Adequate clinical management of infectious diseases relies primarily on the accurate identification of the causal micro-organism and the production of reliable information on its antimicrobial susceptibility.1 Traditional diagnostic methods in microbiology have limited the ability of laboratories to provide doctors with timely and clinically relevant information, but recent technology provides results in minutes or hours rather than days or weeks. In particular, molecular biological techniques have increased the speed and sensitivity of detection methods, as well as allowing laboratories to identify organisms that do not grow or grow slowly in culture. These techniques also allow microbiologists to identify genes that result in resistance to antibiotics and to “fingerprint” individual isolates for epidemiological tracking.2 Recognition of newly emerging infectious diseases and control of antibiotic resistance in Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, and common Gram negative bacilli will rely heavily on these new technologies.
Method
We have included references that provide critical information on new approaches to the laboratory diagnosis of infectious diseases. Most were identified by us during our review of the literature, with additional references being found with a Medline search using Grateful Med as the search engine. We searched under the terms infectious diseases, diagnosis, and laboratory.
We have included citations to reviews and to studies that critically compared a new method with an established standard method. Trials in diagnostic microbiology often do not comply with a randomised, double blind design, and we have included only those that meet currently acceptable study design.
New diagnostic methods
Immunoassays
Detection of microbial antigens in clinical samples has great potential for rapid diagnosis. Latex particle agglutination and coagglutination tests, enzyme linked immunoassays, and direct immunofluorescence antibody assays have been available for some years. Although medical microbiology laboratories have recognised the benefits of using these tests (technical simplicity, rapidity, specificity, and cost effectiveness), they have generally continued to use culture methods.3 In spite of their many advantages, immunoassays have poor sensitivity and low negative predictive value. The next generation of optical immunoassays may be more useful diagnostically.
Automated and semiautomated systems
Automated and semiautomated systems have been available for some years but without full realisation of their potential for rapid diagnosis. They fall into two main groups: identification and susceptibility testing instruments and blood culture systems. Whereas some identification and susceptibility testing instruments take as long as traditional methods, others provide results within a single working day.4 The full healthcare benefits are seen when a laboratory is staffed 24 hours each day and doctors are available to receive and act on the information day or night.
Recent advances
New technologies enable microbiology results to be available in minutes or hours rather than days
Immunoassays have benefits of technical simplicity, rapidity, specificity, and cost effectiveness but often have poor sensitivity and low negative predictive value
An ever increasing range of viruses, bacteria, fungi, and protozoa can be detected and characterised by molecular biological methods
Blood culture systems have had considerable impact on the ability to detect bacteraemia. Growth is detected through generation of a radiometric signal or a fluorescent or colorimetric indicator. Most true positive results are detected within 24 to 36 hours. Identification and susceptibility results may be obtained in many blood culture isolates within the same time when a blood culture system is combined with an automated identification or susceptibility testing instrument. Some of the blood culture systems have been adapted for the automated or semiautomated culture of Mycobacterium tuberculosis and other mycobacteria. These commercial systems reduce the traditional dependence on biochemical reactions to identify organisms; avoid the many tedious, labour intensive steps between isolating and reporting clinically significant bacteria; provide rapid results; and perform tests more reproduibly.5,6
Commercial microbiology systems have limitations. Organisms may be incorrectly identified when the isolate shows unusual biochemical reactions or when the database does not include the correct identification. Most systems give the probability of the result being correct, and low probability should not be ignored. The short incubation time of susceptibility tests in some systems may cause problems. Bacteria with heteroresistance to β lactam drugs, inducible resistance mechanisms, or susceptibility gene mutation may be misclassified. The resistance of pneumococci to penicillin, enterococci to glycopeptides, staphylococci resistance to oxacillin, and Enterobacteriaceae to β lactam drugs may be missed.5,7–9 When commercial systems are known to have difficulties, laboratories should use supplemental testing with manual methods that have been proved to be satisfactory for problematic combinations of organisms and drugs.
