Abstract
The development of therapeutic approaches for the induction of robust, long-lasting and antigen-specific immune tolerance remains an important unmet clinical need for the management of autoimmunity, allergy, organ transplantation and gene therapy. Recent breakthroughs in our understanding of immune tolerance mechanisms have opened new research avenues and therapeutic opportunities in this area. Here, we review mechanisms of immune tolerance and novel methods for its therapeutic induction.
Introduction
Immune system activation is vital to the control of pathogens and cancer, but regulatory mechanisms are needed to prevent immunopathology resulting from excessive immune activity. Perturbations of this balance result in infections, cancer, inflammatory diseases or allergy. Indeed, autoimmune diseases affect as much as 5–10% of the population and are on the rise1. Similarly, inefficacious immune modulation results in graft rejection and graft-versus-host disease (GVHD) in 20–70% of transplant recipients, and pre-existing immunity to viral vectors limits gene therapy efficacy. The development of antigen-specific immunotherapies is an important unmet clinical need.
Key advances have been made in our understanding of immune tolerance and its regulation. Indeed, new technologies for antigen discovery, drug delivery and cell targeting have opened new avenues for the development of therapies for the induction of antigen-specific tolerance. Here we review mechanisms of immune tolerance and discuss strategies for its therapeutic modulation.
Mechanisms of immune tolerance
Immune tolerance is an active state of unresponsiveness towards a specific antigen, which involves both innate and adaptive immune cells. The breakdown of self-tolerance can result in the development of autoimmune disorders, whereas dysregulated immune responses to foreign antigens may lead to hypersensitivity and allergic disease. Thus it is important to define the multiple mechanisms involved in its establishment and maintenance.
Central tolerance
Central tolerance is established during T and B cell development in the thymus and bone marrow, respectively. Bone marrow-derived CD34+ T cell progenitors home to the thymus, where they acquire T cell receptor (TCR) expression. Random V(D)J rearrangements generate a diverse TCR repertoire that is reactive against a wide array of antigens. T cells harbouring TCRs that do not recognize MHC-presented self-peptides die by neglect, whereas those with low affinity for peptide–MHC complexes differentiate into CD4+ or CD8+ single-positive T cells. The randomness of V(D)J rearrangements inevitably generates some TCR clones with high affinity for self-antigen–MHC complexes. High-affinity TCR clones are controlled by various mechanisms of central tolerance including clonal deletion and receptor editing. Some self-reactive T cells escape deletion and leave the thymus but show functional impairment and/or expression of molecules associated with tolerance2, whereas others develop into self-reactive thymus-differentiated regulatory T cells (tTreg cells), which migrate to peripheral lymphoid and nonlymphoid tissues3.
Self-antigen–MHC complexes are expressed by thymic antigen-presenting cells (APCs) including specialized medullary thymic epithelial cells (mTECs), dendritic cells (DCs) and B cells. The transcriptional factor autoimmune regulator (AIRE) promotes the expression of peripheral tissue antigens by mTECs4; mutations in AIRE are linked to autoimmune pathology. However not all tissue-specific antigens expressed by mTECs are controlled by AIRE. Indeed recent studies identified mTECs that express transcription factors such as FEZF2 (ref. 5) or that co-opt lineage-defining transcription factors from peripheral cell types, termed mimetic cells6. These AIRE+, FEZF2+ and mimetic mTECs collaborate with thymic B cells and DCs to promote central tolerance through clonal T cell deletion and Treg cell induction. This process is further aided by the transfer of tissue-specific antigens from mTECs to DCs through a process termed cooperative antigen transfer7. Of note, it was recently reported that intestinal DCs travel to the thymus to present microbiota-derived antigens, highlighting the contribution of peripheral DCs to central tolerance8.
In the bone marrow, developing B cells acquire the expression of a B cell antigen receptor (BCR) that randomly rearranges its V, D and J gene regions to generate a diverse BCR repertoire. Up to 75% of early immature B cells are self-reactive9, but a third of them undergo immunoglobulin gene rearrangements that reduce autoantigen reactivity10. Additional self-reactive B cells are removed by clonal deletion11. However central tolerance does not eliminate all self-reactive clones, for example those reactive to developmentally restricted or inducible antigens that are not expressed by the thymus or the bone marrow. Thus, self-reactive lymphocytes escape central tolerance and are actively controlled by peripheral tolerance mechanisms.
Peripheral tolerance
About 25–40% of self-reactive T cells12 and approximately 40% of autoreactive B cells9 escape central tolerance. Thus peripheral tolerance mechanisms, including anergy, deletion and suppression by Treg cells, are crucial for the prevention of autoimmune diseases or hypersensitivity to antigens first encountered outside the thymus or bone marrow, including food allergens or antigens displayed during infection or pregnancy.
Three signals are required for T cell activation. Signal 1 involves the interaction of the TCR with peptide–MHC molecules. Signal 2 involves the binding of co-stimulatory receptors to their ligands on APCs, most commonly CD28 on T cells and CD80 or CD86 on APCs, but also other co-stimulatory molecules, including inducible T cell co-stimulator (ICOS) and CD40 (ref. 13). Signal 3 involves the activation of cytokine receptors. The activation of TCR signalling (signal 1) in the absence of co-stimulation (signal 2), or strong pre-exposure to cytokines (signal 3) before signals 1 and 2, induces T cell anergy, a state in which the T cell is functionally inactivated, incapable of proliferating or producing IL-2 (ref. 14). T cell anergy can also be induced by repeated antigen stimulation15, exposure to anti-inflammatory cytokines such as IL-10 (ref. 16), or signalling via co-inhibitory receptors such as programmed cell death 1 (PD1) and cytotoxic T lymphocyte associated protein 4 (CTLA4)17. Similarly, B cells require BCR engagement concomitant with Toll-like receptor (TLR) signalling or interactions with T helper cells to be fully activated. High avidity BCR interactions with antigens in the absence of TLRs or T helper cell co-stimulation induce clonal deletion or anergy, inhibiting B cell proliferation and differentiation into antibody-secreting cells and overall shortening B cell lifespan18.
Long-term T cell anergy is associated with epigenetic modifications that render cells more sensitive to inhibitory signals19, while altering gene and surface marker expression and inducing functional changes similar to those observed in exhausted T cells induced during chronic infection or cancer15. However T and B cell anergy is a dynamic process, and the removal of antigen exposure can restore T or B cell functionality15,20. Furthermore, a subset of naturally occurring anergic T cells expressing CD73 and FR4, capable of differentiating into functional FOXP3+ Treg cells and FOXP3−IL-10+ type 1 regulatory T (TR1) cells, has been described21,22, although it is not clear whether this process involves specific APC types or anatomical niches.
The peripheral deletion of T and B cells through apoptosis also controls self-reactive cells. Intrinsic T cell apoptosis largely depends on the pro-apoptotic protein BIM, upregulated during T cell deletion, which inhibits the anti-apoptotic proteins BCL-2 and BCL-xL, activating pro-apoptotic BAX and BAK to permeabilize the mitochondrial membrane23,24. Extrinsic T cell apoptosis involves FAS25 or tumour necrosis factor (TNF) receptor26 signalling, which ultimately triggers caspase activation to induce apoptosis. Signalling through these death receptors limits self-reactive pathogenic T cell and B cell responses. For example, central nervous system (CNS)-resident astrocytes expressing the TNF receptor ligand TRAIL induce T cell apoptosis and limit autoimmune neuroinflammation27. Other forms of peripheral immune cell death (necroptosis, ferroptosis and pyroptosis) also contribute to peripheral immune tolerance28-30.
The mechanisms determining whether self-reactive T or B cells undergo anergy versus cell death following TCR or BCR activation without co-stimulation are still not fully understood. Antigen levels have been postulated to control cell fate, with higher levels triggering anergy and lower levels triggering cell death31. In addition, checkpoint molecule signalling (for example, through PD1, TIGIT, TIM3, LAG3 and VISTA) can induce T cell death or dysfunction32-34.
Finally Treg cells play central roles in peripheral tolerance. Major Treg cell subtypes include FOXP3+ cells and IL-10-producing FOXP3− TR1 cells, but additional subsets have been linked to immune tolerance, including CD8+ Treg cells35, regulatory γδ T cells36 and regulatory invariant natural killer T cells (iNKT cells)37.
FOXP3+ Treg cells differentiate in the thymus (FOXP3+ tTreg cells) in response to self-antigen expression38 and then migrate to peripheral lymphoid and nonlymphoid tissues to limit pathogenic autoreactivity and promote tissue repair39. Some FOXP3+ Treg cells differentiate from naive CD4+ T cells in the periphery (FOXP3+ pTreg cells), enforcing tolerance to antigens not expressed in the thymus, including food antigens, allergens, microbial antigens or pregnancy-linked fetal antigens40. In addition, tissue-resident Treg cells in the skin41, muscle42, visceral adipose tissue43,44 and mucosal tissues, such as intestine45,46 and lungs39, display specialized phenotypes and functions, as recently reviewed47,48.
TR1 cells are IL-10+FOXP3−CD4+ T cells that were initially described following chronic stimulation in the presence of IL-10 (ref. 49). IL-27 was later found to be a stronger TR1 cell differentiation inducer50, with IFNα51, hyaluronic acid52, ICOSL53, CD2 (ref. 54) and CD55 (ref. 55) expression on APCs also displaying important roles (see Box 1). FOXP3+ pTreg and TR1 cell differentiation and function are modulated by host and microbial metabolites, such as aryl hydrocarbon receptor (AHR) agonists56. TR1 cells produce IL-10 and transforming growth factor-β (TGFβ), as well as perforin and granzyme B, which can kill APCs57,58. TR1 cells also express the inhibitory molecules CTLA4 and PD1, enabling contact-dependent T cell suppression, and CD39 (ref. 59), which degrades pro-inflammatory extracellular ATP while promoting the production of anti-inflammatory adenosine.
Box 1. Tolerogenic dendritic cells and the induction of peripheral regulatory T cells.
Tolerogenic dendritic cells (DCs) expand the peripheral regulatory T cell (Treg cell) compartment through multiple mechanisms. Inhibitory molecules on tolerogenic DCs such as programmed cell death ligand 1 (PD-L1) and PD-L2 engage programmed cell death 1 (PD1) on T cells, boosting the differentiation of FOXP3+ Treg cells through the downregulation of phosphorylated AKT, mTOR, S6 and ERK2 and simultaneous upregulation of the phosphatase PTEN274. Additionally, DC expression of inducible T cell co-stimulatory ligand (ICOSL) activates its receptor ICOS on T cells, also promoting the development of FOXP3+ Treg cells and type 1 regulatory T cells (TR1 cells), although ICOS signalling is also critical for the polarization of T helper 1 and T helper 2 effector cells53,70. Finally, binding of the surface receptor B and T lymphocyte attenuator (BTLA) expressed on DCs to herpesvirus entry mediatory (HVEM) on CD4+ T cells is reported to upregulate CD5 and induce FOXP3 expression275,276.
Several secreted factors released by DCs promote Treg cell differentiation. Transforming growth factor-β (TGFβ) induces FOXP3+ Treg cell differentiation but promotes T helper 17 cell development in the presence of IL-6 or IL-21 (ref. 277). In the presence of TGFβ, IL-10 promotes FOXP3 and cytotoxic T lymphocyte associated protein 4 (CTLA4) expression278. IL-10 was also described to induce TR1 cell differentiation96,97. IL-27 is a strong inducer of TR1 cell differentiation through the induction of MAF, aryl hydrocarbon receptor (AHR) and IL-21 (refs. 59,279,280) and has been shown to control specific transcriptional programmes in FOXP3+ Treg cells281. Moreover, IL-27 signalling in DCs and T cells induces the expression of CD39, which degrades extracellular ATP, limiting its pro-inflammatory effects107. Besides cytokines, metabolites produced by DCs such as kynurenine, retinoic acid and lactate have important roles in modulating T cell responses. For example, indoleamine 2,3-dioxygenase limits T cell responses via the production of anti-inflammatory tryptophan metabolites such as kynurenine, many of which activate AHR to promote FOXP3+ Treg cell and TR1 cell differentiation56. Retinoic acid promotes the development of FOXP3+ Treg cells and TR1 cells, enhancing the effects of TGFβ and IL-10 (ref. 112). Finally, lactate produced by DCs can suppress effector T cell differentiation115.
Multiple cell types participate in central and peripheral immune tolerance. DCs play a central role because they process and present antigen, while providing cytokines and stimulatory or inhibitory molecules to modulate T cell differentiation or trigger anergy or deletion. Thus, DCs are frequently targeted for the therapeutic induction of antigen-specific immune tolerance.
