Abstract
Objectives
Myotonia is a clinical sign typical of a group of skeletal muscle channelopathies, the non‐dystrophic myotonias. These disorders are electrophysiologically characterized by altered membrane excitability, due to specific genetic variants in known causative genes (CLCN1 and SCN4A). Juvenile Myoclonic Epilepsy (JME) is an epileptic syndrome identified as idiopathic generalized epilepsy, its genetics is complex and still unclarified. The co‐occurrence of these two phenotypes is rare and the causes likely have a genetic background. In this study, we have genetically investigated an Italian family in which co‐segregates myotonia, JME, or abnormal EEG without seizures was observed.
Methods
All six individuals of the family, 4 affected and 2 unaffected, were clinically evaluated; EMG and EEG examinations were performed. For genetic testing, Exome Sequencing was performed for the six family members and Sanger sequencing was used to confirm the candidate variant.
Results
Four family members, the mother and three siblings, were affected by myotonia. Moreover, EEG recordings revealed interictal generalized sharp‐wave discharges in all affected individuals, and two siblings were affected by JME. All four affected members share the same identified variant, c.644 T > C, p.Ile215Thr, in SCN4A gene. Variants that could account for the epileptic phenotype alone, separately from the myotonic one, were not identified.
Significance
These results provide supporting evidence that both myotonic and epileptic phenotypes could share a common genetic background, due to variants in SCN4A gene. SCN4A pathogenic variants, already known to be causative of myotonia, likely increase the susceptibility to epilepsy in our family.
Plain Language Summary
This study analyzed all members of an Italian family, in which the mother and three siblings had myotonia and epilepsy. Genetic analysis allowed to identify a variant in the SCN4A gene, which appears to be the cause of both clinical signs in this family.
Keywords: channelopathy, epilepsy, juvenile myoclonic epilepsy, myotonia, SCN4A
Key points.
The co‐occurrence of Myotonia and Juvenile Myoclonic Epilepsy is rare and the causes likely have a genetic background.
Exome Sequencing revealed a missense variant in SCN4A gene, in an Italian family in which co‐segregates myotonia, JME or abnormal EEG without seizures.
SCN4A pathogenic variants, already known to be causative of myotonia, likely increase the susceptibility to epilepsy.
1. INTRODUCTION
The term “myotonia” refers to a condition in which it is experienced a slow muscle relaxation after contraction, often presenting with stiffness and cramps. This distinctive sign characterizes a group of skeletal muscle channelopathies, the non‐dystrophic myotonia. 1 Non‐dystrophic myotonia comprises rare genetic conditions, determined by known variants in the principal skeletal muscle voltage‐gated chloride channel gene (CLCN1) and in the voltage‐gated sodium channel gene (SCN4A). Because of these variants, the excitability of the muscle membrane is altered, leading to the myotonic phenomenon. Electromyography and genetic testing are currently the gold standard to confirm the diagnosis. 1 , 2
Juvenile myoclonic epilepsy (JME) is an epileptic syndrome classified as a type of idiopathic generalized epilepsy (IGE) affecting adolescents and adults. 3 It is primarily characterized by generalized tonic–clonic seizures, myoclonic jerks and less frequently absences, occurring commonly after morning awakening. 4 , 5 The genetics of JME is complex, heterogeneous, and still not fully elucidated. 6 By the years, a few Mendelian genes (i.e., CACNB4, EFHC1, GABRA1, and CLCN2) have been sporadically identified as causative in certain family subsets, although their status remains a subject of debate. Additionally, SNPs in BRD2, CX36, and ME2 have been reported as potential susceptibility factors; however, their confirmation is still pending. 6 , 7 , 8 Genome Wide Association Studies (GWAS) have demonstrated an enrichment of common variants for monogenic epileptic genes, especially in generalized epilepsies like JME. 9
The co‐occurrence of myotonia and epilepsy is not frequent and not well‐studied and few cases have been reported in literature. 10 , 11 The possible link between these two phenotypes is probably to be investigated through the genetic sodium channelopathies. 12 , 13 , 14 , 15 During the last decades, next‐generation sequencing (NGS) has improved diagnostic rates and genes identification, passing from gene panel sequencing, for diagnostic purposes, to exome sequencing and whole genome sequencing, for research aims. 2
In the present study, we investigate a family of six individuals, in which the myotonic feature is inherited through two generations in an autosomal dominant way. Two of the affected patients also have Juvenile Myoclonic Epilepsy, and the other two affected individuals present with an abnormal EEG without seizures. The peculiar phenotype of the family led to a genetic investigation to identify a molecular diagnosis, and to research and better understand the possible genetic causes underlying the co‐occurrence of these phenomena.
