell membrane to form a pore which effector proteins can pass through to gain access to the cytosolic host targets (28, 36, 46). The fourth class of proteins, type III chaperones, are responsible for efficient secretion and the translocation of specific effector proteins to which they bind (3). Type III chaperones do not necessarily share primary amino acid homology, but all have several similar characteristics including small size (between 8 and 25 kDa), acidic pIs (∼4.5), and a predicted alpha-helical structure (3).
At least some proteins secreted by TTSS contain two secretion signals. Both are found in the amino terminus of type III effector proteins; one is within the mRNA structure, and the other is within the amino acid sequence. The YopE protein of Yersinia enterocolitica has an mRNA secretion signal within the region encoding the first 15 amino acids (1, 5). The other signal is located within amino acids 15 to 100 and is required for the translocation of YopE into eukaryotic cells (5). This second signal sequence is also the region where the type III chaperone SycE binds to YopE (7, 24, 37, 43). This leads to the hypothesis that chaperone binding mediates translocation of these type III effector proteins not only in Yersinia but in all TTSS-expressing bacteria.
In this study, the yeast two-hybrid assay was used to identify new type III chaperones for SPI-1 effector proteins.
MATERIALS AND METHODS
Bacterial strains, eukaryotic strains, Saccharomyces cerevisiae strains, and culture conditions.
The bacterial and yeast strains as well as plasmids used in this study are listed in Table 1. All bacteria and RAW264.7 macrophages were grown as previously described (28). The yeast strains were grown in yeast extract-peptone-dextrose or minimal media lacking amino acids to select for yeast plasmids.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Genotype and relevant markers | Reference or source |
|---|---|---|
| Salmonella serovar Typhimurium strains | ||
| ATCC 14028s | Wild type | ATCCa |
| CS019 | 14028s phoN2 zxx::6251 Tn10d-Cm | Miller et al. (1989) |
| CS401 | CS019 Strr | S. I. Miller lab |
| TK164 | CS401 ΔprgH | T. G. Kimbrough and S. I. Millerb |
| TK91 | CS401 ΔprgH-K | T. G. Kimbrough and S. I. Millerb |
| PB502 | CS401 ΔinvB | This study |
| PB509 | CS401 ΔinvB ΔprgH | This study |
| EE633 | SL1344 sspA::lacZY4 | 19 |
| PB525 | CS401 sspA::lacZY4 | This study |
| PB523 | CS401 sspA::lacZY4 ΔinvB | This study |
| CAS108 | CS401 ΔsspC | 36 |
| EM323 | CS401 ssaT::mTn5 | 28 |
| Yeast strains | ||
| CBY14α | MATα his3 leu2 trp1 URA3::UASGAL1-lacZ gal4Δ gal80Δ LYS2::UASGAL1-HIS3 | Bendixen et al. (1994) |
| CBY14a | MATa his3 leu2 trp1 URA3::UASGAL1-lacZ gal4Δ gal80Δ LYS2::UASGAL1-HIS3 | Bendixen et al. (1994) |
| Plasmids | ||
| pET15-b | Ampr, T7 promoter, used to make six-His amino-terminal fusions | Novagen |
| pBAD18 | Ampr, arabinose-inducible promoter | 15 |
| pBAD24 | Ampr, arabinose-inducible promoter with Shine-Dalgarno sequence | 15 |
| pBAD18KAN | Kanr, arabinose-inducible promoter | 15 |
| pBAD24KAN | Kanr, arabinose-inducible promoter with Shine-Dalgarno sequence | This study |
| pGAD424 | LEU2, GAL4 activation domain fusion vector | Clontech |
| pGBT9 | TRP1, GAL4 binding domain fusion vector | Clontech |
| pKAS32 | Positive selection (rpsL) suicide vector for allelic exchange | 38 |
| pWSK29 | Low-copy expression vector | Wang and Kushner (1991) |
| pMJH20 | pWSK29 containing cyaA catalytic domain | 28 |
| pB507 | pET15-b expressing six-His-tagged InvB | This study |
| pB501 | Kanr, sspA cloned into pBAD18-Kan | This study |
| pB502 | Ampr, invB cloned into pBAD24 | This study |
| pB500 | pMJH20 expressing SspA-CyaA | This study |
| pEM25 | pMJH20 expressing SspH1-cyaA | 28 |
| pB503 | pKAS32 vector containing DNA used to create ΔinvB in CS401 | This study |
| pEM120 | pMJH20 expressing SspA-CyaA and InvB | This study |
| pB01 | pGBT9 expressing SspA-binding domain fusion | This study |
| pB02 | pGAD424 expressing SicA-activation domain fusion | This study |
| pB05 | pGAD424 expressing SptP-activation domain fusion | This study |
| pB06 | pGBT9 expressing SptP-binding domain fusion | This study |
| pB07 | pGAD424 expressing InvB-activation domain fusion | This study |
| pB10 | pGAD424 expressing PrgJ-activation domain fusion | This study |
| pB17 | pGAD424 expressing SspA-activation domain fusion | This study |
| pB23 | pGAD424 expressing PrgI-activation domain fusion | This study |
| pB24 | pGBT9 expressing SspA-binding domain fusion | This study |
| pB25 | pGBT9 expressing SspA-binding domain fusion | This study |
| pB26 | pGAD424 expressing SicP-activation domain fusion | This study |
| pB30 | pGBT9 expressing InvB-binding domain fusion | This study |
| pB34 | pGBT9 expressing SspA-binding domain fusion | This study |
| pB44 | pGBT9 expressing SspA-binding domain fusion | This study |
| pCAS06 | pGBT9 expressing SspB-binding domain fusion | 36 |
| pCAS07 | pGBT9 expressing SspC-binding domain fusion | 36 |
| pCAS08 | pGBT9 expressing SspA-binding domain fusion | 36 |
| pCAS14 | pGAD424 expressing SspB-activation domain fusion | 36 |
| pCAS15 | pGAD424 expressing SspC-activation domain fusion | 36 |
| pCAS16 | pGAD424 expressing SspD-activation domain fusion | 36 |
ATCC, American Type Culture Collection.
