ABSTRACT
Arboviruses are an existing and expanding threat globally, with the potential for causing devastating health and socioeconomic impacts. Mitigating this threat necessitates a One Health approach that integrates vector surveillance, rapid disease detection, and innovative prevention and control measures. In Southern Africa, limited data on the epidemiology of arboviruses, their vectors, and their hosts prevent an effective response. We reviewed the current knowledge on arboviruses in Southern Africa and identified opportunities for further research. A literature search was conducted to identify studies published on arboviruses in 10 tropical and temperate countries of the Southern African Development Community (SADC) from 1900 onward. We identified 280 studies, half (51.1%) originating from South Africa, that described 31 arboviral species, their vectors, and their clinical effects on hosts reported in the region. Arboviral research flourished in the SADC in the mid-20th century but then declined, before reemerging in the last two decades. Recent research consists largely of case reports describing outbreaks. Historical vector surveillance and serosurveys from the mid-20th century suggest that arboviruses are plentiful across Southern Africa, but large gaps remain in the current understanding of arboviral distribution, transmission dynamics, and public health impact.
KEYWORDS: Arboviruses, vectors, public health, zoonoses, Southern Africa
Introduction
The effects of emerging and reemerging pathogenic infections can be catastrophic and are therefore of increasing global health concern. Prime recent examples include the global coronavirus pandemic and the Marburg virus outbreak in Tanzania [1]. Naturally, these infections result from cross-species viral transmission from animals to humans. Arthropod-borne viruses (arboviruses), which have their sylvatic cycle in vertebrates and are capable of transmission from animals to humans, are notable causes of many vector-borne human and veterinary diseases including zika (ZIKV), chikungunya (CHIKV), yellow fever (YFV), and dengue (DENV) [2,3]. Arboviruses’ global spread has been attributed predominantly to increased proximity between sylvatic hosts and immunologically naive hosts, in the presence of competent hematophagous arthropod vectors [4]. This triad forms the basic conditions necessary to facilitate viral transmission and spillover events.
Although arboviruses frequently circulate in tropical, subtropical and temperate regions of the world, most arboviral research conducted in the African continent has been concentrated in Central, East, and West Africa, with relatively little research done in Southern Africa [5]. Anthropogenic perturbances and environmental fluctuations contribute to dynamic shifts in the geographic distribution of arboviral reservoirs and their vectors into novel areas [5,6]. Climate change has also led to recent changes in vector behavioral patterns [7]. Consequently, arboviral disease outbreaks have expanded and may continue to spread to new areas, with devastating results [8,9]. In 2022, to support the global response to arboviruses, the World Health Organisation (WHO) established the Global Arbovirus Initiative, which aims, in part, to address gaps in arboviral research [10]. This scoping review aims to aggregate and assess the knowledge achieved, and the gaps that remain in arboviral research in Southern Africa. Addressing these gaps will be critical to improving disease surveillance and emergency response systems to mitigate the detrimental health and economic effects of future arboviral outbreaks.
Arboviruses
Arboviruses are obligate viruses that depend on hematophagous arthropod vectors for transmission between vertebrate hosts. The term arbovirus is not a taxonomic indicator but refers to a polyphyletic group of RNA viruses and one DNA virus, African Swine Fever virus [11]. RNA viruses lack a proof-reading RNA-dependent RNA polymerase, resulting in high mutation rates (averaging one mutation per genome replication round) that spread rapidly in large populations [12,13]. Consequently, arboviruses have vast genetic variability that has been associated with high adaptive and phenotypic plasticity and with the emergence of novel strains [14–16]. Additionally, a myriad of external factors, simply categorized as environmental and anthropogenic activities, have influenced and continue to contribute to the global distribution and transmission of arboviruses (Figure 1). Generally, arboviruses are host specific, but opportunistic virus species arise in response to environmental perturbance, adapt to new hosts, and become catalysts of both human and veterinary spill over events [8,18].
Figure 1.
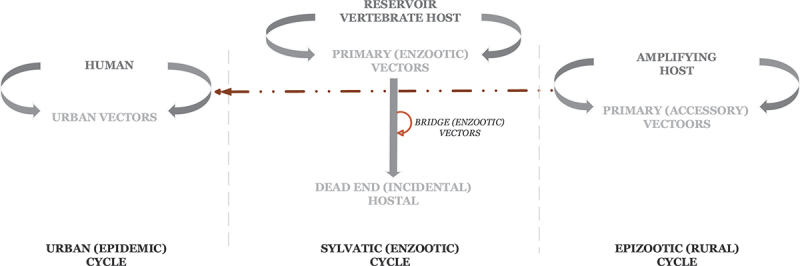
A schematic diagram of arboviral cycles. The schematic diagram adapted from Go et al. [17] illustrates the replicative cycles of arboviruses, how each cycle is maintained and the possible interaction between cycles.
Factors such as urbanization, migration, and climate change have contributed to the expanded and overlapping distribution of arboviruses, their vectors, and hosts [4,6,9]. Particularly concerning are the Flaviviridae (DENV and ZIKV) and Togaviridae (CHIKV) families, which can cause deadly outbreaks in humans [19–22]. Clinical manifestations of these infections in humans range from asymptomatic cases to severe illness and even death. As mild symptoms resemble other common infections such as malaria and influenza, misdiagnosis becomes common and may lead to incorrect treatment [5,23]. Conversely, severe symptoms can manifest as febrile illness, neurological syndromes, debilitating arthralgia, and/or hemorrhagic fever, imposing a significant socioeconomic burden on affected communities and countries [24–26]. Developing nations in sub-Saharan Africa, where competent vector species are abundant, may bear a greater economic impact from arboviral disease outbreaks [27–29].
Transmission of arboviruses
Pathogenesis begins with transmission, a process that facilitates the perpetual preservation of a pathogen’s genetic specificity and identity [30]. Several transmission mechanisms, broadly categorized as either non-biological or biological, exist for arboviruses. In the first category, an opportunistic species enters a susceptible host directly, leading to infection. The latter category requires the presence of both a competent vector and a susceptible host. Unsurprisingly, regardless of the mode of transmission, pathogens use any means necessary to ensure their survival. In general, arboviral transmission results in an array of interactions with direct pathologic outcomes that determine the overall eco-epidemiological impact of these viruses [2,31].
Non-biological transmission
Direct transmission is a widespread non-biological transmission mechanism amongst major arboviral groups as it is versatile and facilitates arboviral entry through innumerable circumstances (e.g. contamination of food and beverages; behaviors that cause injury, abrasions, and lesions) [3].
Another form of non-biological transmission is mechanical transmission, in which viral material is spread via contact with contaminated arthropod mouthparts. Mechanical transmission is assumed to have arisen independently and simultaneously with the evolution of hematophagy in arthropods [3]. Transmission efficiency depends on the arthropod vector’s feeding behaviors and patterns. Compared to insects, acarines (ticks) are less efficient mechanical transmitters as they feed for long uninterrupted periods and tend to remain on the same host [3]. Effective mechanical transmission also requires high virus titers due to low blood volumes on mouthparts [3]. Mechanical transmission occurs with greatest efficiency when vector and host populations are densely concentrated [3].
Biological transmission
Successful biological transmission of pathogens hinges on the complicated network formed by the triad of pathogen, vector, and host. Interactions are largely influenced by ecological factors that either promote or demote transmission. Pathogens must adapt to at least one of the two possible modes of biological transmission: vertical and horizontal [32]. Vertical transmission (also known as hereditary or transovarial transmission) transfers pathogenic agents from parent to progeny, whilst horizontal transmission refers to all other non-parental modes of transmission (e.g. sexual, vector-borne) between hosts and/or vectors [5,33]. These two modes can and often do occur in combination (mixed-mode transmission) to increase survivability, especially during adverse events [34]. Pathogens tend to use vertical transmission to persist in an environment when conditions for available hosts are unfavorable; in more optimal conditions, pathogens can adopt horizontal transmission, which allows for increased genetic variation by introducing mutations through recombination and reassortment [33]. For arboviruses, the pathogen-vector-host network is primarily maintained by horizontal transmission [5,33,35].
Hematophagy is a vital component of the arthropod gonotrophic cycle, as blood meals provide a source of protein. Arthropods may become infected with arboviruses when they ingest a blood meal from an infected vertebrate. Viral transmission to a feeding arthropod is more likely when the vertebrate has a higher blood concentration of virus and a longer duration of viremia [3]. The arbovirus develops in the arthropod, and viral transmission from the arthropod vector to a new, uninfected vertebrate may transpire during blood feeding. This becomes a naturally maintained cycle [35]. It is worth noting that arboviral transmission cycles are disparate because a vector’s biology, host range, geographical spread, and dispersal patterns also affect the geographic distribution of arboviruses [11].
Three transmission cycles have been well described: enzootic sylvatic, epizootic, and urban cycles. In the enzootic sylvatic cycle, arboviruses amplify within and are transmitted to the sylvatic host by a primary vector. The virus can be harbored indefinitely in the sylvatic host, enabling the virus to persist at low concentrations within sylvatic populations. Reinfection occurs with little to no ill effect on the sylvatic host. In addition, in the presence of an enzootic and/or bridge vector (an arthropod that has a broad host range and facilitates transmission to nearby susceptible populations), arboviral transmission to a dead-end or incidental host is possible [6,17]. This incidental transmission is termed a spillover event. Humans are not central in this transmission cycle; they often are dead-end hosts, incapable of transmitting the virus back to its definitive or sylvatic host. However, when human populations are capable of re-infecting wild populations, a spillback event may occur [17]. In summary, humans could be infected during sylvatic amplification when they enter the forest for their activities, or directly by some vector species that could enter and feed in villages, even indoors. This has been demonstrated in Senegal, for example [36]). Moreover, the co-feeding of vectors on the same infected host may result in vector-to-vector transmission and has been at least described for West Nile virus (WNV) [2,37,38].
In the epizootic cycle, transmission occurs amongst domestic and non-domestic animals via a primary or accessory arthropod vector. Veterinary outbreaks occur when arboviruses are amplified within a domestic animal population with viral levels high enough to infect vectors. Human populations in proximity to these animals may be at risk of infection [11,17]. Furthermore, humans with high viremia levels can themselves become amplification hosts, serving as the viral source for mosquitoes that then become the main vector in an urban cycle. It is the urban cycle that temporarily exploits humans as amplification hosts during epidemics and thus has a global impact. Arboviruses like CHIKV, YFV, and DENV use this cycle, causing a varying range of clinical manifestations in humans that can result in high morbidity and mortality [11,39,40].
The greatest threat to pathogens is herd immunity. This occurs when a high proportion of community members develop protective antibodies against a disease, leading to a decline in the spread of the disease between individuals [41,42]. To circumvent this threat, arboviruses have devised three major strategies. In the first, arboviruses move to a novel area that has a small, immunologically naïve population with low immunity. The second strategy makes use of vertebrate species with shorter life spans and high fecundity rates (e.g. rodents, birds) to ensure an incessant supply of susceptible hosts [43]. The last strategy adopted is immune evasion. Through immune evasion, viruses hide in specific tissues and organs, remaining infectious for undetermined periods regardless of high concentrations of antibodies circulating within the vertebrate’s blood [2,44]. Often, the first and second strategies are associated with acute, veterinary infections whilst immune evasion is characteristic of chronic infections in humans [30].
Arboviral vectors
Hematophagy in arthropods has independently arisen in four insect orders (Anoplura, Diptera, Hemiptera and Siphonaptera) and among ticks (order Ixodida) [3]. Vector feeding behavioral patterns have evolved to synchronize feeding activities with times optimal for successfully attaining a blood meal from a vertebrate host. Consequently, blood feeding behaviors vary. Moreover, vectors possess host preferences influenced by genetic and ecological factors. Notably, certain vector species exhibit opportunistic behaviors, adjusting their host selection depending on the availability of vertebrates in a given ecological environment [40,45].
The primary arbovirus vectors are mosquito species from the Culex and Aedes genera [6]. Culex species are primarily zoophilic. In particular, they are the main ornithophilic mosquito species [46,47], and thus have been associated with veterinary arboviral disease outbreaks [45]. However, they do also act as bridge vectors for WNV causing human epidemics [48]. Aedes species exhibit variable degrees of feeding propensity and host specificity, with both zoophilic and anthropophilic tendencies, and are thus associated with most human outbreaks [45]. Globally, Ae. aegypti and Ae. albopictus are the dominant arboviral vectors in tropical and Mediterranean regions of the world [49]. Aedes aegypti exhibits particular phenotypic and behavioral plasticity that enables it to thrive in human populated settlements and modern urban environments. As a result, most human arboviral outbreaks in Africa, including Southern Africa, are caused by Ae. aegypti [50]. Notably, mosquito species have different vector competencies for transmitting viruses to humans [51].
Sylvatic hosts of arboviruses
In the natural habitats of non-human primates (NHP), arboreal mosquitoes transmit arboviruses from infected to naïve animals through a sylvatic transmission cycle. By the same modality, arbovirus infections transmitted by highly anthropophilic mosquitoes can spread rapidly amongst humans [40]. Sylvatic hosts have been well documented for DENV, ZIKV, CHIKV and YFV whilst hosts for other arboviruses are still yet to be determined [52]. Urbanization and deforestation are two critical factors that have increased habitat loss for wildlife and have led to greater co-occurrence between NHP and humans. These factors create interactions with biomedical and epidemiological consequences [53]. Among NHP of interest, Chlorocebus pygerythrus (vervet monkey) has an extensive geographical distribution in Africa, including the Southern regions of the continent, which encroaches into human territories [54]. Vervet monkeys have great biomedical importance as natural hosts of Simian Immunodeficiency Virus, a close relative of Human Immunodeficiency Virus (HIV) [53,55]. One of the first reports alluding to the possibility of vervet monkeys harboring arboviruses followed a viral endemic that erupted from lab animals in Germany [56]. The association between arboviruses and vervet monkeys aroused global interest, resulting in further characterization of members of the Chlorocebus genus from various African countries. Using Southern African vervet monkey biomaterials, McIntosh identified myriad viral species including Marburg, CHIKV and Bunyamwera virus (BUNV) [57]. McIntosh’s discovery was, and still is, pivotal to epidemiological and viral research, as the findings show that NHP harbor many unidentified arbovirus species. The other known species of NHP from Southern Africa found infected with arboviruses (particularly CHIKV) is the Cape baboon (Papio ursinus) [52]. These baboons are widespread in the region and also live in close proximity to humans [57,58].
Birds have also been found to be sylvatic hosts of some zoonotic pathogens including arboviruses [4]. However, birds are less susceptible to pathogens because of their high body temperature, specificity to strains (among arboviruses, predominantly associated with WNV transmission) and excellent immune system [59,60]. Despite their lower susceptibility, birds are fundamental to the global spread of zoonotic pathogens by acting as natural hosts, reservoirs or amplifying hosts. Birds’ various roles in arboviral transmission have yet to be thoroughly investigated, but in Southern Africa, they have been identified as sylvatic and/or amplifying hosts of Shuni (SHUV), Usutu (USUV), WNV, BUNV and Sindbis (SINV) viruses [5].
Impact of arboviruses in Southern Africa
A plethora of arboviruses of African origin currently circulate on the continent, yet the public health impacts remain undetermined in many respects (Table 1). Understanding arboviral transmission dynamics and improving surveillance for the timely detection of arboviral diseases are key components of an effective, integrated response [27]. Extensive reviews have been conducted on arboviral diseases and outbreaks in Central, East and West Africa by Gould et al. [61], Mbanzulu et al. [62], Marchi et al. [63], Nyaruaba et al. [64] and Agboli et al. [65]. However, comprehensive data from the Southern African Development Community (SADC), a region that consists of 16 countries in Southern Africa, are lacking [66]. This knowledge gap hinders public health preparedness and implementation of effective emergency response strategies in Southern Africa, with the potential for significant health and economic consequences [27,28]. Southern Africa has demonstrated a greater level of preparedness for zoonotic diseases compared to other sub-Saharan countries, but current emergency response strategies are still limited in scope, and current capacities for arboviral surveillance, detection, and control remain inadequate [28,67]. The SADC countries, like other sub-Saharan countries, already faces challenges from other pathogens including HIV/Acquired Immunodeficiency Syndrome (AIDS), malaria, and tuberculosis which further strain the ability to respond to potential new arbovirus outbreaks. Consequently, strategies against ‘pathogen x’ remain rudimentary. Expanded research on arboviruses in the SADC, conducted with data-driven approaches within a One Health framework, is therefore critical.
Table 1.
Polyphyletic classification of arboviruses.
