Abstract
Recent evidence indicates activated mitogen-activated protein kinase (MAPK) p38 has a critical function in human cytomegalovirus (HCMV) viral DNA replication in infected human fibroblasts. To elucidate the mechanism of HCMV-mediated p38 activation, we have performed a detailed analysis of p38 activation and the kinases associated with this activation at different times postinfection. We demonstrate that p38 kinase activity is strongly increased following viral infection. Inhibition of this activity significantly inhibited HCMV-induced hyperphosphorylation of pRb and phosphorylation of heat shock protein 27, suggesting that p38 activation is involved in virus-mediated changes in host cell metabolism throughout the course of infection. We then provide evidence that p38 activation is mediated by different mechanisms at early times versus later times of infection. At early times of infection (8 to 14 h postinfection [hpi]), when p38 activation is first observed, no significant activation of the three kinases which can directly phosphorylate p38 (namely, MKK3, MKK6, and MKK4) is detected. Using vectors which express dominant negative proteins, we demonstrate that basal MKK6 kinase activity is necessary for HCMV-mediated p38 activation at these early times of infection (12 hpi). Then, we use ATP depletion to show that at 12 hpi, HCMV inhibits dephosphorylation of activated p38. These two experiments suggest that HCMV activates p38 by inhibition of dephosphorylation of p38. In contrast to early times of infection, at later times of infection (48 to 72 hpi), increased MKK3/6, but not MKK4, activity is observed. These results indicate that at early times of HCMV infection, increased steady-state levels of activated p38 is mediated at least in part by inhibition of dephosphorylation of p38, while at later times of infection p38 activation is due to increased activity of the upstream kinases MKK3 and MKK6. These findings indicate that HCMV has developed multiple mechanisms to ensure activation of the MAPK p38, a kinase critical to viral infection.
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus that is found in over 80% of the population. While asymptomatic in most immunocompetent hosts, in immunocompromised individuals, such as transplant recipients and AIDS patients, HCMV causes a wide range of clinical symptoms which, if left untreated, are often fatal (6, 17). Currently, patients are treated with antiviral drugs, such as ganciclovir and Foscarnet, which inhibit HCMV-permissive infection (33, 37, 40). However, with the dramatic rise in immunocompromised individuals who require long-term antiviral treatment, drug-resistant strains of HCMV are becoming more common, resulting in a loss of ability to control infection (reviewed in reference 12). This problem has resulted in the need to identify and characterize new antiviral targets which can be used to inhibit the life cycle of HCMV.
Several reports have demonstrated that HCMV infection induces activation of numerous host cell transcription factors such as Sp-1, CREB/ATF family members, and NF-κB (7, 20–22, 30, 56–58). This activation ensures high levels of expression of the many viral and cellular genes which are required for completion of the lytic life cycle. Since many of these transcription factors are required for expression of certain genes, and hence are necessary for completion of the viral life cycle, inhibiting their activation represents one mechanism to inhibit viral infection.
One way to inhibit the activation of cellular transcription factors may be to inhibit upstream signaling events which control their activity. Since the transactivation function of many cellular transcription factors is at least partially regulated by phosphorylation events, identifying and inhibiting cellular kinases which phosphorylate transcription factors may represent one mechanism to inhibit HCMV permissiveness (2, 49). This has led members of our laboratory and others to search for specific kinase pathways which are activated following HCMV infection and which activate transcription factors. Mitogen-activated protein kinases (MAPKs) are examples of kinases which activate numerous transcription factors, and some members of the MAPK family are strongly activated following HCMV infection (19, 46).
MAPKs are important cellular signaling kinases which are activated by dual phosphorylation on specific tyrosine and threonine residues in response to various external and internal stimuli (reviewed in references 10 and 45). In mammalian cells, three general groups of MAPKs have been identified: extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38/Hog. Each MAPK is positioned at the bottom of a distinct kinase pathway composed of three sequential dual-specificity kinases, generically termed the MAPKKK (MKKK or MEKK), MAPKK (MKK or MEK), and MAPK. Following stimulation, the MKKK is dually phosphorylated on specific residues by cellular signaling molecules. This activated MKKK then phosphorylates the MKK. Once activated, the MKK phosphorylates the MAPK on the appropriate threonine and tyrosine residues, resulting in MAPK activation (10, 45).
Activated MAPKs can phosphorylate numerous substrates, including a variety of transcription factors (2, 28, 52). In the case of transcription factors, MAPK-mediated phosphorylation is a common mechanism utilized by the cell to induce gene expression (28, 52). Largely through their ability to regulate transcription factors, MAPKs regulate changes in the cell ranging from cell growth to apoptosis to senescence.
Because of the many effects of MAPK signaling on cell growth and viability, the level of phosphorylated, and hence active, MAPK is tightly regulated by the cell. Phosphorylation of MAPK is determined by two processes, MKK kinase activity and phosphatase activity. In the absence of stimulation, there is a low level of basal MKK kinase activity, which phosphorylates the MAPK. This basal activity is counteracted by an equal low level of basal MAPK phosphatase activity. This balance prevents an accumulation of activated MAPK in the absence of stimulation. Following stimulation, MAPK is dramatically activated (by phosphorylation via the MAPK pathway). In many instances, shortly after MAPK activation, MAPK phosphatases are activated. However, the high levels of MKK activity prevents a decrease in the amount of activated MAPK. Once the upstream signal subsides, MKK activity drops, and the MAPK is quickly dephosphorylated by the activated phosphatases (reviewed in reference 16).
Numerous viruses activate one or more of the MAPKs. For example, simian virus 40 (SV40) activates ERK1/2, herpes simplex virus (HSV) activates both JNK and p38, and simian immunodeficiency virus activates all three MAPKs (34, 42, 54, 59). In addition, MAPK activation can be induced by viral binding to the host cell, such as is the case with simian immunodeficiency virus activation of ERK1/2, JNK, and p38, or it can require viral protein synthesis, as is the case with HSV activation of JNK (42).
While there are many reports which demonstrate MAPK activation following viral infection, few have determined whether this activation is important for a viral permissive infection. The recent identification of dominant negative proteins and chemical compounds which specifically inhibit different MAPK kinases have allowed more detailed analysis of the function of MAPK activation in virally infected cells (26, 27, 44). Using 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl) 1 H-imidazole (FHPI), a drug which inhibits p38 kinase activity, investigators demonstrated that p38 kinase activity was necessary for interleukin-1-mediated increased in human immunodeficiency virus replication (11), as measured by increases in pp24 levels (27, 48). In another study, dominant negative proteins were used to demonstrate that inhibition of HSV-mediated JNK activation significantly decreases viral titers (34). However, even though p38 activation was observed following HSV infection, FHPI had no effect on viral titers, indicating that not all MAPKs which are activated following infection have a critical role in viral permissive infection (T. I. McLean and S. L. Bachenheimer, personal communication).
Even in cases where it has been determined that MAPK activation has a function in viral infection, little is known about how MAPK mediates this effect. For example, MAPK substrates which are activated following viral infection have not been identified. Furthermore, little is known about cellular proteins that are involved in virus-mediated MAPK activation. An understanding of these points is of great interest since it will allow investigators to better examine the antiviral potential of MAPKs, in addition to greatly increasing our knowledge of how the virus regulates host cell machinery to ensure a permissive infection.
Interestingly, HCMV infection has been found to activate both ERK1/2 and p38. In the absence of prestimulation, ERK1/2 activation is observed at 5 to 15 min following viral binding to the cell (3) and at 4 to 8 h postinfection (hpi) following viral gene expression (46). Increased p38 activity is detected at 8 hpi and maintained through 48 hpi (19).