Submitted for publication.
Yeast two-hybrid experiments.
Plasmids expressing fusion proteins with either the GAL4 binding or the GAL4 activating domain were transformed into CBY14α and CBY14a, respectively. Strains transformed with the plasmids were then mated in a pairwise fashion by coculturing the two strains in yeast extract-peptone-dextrose, grown overnight at 30°C, and plated on media which selected for both plasmids. Two-hybrid interactions were then identified by assaying for the activation of lacZ and HIS3, both of which are under the control of the Gal1 promoter in the yeast chromosome. All assays were performed as described in the Clontech (Palo Alto, Calif.) protocols or by Bartel and Fields (2).
Construction of Salmonella enterica serovar Typhimurium chromosomal deletion strains.
An in-frame chromosomal deletion leaving the first 12 nucleotides and the last 18 nucleotides of invB was created by using PCR to amplify flanking DNA with Deep Vent (New England Biolabs, Beverly, Mass.) as per the manufacturer's instructions. Then the upstream and downstream DNA was ligated into an allelic-exchange vector, pKAS32 (38). Allelic exchange was performed in strain CS401 as described previously (33). Briefly, the pKAS32 deletion construct is transferred via conjugation into Salmonella serovar Typhimurium, where it is integrated into the chromosome by homologous recombination. Plasmid excision events are selected for by applying counterselection. The strain containing ΔprgH and ΔinvB was made by transducing PB502 with P22 grown on the ΔprgH integrant strain. Counterselection was then applied to select for the excision of plasmid sequences. All deletion strains were verified using PCR and Southern blot analysis.
Construction of InvB- and SspA-inducible plasmids.
To generate inducible expression vectors for InvB and SspA, pBAD18 and pBAD24 plasmids were used. Primers were used to PCR amplify the invB gene from the Salmonella serovar Typhimurium chromosome with an upstream KpnI site and a downstream PstI site. The PCR product and pBAD24 were enzymatically digested, and the products were ligated to make pB502. To generate the arabinose-controlled expression vector pB501, a SacI-SalI fragment containing sspA and its ribosome binding site were PCR amplified and cloned into the same sites within pBAD18KAN (15).
Construction of yeast two-hybrid vectors.
To generate the GAL4 DNA activation and binding domain protein fusions, gene sequences were PCR amplified and cloned into pGAD424 and pGBT9 (Clontech), respectively, using engineered SmaI (sptP, prgJ, sicA, prgI, sicP, invB, and sspA) or EcoRI (sspC, sspB, and sspD) sites at the 5′ end of the gene and PstI (sptP, prgJ, and sicA) or SalI (prgI, sicP, invB, and sspA) sites at the 3′ end. All fusions contain the entire coding sequence of the genes, with the exception of the initial ATG.
Construction of sspA::Tn5lac strains.
To construct strains containing sspA transcriptional fusions in a CS401 background, P22HT int phage was grown on EE633 and transduced into CS401 or PB502. Transductants receiving sspA::Tn5lac were identified by growth in the presence of tetracycline (10 μg/ml) and verified by PCR and Western blot analysis.
Generation of InvB-specific antisera.
invB was PCR amplified from the Salmonella serovar Typhimurium chromosome using primers with restriction enzyme sites so that an amino-terminal fusion with a six-histidine tag could be generated by cloning into the pET15-b vector (Novagen, Madison, Wis.). This plasmid was transformed into Escherichia coli, and cultures were grown overnight, diluted 1:100, and grown to an optical density at 600 nm (OD600) of 0.4. The cultures were then induced with 0.5% IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h, pelleted, and stored at −80°C overnight. The histidine-tagged proteins were then collected using protocols supplied by Qiagen, Valencia, Calif.