| Nucleic acid | Family | Genus | Examples of species |
|---|---|---|---|
| Positive sense single stranded RNA | Flaviviridae (4 genera and 89 species) | Alphavirus | Chikungunya virus |
| O’nyong-nyong virus | |||
| Flaviviridae (4 genera and 89 species) | Flavivirus | Yellow fever virus | |
| Dengue virus | |||
| West Nile virus | |||
| Hepacivirus | Hepatitis C virus | ||
| Hepatitis B virus | |||
| Pegivirus | Pegivirus A | ||
| Pegivirus B | |||
| Pegivirus | Pegivirus D | ||
| Pegivirus E | |||
| Negative sense single stranded | Bunyaviridae (5 genera and 95 species) | Othorbunyavirus | Bunyamwera virus |
| Bwamba virus | |||
| Nairovirus | Dugbe virus | ||
| Crimean-Congo haemorrhagic fever virus | |||
| Phlebovirus | Rift Valley Fever virus | ||
| Sandfly fever Naples virus | |||
| Hantavirus | Hantaan virus | ||
| Puumala virus | |||
| Tospovirus | Tomato spotted wilt virus | ||
| Impatiens necrotic spot virus | |||
| Orthomyxoviridae (7 genera and 9 speices) | Thogotovirus | Dhori thogotovirus | |
| Thogoto thogotovirus | |||
| Alhpainfluenzavirus | Influenza A virus | ||
| Betainflunezavirus | Influenza B virus | ||
| Deltainfluenzavirus | Influenza D virus | ||
| Gammainfluenzavirus | Influenza C virus | ||
| Isavirus | Salmon isavirus | ||
| Quaranjavirus | Johnston Atoll quaranjavirus | ||
| Quaranfil quaranjavirus | |||
| Rhabdoviridae: Subfamily Alpharhabdovirinae (26 genera and 169 species) | Almendravirus | Balsa almendravirus | |
| Coot Bay almendravirus | |||
| Alphanemrhavirus | Xingshan alphanemrhavirus | ||
| Xingzhou alphanemrhavirus | |||
| Alphapaprhabdovirus | Hubei alphapaprhabdovirus | ||
| Pararge alphapaprhabdovirus | |||
| Alpharicinrhavirus | Blanchesco alpharicinrhavirus | ||
| Bole alpharicinrhavirus | |||
| Arurhavirus | Inhangapi arurhavirus | ||
| Xiburema arurhavirus | |||
| Barhavirus | Bahia barhavirus | ||
| Muir barhavirus | |||
| Caligrhavirus | Caligus caligrhavirus | ||
| Lepeophtheirus caligrhavirus | |||
| Curiovirus | Iriri curiovirus | ||
| Iriri curiovirus | |||
| Ephemerovirus | Ephemerovirus | ||
| Hayes ephemerovirus | |||
| Hapavirus | Flanders hapavirus | ||
| Joinjakaka hapavirus | |||
| Ledantevirus | Fikirini ledantevirus | ||
| Fikirini ledantevirus | |||
| Lostrhavirus | Hyalomma lostrhavirus | ||
| Lonestar lostrhavirus | |||
| Lyssavirus | Rabies Lyssavirus | ||
| Taiwan bat lyssavirus | |||
| Merhavirus | Merida merhavirus | ||
| Tritaeniorhynchus merhavirus | |||
| Mousrhavirus | Moussa mousrhavirus | ||
| Ohlsrhavirus | Culex ohlsrhavirus | ||
| Ohlsdorf ohlsrhavirus | |||
| Perhabdovirus | Perch perhabdovirus | ||
| Sea trout perhabdovirus | |||
| Sawgrhavirus | Island sawgrhavirus | ||
| Minto sawgrhavirus | |||
| Sigmavirus | Lousefly sigmavirus | ||
| Drosophila melanogaster sigmavirus | |||
| Sprivivirus | Carp sprivvirus | ||
| Pike fry sprivivirus | |||
| Sripuvirus | Chaco sripuvirus | ||
| Chaco sripuvirus | |||
| Sunrhavirus | Dillard sunrhavirus | ||
| Garba sunrhavirus | |||
| Tibrovirus | Bas-Conga tibrovirus | ||
| Ekpoma 1 tibrovirus | |||
| Tupavirus | Durham tupavirus | ||
| Klamath tupavirus | |||
| Vesiculovirus | Jurona vesiculovirus | ||
| Yug Bogdanovac vesiculovirus | |||
| Rhabdoviridae: Subfamily Betarhabdovirinae (6 genera and 57 species) | lphanucleorhabdovirus | Peach alphanucleorhabdovirus | |
| Eggplant mottled dwarf alphanucleorhabdovirus | |||
| Betanucleorhabdovirus | Sowthistle yellow vein betanucleorhabdhovirus | ||
| Trefoil betanucleorhabdovirus | |||
| Cytorhabdovirus | Wuhan 4 insect cytorhabdovirus | ||
| Yerba mate cytorhabdovirus | |||
| Dichorhavirus | Orchid fleck dichorhavirus | ||
| Coffee ringspot dichorhavirus | |||
| Gammanucleorhabdovirus | Maize fine streak gammanucleorhabdovirus | ||
| Varicosavirus | Alopecurus varicosavirus | ||
| Trifolium varicosavirus | |||
| Rhabdoviridae: Subfamily Gammarhabdovirinae (8 genera and 20 species) | Alphacrustrhavirus | Wenling alphacrurhavirus | |
| Zhejiang alphacrustrhavirus | |||
| Alphadrosrhavirus | Hubei alphadrosrhavirus | ||
| Shayang alphadrosrhavirus | |||
| Alphahymrhavirus | Cinereus alphahymrhavirus | ||
| Hirtum alphahymrhavirus | |||
| Betahymrhavirus | Austriaca betahymrhavirus | ||
| Heterodontonyx betahymrhavirus | |||
| Betanemrhavirus | Hubei betanemrhavirus | ||
| Shayang betanemrhavirus | |||
| Betapaprhavirus | Frugiperda betapaprhavirus | ||
| Sylvina betapaprhavirus | |||
| Betaricinrhavirus | Chimay betaricinrhavirus | ||
| Scapularis betaricinrhavirus | |||
| Novirhabdovirus | Piscine novirhabdovirus | ||
| Salmonid novirhabdovirus | |||
| Double stranded RNA | Reoviridae: Subfamily Sedoreovirinae (6 genera and 35 species) | Cardoreovirus | Eriorcheir sinensis reovirus |
| Mimoreovirus | Microminas pusilla reovirus | ||
| Orbivirus | Bluetongue virus | ||
| Chenuda virus | |||
| Phytoreovirus | Rice dwarf virus | ||
| Wound tumor virus | |||
| Rotavirus | Rotavirus A | ||
| Rotavirus B | |||
| Seadornavirus | Banna virus | ||
| Kadipiro virus | |||
| Reoviridae: Subfamily Spinareovirinae (9 genera and 49 species) | Aquareovirus | Aquareovirus B | |
| Aquareovirus G | |||
| Coltivirus | Colorado tick fever virus | ||
| Eyach virus | |||
| Cypovirus | Cypovirus 1 | ||
| Cypovirus 2 | |||
| Dinovernavirus | Aedes pdeudoscutellaris reovirus | ||
| Fijivirus | Maize rough dwarf virus | ||
| Pangola stunt virus | |||
| Idnoreovirus | Idnoreovirus 1 | ||
| Idnoreovirus 2 | |||
| Mycoreovirus | Mycoreovirus 1 | ||
| Mycoreovirus 2 | |||
| Orthoreovirus | Avian orthoreovirus | ||
| Mammalian orthoreovirus | |||
| Oryzavirus | Echinochloa ragges stunt virus | ||
| Rice ragged stunt virus | |||
| Double stranded DNA | Asfarviridae (1 genus and 1 species) | Asfarvirus | African Swine Fever Virus |
This scoping review aims to provide a historical overview of reported arboviral species in the SADC region. The outcomes of such research will contribute to raising regional awareness about arboviruses, highlight opportunities for improving surveillance and intersectoral collaborations, and guide emergency management strategies. Recognition of existing research and knowledge gaps is essential to enhance our understanding of arboviruses across all sectors, as zoonotic diseases know no borders and pose a constant threat.
Methods
A literary search was conducted to identify articles reporting on arboviral diseases found in ten of the sixteen SADC countries [66]. The following 20 SADC countries were included due to their sub-tropical and temperate climates, which create relatively similar climatic conditions for arbovirus vectors: Angola, Botswana, Eswatini, Lesotho, Malawi, Mozambique, Namibia, South Africa, Zambia, and Zimbabwe. The Democratic Republic of Congo and Tanzania were excluded due to their equatorial climates. Comoros, Madagascar, Mauritius, and Seychelles were excluded because they are islands, thus are geographically isolated and more prone to extreme weather conditions, such as hurricanes, when compared to conditions of the continental mainland.
Relevant studies were searched in health-related databases (PubMed, Google Scholar, and SABINET) using the keywords: arboviruses OR arboviral disease AND Southern Africa OR both the old (pre-independence) and the current name of a Southern African country, as shown in Table 2. The search was limited to studies published in English or at least having an English abstract from the 1st of January 1900 until NaN Invalid Date . Case reports and cross-sectional, retrospective, prospective, and observational studies were included in the assessment criteria. Titles and abstracts of the selected articles were examined to identify reported arboviral disease cases. Relevant papers were thereafter manually crosschecked to identify further references. The taxonomy of arboviruses was cross-referenced with the International Committee on Taxonomy of Viruses (ICTV) for accurate and updated classifications [68].
Table 2.
Southern African countries names pre- and post-independence.
| Country: Old Name Pre-Independence |
Country: New Name Post-Independence |
Year of Independence | Country: Old Name Pre-Independence |
Country: New Name Post-Independence |
Year of Independence |
|---|---|---|---|---|---|
| Reino de Angola | Angola | 1975 | Portuguese East Africa | Mozambique | 1975 |
| Bechuanaland Protectorate* | Botswana | 1966 | South-West Africa | Namibia | 1990 |
| Swaziland | Eswatini** | 1968 | Union of South Africa | South Africa | 1961 |
| Basutoland | Lesotho | 1966 | Northern Rhodesia | Zambia | 1964 |
| Nyasaland | Malawi | 1966 | (Southern) Rhodesia | Zimbabwe | 1980 |
*‘Protectorate’ is dropped to make communication easy to read in the main text.
**The name Swaziland was changed to Eswatini during its 50th post-independence celebration (2018).
For consistency in reporting the geographic locations of historical data, here we use the country names that were given in the original publications, which do not necessarily match the current country names.
Results
From the literature reviewed, we identified 266 publications that described arboviruses in the SADC region. Publications most often referenced South Africa (143, 51.1%), followed by Mozambique (34, 12.1%), multiple countries of the SADC (36, 12.9%), Angola (28, 10.0%), Zimbabwe (15, 5.4%), Zambia (11, 3.9%), Botswana (7, 2.5%), Namibia (5, 1.8%), and Malawi (1, 0.4%) (Figure 2).
Figure 2.
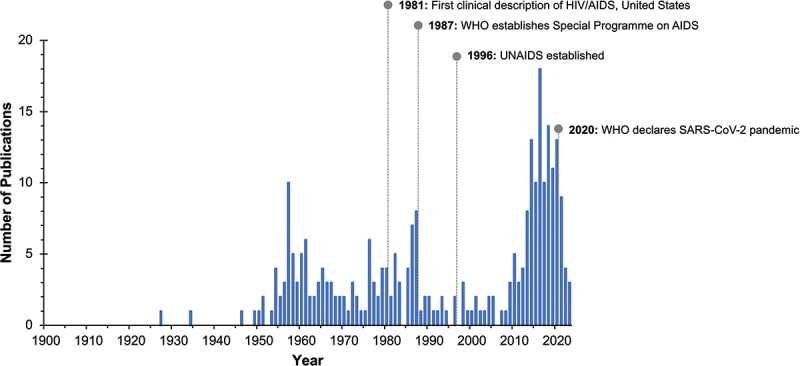
Publications related to arboviruses in 10 countries of the Southern African Development community (SADC), 1900–2023, and other key public health events. Abbreviations: AIDS, acquired Immunodeficiency syndrome; HIV, human Immunodeficiency virus; UNAIDS, joint united nations programme on HIV/AIDS; WHO, World Health Organisation.
A total of 31 arboviral species were described within the SADC region. Ten species have only been reported in limited fashion in Southern Africa. The majority of the identified species were last intensively studied prior to the 1990s. After an approximate 20-year gap with minimal published arbovirus work within the SADC region, research gradually resumed from 2009 onwards (Figure 3). These recent studies primarily focused on reported cases of DENV, YFV, CHIKV, ZIKV, Rift Valley fever virus (RVFV), WNV, and SINV. In all serosurveys, cross-reactivity amongst arboviral groups was a confounding factor that complicated result interpretation, and necessary precautions were implemented to prevent misinterpretation and/or misdiagnosis.
Figure 3.
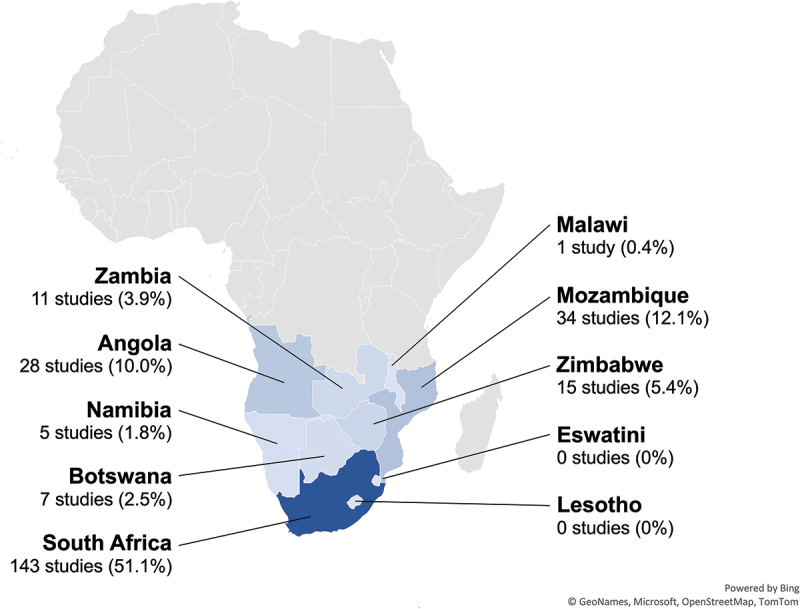
Publications related to arboviruses in 10 countries of the Southern African Development community (SADC), 1900–2023, by country. An additional 36 studies (12.9%) included data from multiple SADC countries.
Published vector surveillance studies within the SADC identified numerous arboviral vector species, mostly mosquitoes from the Aedes and Culex genera, and have contributed greatly to the general understanding of arboviral vector competence. Importantly, recent surveillance has also revealed the presence of vectors previously thought to be absent from the SADC, namely Ae. albopictus [69].
Family: bunyaviridae
Bunyamwera virus
Bunyamwera virus (BUNV) was first isolated from Aedes mosquitoes caught in the Semliki Forest of Uganda [70]. A second strain, isolated in 1955 from Ae. circumluteolus captured in Tongaland, Union of South Africa, was the first report of BUNV in Southern Africa [71]. Aedes circumluteolus appears to serve as the primary vector species for BUNV in the region [71,72]. In humans, BUNV infection tends to cause a febrile illness with mild symptoms (headache, rash, joint pains), though symptoms may be more pronounced in children and immunocompromised hosts [73]. The first described case of human BUNV infection in the SADC was that of a 13-year-old boy who developed fever, headache, and neck stiffness after assisting with mosquito captures in coastal Natal Province, Union of South Africa, in 1957 [72]. Serosurveys subsequently identified antibodies in residents of the Okavango Basin (Bechuanaland Protectorate) and Caprivi Strip (South-West Africa), in addition to the Natal Province [72,74]. No published data exist on more recent BUNV presence in the SADC.
Shuni virus
Shuni virus (SHUV) was first isolated in Nigeria in the 1960s from cattle, Culicoides biting midges, and humans [75–77]. Since then, several mosquito species belonging to the genera Mansonia, Culex, Anopheles, and Aedes have been identified as potential vectors of SHUV [78,79]. Due to their wide host and vector ranges, identifying epidemiological vectors of SHUV has proven difficult. In their study based in the North-Eastern portion of South Africa, Guarido et al. [78] found that Ma. uniformis, Ae. mcintoshi and Ae. subargenteus mosquitoes were positive for SHUV and may potentially act as bridge vectors for the virus. However, SHUV was easily detected in Culicoides midges from Mpumalanga and Limpopo provinces with a higher infection rate when compared to other arboviruses [80]. This may allude to Culicoides midges being better SHUV transmitters than mosquitoes. In South Africa, SHUV appears to circulate annually as an equine virus which causes severe neurological disease and death in horses [79,81], and [82]. SHUV has also been isolated in South Africa from multiple species of nonequine domestic animals, birds, and wildlife, including springboks, giraffes, and crocodiles [83]. The virus’s impact on humans is less well characterized. SHUV was recently detected in the cerebral spinal fluid of humans, most of them children, who were hospitalized in South Africa with neurologic symptoms [84]; however, there have been no other confirmed human cases of SHUV infection since the 1960s. Examining the potential risk to humans in contact with SHUV-infected animals, van Eeden et al. [82] found that 5 of 123 veterinarians had detectable SHUV antibodies [82]. No reports have been made on SHUV transmission out of this context. Overall, much work needs to be conducted to elucidate how SHUV transmission to humans occurs, together with its potential to cause clinical disease in humans.
Family: flaviviridae
Banzi virus
Banzi virus (BANV), originally named strain H336, was first isolated from the serum of a febrile child in Tongaland, Union of South Africa, in 1956 [85,86]. Working in South Africa and Rhodesia, McIntosh and colleagues isolated BANV from wild Cx. rubinotus, identifying that species as the primary vector [87–90]. Culex quinquefasciatus, by contrast, was an inefficient vector for BANV [91]. Serosurveys conducted in subjects across South Africa during the following decade identified communities with a high prevalence of BANV antibodies, interspersed with communities who had no evidence of BANV exposure [85]. Residents of Reino de Angola, the Caprivi Strip, and the Okavango Basin also had sera with protective antibodies to BANV [74,92]. BANV infection in sheep causes febrile illness and severe manifestations, including abortion and neonatal congenital abnormalities, in pregnant ewes [93]. Clinical manifestations of BANV infection in humans have not been well-described but appear to be mild and self-limiting [86,94]. Apart from recent vector surveillance that detected BANV in pools of Cx. rubinotus and Cx. annulioris in South Africa, no current data exist on the presence of BANV in Southern Africa [95].
Dengue virus
Dengue virus (DENV), which has four distinct serotypes (DENV-1, DENV-2, DENV-3 and DENV-4) and unclear geographic origins, is the leading arboviral cause of illness and death in humans worldwide [26,96]. Clinical features of dengue in humans range from asymptomatic infection to a nonspecific febrile illness that may include myalgias, vomiting, or rash, to potentially fatal shock or hemorrhage. DENV is primarily transmitted by Ae. aegypti mosquitoes in Africa as an urban vector. In temperate regions, Ae. albopictus mosquitoes can act as a secondary vector [49].
Although dengue has been a recognized illness for centuries, its etiology and transmission remained uncertain until the virus was isolated in 1942 [97,98]. Dengue-like illness was first described on the African continent in 1779, when an outbreak affected residents of Cairo [99]. In 1926–1927, a large outbreak in Durban, Union of South Africa, marked the first documented cases of dengue in Southern Africa [100]. Serosurveys of South African residents done by Kokernot and colleagues later showed that this was a localized DENV-1 serotype outbreak [101]. Over the next 50 years, circulation of DENV in the SADC region appears to have been either unrecognized or entirely absent, with no published cases within this period. Interval serosurveys conducted among residents of Reino de Angola (1960, 1971–1972), the Okavango Basin (1959), and the Caprivi Strip (1959) demonstrated that no residents had antibodies to DENV-1 or DENV-2 [74,92,102].
In 1984, a DENV outbreak in urban northern Mozambique introduced the current era of DENV circulation in Southern Africa. This outbreak was the first described in Mozambique as well as the first documented occurrence of the DENV-3 serotype in Africa. An estimated 45% of residents were infected over four months, suggesting a highly susceptible population [103]. Low-level DENV transmission may have persisted in Mozambique after this outbreak. In 2012, a small serosurvey among residents of southern Mozambique found evidence of DENV exposure, though that region had not documented outbreaks [104]. Consecutive bouts of DENV-2 serotype outbreaks were reported in northern Mozambique in 2013 and 2014 [105,106]. Limited surveillance to monitor DENV circulation has occurred in Mozambique since late 2016 [107]).
Data on the current presence of DENV in other Southern African countries remains scant. In 2013, a DENV outbreak occurred in Angola [108]. Retrospective PCR-based serotyping identified all four DENV serotypes, suggesting multiple introduction events and emphasizing the possibility of unrecognized low-level transmission [108]. In Namibia, although no autochthonous cases of DENV have been documented, probable or confirmed dengue has been reported in returning travelers captured by global travel surveillance networks [109,110]. A serosurvey of prospective blood donors in Namibia using DENV antibodies, identified some individuals with positive results, which could also reflect cross-reactive exposure to other flaviviruses such as WNV known to circulate in the country [111].
Vector studies specific to DENV in the SADC region, while very limited in number and geographic range, indicate that regional variation in Aedes mosquitoes may restrict DENV transmission. The only study of vector competence for DENV in the SADC region, which was performed in eastern South Africa, identified populations of Ae. aegypti that were susceptible to and capable of transmitting DENV-1 and DENV-2 [112]. Moreover, the same study identified populations of Ae. furcifer and Ae. streliziae from South Africa that were susceptible to DENV-1 and 2. The transmission rates were 0 to 50% for Ae. furcifer and 0 to 29% for Ae. streliziae [112]. Vector sampling in Mozambique noted a predominance of Ae. aegypti aegypti in the north and the predominance of Ae. aegypti formosus in the south, a difference that may have contributed to the DENV outbreaks in the north [113].
Spondweni virus
Spondweni virus (SPOV) was first described in 1955 after isolation from Ma. uniformis mosquitoes in northern Zululand, Union of South Africa [114]. Subsequently, another survey in 1958 in the same area, identified the virus in the following mosquito species: Ae. circumluteolus, Ma. africana, Ae. cumminsii (subspecies mediopunctatus) and Eretmapodites silvestris [115]. Recently it has been demonstrated in laboratory-based experiments on mice that Ae. aegypti can efficiently transmit SPONV, whereas Cx. quinquefasciatus could not [116]. SPOV generally causes mild, nonspecific febrile illness in humans with symptoms similar to ZIKV, leading to potential misclassification [117]. Unlike ZIKV, the transmission cycle and geographical distribution of SPOV remains poorly understood [117]. Available data on SPOV in Southern Africa suggests that low level transmission occurs at irregular times and in variable regions. Serosurveys conducted in the mid-20th century among residents of Reino de Angola, the Okavango Basin, and the Caprivi Strip identified a low prevalence of exposure to SPOV [74,92]. Serosurveys in Natal found evidence of limited exposure among livestock and a high prevalence of exposure among some human residents, albeit variably distributed [85,118]. Mosquito surveillance conducted between 1956 and 1968 in Natal identified mosquitoes carrying SPOV [119] and found similar prevalence and distribution patterns to the results previously obtained by Smithburn et al. [85] and Kokernot et al. [118].