DCs as the central mediators of immune tolerance
DC subsets and their functions
DCs display phenotypic and functional heterogeneity60,61. DCs are classified into plasmacytoid DCs (pDCs), classical (or conventional) type 1 DCs (cDC1s) and type 2 DCs (cDC2s). In addition, monocyte-derived DCs (moDCs), sometimes called TipDCs (TNF-producing and iNOS-producing DCs), adopt a DC-like phenotype under inflammatory conditions62, although recent works call into question their ability to migrate to lymph nodes and prime CD4+ and CD8+ T cells63. A DC3 subtype displaying cDC2 and moDC features was also identified in humans64. Additional heterogeneity within DC subsets has been described. For example, cDC2s are classified into cDC2As and cDC2Bs controlled by the transcription factors T-bet and RORγt, respectively65. In addition CD103 and CD11b distinguish functional cDC subsets in mucosal tissues66.
pDCs are primarily located in the blood and lymphoid tissues but migrate to nonlymphoid tissues during inflammation67. When activated, mainly via TLR7 or TLR9 signalling, pDCs produce large amounts of type I interferons, including IFNα and IFNβ68. Under homeostatic conditions, pDCs are poor activators of naive CD4+ and CD8+ T cells. However, a subpopulation of pDCs stimulates CD4+ T helper 1 (TH1) cells during infection69. pDCs also promote tolerance and Treg cell induction via the expression of ICOSL70, TGFβ71 and inhibitory indoleamine 2,3-dioxygenase (IDO)72. Indeed recent findings suggest that pDC deficits contribute to GVHD following organ transplantation73 and that pDCs contribute to oral tolerance induction74.
cDCs are present in both lymphoid and nonlymphoid tissues at the steady state. cDC1 and cDC2 distribution varies in different tissues, and although both subsets migrate between tissues and lymph nodes, cDC2s appear to have a higher migratory potential and are enriched at mucosal-associated sites such as the lungs and intestine75. Of note, at the steady state cDC1s, cDC2s and pDCs are detected in the CNS choroid plexus and meninges, but they are virtually undetectable in the brain parenchyma and perivascular space76,77. Indeed, cDC1s are the primary subtype present in the choroid plexus, whereas cDC2s are most abundant in the leptomeninges and dura mater76,77. Under inflammation cDC1s, cDC2s, moDCs and pDCs infiltrate the brain parenchyma and present CNS-specific antigens to T cells76-78. Although both cDC1s and cDC2s can present antigen to either CD4+ or CD8+ T cells, cDC1s are better at antigen cross-presentation79 and type III interferon production80. Within the cDC2 subset, cDC2As appear to be less pro-inflammatory than cDC2Bs, expressing higher levels of amphiregulin and matrix metalloproteinase 9, whereas cDC2Bs produce higher levels of TNF and IL-6 (ref. 65). Of note, cDC2s in the intestine have been shown to promote T helper 17 (TH17) cell differentiation81,82. However, both cDC1s and cDC2s are reported to promote the differentiation of FOXP3+ Treg cells and IL-10+ TR1 cells83,84.
Tolerogenic DC phenotype
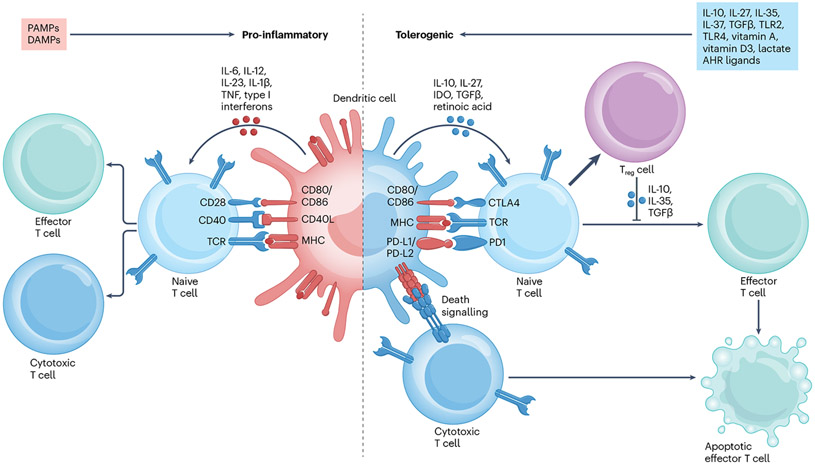
Activation and maturation states dictate the effects of DCs on the immune response. Before their activation via pattern recognition receptors (PRRs), DCs reside at mucosal sites, lymphoid and peripheral tissues or in the blood in an immature state. Activation by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) upregulates DC expression of MHC class I and II, co-stimulatory and adhesion molecules such as CC-chemokine receptor 7 (CCR7). These mature DCs migrate to lymphoid tissues to promote effector T cell differentiation. Immature DCs, conversely, exhibit low expression of MHC class I, MHC class II and co-stimulatory molecules and are capable of inducing T cell anergy, Treg cell differentiation and effector T cell deletion85. It was originally postulated that tolerogenic DCs were essentially immature DCs, but this paradigm was challenged early on86. It has since been proposed that specific stimuli can induce a tolerogenic DC phenotype87 and that tolerogenic DCs undergo some level of maturation and/or activation88. Indeed, specific transcriptional programmes in DCs drive immunogenic versus tolerogenic states87,89. For example, β-catenin signalling90 or phagocytosis of apoptotic material91 under steady-state conditions activate tolerogenic programmes in DCs, which migrate to lymph nodes to present self-antigens and maintain peripheral tolerance. Moreover, a tolerogenic DC phenotype can also be induced in semimature and mature DCs92. For example, an IL-10+ DC-10 subtype was identified in human peripheral blood and the spleen, displaying cDC and moDC surface markers but capable of inducing CD4+ T cell hyporesponsiveness and TR1 cell expansion93 (see Box 1); DC-10s can be induced in vitro by monocyte differentiation in the presence of IL-10. In addition, intestinal CD103+ DCs contribute to tolerance to dietary antigens and the induction of oral tolerance94,95. Regardless of their origin and maturation state, DCs contribute to immune regulation via multiple mechanisms, including co-stimulatory molecule downregulation (CD80, CD86 and CD40), inhibitory molecule expression (PD-L1, ICOSL and BTLA), suppression of pro-inflammatory cytokine production (IL-6, IL-12, IL-23 and TNF) and production of anti-inflammatory cytokines (IL-10, TGFβ and IL-27) and metabolites (IDO, retinoic acid and lactate) (Box 1 and Fig. 1).
Fig. 1 ∣. Mechanisms and features in pro-inflammatory dendritic cells compared with tolerogenic dendritic cells.
Pro-inflammatory dendritic cells (DCs) can be induced via activation by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) and upregulate the expression of surface molecules including MHC molecules, CD80 and CD86. These surface molecules, in addition to secreted pro-inflammatory cytokines, such as IL-1β, IL-6, IL-12, IL-23, tumour necrosis factor (TNF) and type I interferons, induce the differentiation of cytotoxic and effector T cells from naive T cells. Conversely, tolerogenic DCs can be induced via several mechanisms, including exposure to cytokines such as IL-10, IL-27, IL-35, IL-37 or transforming growth factor-β (TGFβ); signalling via Toll-like receptor 2 (TLR2), TLR4 or aryl hydrocarbon receptor (AHR); or exposure to molecules such as vitamin D3, vitamin A or lactate. Tolerogenic DCs express lower levels of MHC molecules, CD80 and CD86 and secrete anti-inflammatory cytokines and molecules such as IL-10, TGFβ, IL-27, indoleamine 2,3-dioxygenase (IDO) and retinoic acid. Tolerogenic DC interactions with T cells induce the differentiation and expansion of anti-inflammatory regulatory T cells (Treg cells) from naive T cells and the apoptosis of cytotoxic T cells through death receptor signalling interactions, such as between programmed cell death 1 (PD1) and PD1 ligand 1 (PD-L1) or PD-L2. CTLA4, cytotoxic T lymphocyte associated protein 4; TCR, T cell receptor.
Numerous stimuli induce a tolerogenic DC phenotype. For example, IL-10 reduces DC expression of MHC and co-stimulatory molecules, decreases pro-inflammatory cytokine production and promotes T cell anergy and Treg cell expansion96,97. These anti-inflammatory effects of IL-10 on DCs are AHR dependent98, recapitulating previous reports of the tolerogenic effects of AHR signalling in DCs99-105. Additional cytokines such as TGFβ106, IL-27 (ref. 107) and IL-37 (ref. 108) also promote an anti-inflammatory DC phenotype. Similarly the exposure of monocytes or bone marrow cells to low concentrations of granulocyte-monocyte colony-stimulating factor (GM-CSF) induces the differentiation of DCs with a tolerogenic phenotype, whereas exposure to higher GM-CSF doses induces a pro-inflammatory DC phenotype109,110. Moreover, commensal bacteria signalling through certain PRRs such as TLR2 (ref. 111) promotes tolerogenic DC induction. Indeed, some microbial metabolites induce tolerogenic DCs, for example via AHR activation99,100. Indeed, AHR agonists inhibit nuclear factor-κB (NF-κB) activation in DCs and drive the expression of IL-10 and IDO, while reducing the expression of MHC molecules, co-stimulatory molecules and pro-inflammatory cytokines such as IL-6 and IL-12. These changes in DCs result in increased FOXP3+ and IL-10+ Treg cells and the suppression of TH1, TH17 and CD8+ effector T cells101-104.
Additional inducers of a tolerogenic DC phenotype include vitamin A, which is metabolized into retinoic acid, a booster of FOXP3+ Treg cell induction112 and vitamin D3 that increases IL-10 production while decreasing IL-12 and co-stimulatory molecule expression113,114. Moreover, lactate, produced by microbiota, activated DCs or other immune cells, regulates DC function via a hypoxia inducible factor 1α (HIF1α)-driven increase in the expression of NADH dehydrogenase NDUFA4L2 that ultimately limits effector T cell activation115.
Finally the uptake of apoptotic cells induces a tolerogenic DC phenotype via mechanisms involving AHR activation116, prostaglandin E2 production117 and signalling via scavenger receptors such as MARCO118. Indeed, both cDCs and pDCs express IL-10, reduce co-stimulatory molecule expression and promote Treg cell expansion following apoptotic cell uptake91.
These and other pathways linked to the tolerogenic DC phenotype offer opportunities for the development of therapeutic immunomodulatory strategies, as discussed below.
Antigen-specific therapeutic strategies to induce immune tolerance
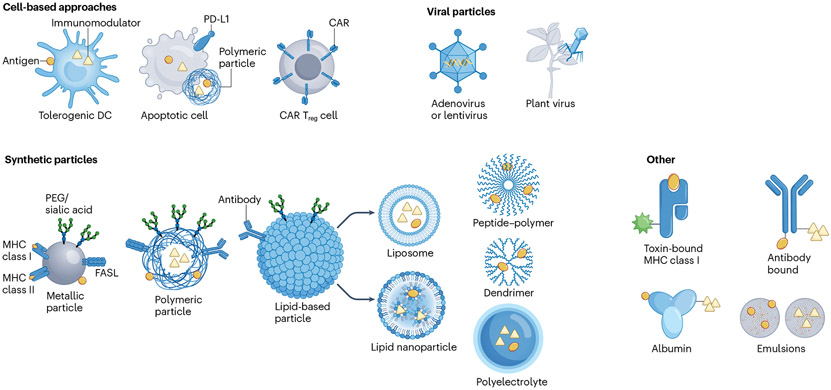
Current therapies for autoimmune diseases, transplant rejection and other pathologies driven by dysregulated immune responses are mostly based on untargeted immunosuppression and consequently are linked to significant side effects. Thus novel approaches to induce antigen-specific immune tolerance are needed, targeting improperly activated T cells but not interfering with protective immunity to pathogens and cancer. Consequently, numerous technologies have been developed to induce antigen-specific tolerance (Fig. 2 and Table 1). In the next section, we discuss strategies for the induction of antigen-specific immune tolerance in autoimmunity, organ transplantation and gene therapy (Fig. 3).
Fig. 2 ∣. Approaches for the induction of antigen-specific immune tolerance.
Cell-based approaches include the ex vivo induction of tolerogenic dendritic cells (DCs), apoptotic cells or regulatory T cells engineered to express chimeric antigen receptors (CAR Treg cells), all of which can be designed to deliver antigen with or without an immunomodulatory signal. Viral particle approaches include the delivery of DNA-encoded or RNA-encoded antigen via adenoviruses, lentiviruses or plant viruses. Synthetic particles, including metallic, polymeric, lipid-based (including liposomes or lipid nanoparticles), peptide–polymer, dendrimer or polyelectrolyte particles, can be designed to co-deliver antigens, antibodies and immunomodulators, in various combinations. Alternatively, antigens can be delivered via toxin-bound MHC molecules to induce the death of antigen-specific cells, and albumin, antibodies or nanoemulsions can deliver antigens and immunomodulators to induce antigen-specific immune tolerance. FASL, FAS ligand; PEG, polyethylene glycol.
Table 1 ∣.