Eluted protein was gel purified and injected into rabbits at Pocono Rabbit Farm and Laboratories. Antisera were further purified by absorption with an acetone powder containing proteins from a culture of PB502 (16).
Collection and analysis of culture supernatants.
Secreted proteins were purified as previously described (32). Briefly, bacterial cultures were grown overnight, and secreted proteins were separated from bacterial cells by centrifugation. Secreted proteins were collected by precipitation with 10% trichloroacetic acid and resuspended in protein loading sample buffer. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot techniques were applied as described earlier (32).
Analysis of SPI-1 and SPI-2 type III-dependent translocation by CyaA fusions.
Cultured RAW264.7 macrophages were infected using Salmonella serovar Typhimurium strains at a multiplicity of infection of 10, and protein translocation was determined as previously described (28). To assay for SPI-1-dependent translocation, exponentially growing bacteria were used to infect macrophages for 1 h. SPI-2-dependent translocation was measured by infecting RAW264.7 cells with stationary-phase bacteria for 1 h followed by 5 h of gentamicin treatment. In both assays, infected macrophages were lysed in 0.1 M HCl and heated to 95°C for 5 min and cyclic AMP (cAMP) levels were determined using the Direct Cyclic AMP Correlate-EIA kit (Assay Designs Inc., Ann Arbor, Mich.). cAMP levels were normalized for the protein content of each sample as determined by the Bradford assay.
Analysis of sspA transcription by LacZ expression.
The desired strains of Salmonella serovar Typhimurium were grown overnight in Luria-Bertani (LB) medium, diluted 1:100, and grown for 2 h. Samples were then taken approximately every 30 min. β-Galactosidase assays were performed by normalizing enzymatic activity to cell number as previously described (29).
Immunoprecipitation.
Bacterial overnight cultures were diluted 1:100 in 20 ml of LB medium and grown to an OD600 of ∼0.8. The cells were then washed and resuspended in 1.5 ml of phosphate-buffered saline (PBS). The samples were kept on ice and lysed by sonicating them for four 20-s bursts. Unlysed cells were removed by centrifugation for 10 min at 4°C. A portion of the lysate (0.5 ml) was then transferred to a new microcentrifuge tube and brought up to 1.0 ml with PBS. To preclear the lysate, 50 μl of a 50% slurry of protein A-Sepharose (Amersham Pharmacia, Piscataway, N.J.) in PBS was added to each sample and rotated end over end at 4°C for 1 h. The cleared lysate was then collected by pelleting the protein A-Sepharose by centrifugation for 30 s at 4°C. Preimmune sera or polyclonal antibodies specific for SspA or SspC or monoclonal antibodies raised to CyaA were added to the samples along with 50 μl of protein A-Sepharose. The samples were then rotated end over end for 1 h at 4°C. The protein A-Sepharose was again collected by centrifugation, and the supernatant was removed. The Sepharose beads were then washed four times with PBS and resuspended in 50 μl of sample buffer. The protein A-Sepharose complexes were then boiled for 5 min and resolved by SDS-PAGE.
RESULTS
SspA and InvB interact in the yeast two-hybrid assay.
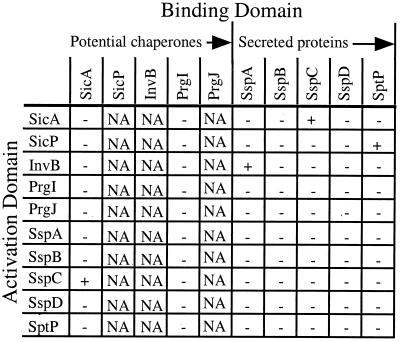
Many chaperone-effector pairs have been identified and studied in Yersinia (9, 13, 20, 30, 42–44); however, only two of these protein pairs have been identified in Salmonella (14, 41). In an effort to find new type III chaperones within SPI-1, DNA sequences were scanned for genes predicted to encode small, acidic proteins. Proteins in SPI-1 which met criteria included SicA, SicP, PrgI, PrgJ, and InvB. These putative chaperones and a subset of SPI-1-secreted proteins were cloned into yeast two-hybrid vectors to create proteins containing amino-terminal fusions with the GAL4 activation or binding domains. The ability of these fusion proteins to interact with each other was determined by mating yeast strains expressing an activation domain fusion with a strain expressing a binding domain fusion in a pairwise fashion. Protein-protein interactions were then determined by measuring lacZ and HIS3 expression in the yeast two-hybrid assay (Fig. 1). Several interactions were identified including interactions between recently identified effector-chaperone protein pairs, SptP-SicP and SspC (SipC)-SicA (14, 41). A third, novel interaction was found between SspA and InvB. SspA is a translocated effector protein which participates in the induction of macropinocytosis by facilitating actin rearrangements within infected eukaryotic cells, and InvB is predicted to be a mostly alpha-helical 128-amino-acid protein with a pI of ∼4.5. The predicted characteristics of InvB coupled with its interaction with SspA in the yeast two-hybrid assay suggest that InvB functions as a type III chaperone for SspA.