Usutu virus
Usutu virus (USUV) was first isolated in the Union of South Africa from a Cx. neavei mosquito in 1959 [120,121] and has been found to be transmitted primarily by Culex mosquitoes, with birds acting as amplifying hosts [122]. Phylogenetic analysis suggests that the virus likely emerged in South Africa from an ancestral strain present as early as the 16th century [123]. Cases of USUV infection causing fever, rash, and neurologic symptoms in humans have been well described in northern sub-Saharan Africa and Europe [124]. However, in the SADC, no further data on the presence of USUV or of its vectors exist.
West Nile virus
West Nile virus (WNV) was first isolated in 1937 from a human with febrile illness in the West Nile district of Uganda [125]. The natural sylvatic hosts for WNV are birds, with over 900 species of birds identified as hosts [60,126,127]. Ornithophilic Culex species are the primary vector for WNV. Culex univittatus is the most frequent and efficient transmitter in the SADC, whilst Cx. theileri and Cx. neavei are also competent vectors [128–133]. Aedes species do not appear to be as capable vectors compared as Culex within the region [134].
Two major lineages of WNV have been described: isolates from lineage 1 generally circulate in Northern Africa, Europe, Asia, North and South America, and Australia, and isolates from lineage 2 generally circulate in Africa and Madagascar [135]. Until 2010, all sequenced WNV isolates from the SADC belonged to lineage 2, a distinction that was attributed to bird migration patterns [135–138]. However, isolates from lineage 1 have since also been identified among wildlife in South Africa [139,140].
WNV infection in humans is often asymptomatic and usually mild, with symptoms resembling a dengue-like illness that resolves after 3–6 days [141,142]. Fewer than 1% of infected humans develop severe illness with neuroinvasive disease (encephalitis, meningitis, or flaccid paralysis) [143]. In horses, neuroinvasive disease develops in as many as 20% of the cases and is often fatal [136,138,140]. Progression to severe disease in humans appears to be mediated in part by the neurovirulence of infecting WNV strains and in part by host immune factors [137,144,145]. Humans and other non-avian vertebrates are considered dead end hosts of WNV as their viremia levels are too low to support transmission back to mosquito vectors [130].
In Southern Africa, WNV was first isolated in the Tongaland in 1958; similar WNV strains were isolated from an ill human and from a captured bird that had presumed contact with the human patient [146]. Sporadic WNV infections, punctuated by epidemics that follow periods of heavy rainfall or unusually hot weather, have since been recorded throughout the region especially in South Africa [147,148]. Serosurveys conducted in the 1950s and 1960s identified WNV at low prevalence levels in indigenous residents of the Union of South Africa and the North-Western region of Reino de Angola, as well as in NHP and birds from South Africa [85,92,142]. In 1974, the largest known epidemic of human WNV infections in SADC occurred in the Cape Province, South Africa, after heavy rainfall. Though no deaths were reported 18,000 people developed acute febrile illness, and post-epidemic serosurveys identified WNV antibodies in 55% of residents [138,149]. Additional serosurveys in the 1980s identified antibodies to WNV among one-third of residents of northern South-West Africa, just to the north of the region affected by the 1974 epidemic [111,150,151]. More recent serosurveys among humans have been conducted only in Zambia. A cross-sectional study provided evidence of WNV infections in humans of all ages in the North-Western and Western provinces of Zambia, indicative of WNV prevalence and persistence within the country [152,153].
WNV circulation in mosquito vectors and avian hosts in Southern Africa tends to overlap with that of another arbovirus, SINV [89,94,131,147,154–156]. Similarly, transmission of WNV to humans has been identified during outbreaks of human SINV infection, though at lower rates than SINV, and their indistinguishable symptoms make the two viruses challenging to diagnose by symptoms alone [141,147,149,157].
In Southern Africa, documented severe WNV infections in humans are few, but infections may go unrecognized due to limited awareness and scarce diagnostics [158]. Severe and fatal WNV infections have also occurred in non-human vertebrates. A dog in Botswana developed fatal encephalitis that was initially attributed to infection with Wesselsbron virus (WESV), later confirmed to be WNV [135,159]. Later serosurveys of dogs in South Africa demonstrated that dogs, while susceptible to WNV infection, developed only low levels of viremia and thus were dead end hosts [160]. Currently, WNV is a cause for concern for horse owners in South Africa, causing annual equine outbreaks with a 34.2% fatality rate [139,161]. Laboratory transmission of WNV to humans has occurred in conjunction with equine cases in South Africa [145,162]. WNV was also recently isolated following fatal infections in non-equine wildlife in South Africa and farmed crocodiles from the Southern Province of Zambia [139,163]. Recent published entomological surveillance data for WNV in the SADC are limited to Namibia, South Africa, and Zambia, where WNV has been identified in Culex mosquitoes [95,164,165]. Overall, available data suggest that WNV circulates throughout the SADC, but many knowledge gaps remain.
Yellow fever virus
Yellow Fever virus (YFV) originated in Africa and spread globally through the slave trade in 1650 or even earlier [5]. Due to the extensive research done on YFV globally, the clinical symptoms of infection in humans have been well described. Most humans infected with YFV experience no or only mild symptoms, but those who develop severe disease have a mortality rate as high as 85% in some regions [166]. YFV has a 3- to 6-day incubation period followed by a brief acute febrile phase, which may be followed by a second morbid phase marked by jaundice, hematemesis, hemorrhage and renal injury [167]. The use of YFV vaccines has remarkably reduced the incidence rate in endemic areas. However, with waning immunity and barriers to widespread vaccination, communities in sub-Saharan Africa still experience sporadic YFV outbreaks [167–169].
In the SADC, much work aiming to understand the regional sylvatic transmission cycle of YFV has been conducted in the North-Western Province of South Africa and North-Western Province of Zambia [170]J. H [171; 172]. Key NHP species that have been identified in YFV sylvatic transmission and have habitat in Southern Africa are the green and vervet monkeys (Cercopithecus spp.), Cape baboon (Papio ursinis), and possibly bush babies (Galago spp.) [5,52,173]. Aedes aegypti and in some cases Ae. africanus are primarily responsible for YFV transmission to humans in Southern Africa [5,172,174]. However, earlier reports showed that Ae. simpsoni plays a secondary role in YFV transmission [175] in the SADC region. Other species with experimentally demonstrated, but not proven, roles in natural transmission are Ae. vittatus, Ae. metallicus, Ae. taylori [175]. Importantly, Ae. luteocephalus, a competent vector for YFV [176], has been found to occur in Mozambique [177], though its vectorial role was not assessed.
YFV research in the SADC began as early as the 1930s. Serosurveys conducted among residents of Angola in 1933 found antibodies at very low prevalence, leading the authors to declare the region ‘practically negative’ [178]. In Northern Rhodesia, following a suspected YFV case, serosurveys conducted from 1937–1943 detected YFV antibodies in residents of the Western Province [170,179]. This led to further YFV investigations in other Southern African countries. Serosurveys conducted in 1945, 1949, and 1959 determined minimal incidence rates of YFV in South-West Africa and Bechuanaland whilst the Union of South Africa experienced periodic YFV outbreaks between 1951–1953 [74;180,181]. In 1971, a YFV outbreak developed in Luanda, Angola, and was halted with a combination of mass vaccination and mosquito control [182,183].
Although Southern Africa is now generally regarded as a low-risk region for YFV (currently, only Angola is considered endemic and mandates YFV vaccination), sporadic YFV outbreaks still occur, and additional cases likely go undetected [168,184,185]. Recent serosurveys have confirmed that YFV still circulates in the North-Western and Western Provinces of Zambia [170]. Additionally, a new outbreak in Luanda in 2015–2016 developed into the largest YFV outbreak reported in the last 30 years, with approximately 4,306 suspected cases and as many as 393 deaths [167,169,186]. Travel from Angola during the outbreak led to imported YFV cases in countries outside the SADC including China, causing the first reported cases of YFV in Asia [167,187,188]. These statistics underscore the vulnerability and limited preparedness in the SADC when it comes to YFV response.
Zika virus
Zika virus (ZIKV) was first isolated in 1947 from a rhesus macaque used for YFV monitoring in Uganda [189]. For many years, ZIKV outbreaks were confined to Africa and Asia until the beginning of this century, when the virus emerged in Micronesia, French Polynesia, and then Central and South America [190–192]. Concerning ZIKV vectors, urban vector Ae. aegypti is associated with ZIKV transmission in Southern Africa, while sylvatic vectors such as Ae. furcifer, Ae. luteocephalus, Ae. africanus and Ae. taylori are vectors in most sub-Saharan African countries except the SADC region where some species are present but have not yet been implicated with ZIKV transmission [193]. While Aedes are the primary arthropod vectors for ZIKV, non-biological modes of ZIKV transmission among humans (e.g. sexual, perinatal, through organ transplantation) have also been described [194]. Given the numerous possible modes of ZIKV transmission, factors driving the global expansion of ZIKV are complex [194–196]. ZIKV infection in humans typically causes a mild febrile illness with nonspecific, self-limiting symptoms (rash, headache, arthralgia, myalgia) [197,198]. Severe neurologic complications also occur: a small proportion of adults experience Guillain-Barré syndrome, and maternal-fetal transmission has been associated with the development of microcephaly and other congenital abnormalities [194,197,198].
In Southern Africa, serosurveys conducted prior to 1990 identified ZIKV in the Reino de Angola, Northern Rhodesia, and Portuguese East Africa [194]. These data were used to map regions of ZIKV circulation [194,199]. However, with missing or omitted information, these maps likely understate ZIKV’s presence in some regions [5,194]. For instance, Kokernot et al. [200] documented a 4.0% seroprevalence of ZIKV in Portuguese East Africa, contradicting historical maps which suggest that ZIKV has never circulated in Mozambique [5,199–202]. From 2009–2015, Chelene et al. [203] conducted a nationwide retrospective seroprevalence study in children and found a 4.9% ZIKV antibody positivity, suggesting recent ZIKV transmission in Mozambique.
There is limited recent data on ZIKV in sylvatic hosts in the SADC. A study by Buechler et al. [204] examined the prevalence of ZIKV in NHP in Zambia, South Africa, and elsewhere and found no evidence of active ZIKV infection or prior ZIKV exposure among NHP from the SADC region [204]. While these findings suggest no current circulation of ZIKV in the natural hosts, this does not exclude the possibility of ZIKV circulation within the SADC region.
Imported ZIKV cases also represent a threat to the SADC region. Since the 2016 ZIKV outbreak in Brazil, its spread has become a major concern particularly in Angola, where imported cases have been reported [192]. Angola was the first Southern African country to report circulation of the Asian lineage of ZIKV and ZIKV-associated microcephaly [205,206]. However, the first African country to report the circulation of the Asian lineage of ZIKV was Cabo Verde [207]. Hill et al. [205] conducted a phylogenetic analysis and found that ZIKV may have been circulating in Angola 28 months prior to the reported cases, suggesting that the outbreak was larger than initially reported. In response to these imported cases, scaled-up surveillance and monitoring efforts in Angola have involved tracking passengers’ in-flight data to assess the likelihood of ZIKV introduction and transmission to Angola and other African countries [205,208].
Family: nairoviridae
Crimean-Congo haemorrhagic fever virus
Crimean-Congo Haemorrhagic Fever virus (CCHFV) is a severe tick-borne zoonotic disease with a high case fatality rate in humans. The first case of this hemorrhagic fever reported in Southern Africa was that of a 13-year-old boy from the Transvaal Province, South Africa, who was diagnosed in 1981 with CCHFV, and shortly thereafter died [209]. This was the first reported case of fatal human CCHFV infection in the region. Transmission occurred via a tick belonging to the Hyalomma marginatum species complex (of which H. rufipes is one member that circulates in Southern Africa), and the boy progressed rapidly from symptom onset to hemorrhagic state and death within nine days [209]. In response, a thorough ecological and serosurvey of CCHFV was conducted by Swanepoel et al. [210] in five provinces of South Africa. Their investigation isolated CCHFV from several tick species (H. rufipes, H. truncatum and Rhipicephalus evertsi) and detected CCHFV antibodies in both humans and livestock, indicating that CCHFV was prevalent in the country. The numerous veterinary cases suggest that livestock act as amplifying hosts, enabling sporadic outbreaks and direct transmission to humans [211]. In their study of infected ticks, Swanepoel et al. [210] also confirmed that H. rufipes was capable of transovarial CCHFV transmission.
The Crimean-Congo Haemorrhagic Fever virus generally circulates in nature in unnoticed enzootic tick – vertebrate–tick cycles, and asymptomatic CCHFV infection has been reported in numerous vertebrate species and appears to be pervasive in both wild and domestic animals [212]. Surveys conducted in South Africa showed evidence of the asymptomatic circulation of CCHFV among cattle [213,214] with a seroprevalence between 26.5 and 28%. Moreover, antibodies were found to be highly prevalent in large mammals such as giraffe, rhinoceros, eland, buffalo, kudu and zebra [215]. In small mammals, antibodies were found in the sera of hares, rodents, and wild carnivores [215]. Antibodies were also detected in domestic dogs from South Africa and Zimbabwe [215]. To conclude, some evidence has also been provided on the circulation of CCHFV among wild birds (guinea fowl) in South Africa [216].
Reports of CCHFV coinfections with other pathogens have also been documented, complicating understanding of its interactions. The first coinfection to be documented was that of CCHFV and MARV, with 486 cases reported in Rhodesia [217]. The second was that of CCHFV and malaria detected by Gear [218] in a traveler who had returned to South Africa from Rhodesia [218]. Gear [218] notes that hemorrhagic fever diagnosis becomes even more complex once the patient is coinfected with another pathogen.
Family: peribunyaviridae
Germiston virus
Germiston virus (GERV), first isolated in 1958 from a mixed pool of Culex mosquitoes in Germiston, Union of South Africa [219], appears to be prevalent but of unclear significance in the SADC region. Not all Culex species are capable of efficient GERV transmission: Cx. rubinotus is a competent vector for GERV, but Cx. quinquefasciatus transmits GERV only at a very low rate [87,90,91,220]. Wild rodents have been identified as hosts [88,94]. Serosurveys have detected antibodies to GERV in as many as one-third of residents in the Okavango Basin, three regions of Reino de Angola, and coastal Natal Province in South Africa [74,92]; B. L [221]. McIntosh [94] found that humans with GERV infection experienced brief, nonspecific symptoms of febrile illness. These data suggest that GERV infections are mild but are not enough to draw conclusive remarks about its epidemiology.
Pongola virus
Pongola virus (PONV) was first isolated from Ae. circumluteolus in Tongaland, Union of South Africa [222]. Studies by Jupp and McIntosh [223], also in Tongaland, further identified Ae. circumluteolus as a potential vector but only in an incidental capacity [223]. Serological surveys conducted in 1959 and 1960 indicated high prevalence (9–26%) of PONV infection in humans in Reino de Angola, Northern Natal, the Caprivi Strip, Bechuanaland and Mozambique, primarily in coastal and low-lying areas [74,85,92,200,224]. However, it is worth noting that PONV is highly cross-reactive with Bwamba virus (BWAV) in many serological tests [224], making it difficult to distinguish the distribution and relative abundance of these two viruses. Scant information on PONV exists in Southern African states.
Simbu virus
Simbu virus (SIMV) was first isolated from Ae. circumluteolus mosquitoes captured at Lake Simbu, Union of South Africa, in 1955 [225]. SIMV has since been categorized in the Simbu serogroup as one of 25 phylogenetically related arboviruses distributed worldwide, transmitted by various mosquito species and Culicoides midges [226]. Whilst other viruses in the Simbu serogroup have been identified as important causes of human and veterinary infection outside Southern Africa [226], the significance of SIMV remains unclear. Laboratory infection with SIMV caused viral encephalitis in mice but no apparent signs or symptoms of illness in rabbits, guinea pigs, or vervet monkeys [225]. Clinical features of SIMV infection in humans are unknown. Early serosurveys found positive SIMV antibodies in a few humans in the Okavango Delta and the northern Natal region of South Africa [74,85]. Apart from additional isolation of SIMV from Ae. circumluteolus captured in the Ndumu Game Reserve, South Africa, in 1964 [119], no other reports on SIMV in SADC exist.
Witwatersrand virus
Witwatersrand virus (WITV) was isolated in 1958 from wild Cx. rubinotus during an outbreak of febrile illness among the residents of Germiston, Union of South Africa [227]. After initial investigations established that Cx. rubinotus is a competent vector and wild rodents are likely hosts. Since, WITV has not been further studied in Southern Africa [87,88,90,91,220].
Family: phenuiviridae
Rift valley fever virus
Rift Valley fever virus (RVFV) causes disease primarily in domestic ruminants, leading to febrile illness and abortion, and was first reported as the cause of sheep deaths in Kenya in 1931 [228,229]. RVFV has since been described in livestock, humans, and arthropod vectors throughout Africa and the Middle East [230]. The first report of RVFV in the SADC occurred during a livestock epidemic in 1951 in the Union of South Africa [231]. Subsequent studies done in the same country identified multiple species from the Aedes and Culex genera as potential RVFV vectors, with Cx. theileri and Cx. zombaensis likely responsible for RVFV outbreaks in the region [71,232–237]. Moreover, it has been demonstrated that Ae. juppi can easily be infected with RVFV but has no transmission capabilities, whilst Ae. unidentatus, Ae. dentatus and Cx. poicilipes were identified as potential epizootic vectors in South Africa [238]. More recently, Linthicum et al. [239] curated an extensive and comprehensive list of RVFV vectors found globally including those circulating in Southern Africa. This list shows Aedes and Culex as dominant and effective transmitters of RVFV. The recent confirmation of Ae. durbanensis as a vector of RVFV in the endemic region of Kwazulu-Natal, South Africa, was a result of a thorough genomic and phylogenetic analysis done by van den Bergh et al. [240]. Phylogenetic analysis also revealed that the identified virus is closely related to two isolates from the earliest outbreaks, which occurred in central South Africa more than 60 years ago, indicating long-term endemicity in the region (van de Bergh et al., 2022).
RVFV infection in livestock can be asymptomatic, but its role as a cause of abortion in pregnant livestock and fatal infection in newborn livestock gives it great economic importance [230,241–243]. Numerous RVFV outbreaks have been documented in livestock across Southern Africa, including Mozambique, Northern Rhodesia, Rhodesia, and especially South Africa (where continuous surveillance occurs) [229,230,232,237,244,245]. Serosurveys conducted in livestock in Mozambique, South Africa, and Zambia have also shown evidence of RVFV circulation in herds without signs of clinical disease [244,246,247]. For example, in Mozambique, serosurveys in cattle identified RVFV seroprevalence as high as 62.2% in a province that had no documented RVFV outbreaks in the previous four decades [246].