2. PATIENTS AND METHODS
2.1. Family
The family was enrolled at the Institute of Neurology, University Magna Graecia of Catanzaro, Italy. Clinical data were collected from all family members and pedigree was constructed (Figure 1). All patients were Caucasian, originated in Calabria, south of Italy. The family is composed by six members, two non‐consanguineous parents and four siblings. The affected mother is now 61 years old, while the unaffected father is 64. Through the siblings, two males and one female are affected, 36, 35, and 34 years, respectively, while the last 31 years old sister is unaffected. At the time of investigation, an informed consent was signed from each patient, and a detailed comprehensive clinical and laboratory evaluation was obtained from all the members of the family. All individuals performed awake and sleep EEG recordings and EMG examinations.
FIGURE 1.
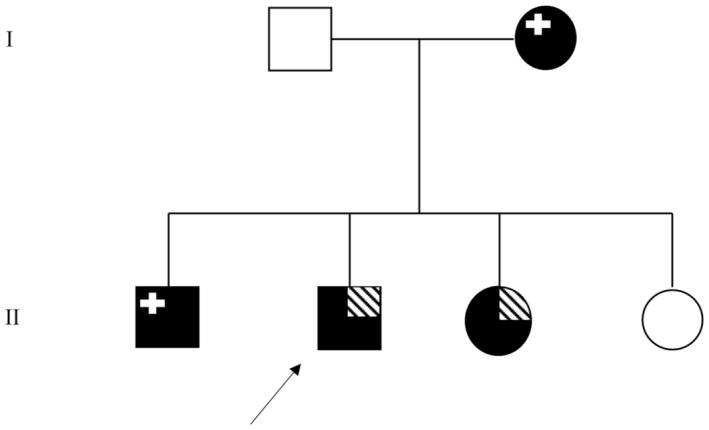
Pedigree of the family. The black symbols indicate the myotonic phenotype. The striated, upper, right corner represents the epileptic phenotype. The upper, left, white cross indicates an abnormal EEG. The arrow shows the proband. Variant localization and conservation.
2.2. Genetic testing
To carry out the genetic testing, peripheral blood was drawn and DNA was extracted by salting‐out method in the Italian center.
Exome Sequencing (ES) was performed on all the family members in the French Civil Hospital of Lyon, Laboratory of Genetics and Cytogenetics. The KAPA Hyper Prep kit (Roche) was used for library preparation, following the manufactures guidelines. Sequencing was performed with NextSeq500 (Illumina) sequencer. Genomic alignment was realized against the hg19/GRCh37 assembly using BWA‐MEM v.0.7.12 (Li and Durbin, 2009). Variant calling was done with GATK HaplotypeCaller v.3.4 (Broad Institute, Boston, MA, USA). For the analysis, high confident rare variants were kept with these features: total depth >9; alternative allele depth >4; no strand bias; mosaicism >10%; gnomAD database frequency <1%.
2.3. Raw data analysis
Raw data were filtered following two main hypotheses: autosomal dominant inheritance hypothesis, choosing “occurrence 4” through the family and eliminating the ones that included also unaffected patients; direct research for epilepsy‐related genes. All the filtered variants were individually analyzed, and bioinformatics tools were applied to predict their pathogenicity. A PubMed research was performed to clarify the function of identified genes, and to evaluate their possible involvement in the phenotypic manifestation.
Sanger sequencing was performed on an ABI 3500Dx Genetic Analyzer (Life Technologies, Carlsbad, CA, USA) to confirm the candidate variant.