Excitingly, treatment of infected cells with drugs which inhibit either ERK1/2 activation or p38 kinase activity significantly delays viral DNA replication and subsequent plaque formation (19; R. A. Johnson, A. D. Yurochko, S.-M. Huong, and E.-S. Huang, unpublished data). Additional studies have shown that inhibition of either p38 or ERK1/2 kinase activity does not affect expression of the two viral major immediate-early (IE) genes, IE1-72 and IE2-86 (19, 46). However, while inhibition of ERK1/2 activation reduced expression of several viral E genes necessary for initiation of viral DNA replication, inhibition of p38 kinase activity did not, suggesting that rather than having redundant roles, each has a distinct function in HCMV infection (19, 48; Johnson et al., unpublished data).
As with other viruses, little is known about the mechanism of p38 activation following HCMV infection or which cellular proteins are phosphorylated in a p38-dependent manner following infection. Therefore, we have undertaken a detailed study into the mechanism of p38 activation following HCMV infection. Initially, we provide evidence that p38 is not activated immediately following viral binding to the host cell. Rather, p38 is first activated at 8 to 10 hpi, and this activation is maintained throughout infection (72 hpi), though a decrease in activity is often detected at 24 hpi. Since extended activation of p38 is unusual, we next examined whether any p38 substrates were also activated for extended periods of time in a p38-dependent manner. We present data suggesting that activation of p38 for extended periods of time is necessary for HCMV-mediated hyperphosphorylation of pRb, which is observed at 48 to 72 hpi, and for phosphorylation of heat shock protein 27 (HSP27), which is observed from 12 to 48 hpi.
We then focused on the mechanism by which HCMV-mediated this unusually long-term activation of p38. We provide evidence that HCMV utilizes two mechanisms to activate p38. Our results suggest that at early times of infection, HCMV inhibits dephosphorylation of activated p38, allowing an accumulation of phosphorylated p38 in the absence of increased MKK3/6 activity. At later times of infection (48 to 72 hpi), increased activation of MKK3/6 allows continued p38 activation. To our knowledge, this is the first report of any stimulus or virus which activates p38 by inhibiting dephosphorylation, underscoring the complex interaction between HCMV and infected host cells.
Collectively, the results of this study demonstrate that p38 is activated for extended periods of time following infection, and they also identify proteins phosphorylated in a p38-dependent manner in the context of viral infection. Identifying these substrates is significant since it will allow future investigation into the biological function of p38 in HCMV infection. Finally, several lines of evidence presented here suggest that HCMV has developed multiple mechanisms to ensure activation of a cellular kinase which is critical to initiation of viral DNA replication. Targeting these mechanisms may provide a means to inhibit HCMV-mediated p38 activation and viral permissiveness.
MATERIALS AND METHODS
Cell culture and viral passage.
Human embryonic lung (HEL) fibroblasts were cultured in Eagle's minimal essential media (EMEM) (Gibco, BRL) supplemented with 10% fetal bovine serum (FBS) plus antibiotics. The human astrocytoma cell line U373MG was grown in Dulbecco's modified Eagle medium (DMEM) (Gibco, BRL) supplemented with 10% FBS plus antibiotics. Towne strain HCMV (passages 36 to 40) was propagated in HEL fibroblasts as previously described (22).
Expression vectors and cell lines.
FLAG-pCDNA3-MKK3 dominant negative (MKK3dn) and FLAG-pCDNA3-MKK6 dominant negative (MKK6dn) expression vectors were kindly provided by Roger Davis and have been previously described (44). To obtain cell lines, U373 cells were seeded in 35-mm-diameter six-well plates at 106 cells/well. The following day they were transfected with the indicated plasmid, using Fugene 6 as specified by the manufacturer (Boehringer GmbH, Mannheim, Germany). Forty-eight hours later, fresh DMEM containing 10% FBS, antibiotics, and G418 (800 μg/ml) was added to each well. The medium was changed every 5 days. After G418 selection, the expression of transfected protein in U373 was confirmed by Western blot analysis, immunoprecipitation, and immunofluorescence.
Antibodies and inhibitor drugs.
All phosphospecific antibodies and the p38 nonphosphospecific antibody were purchased from New England Biolabs (NEB; Beverly, Mass.). FLAG antibody M2 was obtained from Kodak (Rochester, N.Y.). pRb antibody was from Santa Cruz Biotechnology (Santa Cruz, Calif.), and cyclin D and cyclin E antibodies were from Oncogene Science (Cambridge, Mass.). HSP27 antibody was from Sigma (St. Louis, Mo.). Anisomycin, rotenone, and 2-deoxyglucose were purchased from Sigma. The specific p38 inhibitor drug FHPI was purchased from Calbiochem (La Jolla, Calif.).
Western blot analysis.
Fibroblasts were grown to confluence, serum starved for 48 h, and then infected with HCMV at a multiplicity of infection (MOI) of 2 to 5 PFU per cell as described elsewhere (19). In experiments which utilized FHPI, cells were pretreated with 10 μM FHPI for 1 h prior to infection. Following pretreatment, cells were infected and incubated in the presence of 10 μM FHPI as previously described (19). At the indicated times, cells were harvested in 2× Laemmli sodium dodecyl (SDS) sample buffer, boiled, and loaded onto SDS-polyacrylamide gels. Mock-infected samples were treated and harvested in the same manner as the infected samples, except that EMEM without virus was used during the infection. Proteins were separated by polyacrylamide gel electrophoresis (PAGE) and transferred overnight at 14 V to Immobilon-P transfer membranes (Millipore, Bedford, Mass.). Blots were blocked for 30 min in 10% (wt/vol) Carnation nonfat dry milk dissolved in phosphate-buffered saline (PBS) with 0.1% Tween 20 (PBS+T). Blots were then probed with primary antibody (1:1,000 dilution) for 2 h at room temperature or overnight at 4°C in PBS+T. Blots were washed three times with PBS+T. After washing, the blots were probed with secondary antibody (horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G [Sigma or NEB, respectively]). Blots were washed in PBS+T and developed by enhanced chemiluminescence as specified by the manufacturer (NEB).
Immunoprecipitations and kinase assays.
Glutathione S-transferase (GST)–c-Jun (amino acids [aa] 1 to 79) and GST–ATF-2 (full length) were purchased from NEB. p38 and JNK kinase assays were performed as previously described (43). Briefly, cells were pretreated and infected as described above. At the indicated times, cells were harvested in lysis buffer (150 mM NaCl, 20 mM Tris-HCl [pH 7.5], 1.0% Triton X-100, 0.5 mM EDTA, 50 mM NaF, 10% glycerol, 20 μg of leupeptin per ml, 20 μg of phenylmethylsulfonyl fluoride [PMSF] per ml, 1 mM sodium vanadate [Na3VO4]), rocked for 15 min at 4°C, and then vortexed for 15 seconds. Cell debris was pelleted by centrifugation, and the concentration of the supernatant was determined by the Bio-Rad protein assay. For the JNK kinase assay, 75 μg of supernatant was rocked for 4 h at 4°C with 1 μg of GST–c-Jun bound to GST beads (Biochem Pharmacia). For the p38 assay, p38 was immunoprecipitated overnight from 200 μg of supernatant in ELB+ (0.25 M NaCl, 0.1% NP-40, 0.05 M HEPES [pH 7.0], 1 mM PMSF, 0.5 mM EDTA, 0.05 mM dithiothreitol [DTT]) plus 0.1 mM Na3VO4 with a p38 polyclonal antibody and 15 μl of protein G-Sepharose beads (Biochem Pharmacia). After the appropriate incubation, the beads were washed three times in lysis buffer, and once in kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 50 mM NaCl2, 5 mM β-glycerophosphate, 1.5 mM EGTA, 1 mM PMSF, 0.5 mM EDTA, 0.05 mM DTT, 0.1 mM Na3VO4). Kinase assays were then performed, using 5 μCi of [γ-32P]ATP/reaction and, in the case of p38, addition of 1 μg of GST–ATF-2. After a 20-min incubation at 30°C, the beads were washed once and then denatured with 2× SDS sample buffer. After boiling, the samples were separated by SDS-PAGE on a 12% polyacrylamide gel, dried, and subjected to autoradiography. Bands were quantitated with a densitometer.