In humans, RVFV infection may be asymptomatic, uncomplicated (with acute influenza-like fever, headaches, and muscle pains), or complicated (with gastrointestinal symptoms, hepatic or renal failure, encephalitis, visual symptoms, and/or hemorrhagic manifestations) [248]. Human RVFV infection was first recognized in farmers and veterinarians who directly handled the tissues of infected animals [228]. The first documented human infections in Southern Africa occurred in 1951, when farmers and veterinarians in the Union of South Africa developed febrile illness after performing an autopsy on an infected bull; all recovered, except for a few individuals who had residual visual defects [232,249,250]. Similar self-limited infections occurred in Rhodesia in 1957 among farmers and a laboratory worker [251,252]. Descriptions of severe and fatal human RVFV infections, including hemorrhagic fevers, began to emerge in Southern Africa in the 1970s [253,254]. In 1975, an RVFV outbreak in South Africa led to seven human deaths from gastrointestinal hemorrhage [218]. Serosurveys among asymptomatic humans in the Caprivi Strip and Okavango Basin (1959), Angola (1971–1972), South Africa (1981–1982), northern South-West Africa (1983), and Botswana (1984–1986) also demonstrated evidence of RVFV exposure separate from documented livestock outbreaks, suggesting vector-borne transmission [74,102,150]; B. L [221; 255]. Niklasson et al. [256] examined the effects of RVFV infection in pregnant women in Mozambique; the authors identified RVFV antibodies in 2% and found that women with evidence of past RVFV exposure experienced no significant difference in pregnancy outcomes. However, studies elsewhere in Africa have linked acute RVFV infection to an increased risk for miscarriage in pregnant women [257].
More recent studies have demonstrated ongoing low-level circulation of RVFV among humans across Southern Africa. Serosurveys of humans in northern Botswana (2013–2014) and KwaZulu-Natal, South Africa (2018–2019), found overall RVFV seroprevalences of 5% and 2.8%, respectively [258,259]. A cross-sectional study of febrile patients in Mozambique identified RVFV as the causative agent in 5% of them [260].
Overall, these studies indicate that RVFV circulates in Southern Africa in a heterogeneous fashion, characterized by low-level maintenance transmission punctuated by periodic and sporadic outbreaks. In the last decade, genome sequencing and spatiotemporal modeling techniques have begun to better describe the factors influencing RVFV transmission in the SADC, but much is still unclear. Sequencing of RVFV from infected livestock in Mozambique isolated RVFV belonging to lineage C, which is also a known cause of livestock deaths along the eastern coastline of Africa, Madagascar, and some Middle Eastern countries [245]. Recently sequenced RVFV isolates in South Africa, however, belonged to multiple other lineages, indicating high genetic diversity within a relatively small geographic area [261]. In Botswana, Pachka et al. [262] developed a mechanistic model to explore how environmental factors influence the dynamics of Cx. pipiens as a potential RVFV vector. In South Africa, other models have explored the impact of temperature, rainfall, soil conditions, and other local environmental characteristics on the likelihood of RVFV transmission [263–265]. Such models can aid in vector control. Vaccination provides another approach for outbreak prevention; vaccines against RVFV have been developed for livestock and humans, but problems with vaccine safety, efficacy, and delivery have hindered widespread use [236,266].
Lunyo virus
Lung virus (LUNV), an atypical strain of RVFV, was first isolated in 1955 from a pool of collected Aedes mosquitoes in Uganda [267]. Recent genomic and phylogenetic analysis of LUNV by Lumley et al. [268] confirm LUNV is indeed a strain of RVFV.
Family: togaviridae
Chikungunya virus
Chikungunya virus (CHIKV) was first isolated in Tanganyika (mainland part of present-day Tanzania) in 1952 [269,270]. However, evolutionary studies conducted by Weaver and Forrester [271] suggest that CHIKV may have existed for at least a century prior to its discovery. In the SADC, baboons (Papio ursinus) and vervet monkeys (Cercopithecus aethiops) have been identified as sylvatic hosts [57,272]. Sylvatic vectors are important for CHIKV transmission in Africa. In particular, Ae. furcifer, and possibly also Ae. cordellieri, have been identified in South Africa [272,273] as primary vectors of the virus.
Ae. aegypti and Ae. albopictus are urban vectors. Domestic animals and humans are both dead-end hosts [274]. Clinical symptoms of CHIKV in humans are often self-limiting and include fever, rash, and severe arthralgia, with symptoms typically lasting up to 7 days; however, as many as 40% of infected individuals develop chronic, recurrent arthralgia, and more severe manifestations have been reported [274,275].
Regional serosurveys in Reino de Angola, the Okavango Basin, the Caprivi Strip, and the Union of South Africa by Smithburn et al. [85], Kokernot, Casaca, et al. [92] and Kokernot, Szlamp, et al. [74] in the 1950s and 1960s found CHIKV antibodies in adults and some children, indicating CHIKV circulation within the surveyed regions. Children in coastal Natal Province, Union of South Africa, lacked protective sera, highlighting the possibility of a future CHIKV epidemic [85]. During the same period in Rhodesia, several serosurveys were conducted in response to an outbreak in the south of the country [58,276–278]. Swanepoel and Cruickshank [237] also found high CHIKV epidemiological patterns that were seasonal and specific to different areas in Rhodesia. These results suggest that CHIKV was previously an endemic disease in Rhodesia. In Luanda, Angola, in 1970–1971, CHIKV co-circulated with YFV and produced a dual arboviral outbreak [183,279]. In Malawi in the 1980s, a serosurvey conducted among children identified CHIKV antibodies in approximately half of children, suggesting active CHIKV circulation [280].
More recently, a report of a severe case of human CHIKV infection in Pemba, Mozambique, describes well the current knowledge of CHIKV in Southern Africa [281]. In their report, the authors clearly show the barriers that regional healthcare systems face in accurately diagnosing and treating patients with CHIKV and other arboviral infections. Much effort has been placed on understanding the current prevalence and spatiotemporal distribution of CHIKV in Mozambique [282–288]. In Zambia, a recent cross-sectional serosurvey also identified CHIKV antibodies in 36.9% of residents of a wetland area [289]. Generally, scant information is available on recent CHIKV circulation in the rest of the SADC. CHIKV infection has been diagnosed in travelers returning from Angola, yet no recent data from within Angola have been published [290,291]. The current range of CHIKV circulation in Southern Africa needs further investigation.
Middelburg virus
Middelburg virus (MIDV) was first isolated from Aedes species in the Eastern Cape Province, Union of South Africa, in 1957 [292], with a single human case later reported in a resident of Natal [247]. Vector surveillance conducted from 1960–1968 in South Africa by McIntosh [233], confirmed MIDV in Aedes species. More recently, Ae. callabus and Ma. africana have been implicated as vectors that are primarily found to be positive for MIDV [293]. Early serosurveys among humans did not identify any neutralizing antibodies to MIDV in residents of Reino de Angola, the Okavango Basin, or the Caprivi Strip [74,92]. Any link between MIDV infection and disease in either humans or other animals was initially unclear. However, isolation of MIDV from horses in South Africa and Zimbabwe has since confirmed MIDV as a cause of severe and fatal equine disease [294–296]. In addition, MIDV has been isolated in South Africa from a variety of non-equine wildlife and birds who died of otherwise-unexplained neurologic or febrile disease [297]. Emerging case reports from South Africa also suggest that MIDV infection may be the cause of neurologic manifestations in humans [298]. Despite this knowledge, the host ranges for MIDV remain unknown. Whether MIDV transmission currently occurs in other SADC nations is undetermined.
Semliki forest virus
Semliki Forest virus (SFV) was first isolated from the Ae. abnormalis group of mosquitoes in 1942 in Uganda [299]. It was subsequently isolated in coastal central Portuguese East Africa from Ae. argenteopunctatus in 1959 by McIntosh et al. [300]. In addition, in a lab model it has been shown that Ae. aegypti is capable of acting as an efficient vector of SFV, both via biological and mechanical transmission [301]. Serosurveys have since detected SFV throughout the SADC region. Evidence points to SFV virus being most prevalent near large bodies of surface water in temperate or tropical areas, such as in northern Reino de Angola, the Caprivi Strip, and in the provinces of Natal, Cape, and Transvaal, Union of South Africa [74,92,101]. For example, serosurveys conducted in coastal north-western Reino de Angola identified neutralizing antibodies to SFV in 33% of adults [92]. Limited information is available regarding the clinical symptoms of SFV infection in humans. The first reported human outbreak of SFV infection, which occurred in the Central African Republic in 1987, described fever, severe headache, myalgia, and arthralgia that lasted 2–4 days with a slow recovery [302]. To date, no human SFV infections have been documented in the SADC.
Sindbis virus
Sindbis virus (SINV) was first isolated in 1952 from Culex and Culiseta mosquitoes in Egypt [126]. Birds act as the natural host, and serosurveys and vector surveillance have identified a broad distribution globally [303]. In the SADC, SINV was first clinically diagnosed and isolated in South Africa in 1963 [304]. The primary vectors of SINV in Southern Africa are Cx. univattatus and Cx. neavei, and both are capable of transmitting SINV to humans [89,131,134,155,305]. In a recent survey (2014–2017) in several provinces of South Africa [306], the following mosquito species have been found infected with SINV: Cx. univittatus, Cx. pipens s.l., Cx. zombaensis, Cx. theileri, Cx. annulioris, Ae. tarsalis/aerarius, Ae. durbanensis, Ae. mcintoshi, Ma. uniformis. Sindbis virus was found to be detected slightly more frequently in peri-urban sites than in conservation and rural areas [306].
As previously noted, symptoms of SINV infection in humans are similar to those of WNV infection and include fever, arthralgia, rash, and malaise [94]. Many studies in the SADC have shown direct or indirect evidence of SINV infection in humans, including the Reino de Angola, the Okavango Basin, the Caprivi Strip, Rhodesia, Zambia, and particularly South Africa, where SINV outbreaks occurred in 1974 and 1984 [74,85,92,237,303,307,308]. Recent passive surveillance in South Africa has continued to identify human SINV infections, and no fatalities have been reported to date [303]. Latterly, SINV (2019–2020) cases were detected from hospitalized patient samples by PCR and serological methods in two northern provinces of South Africa [309].
Wesselsbron virus
Wesselsbron virus (WESV) was first isolated from an aborted lamb at Wesselsbron Farm in the Orange Free State Province, in the Union of South Africa [310,311]. This discovery spurred an onset of investigative studies across Southern Africa to determine the characteristics of WESV. Aedes circumluteolus was identified as a natural vector [312,313], while Ae. caballus was a competent vector under laboratory conditions [219]. In sheep and goats, WESV infection causes febrile illness and high rates of abortion in pregnant animals [314–316]. Cattle experience mild or asymptomatic WESV infection, without abortion [317]. WESV infection in humans, which can occur via vector or via direct transmission when handling tissues from infected livestock, causes a nonspecific, self-limited febrile illness [237,318]. Serosurveys conducted in the 1950s and 1960s in Bechuanaland, the Caprivi Strip, Northern Rhodesia, Nyasaland, Reino de Angola, Rhodesia, Mozambique, and the Union of South Africa showed presence of WESV antibodies in humans, cattle, and sheep, suggesting that WESV was endemic in Southern Africa at the time [74,92,218,237,316]. Currently, there is no active surveillance for WESV in the SADC, but occasional reports appear of WESV outbreaks among humans and livestock [315,319,320].
Other arbovirus species identified in Southern Africa
Vector and host surveys conducted in the SADC from the 1950s through the 1960s identified multiple other arboviruses that have not been well-studied in the region since. The current impact of these arboviruses on humans and animals in Southern Africa is thus unknown.
Kokernot and colleagues found sera positive for antibodies to BWAV in humans, donkeys, and a bird in Mozambique and the Union of South Africa [101,200,321]. In the low-lying region of Simbu Pan, Natal Province, Union of South Africa, BWAV was the predominant arbovirus identified among humans; 80% of those tested had neutralizing antibodies to BWAV [101]. Bwamba virus circulation appears to have been localized, in fact humans, NHP, domestic animals, and birds in other areas of the Union of South Africa had no or very infrequent antibody response [101]. Kokernot and colleagues also tested human and animal sera from the Union of South Africa for antibodies to Ntaya virus (NTAV) and found no positive sera [101]. In Reino de Angola, however, they identified a low prevalence of NTAV exposure in humans (corroborated by a later serosurvey conducted by Filipe and colleagues), as well as sera positive for antibodies to Lumbo (LUMV), Mayaro (MAYV), and Oriboca (ORIV) viruses [92,102]. Serosurveys in the neighboring Caprivi Strip and Okavango Basin also found a low prevalence of ORIV exposure in humans but did not evaluate for exposure to BWAV, NTAV, LUMV or MAYV [74].
In an extensive field survey in the Ndumu Game Reserve (Union of South Africa) from 1956–1968, McIntosh and colleagues conducted annual mosquito catches, isolated viruses and described their viral vectors, and discussed the epidemiological implications of their results [119,322]. Their work detected Ingwavuma (INGV), Lebombo (LEBV), Shokwe (SHOV), and Ndumu (NDUV) viruses for the first time in Southern Africa [119,322]. They also identified Mossuril virus (MOSV), which had previously been isolated once from a pool of Cx. sitiens in Mossuril, Portuguese East Africa, in 1959 [119,323]. Field collections showed that Ae. circumluteolus was the dominant species but was only an incidental vector that was unable to maintain viral survivability [119].
Discussion
This review provides evidence of the significant presence, both past and current, of arboviruses with medical and veterinary importance within the SADC. Environmental conditions in Southern Africa support the circulation of arboviruses and their vectors. Ecological and anthropological factors have increased the proximity between sylvatic reservoirs and humans, increasing the likelihood of spillover events and outbreaks in ecologically naïve and niche areas. Recent outbreaks of CHIKV, DENV, YFV, and RVFV, accompanied by multisectoral challenges in recognizing and controlling arboviral transmission, illustrate Southern Africa’s current vulnerability [187,261,281].
Arboviral research flourished in Southern Africa in the 1950s, led by researchers at the Rockefeller Foundation Virus Program in Johannesburg, Union of South Africa, and continued intensively until the late 1980s [324]. However, a notable 20-year lull occurred before research began to resume around 2010 (Figure 3). A myriad of reasons likely contributed to the decline of arboviral research in the region. The emergence of the HIV/AIDS pandemic in the 1980s diverted scientific attention and resources [325]. Lack of recognition and underreporting of arbovirus infections, due in part to the self-limited and benign nature of most cases, may have added to a lack of research interest [281]. Concurrently, an intensive public health focus on malaria and its vectors, which has resulted in admirable progress toward malaria elimination, prioritized research in malaria over work in arboviruses [23,67]. The geopolitical turbulence and conflicts experienced when Southern African countries fought for independence also could have hindered research efforts, with limited resources reallocated to other critical needs. Indeed, social conflict is a long-recognized driver of infectious diseases, and this link is particularly true in Africa [326]. Finally, funding for arboviral research in the region has not recovered since the closure of the Rockefeller Foundation Virus Program [324]. A search of the National Institutes of Health (NIH) Research Portfolio Online Reporting Tools Expenditures and Results (RePORTER) datasets, for example, identifies no NIH-funded studies on arboviruses in the 10 SADC countries examined here from 1985 onward [327].
While an effective response to arboviruses should prioritize risk monitoring, the current infrastructure for arboviral surveillance in the SADC is inadequate and largely relies on passive rather than active measures [10,27,67]. Southern Africa is not unique in its struggle to develop a sustained arboviral surveillance network; in high-income countries like the United States, capacity for arboviral surveillance also fluctuates in reaction to outbreaks [328].
Finally, there is growing concern that climate change will alter the distribution and burden of vector-borne diseases, potentially reversing the gains of vector control programs (as achieved for malaria in Africa) and increasing the threat of emerging diseases. In this context, Ae. aegypti has shown ecophysiological plasticity in adapting to varying ecological conditions and has potential to expand the reach of arboviruses [7,329]. Prompt intersectoral action from governments and research institutions is needed to address the effects of climate change.
We strove here to compile a comprehensive assessment of arboviral research conducted in Southern Africa to date, but we may have overlooked some work. Incomplete cataloging terms, especially for older studies, make some arboviral research challenging to locate. Studies published in languages other than English were excluded. We also included only published data, not any findings that may have only been reported to local health systems or surveillance teams. This is a limitation of our assessment but also emphasizes the importance of developing intersectoral and inter-country information networks to readily share data on arboviral transmission and spread.
Future directions
As most Southern African states are low-middle income countries, determining transmission patterns and evaluating epidemiological data to conduct informative diagnostic investigations have been severely hampered by poor laboratory capacity [27,67,260]. In this context, new and improved diagnostics, including low-cost and point-of-care tests, will also be essential to allow rapid identification of arboviral infections [330]. Undoubtedly, these advances will shift the limits of arboviral surveillance and detection. However, vector control is currently the most effective preventative tool available to reduce the risks of arboviral transmission and associated pathological effects. With insecticide resistance threatening current control methods, biotechnological advances in modeling arbovirus-vector interactions will be essential to provide innovative and sustainable vector control solutions [331,332]. For example, we would like to highlight how Wolbachia, a bacterium, has been found to reduce transmission of several arboviruses including ZIKV, CHIKV and DENV [333,334]. However, unlike in most mosquito species, Wolbachia does not naturally occur in Ae. aegypti [335] and must be introduced introgressively into the Ae. aegypti population [336,337]. Promising efforts supported by the World Mosquito Programme that have been successively introduced Wolbachia into Ae. aegypti populations in 11 countries, on 3 continents (Oceania, South America and Southeastern Asia). Current efforts are looking at feasibly scaling up these approaches [337], and assessing the associated risks [336,338] prior to releasing Wolbachia infected Ae. aegypti into sub-Saharan Africa and other parts of the world [337,339].
To reduce arboviral transmission (together with other vector-borne diseases, such as malaria), and in response to the rise of insecticide resistance, the WHO and the international community have promoted the use of integrated vector management (IVM) as a progressive approach toward sustainable, cost-effective and enhanced vector control. The key elements for the successful implementation of IVM are: i) advocacy for community engagement and empowerment; ii) multisectoral collaboration and resource sharing; iii) integration of conventional approaches with novel modalities; iv) evidence-based decision making to guide implementation strategies; and, lastly, v) capacity building at local, national and regional levels [340]. Also, very recently, another WHO strategy was the launch of the Global Arbovirus Initiative on 31 March 2022 [10]. The Global Arbovirus Initiative is an integrated strategic plan to tackle emerging and reemerging arboviruses with epidemic and pandemic potential focusing on monitoring risk, pandemic prevention, preparedness, detection and response, and building a coalition of partners (https://www.who.int/news-room/events/detail/2022/03/31/default-calendar/global-arbovirus-initiative).
Additionally, ongoing efforts are taking place to understand the molecular mechanisms of transmission, disease pathogenesis and epidemiology of arboviruses using recent advances in biotechnology. These methods are high-throughput and make use of predictive abilities to reduce the gap between experimental knowledge and available resources. Combining predictive and experimental data is quintessential to providing innovative and sustainable solutions [339]. Predictive models, such as AlphaFold2 and machine learning, in combination with other bioinformatic tools, will aid in developing much improved omic results that will elucidate on underlying mechanisms [331,332]. CLIMADE, a global consortium based in South Africa, makes use of bioinformatic tools to enhance our global understanding of arboviral pathogenesis and epidemiology (https://climade.health/). Furthermore, with the AU-EU Innovation Agenda being established in 2023 (https://research-and-innovation.ec.europa.eu/system/files/2023–07/ec_rtd_au-eu-innovation-agenda-final-version.pdf), we anticipate that the many partnerships and collaborations formed will facilitate high-throughput analysis to be possible in circumstances where such analysis would never have been thought possible.
Another important recent initiative is the Quadripartite Secretariat for One Health. This multinational, multi-sectorial, multidisciplinary collaboration of four main agencies – FAO, UNEP, WHO, and WOAH – aims to achieve together what no one sector can achieve alone, thus providing a framework for collective and coordinated action to mainstream the One Health approach at all levels and provide policy and legislative advice and technical assistance to help set national targets and priorities (https://www.who.int/teams/one-health-initiative/quadripartite-secretariat-for-one-health).