Antigen-specific immunotherapy approaches
| Material | Mediator of antigen specificity |
Immunomodulator | Disease model | Findings | Refs. |
|---|---|---|---|---|---|
| Cell-based approaches | |||||
| IL-10-moDCs | Lentiviral vectors | NOD | Induction of antigen-specific T cell tolerance and prevention of disease | 122 | |
| Schwann cells+ PEG–PLGA NPs | Natural | PD-L1–Fc, CD86–Fc, leflunomide | EAE | Inhibition of TH1 cell responses, leflunomide loading increased myelin repair | 256 |
| Erythrocytes | Peptide mimotope | NOD | Reduced trafficking of effector T cells into organs | 136 | |
| Erythrocytes | Recombinant antigen | EAE | Persistent antigen exposure via erythrocytes induced T cell exhaustion and dysfunction | 135 | |
| BAR Treg cells | Retroviral | ADAs | ADA suppression | 146 | |
| CAR Treg cells | Retroviral | EAE | Suppression of antigen-specific and other T cells even in a pro-inflammatory environment | 147 | |
| CAR Treg cells | Plasmid | Allograft rejection | Protection of allografts better than polyclonal Treg cells | 142 | |
| CAR/FOXP3+ Treg cells | CAR | EAE | Intranasally administered cells accessed various brain regions and suppressed disease | 144 | |
| Vitamin D3–IL-4–IL-10–GM-CSF moDCs | None | CTLA4–Ig | Alloimmune reactivity | Only in addition with CTLA4–Ig: alloreactive Treg cell induction and reduction in T cell activity | 126 |
| Apoptotic cells | Peptide | CFA | Arthritis | Induction of antigen-specific tolerance via B cell-mediated Treg cell induction | 134 |
| Viral-based approaches | |||||
| Cowpea mosaic virus | Plasmid | EAE | Safe and efficient gene delivery | 220 | |
| Adeno-associated virus | Plasmid | EAE | Liver-targeted expression of protein induced Treg cells, regardless of epitope or HLA background | 219 | |
| Lentivirus | Plasmid | NOD | Transient expression of antigen with integrase-incompetent lentivirus protected from disease | 217 | |
| Nanoparticles without adjuvant | |||||
| PLGA NPs | EAE, DTH | Tolerance dependent on dose and antigen loading | 157 | ||
| PLGA NPs | Multiple proteins | NOD | Encapsulation of multiple epitopes broadened spectrum of induced tolerance | 257 | |
| PEMA-PLGA NPs | Protein | EAE | Kupffer cells and LSECs induced tolerance after antigen uptake | 194 | |
| Liposome | mRNA | EAE | Bystander tolerance by induction of Treg cells | 237 | |
| Modified PLGA NPs | Peptide | EAE, NOD, colitis | Optimized NPs can be effectively loaded with a variety of antigens | 258 | |
| PEG–PLGA NPs | Peptide | EAE | PEGylation increased bioavailability of subcutaneously injected NPs | 192 | |
| Iron oxide NPs | MHC class II-bound ubiquitous antigen | EAE | Ubiquitous liver autoantigens are involved in extrahepatic immune diseases and tolerance induction mitigates extrahepatic autoimmunity | 259 | |
| PEGylated iron oxide NPs | MHC class II-bound peptide | PBC, AIH, PSC | Ubiquitous antigens are involved in autoimmune liver diseases | 260 | |
| PLGA NPs | Hybrid peptide | NOD | Effector T cell anergy induced against several epitopes | 154 | |
| PLGA NPs | Peptides | NOD | Antigen-coupled NPs induced Treg cells | 155 | |
| DSPG liposomes | Peptide | Atherosclerosis | C1q-dependent update via scavenger receptors induced tolerance | 173 | |
| PLGA NPs | Peptide | R-EAE | Uptake of antigen by APCs led to PD-L1-dependent tolerance induction | 182 | |
| CdSe-ZnS-quantum dots | Protein | EAE | Antigen density dictates disease suppression | 158 | |
| Dextran-coated or PEGylated iron oxide | MHC class II-bound peptide | EAE, NOD | Expansion of TR1 cells | 149 | |
| PSL NPs | None | Atherosclerosis | Apoptotic cell mimicry induced IgM and reduced inflammation | 181 | |
| PLGA-PEMA NPs | Protein | R-EAE | Surfactant modification increased efficacy of NPs, reduced CNS infiltration of effector T cells | 156 | |
| PEI NPs | Plasmid | Arthritis | Reduction of TLR9 activation by DNA promoted IDO-mediated induction of tolerance | 261 | |
| Iron oxide NPs | MHC class I-bound peptide | NOD | Induction of Treg cells that suppressed APCs via IDO and perforin | 198 | |
| Dendrimer branched lysine core particles | Multiple antigen peptides | EAE | Non-inflammatory presentation of antigen decreased effector T cell but not Treg cell CNS infiltration | 262 | |
| Peptide–polymer | Peptide | Cholangitis | LSECs presented antigen on MHC class I, decreasing liver infiltration of antigen-specific CD8+ T cells | 263 | |
| Particles with adjuvant | |||||
| PLGA NPs | Peptide | ICAM1 inhibitor | EAE | Dual-peptide NPs have stronger inhibitory effect | 264 |
| Dual size PLGA | Protein | GM-CSF, TGFβ, vitamin D3 | NOD | Long-lasting protection in advanced disease states | 210,211 |
| Liposomes/serum-albumin–bound NPs | Protein | Treg cell epitopes | NOD | Tregitopes induced tolerance via specific Treg cell activation | 265 |
| Lipid-coated salt NPs | Citrullinated peptides | Rapamycin | RA | Induction of immune tolerance in advanced disease | 213 |
| PEG–PLGA NPs | Peptide | Knockdown of CD40, CD80, CD86 | NOD | Expansion of antigen-specific Treg cells | 266 |
| Liposomes | Mimotope | Vitamin D3 | NOD | Co-encapsulation of vitamin D3 can mediate bystander tolerance | 214 |
| Liposomes | Peptide | ITE | EAE | Co-encapsulation of immunomodulator induced Treg cells and bystander tolerance | 104 |
| Liposomes | Peptide | Vitamin D3 | RA, Goodpasture’s vasculitis | Calcitriol-antigen loaded NPs increased Treg cells and suppressed effector T cells in a PD-L1-dependent manner | 206 |
| Liposomes | Peptide | CD22 ligands, rapamycin | Hypersensitivity | Rapamycin enhanced tolerance induction in naive but not in presensitized mice | 208 |
| PEG–Gold NPs | Peptide | ITE | EAE, NOD | Co-encapsulation of immunomodulators expanded Treg cells and increased efficacy | 102,103 |
| PEG–PLA NPs, PLGA NPs | Peptides/drug | Rapamycin | EAE, DTH, ADAs | Co-encapsulation of rapamycin induced durable B and T cell tolerance | 202 |
| Dextran NPs | Peptide | Dexamethasone | EAE | Immunomodulator increased effectiveness | 267 |
| PLGA NPs | Peptide | Recombinant IL-10 | EAE | NPs release antigen and immunomodulator constantly for several weeks, addition of IL-10 decreased IL-17 and IFNγ | 205 |
| PEG–liposomes | Multivalent peptide | CD22 ligands | ADAs | Tolerance induction towards presented alloantigen | 268 |
| Liposomes | Protein | NF-κB inhibitors | RA | Co-encapsulation of immunomodulators increased efficacy of tolerance induction | 203 |
| Particle free | |||||
| Anti-MHC class II antibodies | Peptide | Dexamethasone | EAE, NOD, RA | Immunomodulator reduced adverse effects and increased effectiveness | 207 |
| Nanoemulsion | Citrullinated self-antigen | Rapamycin | RA | Nanoemulsion accumulated in inflamed regions and suppressed disease activity | 207 |
| Mannosylated antigen | Peptide | EAE, R-EAE | Amelioration of EAE, reduced CNS infiltration of immune cells | 196 | |
ADA, antidrug antibody; AIH, autoimmune hepatitis; APC, antigen-presenting cell; BAR, B cell-targeting antibody receptor; CAR, chimeric antigen receptor; CFA, complete Freunds adjuvant; CNS, central nervous system; CTLA4, cytotoxic T lymphocyte associated protein 4; DC, dendritic cell; DSPG, 1,2-distearoyl-sn-glycero-3-phosphoglycerol; DTH, delayed-type hypersensitivity; EAE, experimental autoimmune encephalitis; ICAM1, intercellular adhesion molecule 1; IFNγ, interferon-γ; GM-CSF, granulocyte-monocyte colony-stimulating factor; IDO, indoleamine-pyrrole 2,3-dioxygenase; LSECs, liver sinusoidal endothelial cells; moDC, monocyte-derived dendritic cell; NF-κB, nuclear factor-κB; NOD, non-obese diabetes; NP, nanoparticle; PBC, primary biliary cholangitis; PD-L1, programmed cell death ligand 1; PEG, polyethylene glycol; PEI, polyethyleneimine; PEMA, poly(ethylene-maleic acid); PLA, poly(lactic acid); PLGA, poly(lactic-co-glycolic acid); PSC, primary sclerosing cholangitis; PSL, phosphatidyl serine liposome; RA, rheumatoid arthritis; R-EAE, relapsing–remitting EAE; TGFβ, transforming growth factor-β.
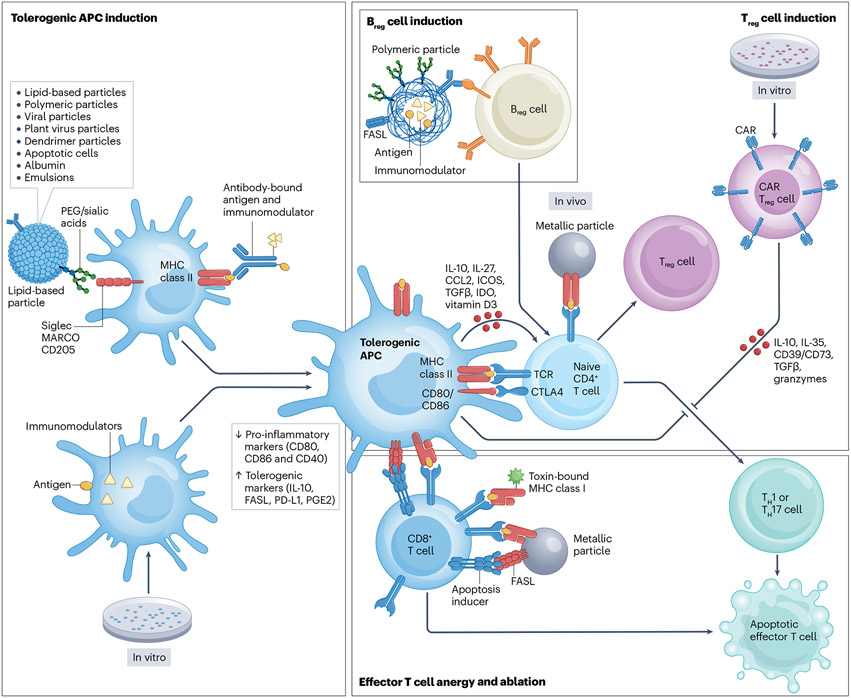
Fig. 3 ∣. Mechanisms for the induction of antigen-specific immune tolerance.
Tolerogenic antigen-specific antigen-presenting cells (APCs) can be induced in vivo through the delivery of synthetic particles, viral particles or cell-based approaches, or induced in vitro and engineered to express disease-specific antigens and an immunomodulatory signal. Tolerogenic APCs are characterized by reduced expression of pro-inflammatory markers including CD80, CD86 and CD40 and an increased expression or production of tolerogenic molecules such as IL-10, FAS ligand (FASL), programmed cell death ligand 1 (PD-L1) and prostaglandin E2 (PGE2). Tolerogenic APCs can in turn induce naive CD4+ T cells to differentiate into regulatory T (Treg) cells or can induce effector T cell anergy and ablation. Similarly, the induction of regulatory B (Breg) cells via synthetic particle administration or Treg cells via in vivo delivery of particles or in vitro engineering of chimeric antigen receptor (CAR) Treg cells results in the reduction of effector T cells by the induction of anergy or cell death. PEG, polyethylene glycol.
Cell-based tolerogenic therapies
The identification of stimuli inducing a tolerogenic phenotype in DCs guided cellular therapeutic approaches, commonly based on DCs generated ex vivo from peripheral blood-derived monocytes and loaded with disease-relevant antigens. However, there is not yet a standardized method to generate tolerogenic DCs ex vivo, and multiple protocols and tolerogenic molecules have been explored. For example, moDCs differentiated in vitro in the presence of low GM-CSF concentrations, termed autologous tolerogenic DCs (ATDCs), display an immature phenotype with a low expression of MHC class II, CD80, CD86 and CD40 and high IL-10 and lactate production119. ATDCs were well tolerated in a phase I/IIA clinical trial to prevent graft rejection following kidney transplantation, and additional trials are needed to evaluate their clinical efficacy120,121. Similarly, IL-10-induced DC-10s loaded with disease-specific antigens induce antigen-specific immune tolerance122; their clinical efficacy remains to be evaluated.
Vitamin D3 also induces a tolerogenic DC phenotype ex vivo113,114. Autologous vitamin D3-treated tolerogenic DCs loaded with disease-specific antigens have been tested in phase I clinical trials, including studies focused on type 1 diabetes (T1D)123,124 and multiple sclerosis (MS)125 (Table 2). Moreover, moDCs differentiated in the presence of vitamin D3 and IL-10 were shown to be tolerogenic and induce IL-10-producing T cells in a nonhuman primate alloimmune reactivity model126. Similarly, moDCs treated with dexamethasone display a tolerogenic phenotype characterized by high IL-10 and TGFβ secretion and low pro-inflammatory cytokine production127,128. Dexamethasone-induced tolerogenic DCs loaded with disease-specific peptides were well tolerated in phase I clinical trials in RA, MS and neuromyelitis optica129,130. Moreover, tolerogenic DCs induced with dexamethasone and vitamin A were tested in a phase I trial in Crohn’s disease131.
Table 2 ∣.