3. RESULTS
3.1. Clinical analysis
The proband is the patient II‐2, a 35‐year‐old man, who developed both the myotonic and epileptic phenotypes (Figure 1). Since infancy, he reported difficulty in relaxing all muscle groups and occasional muscle cramps both at rest and after exercise. At the age of 15 years, he began to manifest epileptic seizures, mainly in the early morning, characterized by daily massive myoclonic jerks of upper limbs. At age 17, he experienced the first generalized clonic–tonic–clonic seizure, triggered by intermittent exposition to strobe lights. For this reason, he performed a detailed clinical evaluation with EEG, which showed generalized spike and wave discharges with a photoparoxysmal response (Figure 2). A diagnosis of JME was made and he started valproate (VPA) 500 mg/day with complete seizure freedom. After an 8‐year period of seizure freedom, VPA was gradually reduced and stopped at the age of 26 years. During the same period, he began to complain diffuse pain muscle cramps with an exacerbation of difficulty in muscle relaxing after contraction. At this time, neurological examination revealed right winged scapula (Figure 3). Routine blood tests resulted in high levels of CK (210 UI/l), with normal levels of PCR and VES. Electromyography study showed the characteristic myotonic discharges (Figure 4).
FIGURE 2.
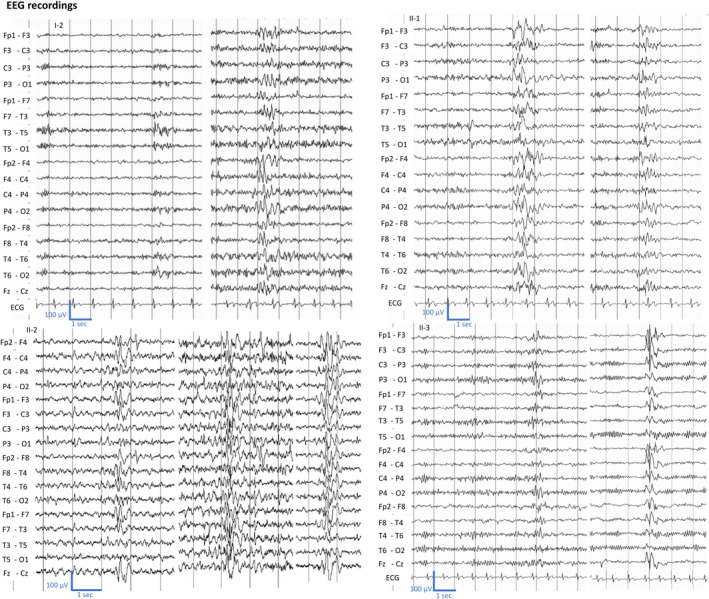
Shared interictal EEG abnormalities of generalized sharp‐wave discharges across different members of the family: proband (II‐2), II‐3 and II‐1 show during wakefulness. The EEG of the proband mother (I‐2) shows diffuse sharp‐wave discharges.
FIGURE 3.
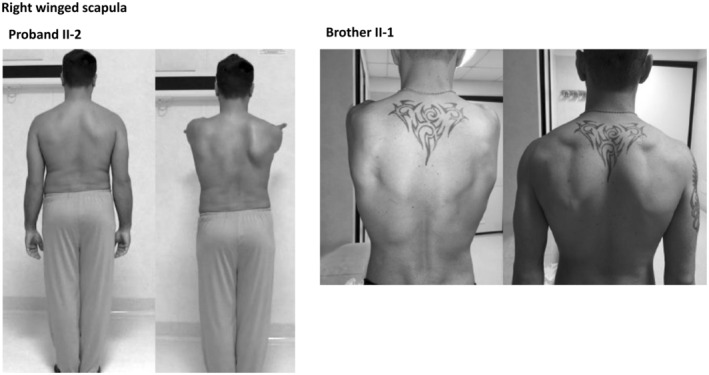
The following figure shows the clinical sign, right winged scapula, typical of myotonic phenotype. In both brother, the left photo shows the back at rest, while the right photo shows the winged scapula seen when arms are raised forward. This phenomenon indicates the muscle disorder, so the muscle fails to hold the scapula in place.
FIGURE 4.