Our approach to addressing these challenges ought to extend beyond mitigating the effects of climate change and should look at how we can achieve sustainable health (defined by [341] to better the lives of community members in the long run.
Conclusion
This review underscores the urgent need for heightened surveillance and control measures within the SADC region to effectively manage the risk posed by arboviruses. By understanding the factors that drive arboviral persistence and transmission in Southern Africa, public health officials and policymakers can develop more targeted and efficient strategies to mitigate the impact of arboviruses on both human and animal populations. Particularly, it is indicated that i) data-driven approaches with a One Health framework are critical; ii) vector control is currently the most effective preventive tool available to combat arbovirus transmission (despite the issue of insecticide resistance); and iii) strengthening surveillance is crucial to guide interventions and management of emergencies. In conclusion, continued research and collaboration across disciplines will be key in improving our understanding of these complex ecological interactions and enhancing preparedness against future arboviral outbreaks.
Acknowledgements
We would like to thanks the two anonymous reviewers that really helped in improving the manuscript. In addition, we would also thank the Soulsby Foundation under a Soulsby Travelling Fellowship, and the Penn Center for AIDS Research under Grant #5-P30-AI-045008-17 for supporting this work.
Funding Statement
The work was supported by the Penn Center for AIDS Research [5-P30-AI-045008-17]; Soulsby Foundation .
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].World Health Organization . Tanzania Confirms First-Ever Outbreak Of Marburg Virus Disease; 2023. https://www.afro.who.int/countries/united-republic-of-tanzania/news/tanzania-confirms-first-ever-outbreak-marburg-virus-disease
- [2].Huang Y-JS, Higgs S, Vanlandingham DL.. Arbovirus-mosquito vector-host interactions and the impact on transmission and disease pathogenesis of arboviruses. Front Microbiol. 2019;10:22. doi: 10.3389/fmicb.2019.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kuno G, Chang G-J-J. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clinical Microbiology Reviews. 2005;18(4):608–637. doi: 10.1128/CMR.18.4.608-637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weaver SC, Barrett ADT. Transmission cycles, host range, evolution and emergence of arboviral disease. Nature Rev Microbiol. 2004;2(10):789–801. doi: 10.1038/nrmicro1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Braack L, Gouveia de Almeida AP, Cornel AJ, et al. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasites Vectors. 2018;11(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–345. doi: 10.1016/j.antiviral.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Couper LI, Farner JE, Caldwell JM, et al. How will mosquitoes adapt to climate warming? Elife. 2021;10:e69630. doi: 10.7554/eLife.69630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Girard M, Nelson CB, Picot V, et al. Arboviruses: a global public health threat. Vaccine. 2020;38(24):3989–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wilder-Smith A, Gubler DJ, Weaver SC, et al. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17(3):e101–e106. [DOI] [PubMed] [Google Scholar]
- [10].World Healths Organization . Launch Of The Global Arbovirus Initiative; 2022c. https://www.who.int/news-room/events/detail/2022/03/31/default-calendar/global-arbovirus-initiative [Google Scholar]
- [11].Weaver SC. Evolutionary influences in arboviral disease. Curr Top Microbiol Immunol. 2006;299:285–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Drake JW. Rates of spontaneous mutation among RNA viruses. Proc Natl Acad Sci USA. 1993;90(9):4171–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Picarazzi F, Vicenti I, Saladini F, et al. Targeting the RdRp of emerging RNA viruses: the structure-based drug design challenge. Molecul (Basel, Switzerland). 2020;25(23):5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coffey LL, Forrester N, Tsetsarkin K, et al. Factors shaping the adaptive landscape for arboviruses: implications for the emergence of disease. Future Microbiol. 2013;8(2):155–176. doi: 10.2217/fmb.12.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li H, Roossinck MJ. Genetic bottlenecks reduce population variation in an experimental RNA virus population. J Virol. 2004;78(19):10582–10587. doi: 10.1128/JVI.78.19.10582-10587.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Migné CV, Moutailler S, Attoui H. Strategies for assessing arbovirus genetic variability in vectors and/or mammals. Pathogens. 2020;9(11):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Go YY, Balasuriya UBR, Lee C. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clin Exp Vaccine Res. 2014;3(1):58. doi: 10.7774/cevr.2014.3.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weaver SC. Prediction and prevention of urban arbovirus epidemics: a challenge for the global virology community. Antiviral Res. 2018;156:80–84. doi: 10.1016/j.antiviral.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Araf Y, Ullah M, Faruqui A, et al. Dengue outbreak is a global recurrent crisis: review of the literature. Electron J Gen Med. 2020;18(1):em267. [Google Scholar]
- [20].Higgs S, Vanlandingham D. Chikungunya virus and its mosquito vectors. Vector-Borne Zoonotic Dis. 2015;15(4):231–240. doi: 10.1089/vbz.2014.1745 [DOI] [PubMed] [Google Scholar]
- [21].Higgs S, Vanlandingham DL, Powers AM. Chikungunya and Zika viruses: global emerging health threats. In: Stephen , Dana LV, An. 1st editors. London (UK): Academic Press; 2018. [Google Scholar]
- [22].Hoch AL, Gargan TP, Bailey CL. Mechanical transmission of rift valley fever virus by hematophagous diptera. Am J Trop Med Hyg. 1985;34(1):188–193. doi: 10.4269/ajtmh.1985.34.188 [DOI] [PubMed] [Google Scholar]
- [23].Mordecai EA, Ryan SJ, Caldwell JM, et al. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet Health. 2020;4(9):e416–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fredericks AC, Fernandez-Sesma A. The burden of dengue and chikungunya worldwide: implications for the southern United States and California. Ann Glob Health. 2014;80(6):466–475. doi: 10.1016/j.aogh.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Näslund J, Ahlm C, Islam K, et al. Emerging mosquito-borne viruses linked to Aedes aegypti and Aedes albopictus: global status and preventive strategies. Vector Borne Zoonotic Dis. 2021;21(10):731–746 [DOI] [PubMed] [Google Scholar]
- [26].Shepard DS, Undurraga EA, Halasa YA, et al. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16(8):935–941. [DOI] [PubMed] [Google Scholar]
- [27].Braack L, Wulandhari SA, Chanda E, et al. Developing African arbovirus networks and capacity strengthening in arbovirus surveillance and response: findings from a virtual workshop. Parasites Vectors. 2023;16(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].And the PANDORA-ID-NET consortium, Elton L, Haider N, et al. Zoonotic disease preparedness in sub-Saharan African countries. One Health Outlook. 2021;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Power GM, Vaughan AM, Qiao L, et al. Socioeconomic risk markers of arthropod-borne virus (arbovirus) infections: a systematic literature review and meta-analysis. BMJ Global Health. 2022;7(4):e007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hilleman MR. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc Nat Acad Sci. 2004;101(suppl_2):14560–14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med. 2008;102(5):400–408. doi: 10.1016/j.trstmh.2008.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fine PEM. Vectors and vertical transmission: an epidemiologic perspective. Ann N Y Acad Sci. 1975;266(1):173–194. doi: 10.1111/j.1749-6632.1975.tb35099.x [DOI] [PubMed] [Google Scholar]
- [33].Lequime S, Paul RE, Lambrechts L, et al. Determinants of arbovirus vertical transmission in mosquitoes. PLOS Pathogens. 2016;12(5):e1005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ebert D. The epidemiology and evolution of symbionts with mixed-mode transmission. Annu Rev Ecol Evol Syst. 2013;44(1):623–643. doi: 10.1146/annurev-ecolsys-032513-100555 [DOI] [Google Scholar]
- [35].Diaz LA, Flores FS, Quaglia A, et al. Intertwined arbovirus transmission activity: reassessing the transmission cycle paradigm. Front Physiol. 2013;3:3. doi: 10.3389/fphys.2012.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Buenemann D, Sall AA, Diagne CT, et al. Patterns of a sylvatic yellow fever virus amplification in southeastern Senegal, 2010. Am J Trop Med Hyg. 2014;90(6):1003–1013. doi: 10.4269/ajtmh.13-0404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schneider CA, Calvo E, Peterson KE. Arboviruses: how saliva impacts the journey from vector to host. Int J Mol Sci. 2021;22(17):9173. doi: 10.3390/ijms22179173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Viglietta M, Bellone R, Blisnick AA, et al. Vector specificity of arbovirus transmission. Front Microbiol. 2021;12:773211. doi: 10.3389/fmicb.2021.773211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Figueiredo LTM. Human urban arboviruses can Infect wild animals and jump to sylvatic maintenance cycles in South America. Front Cell Infect Microbiol. 2019;9:259. doi: 10.3389/fcimb.2019.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Martina BE, Barzon L, Pijlman GP, et al. Human to human transmission of arthropod-borne pathogens. Curr Opin Virol. 2017;22:13–21. doi: 10.1016/j.coviro.2016.11.005 [DOI] [PubMed] [Google Scholar]
- [41].Desai AN, Majumder MS. What is herd immunity? JAMA. 2020;324(20):2113. doi: 10.1001/jama.2020.20895 [DOI] [PubMed] [Google Scholar]
- [42].Metcalf CJE, Ferrari M, Graham AL, et al. Understanding herd immunity. Trends Immunol. 2015;36(12):753–755. doi: 10.1016/j.it.2015.10.004 [DOI] [PubMed] [Google Scholar]
- [43].Ribeiro GS, Hamer GL, Diallo M, et al. Influence of herd immunity in the cyclical nature of arboviruses. Curr Opin Virol. 2020;40:1–10. doi: 10.1016/j.coviro.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nuttall PA, Labuda M. Dynamics of infection in tick vectors and at the tick–host interface. Adv Virus Res. 2003;60:233–272. Elsevier. [DOI] [PubMed] [Google Scholar]
- [45].Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58(1):433–453. doi: 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- [46].Chathuranga WGD, Karunaratne SHPP, Fernando BR, et al. Diversity, distribution, abundance, and feeding pattern of tropical ornithophilic mosquitoes. J Vector Ecol. 2018;43(1):158–167. doi: 10.1111/jvec.12295 [DOI] [PubMed] [Google Scholar]
- [47].Farajollahi A, Fonseca DM, Kramer LD, et al. “Bird biting” mosquitoes and human disease: a review of the role of culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11(7):1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hamer GL, Kitron UD, Brawn JD, et al. Culex pipiens (Diptera: Culicidae): A bridge vector of west Nile virus to humans. J Med Entomol. 2008;45(1):125–128. doi: 10.1093/jmedent/45.1.125 [DOI] [PubMed] [Google Scholar]
- [49].Lounibos LP, Kramer LD. Invasiveness of Aedes aegypti and Aedes albopictus and vectorial capacity for chikungunya virus. J Infect Dis. 2016;214(suppl 5):S453–S458. doi: 10.1093/infdis/jiw285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti—a review. Memórias Inst Oswaldo Cruz. 2013;108(suppl 1):11–17. doi: 10.1590/0074-0276130395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jansen van Vuren P, Weyer J, Kemp A, et al. Is South Africa at risk for Zika virus disease? S Afr Med J. 2016;106(3):232–233. [DOI] [PubMed] [Google Scholar]
- [52].Valentine MJ, Murdock CC, Kelly PJ. Sylvatic cycles of arboviruses in non-human primates. Parasites Vectors. 2019;12(1):463. doi: 10.1186/s13071-019-3732-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jasinska AJ, Schmitt CA, Service SK, et al. Systems biology of the vervet monkey. ILAR J. 2013;54(2):122–143. doi: 10.1093/ilar/ilt049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Thatcher HR, Downs CT, Koyama NF. Understanding foraging flexibility in urban vervet monkeys, chlorocebus pygerythrus, for the benefit of human-wildlife coexistence. Urban Ecosyst. 2020;23(6):1349–1357. doi: 10.1007/s11252-020-01014-1 [DOI] [Google Scholar]
- [55].Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1(1):a006841–a006841. doi: 10.1101/cshperspect.a006841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Luby JP, Sanders CV. Green monkey disease (“Marburg virus” disease): A new zoonosis. Ann internal med. 1969;71(3):657–660. doi: 10.7326/0003-4819-71-3-657 [DOI] [PubMed] [Google Scholar]
- [57].McIntosh BM. Antibody against chikungunya virus in wild primates in Southern Africa. South African J Med Sci. 1970;35(3):65–74. [PubMed] [Google Scholar]
- [58].Mcintosh BM, Paterson HE, Mcgillivray G, et al. Further studies on the chikungunya outbreak in Southern Rhodesia in 1962. I. Mosquitoes, wild primates and birds in relation to the epidemic. Ann Trop Med Parasitol. 1964;58(1):45–51. [DOI] [PubMed] [Google Scholar]
- [59].Contreras A, Gómez-Martín A, Paterna A, et al. Papel epidemiológico de las aves en la transmisión y mantenimiento de zoonosis. Rev Sci Tech OIE. 2016;35(3):845–862. doi: 10.20506/rst.35.3.2574 [DOI] [PubMed] [Google Scholar]
- [60].Tolsá MJ, García-Peña GE, Rico-Chávez O, et al. Macroecology of birds potentially susceptible to West Nile virus. Proc R Soc B. 2018;285(1893):20182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gould E, Pettersson J, Higgs S, et al. Emerging arboviruses: why today? One Health. 2017;4:1–13. doi: 10.1016/j.onehlt.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mbanzulu KM, Wumba R, Mukendi J-PK, et al. Mosquito-borne viruses circulating in Kinshasa, democratic republic of the congo. Inter J Infect Dis. 2017;57:32–37. doi: 10.1016/j.ijid.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Marchi S, Trombetta CM, Montomoli E. Emerging and re-emerging arboviral diseases as a global health problem. In: Majumder AA, Kabir RRahman S, editors. Public Health—emerging and re-emerging issues. InTech; 2018. [Google Scholar]
- [64].Nyaruaba R, Mwaliko C, Mwau M, et al. Arboviruses in the East African community partner states: a review of medically important mosquito-borne arboviruses. Pathog Glob Health. 2019;113(5):209–228. doi: 10.1080/20477724.2019.1678939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Agboli E, Zahouli JBZ, Badolo A, et al. Mosquito-associated viruses and their related mosquitoes in West Africa. Viruses. 2021;13(5):891. doi: 10.3390/v13050891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Southern African Development Community . Member States. 2022. https://www.sadc.int/member-states
- [67].World Health Organization . Surveillance and control of arboviral diseases in the WHO African region: assessment of country capacities. World Health Organization; 2022b. https://fctc.who.int/publications/i/item/9789240052918 [Google Scholar]
- [68].International Committee on Taxonomy of Viruses: ICTV . [dataset]; 2022. https://ictv.global
- [69].Kampango A, Abílio AP. The Asian tiger hunts in Maputo city—the first confirmed report of Aedes (stegomyia) albopictus (skuse, 1895) in mozambique. Parasites Vectors. 2016;9(1):76. doi: 10.1186/s13071-016-1361-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Smithburn KC, Haddow AJ, Mahaffy AF. A neurotropic virus isolated from Aedes mosquitoes caught in the semliki forest. Am J Trop Med Hyg. 1946;s1-26(2):189–208. [DOI] [PubMed] [Google Scholar]
- [71].Kokernot RH, Heymann CS, Muspratt J, et al. Studies on arthropod-borne viruses of tongaland. V. Isolation of bunyamwera and rift valley fever viruses from mosquitoes. South African J Med Sci. 1957;22(2–3):71–80. [PubMed] [Google Scholar]
- [72].Kokernot RH, Smithburn KC, De Meillon B, et al. Isolation of Bunyamwera virus from a naturally infected human being and further isolations from Aedes (banksinella) circumluteolus theo. 1. Am J Trop Med Hyg. 1958;7(6):579–584. doi: 10.4269/ajtmh.1958.7.579 [DOI] [PubMed] [Google Scholar]
- [73].Dutuze MF, Nzayirambaho M, Mores CN, et al. A review of bunyamwera, batai, and ngari viruses: understudied orthobunyaviruses with potential one health implications. Front Vet Sci. 2018;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kokernot RH, Szlamp EL, Levitt J, et al. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of the caprivi strip and bechuanaland protectorate. Trans R Soc Trop Med. 1965;59(5):553–562. [DOI] [PubMed] [Google Scholar]
- [75].Causey OR, Kemp GE, Causey CE, et al. Isolations of simbu-group viruses in Ibadan, Nigeria 1964–69, including the new types sango, shamonda, sabo and shuni. Ann Trop Med Parasitol. 1972;66(3):357–362. [DOI] [PubMed] [Google Scholar]
- [76].Lee VH. Isolation of viruses from field populations of Culicoides (Diptera: Ceratopogonidae) in Nigeria. J Med Entomol. 1979;16(1):76–79. doi: 10.1093/jmedent/16.1.76 [DOI] [PubMed] [Google Scholar]
- [77].Moore DL, Causey OR, Carey DE, et al. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol. 1975;69(1):49–64. [DOI] [PubMed] [Google Scholar]
- [78].Guarido MM, Motlou T, Riddin MA, et al. Potential mosquito vectors for shuni virus, South Africa, 2014–2018. Emerg Infect Dis. 2021;27(12):3142–3146. doi: 10.3201/eid2712.203426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].van Eeden C, Williams JH, Gerdes TGH, et al. Shuni virus as cause of neurologic disease in horses. Emerg Infect Dis. 2012;18(2):318–321. doi: 10.3201/eid1802.111403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Snyman J, Venter GJ, Venter M. An investigation of Culicoides (Diptera: Ceratopogonidae) as potential vectors of medically and veterinary important arboviruses in South Africa. Viruses. 2021;13(10):1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Motlou TP, Williams J, Venter M. Epidemiology of shuni virus in horses in South Africa. Viruses. 2021;13(5):937. doi: 10.3390/v13050937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].van Eeden C, Swanepoel R, Venter M. Antibodies against West Nile and shuni viruses in veterinarians, South Africa. Emerg Infect Dis. 2014;20(8):1409–1411. doi: 10.3201/eid2008.131724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Steyn J, Motlou P, van Eeden C, et al. Shuni virus in wildlife and nonequine domestic animals, South Africa. Emerg Infect Dis. 2020;26(7):1521–1525. doi: 10.3201/eid2607.190770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Motlou TP, Venter M. Shuni virus in cases of neurologic disease in humans, South Africa. Emerg Infect Dis. 2021;27(2):565–569. doi: 10.3201/eid2702.191551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Smithburn KC, Kokernot RH, Heymann CS, et al. Neutralizing antibodies for certain viruses in the sera of human beings residing in northern natal. S Afr Med J. 1959;33(27):555–561. [PubMed] [Google Scholar]