FIG. 1.
Yeast two-hybrid interactions. A subset of genes encoding SPI-1-secreted proteins and potential chaperones were cloned into pGBT9 and pGAD424 to create fusion proteins with the GAL4 binding and activation domains, respectively. These fusion proteins were expressed in yeast, and positive interactions (+) were identified by growth on histidine-deficient medium and β-galactosidase activity. When fused to the GAL4 binding domain, some proteins caused nonspecific activation of Gal1-regulated genes (NA). All results were confirmed by testing interactions in fresh background strains.
InvB and SspA can be coimmunoprecipitated from the cytoplasm of Salmonella serovar Typhimurium.
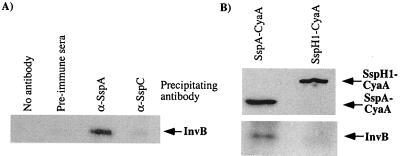
To determine if the SspA-InvB yeast two-hybrid result was predictive of a physiologically relevant interaction, coimmunoprecipitation experiments were performed. Wild-type S. enterica serovar Typhimurium was grown to exponential growth phase, when SPI-1 TTSS is expressed, and cytoplasmic lysates were collected by sonication. The lysates were precleared, and various antisera were added to immunoprecipitate proteins. Protein-antibody complexes precipitated by protein A-Sepharose were then analyzed by Western blotting. Antisera specific for SspA immunoprecipitated both SspA (data not shown) and InvB, while antisera raised against other secreted proteins (SspC) failed to coimmunoprecipitate InvB (Fig. 2A).
FIG. 2.
Immunoprecipitation of InvB from Salmonella serovar Typhimurium. Either wild-type Salmonella serovar Typhimurium (A) (CS401) or wild-type bacteria expressing either SspA-CyaA (pB500) or SspH1-CyaA (pEM25) (B) were grown to logarithmic phase, and cytoplasmic fractions were isolated after sonication. The resulting lysate was precleared with protein A-Sepharose, and proteins were immunoprecipitated by the addition of various sera and protein A-Sepharose. Proteins associated with the Sepharose beads were collected by centrifugation and solubilized by boiling in SDS-PAGE sample buffer. All protein samples were resolved by SDS-PAGE and analyzed with Western blotting with sera specific for InvB. Results shown are representative of multiple experiments.
Type III chaperones typically bind to the amino-terminal domain of effector proteins. To further define the InvB binding site on SspA, a fusion protein which contained the amino-terminal 158 residues of SspA fused to the CyaA gene product from Bordetella pertussis was constructed. As a negative control, a CyaA fusion to another SPI-1 TTSS-translocated protein, SspH1 (28), was included in these experiments. Protein complexes were immunoprecipitated from cell lysates of Salmonella serovar Typhimurium expressing these fusion proteins. Both fusion proteins were immunoprecipitated by monoclonal antibodies to CyaA, while only the SspA-CyaA fusion protein was able to coimmunoprecipitate InvB (Fig. 2B). These results indicate that InvB binds to the amino-terminal 158 amino acids of SspA and forms a stable complex in the bacterial cytosol. Additionally, these data confirm the validity of the two-hybrid interactions, as they strongly indicate a physiologically significant interaction between InvB and SspA in Salmonella serovar Typhimurium.
Deletion of invB affects secretion of SspA.
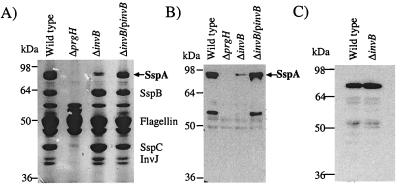
Many proteins can be isolated from overnight cultures of Salmonella serovar Typhimurium. Some of these proteins are SPI-1-dependent effector and translocon proteins which have been secreted into culture media. These secreted protein profiles can be analyzed to assay for fully functional SPI-1 TTSS apparatus (19). To determine if InvB acts as a chaperone for SspA secretion in Salmonella serovar Typhimurium, a strain containing a nonpolar chromosomal deletion of invB was created by allelic exchange. Culture supernatants from the ΔinvB strain were assayed for the presence of proteins secreted by SPI-1 TTSS. This analysis showed reduced levels of secreted SspA in the supernatant, while other type III secreted proteins were not significantly affected (Fig. 3A). Specifically, the SPI-1 type III-secreted protein SspH1-CyaA was found in culture supernatant even in the absence of InvB (Fig. 3C). Further analysis of Western blots by quantitative phosphorimaging indicated that the ΔinvB strain secreted only 10 to 35% of the wild-type levels of SspA (Fig. 3B). These results shows that the secretion defect is specific for SspA and further support the hypothesis that InvB functions as a type III chaperone for SspA.
FIG. 3.