Heat treatment and ATP depletion.
Confluent, serum-starved cells were washed once with EMEM which had been prewarmed to 45°C and then incubated in EMEM at 45°C for 20 min. ATP depletion was performed as previously described (35). Briefly, following infection or heat treatment, cells were washed twice with PBS which was preheated to 37°C. Cells were then incubated with PBS supplemented with 5 μM rotenone and 10 mM 2-deoxyglucose at 37°C for the indicated times. Following the appropriated incubation, cells were washed twice with PBS and harvested in 2× SDS lysis buffer.
In vivo analysis of HSP27 phosphorylation.
Confluent, serum-starved fibroblasts were infected as described above. Three hours prior to harvesting, cells were washed twice with phosphate-free EMEM prewarmed to 37°C and then incubated with 0.4 mCi of [32P]orthophosphate per ml in phosphate-free EMEM (ICN). Following a 3-h incubation, cells were harvested and protein concentration was determined by the Bio-Rad protein assay as described above. HSP27 was immunoprecipitated as described above and separated by SDS-PAGE on a 12% gel. HSP27 phosphorylation was then determined by autoradiography.
RESULTS
Kinetics of p38 activation following viral infection.
Inhibition of p38 kinase activity by treatment of infected cells with FHPI significantly inhibits HCMV DNA replication and decreases viral titers (19). Determining the mechanism of HCMV-mediated p38 activation could identify new targets to inhibit this activation and, therefore, viral permissiveness. Since little is known about p38 activation following HCMV infection, it was first necessary to determine the kinetics of p38 activation during HCMV infection. With this information, we could focus our investigation of the mechanism of HCMV-mediated p38 activation on those times which correlated with increased levels of p38 activity.
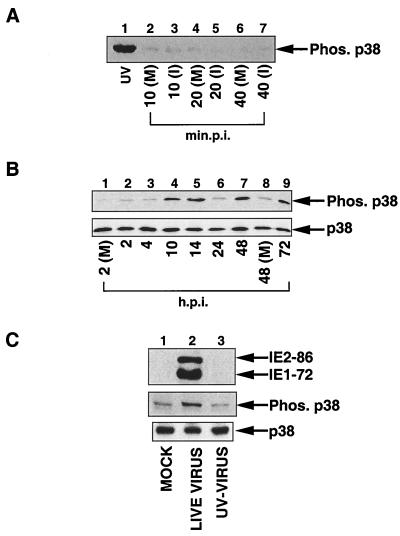
To determine the kinetics of activation, an extended time course was performed. Confluent fibroblasts were serum starved for 48 h, infected with HCMV at an MOI of 2 to 5 PFU, and harvested at the indicated times postinfection. p38 activation was examined by Western blot analysis using a phosphospecific antibody which recognizes only the dually phosphorylated, and hence active, form of p38 (43). As shown in Fig. 1A, HEL cells infected with HCMV did not display increased p38 activity from 10 to 40 min following infection (lanes 2 to 7). However, a strong increase in p38 phosphorylation was detected by 10 hpi (Fig. 1B, lane 4), which is consistent with previously published reports (19). p38 phosphorylation decreased between 14 and 24 hpi, then increased dramatically between 24 and 48 hpi, and remained at this level through 72 hpi (Fig. 4B, lanes 5 to 9). Western blot analysis using an antibody which recognizes both phosphorylated and nonphosphorylated p38 demonstrated that the overall levels of p38 protein were not significantly altered during the course of infection, suggesting that the increase in phosphorylated p38 was not due to an elevation in the amount of p38 analyzed (Fig. 1B, lower blot).
FIG. 1.
Activation of p38 following HCMV infection. HEL fibroblasts were infected with sucrose gradient-purified virus at an MOI of 2 to 5 PFU. Cells were then harvested in 2× SDS sample buffer at the indicated time points, and Western blot analysis using a p38 phosphospecific antibody was performed. (A) Time course examining the effect of HCMV infection on p38 activation at times corresponding to the first tier of activation of host cell transcription factors. (B) (Top) Western blot demonstrating that p38 is phosphorylated on activating residues beginning at 10 hpi; (bottom) Western blot using p38 antibody, which recognizes all forms of p38, demonstrating that the overall levels of p38 do not fluctuate. (C) Viral protein synthesis is necessary for HCMV-mediated p38 activation. Fibroblasts were infected with unpurified virus stock at an MOI of 2 to 5 PFU. Where indicated, virus was inactivated by UV irradiation prior to infection. Blots were also probed for IE1-72 and IE2-86 gene expression to demonstrate inactivation of virus by UV irradiation (UV-VIRUS). p.i., postinfection; (M), mock-infected cells; (I), infected cells; UV, UV-irradiated HEL fibroblasts [positive control]); Phos., phosphorylated. The results are representative of five experiments.
FIG. 4.
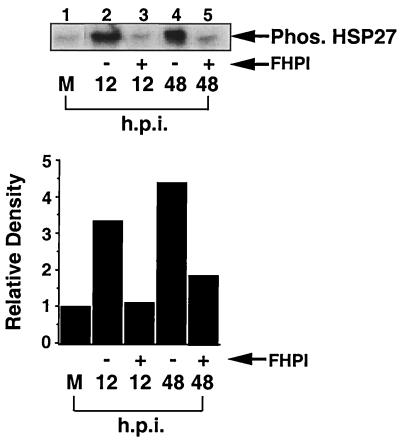
p38 kinase activity is necessary for phosphorylation of HSP27 following viral infection. Confluent, serum-starved fibroblasts were infected and then labeled with 0.4 mCi of [32P]orthophosphate per ml in the presence or absence of FHPI 3 h prior to being harvested. At the indicated times, cell extracts were harvested, and HSP27 was immunoprecipitated from 500 μg of whole-cell extract per sample, separated by SDS-PAGE on a 12% gel, and subjected to autoradiography. M, mock infected; Phos., phosphorylated.
To further characterize HCMV-mediated activation of p38, fibroblasts were infected with unpurified virus stock which was either untreated or UV irradiated prior to infection. UV irradiation creates thymidine dimers which prevents transcription of viral genes without inhibiting the ability of the virus to bind to and enter the host cell. As Fig. 1C shows, p38 activation was observed only following infection with live virus, indicating that viral protein synthesis is necessary for p38 activation following HCMV infection. Similar results were obtained when fibroblasts were infected with sucrose gradient-purified live or UV-irradiated virus (data not shown).
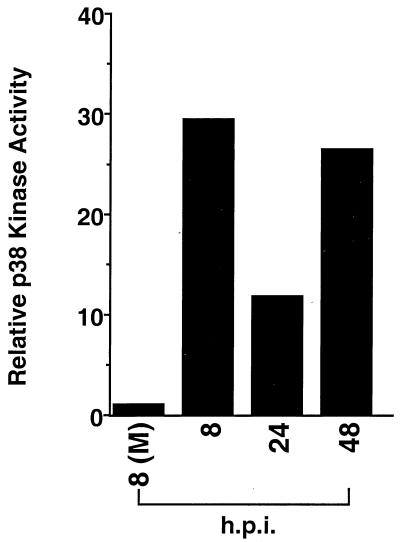
To verify that p38 phosphorylation correlated with p38 kinase activity, a p38 kinase immunocomplex assay was performed with GST–ATF-2 as a substrate. As shown in Fig. 2, quantitation of band intensity demonstrates that p38 kinase activity correlated well with p38 phosphorylation (compare Fig. 1B with Fig. 2), in which activation was observed at early times of infection, decreased at 24 hpi, and then rose again at 48 hpi. The drop in p38 activation at 24 hpi, which was found in most, though not all, time courses, suggested that p38 activation may be biphasic.