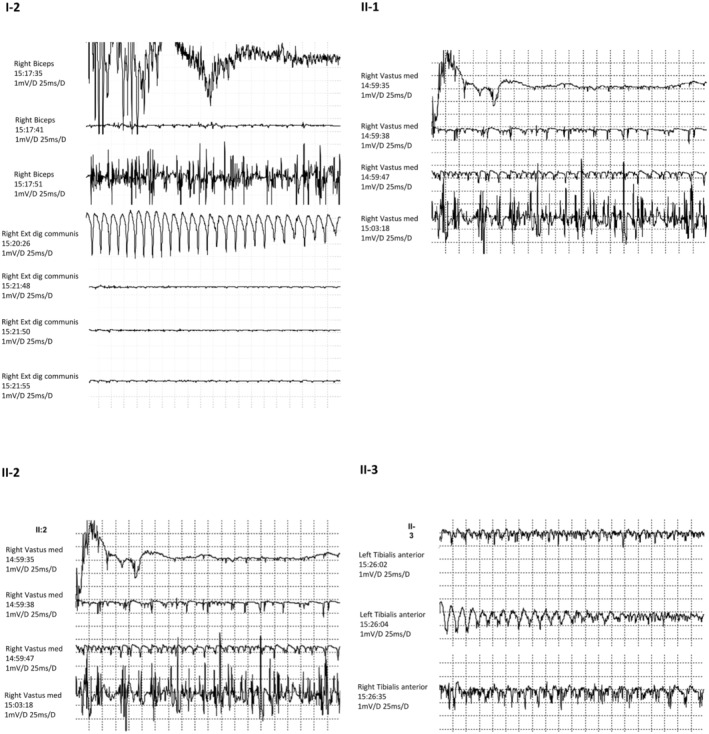
Needle EMG revealed myotonic potentials characterized by repetitive discharges, firing at a rate of 20–80 Hz, in which the amplitude and frequency of the potentials wax and wane, with the characteristic “dive bomber” sound.
Patient II‐3 was the affected 34‐year‐old sister that manifested both myotonia and epilepsy. She experienced myoclonic and tonic–clonic seizures since the age of 17, with an electro‐clinical diagnosis highly characteristic of JME. Interictal EEG recordings showed rare generalized sharp‐wave complexes. She firstly started levetiracetam, 1 g/day, but it was withdrawn after 1 year due to irritability and aggressive behavior. Then, she became seizure free with VPA up to 750 mg/day at age of 25, except for occasional subtle jerks while she is in front of computer screen. After close questioning, it emerged that she also presented the same muscular difficulties of her brother (II‐2) since childhood, then EMG proved the myotonic phenomenon as well (Figure 4).
Patient II‐1 was the oldest 36‐year‐old brother affected by myotonia. He first presented with difficulty in relaxing all muscular districts, including the cranium, and muscle cramps with occurrence during the night and after efforts, since teen age. He showed a light winged scapula (Figure 3), and the EMG examination revealed the myotonic phenomenon (Figure 4). Despite he has never experienced seizures, even if he referred subtle transient episodes of behavioral arrest with sporadic frequency in infancy. EEG in adulthood showed interictal generalized spike‐waves discharges (Figure 2). He did not receive any anti‐seizure medication and at last follow‐up no seizure has occurred.
The 61‐year‐old mother (patient I‐2) also complained difficulty in relaxing all muscle districts and occasional muscle cramps either at rest or after exercise since adolescence. At the time of the investigation of the whole family, she was diagnosed with myotonia, and the EMG examination revealed the myotonic phenomenon (Figure 4). Even though she has never had seizures, her EEG showed rare diffuse sharp‐wave discharges (Figure 2).
Both I‐1 and II‐4 unaffected individuals, respectively the 64‐year‐old father and the youngest 31‐year‐old sister were clinically unaffected, they have never referred seizures and their EMG and EEG recordings were normal.
3.2. Genetic analysis
Raw ES data were filtered as described in the Methods and Materials section, leading to the identification of 9 rare variants shared by the four affected patients and absent from the two unaffected (Table 1). Through these variants, six were excluded because the genes were not considered possibly related to the phenotypes, according to OMIM and PubMed research. Two others were excluded because they were found in the 5′ UTR region without creation of alternative start site, and thus were not considered disruptive for the protein.
TABLE 1.