- [86].Smithburn KC, Paterson HE, Heymann CS, et al. An agent related to Uganda S virus from man and mosquitoes in South Africa. S Afr Med J. 1959;33:959–962. [PubMed] [Google Scholar]
- [87].Jupp PG, McIntosh BM, Anderson D. Culex (eumelanomyia) rubinotus Theobald as vector of banzi, Germiston and Witwatersrand viruses. IV. Observations on the biology of C. rubinotus. J Med Entomol. 1976;12(6):647–651. doi: 10.1093/jmedent/12.6.647 [DOI] [PubMed] [Google Scholar]
- [88].McIntosh BM, Dickinson DB, Meenehan GM, et al. Culex (eumelanomyia) rubinotus Theobald as vector of banzi, Germiston and Witwatersrand viruses. II. Infections in sentinel hamsters and wild rodents. J Med Entomol. 1976;12(6):641–644. doi: 10.1093/jmedent/12.6.641 [DOI] [PubMed] [Google Scholar]
- [89].McIntosh BM, Jupp PG. Infections in sentinel pigeons by Sindbis and West Nile viruses in South Africa, with observations on culex (culex) univittatus (Diptera: Culicidae) attracted to these birds. J Med Entomol. 1979;16(3):234–239. doi: 10.1093/jmedent/16.3.234 [DOI] [PubMed] [Google Scholar]
- [90].McIntosh BM, Jupp PG, Dos Santos IS, et al. Culex (eumelanomyia) rubinotus Theobald as vector of banzi, Germiston and Witwatersrand viruses. I. Isolation of virus from wild populations of C. rubinotus. J Med Entomol. 1976b;12(6):637–640. doi: 10.1093/jmedent/12.6.637 [DOI] [PubMed] [Google Scholar]
- [91].Donaldson JM. An assessment of culex pipiens quinquefasciatus say as a vector of virus diseases in the Witwatersrand region of the Transvaal. 3. Witwatersrand, Germiston and H336 viruses. South African J Med Sci. 1966;31(1):16–20. [PubMed] [Google Scholar]
- [92].Kokernot RH, Casaca VMR, Weinbren MP, et al. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of angola. Trans R Soc Trop Med. 1965;59(5):563–570. [DOI] [PubMed] [Google Scholar]
- [93].Barnard BJ, Voges SF. Flaviviruses in South Africa: pathogenicity for sheep. Onderstepoort J Vet Res. 1986;53(4):235–238. [PubMed] [Google Scholar]
- [94].McIntosh BM. Mosquito-borne virus diseases of man in southern Africa. S Afr Med J. 1986;Suppl:69–72. [PubMed] [Google Scholar]
- [95].MacIntyre C, Guarido MM, Riddin MA, et al. Survey of West Nile and banzi viruses in mosquitoes, South Africa, 2011–2018. Emerg Infect Dis. 2023;29(1):164–169. doi: 10.3201/eid2901.220036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol. 2003;3(1):19–28. doi: 10.1016/S1567-1348(03)00004-2 [DOI] [PubMed] [Google Scholar]
- [97].Ashburn PM, Craig CF. Experimental investigations regarding the etiology of dengue fever. J Infect Dis. 1907;4(3):440–475. doi: 10.1093/infdis/4.3.440 [DOI] [PubMed] [Google Scholar]
- [98].Hotta S. Experimental studies on dengue. I. Isolation, identification and modification of the virus. J Infect Dis. 1952;90(1):1–9. doi: 10.1093/infdis/90.1.1 [DOI] [PubMed] [Google Scholar]
- [99].Christie J. On epidemics of dengue fever: their diffusion and etiology. Glasgow Med J. 1881;16(3):161–176. [PMC free article] [PubMed] [Google Scholar]
- [100].Edington AD. “Dengue”, as seen in the recent epidemic in durban. J Med Associat South Africa. 1927;1:446–448. [Google Scholar]
- [101].Kokernot RH, Smithburn KC, Weinbren MP. Neutralizing antibodies to arthropod-borne viruses in human beings and animals in the Union of South Africa. J Obstet Gynaecol. 1956;77(5):313–323. [PubMed] [Google Scholar]
- [102].Filipe AR, De Carvalho RG, Relvas A, et al. Arbovirus studies in Angola: Serological survey for antibodies to arboviruses. Am J Trop Med Hyg. 1975;24(3):516–520. doi: 10.4269/ajtmh.1975.24.516 [DOI] [PubMed] [Google Scholar]
- [103].Gubler DJ, Sather GE, Kuno G, et al. Dengue 3 virus transmission in Africa. Am J Trop Med Hyg. 1986;35(6):1280–1284. doi: 10.4269/ajtmh.1986.35.1280 [DOI] [PubMed] [Google Scholar]
- [104].Gudo ES, Lesko B, Vene S, et al. Seroepidemiologic screening for zoonotic viral infections, Maputo, mozambique. Emerg Infect Dis. 2016;22(5):915–917. doi: 10.3201/eid2205.151002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Massangaie M, Pinto G, Padama F, et al. Clinical and epidemiological characterization of the first recognized outbreak of dengue virus-type 2 in Mozambique, 2014. Am J Trop Med Hyg. 2016;94(2):413–416. doi: 10.4269/ajtmh.15-0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Muianga A, Pinto G, Ali S, et al. Occurrence of dengue in 2013 and 2014 in northern Mozambique: is dengue an endemic disease in Mozambique? Inter J Infect Dis. 2016;45:174. doi: 10.1016/j.ijid.2016.02.411 [DOI] [Google Scholar]
- [107].Gudo J, Muianga B, Mahumane Gundane I, et al. Dengue virus serotype 2 established in northern Mozambique (2015–2016). Am J Trop Med Hyg. 2017;97(5):1418–1422. doi: 10.4269/ajtmh.17-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Abreu C, Silva-Pinto A, Lazzara D, et al. Imported dengue from 2013 Angola outbreak: not just serotype 1 was detected. J Clin Virol. 2016;79:77–79. doi: 10.1016/j.jcv.2016.04.011 [DOI] [PubMed] [Google Scholar]
- [109].Amarasinghe A. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17(8):1349–1354. doi: 10.3201/eid1708.101515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Noden BH, van der Colf BE. Neglected tropical diseases of Namibia: unsolved mysteries. Acta tropica. 2013;125(1):1–17. doi: 10.1016/j.actatropica.2012.09.007 [DOI] [PubMed] [Google Scholar]
- [111].Noden BH, Musuuo M, Aku-Akai L, et al. Risk assessment of flavivirus transmission in namibia. Acta tropica. 2014;137:123–129. doi: 10.1016/j.actatropica.2014.05.010 [DOI] [PubMed] [Google Scholar]
- [112].Jupp PG, Kemp A. The potential for dengue in South Africa: vector competence tests with dengue 1 and 2 viruses and 6 mosquito species. Trans R Soc Trop Med. 1993;87(6):639–643. doi: 10.1016/0035-9203(93)90271-Q [DOI] [PubMed] [Google Scholar]
- [113].Higa Y, Abílio AP, Futami K, et al. Abundant Aedes (stegomyia) aegypti aegypti mosquitoes in the 2014 dengue outbreak area of mozambique. Trop Med Int Health. 2015;43(2):107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kokernot RH, Smithburn KC, Muspratt J, et al. Studies on arthropod-borne viruses of Tongaland. VIII. Spondweni virus, an agent previously unknown, isolated from taeniorhynchus (mansonioides) uniformis. South African J Med Sci. 1957;22(2–3):103–112. [PubMed] [Google Scholar]
- [115].Mcintosh BM, Kokernot RH, Paterson HE, et al. Isolation of Spondweni virus from four species of culicine mosquitoes and a report of two laboratory infections with the virus. S Afr Med J. 1961 Aug 5;35:647–50. [PubMed] [Google Scholar]
- [116].Jaeger AS, Weiler AM, Moriarty RV, et al. Spondweni virus causes fetal harm in Ifnar1-/- mice and is transmitted by Aedes aegypti mosquitoes. Virol. 2020;547:35–46. doi: 10.1016/j.virol.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Haddow AD, Woodall JP. Distinguishing between zika and spondweni viruses. Bullet World Health Organ. 2016;94(10):711–711A. doi: 10.2471/BLT.16.181503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kokernot RH, Smithburn KC, Kluge E. Neutralizing antibodies against arthropod-borne viruses in the sera of domestic quadrupeds ranging in Tongland, Union of South Africa. Ann Trop Med Parasitol. 1961;55(1):73–85. doi: 10.1080/00034983.1961.11686021 [DOI] [PubMed] [Google Scholar]
- [119].McIntosh BM, Jupp PG, De Sousa J. Further Isolations of arboviruses from mosquitoes collected in Tongaland, South Africa, 1960–19681. J Med Entomol. 1972;9(2):155–159. doi: 10.1093/jmedent/9.2.155 [DOI] [PubMed] [Google Scholar]
- [120].McIntosh BM. Usutu (SAAr 1776), nouvel arbovirus du groupe B. Int Cat Arboviruses. 1985;3:1059–1060. [Google Scholar]
- [121].Nikolay B, Diallo M, Boye CSB, et al. Usutu virus in Africa. Vector Borne Zoonotic Dis. 2011;11(11):1417–1423. doi: 10.1089/vbz.2011.0631 [DOI] [PubMed] [Google Scholar]
- [122].Ndione MHD, Ndiaye EH, Thiam MS, et al. Impact of genetic diversity on biological characteristics of usutu virus strains in Africa. Virus res. 2019;273:197753. doi: 10.1016/j.virusres.2019.197753 [DOI] [PubMed] [Google Scholar]
- [123].Engel D, Jöst H, Wink M, et al. Reconstruction of the evolutionary history and dispersal of usutu virus, a neglected emerging arbovirus in Europe and Africa. MBio. 2016;7(1):e01938–15. doi: 10.1128/mBio.01938-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Benzarti E, Garigliany M. In vitro and in vivo models to study the zoonotic mosquito-borne Usutu virus. Viruses. 2020;12(10):1116. doi: 10.3390/v12101116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Smithburn KC, Hughes TP, Burke AW, et al. A neurotropic virus isolated from the blood of a native of Uganda 1. Am J Trop Med Hyg. 1940;s1-20(4):471–492. [Google Scholar]
- [126].Taylor RM, Rizk F, Work TH, et al. A study of the ecology of West Nile virus in Egypt 1. Am J Trop Med Hyg. 1956;5(4):579–620. [DOI] [PubMed] [Google Scholar]
- [127].Work TH, Hurlbut HS, Taylor RM. Indigenous wild birds of the Nile Delta as potential West Nile virus circulating reservoirs 1. Am J Trop Med Hyg. 1955;4(5):872–888. doi: 10.4269/ajtmh.1955.4.872 [DOI] [PubMed] [Google Scholar]
- [128].Anderson D. Ecological studies on Sindbis and West Nile viruses in South Africa. 3. Host preferences of mosquitoes as determined by the precipitin test. S Afr J Med Sci. 1967;32(1):34–39. [PubMed] [Google Scholar]
- [129].Cornel AJ, Jupp PG. Comparison of three methods for determining transmission rates in vector competence studies with culex univittatus and West Nile and Sindbis viruses. J Am Mosq Control Assoc. 1989;5(1):70–72. [PubMed] [Google Scholar]
- [130].Jupp PG. The ecology of West Nile virus in South Africa and the occurrence of outbreaks in humans. Ann N Y Acad Sci. 2001;951(1):143–152. doi: 10.1111/j.1749-6632.2001.tb02692.x [DOI] [PubMed] [Google Scholar]
- [131].Jupp PG, McIntosh BM. Ecological studies on Sindbis and West Nile viruses in South Africa. II. Mosquito bionomics. South African J Med Sci. 1967;32(1):15–33. [PubMed] [Google Scholar]
- [132].Jupp PG, McIntosh BM. Quantitative experiments on the vector capability of culex (culex) univittatus Theobald with West Nile and Sindbis viruses. J Med Entomol. 1970;7(3):371–373. doi: 10.1093/jmedent/7.3.371 [DOI] [PubMed] [Google Scholar]
- [133].Jupp PG, McIntosh BM, Blackburn NK. Experimental assessment of the vector competence of culex (culex) neavei Theobald with West Nile and Sindbis viruses in South Africa. Trans R Soc Trop Med. 1986;80(2):226–230. doi: 10.1016/0035-9203(86)90019-2 [DOI] [PubMed] [Google Scholar]
- [134].Jupp PG. Laboratory studies on the vector capability of Aedes (neomelaniconion) unidentatus McIntosh and Aedes (aedimorphus/dentatus (Theobald) with West Nile and Sindbis viruses. S Afr J Med Sci. 1976;41(4):265–269. [PubMed] [Google Scholar]
- [135].Burt FJ, Grobbelaar AA, Leman PA, et al. Phylogenetic relationships of southern African West Nile virus isolates. Emerg Infect Dis. 2002;8(8):820–826. doi: 10.3201/eid0808.020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Venter M, Human S, Zaayman D, et al. Lineage 2 west Nile virus as cause of fatal neurologic disease in horses, South Africa. Emerg Infect Dis. 2009;15(6):877–884. doi: 10.3201/eid1506.081515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Venter M, Myers TG, Wilson MA, et al. Gene expression in mice infected with West Nile virus strains of different neurovirulence. Virology. 2005;342(1):119–140. doi: 10.1016/j.virol.2005.07.013 [DOI] [PubMed] [Google Scholar]
- [138].Venter M, Swanepoel R. West Nile virus lineage 2 as a cause of zoonotic neurological disease in humans and horses in southern Africa. Vector Borne Zoonotic Dis. 2010;10(7):659–664. doi: 10.1089/vbz.2009.0230 [DOI] [PubMed] [Google Scholar]
- [139].Steyn J, Botha E, Stivaktas VI, et al. West Nile virus in wildlife and nonequine domestic animals, South Africa, 2010–2018. Emerg Infect Dis. 2019;25(12):2290–2294. doi: 10.3201/eid2512.190572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Venter M, Human S, van Niekerk S, et al. Fatal neurologic disease and abortion in mare infected with lineage 1 West Nile virus, South Africa. Emerg Infect Dis. 2011;17(8):153–6. doi: 10.3201/eid1708.101794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Mcintosh BM, Mcgillivray GM, Dickinson DB, et al. Illness caused by Sindbis and West Nile viruses in South africa. S Afr Med J. 1964;38:291–294. [PubMed] [Google Scholar]
- [142].Weinbren MP. The occurrence of West Nile virus in South Africa. S Afr Med J. 1955;29(47):1092–1097. [PubMed] [Google Scholar]
- [143].Ouhoumanne N, Lowe A-M, Fortin A, et al. Morbidity, mortality and long-term sequelae of West Nile virus disease in Québec. Epidemiol Infect. 2018;146(7):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Botha EM, Markotter W, Wolfaardt M, et al. Genetic determinants of virulence in pathogenic lineage 2 West Nile virus strains. Emerg Infect Dis. 2008;14(2):222–230. doi: 10.3201/eid1402.070457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Venter M, Burt FJ, Blumberg L, et al. Cytokine induction after laboratory-acquired West Nile virus infection. N Engl J Med. 2009;360(12):1260–1262. [DOI] [PubMed] [Google Scholar]
- [146].Kokernot RH, McIntosh BM. Isolation of West Nile virus from a naturally infected human being and from a bird, sylvietta rufescens (vieillot). S Afr Med J. 1959;33(47):987–989. [PubMed] [Google Scholar]
- [147].Jupp PG, Blackburn NK, Thompson DL, et al. Sindbis and West Nile virus infections in the Witwatersrand-Pretoria region. S Afr Med J. 1986;70(4):218–220. [PubMed] [Google Scholar]
- [148].Uejio CK, Kemp A, Comrie AC. Climatic controls on West Nile virus and Sindbis virus transmission and outbreaks in South Africa. Vector Borne Zoonotic Dis. 2012;12(2):117–125. doi: 10.1089/vbz.2011.0655 [DOI] [PubMed] [Google Scholar]
- [149].McIntosh BM, Jupp PG, Dos Santos I, et al. Epidemics of West Nile and Sindbis viruses in South Africa with culex (culex) univittatus Theobald as vector. South African J Sci. 1976a;72(10):295–300. [Google Scholar]
- [150].Joubert JJ, Prozesky OW, Lourens JG, et al. Prevalence of hepatitis virus and some arbovirus infections in kavango, northern SWA/Namibia. S Afr Med J. 1985;67(13):500–502. [PubMed] [Google Scholar]
- [151].Joubert JJ, van der Merwe CA, Lourens JH, et al. Serological markers of hepatitis B virus and certain other viruses in the population of eastern Caprivi, Namibia. Trans R Soc Trop Med. 1991;85(1):101–103. [DOI] [PubMed] [Google Scholar]
- [152].Mweene-Ndumba I, Siziya S, Monze M, et al. Seroprevalence of West Nile virus specific IgG and IgM antibodies in North-Western and Western provinces of Zambia. Afr H Sci. 2015;15(3):803–809. doi: 10.4314/ahs.v15i3.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Velu RM, Kwenda G, Libonda L, et al. Mosquito-borne viral pathogens detected in Zambia: a systematic review. Pathogens. 2021;10(8):1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].McIntosh BM, Dickinson DB, McGillivray GM. Ecological studies on Sindbis and West Nile viruses in South Africa. V. The response of birds to inoculation of virus. South African J Med Sci. 1969;34(3):77–82. [PubMed] [Google Scholar]
- [155].McIntosh BM, Jupp PG, Dickinson DB, et al. Ecological studies on Sindbis and West Nile viruses in South Africa. I. Viral activity as revealed by infection of mosquitoes and sentinel fowls. South African J Med Sci. 1967;32(1):1–14. [PubMed] [Google Scholar]
- [156].McIntosh BM, Madsen W, Dickinson DB. Ecological studies on Sindbis and West Nile viruses in South Africa. VI. The antibody response of wild birds. South African J Med Sci. 1969;34(3):83–91. [PubMed] [Google Scholar]
- [157].Editors of the South African Medical Journal . Sindbis and West Nile exanthemata in South Africa. S Afr Med J. 1968;42(9):197. [PubMed] [Google Scholar]
- [158].Zaayman D, Venter M. West Nile virus neurologic disease in humans, South Africa, September 2008–May 2009. Emerg Infect Dis. 2012;18(12):2051–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Simpson VR, Kuebart G, Barnard B. A fatal case of wesselsbron disease in a dog. Vet Rec. 1979;105(14):329. doi: 10.1136/vr.105.14.329 [DOI] [PubMed] [Google Scholar]
- [160].Blackburn NK, Reyers F, Berry WL, et al. Susceptibility of dogs to West Nile virus: a survey and pathogenicity trial. J Comp Pathol. 1989;100(1):59–66. doi: 10.1016/0021-9975(89)90090-X [DOI] [PubMed] [Google Scholar]
- [161].Williams JH, van Niekerk S, Human S, et al. Pathology of fatal lineage 1 and 2 West Nile virus infections in horses in South Africa. J S Afr Vet Assoc. 2014;85(1):1105. doi: 10.4102/jsava.v85i1.1105 [DOI] [PubMed] [Google Scholar]
- [162].Venter M, Steyl J, Human S, et al. Transmission of West Nile virus during horse autopsy. Emerg Infect Dis. 2010;16(3):573–575. doi: 10.3201/eid1603.091042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Simulundu E, Ndashe K, Chambaro HM, et al. West Nile virus in farmed crocodiles, Zambia, 2019. Emerg Infect Dis. 2020;26(4):811–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Guggemos HD, Fendt M, Hieke C, et al. Simultaneous circulation of two West Nile virus lineage 2 clades and bagaza virus in the Zambezi region, Namibia. PLoS negl trop dis. 2021;15(4):e0009311. doi: 10.1371/journal.pntd.0009311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Orba Y, Hang’ombe BM, Mweene AS, et al. First isolation of West Nile virus in Zambia from mosquitoes. Transbound Emerg Dis. 2018;65(4):933–938. doi: 10.1111/tbed.12888 [DOI] [PubMed] [Google Scholar]
- [166].Nwaiwu AU, Musekiwa A, Tamuzi JL, et al. The incidence and mortality of yellow fever in Africa: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ahmed QA, Memish ZA. Yellow fever from Angola and Congo: a storm gathers. Trop Doct. 2017;47(2):92–96. doi: 10.1177/0049475517699726 [DOI] [PubMed] [Google Scholar]