Molecular biological methods
Nucleic acid probe hybridisation, the polymerase chain reaction, the ligase chain reaction, transcription mediated amplification, other evolving amplification methods, and nucleic acid sequencing form the basis of detecting and characterising an ever increasing range of viruses, bacteria, fungi, and protozoa. This information is needed to type strains for infection control and other epidemiological purposes and to detect resistance genes or their surrogate markers. Nucleic acid probes are commercially available for cytomegalovirus, human papillomavirus, hepatitis B virus, hepatitis C virus, Chlamydia trachomatis, Neisseria gonorrhoeae, Streptococcus pyogenes, and mycobacteria among others. Nucleic acid amplification systems are available for the direct detection in clinical specimens of hepatitis C virus, HIV, M tuberculosis, C trachomatis, and N gonorrhoeae.4,10,11
With increasing availability of cost effective commercial systems, laboratories will be able to capitalise on the extreme specificity, high sensitivity, and rapidity of these molecular approaches.12 In the mean time, challenges need to be overcome, including the need for specialised equipment and segregated rooms in laboratories, the sampling and purifying of target nucleic acid, the use of polymerase inhibitors in the specimens, and the degradation of nucleic acid targets. Currently, these techniques should be applied only to detect micro-organisms whose available diagnostic approaches are markedly insensitive or non-existent or when turnaround times for existing tests are much longer than can be achieved using molecular methods.13
Application of new diagnostic methods
Respiratory infections
Among the first of the rapid diagnostic approaches was immunoassay detection of group A streptococcal antigen in patients with pharyngitis.14 These systems are intended for use in primary care or other ambulatory care settings; they provide results within minutes and are highly specific. Their greatest drawbacks are cost and lack of sensitivity. Negative results must be confirmed by conventional culture. Even the molecular probe shows only 90% sensitivity compared with conventional culture.15
Lower respiratory viral and bacterial infections are common. Laboratory diagnosis of these infections is notoriously unreliable. Earlier rapid methods such as the detection of circulating bacterial polysaccharide in urine and blood by latex agglutination, counterimmunoelectrophoresis, or enzyme linked immunoabsorbent assay lacked sensitivity and specificity.16 Nucleic acid amplification techniques show more potential for detecting a wide range of pathogens, including rhinoviruses, coronaviruses, hantaviruses, respiratory syncytial virus, Bordetella pertussis, C pneumoniae, M pneumoniae, and Coxiella burnetii.13 Infections due to Legionella species, fungi, and Pneumocystis carinii can be detected using molecular techniques but are probably better diagnosed using immunofluorescent methods.
There has been particular interest in the direct detection of M tuberculosis in sputum by nucleic acid amplification. Early diagnosis facilitates effective infection control and initiation of specific treatment. The available commercial systems have excellent specificity. Sensitivity is around 95% for acid fast smear positive specimens but drops to around 65% for smear negative samples.17 At present, amplification methods cannot replace culture methods because of their lack of sensitivity and because molecular methods currently detect only resistance to rifampicin. More conventional systems are needed to detect resistance to other antimycobacterial drugs.
Central nervous system
Detection of the causal agents of meningitis on the basis of immunoassay has been available for some years. The clinical value and usefulness of these assays has been controversial. Generally they are insensitive when compared with culture, and they have been largely abandoned except possibly for the detection of Cryptococcus neoformans.18
Nucleic acid amplification, mainly by the polymerase chain reaction, has been applied to the diagnosis of bacterial and viral meningitis and to viral encephalitis.19 As yet, much of work is in the hands of individual investigators and the methods are not developed to the point where they can be implemented in routine microbiological testing of patients with meningitis.
Viral diseases
Molecular diagnosis of an ever increasing scope of viral infections fills much of the microbiology literature. Included are human papillomavirus, cytomegalovirus, hepatitis B virus, hepatitis C virus, and herpesvirus, to name but a few. Molecular techniques will permit rapid diagnosis in otherwise difficult circumstances, as well as the rational use of specific antiviral therapeutic agents.
Sexually transmitted diseases
Commercially available panels of reagents to detect the common organisms in sexually transmitted diseases are either available or will become so soon. Individual amplification and detection of specific agents has been available for some time. Perhaps the greatest interest has been in the detection of C trachomatis in cervical or urethral swabs and in urine. When compared with enzyme immunoassays and culture, nucleic acid hybridisation assays for this organism have greater sensitivity.20
Molecular methods undoubtedly have enormous potential in diagnosing infectious diseases. New molecular methods will be widely accepted and implemented routinely within the next decade.21
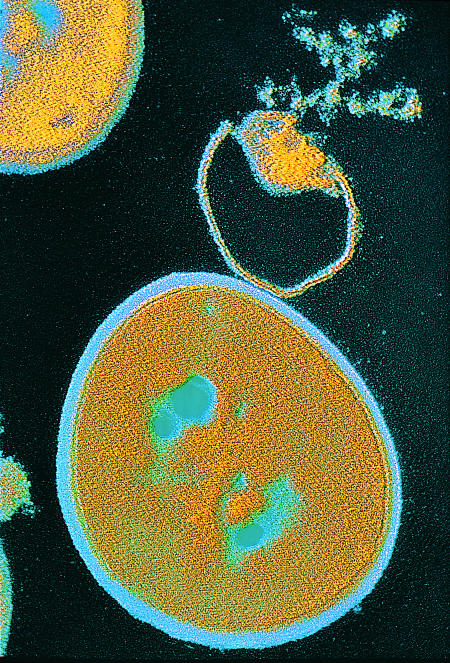
Figure 1.
Lysis of staphylococcus aureus
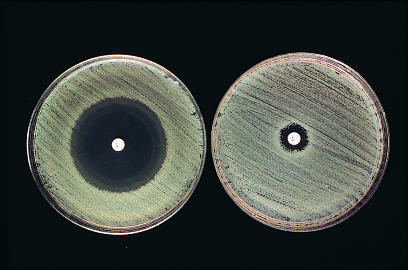
Figure 2.
A clear zone of inhibited bacterial growth (left) shows sensitivity to penicillin
Footnotes
Conflict of interest: None.