Filtered variants form exome sequencing.
| Variant | Gene | HGVS_C | HGVS_P | Effects | Transcript | Status | dbSNP ID | ClinVar significance | PubMed research for function |
|---|---|---|---|---|---|---|---|---|---|
| chr1:20915549G > A | CDA | c.‐74G > A | NA | 5_prime_UTR | NM_001785.2 | Het | rs1306583369 | NA | |
| chr4:187629831G > A | FAT1 | c.1151C > T | p.Thr384Ile | Missense | NM_005245.3 | Het | rs961756374 | NA | Tumor suppressor |
| chr6:31239902C > A | HLA‐C | c.‐54G > T | NA | 5_prime_UTR | NM_001243042.1.7 | Het | rs908960430 | NA | |
| chr6:46657430G > T | TDRD6 | c.1565G > T | p.Gly522Val | Missense | NM_001010870.2 | Het | rs766934242 | NA | Oligoasthenoteratozoospermia |
| chr6:137528173 T > A | IFNGR1 | c.127A > T | p.Asn43Tyr | Missense | NM_000416.2 | Het | rs1404651605 | VUS | |
| chr14:74960976A > G | ISCA2 | c.175‐2A > G | NA | Splice acceptor variant & intron variant | NM_194279.3 | Het | rs753284570 | NA | |
| chr17:62048581A > G | SCN4A | c.644 T > C | p.Ile215Thr | Missense | NM_000334.4 | Het | rs1555604867 | Pathogenic | |
| chr19:14200062G > A | SAMD1 | c.749C > T | p.Pro250Leu | Missense | NM_138352.1 | Het | rs749734399 | NA | LDL‐Binding protein |
| chr19:16236344G > A | RAB8A | c.311G > A | p.Arg104His | Missense | NM_005370.4 | Het | rs751842773 | NA | RAS‐Associated protein |
Note: The following table presents the 9 filtered variants carried by all four affected individuals. Both CDA and HLA‐C variants were excluded because of the occurrence in 5‐prime‐UTR region, so they are not considered disruptive for the protein. The other six variants were excluded due to the phenotype they are associated with, according to OMIM and PubMed research. The bold variant is the candidate one, described in this study.
The identified candidate variant is the missense c.644 T > C p.Ile215Thr (rs1555604867), present in the exon 5 of SCN4A gene (NM_000334.4). It is present in the heterozygous state in all affected individuals of the family and absent from the unaffected, with a maternal inheritance. SCN4A encodes the α‐subunit of the sodium channel complex, Nav1.4, primarily expressed in the skeletal muscle and also in brain. 12 It is formed by four domains (D1–D4), each composed of six transmembrane segments (S1‐S6). The p.Ile215Thr cause an aminoacidic change in the extracellular S3–S4 loop, in the first domain of the protein (Figure 5). The applied predictive tools results are as follow: disease causing for MutationTaster; probably damaging with a score of 0.972 for Polyphen2; Revel score 0.802; CADD‐phred score 24.40; slightly intolerant for MetaDome with score 0.62. We classified this variant as Pathogenic, according to the ACMG/AMP guidelines. We applied the “fuNCtion” tool (https://funnc.shinyapps.io/shinyappweb/) that predicted this variant as pathogenic with a gain‐of‐function effect. 16 This variant has been reported in one study as pathogenic 17 and as VUS in another one, 18 and it has five submissions on ClinVar (National Center for Biotechnology Information. ClinVar; [VCV000432742.21], https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000432742.21 (accessed April 25, 2023)).
FIGURE 5.
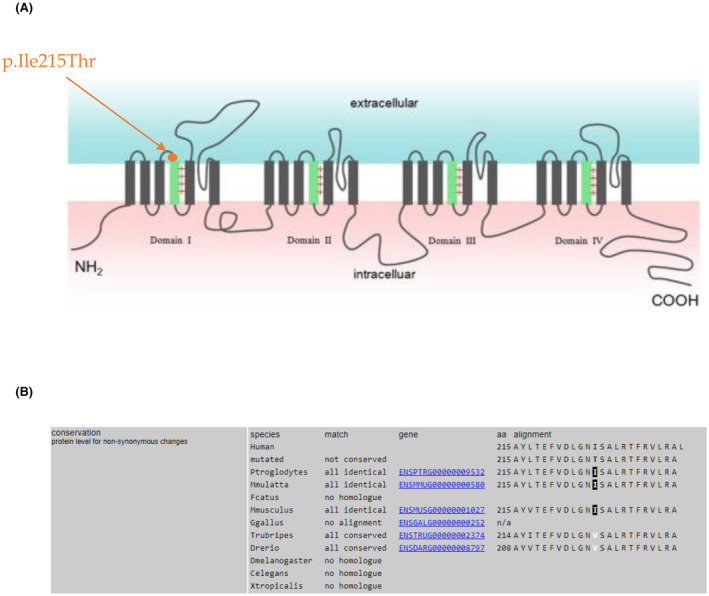
(A) Variant localization. Here is shown the localization of the variant p.Ile215Thr in Nav1.4 sodium channel. The identified variant occurs in the first domain, in the extracellular loop between S3 and S4 segments (D1, S3–S4 linker). Protein image was realized by Li et al., 24 and then modified to show our variant localization. (B) Here is reported the aminoacidic conservation through the species.