FIG. 2.
HCMV-induced p38 phosphorylation correlates with increases in p38 kinase activity. Fibroblasts were treated and infected as for Fig. 1. At the indicated time points, cells were harvested in lysis buffer, and p38 kinase activity was determined by using GST–ATF-2 (aa 1 to 254) as a substrate. Autoradiography was analyzed with a densitometer, and the quantitation is shown. (M), mock infected. The data are representative of two separate experiments.
The results in Fig. 1 and 2 demonstrate that p38 is activated for an extended period of time following HCMV infection. It is very unusual for stimuli to induce such a prolonged activation of p38, and the fact that HCMV does supports our hypothesis that p38 activation has a very important role in HCMV infection for extended periods of time.
HCMV infection results in hyperphosphorylation of pRb and phosphorylation of HSP27 in a p38-dependent manner.
The results in Fig. 1 and 2 suggest that p38 has a role in HCMV infection throughout the course of infection. However, no one has yet examined if the extended activation of p38 correlates with activation of any p38 substrates in the context of viral infection. For this reason, we sought to identify substrates which are phosphorylated in a p38-dependent manner following viral infection. Following activation by certain stimuli, p38 phosphorylates the pocket protein pRb and HSP27 (13, 47, 50). Furthermore, under specific conditions, increased p38 kinase activity can regulate expression of the cell cycle-regulating protein cyclin D (25).
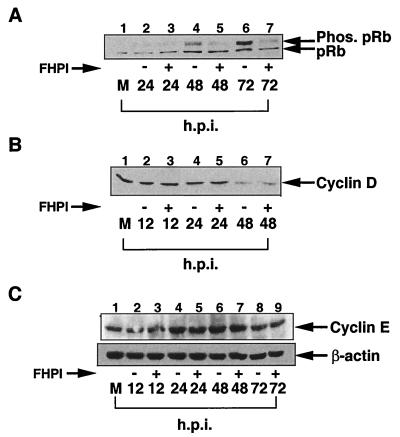
We therefore examined whether under our conditions HCMV infection can induce hyperphosphorylation of pRb and whether inhibiting p38 kinase activity affected pRb phosphorylation following infection. Fibroblasts were infected in the presence or absence of FHPI, a drug that specifically inhibits of p38 kinase activity and that we have previously used to examined the role of p38 in HCMV infection (19, 27). We then examined by Western blot analysis the level of pRb hyperphosphorylation at various times following infection. As shown in Fig. 3A, HCMV induced pRb hyperphosphorylation by 48 hpi (lane 4). In the presence of FHPI, the amount of hyperphosphorylated pRb was significantly less (lane 5). This finding indicates that p38 kinase activity effects pRb phosphorylation following viral infection.
FIG. 3.
Inhibition of p38 kinase activity inhibits HCMV-mediated pRb hyperphosphorylation but does not effect HCMV mediated changes in cyclin E or cyclin D protein levels. Fibroblasts were grown to confluence and then serum starved for 48 h. Cells were pretreated for 1 h with 10 μM FHPI prior to infection with HCMV. HCMV infection was done in the presence of FHPI, and extracts were harvested at the indicated times. Western blot analysis was performed with antibody for pRb (A), cyclin D (B), and cyclin E (C). M, mock infected; Phos., phosphorylated. Each experiment was performed at least three times, and representative blots are shown.
We next examined if treatment of FHPI altered cyclin D and, as a control, cyclin E, protein levels in HCMV-infected cells. HEL cells were infected in the presence or absence of FHPI, and the levels of cyclin D and cyclin E proteins were determined at the indicated times of infection by Western blot analysis. As previously reported, we found that cyclin D levels began to drop at 24 to 28 hpi (Fig. 3B, lanes 5 and 7) (5). As was also previously demonstrated, cyclin E protein levels increased slightly at 24 to 48 h (Fig. 3C, lanes 2 and 4) and then decreased at 48 to 72 hpi (lanes 6 and 8) (5). In both cases, FHPI had no effect on HCMV-mediated changes in protein levels. This indicates that following HCMV infection, p38 activity does not have a role in regulating cyclin D or cyclin E protein levels.
Finally, we examined the ability of p38 to regulate phosphorylation of HSP27 following viral infection. HEL cells were infected in the presence or absence of FHPI and then labeled with [32P]orthophosphate for 3 h prior to harvesting. HSP27 was immunoprecipitated from whole cell extract and separated by SDS-PAGE, and the amount of phosphorylated HSP27 was determined by autoradiography. As shown in Fig. 4, HSP27 was strongly phosphorylated at early (12 hpi) and late (48 hpi) times of infection, and this increase in phosphorylation was blocked by the presence of the p38 inhibitor compound FHPI (Fig. 4; compare lanes 2 and 4 with lanes 3 and 5).
These data indicate that activated p38 regulates phosphorylation of specific substrates throughout the course of infection. As discussed below, these results also identify biological mechanism by which p38 may affect HCMV viral DNA replication.
HCMV infection does not activate MKK4 or JNK.
The above results confirm that p38 has an active role in phosphorylating specific substrates for extended periods of time following infection. Since extended activation of p38 is unusual, we were interested in how HCMV was able to maintain p38 activation. This would further our understanding of the interaction between HCMV and the host cell, with the long-term view of identifying novel targets to inhibit HCMV-mediated p38 activation and, subsequently, viral permissive infection.
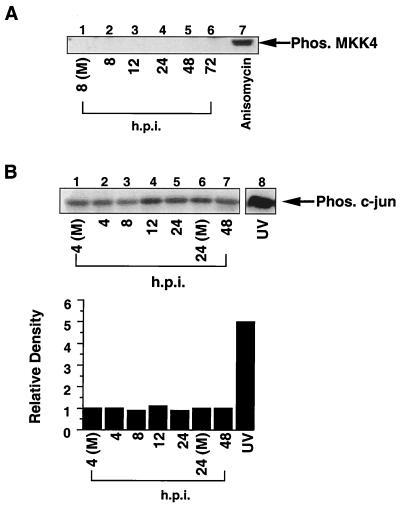
Following exposure to stimuli, increases in the level of phosphorylated p38 are due to increased phosphorylation or kinase activity of MKK3/6 and/or MKK4 (reviewed in references 10 and 38). Based on this knowledge, we first examined if MKK4 was activated following infection. Again, we used a phosphospecific antibody, in this case one which recognizes only phosphorylated, and hence activated, MKK4. Having determined the kinetics of p38 activation (Fig. 1), we could focus on times of infection which corresponded to p38 activation. As shown in Fig. 5A, HCMV infection did not activate MKK4 (lanes 2 to 6), while treatment of HEL fibroblasts with anisomycin (a strong MKK4 activator) strongly induced MKK4 phosphorylation (lane 5).
FIG. 5.