- [168].Garske T, Van Kerkhove MD, Yactayo S, et al. Yellow fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLOS Med. 2014;11(5):e1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Grobbelaar AA, Weyer J, Moolla N, et al. Resurgence of yellow fever in Angola, 2015–2016. Emerg Infect Dis. 2016;22(10):1854–1855. doi: 10.3201/eid2210.160818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Babaniyi O, Chizema E, Eshetu-Shibeshi M, et al. Risk assessment for yellow fever in western and North-Western provinces of Zambia. J Glob Infect Dis. 2015;7(1):11. doi: 10.4103/0974-777X.150884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Gear JH. The history of virology in South Africa. S Afr Med J. 1986;Suppl:7–10. [PubMed] [Google Scholar]
- [172].Masaninga F, Muleba M, Masendu H, et al. Distribution of yellow fever vectors in Northwestern and Western provinces, Zambia. Asian Pac J Trop Med. 2014;7S(1):S88–92. doi: 10.1016/S1995-7645(14)60210-8 [DOI] [PubMed] [Google Scholar]
- [173].Hanley KA, Monath TP, Weaver SC, et al. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292–311. doi: 10.1016/j.meegid.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comp Immunol Microbiol Infect Dis. 2004;27(5):319–330. doi: 10.1016/j.cimid.2004.03.013 [DOI] [PubMed] [Google Scholar]
- [175].De Meillon B. Proved and potential vectors of yellow fever in South Africa. Bullet World Health Organ. 1954;11(3):443–451. [PMC free article] [PubMed] [Google Scholar]
- [176].Bauer JH. The transmission of yellow fever by mosquitoes other than Aedes aegypti. JAMA. 1928;90(26):2091. doi: 10.1001/jama.1928.02690530019007 [DOI] [Google Scholar]
- [177].Abílio AP, Kampango A, Armando EJ, et al. First confirmed occurrence of the yellow fever virus and dengue virus vector Aedes (stegomyia) luteocephalus (Newstead, 1907) in Mozambique. Parasites Vectors. 2020;13(1):350. doi: 10.1186/s13071-020-04217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Beeuwkes H, Mahaffy AF, Burke AW, et al. Yellow fever protection test surveys in the French cameroons, French equatorial Africa, the Belgian Congo, and Angola. Trans R Soc Trop Med. 1934;28(3):233–258. [Google Scholar]
- [179].Mahaffy AF, Smithburn KC, Hughes TP. The distribution of immunity to yellow fever in Central and East Africa. Trans R Soc Trop Med. 1946;40(1):57–82. doi: 10.1016/0035-9203(46)90062-4 [DOI] [PubMed] [Google Scholar]
- [180].Freedman ML. The yellow fever situation in the bechuanaland protectorate. Bullet World Health Organ. 1954;11(3):487–492. [PMC free article] [PubMed] [Google Scholar]
- [181].Smithburn KC, Goodner K, Dick GWA. Further studies on the distribution of immunity to yellow fever in east and south-east Africa. Ann Trop Med Parasitol. 1949;43(2):182–193. doi: 10.1080/00034983.1949.11685404 [DOI] [PubMed] [Google Scholar]
- [182].Pinto MR, Filipe AR. The yellow fever epidemic in Luanda in 1971. Bull Soc Pathol Exot Filiales. 1971;64(5):708–710. [PubMed] [Google Scholar]
- [183].Pinto MR, Filipe AR. Arbovirus studies in Luanda, Angola. 1. Virological and serological studies during a yellow fever epidemic. Bullet World Health Organ. 1973;49(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- [184].Jupp PG, Kemp A. What is the potential for future outbreaks of chikungunya, dengue and yellow fever in southern Africa? S Afr Med J. 1996;86(1):35–37. [PubMed] [Google Scholar]
- [185].World Health Organization . Countries with risk of yellow fever transmission and countries requiring yellow fever vaccination (November 2022). World Health Organization; 2022a. https://www.who.int/publications/m/item/countries-with-risk-of-yellow-fever-transmission-and-countries-requiring-yellow-fever-vaccination-(november-2022) [Google Scholar]
- [186].Zhao S, Stone L, Gao D, et al. Modelling the large-scale yellow fever outbreak in Luanda, Angola, and the impact of vaccination. PLoS negl trop dis. 2018;12(1):e0006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Wilder-Smith A, Massad E. Estimating the number of unvaccinated Chinese workers against yellow fever in Angola. BMC Infect Dis. 2018;18(1):185. doi: 10.1186/s12879-018-3084-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Woodall JP, Yuill TM. Why is the yellow fever outbreak in Angola a “threat to the entire world”? Internat J Infect Dis: IJID: Official Publicat Internat Soc Infectious Dis. 2016;48:96–97. [DOI] [PubMed] [Google Scholar]
- [189].Dick GWA, Kitchen SF, Haddow AJ. Zika Virus (I). Isolations and serological specificity. Trans R Soc Trop Med. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- [190].Cao-Lormeau V-M, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20(6):1084–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Lowe R, Barcellos C, Brasil P, et al. The zika virus epidemic in Brazil: from discovery to future implications. Int J Environ Res Public Health. 2018;15(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Gutiérrez-Bugallo G, Piedra LA, Rodriguez M, et al. Vector-borne transmission and evolution of zika virus. Nat Ecol Evol. 2019;3(4):561–569. doi: 10.1038/s41559-019-0836-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Musso D, Gubler DJ. Zika virus. Clin Microbiol Revi. 2016;29(3):487–524. doi: 10.1128/CMR.00072-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Gyawali N, Bradbury RS, Taylor-Robinson AW. The global spread of Zika virus: is public and media concern justified in regions currently unaffected? Infect Dis Poverty. 2016;5(1):37. doi: 10.1186/s40249-016-0132-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: Following the path of dengue and chikungunya? Lancet. 2015;386(9990):243–244. doi: 10.1016/S0140-6736(15)61273-9 [DOI] [PubMed] [Google Scholar]
- [197].Halani S, Tombindo PE, O’Reilly R, et al. Clinical manifestations and health outcomes associated with zika virus infections in adults: a systematic review. PLoS negl trop dis. 2021;15(7):e0009516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Song B-H, Yun S-I, Woolley M, et al. Zika virus: History, epidemiology, transmission, and clinical presentation. J Neuroimmunol. 2017;308:50–64. doi: 10.1016/j.jneuroim.2017.03.001 [DOI] [PubMed] [Google Scholar]
- [199].National Center for Emerging and Zoonotic Infectious Diseases (U.S.). Division of Vector-Borne Diseases . Countries That Have Past Or Current Evidence Of Zika Virus Transmission (As Of January 2016) [Map]; 2016. https://stacks.cdc.gov/view/cdc/37509
- [200].Kokernot RH, Smithburn KC, Gandara AF, et al. Neutralization tests with sera from individuals residing in Mozambique against specific viruses isolated in Africa, transmitted by arthropods. Anais Do Instituto De Medicina Tropical. 1960;17:201–230. [PubMed] [Google Scholar]
- [201].Gudo ES, Falk KI, Ali S, et al. A historic report of zika in Mozambique: implications for assessing current risk. PLoS negl trop dis. 2016;10(12):e0005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Rückert C, Weger-Lucarelli J, Garcia-Luna SM, et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun. 2017;8(1):15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Chelene IR, Ali S, Mula FI, et al. Retrospective investigation of IgM antibodies against Zika virus in serum from febrile patients in Mozambique, 2009-2015. BMC Res Notes. 2019;12(1):469. doi: 10.1186/s13104-019-4511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Buechler CR, Bailey AL, Weiler AM, et al. Seroprevalence of Zika virus in wild African green monkeys and baboons. mSphere. 2017;2(2):e00392–16. doi: 10.1128/mSphere.00392-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Hill SC, Vasconcelos J, Neto Z, et al. Emergence of the Asian lineage of Zika virus in Angola: an outbreak investigation. Lancet Infect Dis. 2019;19(10):1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Sassetti M, Zé-Zé L, Franco J, et al. First case of confirmed congenital Zika syndrome in continental Africa. Trans R Soc Trop Med. 2018;112(10):458–462. [DOI] [PubMed] [Google Scholar]
- [207].Faye O, De Lourdes Monteiro M, Vrancken B, et al. Genomic epidemiology of 2015–2016 zika virus outbreak in Cape verde. Emerg Infect Dis. 2020;26(6):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Kraemer MUG, Brady OJ, Watts A, et al. Zika virus transmission in Angola and the potential for further spread to other African settings. Trans R Soc Trop Med. 2017;111(11):527–529. doi: 10.1093/trstmh/try001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Gear JH, Thomson PD, Hopp M, et al. Congo-Crimean haemorrhagic fever in South Africa. Report of a fatal case in the Transvaal. S Afr Med J. 1982;62(16):576–580. [PubMed] [Google Scholar]
- [210].Swanepoel R, Struthers JK, Shepherd AJ, et al. Crimean-Congo hemorrhagic fever in South Africa. Am J Trop Med Hyg. 1983;32(6):1407–1415. doi: 10.4269/ajtmh.1983.32.1407 [DOI] [PubMed] [Google Scholar]
- [211].Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15(4):307–417. doi: 10.1093/jmedent/15.4.307 [DOI] [PubMed] [Google Scholar]
- [212].Spengler JR, Bergeron É, Rollin PE, et al. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS negl trop dis. 2016;10(1):e0004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Swanepoel R, Shepherd AJ, Leman PA, et al. Investigations following initial recognition of Crimean-Congo haemorrhagic fever in South Africa and the diagnosis of 2 further cases. S Afr Med J. 1985;68(9):638–641. [PubMed] [Google Scholar]
- [214].Swanepoel R, Shepherd AJ, Leman PA, et al. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am J Trop Med Hyg. 1987;36(1):120–132. doi: 10.4269/ajtmh.1987.36.120 [DOI] [PubMed] [Google Scholar]
- [215].Shepherd AJ, Swanepoel R, Shepherd SP, et al. Antibody to Crimean-Congo hemorrhagic fever virus in wild mammals from southern Africa. Am J Trop Med Hyg. 1987;36(1):133–142. doi: 10.4269/ajtmh.1987.36.133 [DOI] [PubMed] [Google Scholar]
- [216].Burt FJ, Swanepoel R, Braack LE. Enzyme-linked immunosorbent assays for the detection of antibody to Crimean-Congo haemorrhagic fever virus in the sera of livestock and wild vertebrates. Epidemiol Infect. 1993;111(3):547–557. doi: 10.1017/S0950268800057277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Blackburn NK, Searle L, Taylor P. Viral haemorrhagic fever antibodies in Zimbabwe schoolchildren. Trans R Soc Trop Med. 1982;76(6):803–805. doi: 10.1016/0035-9203(82)90113-4 [DOI] [PubMed] [Google Scholar]
- [218].Gear JH. The hemorrhagic fevers of Southern Africa with special reference to studies in the South African institute for medical research. Yale J Biol Med. 1982;55(3–4):207–212. [PMC free article] [PubMed] [Google Scholar]
- [219].Kokernot RH, Smithburn KC, Paterson HE, et al. Further isolations of wesselsbron virus from mosquitoes. S Afr Med J. 1960;34:871–874. [PubMed] [Google Scholar]
- [220].McIntosh BM, Dos Santos IS, Meenehan GM. Culex (eumelanomyia) rubinotus Theobald as vector of banzi, Germiston and Witwatersrand viruses. III. Transmission of virus to hamsters by wild-caught infected C. rubinotus. J Med Entomol. 1976;12(6):645–646. doi: 10.1093/jmedent/12.6.645 [DOI] [PubMed] [Google Scholar]
- [221].Sharp BL, Appleton CC, Thompson DL, et al. Anthropophilic mosquitoes at Richards Bay, Natal, and arbovirus antibodies in human residents. Trans R Soc Trop Med. 1987;81(2):197–201. [DOI] [PubMed] [Google Scholar]
- [222].Kokernot RH, Smithburn KC, Weinbren MP, et al. Studies on arthropod-borne viruses of Tongaland. VI. Isolation of pongola virus from Aedes (Banksinella) circumluteolus Theo. South African J Med Sci. 1957;22(2–3):81–92. [PubMed] [Google Scholar]
- [223].Jupp PG, McIntosh BM. A bionomic study of adult Aedes (Neomelaniconion) circumluteolus in northern Kwazulu, South Africa. J Am Mosq Control Assoc. 1987;3(2):131–136. [PubMed] [Google Scholar]
- [224].Groseth A, Mampilli V, Weisend C, et al. Molecular characterization of human pathogenic bunyaviruses of the nyando and Bwamba/Pongola virus groups leads to the genetic identification of Mojuí dos Campos and Kaeng Khoi virus. PLoS negl trop dis. 2014;8(9):e3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Weinbren MP, Heymann CS, Kokernot RH, et al. Studies on arthropod-borne viruses of Tongaland. VII. Simbu virus, a hitherto unknown agent isolated from Aedes (Banksinella) circumluteolus theo. S Afr J Med Sci. 1957;22(2–3):93–102. [PubMed] [Google Scholar]
- [226].Saeed MF, Li L, Wang H, et al. Phylogeny of the simbu serogroup of the genus bunyavirus. J Gen Virol. 2001;82(Pt 9):2173–2181. doi: 10.1099/0022-1317-82-9-2173 [DOI] [PubMed] [Google Scholar]
- [227].McIntosh BM, Smithburn KC, Paterson HE, et al. Isolation of Germiston virus, a hitherto unknown agent, from culicine mosquitoes, and a report of infection in two laboratory workers *. Am J Trop Med Hyg. 1960;9(1):62–69. doi: 10.4269/ajtmh.1960.9.62 [DOI] [PubMed] [Google Scholar]
- [228].Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J Pathol Bacteriol. 1931;34(4):545–579. doi: 10.1002/path.1700340418 [DOI] [Google Scholar]
- [229].Pienaar NJ, Thompson PN. Temporal and spatial history of rift valley fever in South Africa: 1950 to 2011. Onderstepoort J Vet Res. 2013;80(1):384. doi: 10.4102/ojvr.v80i1.384 [DOI] [PubMed] [Google Scholar]
- [230].Nanyingi MO, Munyua P, Kiama SG, et al. A systematic review of rift valley fever epidemiology 1931-2014. Infect Ecol Epidemiol. 2015;5:28024. doi: 10.3402/iee.v5.28024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Alexander RA. Rift valley fever in the Union. J S Afr Vet Assoc. 1951;22(3):105–112. [Google Scholar]
- [232].Gear J, De Meillon B, Le Roux AF, et al. Rift valley fever in South Africa; a study of the 1953 outbreak in the orange free state, with special reference to the vectors and possible reservoir hosts. S Afr Med J. 1955;29(22):514–518. [PubMed] [Google Scholar]
- [233].McIntosh BM. Rift valley fever. 1. Vector studies in the field. J S Afr Vet Med Assoc. 1972;43(4):391–395. [PubMed] [Google Scholar]
- [234].McIntosh BM, Jupp PG, dos Santos I, et al. Vector studies on rift valley fever virus in South Africa. S Afr Med J. 1980;58(3):127–132. [PubMed] [Google Scholar]
- [235].McIntosh BM, Jupp PG, Dos Santos I, et al. Field and laboratory evidence implicating culex zombaensis and Aedes circumluteolus as vectors of rift valley fever virus in coastal South Africa. South African J Sci. 1983;79(2):61–64. [Google Scholar]
- [236].Pepin M, Bouloy M, Bird BH, et al. Rift valley fever virus (Bunyaviridae: phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41(6):61. doi: 10.1051/vetres/2010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Swanepoel R, Cruickshank JG. Arthropod borne viruses of medical importance in Rhodesia 1968-1973. Cent Afr J Med. 1974;20(4):71–79. [PubMed] [Google Scholar]
- [238].Jupp PG, Cornel AJ. Vector competence tests with rift valley fever virus and five South African species of mosquito. J Am Mosq Control Assoc. 1988;4(1):4–8. [PubMed] [Google Scholar]
- [239].Linthicum KJ, Britch SC, Anyamba A. Rift valley fever: an emerging mosquito-borne disease. Annu Rev Entomol. 2016;61(1):395–415. doi: 10.1146/annurev-ento-010715-023819 [DOI] [PubMed] [Google Scholar]
- [240].van den Bergh C, Thompson PN, Swanepoel R, et al. Detection of Rift Valley Fever Virus in aedes (aedimorphus) durbanensis, South Africa. Pathogens. 2022 Jan 21;11(2):125. doi: 10.3390/pathogens11020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Coetzer JA. The pathology of rift valley fever. I. Lesions occurring in natural cases in new-born lambs. Onderstepoort J Vet Res. 1977;44(4):205–211. [PubMed] [Google Scholar]
- [242].Coetzer JA. The pathology of Rift Valley fever. II. Lesions occurring in field cases in adult cattle, calves and aborted foetuses. Onderstepoort J Vet Res. 1982;49(1):11–17. [PubMed] [Google Scholar]
- [243].Coetzer JA, Barnard BJ. Hydrops amnii in sheep associated with hydranencephaly and arthrogryposis with wesselsbron disease and rift valley fever viruses as aetiological agents. Onderstepoort J Vet Res. 1977;44(2):119–126. [PubMed] [Google Scholar]
- [244].Davies FG, Kilelu E, Linthicum KJ, et al. Patterns of rift valley fever activity in Zambia. Epidemiol Infect. 1992;108(1):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Fafetine JM, Coetzee P, Mubemba B, et al. Rift valley fever outbreak in livestock, Mozambique, 2014. Emerg Infect Dis. 2016;22(12):2165–2167. doi: 10.3201/eid2212.160310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Lagerqvist N, Moiane B, Mapaco L, et al. Antibodies against rift valley fever virus in cattle, Mozambique. Emerg Infect Dis. 2013;19(7):1177–1179. doi: 10.3201/eid1907.130332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].van der Riet FD, Sayed AR, Barnard BJ, et al. Arthropod-borne virus zoonosis surveillance in the cape province: 1. Prospective serological investigations for virus activity in the Beaufort West and Middelburg districts during 1981. J S Afr Vet Assoc. 1985;56(1):25–29. [PubMed] [Google Scholar]
- [248].Anywaine Z, Lule SA, Hansen C, et al. Clinical manifestations of rift valley fever in humans: systematic review and meta-analysis. PLoS negl trop dis. 2022;16(3):e0010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Mundel B, Gear J. Rift valley fever; I. The occurrence of human cases in Johannesburg. S Afr Med J. 1951;25(44):797–800. [PubMed] [Google Scholar]
- [250].van der Linde NT. A recent epidemic of rift valley fever in the orange free state. J S Afr Vet Assoc. 1953;24(3):145–148. [Google Scholar]
- [251].Shone DK. Rift Valley fever in Southern Rhodesia. Cent Afr J Med. 1958;4(7):284–286. [PubMed] [Google Scholar]
- [252].Stern L. Rift Valley fever in Rhodesia; report of a case in a laboratory worker. Cent Afr J Med. 1958;4(7):281–284. [PubMed] [Google Scholar]
- [253].McIntosh BM, Russell D, dos Santos I, et al. Rift Valley fever in humans in South Africa. S Afr Med J. 1980;58(20):803–806. [PubMed] [Google Scholar]
- [254].Swanepoel R, Manning B, Watt JA. Fatal Rift Valley fever of man in Rhodesia. Cent Afr J Med. 1979;25(1):1–8. [PubMed] [Google Scholar]
- [255].Tessier SF, Rollin PE, Sureau P. Viral haemorrhagic fever survey in Chobe (Botswana). Trans R Soc Trop Med. 1987;81(4):699–700. doi: 10.1016/0035-9203(87)90462-7 [DOI] [PubMed] [Google Scholar]
- [256].Niklasson B, Liljestrand J, Bergström S, et al. Rift Valley fever: A sero-epidemiological survey among pregnant women in Mozambique. Epidemiol Infect. 1987;99(2):517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Baudin M, Jumaa AM, Jomma HJE, et al. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health. 2016;4(11):e864–e871. doi: 10.1016/S2214-109X(16)30176-0 [DOI] [PubMed] [Google Scholar]