Secretion defects of a nonpolar chromosomal deletion of invB in Salmonella serovar Typhimurium. Culture supernatants were collected from either the wild-type strain (CS401), the ΔinvB strain (PB502), or the ΔinvB strain with a plasmid-borne copy of invB (pB502). Bacterial cultures were grown overnight, and supernatants were collected after centrifugation. Secreted proteins were precipitated with trichloroacetic acid and separated by SDS-PAGE. Proteins were visualized either by Coomassie blue staining (A) or by Western blotting with antibodies specific to SspA (B). Culture supernatants were also collected from wild-type (CS401) and ΔinvB (PB502) strains expressing the SPI-1-secreted fusion protein SspH1-CyaA. Supernatant proteins were separated by SDS-PAGE and analyzed by Western blotting with monoclonal antibodies specific for CyaA (C). Results presented are representative of multiple supernatant preparations.
InvB is required for efficient translocation of SspA through SPI-1 TTSS.
Although SPI-1 TTSS secretes effectors in liquid cultures, the in vivo function of SPI-1 TTSS is to translocate effector proteins into the eukaryotic cytosol. To analyze SspA translocation, an adenylate cyclase reporter system which has been employed widely in the examination of TTSS translocation in Yersinia, Salmonella, and E. coli was utilized (4, 21, 27, 28, 37, 39, 40, 45). The activity of the B. pertussis CyaA adenylate cyclase toxin is dependent upon the eukaryotic cytosolic protein calmodulin. Putative type III translocation signals were fused to the catalytic domain of CyaA and expressed in Salmonella. Eukaryotic cells were infected with these bacteria, and increases in intracellular cAMP were measured as a quantitative indicator of TTSS-dependent protein translocation.
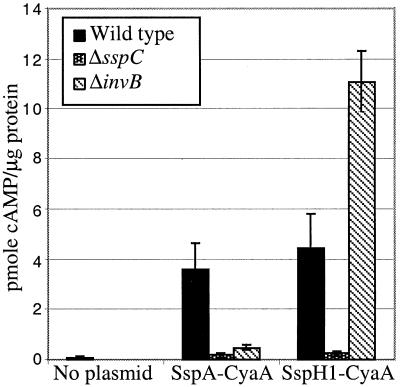
A reporter fusion which contained the first 158 amino acids of SspA fused to the catalytic domain of CyaA was constructed. This fusion was expressed in wild-type, ΔinvB, and ΔsspC (SPI-1 translocon-negative) strains, and their ability to translocate the reporter into RAW264.7 cells was analyzed after a 1-h infection. cAMP levels increased in an SspC-dependent fashion, consistent with the translocation of SspA by SPI-1 TTSS (Fig. 4). Additionally, deletion of invB was found to significantly reduce the amount of SspA-CyaA translocated into the eukaryotic cytosol. In contrast, the invB deletion did not reduce the amount of another known SPI-1-translocated protein, SspH1-CyaA. In fact, the amount of SspH1 translocation seems to have increased. This could be due to the fact that SspA is probably the most abundant SPI-1-secreted protein, and in the ΔinvB strains, the inability of SspA to be translocated could permit other proteins to more readily compete for translocation by SPI-1 TTSS. These experiments not only demonstrate that InvB is necessary for SspA translocation but also suggest that an interaction between InvB and the first 158 amino acids of SspA is sufficient to mediate translocation by SPI-1 TTSS.
FIG. 4.
SPI-1 TTSS translocation phenotype of ΔinvB strain. Intracellular cAMP levels were determined in RAW264.7 macrophages after a 1-h infection with the wild-type strain (CS401), the ΔsspC strain (CAS108), or the ΔinvB strain (PB502) expressing SspA-CyaA (pB500) or SspH1-CyaA (pEM25). cAMP levels were determined by enzyme immunoassay and normalized for protein content. Standard deviations of triplicate wells are shown for an assay representative of three performed.
InvB expression facilitates translocation of SspA by SPI-2 TTSS.
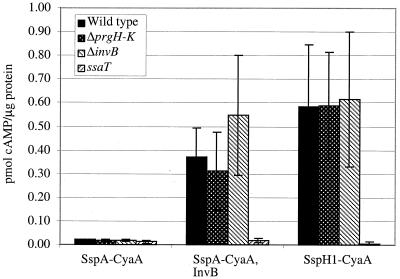
Previous studies have shown that expression of effector proteins along with their cognate chaperones is sufficient for targeting of these proteins for secretion-translocation through heterologous TTSS (11, 12, 34, 35). The two TTSS found in Salmonella serovar Typhimurium, SPI-1 and SPI-2 TTSS, are essentially heterologous; they are expressed under different conditions and appear to be expressed completely independently. Previously, we have shown that SspA-CyaA is not translocated by SPI-2 TTSS in wild-type bacteria (26). We wished to determine if heterologous expression of InvB could confer upon SspA-CyaA the ability to be translocated through SPI-2 TTSS. Salmonella serovar Typhimurium strains containing a plasmid expressing SspA-CyaA or SspA-CyaA and InvB were used to infect RAW264.7 cells for 6 h under conditions which induce SPI-2 but not SPI-1 TTSS activity (28), and intracellular cAMP levels were determined. Increases in cAMP due to the SspA-CyaA fusion were detected when InvB was expressed in either wild-type, SPI-1-defective (ΔprgH), or ΔinvB strains. These increases were not seen in a strain that did not heterologously express InvB or in strains carrying a mutation within an SPI-2 TTSS apparatus gene (ssaT) (Fig. 5). The data show that InvB permits SspA translocation in an SPI-2 TTSS-dependent manner in Salmonella serovar Typhimurium and indicate that InvB is an important regulatory element for SspA translocation.