HCMV infection does not activate MKK4 or JNK. (A) Western blot analysis of infected whole-cell extract with an antibody which recognizes only active MKK4. For the positive control, HEL fibroblasts were treated with anisomycin (10 μg/ml) for 20 min and harvested in the same manner as the HCMV-infected cells. (B) JNK is not activated following HCMV infection. Cells were infected with HCMV and harvested in JNK lysis buffer, and JNK kinase activity was determined with GST–c-Jun (aa 1 to 79) as a substrate. Following autoradiography, band intensity was determined with a densitometer, and the quantitated results are shown. UV-irradiated fibroblasts were used as a positive control. (M), mock infected; Phos., phosphorylated. Each panel shows representative data from at least three separate experiments.
To further demonstrate that MKK4 was not activated following HCMV infection, we examined the effect of HCMV infection on activation of a second MKK4 substrate, JNK (9, 29, 38). A JNK kinase assay was performed with GST–c-Jun as a substrate. Quantitation of band intensity (Fig. 5B) demonstrates that JNK activity does not increase significantly following HCMV infection. Together, these data indicate that MKK4 kinase activity is not increased during HCMV infection and therefore cannot account for the increases in p38 activation.
MKK3 and MKK6 are activated at late times of HCMV infection.
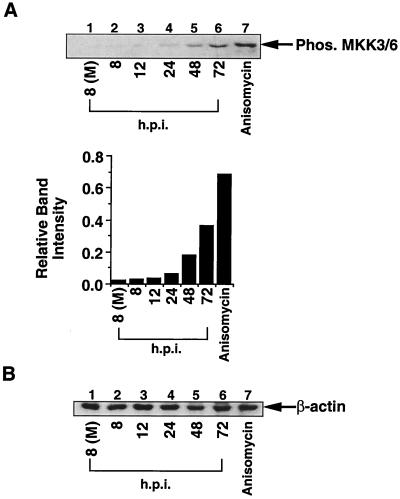
Next, we determined if viral infection activated MKK3 and MKK6, the remaining two cellular kinases which are known to phosphorylate p38 (10). Interestingly, while we observed a strong increase in MKK3/6 phosphorylation at 48 and 72 hpi (Fig. 6A, lanes 5 and 6), only a very slight increase in phosphorylation was observed at 12 hpi (lanes 2 and 3). Again, Western blot analysis for β-actin demonstrated that equal amounts of protein were loaded in each lane (Fig. 6B). Thus, increased MKK3/6 activity accounts for p38 activation at later times of infection (48 to 72 hpi). Since HCMV infection results in the activation of p38 as early as 8 hpi, we postulated that there must exist another mechanism to account for the strong activation of p38 at the earlier time points.
FIG. 6.
HCMV infection strongly activates MKK3/6 at later times of infection. (A) Fibroblast extract used in Fig. 5 were probed for MKK3/6 activation using an MKK3/6 phosphospecific antibody. Band intensity was quantitated with a densitometer. (B) Western blot analysis for β-actin demonstrates equal protein loading in all samples. (M), mock infected; Phos., phosphorylated. The data shown are representative of results from four separate experiments.
Basal kinase activity of MKK3/6 is needed for p38 phosphorylation at early times of infection.
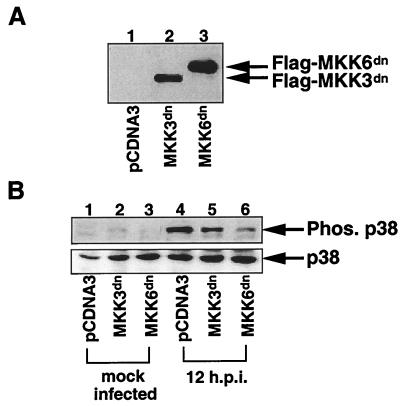
In response to heat shock, JNK is activated through inhibition of dephosphorylation rather than increased activity of upstream kinases (35). This activation requires basal upstream kinase activity (in the case of JNK, basal MKK4 activity is required) and inhibition of dephosphorylation of activated JNK. We were interested in determining whether HCMV activated p38 in a similar mechanism, since we were unable to detect an increase in MKK activity at early times postinfection. To test this theory, it was first necessary to demonstrate that basal activity of an MKK is necessary for p38 activation following HCMV infection. Since there are no drugs available to inhibit MKK3/6 kinase activity, we made cell lines which expressed a nonphosphorylated MKK6 or MKK3 (MKK3dn and MKK6dn). These are dominant negative proteins which have their phosphorylation sites mutated, and when overexpressed, they prevent the wild-type protein from becoming phosphorylated (44).
Due to their low transfectability and ability to be passaged only a limited number of times in tissue culture, HEL fibroblasts are unsuitable for making stable expressing cell lines by transient transfection assay. Therefore, MKK3dn-, MKK6dn-, and vector control (pCDNA3)-expressing cell lines were made in the U373MG cells, which like fibroblasts are fully permissive for HCMV infection in tissue culture. Importantly, the pattern of p38 and MKK3/6 activation in U373MG cells following HCMV infection mirrors that in infected fibroblasts (data not shown). After transfection and subsequent selection, protein expression in the cell lines was confirmed by immunofluorescence, immunoprecipitation, and Western blot analysis (Fig. 7A and data not shown). Following establishment of the cell lines, the cells were infected, harvested, and analyzed for phosphorylation of p38 by Western blot analysis. As shown in Fig. 7B, expression of MKK6dn strongly inhibited the ability of HCMV infection to induce p38 phosphorylation at 12 hpi (compare lanes 4 and 6), while MKK3dn had a much less significant effect on virus-mediated p38 activation (compare lanes 4 and 5). This finding suggests that kinase activity of MKK6 is necessary for HCMV to induce p38 phosphorylation at early times of infection, even though only a slight increase in MKK6 phosphorylation is observed at this time of infection (Fig. 6).
FIG. 7.
Basal levels of MKK3/6 kinase activity are necessary and sufficient for HCMV-mediated p38 activation. U373 cells were transfected with an expression vector for either MKK3dn or MKK6dn. (A) After selection in medium containing G418 (800 μg/ml), Western blot analysis using a FLAG monoclonal antibody was performed to verify protein expression. (B) Cells were pretreated, infected, and harvested as for Fig. 1. Western blot analysis was performed with polyclonal phosphospecific (to demonstrate p38 activation) or nonphosphospecific (to demonstrate that overall levels of p38 were equal) p38 antibodies. Phos., phosphorylated. The data are representative of results from three separate experiments.
HCMV inhibits dephosphorylation of p38 at early but not late times of infection.
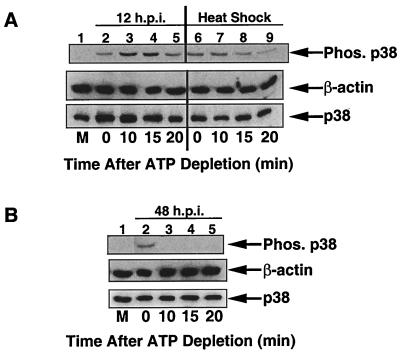
The results in Fig. 7 suggest that p38 is activated by the low levels of MKK6 basal kinase activity that is present in unstimulated cells. For this basal kinase activity to increase the steady-state level of phosphorylated p38, dephosphorylation of active p38 needs to be inhibited. This would allow a buildup of activated p38. Therefore, the next step was to determine the effect of HCMV infection on dephosphorylation of active p38. Traditional pulse-chase assays are not sensitive enough for examining the inhibition of dephosphorylation under these experimental conditions (35). Therefore, to assess the effect of HCMV infection on dephosphorylation of p38, it was necessary to inhibit the ability of upstream kinases (such as MKK3 and MKK6) to phosphorylate p38 and then monitor the rate of decline in the levels of phosphorylated p38. To accomplish this task, we used the technique of ATP depletion, which was successfully used by Meriin et al. to examine the rate of dephosphorylation of p38 and JNK following various stress treatments (35). In this experiment, cells are treated with the indicated stress. ATP is then depleted from the cells, which inhibits upstream kinase activity. This prevents the cell from further phosphorylating p38, and therefore the rate of dephosphorylation is the sole determinant of the level of phosphorylated p38. Following ATP depletion, time points are taken, and the amount of phosphorylated protein is determined by Western blot analysis with phosphospecific antibodies. Meriine et al. also demonstrated that heat shock-mediated p38 activation was not mediated through inhibition of dephosphorylation (35). Therefore, we used heat shock treatment as our control for the rate of dephosphorylation.