The direct research for additional variants in epilepsy‐related genes was firstly conducted on the two, brother and sister, sharing the epileptic phenotype (II‐2 and II‐3), then on the two mother and son showing the electric phenomenon without epileptic seizures (I‐2 and II‐1), lastly on all four individuals together. This analysis was conducted to research other variants that could explain the JME phenotype independently of the muscular phenotype, and in the end led to negative results.
4. DISCUSSION
We present here the characteristics of a family featuring myotonia and epilepsy phenotypes. The myotonic phenomenon arose during childhood in all four affected patients and worsened only in the proband. EMG examination showed the same myotonic phenotype in all affected members providing further confirmation of the diagnosis. The age of onset of the epilepsy was the same for both II‐2 and II‐3 and the electroclinical features were characteristic of JME. As seen in IGE families, the other two affected individuals only had abnormal EEGs that is genetically related to the epilepsy phenotype. All four affected siblings of the family shared the identified variant in SCN4A gene, inherited in heterozygous state from the affected mother, while no other variants that could explain the epileptic phenotype have been identified. Due to these genetic results, we hypothesized that obtaining a dual molecular diagnosis in this family was unlikely. However, we are aware that rare cases of co‐occurrence of two variants in dominant genes have been reported, and this circumstance leads the variants to interfere with the classical phenotypic manifestation. 19 , 20 Although this is not our case, we cannot exclude the presence of variants in non‐coding regions that eluded detection through Exome Sequencing, which could influence gene expression and contribute to susceptibility to epilepsy.
SCN4A is physiologically expressed in the skeletal muscle and in cerebral cortex, 12 it encodes the Nav1.4 sodium channel and it is responsible of the control of muscular excitability. To date, several variants in this gene were related to skeletal muscle channelopathies, 1 , 13 , 16 , 17 and there have been reports of few cases in which SCN4A variants could represent a risk factor of other rare phenotypes such as essential tremor, 12 apnea, 21 severe neonatal episodic laryngospasm, 22 and Sudden Unexpected Death in Epilepsy. 23 The most frequent pathogenic variants are missenses, inherited in autosomal dominant way, and related to the sodium channel myotonia phenotype. The widespread pathological molecular mechanism is an enhanced activation and impaired fast inactivation of the mutant channel. 1 These data are concordant with the myotonic phenotypic manifestation of our family carrying the SCN4A variant. The localization of the pathogenic variants is more frequent in the cytosolic D3‐D4 linker, with a gain‐of‐function effect. 16 , 18 Our variant occurs in the D1, and the other reported patients carrying variants in this domain manifested variable and non‐severe symptoms. This is probably the cause of an underestimated frequency of variants in this site. 17
The p.Ile215Thr variation has been reported in families with myotonia, but none of the affected individuals had epileptic manifestations. 17 , 18 Pagliarani et al. investigated it by functional studies: they demonstrated that the aminoacidic substitution cause a gain of function of the channel, as predicted by the in silico tools. Their patients showed a variability in the myotonic phenotype that was associated with myotonic discharges on the EMG recordings, the same shared by our patients. Moreover, in their cohort, the variant was shared by affected individuals of six unrelated families, all originating from South of Italy (5 from Sicily and 1 from Calabria). Microsatellite marker analysis demonstrated a founder effect. Due to the Calabrian origin of our patients, it is very likely that our family may belongs to the same founder effect. Despite sharing the same variant, myotonic phenotype and founder effect, their patients do not exhibit epileptic phenotypes or altered EEG, contrasting with the distinctive co‐occurrence of myotonia and epilepsy evident in our family. This discrepancy suggests a potential specificity of the epileptic susceptibility gene to our family.