FIG. 5.
Effects of InvB expression on SPI-2 TTSS translocation. Intracellular cAMP levels were determined in RAW264.7 macrophages after a 6-h infection with the wild-type strain (CS401), the ΔprgH-K strain (TK91), the ssaT strain (EM323), or the ΔinvB strain (PB502) expressing SspA-CyaA (pB500), SspA-CyaA and InvB (pEM120), or SspH1-CyaA (pEM25). cAMP levels were determined as for Fig. 4. Results shown are representative of three assays.
invB alters SspA protein levels without changing transcription levels.
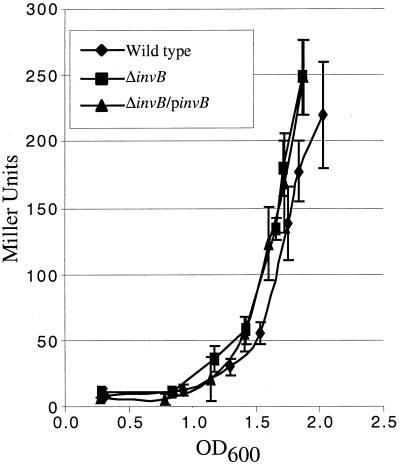
In order to further examine the mechanism by which type III chaperones affect the secretion and translocation of their cognate translocated effector, the effects of a chromosomal invB deletion on various characteristics of SspA were examined. One possible effect that InvB could exert on SspA is to increase the level of sspA mRNA produced. In order to determine the effect of InvB on sspA transcription, expression of an sspA::Tn5lac transcriptional fusion was analyzed in wild-type and ΔinvB strains. β-Galactosidase activity was measured in bacterial cultures over a period when SPI-1 TTSS genes are expressed. No significant difference in β-galactosidase activity was detected between strains (Fig. 6). This implies that the deletion of invB does not affect the transcription of sspA mRNA and cannot account for the differences seen in secretion and translocation.
FIG. 6.
Effect of invB deletion on transcription of sspA. Transcription of sspA was measured in wild-type strains (PB525), ΔinvB strains (PB523), and ΔinvB strains containing a plasmid expressing invB (pB502). All strains contain sspA::Tn5lac chromosomal fusions. Stationary-phase bacteria were diluted in LB medium and grown for 3 h. Samples were assayed for β-galactosidase activity every 30 min from an OD600 of ∼0.3 to ∼2.0. Standard deviations of triplicate samples are shown. These experiments were performed three times with similar results.
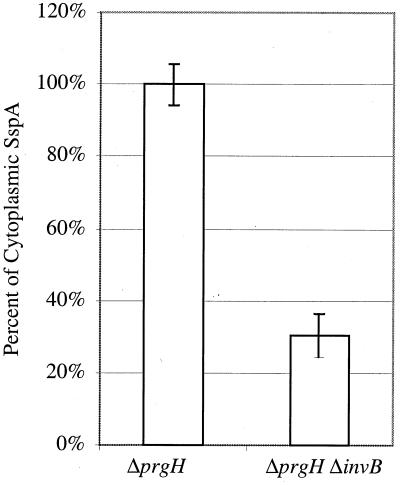
A second possible mechanism by which InvB could affect the secretion-translocation of SspA is to alter the amount of SspA in the bacterial cytoplasm. Decreased SspA cytoplasmic protein levels could result in decreased amounts of protein available for secretion by SPI-1. In an effort to determine the effects of InvB on the total amount of SspA available for secretion, differential protein stability had to be taken into account. Proteins in the supernatant and cellular fractions cannot simply be summed in strains with potentially altered secretion patterns because proteins which have been secreted into the supernatant are protected from degradation by cytoplasmic proteases. Thus, in order to determine the effect of InvB on the cytoplasmic SspA levels, we examined Salmonella serovar Typhimurium strains which were defective for SPI-1-dependent secretion (ΔprgH). The ΔprgH and ΔprgH ΔinvB strains were grown under conditions which induce SPI-1 TTSS genes, and bacterial lysates were collected and analyzed by Western blotting with antibodies specific to SspA. Quantification of SspA revealed that the deletion of invB results in approximately a 70% reduction of SspA within the bacterial cell (Fig. 7). This implies that InvB is needed to achieve optimal levels of SspA in the bacterial cytoplasm.