Cells were either infected for 12 h or heat shocked for 20 min and then subjected to ATP depletion. Cell extracts were harvested at 0, 10, 15, and 20 min following addition of ATP-depleting reagents. Subsequent Western blot analysis demonstrated that the amount of phosphorylated p38 decreased significantly in heat-shocked cells between 10 and 15 min after addition of ATP-depleting reagents (Fig. 8A, compare lanes 7 and 8). This indicated that if phosphatase activity is not inhibited, a decrease in the amount of phosphorylated p38 should be observed between 10 and 15 min after addition of ATP-depleting reagents. When cells infected for 12 h with HCMV were subjected to ATP depletion, only a slight drop in the amount of phosphorylated p38 was detected between 10 and 15 min after addition of ATP-depleting reagents (compare lanes 3 and 4). This indicates that HCMV infection is able to inhibit dephosphorylation of active p38. To our knowledge, this is the first report of p38 activation via inhibition of dephosphorylation of p38 by any stimulus.
FIG. 8.
HCMV infection inhibits dephosphorylation of active p38 at early, but not late, times of infection. (A) U373 cells were infected for 12 h or subjected to heat shock (45°C for 20 min). ATP was depleted by the addition of rotenone and 2-deoxyglucose, and cells were harvested at the indicated times after depletion. The amount of phosphorylated p38 was determined by Western blot analysis. (B) Cells were infected for 48 h and then subjected to ATP depletion and Western blot analysis as for panel A. Time 0, start of ATP depletion; M, mock-infected cells; Phos., phosphorylated.
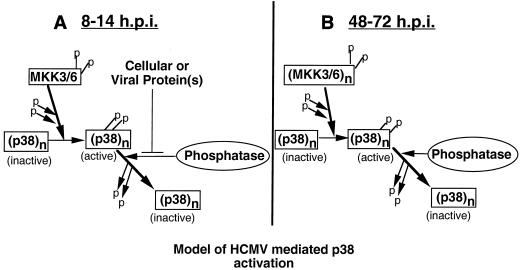
We next examined whether dephosphorylation of activated p38 was also inhibited at later times of infection, when phosphorylation of both p38 and MKK3/6 is observed. To answer this question, ATP depletion was performed on cells which had been infected for 48 h. As shown in Fig. 8B, following addition of ATP-depleting reagents, dephosphorylation of activated p38 occurred extremely rapidly. No phosphorylated p38 could be detected by 10 min after the start of ATP depletion. This finding indicates that at 48 hpi, HCMV can no longer inhibit dephosphorylation of active p38. Based on this observation, we have developed a model in which HCMV utilizes two mechanisms to ensure activation of p38 for extended periods of time (Fig. 9). At early times of HCMV infection, dephosphorylation of active p38 is inhibited, resulting in an accumulation of activated p38 (Fig. 9A). In contrast, at later times of infection, the ability of HCMV to inhibit p38 dephosphorylation is lost, indicating another mechanism is responsible for p38 activation. This mechanism is likely the significant increase in MKK3/6 activity that we observed at 48 to 72 hpi (Fig. 6 and 9B). It is important to remember that in the ATP depletion experiment, MKK3/6 cannot phosphorylate p38 (since no ATP is present), and therefore MKK3/6 is unable to maintain phosphorylation of p38. However, in the presence of ATP, the increased MKK3/6 activity is sufficient to activate p38.
FIG. 9.
Model for HCMV-mediated p38 activation. (A) At early times of infection, when no increase in MKK3/6 or MKK4 kinase activity is observed, the virus prevents phosphatases from recognizing activated p38. As basal MKK3/6 kinase activity continues to phosphorylate p38, phosphatases are unable to dephosphorylate p38, and so the amount of activated p38 accumulates. (B) At later times of infection, MKK3/6 kinase activity is increased, resulting in p38 activation.
Again, the findings presented here underscore the importance of p38 activation in viral infection. The virus has developed two mechanisms to ensure activation of p38. These findings are exciting because the unusual mechanism of p38 activation at 12 hpi may provide an antiviral target which inhibits HCMV-mediated p38 activation without affecting p38 activation in response to other stimuli.
DISCUSSION
In vivo, primary targets for HCMV lytic infection are terminally differentiated cells in which many macromolecules and cellular pathways, including kinase pathways such as p38, which HCMV needs to undergo a lytic infection, are present at low levels or are inactive (53). Several recent studies have shown that HCMV infection elicits a mitogenic-like response which results in activation of these critical cellular components (see the introduction). However, little is known about the mechanism by which HCMV induces this activation. In this report, we have examined the kinetics and mechanisms of p38 activation following HCMV infection. In fibroblasts, viral protein expression is necessary to obtain p38 activation, which is first observed at 8 to 10 hpi (Fig. 1 and 2). This is somewhat surprising, since our laboratory recently reported that HCMV infection of monocytes results in a dramatic up regulation of p38 kinase activity by 15 min postinfection, independent of viral protein expression (55). One explanation for these differences is that HCMV may utilize different receptors to bind fibroblasts and monocytes. Alternatively, HCMV infection may regulate p38 activation differently in permissive cell types (such as fibroblasts) compared to nonpermissive cell types (such as monocytes). Currently, we are trying to address this issue by first identifying HCMV receptors on monocytes and fibroblasts. In addition, we are comparing activation of p38 following HCMV infection of other permissive and nonpermissive cell types.
We were also unable to detect p38 activation following infection of unpurified viral stock in which HCMV had been inactivated by UV irradiation prior to infection (Fig. 1C). This suggests that secreted cytokines released by infected cells into the media are not responsible for p38 activation in this case. Based on these results, we believe it most likely that viral proteins themselves are responsible for p38 activation throughout the course of infection.
We then provide evidence that HCMV infection induces hyperphosphorylation of pRb and HSP27 in a p38-dependent manner. These results not only demonstrate that p38 is functionally active throughout infection but also provide insight into how p38 may mediate its biological effects on viral DNA replication. pRb regulates expression of many cellular proteins associated with DNA synthesis, and many of these proteins are thought to be necessary for viral DNA replication (51). At early stages of the cell cycle, pRb is hypophosphorylated, and in this form it acts as an inhibitor of gene expression, in most instances by directly complexing with transcription factors such as E2F and suppressing their transactivation function (51). Upon exposure to a proliferative signal, the cell induces hyperphosphorylation of pRb. Once hyperphosphorylated, pRb loses its ability to suppress transactivation function, which results in transcription of the genes necessary for progression into S phase and subsequent cellular DNA synthesis.
The classical binding partner for pRb is the E2F family of transcription factors, and hyperphosphorylation of pRb is necessary for relieving pRb-mediated suppression of E2F, which results in cell cycle progression past the G1/S phase transition point (reviewed in reference 39). However, the HCMV IE2-86 protein binds to pRb and alleviates pRb-mediated suppression of E2F transactivation function (15). Furthermore, IE2-86 has a high affinity for hypophosphorylated pRb, suggesting that HCMV can induce E2F transactivation function in the absence of pRb hyperphosphorylation (15). Therefore, while FHPI inhibits the pRb hyperphosphorylation observed following HCMV infection, it is likely that it does not inhibit pRb-mediated suppression of E2F transactivation function in the context of viral infection. Indeed, our preliminary results indicate this is the case (data not shown). However, pRb also regulates the activity of several other proteins in a phosphorylation-dependent manner. As with E2F, these proteins, which include histone deacetylases and RNA polymerase III, regulate expression of a variety of cellular genes involved in cell cycle progression and likely have important functions in viral infection (4, 24, 25, 32, 33). Currently, we are investigating the role of these other proteins in viral infection.