Antigen-specific immunotherapy approaches in phase I or II clinical trials
| Study type | Vehicle or reagent | Major findings | Clinical trial ID |
|---|---|---|---|
| Cell-based approaches | |||
| Neuroinflammation | |||
| Tolerogenic fibroblasts | Tolerogenic fibroblasts | No significant safety concerns or side effects up to 16 weeks after infusion | NCT05080270 |
| Tolerogenic DCs (TOLERVIT-MS) | Vitamin D3-moDCs + IFNβ | Interim report: no safety concerns | NCT02903537 |
| “Negative” DC vaccine (MS-tolDC) | Vitamin D3-moDCs | Interim report: no safety concerns | NCT02618902 |
| Regulatory DCs (TolDec-EM-NMO) | Dexamethasone–GM-CSF–IL-4-moDCs with seven peptides from MBP, MOG, PLP, AQP4 | Safe; induction of TR1 cells and decreased CD8+ T cells, NK cells, CD14+CD56+ cells | NCT02283671 |
| Peptide-coupled PBMCs (ETIMS) | Autologous PBMCs coupled with seven peptides | No adverse effects, reduced antigen-specific T cell response, no effects on immunoglobulins or recall antibody effect | NCT01414634 |
| Mesenchymal stem cells (MSCIMS) | Mesenchymal stem cells | Safe; neuroprotective; met some secondary visual end points | NCT00395200 |
| T cell vaccine | Irradiated MBP-reactive T cells against nine epitopes | Reduction in EDSS, walking time and relapses | NCT01448252 |
| T cell vaccine | Irradiated MBP-reactive T cells | Depletion of autoreactive T cells; smaller lesion volume | 269 |
| Rheumatoid arthritis | |||
| Autologous tolerogenic DCs (AutoDECRA) | Dexamethasone–vitamin D-moDCs with synovia | Safe, but no systemic effects detected | NCT01352858 |
| Autologous tolerogenic DCs (TolDCfoRA) | Dexamethasone-moDCs | Well tolerated | NCT03337165 |
| DC vaccine to suppress the immune response to citrullinated antigen (BAY11-7082; Rheumavax) | NF-κB-inhibitor-moDCs | Safe; fewer effector T cells and decreased pro-inflammatory cytokines | ACTRN12610000373077 |
| Autologous DCs (CreaVax-RA) | moDCs pulsed with PAD4, citrullinated vimentin and fillagrin. HNRPA2/B1 | Safe; reduction in autoantibody and IFNγ-producing T cells | CRiSKCT0000035 |
| Inflammatory bowel disease | |||
| Intralesional tolerogenic DCs (TolDecCDintra) | Dexamethasone-moDCs | Terminated (low recruitment) | NCT02622763 |
| Antigen-specific Treg cell therapy | Treg cells with ovalbumin | No adverse effects; symptom reduction | 2006-004712-44 |
| Autologous tolerogenic DCs | Dexamethasone–vitamin D-moDCs | Mixed clinical response | 2007-003469-42 |
| Type 1 diabetes | |||
| Immunotherapy vaccine (PIpepTolDC) | Autologous tolerogenic DCs loaded with proinsulin peptides | Ongoing | NCT04590872 |
| Autologous tolerogenic DCs | Autologous moDCs primed with peptides | Ongoing | NCT05207995 |
| AVT001 | Autologous moDCs | Ongoing | NCT03895996 |
| Tolerogenic DCs (D-Sense) | Vitamin D3-moDCs with proinsulin | Not published | NTR5542 |
| Polyclonal Treg cells + IL-2 (TILT) | Polyclonal Treg cells | No adverse effects but poor Treg cell survival | NCT02772679 |
| Tolerogenic DCs | Dexamethasone-vitamin D3-moDCs pulsed with islet antigen | Long-lasting CD4+ T cell tolerance and temporary bystander tolerance | 2013-005476-18 |
| Autologous immunoregulatory DCs | Anti-CD40/CD80/CD8-moDCs with six ODNs | Not published | NCT02354911 |
| CD4+CD127lowCD25+ polyclonal Treg cells | Polyclonal Treg cells + IL-2 | Treg cell survival but also expansion of cytotoxic T cells | NCT01210664 |
| Autologous DCs | Antisense CD40, CD80, CD86 ODNs-moDCs | Safe; induction of B220+CD11c+ B cells | NCT00445913 |
| Treg cells | Autologous ex vivo-expanded Treg cells | No adverse effects; elevated C-peptide levels and lower insulin dependence after 1 year | ISRCTN06128462 |
| Systemic lupus erythematosus | |||
| Autologous polyclonal Treg cells | Treg cells | Increased Treg cells in inflamed tissue | NCT02428309 |
| Graft rejection after kidney transplantation | |||
| DCreg | Donor-derived tolerogenic DCs | Ongoing | NCT03164265 |
| Donor alloantigen reactive Treg cells (ARTEMIS) | Donor alloantigen reactive Treg cells | No adverse effects | NCT02474199 |
| Autologous tolerogenic DCs (ONEatDC) | GM-CSF-moDCs | No adverse effects, fewer infections | NCT02252055 |
| Regulatory macrophages (ONEmreg12) | Donor-derived regulatory macrophages induced with GM-CSF + IFNγ | No adverse effects, fewer infections | NCT02085629 |
| Donor alloantigen-reactive Treg cells (The ONE Study) (DART) | Donor-alloantigen-reactive Treg cells with donor antigen | No adverse effects, fewer infections | NCT02244801 |
| Natural Treg cells (ONEnTreg13) | CD4+CD25+FOXP3+ Treg cells | No adverse effects, fewer infections | NCT02371434 |
| Treg cells (ONETreg1) | CD4+CD25+FOXP3+ Treg cells | No adverse effects, fewer infections | NCT02129881 |
| Treg cells (The ONE Study) | Treg cells induced with belatacept ex vivo with donor antigen | No adverse effects, fewer infections | NCT02091232 |
| Graft-versus-host disease | |||
| Ex vivo expanded donor Treg cells | Donor Treg cells cultured with recipient DCs | Not published | NCT01795573 |
| T cell-depleted graft with simultaneous infusion T cells | Conventional T cells and Treg cells in predefined ratio | Safe, no adverse effects | NCT01660607 |
| Treg cells | Induced Treg cells | No adverse effects | NCT01634217 |
| Treg cells | Induced Treg cells | Safe, low risk of acute GVHD | NCT00602693 |
| Free antigen approaches | |||
| Multiple sclerosis | |||
| ATX-MS-1467 | Synthetic peptides of four epitopes of MBP | Study 1 showed temporary lesion reduction; study 2 showed lesion reduction up to 48 weeks with higher doses | NCT01973491 |
| Myelin peptides | Transdermal peptide mix | Reduction of gadolinium-enhancing lesions | 270 |
| Myelin peptides | Transdermal peptide mix | Induction of TR1 cells but not FOXP3+ Treg cells | 271 |
| MBP8298 (MAESTRO-03) | MBP8298 | No difference to placebo | NCT00468611 |
| BHT-3009 | MOG-DNA | Fewer lesions and reduction of a spectrum of antibodies | NCT00382629 |
| Glatiramer acetate | Random peptides resembling MBP | Reduced number of lesions | 272 |
| Type 1 diabetes | |||
| TOL-3021 in new onset disease (DAWN) | DNA-plasmid encoding proinsulin | Ongoing | NCT03794973 |
| TOL-3021 in established disease (DAY) | DNA-plasmid encoding proinsulin | Ongoing | NCT03794960 |
| Proinsulin peptide (MonoPepT1De) | Proinsulin | No adverse effects, reduced insulin use increase, FOXP3 and IL-10 induction | NCT01536431 |
| BHT-3021 | DNA-plasmid encoding proinsulin | No adverse effects, C-peptide level increased but no effect on insulin requirement | NCT00453375 |
| DPT-1 | Insulin, GAD65 | No effect on prevention of familial disease development | |
| Rheumatoid arthritis | |||
| Chicken type II collagen | Chicken type II collagen | Improvement of symptoms, albeit less than methotrexate-treated control group | ChiCTR-TRC-00000093 |
| Altered peptide ligand approaches | |||
| Multiple sclerosis | |||
| RTL1000 | TCR ligand | No adverse effects at <100 mg; no worsening of disease | NCT00411723 |
| NBI-5788 | Altered peptide ligand | Suspended after detection of increased brain lesions in some patients | NCT00079495 |
| CGP77116 | Altered peptide ligand | Aborted owing to exacerbation of disease in three patients | NCT00001781 |
| NBI-5788 | Altered peptide ligand | Hypersensitivity reactions, reduced lesion load | 200 |
| Type 1 diabetes | |||
| NBI-6024 | Altered peptide ligand | No effect compared with placebo | NCT00873561 |
| Antigen and adjuvant approaches | |||
| Multiple sclerosis | |||
| NeuroVax | TCR peptides in IFA | Ongoing | NCT02057159 |
| Type 1 diabetes | |||
| Diamyd (DIAGNODE-3) | rhGAD65–Alum+vitamin D HLA-DR3-DQ2+ haplotype | Ongoing | NCT05018585 |
| Diamyd booster | Booster Diamyd+vitamin D3 | Ongoing | NCT05351879 |
| MER3101: MAS-1 adjuvanted (MER3101) | β-chain + MER3101 administered into lymph nodes | Ongoing | NCT03624062 |
| Diamyd (DIAGNODE-2) | rhGAD65–alum+vitamin D3 administered into lymph nodes | No significant improvement in overall groups but increased glycaemic control in HLA-DR3-DQ+ subjects | NCT03345004 |
| Diamyd (DIAGNODE-1) | rhGAD65–alum+vitamin D | No adverse effects; TH2 cell induction; TH1 cell reduction; C-peptide baseline increased | NCT02352974 |
| Islet β-chain | IFA–β-chain emulsion | Insulin-specific antibody and T cell induction; induction of long-lasting antigen-specific Treg cells, no HBA1C or insulin use changes | NCT00057499 |
| Diamyd in newly diagnosed disease (DIAPREVENT) | rhGAD65–alum | No change in C-peptide levels | NCT00751842 |
| Diamyd in newly diagnosed disease (DIAPREVENT) | GAD–alum | No adverse effects; no difference in insulin secretion | NCT00529399 |
| Nanoparticle-based approaches | |||
| Multiple sclerosis | |||
| Xemys | CD206-targeted liposomes with three MBP peptides | Cytokine normalization | #930 [FASEMS-01/01] |
| Rheumatoid arthritis | |||
| DEN-181 | Liposomes with collagen peptide + NF-κB inhibitor | Reduced effector T cells, increased Treg cells | 176 |
| AG4263 | Iron particles coated with peptide–MHC class II-bound antigen | No long-lasting effects | 273 |
| Coeliac disease | |||
| TIMP-GLIA | PLGA nanoparticles with gliadin TAK-101 | Reduction of IFNγ-producing cells on challenges | NCT03738475 |
AQP4, aquaporin 4; DC, dendritic cell; EDSS, expanded disability status scale; GAD, glutamic acid decarboxylase; GVHD, graft-versus-host disease; GM-CSF, granulocyte-monocyte colony-stimulating factor; IFA, incomplete Freunds adjuvant; IFNβ, interferon-β; IFNγ, interferon-γ; MBP, myelin basic protein; moDC, monocyte-derived dendritic cell; MOG, myelin oligodendrocyte glycoprotein; NF-κB, nuclear factor-κB; ODNs, oligodeoxynucleotides; PAD4, peptidylarginine deiminase 4; PLGA, poly(lactic-co-glycolic acid); PLP, proteolipid protein; TCR, T cell receptor; Treg cell, regulatory T cell.
Alternatively, lymphocytes and red blood cells coupled with antigens ex vivo have been used to induce antigen-specific tolerance132,133. This approach is thought to induce tolerance as a result of the apoptosis of the antigen-coupled cells and their subsequent uptake by APCs, which acquire a tolerogenic phenotype following apoptotic cell uptake134. For example, in a study by Watkins et al. antigen-conjugated erythrocytes were taken up by BATF3+ cDC1s, inducing antigen-specific T cell dysfunction via PD1, CTLA4, LAG3 and TOX expression135. Building on these findings, Raposo et al. developed a microfluidic loading technique to produce antigen-loaded erythrocytes, which reduce effector T cell trafficking into target organs136. In addition, antigen-loaded erythrocytes induced bystander tolerance136, inhibiting effector T cell responses against the antigen loaded in erythrocytes and also other antigens expressed in the same tissue. Bystander tolerance induction is critical to the success of antigen-specific immunotherapies because multiple antigens, many of them unknown, are targeted in most autoimmune disorders and different antigens may be targeted in different patients.
Because of their ability to traffic to inflamed tissues, suppress pathogenic T cells and promote tissue repair39, multiple tolerance-inducing approaches rely on FOXP3+ Treg cells or TR1 cells. Indeed, more than 25 clinical trials have tested Treg cell-based therapies in T1D, systemic lupus erythematosus, Crohn’s disease, organ transplantation and GVHD120,137-140 (Table 2). These therapies usually involve autologous polyclonal Treg cells isolated from peripheral blood and expanded ex vivo in the presence of IL-2 (ref. 141). Treg cell therapies are well tolerated and Treg cells are stable in vivo. Indeed, in one clinical trial, 25% of ex vivo-expanded autologous polyclonal Treg cells could still be detected 1 year after transfer into patients, pointing to a surprisingly long half-life for these cells141. However, although several studies provide early indications of clinical efficacy of Treg cell therapies in phase I and I/II trials, larger clinical trials are still needed140. Moreover, concerns regarding nonspecific immunosuppression led to the development of antigen-specific Treg cell therapies.
A further development of Treg cell-based approaches has been the engineering of Treg cells with chimeric antigen receptors (CARs). CAR Treg cells were reported to ameliorate GVHD142 and other immune-mediated disorders143, and myelin oligodendrocyte glycoprotein (MOG)-targeting CAR Treg cells homed to the CNS in a mouse model of MS144. Treg cells engineered to target pro-inflammatory molecules such as TNF recently showed promising results in a mouse model of GVHD and may be useful when the pathology-driving antigens are not well known or where many antigens are targeted145. Similarly, CAR Treg cells targeting B cells suppress antibody responses in a mouse model of haemophilia A146, pointing to the versatility of engineered T cell therapies. Importantly, CAR Treg cells have been shown to remain tolerogenic in highly pro-inflammatory environments, alleviating concerns about their potential conversion into pathogenic effector T cells147. CAR Treg cells were also shown to induce bystander tolerance147.
The widespread use of cell-based strategies to induce antigen-specific tolerance faces important challenges, particularly related to their patient-specific production in a clinical setup. Strategies based on gene-edited stem cells may overcome some of these challenges by enabling the production of off-the-shelf universal cell lines for tolerance induction in multiple individuals.
Synthetic particle-based delivery systems
An exciting approach for antigen-specific immunomodulation is the use of nanoparticles. Nanoparticles offer an attractive platform for antigen-specific tolerance induction as they do not rely on patient-derived cells, are made with safe biodegradable materials and can be produced at large scale with little batch-to-batch variation. In addition, nanoparticles can be targeted to specific cells of interest and deliver multiple cargos, while improving small-molecule and antigen solubility and bioavailability. Numerous types of nanoparticles have been used for immunomodulation, including metallic, polymeric, lipid-based and peptide–polymer particles, each with its own advantages and limitations (Fig. 2).