FIG. 7.
Western blot analysis of cytoplasmic levels of SspA. Bacterial strains deficient for secretion were grown overnight, back diluted, and grown to exponential growth phase. Samples from ΔprgH (TK164) and ΔprgH ΔinvB (PB509) strains were collected, resolved using SDS-PAGE, and analyzed by Western blotting using antibodies specific for SspA. The amount of SspA present in these strains was quantitated using a STORM840 Phosphoimaging System and by analyzing the data with Imagequant V1.2. Percent SspA levels are presented with standard deviations of triplicate samples. These experiments were repeated multiple times.
DISCUSSION
Many proteins secreted by TTSS require specific type III chaperones for efficient secretion and translocation. In this work, the yeast two-hybrid assay was used to identify protein-protein interactions between type III chaperones and secreted proteins. Two interactions between the known type III chaperone-secreted protein pairs SicA-SspC and SicP-SptP were identified along with a third, novel interaction between SspA and InvB (14, 41). The InvB-SspA interaction was subsequently confirmed to be physiologically relevant by isolating a protein complex from the cytoplasm of Salmonella serovar Typhimurium by coimmunoprecipitation. Further coimmunoprecipitations with additional constructs showed that InvB binds to the first 158 amino acids of SspA. Additionally, it was found that invB mutants were specifically defective in the secretion and translocation of SspA. Therefore, in this study we have determined that InvB is the previously unidentified chaperone for SspA. These findings suggest that the homolog of InvB in Shigella species, SpaK, may be the type III chaperone for IpaA and that the yeast two-hybrid assay is a good method for the identification of novel type III chaperone-effector protein pairs.
In addition to facilitating optimal secretion and translocation of its cognate effector proteins through native TTSS, type III chaperones have been shown to mediate secretion and translocation through heterologous TTSS (11, 12, 34, 35). In this study, we have shown that InvB is able to mediate the translocation of SspA-CyaA by the heterologous SPI-2 TTSS. The ability of type III chaperones to mediate translocation in heterologous systems suggests two mechanisms by which type III chaperones may permit translocation by any TTSS. First, the chaperone may physically interact with the TTSS and the secreted protein. In this model, the chaperone recognizes homologous features of TTSS apparatuses and delivers the effector proteins to the TTSS for secretion-translocation. A second hypothesis is that the type III chaperone does not interact directly with the apparatus but binds to the effector protein and stabilizes the conformation of the secretion signal such that it is available for recognition by the TTSS.
In addition to the potential to mediate recognition of secreted proteins by TTSS apparatuses, evidence suggests that type III chaperones are also required for secreted protein production and/or stability. Several mechanisms by which type III chaperones could affect the amount of protein available in the cell for secretion and/or translocation have been suggested. These effects include altering transcription, mRNA stability, translation, and protein stability. For example, one study presents evidence that the type III chaperone SicA is required for transcription of its cognate secreted protein SspC (SipC) (8). More typically, type III chaperones are reported to maintain the cytoplasmic level of their target protein, as was previously shown for the chaperone-effector protein pair SicP-SptP, without altering gene transcription (14). Similar to SicP, InvB was not found to affect the transcription of sspA but was found to affect the amount of SspA protein found in the bacterial cytoplasm. A 70% reduction of SspA was seen in Salmonella serovar Typhimurium when InvB was not present. This decrease is similar to the reduction in the amount of secretion observed, suggesting that the alteration of SspA secretion in the ΔinvB strain may result, at least in part, from a reduction in the cytoplasmic SspA protein pool.
A recent report from Karlinsey et al. illustrates another hypothesis of how type III chaperones act. These authors report that FlgN, the type III chaperone for FlgM, affects the amount of protein available for secretion by regulating translation of FlgM in the flagellar TTSS (22). This exciting result complements previous hypotheses which suggest that an important function of type III chaperones could be to couple translation of the effector protein and secretion through the TTSS (6). In addition, such results could begin to provide a unifying theory of secretion where mRNA and chaperone secretion signals work together to control translational coupling with secretion.
ACKNOWLEDGMENTS
We are grateful to Tyler G. Kimbrough for critically reviewing the manuscript and Kelly Hughes for sharing unpublished data during the preparation of the paper.
This work was supported in part by a Molecular and Cellular Training Grant (GM 07270) to P.A.B., the Poncin Scholarship Fund (E.A.M.), and grant RO1 AI41069-O1A2 (S.I.M.) from the National Institutes of Health.
