Abstract
Objectives
Adult-onset Still’s disease (AOSD) is a rare condition characterized by fevers, rash, and arthralgia/arthritis; most doctors treating AOSD in the Netherlands treat <5 patients per year. Currently, there is no internationally accepted treatment guideline for AOSD. The objectives of this study were to conduct a Delphi panel aimed at reaching consensus about diagnostic and treatment strategies for patients with AOSD and to use the outcomes as a basis for a treatment algorithm.
Methods
The Delphi panel brought together 18 AOSD experts: rheumatologists, internists and paediatricians. The Delphi process consisted of three rounds. In the first two rounds, online lists of questions and statements were completed. In the third round, final statements were discussed during a virtual meeting and a final vote took place. Consensus threshold was set at 80%. Two targeted literature searches were performed identifying the level of evidence of the consensus-based statements.
Results
Consensus was reached on 29 statements, including statements related to diagnosis and diagnostic tests, definition of response and remission, the therapy, the use of methotrexate and tapering of treatment. The panel consented on reduction of the use of glucocorticoids to avoid side effects, and preferred the use of biologics over conventional treatment. The role of IL-1 and IL-6 blocking agents was considered important in the treatment of AOSD.
Conclusion
In this Delphi panel, a high level of consensus was achieved on recommendations for diagnosis and therapy of AOSD that can serve as a basis for a treatment guideline.
Keywords: Adult-onset Still’s disease, Delphi, IL-1, Still’s disease, treatment, guideline, diagnosis
Rheumatology key messages.
Corticosteroids are effective in AOSD but should be avoided because of side effects.
Early initiation of biologics is preferred over conventional treatment such as methotrexate.
There is an important role for early use of IL-1 and IL-6 blockade in AOSD.
Introduction
Adult onset Still’s disease (AOSD) is a rare systemic autoinflammatory condition characterized by fever, arthralgia/(poly)arthritis and a salmon-coloured skin rash. Other symptoms include hepatosplenomegaly, lymphadenopathy and neutrophilia [1]. Systemic juvenile idiopathic arthritis (sJIA, Still’s disease) shows many similarities and SJIA and AOSD are increasingly seen as part of the same clinical disease entity. Their aetiology is largely unknown.
As AOSD is primarily a diagnosis of exclusion, a significant diagnostic delay is often present, increasing the chance of complications. To shorten the time until diagnosis, there is a need for a clear diagnostic workflow.
Anti-inflammatory drugs form the cornerstone of treatment of AOSD. Treatment is often based on expert opinion through the lack of international guidelines. Historically, glucocorticoids (GC) are considered the backbone of treatment. MTX is often the first conventional DMARD used to spare GC [2]. More recently, IL-1β and IL-6 were identified as important mediators of inflammation in AOSD. Biologics targeting these pathways are approved by both the FDA and the EMA for the treatment of sJIA and AOSD.
Given the rarity of AOSD, most Dutch physicians who treat these patients treat <5 patients a year, resulting in limited treatment experience. There is no internationally accepted guideline for the treatment of AOSD. The optimal timing of initiation of conventional DMARDs and interleukin-blocking therapy remains unresolved [3]. To fill the remaining gap, we performed a targeted literature review and Delphi-based study and propose a treatment algorithm for AOSD.
Methods
Design
The aim of a (modified) Delphi exercise is to reach consensus between experts on a specific topic where insufficient evidence-based proof exist [4] by systematically synthesizing the knowledge and opinions of a large group of individuals with diverse expertise and from different geographic locations [5].
A three-member core group (H.L., P.D. and S.T.), who have all treated over 15 AOSD patients in the previous 15 years, initiated the Delphi panel study. All three core members work in a university hospital. Two core members are internist-clinical immunologists and one is an internist- rheumatologist. They were supported by one paediatric rheumatologist (B.V.) who led the design of treatment guidelines for sJIA [6].