Metallic nanoparticles, including gold, silver and iron oxide particles, have been used for simultaneous diagnostic and therapeutic purposes, for example as contrast-enhancing agents and for the delivery of surface-conjugated cargo148. Interestingly, iron oxide nanoparticles conjugated to MHC class II-bound peptides induce TR1 cells, which in turn induce regulatory B cells and limit inflammation in numerous preclinical mouse models149. In this case, TR1 cell induction depends on the high density of MHC molecules in the nanoparticles, which induces TCR microclusters devoid of co-stimulatory molecules on antigen-specific CD4+ T cells149,150. In addition, regulatory B and T cells in the liver induce immunosuppressive neutrophils, limiting liver autoimmunity and fibrosis151. Metallic nanoparticles can be modified to improve their performance, but the resulting particles may be unstable. Indeed, surface conjugation can make metallic nanoparticles prone to aggregation during production, limiting the type of loadable cargo and interfering with scale-up efforts152. In addition, metal particles are not easily biodegradable and their accumulation in tissues may trigger adverse effects.
Conversely, polymeric particles made from carbohydrate acids, such as poly(lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) nanoparticles, are easily modifiable, relatively simple to manufacture and quickly degraded, although some by-products induce adverse effects153. Polymeric particles delivering disease-specific antigens showed therapeutic effects in preclinical autoimmune disease models of MS, rheumatoid arthritis (RA) and T1D mediated by the induction of CTLA4+PD1+ Treg cells, the reduction of effector T cells and decreased expression of IL-12, microRNA-155 and vascular endothelial growth factor154-158. Moreover, phase I and phase IIa clinical trials in coeliac disease showed that PLGA particles encapsulating a gliadin antigen were well tolerated and reduced gliadin-specific IFNγ production and effector memory T cells159. However, additional trials are needed to fully evaluate their therapeutic effects. Of note, PLGA particles have shown context-specific anti-inflammatory and pro-inflammatory effects independent of their cargo. Indeed, one of the primary degradation products of PLA and PLGA particles is l-lactate, which inhibits DC maturation and pro-inflammatory responses via HIF1α activation and NF-κB inhibition115,160. Conversely, PLGA particles can activate the NBD, LRR and pyrin domain-containing protein 3 (NLRP3) inflammasome in DCs161 and polarize macrophages towards a pro-inflammatory phenotype162. PLGA particles are also reported to induce effector CD8+ T cell activation and IFNγ production163 and also act as TH2 cell adjuvants164.
Lipid-based nanoparticles are widely used in cosmetics165, as well as US Food and Drug Administration (FDA)-approved cancer treatments166 and mRNA coronavirus vaccines167,168. Depending on the production method and formulation physicochemical properties, lipid nanoparticles can be classified into various categories, including liposomes, lipid nanoparticles and cubosomes. Owing to the amphipathic nature of fatty acids, lipid nanoparticles can carry hydrophobic molecules intercalated in the membrane and hydrophilic substances in an aqueous core or conjugated to the surface. Furthermore, lipids can be engineered to be easily degraded169. Moreover, the incorporation of lipids such as dioleoylphosphatidylethanolamine or cholesterol can modulate the fusogenic properties of nanoliposomes to improve endosomal drug release170. Indeed, intracellular cholesterol accumulation can induce DC tolerance via liver X receptor activation171. Lipid nanoparticles have been successfully used to deliver autoantigens, with therapeutic effects in numerous preclinical models of T1D, MS, RA and myasthenia gravis linked to the induction of tolerogenic DCs, Treg cell expansion and suppression of pathogenic effector T cells172-175. Moreover, in a phase Ib clinical trial in patients with RA, liposomes co-encapsulating a collagen peptide and an NF-κB inhibitor were well tolerated, inducing an increase in circulating collagen-specific PD1+ T cells and a decrease in disease activity176.
Protein-based nanoparticles offer a biodegradable, nontoxic and stable delivery platform but are rarely used for antigen-specific tolerance induction because of their highly immunogenic nature associated with their structural similarities to virus particles177.
The physicochemical characteristics of nanoparticles, including size, charge, structure, hydrophobicity and rigidity influence their immunomodulatory effects and can be modified to alter nanoparticle circulation, cell targeting and uptake, and immunomodulatory function to maximize therapeutic activity153,178,179. In general, nanoparticle surface charge is an important determinant of cellular uptake and immunomodulation. Nanoparticles with a negative surface charge have been proposed to mimic tolerogenic apoptotic cells180,181 and be preferentially taken up by phagocytic cells via scavenger receptors such as MARCO in macrophages182. Conversely, positively charged nanoparticles are thought to interact directly with negatively charged cell membranes and thus be taken up more rapidly by a wider variety of cell types183, although this property is also linked to an increased potential to disrupt lipid bilayers and cause cytotoxicity184. Positively charged nanoparticles can also promote inflammation via CD80 and CD86 upregulation and the production of reactive oxygen species185,186. However, widespread consensus about the effects of particle charge on uptake, toxicity and inflammation is still lacking.
Particle size also influences particle biodistribution, targeting, uptake and toxicity. In general, particles of <200 nm are taken up by DCs and >500 nm by macrophages187,188. Indeed, it was suggested that the size of antigens can dictate immune responses, promoting TH1, TH2 or Treg cell induction189. Moreover, particle size and rigidity affect the immune response, skewing DCs and macrophages towards pro-inflammatory or anti-inflammatory phenotypes190,191. Polyethylene glycol is commonly used as a shielding agent to reduce interactions with serum proteins, decrease uptake by the reticuloendothelial system and increase circulation time and bioavailability. The attachment of polyethylene glycol chains to a protein may also be critical for subcutaneous uptake, reducing complement activation and granulocyte recruitment192. Finally, it is important to consider that manufacturing processes used in basic research often differ from those used in FDA-approved therapies. Consequently, charge, size and other features may be altered during nanoparticle production scale up for clinical testing, affecting immunomodulatory activity.
Targeting of specific cell types
Most untargeted nanoparticles are taken up by DCs and macrophages via scavenger receptors and complement factor binding. This passive targeting of DCs generally results in the presentation of nanoparticle-delivered antigens on MHC class II molecules193. CD4+ T cell recognition of MHC class II–presented antigens in the absence of co-stimulatory molecules induces clonal T cell deletion and inhibition via PD-L1 and induction of FOXP3+ and IL-10+ Treg cells182,194.
Nanoparticles can also be targeted to specific cell types using antibodies or other molecules reactive with specific cell populations (Table 1). For example, mannosylated antigens target the mannose receptor in DCs, inducing IL-10 production and antigen-specific tolerance195,196. Mannosylated liposomes encapsulating myelin peptide antigens reduced pro-inflammatory cytokines in blood in a phase I clinical trial in patients with MS197, but their therapeutic value is still unknown.
An alternative approach is to target nanoparticles based on the antigen specificity of immune receptors in the cells they aim to modulate. For example, metallic nanoparticles displaying peptides loaded in recombinant MHC class I molecules in the absence of signals 2 and 3 induce antigen-specific CD8+ effector T cell anergy and a memory-like regulatory phenotype, which inhibits DCs via IFNγ, IDO and perforin198. Thus, targeting nanoparticles to specific immune cells, defined by their surface molecule expression or antigenic reactivity, is an attractive approach for targeted immunotherapy. However, the incorporation of additional components to the therapeutic nanoparticles (for example, surface antibodies) may interfere with their manufacturing.
Introducing immunosuppressive agents into nanoparticles
A major risk of immunomodulation is the potential exacerbation of pathogenic immune responses. Indeed, adverse effects ranging from local reactions to anaphylactic shock and lethality have been documented while testing immunomodulatory approaches199; clinical trials have been interrupted because of the induction of hypersensitivity reactions200 and autoimmune disease relapses201. These adverse reactions suggest that safe antigen-specific immunomodulation requires the activation of tolerogenic pathways. This concept is exemplified by a recent report on the evaluation of antigen–MHC class II complexes, which triggered inflammation in one-third of treated mice; this pro-inflammatory effect was abrogated by attaching dexamethasone to the antigen–MHC class II complex at doses 200-fold lower than those used in dexamethasone-alone treatment schemes193. Interestingly, self-antigen administration using nanoparticles and nanoliposomes does not seem to trigger or boost pro-inflammatory responses102-104,202, suggesting that intrinsic properties make some platforms safer for clinical use. However, therapeutic tolerance induction in the clinic will probably require the activation of anti-inflammatory pathways to improve both safety and efficacy.
One of the first attempts to combine autoantigens and immunomodulatory drugs used liposomes to co-deliver an antigen and an NF-κB inhibitor, ameliorating experimental arthritis in a FOXP3+ Treg cell-dependent manner203. Similarly, based on the role of AHR in the suppression of NF-κB signalling and the control of adaptive and innate immunity204, nanoparticles engineered to co-deliver the AHR agonist 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) with disease-relevant antigens re-established antigen-specific tolerance in preclinical models of MS and T1D102-104. Other immunomodulatory agents co-encapsulated with antigens include IL-10 (ref. 205), vitamin D3 (ref. 206) and the mTOR inhibitor rapamycin202,207-209, with encouraging results in experimental autoimmune encephalomyelitis, allergy and the suppression of antidrug antibodies. Indeed, the co-administration of a disease-relevant antigen with multiple immunomodulators (vitamin D3, GM-CSF or TGFβ) in T1D, RA and MS models showed significant therapeutic effects linked to the induction of IL-10 and PD1, as well as of regulatory T and B cells210-212.
Human autoimmune diseases usually target multiple autoantigens, which may differ between patients and disease stages, posing a significant challenge to immunomodulatory interventions targeting one or a few antigens or epitopes. However, approaches based on the co-delivery of self-antigens and immunomodulatory agents are reported to induce bystander suppression. Nanoparticle-based co-delivery of antigen and ITE induced bystander tolerance via the induction of FOXP3+ and IL-10+ Treg cells that migrate to the site of inflammation, also suppressing pathology driven by local innate immune responses104. Similarly, lipid-coated calcium phosphate nanoparticles loaded with citrullinated autoantigen and rapamycin induced bystander tolerance in an RA model213, and liposomal co-delivery of vitamin D3 and autoantigen induced bystander tolerance in a T1D model214. Collectively, these findings suggest that the co-administration of immunomodulatory molecules with self-antigens is needed not only to boost the therapeutic activity of antigen-specific tolerogenic approaches but also to prevent the unwanted exacerbation of autoimmune pathology particularly associated with some therapeutic modalities.
Nucleic acid–based and viral particle–based approaches to antigen-specific immunotherapy
Nucleic acid–based approaches, including those based on DNA and mRNA, are attractive methods for antigen-specific immunomodulation. These methods offer several advantages over peptide-based or protein-based approaches including the ease of manufacturing and cargo alteration (both antigen and immunomodulator) and the fact that the encoded antigens can be posttranslationally modified in the host and have relatively low production costs215.
Viral particles provide an effective platform for antigen delivery216. Viral particles are used as gene therapy vectors and have been used to deliver autoantigen to the liver217 and thymus218, inducing antigen-specific Treg cell expansion, effector T cell suppression and bystander tolerance epitopes219. In response to safety concerns, plant virus particles have also been tested in preclinical models of T1D and RA220. However, risks linked to viral gene therapy, pre-existing antibodies against adeno-associated viruses and the induction of antivector antibodies by repeated treatment limit the utility of virus-based approaches for antigen-specific immunomodulation.
Nucleic acid vaccines circumvent some of the risks linked to viral-based approaches. In pioneering work, Waisman et al. used a plasmid encoding the TCR from a pathogenic T cell clone, depleting TCR-specific pathogenic CD4+ T cells and ameliorating disease in a mouse model of MS221. Similar encouraging results were obtained with vaccines encoding other antigens in preclinical models of systemic lupus erythematosus, T1D and RA222-225. Following these initial findings, DNA vaccines encoding disease-associated antigens were tested in MS and T1D clinical trials226-228. An important feature of the DNA vectors used for tolerance induction was the removal of TLR9-activating CpG motifs in the plasmid to minimize the activation of innate immunity. Despite showing reductions in disease-associated biomarkers and evidence of some bystander tolerance, these trials did not meet clinical end points. Thus, although DNA vaccines represent a promising approach and additional clinical trials are ongoing (Table 2), further developments may be needed for the success of this approach, including the co-administration of plasmids encoding tolerogenic immunomodulators229. It is also possible that the intrinsic immunostimulatory properties of plasmid DNA in combination with the limited control over its half-life, biodistribution and uptake impose unsurmountable challenges for the clinical use of antigen-encoding DNA vaccines for immunomodulation.
mRNA is less stable than DNA, requiring appropriate delivery platforms and modifications to prevent the activation of innate immunity230. Nanoliposomes provide a unique platform for controlled mRNA delivery. In addition, unlike peptide-based vaccines, nanoliposome mRNA vaccines do not need to be extensively optimized to accommodate each nucleic acid–encoded antigen. Moreover, mRNA is quickly degraded in vivo, diminishing concerns about long-term detrimental effects and tumorigenesis previously linked to some DNA-based approaches. Furthermore, mRNA vaccines offer a safer alternative for the treatment of patients who are immunosuppressed than attenuated viral or bacterial vaccines231.
mRNA is a potent pro-inflammatory adjuvant because of its ability to activate innate immunity via TLR3, TLR7 and other immune receptors involved in sensing viral infection232. Consequently, vaccination with mRNA-encoded epitopes induces potent antigen-specific CD4+ and CD8+ effector T cells233. mRNA vaccines have been developed to induce protective immunity against pathogens such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)167,168. Similar exciting results have been described in the context of cancer immunotherapy234,235.