REFERENCES
- 1.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 2.Bartel P L, Fields S. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–263. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- 3.Bennett J C, Hughes C. From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol. 2000;8:202–204. doi: 10.1016/s0966-842x(00)01751-0. [DOI] [PubMed] [Google Scholar]
- 4.Boland A, Sory M P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5–1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L W, Schneewind O. Type III machines of gram-negative bacteria: delivering the goods. Trends Microbiol. 2000;8:214–220. doi: 10.1016/s0966-842x(99)01665-0. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L W, Schneewind O. Yersinia enterocolitica type III secretion. On the role of SycE in targeting YopE into HeLa cells. J Biol Chem. 1999;274:22102–22108. doi: 10.1074/jbc.274.31.22102. [DOI] [PubMed] [Google Scholar]
- 8.Darwin K H, Miller V L. The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol Microbiol. 2000;35:949–960. doi: 10.1046/j.1365-2958.2000.01772.x. [DOI] [PubMed] [Google Scholar]
- 9.Day J B, Plano G V. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol Microbiol. 1998;30:777–788. doi: 10.1046/j.1365-2958.1998.01110.x. [DOI] [PubMed] [Google Scholar]
- 10.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 11.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 12.Frithz-Lindsten E, Holmstrom A, Jacobsson L, Soltani M, Olsson J, Rosqvist R, Forsberg A. Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol Microbiol. 1998;29:1155–1165. doi: 10.1046/j.1365-2958.1998.00994.x. [DOI] [PubMed] [Google Scholar]
- 13.Frithz-Lindsten E, Rosqvist R, Johansson L, Forsberg A. The chaperone-like protein YerA of Yersinia pseudotuberculosis stabilizes YopE in the cytoplasm but is dispensible for targeting to the secretion loci. Mol Microbiol. 1995;16:635–647. doi: 10.1111/j.1365-2958.1995.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Galán J E. Identification of a specific chaperone for SptP, a substrate of the centisome 63 type III secretion system of Salmonella typhimurium. J Bacteriol. 1998;180:3393–3399. doi: 10.1128/jb.180.13.3393-3399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman L M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 17.Hensel M, Shea J E, Raupach B, Monack D, Falkow S, Gleeson C, Kubo T, Holden D W. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella pathogenicity island 2. Mol Microbiol. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 18.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 20.Iriarte M, Sory M P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones M A, Wood M W, Mullan P B, Watson P R, Wallis T S, Galyov E E. Secreted effector proteins of Salmonella dublin act in concert to induce enteritis. Infect Immun. 1998;66:5799–5804. doi: 10.1128/iai.66.12.5799-5804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlinsey J E, Lonner J, Brown K L, Hughes K T. Translation/secretion coupling by type III secretion systems. Cell. 2000;102:487–497. doi: 10.1016/s0092-8674(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 23.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 24.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee V T, Schneewind O. Type III secretion machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol Rev. 1999;168:241–255. doi: 10.1111/j.1600-065x.1999.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 26.Miao E A, Miller S I. Bacteriophages in the evolution of pathogen-host interactions. Proc Natl Acad Sci USA. 1999;96:9452–9454. doi: 10.1073/pnas.96.17.9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao E A, Miller S I. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc Natl Acad Sci USA. 2000;97:7539–7544. doi: 10.1073/pnas.97.13.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao E A, Scherer C A, Tsolis R M, Kingsley R A, Adams L G, Bäumler A J, Miller S I. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol Microbiol. 1999;34:850–864. doi: 10.1046/j.1365-2958.1999.01651.x. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis S, Inouye H, Oliver D, Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol. 1983;154:366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neyt C, Cornelis G R. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol Microbiol. 1999;31:143–156. doi: 10.1046/j.1365-2958.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 31.Ochman H, Soncini F C, Solomon F, Groisman E A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 33.Rakeman J L, Bonifield H R, Miller S I. A HilA-independent pathway to Salmonella typhimurium invasion gene transcription. J Bacteriol. 1999;181:3096–3104. doi: 10.1128/jb.181.10.3096-3104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossier O, Wengelnik K, Hahn K, Bonas U. The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens. Proc Natl Acad Sci USA. 1999;96:9368–9373. doi: 10.1073/pnas.96.16.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherer C A, Cooper E, Miller S I. Association of the type III secretion translocon protein SspC with the plasma membrane upon Salmonella infection of epithelial cells. Mol Microbiol. 2000;37:1133–1145. doi: 10.1046/j.1365-2958.2000.02066.x. [DOI] [PubMed] [Google Scholar]
- 37.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 39.Sory M P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sory M P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 41.Tucker S C, Galan J E. Complex function for SicA, a Salmonella enterica serovar Typhimurium type III secretion-associated chaperone. J Bacteriol. 2000;182:2262–2268. doi: 10.1128/jb.182.8.2262-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in Ohe secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 44.Woestyn S, Sory M P, Boland A, Lequenne O, Cornelis G R. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol Microbiol. 1996;20:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 45.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 46.Wood M W, Rosqvist R, Mullan P B, Edwards M H, Galyov E E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 47.Zierler M K, Galan J E. Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect Immun. 1995;63:4024–4028. doi: 10.1128/iai.63.10.4024-4028.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]