In the literature, we have found two reports of SCN4A variants contributing to the epilepsy phenotype. 11 , 12 In one SCN4A family, two of the five mutation carriers also had generalized seizures since adolescence, which led the authors to conclude that SCN4A pathogenic variants may lead to increased seizure susceptibility, given the SCN4A‐associated phenotypic heterogeneity and the known role of the SCNs family in epilepsies. 12 The other report describes a patient with normo‐kalemic periodic paralysis and generalized epilepsy, in which were present together two missense variants in the third domain (D3) of SCN4A. 11 A double‐mutation in the same individual is rare, but not impossible, 19 for a dominant inherited disease, although no functional studies supported their data. They concluded that probably a synergic effect of the variants contributed to the phenotypes. Although the phenotypic similarity, our variant has been well studied and it appears to aligns with the clinical features.
In conclusion, the co‐occurrence of myotonia and epilepsy is quite rare, and our findings are an additional evidence that supports the hypothesis of a link between these two phenomena. This connection is likely to be found in the genetic background, through channelopathies. It is probable that variants in SCN4A, already related to myotonia, play a role in conferring susceptibility to epilepsy, due to its role in regulating membrane excitability, primarily in muscle and perhaps partially also in the brain. 12 Our two patients with EEG abnormalities and no seizures represent evidence that the epilepsy phenotype related to SCN4A may be very mild, and for this reason underestimated. Based on our findings, it is plausible to hypothesize that patients with IGE/JME phenotype might occasionally have subtle myotonia that may pass unrecognized. In our family, indeed, we carried out a thorough history and clinical examination for muscle disorders only after the proband began to complain painful muscle cramps with the appearance of a right winged scapula. Perhaps, patients with IGE/JME should be screened for subtle myotonia and vice versa. Further in‐depth studies are needed to evaluate the role of SNC4A in epileptic susceptibility.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Talarico M, Fortunato F, Labalme A, Januel L, Chatron N, Sanlaville D, et al. Idiopathic generalized epilepsy in a family with SCN4A‐related myotonia. Epilepsia Open. 2024;9:951–959. 10.1002/epi4.12920
Mariagrazia Talarico and Francesco Fortunato equally contributed to the study.
REFERENCES
- 1. Matthews E, Fialho D, Tan SV, Venance SL, Cannon SC, Sternberg D, et al. The non‐dystrophic myotonias: molecular pathogenesis, diagnosis and treatment. Brain. 2010;133:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vivekanandam V, Männikkö R, Matthews E, Hanna MG. Improving genetic diagnostics of skeletal muscle channelopathies. Expert Rev Mol Diagn. 2020;20(7):725–736. [DOI] [PubMed] [Google Scholar]
- 3. Hirsch E, French J, Scheffer IE, Bogacz A, Alsaadi T, Sperling MR, et al. ILAE definition of the idiopathic generalized epilepsy syndromes: position statement by the ILAE task force on nosology and definitions. Epilepsia. 2022;63(6):1475–1499. [DOI] [PubMed] [Google Scholar]
- 4. Wolf P, Yacubian EM, Avanzini G, Sander T, Schmitz B, Wandschneider B, et al. Juvenile myoclonic epilepsy: a system disorder of the brain. Epilepsy Res. 2015;114:2–12. [DOI] [PubMed] [Google Scholar]
- 5. Baykan B, Wolf P. Juvenile myoclonic epilepsy as a spectrum disorder: a focused review. Seizure. 2017;49:36–41. [DOI] [PubMed] [Google Scholar]
- 6. Stefani S, Kousiappa I, Nicolaou N, Papathanasiou ES, Oulas A, Fanis P, et al. Neurophysiological and genetic findings in patients with juvenile myoclonic epilepsy. Front Integr Neurosci. 2020;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailey JN, Patterson C, de Nijs L, Durón RM, Nguyen VH, Tanaka M, et al. EFHC1 variants in juvenile myoclonic epilepsy: reanalysis according to NHGRI and ACMG guidelines for assigning disease causality. Genet Med. 2017;19(2):144–156. [DOI] [PubMed] [Google Scholar]