Eukaryotic RNA is heavily edited, facilitating the discrimination between self and microbial mRNAs. Thus, RNA modification has been actively pursued to minimize the activation of innate immunity and develop tolerogenic vaccines236. For example, nanoliposome-delivered mRNA vaccines using pseudo-UTP and encoding the myelin autoantigen MOG suppressed disease development in MS models, inducing bystander tolerance against additional myelin antigens237. Mechanistically, these therapeutic effects were linked to the PD1- and CTLA4-dependent induction of antigen-specific Treg cells237. Of note, mRNA has also been used to transfect T cells with autoantigen-specific CARs, with promising effects in suppressing pathogenic CD4+ and CD8+ effector T cells in the non-obese-diabetes mouse model238,239. Together, these findings suggest that vaccines containing mRNA-encoded antigens may provide efficacious platforms for the treatment of inflammatory disorders.
Conclusions, challenges and outlook
The induction of antigen-specific immune tolerance is considered the “holy grail” of disease management for autoimmunity and organ transplantation. Decades of research have resulted in numerous promising advances. Yet despite the encouraging preclinical results, no truly antigen-specific immunotherapies are currently approved for the treatment of autoimmune diseases or organ transplantation, and few approaches have been tested beyond phase I or II clinical trials.
One important challenge is our limited understanding of the breadth of immune targets recognized in autoimmune diseases. Indeed, antigen targets may vary from a single autoantigen in Graves disease240 to multiple antigens in RA and systemic lupus erythematosus241. Epitope spreading remains a significant challenge, suggesting that successful antigen-specific immunotherapy must either halt epitope spreading, incorporate a method for the repeated unbiased evaluation of the specificity of the autoimmune response and/or induce bystander tolerance. In addition, it should be kept in mind that most studies of the therapeutic induction of antigen-specific tolerance assume that the modulation of T cell-mediated autoimmunity results in a concomitant decrease in pathogenic B cell responses. However, it is not clear whether the magnitude, breadth and kinetics of this indirect B cell modulation are enough to result in significant clinical improvement of B cell-driven pathology. Moreover, patient-to-patient variability, stage-specific autoimmune responses and HLA allelic diversity further complicate the design of antigen-specific therapies. Still, significant advances have been made in immune repertoire analysis, including the development of antigen microarrays241,242, high-throughput BCR and TCR sequencing243,244, multiplexed monitoring with barcoded tetramers245 and bioinformatic approaches for epitope prediction246. These methods may enable not only the identification of candidate antigens for the induction of antigen-specific tolerance but also the monitoring of response to therapy, providing personalized approaches like those being developed for cancer immunotherapy234.
An additional challenge is that often immunotherapeutic interventions for autoimmune diseases are initiated after years of subclinical and clinical disease, resulting in the accumulation of tissue damage, immunological memory and the triggering of local mechanisms of inflammation and disease pathology. Thus, although developments in this area have been made for some diseases, such as T1D247, the identification of effective biomarkers for patient identification and stratification remains an important need for the development of antigen-specific immunotherapy. Indeed, these limitations highlight the challenges of translating exciting findings in preclinical models into efficacious therapies for human diseases. In this context, the selection of autoimmune diseases in which to test antigen-specific immunomodulatory approaches remains critical. Coeliac disease, for example, offers unique opportunities for clinical trial design, as patients on a gluten-free diet may receive experimental antigen-specific immunotherapies before dietary challenge.
Finally, how can we identify target signalling pathways to increase the therapeutic activity of immunomodulatory approaches while preventing adverse events? Novel platforms may guide the identification of candidate signalling pathways for the therapeutic induction of tolerance, including the use of new methods to study cell–cell interactions involved in the regulation of inflammation248-250, CRISPR-based platforms to study immune regulation in vivo251 and the use of experimental systems such as zebrafish in combination with artificial intelligence252. These approaches have already identified novel immunoregulatory mechanisms with therapeutic potential. In addition, recently identified populations of tolerogenic APCs may offer additional targets for immune tolerance induction253-255. Provided these important challenges are addressed, recent advances in methods for the induction of antigen-specific immune tolerance, combined with novel methods for the identification of target antigens and regulatory pathways, will probably guide the development of platforms for personalized antigen-specific immunomodulation in autoimmune diseases, allergy, transplantation and gene therapy.
Acknowledgements
Research in the Quintana lab was supported by grants NS102807, ES02530, ES029136, AI126880 from the NIH; RG4111A1 andJF2161-A-5 from the NMSS; and PA-1604-08459 from the International Progressive MS Alliance. During the writing of this article, J.E.K. was supported by a T32 Cancer Neuroscience training grant (T32CA27386); N.A.S. was supported by funding from the Boehringer Ingelheim Fonds.
Footnotes
Competing interests
F.J.Q. is the Scientific Founder of AnTolRx, a company developing novel therapies for inflammatory disorders. The other authors have no competing interests.
References
- 1.Conrad N. et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet 401, 1878–1890 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Ramsdell F, Lantz T & Fowlkes BJ A nondeletional mechanism of thymic self tolerance. Science 246, 1038–1041 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Owen DL, Sjaastad LE & Farrar MA Regulatory T cell development in the thymus. J. Immunol 203, 2031–2041 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson MS et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Takaba H. et al. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell 163, 975–987 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Michelson DA, Hase K, Kaisho T, Benoist C & Mathis D Thymic epithelial cells co-opt lineage-defining transcription factors to eliminate autoreactive T cells. Cell 185, 2542–2558 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry JSA et al. Transfer of cell-surface antigens by scavenger receptor CD36 promotes thymic regulatory T cell receptor repertoire development and allo-tolerance. Immunity 48, 1271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zegarra-Ruiz DF et al. Thymic development of gut-microbiota-specific T cells. Nature 594, 413–417 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardemann H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Halverson R, Torres RM & Pelanda R Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol 5, 645–650 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Nemazee DA & Bürki K Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337, 562–566 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Bouneaud C, Kourilsky P & Bousso P Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity 13, 829–840 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Chen L & Flies DB Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol 13, 227–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sckisel GD et al. Out-of-sequence signal 3 paralyzes primary CD4+ T-cell-dependent immunity. Immunity 43, 240–250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trefzer A. et al. Dynamic adoption of anergy by antigen-exhausted CD4+ T cells. Cell Rep. 34, 108748 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Groux H, Bigler M, de Vries JE & Roncarolo MG Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med 184, 19–29 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK & Sharpe AH CTLA-4 regulates induction of anergy in vivo. Immunity 14, 145–155 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Goodnow CC et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334, 676–682 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Bevington SL et al. Chromatin priming renders T cell tolerance-associated genes sensitive to activation below the signaling threshold for immune response genes. Cell Rep. 31, 107748 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauld SB, Benschop RJ, Merrell KT & Cambier JC Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat. Immunol 6, 1160–1167 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Kalekar LA et al. CD4+ T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol 17, 304–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong S-W et al. Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction. Nature 607, 762–768 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davey GM et al. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J. Exp. Med 196, 947–955 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouillet P. et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415, 922–926 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Dhein J, Walczak H, Bäumler C, Debatin KM & Krammer PH Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373, 438–441 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Tartaglia LA, Ayres TM, Wong GH & Goeddel DV A novel domain within the 55 kd TNF receptor signals cell death. Cell 74, 845–853 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Sanmarco LM et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 590, 473–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Kang R, Kroemer G & Tang D Ferroptosis in infection, inflammation, and immunity. J. Exp. Med 218, e20210518 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalkavan H, Rühl S, Shaw JJP & Green DR Non-lethal outcomes of engaging regulated cell death pathways in cancer. Nat. Cancer 4, 795–806 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legrand AJ, Konstantinou M, Goode EF & Meier P The diversification of cell death and immunity: memento mori. Mol. Cell 76, 232–242 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Redmond WL, Marincek BC & Sherman LA Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J. Immunol 174, 2046–2053 (2005). [DOI] [PubMed] [Google Scholar]
- 32.ElTanbouly MA et al. VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science 367, eaay0524 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson AC, Joller N & Kuchroo VK Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharpe AH & Pauken KE The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol 18, 153–167 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Verbinnen B, Tang X, Lu L & Cantor H Inhibition of follicular T-helper cells by CD8+ regulatory T cells is essential for self tolerance. Nature 467, 328–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dart RJ et al. Conserved γδ T cell selection by BTNL proteins limits progression of human inflammatory bowel disease. Science 381, eadh0301 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto K, Miyake S & Yamamura T A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Malchow S. et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44, 1102–1113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arpaia N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun CM et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med 204, 1775–1785 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali N. et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burzyn D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasanthakumar A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol 16, 276–285 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Li C. et al. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive fat-Treg phenotype. Cell 174, 285–299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohnmacht C. et al. The microbiota regulates type 2 immunity through RORgammat+ T cells. Science 349, 989–993 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Hadis U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Munoz-Rojas AR & Mathis D Tissue regulatory T cells: regulatory chameleons. Nat. Rev. Immunol 21, 597–611 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown CC & Rudensky AY Spatiotemporal regulation of peripheral T cell tolerance. Science 380, 472–478 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Groux H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Awasthi A. et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol 8, 1380–1389 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Levings MK et al. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol 166, 5530–5539 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Bollyky PL et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc. Natl Acad. Sci. USA 108, 7938–7943 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akbari O. et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med 8, 1024–1032 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Wakkach A, Cottrez F & Groux H Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J. Immunol 167, 3107–3113 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Sutavani RV et al. CD55 costimulation induces differentiation of a discrete T regulatory type 1 cell population with a stable phenotype. J. Immunol 191, 5895–5903 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Rothhammer V & Quintana FJ The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol 19, 184–197 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Magnani CF et al. Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur. J. Immunol 41, 1652–1662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M & Gagliani N The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity 49, 1004–1019 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Mascanfroni ID et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med 21, 638–646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson DA III, Dutertre CA, Ginhoux F & Murphy KM Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol 21, 101–115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M & Reis e Sousa C Dendritic cells revisited. Annu. Rev. Immunol 39, 131–166 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM & Muller WA Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Bosteels C. et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 52, 1039–1056 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villani A-C et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown CC et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 179, 846–863 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun T, Nguyen A & Gommerman JL Dendritic cell subsets in intestinal immunity and inflammation. J. Immunol 204, 1075–1083 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Reizis B. Plasmacytoid dendritic cells: development, regulation, and function. Immunity 50, 37–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cella M. et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med 5, 919–923 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Alculumbre SG et al. Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat. Immunol 19, 63–75 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Ito T. et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med 204, 105–115 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diana J. et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J. Exp. Med 208, 729–745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munn DH et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest 114, 280–290 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian Y. et al. Graft-versus-host disease depletes plasmacytoid dendritic cell progenitors to impair tolerance induction. J. Clin. Invest 131, e136774 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uto T. et al. Critical role of plasmacytoid dendritic cells in induction of oral tolerance. J. Allergy Clin. Immunol 141, 2156–2167 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Granot T. et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 46, 504–515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mrdjen D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Mundt S. et al. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol 4, eaau8380 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Gallizioli M. et al. Dendritic cells and microglia have non-redundant functions in the inflamed brain with protective effects of type 1 cDCs. Cell Rep. 33, 108291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jongbloed SL et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med 207, 1247–1260 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hubert M. et al. IFN-III is selectively produced by cDC1 and predicts good clinical outcome in breast cancer. Sci. Immunol 5, eaav3942 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Liu H. et al. TLR5 mediates CD172α+ intestinal lamina propria dendritic cell induction of Th17 cells. Sci. Rep 6, 22040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott CL et al. CCR2+CD103− intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol. 8, 327–339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joeris T. et al. Intestinal cDC1 drive cross-tolerance to epithelial-derived antigen via induction of FoxP3+CD8+ Tregs. Sci. Immunol 6, eabd3774 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Akbari O, DeKruyff RH & Umetsu DT Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol 2, 725–731 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Steinman RM et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann. N. Y. Acad. Sci 987, 15–25 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Lutz MB & Schuler G Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 23, 445–449 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Ardouin L. et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity 45, 305–318 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Lutz MB, Backer RA & Clausen BE Revisiting current concepts on the tolerogenicity of steady-state dendritic cell subsets and their maturation stages. J. Immunol 206, 1681–1689 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Baratin M. et al. Homeostatic NF-kappaB signaling in steady-state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity 42, 627–639 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Jiang A. et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 27, 610–624 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kushwah R. et al. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur. J. Immunol 40, 1022–1035 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iberg CA & Hawiger D Natural and induced tolerogenic dendritic cells. J. Immunol 204, 733–744 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gregori S. et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 116, 935–944 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Coombes JL et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med 204, 1757–1764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esterhazy D. et al. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat. Immunol 17, 545–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steinbrink K, Wölfl M, Jonuleit H, Knop J & Enk AH Induction of tolerance by IL-10-treated dendritic cells. J. Immunol 159, 4772–4780 (1997). [PubMed] [Google Scholar]
- 97.Steinbrink K, Graulich E, Kubsch S, Knop J & Enk AH CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood 99, 2468–2476 (2002). [DOI] [PubMed] [Google Scholar]
- 98.Avancini D. et al. Aryl hydrocarbon receptor activity downstream of IL-10 signaling is required to promote regulatory functions in human dendritic cells. Cell Rep. 42, 112193 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nguyen NT et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl Acad. Sci. USA 107, 19961–19966 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Q, Harden JL, Anderson CD & Egilmez NK Tolerogenic phenotype of IFN-γ-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop. J. Immunol 197, 962–970 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Hauben E. et al. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood 112, 1214–1222 (2008). [DOI] [PubMed] [Google Scholar]
- 102. Yeste A, Nadeau M, Burns EJ, Weiner HL & Quintana FJ Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109, 11270–11275 (2012). This work describes the co-administration of an antigen with a tolerogenic small molecule using nanoparticles to induce antigen-specfic tolerance.