The other Delphi panel members were selected by nomination by the core group and upon advice of colleagues. Regional spread was a criterion. A second paediatrician with expertise in sJIA was involved. The total panel consisted of 17 participants, of whom 10 (59%) work in a university hospital, the others in a community hospital. There were nine adult rheumatologists (53%), six internists (35%) and two paediatric rheumatologists. For further characteristics of the participants, see Supplementary Tables S1 and S2 (available at Rheumatology online). All participants gave their opinion during the third and final virtual meeting, although three were not present but responded in parallel using e-mail.
Background
Before the first Delphi round, relevant literature on (i) evidence regarding MTX in Still’s disease; (ii) a recent overview on IL-1 blockade in Still’s disease [7], and (iii) the Dutch treatment guideline on (s)JIA [8] was identified. During a teleconference all participants were informed on the background and aim of the Delphi panel and the aim to discuss the literature.
Literature review
A systematic literature review (for search string see Supplementary Data S1–S3, available at Rheumatology online) was performed by the core group, using MEDLINE via PubMed and EMBASE, with an end date of 31 January 2020. A summary was shared with the panellists together with the first round Delphi questions. Evidence was scored using the GRADE system [9].
Consensus development process
The first round consisted of 27 open ended, nominal, and yes or no questions with the subjects of (i) demographics of the participants; (ii) diagnosis of AOSD; (iii) definitions of response and remission in AOSD; and (iv) treatment of AOSD (e.g. optimal timing, reduction of GC use, biologics).
Based on the results, a total of 37 statements were formed, partly inspired by the Italian Delphi panel study [3]. In round 2, aggregated results and the 37 consensus statements were presented for agreement. If a respondent did not agree, they were asked to provide further arguments for their disagreement. A total of 21 of 37 statements reached consensus (between >85% of respondents) after the second round.
All statements not reaching consensus were re-worded for the third round by the core group, using the disagreeing arguments from round 2. An online portal (Dynata) was used for round 1 and 2.
In May 2020 the statements that did not reach consensus during round 2 were discussed during a virtual meeting (Microsoft Teams). The meeting was moderated by J.F., a paediatric rheumatologist with specific expertise in hereditary autoinflammatory syndromes. Using a rotation method three new participants were asked to open the discussion for each statement, after which statements were voted on (raising a virtual hand, via chat function, or verbally). If needed, statements were reworded and/or merged during the virtual meeting. The total adjusted number of statements voted on during round 2 and 3 was 33 (Supplementary Tables S3 and S4, available at Rheumatology online). Consensus level was set at 80% agreement [10].
Ethics
Due to the Delphi design no patient data were used. No ethics approval was obtained for this project, since informed consent was non-applicable.
Results
At the end of round 3, after rewording and merging, a total of 29 of 33 final statements achieved consensus (Table 1, Supplementary Tables S3 and S4, available at Rheumatology online). Based on these statements, we propose diagnostic and treatment algorithms (Figs 1 and 2 and Supplementary Figs S1–S3, available at Rheumatology online).
Table 1.
Main consensus statements used for the formation of a treatment algorithm for AOSD and level of evidence
| Statement | Agreement | Level of evidence | Grade | References | |
|---|---|---|---|---|---|
| 1 | High dose glucocorticoids are effective in AOSD, but due to side effects, the aim should be to limit the frequency and duration of treatment | 100% | 2B | B | [1, 6, 11–13] |
| 2 | IL-1 blockade is an effective treatment early in the AOSD disease course, including in glucocorticoid naïve patients | 88% | 2B | B | [12, 14–17] |
| 3 | In case of life-threatening symptoms of AOSD, treatment with a combination of glucocorticoids with a biologic or glucocorticoid monotherapy should be started as soon as possible | 88% | 2B | C | [2, 12, 13] |
| 4 | If bacterial sepsis is unlikely, starting with short-acting IL-1 blockade in case of life-threatening and rapidly progressing AOSD is justified even if not all test results are known yet | 100% | 1B | B | [15] and expert opinion |
| 5 | In case treatment is started with short-acting IL-1 blockade, the diagnostic process is disrupted less than when starting GC (besides a possible decrease in serum cytokine levels) | 100% | 4 | D | Expert opinion |
| 6 | IL-6 blockade is a treatment option in glucocorticoid-resistant or glucocorticoid-dependent AOSD | 100% | 2B | C | [18–30] |
| 7 | IL-1 or IL-6 blockade is preferred in AOSD over TNF blockade; however, TNF blockade may be useful in some patients with persistent arthritis (without systemic manifestations) in the course of their disease | 100% | 5 | D | [18, 21–25, 27–36] |
| 8 | Adding MTX may be useful for the treatment of arthritis in AOSD, but there is little evidence for treatment in AOSD with systemic features | 94% | 4 | C | [37–47] |
AOSD: adult-onset Still’s disease.