- [258].Pawęska JT, Msimang V, Kgaladi J, et al. Rift Valley fever virus seroprevalence among humans, northern KwaZulu-Natal Province, South Africa, 2018–2019. Emerg Infect Dis. 2021;27(12):3159–3162. doi: 10.3201/eid2712.210643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Sanderson CE, Jori F, Moolla N, et al. Silent circulation of Rift Valley fever in humans, Botswana, 2013–2014. Emerg Infect Dis. 2020;26(10):2453–2456. doi: 10.3201/eid2610.191837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Gudo ES, Pinto G, Weyer J, et al. Serological evidence of rift valley fever virus among acute febrile patients in Southern Mozambique during and after the 2013 heavy rainfall and flooding: implication for the management of febrile illness. Virol J. 2016;13(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Jansen van Vuren P, Kgaladi J, Msimang V, et al. Rift Valley fever reemergence after 7 years of quiescence, South Africa, May 2018. Emerg Infect Dis. 2019;25(2):338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Pachka H, Annelise T, Alan K, et al. Rift Valley fever vector diversity and impact of meteorological and environmental factors on culex pipiens dynamics in the Okavango Delta, Botswana. Parasites Vect. 2016;9(1):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Anyamba A, Linthicum KJ, Small J, et al. Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006-2008 and possible vector control strategies. Am J Trop Med Hyg. 2010;83(2 Suppl):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Glancey MM, Anyamba A, Linthicum KJ. Epidemiologic and environmental risk factors of Rift Valley fever in Southern Africa from 2008 to 2011. Vector Borne Zoonotic Dis. 2015;15(8):502–511. doi: 10.1089/vbz.2015.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Verster AM, Liang JE, Rostal MK, et al. Selected wetland soil properties correlate to Rift Valley fever livestock mortalities reported in 2009-10 in central South Africa. PLoS One. 2020;15(5):e0232481. doi: 10.1371/journal.pone.0232481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Kitandwe PK, McKay PF, Kaleebu P, et al. An overview of Rift Valley fever vaccine development strategies. Vaccines. 2022;10(11):1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Weinbren MP, Williams MC, Haddow AJ. A variant of Rift Valley fever virus. S Afr Med J. 1957;31(38):951–957. [PubMed] [Google Scholar]
- [268].Lumley S, Horton DL, Marston DA, et al. Complete genome sequence of Rift Valley fever virus strain Lunyo. Genome Announc. 2016;4(2):e00170–16. doi: 10.1128/genomeA.00170-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Lumsden WHR. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952–1953 II. General description and epidemiology. Trans R Soc Trop Med. 1955;49(1):33–57. doi: 10.1016/0035-9203(55)90081-X [DOI] [PubMed] [Google Scholar]
- [270].Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952–1953. Trans R Soc Trop Med. 1955;49(1):28–32. doi: 10.1016/0035-9203(55)90080-8 [DOI] [PubMed] [Google Scholar]
- [271].Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016 [DOI] [PubMed] [Google Scholar]
- [272].McIntosh BM, Jupp PG, Dos Santos I. Rural epidemic of chikungunya in South Africa with involvement of Aedes (diceromyia) furcifer (Edwards) and Baboons. South African J Sci. 1977;73(9):267–269. [Google Scholar]
- [273].Jupp PG, McIntosh BM, Dos Santos I, et al. Laboratory vector studies on six mosquito and one tick species with chikungunya virus. Trans R Soc Trop Med. 1981;75(1):15–19. [DOI] [PubMed] [Google Scholar]
- [274].Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nature Rev Microbiol. 2010;8(7):491–500. doi: 10.1038/nrmicro2368 [DOI] [PubMed] [Google Scholar]
- [275].Rajapakse S, Rodrigo C, Rajapakse A. Atypical manifestations of chikungunya infection. Trans R Soc Trop Med. 2010;104(2):89–96. doi: 10.1016/j.trstmh.2009.07.031 [DOI] [PubMed] [Google Scholar]
- [276].Mcintosh BM, Harwin RM, Paterson HE, et al. An epidemic of chikungunya in South-Eastern Rhodesia. Cent Afr J Med. 1963;9:351–359. [PubMed] [Google Scholar]
- [277].Paterson HE, Mcintosh BM. Further studies on the chikungunya outbreak in Southern Rhodesia in 1962. II. transmission experiments with the Aedes furcifer -taylori group of mosquitoes and with a member of the anopheles gambiae complex. Ann Trop Med Parasitol. 1964;58:52–55. [DOI] [PubMed] [Google Scholar]
- [278].Rodger LM. An outbreak of suspected chikungunya fever in Northern Rhodesia. S Afr Med J. 1961;35:126–128. [PubMed] [Google Scholar]
- [279].Filipe AF, Pinto MR. Arbovirus studies in Luanda, Angola. 2. Virological and serological studies during an outbreak of dengue-like disease caused by the Chikungunya virus. Bullet World Health Organ. 1973;49(1):37–40. [PMC free article] [PubMed] [Google Scholar]
- [280].van den Bosch C, Lloyd G. Chikungunya fever as a risk factor for endemic Burkitt’s lymphoma in Malawi. Trans R Soc Trop Med. 2000;94(6):704–705. doi: 10.1016/S0035-9203(00)90240-2 [DOI] [PubMed] [Google Scholar]
- [281].Aly MM, Ali S, Muianga AF, et al. Severe Chikungunya infection in northern Mozambique: a case report. BMC Res Notes. 2017;10(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].António VS, Amade NA, Muianga AF, et al. Retrospective investigation of antibodies against chikungunya virus (CHIKV) in serum from febrile patients in Mozambique, 2009–2015: implications for its prevention and control. PLoS One. 2019;14(3):e0213941. doi: 10.1371/journal.pone.0213941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].António VS, Muianga AF, Wieseler J, et al. Seroepidemiology of Chikungunya virus among febrile patients in eight health facilities in Central and Northern Mozambique, 2015-2016. Vector Borne Zoonotic Dis. 2018;18(6):311–316. [DOI] [PubMed] [Google Scholar]
- [284].Gudo ES, Ali S, António VS, et al. Seroepidemiological studies of arboviruses in Africa. Adv Exp Med Biol. 2018;1062:361–371. [DOI] [PubMed] [Google Scholar]
- [285].Gudo ES, Black JFP, Cliff JL, et al. Chikungunya in Mozambique: A forgotten history. PLoS negl trop dis. 2016;10(11):e0005001. doi: 10.1371/journal.pntd.0005001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Gudo ES, Pinto G, Vene S, et al. Serological evidence of Chikungunya virus among acute febrile patients in Southern Mozambique. PLoS negl trop dis. 2015;9(10):e0004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Mugabe VA, Ali S, Chelene I, et al. Evidence for chikungunya and dengue transmission in Quelimane, Mozambique: results from an investigation of a potential outbreak of chikungunya virus. PLoS One. 2018;13(2):e0192110. doi: 10.1371/journal.pone.0192110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Muianga A, Pinto G, Massangaie M, et al. Antibodies against Chikungunya in Northern Mozambique during Dengue outbreak, 2014. Vector Borne Zoonotic Dis. 2018;18(8):445–449. [DOI] [PubMed] [Google Scholar]
- [289].Chisenga CC, Bosomprah S, Musukuma K, et al. Sero-prevalence of arthropod-borne viral infections among Lukanga swamp residents in Zambia. PLoS One. 2020;15(7):e0235322. doi: 10.1371/journal.pone.0235322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Parreira R, Centeno-Lima S, Lopes A, et al. Dengue virus serotype 4 and chikungunya virus coinfection in a traveller returning from Luanda, Angola, January 2014. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 2014;19(10):20730. [DOI] [PubMed] [Google Scholar]
- [291].Takaya S, Kutsuna S, Nakayama E, et al. Chikungunya fever in traveler from Angola to Japan, 2016. Emerg Infect Dis. 2017;23(1):156–158. doi: 10.3201/eid2301.161395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Kokernot RH, De Meillon B, Paterson HE, et al. Middelburg virus; a hitherto unknown agent isolated from Aedes mosquitoes during an epizootic in sheep in the eastern Cape province. South African J Med Sci. 1957;22(4):145–153. [PubMed] [Google Scholar]
- [293].Hubálek Z, Rudolf I, Nowotny N. Arboviruses pathogenic for domestic and wild animals. Adv Virus Res. 2014;89:201–275. [DOI] [PubMed] [Google Scholar]
- [294].Attoui H, Sailleau C, Mohd Jaafar F, et al. Complete nucleotide sequence of Middelburg virus, isolated from the spleen of a horse with severe clinical disease in Zimbabwe. J Gen Virol. 2007;88(Pt 11):3078–3088. doi: 10.1099/vir.0.83076-0 [DOI] [PubMed] [Google Scholar]
- [295].Fourie I, Snyman J, Williams J, et al. Epidemiological and genomic characterisation of Middelburg and Sindbis alphaviruses identified in horses with febrile and neurological infections, South Africa (2014–2018). Viruses. 2022;14(9):2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].van Niekerk S, Human S, Williams J, et al. Sindbis and Middelburg old World alphaviruses associated with neurologic disease in horses, South Africa. Emerg Infect Dis. 2015;21(12):2225–2229. doi: 10.3201/eid2112.150132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Steyn J, Fourie I, Steyl J, et al. Zoonotic alphaviruses in fatal and neurologic infections in wildlife and nonequine domestic animals, South Africa. Emerg Infect Dis. 2020;26(6):1182–1191. doi: 10.3201/eid2606.191179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Fourie I, Williams J, Ismail A, et al. Detection and genome characterization of Middelburg virus strains isolated from CSF and whole blood samples of humans with neurological manifestations in South Africa. PLoS negl trop dis. 2022;16(1):e0010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Smithburn KC, Haddow AJ. Semliki forest virus. I. Isolation and pathogenic properties. J Immunol. 1944;49(3):141–157. doi: 10.4049/jimmunol.49.3.141 [DOI] [Google Scholar]
- [300].Mcintosh BM, Worth CB, Kokernot RH. Isolation of Semliki Forest virus from Aedes (aedimorphus) argenteopunctatus (Theobald) collected in Portuguese East Africa. Trans R Soc Trop Med. 1961;55(2):192–198. doi: 10.1016/0035-9203(61)90025-6 [DOI] [PubMed] [Google Scholar]
- [301].Woodall JP, Bertram DS. The transmission of Semliki forest virus by Aedes aegypti L. Trans R Soc Trop Med. 1959;53(6):440–444. doi: 10.1016/0035-9203(59)90019-7 [DOI] [PubMed] [Google Scholar]
- [302].Mathiot CC, Grimaud G, Garry P, et al. An outbreak of human Semliki forest virus infections in Central African republic. Am J Trop Med Hyg. 1990;42(4):386–393. doi: 10.4269/ajtmh.1990.42.386 [DOI] [PubMed] [Google Scholar]
- [303].Storm N, Weyer J, Markotter W, et al. Human cases of Sindbis fever in South Africa, 2006-2010. Epidemiol Infect. 2014;142(2):234–238. doi: 10.1017/S0950268813000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Malherbe H, Strickland-Cholmley M. Sindbis virus infection in man. Report of a case with recovery of virus from skin lesions. S Afr Med J. 1963;37(21):547–552. [PubMed] [Google Scholar]
- [305].Jupp PG, Phillips JI. An electron microscopical study of Rift Valley fever and Sindbis viral infection in mosquito salivary glands (Diptera: Culicidae). African Entomol. 1998;6(1):75–81. [Google Scholar]
- [306].Guarido MM, Fourie I, Meno K, et al. Alphaviruses detected in mosquitoes in the North-Eastern regions of South Africa, 2014 to 2018. Viruses. 2023;15(2):414. doi: 10.3390/v15020414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Eisenhut M, Schwarz TF, Hegenscheid B. Seroprevalence of dengue, chikungunya and Sindbis virus infections in German aid workers. Infection. 1999;27(2):82–85. doi: 10.1007/BF02560502 [DOI] [PubMed] [Google Scholar]
- [308].Maar SA. A case of sindbis virus infection in Zimbabwe. Cent Afr J Med. 1980;26(7):161–162. [PubMed] [Google Scholar]
- [309].Meno K, Yah C, Mendes A, et al. Incidence of Sindbis virus in hospitalized patients with acute fevers of unknown cause in South Africa, 2019–2020. Front Microbiol. 2021;12:798810. doi: 10.3389/fmicb.2021.798810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Weinbren MP, Kokernot RH, Smithburn KC. Strains of Sindbis-like virus isolated from culicine mosquitoes in the Union of South Africa. I. Isolation and properties. S Afr Med J. 1956;30(27):631–636. [PubMed] [Google Scholar]
- [311].Weiss KE, Haig DA, Alexander RA, et al. Wesselsbron virus—a virus not previously described, associated with abortion in domestic animals. Onderstepoort J Veterin Res. 1956;27(2):183–195. [Google Scholar]
- [312].Muspratt J, Smithburn KC, Paterson HE, et al. Studies on arthropod-borne viruses of Tongaland. X. The laboratory transmission of Wesselsbron virus by the bite of Aedes (Banksinella) circumluteolus theo. South African J Med Sci. 1957;22(2–3):121–126. [PubMed] [Google Scholar]
- [313].Smithburn KC, Kokernot RH, Weinbren MP, et al. Studies on arthropod-borne viruses of Tongaland. IX. Isolation of Wesselsbron virus from a naturally infected human being and from Aedes (Banksinella) circumluteolus theo. South African J Med Sci. 1957;22(2–3):113–120. [PubMed] [Google Scholar]
- [314].Coetzer JA, Theodoridis A. Clinical and pathological studies in adult sheep and goats experimentally infected with Wesselsbron disease virus. Onderstepoort J Vet Res. 1982;49(1):19–22. [PubMed] [Google Scholar]
- [315].Mushi EZ, Binta MG, Raborokgwe M. Wesselsbron disease virus associated with abortions in goats in Botswana. J Vet Diagn Invest. 1998;10(2):191. doi: 10.1177/104063879801000216 [DOI] [PubMed] [Google Scholar]
- [316].Neitz WO. Wesselsbron disease. Bull Off Int Epizoot. 1966;65(9):1735–1741. [PubMed] [Google Scholar]
- [317].Blackburn NK, Swanepoel R. An investigation of flavivirus infections of cattle in Zimbabwe Rhodesia with particular reference to Wesselsbron virus. J Hyg (Lond). 1980;85(1):1–33. doi: 10.1017/S0022172400027066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Heymann CS, Kokernot RH, De Meillon B. Wesselsbron virus infections in man. S Afr Med J. 1958;32(21):543–545. [PubMed] [Google Scholar]
- [319].Jupp PG, Kemp A. Studies on an outbreak of Wesselsbron virus in the free state Province, South Africa. J Am Mosq Control Assoc. 1998;14(1):40–45. [PubMed] [Google Scholar]
- [320].Weyer J, Thomas J, Leman PA, et al. Human cases of Wesselsbron disease, South Africa 2010-2011. Vector Borne Zoonotic Dis. 2013;13(5):330–336. doi: 10.1089/vbz.2012.1181 [DOI] [PubMed] [Google Scholar]
- [321].Paterson HE, Kokernot RH, Davis SH. Studies of arthropod-borne viruses of Tongaland. IV. The birds of tongaland and their possible role in virus disease. S Afr J Med Sci. 1957;22(2–3):63–69. [PubMed] [Google Scholar]
- [322].Worth CB, Paterson HE, De Meillon B. The incidence of arthropod-borne viruses in a population of culicine mosquitoes in Tongaland, Union of South Africa (January, 1956, through April, 1960). Am J Trop Med Hyg. 1961;10:583–592. [DOI] [PubMed] [Google Scholar]
- [323].Kokernot RH, INTOSH BM, Worth CB, et al. Isolation of viruses from mosquitoes collected at Lumbo, Mozambique. II. Mossuril virus, a new virus isolated from the culex (culex) sitiens Wiedemann group. Am J Trop Med Hyg. 1962;11:683–684. [DOI] [PubMed] [Google Scholar]
- [324].Vasilakis N, Tesh RB, Popov VL, et al. Exploiting the legacy of the arbovirus hunters. Viruses. 2019;11(5):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Kagaayi J, Serwadda D. The history of the HIV/AIDS epidemic in Africa. Current HIV/AIDS Reports. 2016;13(4):187–193. doi: 10.1007/s11904-016-0318-8 [DOI] [PubMed] [Google Scholar]
- [326].Berrang Ford L. Civil conflict and sleeping sickness in Africa in general and Uganda in particular. Confl Health. 2007;1(1):6. doi: 10.1186/1752-1505-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].National Institutes of Health . Research Portfolio Online Reporting Tools Expenditures And Results (RePorter) [Dataset]; 2023. https://reporter.nih.gov
- [328].Kading RC, Cohnstaedt LW, Fall K, et al. Emergence of arboviruses in the United States: the boom and bust of funding, innovation, and capacity. Trop Med Infect Dis. 2020;5(2):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Kramer IM, Pfeiffer M, Steffens O, et al. The ecophysiological plasticity of Aedes aegypti and Aedes albopictus concerning overwintering in cooler ecoregions is driven by local climate and acclimation capacity. Sci Total Environ. 2021;778:146128. doi: 10.1016/j.scitotenv.2021.146128 [DOI] [PubMed] [Google Scholar]
- [330].Venter M, Zaayman D, van Niekerk S, et al. Macroarray assay for differential diagnosis of meningoencephalitis in southern Africa. J Clin Virol. 2014;60(1):50–56. [DOI] [PubMed] [Google Scholar]
- [331].Petit MJ, Shah PS. Mapping arbovirus-vector interactions using systems biology techniques. Front Cell Infect Microbiol. 2019;8:440. doi: 10.3389/fcimb.2018.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Tourlet S, Radjasandirane R, Diharce J, et al. AlphaFold2 update and perspectives. BioMedinformat. 2023;3(2):378–390. doi: 10.3390/biomedinformatics3020025 [DOI] [Google Scholar]
- [333].Gesto JSM, Ribeiro GS, Rocha MN, et al. Reduced competence to arboviruses following the sustainable invasion of Wolbachia into native Aedes aegypti from Southeastern Brazil. Sci Rep. 2021;11(1):10039. doi: 10.1038/s41598-021-89409-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Kamtchum-Tatuene J, Makepeace BL, Benjamin L, et al. The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Curr Opin Infect Dis. 2017;30(1):108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Rainey SM, Shah P, Kohl A, et al. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. J Gen Virol. 2014;95(Pt 3):517–530. doi: 10.1099/vir.0.057422-0 [DOI] [PubMed] [Google Scholar]
- [336].Ant TH, Mancini MV, McNamara CJ, et al. Wolbachia-virus interactions and arbovirus control through population replacement in mosquitoes. Pathog Glob Health. 2023;117(3):245–258. doi: 10.1080/20477724.2022.2117939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Denton JA, Joubert DA, Goundar AA, et al. International shipments of Wolbachia-infected mosquito eggs: towards the scaling-up of World mosquito program operations. Rev Sci Tech. 2022;41(1):91–99. doi: 10.20506/rst.41.1.3306 [DOI] [PubMed] [Google Scholar]
- [338].Buchori D, Mawan A, Nurhayati I, et al. Risk assessment on the Release of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia. Insects. 2022;13(10):924. doi: 10.3390/insects13100924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Koerich LB, Sant’anna MRV, Huits R. Recent technological advances and strategies for arbovirus vector control. Trop Med Infect Dis. 2022;7(9):204. doi: 10.3390/tropicalmed7090204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].World Health Organization . Global strategic framework for integrated vector management. World Health Organization; 2004. https://www.who.int/publications/i/item/WHO-CDS-CPE-PVC-2004.10# [Google Scholar]
- [341].Wanyenze RK, Alfvén T, Ndejjo R, et al. Sustainable health—a call to action. BMC Global and Publ Health. 2023;1(1):3. doi: 10.1186/s44263-023-00007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