- 103.Yeste A. et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci. Signal 9, ra61 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Kenison JE et al. Tolerogenic nanoparticles suppress central nervous system inflammation. Proc. Natl Acad. Sci. USA 117, 32017–32028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quintana FJ et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 107, 20768–20773 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramalingam R. et al. Dendritic cell-specific disruption of TGF-β receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J. Immunol 189, 3878–3893 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mascanfroni ID et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol 14, 1054–1063 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo Y et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc. Natl Acad. Sci. USA 111, 15178–15183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lutz MB et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur. J. Immunol 30, 1813–1822 (2000). [DOI] [PubMed] [Google Scholar]
- 110.Guindi C. et al. Differential role of NF-kappaB, ERK1/2 and AP-1 in modulating the immunoregulatory functions of bone marrow-derived dendritic cells from NOD mice. Cell Immunol. 272, 259–268 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Mazmanian SK, Round JL & Kasper DL A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Mucida D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Ferreira GB et al. Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. Cell Rep. 10, 711–725 (2015). [DOI] [PubMed] [Google Scholar]
- 114.Anderson AE et al. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J. Leukoc. Biol 84, 124–133 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sanmarco LM et al. Lactate limits CNS autoimmunity by stabilizing HIF-1alpha in dendritic cells. Nature 620, 881–889 (2023). This work describes the engineering of bacteria to activate tolerogenic programmes in intestinal DCs and control CNS autoimmunity.
- 116.Shinde R. et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat. Immunol 19, 571–582 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pujol-Autonell I. et al. Efferocytosis promotes suppressive effects on dendritic cells through prostaglandin E2 production in the context of autoimmunity. PLoS ONE 8, e63296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wermeling F. et al. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J. Exp. Med 204, 2259–2265 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hill M. et al. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and Epstein-Barr virus-induced gene 3. Am. J. Transpl 11, 2036–2045 (2011). [DOI] [PubMed] [Google Scholar]
- 120.Sawitzki B. et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 395, 1627–1639 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moreau A. et al. A Phase I/IIa study of autologous tolerogenic dendritic cells immunotherapy in kidney transplant recipients. Kidney Int. 103, 627–637 (2023). [DOI] [PubMed] [Google Scholar]
- 122.Passeri L. et al. Tolerogenic IL-10-engineered dendritic cell-based therapy to restore antigen-specific tolerance in T cell mediated diseases. J. Autoimmun 138, 103051 (2023). [DOI] [PubMed] [Google Scholar]
- 123.Nikolic T. et al. Safety and feasibility of intradermal injection with tolerogenic dendritic cells pulsed with proinsulin peptide-for type 1 diabetes. Lancet Diabetes Endocrinol. 8, 470–472 (2020). [DOI] [PubMed] [Google Scholar]
- 124.Nikolic T. et al. Tolerogenic dendritic cells pulsed with islet antigen induce long-term reduction in T-cell autoreactivity in type 1 diabetes patients. Front. Immunol 13, 1054968 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Willekens B. et al. Tolerogenic dendritic cell-based treatment for multiple sclerosis (MS): a harmonised study protocol for two phase I clinical trials comparing intradermal and intranodal cell administration. BMJ Open. 9, e030309 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zahorchak AF et al. Infusion of stably immature monocyte-derived dendritic cells plus CTLA4Ig modulates alloimmune reactivity in rhesus macaques. Transplantation 84, 196–206 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Falcon-Beas C. et al. Dexamethasone turns tumor antigen-presenting cells into tolerogenic dendritic cells with T cell inhibitory functions. Immunobiology 224, 697–705 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Mainali ES, Kikuchi T & Tew JG Dexamethasone inhibits maturation and alters function of monocyte-derived dendritic cells from cord blood. Pediatr. Res 58, 125–131 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Kurochkina Y et al. SAT0212 The safety and tolerability of intra-articular injection of tolerogenic dendritic cells in patients with rheumatoid arthritis: the preliminary results. Ann. Rheum. Dis 77, 966–967 (2018).29588276 [Google Scholar]
- 130.Florez-Grau G, Zubizarreta I, Cabezon R, Villoslada P & Benitez-Ribas D Tolerogenic dendritic cells as a promising antigen-specific therapy in the treatment of multiple sclerosis and neuromyelitis optica from preclinical to clinical trials. Front. Immunol 9, 1169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jauregui-Amezaga A. et al. Intraperitoneal administration of autologous tolerogenic dendritic cells for refractory Crohn’s disease: a phase I study. J. Crohns Colitis 9, 1071–1078 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Follett DA, Battisto JR & Bloom BR Tolerance to a defined chemical hapten produced in adult guinea-pigs after thymectomy. Immunology 11, 73–76 (1966). [PMC free article] [PubMed] [Google Scholar]
- 133. Miller SD, Wetzig RP & Claman HN The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J. Exp. Med 149, 758–773 (1979). This work describes the induction of immune tolerance after the administration of an antigen coupled to lymphocytes, putting forward an approach that was then mimicked with synthetic particle-based antigen delivery.
- 134.Gray M, Miles K, Salter D, Gray D & Savill J Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc. Natl Acad. Sci. USA 104, 14080–14085 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watkins EA et al. Persistent antigen exposure via the eryptotic pathway drives terminal T cell dysfunction. Sci. Immunol 6, eabe1801 (2021). [DOI] [PubMed] [Google Scholar]
- 136.Raposo CJ et al. Engineered RBCs encapsulating antigen induce multi-modal antigen-specific tolerance and protect against type 1 diabetes. Front. Immunol 13, 869669 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marek-Trzonkowska N. et al. Therapy of type 1 diabetes with CD4+CD25highCD127-regulatory T cells prolongs survival of pancreatic islets—results of one year follow-up. Clin. Immunol 153, 23–30 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Tang Q. et al. Selective decrease of donor-reactive T(regs) after liver transplantation limits T(reg) therapy for promoting allograft tolerance in humans. Sci. Transl Med 14, eabo2628 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Desreumaux P. et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143, 1207–1217 (2012). [DOI] [PubMed] [Google Scholar]
- 140.Bluestone JA, McKenzie BS, Beilke J & Ramsdell F Opportunities for Treg cell therapy for the treatment of human disease. Front. Immunol 14, 1166135 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bluestone JA et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl Med 7, 315ra189 (2015). This work describes the transfer of human Treg cells for the treatment of autoimmunity, paving the way to other cell-based approaches using expanded or CAR-based Treg cells.
- 142.Boardman DA et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transpl 17, 931–943 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Arjomandnejad M, Kopec AL & Keeler AM CAR-T regulatory (CAR-Treg) cells: engineering and applications. Biomedicines 10, 287 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fransson M. et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflammation 9, 112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bittner S. et al. Biosensors for inflammation as a strategy to engineer regulatory T cells for cell therapy. Proc. Natl Acad. Sci. USA 119, e2208436119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang AH, Yoon J, Kim YC & Scott DW Targeting antigen-specific B cells using antigen-expressing transduced regulatory T cells. J. Immunol 201, 1434–1441 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim YC et al. Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells. J. Autoimmun 92, 77–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Santra S, Kaittanis C, Grimm J & Perez JM Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small 5, 1862–1868 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Clemente-Casares X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Singha S. et al. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat. Nanotechnol 12, 701–710 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Umeshappa CS et al. Liver-specific T regulatory type-1 cells program local neutrophils to suppress hepatic autoimmunity via CRAMP. Cell Rep. 34, 108919 (2021). [DOI] [PubMed] [Google Scholar]
- 152.Chandrakala V, Aruna V & Angajala G Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 5, 1593–1615 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andorko JI, Hess KL, Pineault KG & Jewell CM Intrinsic immunogenicity of rapidly-degradable polymers evolves during degradation. Acta Biomater. 32, 24–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jamison BL et al. Nanoparticles containing an insulin-ChgA hybrid peptide protect from transfer of autoimmune diabetes by shifting the balance between effector T cells and regulatory T cells. J. Immunol 203, 48–57 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Prasad S. et al. Tolerogenic Ag-PLG nanoparticles induce Tregs to suppress activated diabetogenic CD4 and CD8 T cells. J. Autoimmun 89, 112–124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hunter Z. et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 8, 2148–2160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Casey LM et al. Nanoparticle dose and antigen loading attenuate antigen-specific T-cell responses. Biotechnol. Bioeng 120, 284–296 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hess KL et al. Engineering immunological tolerance using quantum dots to tune the density of self-antigen display. Adv. Funct. Mater 27, 1700290 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kelly CP et al. TAK-101 nanoparticles induce gluten-specific tolerance in celiac disease: a randomized, double-blind, placebo-controlled study. Gastroenterology 161, 66–80 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Allen RP, Bolandparvaz A, Ma JA, Manickam VA & Lewis JS Latent, immunosuppressive nature of poly(lactic-co-glycolic acid) microparticles. ACS Biomater. Sci. Eng 4, 900–918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sharp FA et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl Acad. Sci. USA 106, 870–875 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ma S. et al. The pro-inflammatory response of macrophages regulated by acid degradation products of poly(lactide-co-glycolide) nanoparticles. Eng. Life Sci 21, 709–720 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Min Y. et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol 12, 877–882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wilson KL et al. Biodegradable PLGA-b-PEG nanoparticles induce T helper 2 (Th2) immune responses and sustained antibody titers via TLR9 stimulation. Vaccines 8, 261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Puglia C & Bonina F Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert. Opin. Drug Deliv 9, 429–441 (2012). [DOI] [PubMed] [Google Scholar]
- 166.Orlowski RZ et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J. Clin. Oncol 25, 3892–3901 (2007). [DOI] [PubMed] [Google Scholar]
- 167.Jackson LA et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med 383, 1920–1931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mulligan MJ et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586, 589–593 (2020). [DOI] [PubMed] [Google Scholar]
- 169.Qiu M, Li Y, Bloomer H & Xu Q Developing biodegradable lipid nanoparticles for intracellular mRNA delivery and genome editing. Acc. Chem. Res 54, 4001–4011 (2021). [DOI] [PubMed] [Google Scholar]
- 170.Du Z, Munye MM, Tagalakis AD, Manunta MDI & Hart SL The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci. Rep 4, 7107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bosteels V. et al. LXR signaling controls homeostatic dendritic cell maturation. Sci. Immunol 8, eadd3955 (2023). [DOI] [PubMed] [Google Scholar]
- 172.Almenara-Fuentes L. et al. A new platform for autoimmune diseases. Inducing tolerance with liposomes encapsulating autoantigens. Nanomedicine 48, 102635 (2023). [DOI] [PubMed] [Google Scholar]
- 173.Benne N. et al. Anionic 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG) liposomes induce antigen-specific regulatory T cells and prevent atherosclerosis in mice. J. Control. Rel 291, 135–146 (2018). [DOI] [PubMed] [Google Scholar]
- 174.Pujol-Autonell I. et al. Liposome-based immunotherapy against autoimmune diseases: therapeutic effect on multiple sclerosis. Nanomedicine 12, 1231–1242 (2017). [DOI] [PubMed] [Google Scholar]
- 175.Pujol-Autonell I. et al. Use of autoantigen-loaded phosphatidylserine-liposomes to arrest autoimmunity in type 1 diabetes. PLoS ONE 10, e0127057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sonigra A. Randomized phase I trial of antigen-specific tolerizing immunotherapy with peptide/calcitriol liposomes in ACPA+ rheumatoid arthritis. JCI Insight 7, e160964 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.López-Sagaseta J, Malito E, Rappuoli R & Bottomley MJ Self-assembling protein nanoparticles in the design of vaccines. Comput. Struct. Biotechnol. J 14, 58–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Casey LM et al. Cargo-less nanoparticles program innate immune cell responses to Toll-like receptor activation. Biomaterials 218, 119333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Truong N, Black SK, Shaw J, Scotland BL & Pearson RM Microfluidic-generated immunomodulatory nanoparticles and formulation-dependent effects on lipopolysaccharide-induced macrophage inflammation. AAPS J. 24, 6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ramos GC et al. Apoptotic mimicry: phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. Br. J. Pharmacol 151, 844–850 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hosseini H. et al. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovasc. Res 106, 443–452 (2015). [DOI] [PubMed] [Google Scholar]
- 182.McCarthy DP et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine 13, 191–200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Longmire M, Choyke PL & Kobayashi H Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 3, 703–717 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tatur S, Maccarini M, Barker R, Nelson A & Fragneto G Effect of functionalized gold nanoparticles on floating lipid bilayers. Langmuir 29, 6606–6614 (2013). [DOI] [PubMed] [Google Scholar]
- 185.Platel A. et al. Influence of the surface charge of PLGA nanoparticles on their in vitro genotoxicity, cytotoxicity, ROS production and endocytosis. J. Appl. Toxicol 36, 434–444 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Vangasseri DP et al. Immunostimulation of dendritic cells by cationic liposomes. Mol. Membr. Biol 23, 385–395 (2006). [DOI] [PubMed] [Google Scholar]
- 187.Sato Y, Hatakeyama H, Hyodo M & Harashima H Relationship between the physicochemical properties of lipid nanoparticles and the quality of siRNA delivery to liver cells. Mol. Ther 24, 788–795 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hoshyar N, Gray S, Han H & Bao G The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 11, 673–692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bacher P. et al. Regulatory T cell specificity directs tolerance versus allergy against aeroantigens in humans. Cell 167, 1067–1078 (2016). [DOI] [PubMed] [Google Scholar]
- 190.Mant A, Chinnery F, Elliott T & Williams AP The pathway of cross-presentation is influenced by the particle size of phagocytosed antigen. Immunology 136, 163–175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Benne N, van Duijn J, Kuiper J, Jiskoot W & Slutter B Orchestrating immune responses: how size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control. Rel 234, 124–134 (2016). [DOI] [PubMed] [Google Scholar]