HSP27 appears to have a critical role in protecting cells from apoptosis following certain stresses (reviewed in references 8 and 14). One mechanism by which it does this is by acting as a molecular chaperone. It binds to proteins and can either prevent their incorrect folding or association with inhibitory proteins and/or ensure their translocation between subcellular compartments (14). In the context of viral infection, p38-mediated activation of HSP27 could have two functions. It might serve to inhibit apoptosis following external stresses. This would ensure that the virus is able to complete its life cycle before the cell dies. Alternatively, infection itself is a type of stress and may cause inactivation of proteins which are necessary for viral permissiveness, due to either misfolding or incorrect compartmental localization. Therefore, HCMV may activate HSP27 to ensure that these proteins are active and able to perform their function as it relates to HCMV-permissive infection. As with the pRb hyperphosphorylation, we are currently addressing these possibilities.
The next series of experiments characterized the mechanism of HCMV-mediated p38 activation. Using ATP depletion, we provided evidence that at early times of infection, HCMV inhibited dephosphorylation of activated p38, allowing an increase in the steady-state levels of phosphorylated p38 in the presence of only basal MKK6 kinase activity. We also consistently detected an increase in the amount of active p38 immediately following addition of ATP-depleting reagents but before ATP had been depleted from the cells (Fig. 8, compare lanes 2 and 3). This increase was not due to the process of ATP depletion, as no increase in the amount of phosphorylated p38 was detected following addition of ATP-depleting reagents to either heat-shocked cells or cells infected with HCMV for 48 h. Little is known about the mechanism which mediates activation of p38 or JNK by inhibition of dephosphorylation, and therefore it is difficult to speculate on why this increase occurs. It may be due to the efficient use of residual ATP by HCMV-infected cells before the ATP depletion is complete. Alternatively, it may be a characteristic of stimuli which activates p38 or JNK by inhibition of dephosphorylation. We are currently addressing these questions experimentally.
While inhibition of dephosphorylation is an uncommon mechanism for inducing MAPK activation, a similar mechanism was recently found for heat shock-mediated activation of JNK (35). In that study, basal MKK4 kinase activity coupled with inhibition of JNK dephosphorylation was sufficient to induce JNK activation. Two other reports have been published, both examining activation of ERK1/2 by inhibition of dephosphorylation. The first report described inhibition of protein phosphatase PP2A activity by SV40 small T antigen (41). This inhibition allowed accumulation of phosphorylated MKK1/2 and ERK in the absence of increased MKKK activity (54). A second report demonstrated that activation of ERK1/2 following HCMV infection of prestimulated fibroblasts may be at least partially due to inhibition of ERK1/2 dephosphorylation (46).
It may be fortuitous for HCMV to use an unusual mechanism to activate p38. For instance, increased MKK6 kinase activity has been implicated in some types of apoptosis (18). By not increasing MKK6 kinase activity, HCMV may avoid this effect, which would be detrimental to a successful viral life cycle. Also, many stimuli that activate p38 by activation of upstream kinases also activate JNK. Recently, it has been suggested that the antiviral property of tumor necrosis factor alpha may be due to its ability to activate JNK (1). By activating p38 through inhibition of dephosphorylation rather than increasing kinase activity of MKKs, perhaps HCMV ensures that JNK is not activated. This theory is supported by our findings that neither MKK4 nor JNK is activated following infection (Fig. 5).
HCMV could inhibit dephosphorylation of activated p38 by two mechanisms. It could directly bind to and inactivate one or more p38-specific phosphatases. This would be analogous to SV40 small T antigen, which binds to a subunit of the PP2A complex, preventing the formation of an active PP2A (41, 54). Alternatively, it could somehow prevent active phosphatases from recognizing p38, perhaps by binding p38 in such a way that it is masked from the phosphatases. We have found that p38 phosphatases are strongly activated following infection (data not shown), which correlates with previously published data showing that PP2A and PP1, both of which can dephosphorylate p38, are activated following HCMV infection (36). These findings suggest that p38 is likely activated by the latter mechanism (activated phosphatases cannot recognize phosphorylated p38) following HCMV infection.
Currently, we are addressing the question of which viral proteins are responsible for the inhibition of dephosphorylation. One problem with identifying these candidates is that it is difficult to determine the exact time at which the infected cell begins accumulating phosphorylated p38. The earliest we have been able to detect p38 phosphorylation is 6 to 8 hpi. However, since activation depends on basal kinase activity, it may be quite some time between the commencement of inhibition of p38 dephosphorylation and the detection of p38 activation. We have looked for the ability of the two major viral IE proteins (IE1-72 and IE2-86) to activate p38 but have been unable to induce p38 activation by overexpression of either IE2-86 or IE1-72 (data not shown). Aside from the possibility that these proteins may not be involved in p38 activation, two alternate explanations for why we have been unable to detect p38 activation by specific viral proteins are that posttranslational modification of IE proteins, such as phosphorylation, and/or expression of multiple viral genes is necessary to obtain p38 activation. Our search has been further complicated by the fact that different mechanisms are utilized to activate p38 at early and late times of infection. Currently, we are developing better techniques to identify the viral proteins necessary for p38 activation.
We went on to show that at later times of infection, HCMV no longer inhibited dephosphorylation of activated p38 (Fig. 8B). However, MKK3/6 activity was increased at these later times of infection (Fig. 6), and this increased activity was sufficient to increase the steady-state level of activated p38. This finding indicates HCMV utilized a different mechanism to activate p38 at later times of infection. These results may also explain why a decrease in p38 phosphorylation was often observed at 24 hpi. The ability of HCMV to inhibit dephosphorylation of p38 may be lost before MKK3/6 is activated. At that time of infection, no mechanism to activate p38 would be present, and the levels of phosphorylated p38 would drop.
This characterization of HCMV-mediated p38 activation provides important new insight into the complex interaction between HCMV and the infected host cell. We have demonstrated that p38 is activated during viral infection. We have also identified two p38 substrates which are phosphorylated in a p38-dependent manner in the context of a viral infection, which will be helpful in future studies of the biology of p38 function in the context of viral infection. Finally, this study provides strong evidence that HCMV activates p38 by two different mechanisms, including the very unusual mechanism of inhibition of dephosphorylation. Since it is unusual, it may provide a future target for preventing p38 activation and, subsequently, HCMV infection.
ACKNOWLEDGMENTS
We are grateful to R. J. Davis for providing the MKK3dn and MKK6dn expression vectors. We also thank Xuli Ma for technical assistance and M. Hiremath, A. Yurochko, M. Mayo, and R.-H.E.S. Bitter for critical reviews of the manuscript.
R.A.J. is a virology training grant (2T32 AI07419) recipient. This work was supported by grants AI12717 and CA19014 from the National Institutes of Health (to E.-S.H.).