- 8. Delgado‐Escueta AV, Koeleman BP, Bailey JN, Medina MT, Durón RM. The quest for juvenile myoclonic epilepsy genes. Epilepsy Behav. 2013;28(Suppl 1):S52–S57. [DOI] [PubMed] [Google Scholar]
- 9. International League Against Epilepsy Consortium on Complex Epilepsies . Genome‐wide mega‐analysis identifies 16 loci and highlights diverse biological mechanisms in the common epilepsies. Nat Commun. 2018;9(1):5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subramanian M, Senthil N, Sujatha S. Idiopathic generalized epilepsy and hypokalemic periodic paralysis in a family of south Indian descent. Case Rep Neurol Med. 2015;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao L, Li X, Hong D. Normokalemic periodic paralysis with involuntary movements and generalized epilepsy associated with two novel mutations in SCN4A gene. Seizure. 2015;24:134–136. [DOI] [PubMed] [Google Scholar]
- 12. Bergareche A, Bednarz M, Sánchez E, Krebs CE, Ruiz‐Martinez J, de la Riva P, et al. SCN4A pore mutation pathogenetically contributes to autosomal dominant essential tremor and may increase susceptibility to epilepsy. Hum Mol Genet. 2015;24(24):7111–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matthews E, Balestrini S, Sisodiya SM, Hanna MG. Muscle and brain sodium channelopathies: genetic causes, clinical phenotypes, and management approaches. Lancet Child Adolesc Health. 2020;4(7):536–547. [DOI] [PubMed] [Google Scholar]
- 14. Berkovic SF, Kapur J. Are myotonia and epilepsy linked by a chloride channel? Neurology. 2013;80(12):1074–1075. [DOI] [PubMed] [Google Scholar]
- 15. Chen TT, Klassen TL, Goldman AM, Marini C, Guerrini R, Noebels JL. Novel brain expression of ClC‐1 chloride channels and enrichment of CLCN1 variants in epilepsy. Neurology. 2013;80(12):1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brunklaus A, Feng T, Brünger T, Perez‐Palma E, Heyne H, Matthews E, et al. Gene variant effects across sodium channelopathies predict function and guide precision therapy. Brain. 2022;145(12):4275–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pagliarani S, Lucchiari S, Scarlato M, Redaelli E, Modoni A, Magri F, et al. Sodium Channel Myotonia due to novel mutations in domain I of Nav1.4. Front Neurol. 2020;11:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maggi L, Brugnoni R, Canioni E, Tonin P, Saletti V, Sola P, et al. Clinical and molecular Spectrum of Myotonia and periodic paralyses associated with mutations in SCN4A in a large cohort of Italian patients. Front Neurol. 2020;11:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furby A, Vicart S, Camdessanché JP, Fournier E, Chabrier S, Lagrue E, et al. Heterozygous CLCN1 mutations can modulate phenotype in sodium channel myotonia. Neuromuscul Disord. 2014;24(11):953–959. 10.1016/j.nmd.2014.06.439 [DOI] [PubMed] [Google Scholar]
- 20. Brugnoni R, Maggi L, Canioni E, Verde F, Gallone A, Ariatti A, et al. Next‐generation sequencing application to investigate skeletal muscle channelopathies in a large cohort of Italian patients. Neuromuscul Disord. 2021;31(4):336–347. 10.1016/j.nmd.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 21. Türkdoğan D, Matthews E, Usluer S, Gündoğdu A, Uluç K, Mannikko R, et al. Possible role of SCN4A skeletal muscle mutation in apnea during seizure. Epilepsia Open. 2019;4(3):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cannon SC. Sodium Channelopathies of skeletal muscle. Handb Exp Pharmacol. 2018;246:309–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rochtus AM, Goldstein RD, Holm IA, Brownstein CA, Pérez‐Palma E, Haynes R, et al. The role of sodium channels in sudden unexpected death in pediatrics. Mol Genet Genomic Med. 2020;8(8):e1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, Huang Q, Ge L, Xu J, Shi X, Xie W, et al. Identification of genetic variations of a Chinese family with paramyotonia congenita via whole exome sequencing. Genom Data. 2015;4:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