- 192.Li PY et al. PEGylation enables subcutaneously administered nanoparticles to induce antigen-specific immune tolerance. J. Control. Rel 331, 164–175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pishesha N. et al. Induction of antigen-specific tolerance by nanobody-antigen adducts that target class-II major histocompatibility complexes. Nat. Biomed. Eng 5, 1389–1401 (2021). [DOI] [PubMed] [Google Scholar]
- 194.Casey LM et al. Mechanistic contributions of Kupffer cells and liver sinusoidal endothelial cells in nanoparticle-induced antigen-specific immune tolerance. Biomaterials 283, 121457 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chieppa M. et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol 171, 4552–4560 (2003). [DOI] [PubMed] [Google Scholar]
- 196.Kel J. et al. Soluble mannosylated myelin peptide inhibits the encephalitogenicity of autoreactive T cells during experimental autoimmune encephalomyelitis. Am. J. Pathol 170, 272–280 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lomakin Y. et al. Administration of myelin basic protein peptides encapsulated in mannosylated liposomes normalizes level of serum TNF-α and IL-2 and chemoattractants CCL2 and CCL4 in multiple sclerosis patients. Mediators Inflamm. 2016, 2847232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsai S. et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32, 568–580 (2010). [DOI] [PubMed] [Google Scholar]
- 199.Bernstein DI et al. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J. Allergy Clin. Immunol 113, 1129–1136 (2004). [DOI] [PubMed] [Google Scholar]
- 200.Kappos L. et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The altered peptide ligand in relapsing MS study group. Nat. Med 6, 1176–1182 (2000). [DOI] [PubMed] [Google Scholar]
- 201.Bielekova B. et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med 6, 1167–1175 (2000). [DOI] [PubMed] [Google Scholar]
- 202.Maldonado RA et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl Acad. Sci. USA 112, E156–E165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Capini C. et al. Antigen-specific suppression of inflammatory arthritis using liposomes. J. Immunol 182, 3556–3565 (2009). [DOI] [PubMed] [Google Scholar]
- 204.Quintana FJ & Sherr DH Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev 65, 1148–1161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cappellano G. et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine 32, 5681–5689 (2014). [DOI] [PubMed] [Google Scholar]
- 206.Galea R. et al. PD-L1- and calcitriol-dependent liposomal antigen-specific regulation of systemic inflammatory autoimmune disease. JCI Insight 4, e126025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li C. et al. Nanoemulsions target to ectopic lymphoids in inflamed joints to restore immune tolerance in rheumatoid arthritis. Nano Lett. 21, 2551–2561 (2021). [DOI] [PubMed] [Google Scholar]
- 208.Pang L, Macauley MS, Arlian BM, Nycholat CM & Paulson JC Encapsulating an immunosuppressant enhances tolerance induction by Siglec-engaging tolerogenic liposomes. Chembiochem 18, 1226–1233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Burke JA et al. Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nat. Nanotechnol 17, 319–330 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lewis JS et al. Dual-sized microparticle system for generating suppressive dendritic cells prevents and reverses type 1 diabetes in the nonobese diabetic mouse model. ACS Biomater. Sci. Eng 5, 2631–2646 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kwiatkowski AJ et al. Treatment with an antigen-specific dual microparticle system reverses advanced multiple sclerosis in mice. Proc. Natl Acad. Sci. USA 119, e2205417119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Allen R, Chizari S, Ma JA, Raychaudhuri S & Lewis JS Combinatorial, microparticle-based delivery of immune modulators reprograms the dendritic cell phenotype and promotes remission of collagen-induced arthritis in mice. ACS Appl. Bio Mater 2, 2388–2404 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Chen X. et al. Restoring immunological tolerance in established experimental arthritis by combinatorial citrullinated peptides and immunomodulatory signals. Nano Today 41, 101307 (2021). [Google Scholar]
- 214.Bergot A-S et al. Regulatory T cells induced by single-peptide liposome immunotherapy suppress islet-specific T cell responses to multiple antigens and protect from autoimmune diabetes. J. Immunol 204, 1787–1797 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kulkarni JA et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol 16, 630–643 (2021). [DOI] [PubMed] [Google Scholar]
- 216.Wang D, Tai PWL & Gao G Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov 18, 358–378 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Akbarpour M. et al. Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci. Transl Med 7, 289ra281 (2015). [DOI] [PubMed] [Google Scholar]
- 218.Siatskas C. et al. Thymic gene transfer of myelin oligodendrocyte glycoprotein ameliorates the onset but not the progression of autoimmune demyelination. Mol. Ther 20, 1349–1359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Keeler GD et al. Induction of antigen-specific tolerance by hepatic AAV immunotherapy regardless of T cell epitope usage or mouse strain background. Mol. Ther. Methods Clin. Dev 28, 177–189 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zampieri R. et al. Prevention and treatment of autoimmune diseases with plant virus nanoparticles. Sci. Adv 6, eaaz0295 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Waisman A. et al. Suppressive vaccination with DNA encoding a variable region gene of the T–cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nat. Med 2, 899–905 (1996). This work describes the use of DNA vaccines to induce antigen-specific tolerance, paving the way for other nucleic-based approaches for the treatment of allergy and autoimmunity.
- 222.Liu A. et al. DNA vaccination with Hsp70 protects against systemic lupus erythematosus in (NZB×NZW)F1 mice. Arthritis Rheumatol. 72, 997–1002 (2020). [DOI] [PubMed] [Google Scholar]
- 223.Quintana FJ, Carmi P & Cohen IR DNA vaccination with heat shock protein 60 inhibits cyclophosphamide-accelerated diabetes. J. Immunol 169, 6030–6035 (2002). [DOI] [PubMed] [Google Scholar]
- 224.Quintana FJ, Carmi P, Mor F & Cohen IR Inhibition of adjuvant arthritis by a DNA vaccine encoding human heat shock protein 60. J. Immunol 169, 3422–3428 (2002). [DOI] [PubMed] [Google Scholar]
- 225.Quintana FJ, Carmi P, Mor F & Cohen IR DNA fragments of the human 60-kDa heat shock protein (HSP60) vaccinate against adjuvant arthritis: identification of a regulatory HSP60 peptide. J. Immunol 171, 3533–3541 (2003). [DOI] [PubMed] [Google Scholar]
- 226.Bar-Or A. et al. Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch. Neurol 64, 1407–1415 (2007). [DOI] [PubMed] [Google Scholar]
- 227.Garren H. et al. Phase 2 trial of a DNA vaccine encoding myelin basic protein for multiple sclerosis. Ann. Neurol 63, 611–620 (2008). [DOI] [PubMed] [Google Scholar]
- 228.Roep BO et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl Med 5, 191ra182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Garren H. et al. Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity 15, 15–22 (2001). [DOI] [PubMed] [Google Scholar]
- 230.Wadhwa A, Aljabbari A, Lokras A, Foged C & Thakur A Opportunities and challenges in the delivery of mRNA-based vaccines. Pharm 12, 102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mrak D. et al. Heterologous vector versus homologous mRNA COVID-19 booster vaccination in non-seroconverted immunosuppressed patients: a randomized controlled trial. Nat. Commun 13, 5362 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kranz LM et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). [DOI] [PubMed] [Google Scholar]
- 233.Kreiter S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rojas LA et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sahin U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020). [DOI] [PubMed] [Google Scholar]
- 236.Karikó K, Buckstein M, Ni H & Weissman D Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005). [DOI] [PubMed] [Google Scholar]
- 237. Krienke C. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 371, 145–153 (2021). This work describes the use of modified mRNA vaccines to induce antigen-specific tolerance in experimental autoimmunity.
- 238.Fishman S. et al. Adoptive transfer of mRNA-transfected T cells redirected against diabetogenic CD8 T cells can prevent diabetes. Mol. Ther 25, 456–464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Perez S. et al. Selective immunotargeting of diabetogenic CD4 T cells by genetically redirected T cells. Immunology 143, 609–617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Smith TJ & Hegedüs L Graves’ disease. N. Engl. J. Med 375, 1552–1565 (2016). [DOI] [PubMed] [Google Scholar]
- 241.Robinson WH et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med 8, 295–301 (2002). [DOI] [PubMed] [Google Scholar]
- 242.Quintana FJ et al. Functional immunomics: microarray analysis of IgG autoantibody repertoires predicts the future response of mice to induced diabetes. Proc. Natl Acad. Sci. USA 101, 14615–14621 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bashford-Rogers RJM et al. Analysis of the B cell receptor repertoire in six immune-mediated diseases. Nature 574, 122–126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dash P. et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547, 89–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bentzen AK et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol 34, 1037–1045 (2016). [DOI] [PubMed] [Google Scholar]
- 246.Sulzer D. et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546, 656–661 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Xu P. et al. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care 35, 1975–1980 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Wheeler MA et al. Droplet-based forward genetic screening of astrocyte-microglia cross-talk. Science 379, 1023–1030 (2023). This work describes a novel platform that enables the identification of candidate mechanisms of DC–T cell communication to be targeted with novel tolerogenic approaches.
- 249.Clark IC et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pasqual G. et al. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature 553, 496–500 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.LaFleur MW et al. A CRISPR-Cas9 delivery system for in vivo screening of genes in the immune system. Nat. Commun 10, 1668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sanmarco LM et al. Identification of environmental factors that promote intestinal inflammation. Nature 611, 801–809 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Akagbosu B. et al. Novel antigen-presenting cell imparts T(reg)-dependent tolerance to gut microbiota. Nature 610, 752–760 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kedmi R. et al. A RORγt+ cell instructs gut microbiota-specific T(reg) cell differentiation. Nature 610, 737–743 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lyu M. et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 610, 744–751 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Au KM, Tisch R & Wang AZ Immune checkpoint ligand bioengineered schwann cells as antigen-specific therapy for experimental autoimmune encephalomyelitis. Adv. Mater 34, e2107392 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Podojil JR et al. Tolerogenic immune-modifying nanoparticles encapsulating multiple recombinant pancreatic β cell proteins prevent onset and progression of type 1 diabetes in nonobese diabetic mice. J. Immunol 209, 465–475 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chen X. et al. Modular immune-homeostatic microparticles promote immune tolerance in mouse autoimmune models. Sci. Transl Med 13, eaaw9668 (2021). [DOI] [PubMed] [Google Scholar]
- 259.Umeshappa CS et al. Ubiquitous antigen-specific T regulatory type 1 cells variably suppress hepatic and extrahepatic autoimmunity. J. Clin. Invest 130, 1823–1829 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Umeshappa CS et al. Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. Nat. Commun 10, 2150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Huang L. et al. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J. Immunol 188, 4913–4920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wegmann KW, Wagner CR, Whitham RH & Hinrichs DJ Synthetic peptide dendrimers block the development and expression of experimental allergic encephalomyelitis. J. Immunol 181, 3301–3309 (2008). [DOI] [PubMed] [Google Scholar]
- 263.Carambia A. et al. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J. Hepatol 62, 1349–1356 (2015). [DOI] [PubMed] [Google Scholar]
- 264.Wang H. et al. Dual peptide nanoparticle platform for enhanced antigen-specific immune tolerance for the treatment of experimental autoimmune encephalomyelitis. Biomater. Sci 10, 3878–3891 (2022). [DOI] [PubMed] [Google Scholar]
- 265.De Groot AS et al. Therapeutic administration of Tregitope-human albumin fusion with insulin peptides to promote antigen-specific adaptive tolerance induction. Sci. Rep 9, 16103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Luo Y-L et al. An all-in-one nanomedicine consisting of CRISPR-Cas9 and an autoantigen peptide for restoring specific immune tolerance. ACS Appl. Mater. Interfaces 12, 48259–48271 (2020). [DOI] [PubMed] [Google Scholar]
- 267.Peine KJ et al. Treatment of experimental autoimmune encephalomyelitis by codelivery of disease associated peptide and dexamethasone in acetalated dextran microparticles. Mol. Pharm 11, 828–835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Macauley MS et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J. Clin. Invest 123, 3074–3083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Medaer R, Stinissen P, Truyen L, Raus J & Zhang J Depletion of myelin-basic-protein autoreactive T cells by T-cell vaccination: pilot trial in multiple sclerosis. Lancet 346, 807–808 (1995). [DOI] [PubMed] [Google Scholar]
- 270.Walczak A, Siger M, Ciach A, Szczepanik M & Selmaj K Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol 70, 1105–1109 (2013). [DOI] [PubMed] [Google Scholar]
- 271.Juryńczyk M. et al. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann. Neurol 68, 593–601 (2010). [DOI] [PubMed] [Google Scholar]
- 272.Wolinsky JS et al. United States open-label glatiramer acetate extension trial for relapsing multiple sclerosis: MRI and clinical correlates. Multiple Sclerosis Study Group and the MRI Analysis Center. Mult. Scler 7, 33–41 (2001). [DOI] [PubMed] [Google Scholar]
- 273.Kavanaugh A. et al. Allele and antigen-specific treatment of rheumatoid arthritis: a double blind, placebo controlled phase 1 trial. J. Rheumatol 30, 449–454 (2003). [PubMed] [Google Scholar]
- 274.Francisco LM et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med 206, 3015–3029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jones A. et al. Immunomodulatory functions of BTLA and HVEM govern induction of extrathymic regulatory T cells and tolerance by dendritic cells. Immunity 45, 1066–1077 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Henderson JG, Opejin A, Jones A, Gross C & Hawiger D CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity 42, 471–483 (2015). [DOI] [PubMed] [Google Scholar]
- 277.Schnell A, Littman DR & Kuchroo VK TH17 cell heterogeneity and its role in tissue inflammation. Nat. Immunol 24, 19–29 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chaudhry A. et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34, 566–578 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Apetoh L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol 11, 854–861 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gandhi R. et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3+ regulatory T cells. Nat. Immunol 11, 846–853 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Do J. et al. Treg-specific IL-27Rα deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc. Natl Acad. Sci. USA 114, 10190–10195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]