References
- 1.Garcia-de-Lomas J, Navarro D. New directions in diagnostics. Pediatr Infect Dis J. 1997;16:S43–S48. doi: 10.1097/00006454-199703001-00004. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Herwaldt LA. The clinical microbiology laboratory and infection control: emerging pathogens, antimicrobial resistance, and new technology. Clin Infect Dis. 1997;25:858–870. doi: 10.1086/515557. [DOI] [PubMed] [Google Scholar]
- 3.La Scolea LJ. Diagnosis of pediatric infections using bacterial antigen detection systems. Clin Microbiol News. 1988;10:21–23. [Google Scholar]
- 4.Bartlett RC, Mazens-Sullivan M, Tetreaulkt JZ, Lobel S, Nivard J. Evolving approaches to the management of quality in clinical microbiology. Clin Microbiol Rev. 1994;7:55–88. doi: 10.1128/cmr.7.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferraro MJ, Jorgensen JH. Instrument-based antibacterial susceptibility testing. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. 6th ed. Washington, DC: American Society of Microbiology; 1995. pp. 1379–1384. [Google Scholar]
- 6.Stager CE, Davis JR. Automated systems for identification of microorganisms. Clin Microbiol Rev. 1992;5:302–327. doi: 10.1128/cmr.5.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishner KK, Linscott A. Comparison of three commercial MIC systems, E test, Fastidious Antimicrobial Susceptibility Panel, and FOX Fastidious Panel for confirmation of penicillin and cephalosporin resistance in Streptococcus pneumoniae. J Clin Microbiol. 1994;32:2242–2245. doi: 10.1128/jcm.32.9.2242-2245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenover FC, Swenson JM, O’Hara CM, Stocker SA. Ability of commercial and reference antimicrobial susceptibility testing methods to detect vancomycin resistance in enterococci. J Clin Microbiol. 1995;33:1524–1527. doi: 10.1128/jcm.33.6.1524-1527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skulnick M, Simor AE, Gregson D, Patel M, Small GW, Kreiswirth B, et al. Evaulation of commercial and standard methodology for determination of oxacillin susceptibility in Staphylococcus aureus. J Clin Microbiol. 1992;30:1985–1988. doi: 10.1128/jcm.30.8.1985-1988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persing DH, editor. PCR protocols for emerging infectious diseases. Washington, DC: American Society for Microbiology; 1996. [Google Scholar]
- 11.Relman DA, Persing DH. Genotypic methods for microbial identification. In: Persing DH, editor. PCR protocols for emerging infectious diseases. Washington, DC: American Society for Microbiology; 1996. pp. 3–31. [Google Scholar]
- 12.Persing DH, Smith RF, Tenover FC, White TJ, editors. Diagnostic molecular microbiology: principles and applications. Washington, DC: American Society for Microbiology; 1993. [Google Scholar]
- 13.Ieven M, Goossens H. Relevance of nucleic acid amplification techniques for diagnosis of respiratory tract infections in the clinical laboratory. Clin Microbiol Rev. 1997;10:242–256. doi: 10.1128/cmr.10.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulman ST. Streptococcal pharyngitis: diagnostic considerations. Pediatr Infect Dis J. 1994;13:567–571. doi: 10.1097/00006454-199406000-00034. [DOI] [PubMed] [Google Scholar]
- 15.Steed LL, Korgenski EK, Daly JA. Rapid detection of Streptoccus pyogenes in pediatric patient specimens by DNA probe. J Clin Microbiol. 1993;31:2996–3000. doi: 10.1128/jcm.31.11.2996-3000.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nohynek H, Eskola J, Kleemola M, Jalonen E, Saikku P, Leinonen M. Bacterial antibody assays in the diagnosis of acute lower respiratory tract infections in children. Pediatr Infect Dis J. 1995;14:478–484. doi: 10.1097/00006454-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 17.D’Amato RF, Wallman AA, Hochstein LH, Colaninno PM, Scardamaglia M, Ardila E, et al. Rapid diagnosis of pulmonary tuberculosis by using Roche Amplicor Mycobacterium tuberculosis PCR test. J Clin Microbiol. 1995;33:1832–1834. doi: 10.1128/jcm.33.7.1832-1834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins MD, Mirret S, Reller B. Rapid bacterial antigen detection is not clinically useful. J Clin Microbiol. 1995;33:1486–1491. doi: 10.1128/jcm.33.6.1486-1491.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong DU, Greisen KS. PCR detection of bacteria found in cerebrospinal fluid. In: Persing DH, Smith TF, Tenover FC, White TJ, editors. Diagnostic molecular microbiology: principles and applications. Washington, DC: American Society for Microbiology; 1993. pp. 300–308. [Google Scholar]
- 20.Farrell DJ, Haran MV, Park BW. Comparison of PCR/nucleic acid hybridization and EIA for the detection of Chlamydia trachomatis in different populations in a regional centre. Pathology. 1996;28:74–78. doi: 10.1080/00313029600169583. [DOI] [PubMed] [Google Scholar]
- 21.Low DE, McGeer A. The use of molecular biology techniques for diagnostic microbiology and hospital epidemiology. New Horiz. 1995;3:161–169. [PubMed] [Google Scholar]