REFERENCES
- 1.Allan-Yorke J, Record M, de Preval C, Davrinche C, Davignon J L. Distinct pathways for tumor necrosis factor alpha and ceramides in human cytomegalovirus infection. J Virol. 1998;72:2316–2322. doi: 10.1128/jvi.72.3.2316-2322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrisani O M. CREB-mediated transcriptional control. Crit Rev Eukaryotic Gene Expr. 1999;9:19–32. [PubMed] [Google Scholar]
- 3.Boyle K A, Pietropaolo R L, Compton T. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature (London) 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 5.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 6.Britt W J, Alford C A. Cytomegalovirus. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 7.Chau N H, Vanson C D, Kerry J A. Transcriptional regulation of the human cytomegalovirus US11 early gene. J Virol. 1999;73:863–870. doi: 10.1128/jvi.73.2.863-870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciocca D R, Oesterreich S, Chamness G C, McGuire W L, Fuqua S A. Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- 9.Derijard B, Raingeaud J, Barrett T, Wu I H, Ulevitch R J, Davis R J. Independent human MAPK kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 10.Dhanasekaran N, Reddy E P. Signaling by dual specificity kinases. Oncogene. 1998;17:1447–1455. doi: 10.1038/sj.onc.1202251. [DOI] [PubMed] [Google Scholar]
- 11.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 12.Field A K, Biron K K. “The end of innocence” revisited: resistance of herpesviruses to antiviral drugs. Clin Microbiol Rev. 1995;7:1–13. doi: 10.1128/cmr.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freshney N W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 14.Georgopoulos C, Welch W J. Role of the major heat shock proteins as molecular chaperones. Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 15.Hagemeier C, Caswell R, Hayhurst G, Sinclair J H, Kouzarides T. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 1994;13:2897–2903. doi: 10.1002/j.1460-2075.1994.tb06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haneda M, Sugimoto T, Kikkawa R. Mitogen-activated protein kinase phosphatase: a negative regulator of the mitogen-activated protein kinase cascade. Eur J Pharmacol. 1999;15:1–7. doi: 10.1016/s0014-2999(98)00857-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang E-S, Kowalik T F. The pathogenicity of human cytomegalovirus: an overview. In: Becker Y, Darai G, Huang E S, editors. Molecular aspects of human cytomegalovirus diseases. Berlin, Germany: Springer-Verlag; 1993. pp. 1–45. [Google Scholar]
- 18.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch R J, Nemerow G R, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAPK kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R A, Huong S, Huang E. Inhibitory effect of 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1 H-imidazole on HCMV DNA replication and permissive infection. Antiviral Res. 1999;41:101–111. doi: 10.1016/s0166-3542(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 20.Kerry J A, Priddy M A, Staley T L, Jones T R, Stenberg R M. The role of ATF in regulating the human cytomegalovirus DNA polymerase (UL54) promoter during viral infection. J Virol. 1997;71:2120–2126. doi: 10.1128/jvi.71.3.2120-2126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerry J A, Priddy M A, Stenberg R M. Identification of sequence elements in the human cytomegalovirus DNA polymerase gene promoter required for activation by viral gene products. J Virol. 1994;68:4167–4176. doi: 10.1128/jvi.68.7.4167-4176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalik T F, Wing B, Haskill J S, Azizkhan J C, Baldwin A S, Jr, Huang E-S. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc Natl Acad Sci USA. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larminie C G, Alzuherri H M, Cairns C A, McLees A, White R J. Transcription by RNA polymerases I and III: a potential link between cell growth, protein synthesis and the retinoblastoma protein. J Mol Med. 1998;76:94–103. doi: 10.1007/s001090050196. [DOI] [PubMed] [Google Scholar]
- 24.Larminie C G, Cairns C A, Mital R, Kouzarides T, Jackson S P, White R J. Mechanistic analysis of RNA polymerase III regulation by the retinoblastoma protein. EMBO J. 1997;16:2061–2071. doi: 10.1093/emboj/16.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavoie J N, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44mapk pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 26.Lee J C, Adams J L. Inhibitors of serine/threonine kinases. Curr Opin Biotechnol. 1995;6:657–661. doi: 10.1016/0958-1669(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 28.Lewis T S, Shapiro P S, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:74–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 29.Lin A, Minden A, Martinetto H, Claret F-X, Lang-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38 MPK. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Stinski M F. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J Virol. 1992;66:4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo R X, Postigo A A, Dean D C. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 32.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Loran S, Le Villain J P, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature (London) 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 33.Mar E-C, Cheng Y-C, Huang E-S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl) guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983;24:518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean T I, Bachenheimer S L. Activation of c-Jun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meriin A B, Yaglom J A, Gabai V L, Mosser D D, Zon L, Sherman M Y. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol. 1999;19:2547–2555. doi: 10.1128/mcb.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelson S, Turowski P, Picard L, Goris J, Landini M P, Topilko A, Hemmings B, Bessia C, Garcia A, Virelizier J L. Human cytomegalovirus carries serine/threonine protein phosphatases PP1 and a host-cell derived PP2A. J Virol. 1996;70:1415–1423. doi: 10.1128/jvi.70.3.1415-1423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills J, Jacombson M A, O'Donnell J J, Cederberg D, Holland G N. Treatment of cytomegalovirus retinitis in patients with AIDS. Rev Infect Dis. 1988;10(Suppl.):S522–S531. doi: 10.1093/clinids/10.supplement_3.s522. [DOI] [PubMed] [Google Scholar]
- 38.Minden A, Karin M. Regulation and function of the JNK subgroup of MAPK kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 39.Nevins J R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 40.Oberg B, Behrnetz S, Eriksson B, Jozwiak H, Larsson A, Lernestedt J D, Aberg V L. Clinical use of Foscarnet (phosphonoformate) In: Clercq E D, editor. Clinical use of antiviral drugs. Boston, Mass: Martinus Nijhoff; 1988. pp. 223–240. [Google Scholar]
- 41.Pallas D C, Shahrik L K, Martin B L, Jaspers S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 42.Popik W, Pitha P M. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology. 1998;252:210–217. doi: 10.1006/viro.1998.9466. [DOI] [PubMed] [Google Scholar]
- 43.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 44.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 46.Rodems S M, Spector D H. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J Virol. 1998;72:9173–9180. doi: 10.1128/jvi.72.11.9173-9180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro L, Heidenreich K A, Meintzer M D, Dinarello C A. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proc Natl Acad Sci USA. 1998;95:7422–7426. doi: 10.1073/pnas.95.13.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson S, Mahadevan L C, Clayton A L. MAP kinase-mediated signalling to nucleosomes and immediate-early gene induction. Semin Cell Dev Biol. 1999;10:205–214. doi: 10.1006/scdb.1999.0302. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Nath N, Minden A, Chellappan S. Regulation of Rb and E2F by signal transduction cascades: divergent effects of JNK1 and p38 kinases. EMBO J. 1999;18:1559–1570. doi: 10.1093/emboj/18.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 52.Weitzman J B, Yaniv M. Signal transduction pathways and modulation of gene activity. Clin Chem Lab Med. 1998;36:535–539. doi: 10.1515/CCLM.1998.091. [DOI] [PubMed] [Google Scholar]
- 53.Weller T H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. N Engl J Med. 1971;285:203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]
- 54.Yang S I, Lickteig R L, Estes R, Rundell K, Walter G, Mumby M C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991;11:1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yurochko A D, Huang E-S. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunol. 1999;162:4806–4816. [PubMed] [Google Scholar]
- 56.Yurochko A D, Hwang E-S, Rasmussen L, Keay S, Pereira L, Huang E-S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yurochko A D, Kowalik T F, Huong S-M, Huang E-S. HCMV upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yurochko A D, Mayo M W, Poma E E, Baldwin A S, Jr, Huang E-S. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-κB promoters. J Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zachos G, Clements B, Conner J. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J Biol Chem. 1999;274:5097–5103. doi: 10.1074/jbc.274.8.5097. [DOI] [PubMed] [Google Scholar]