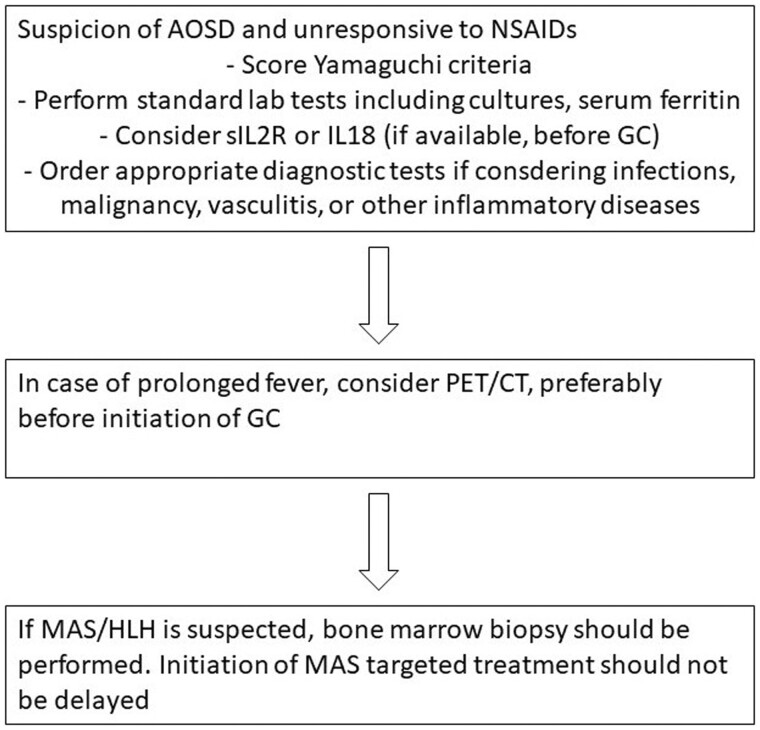
Figure 1.
Diagnostic workup of (suspected) AOSD. AOSD: adult onset Still’s disease; GC: glucocorticoids; HLH: haemophagocytic lymphohistiocytosis; MAS: macrophage activation syndrome
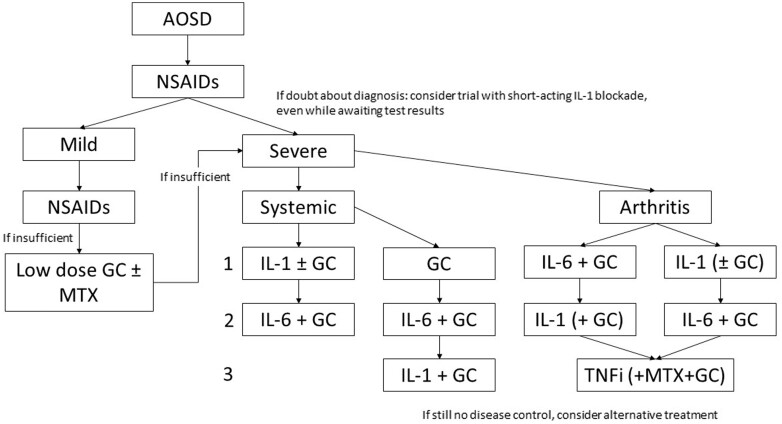
Figure 2.
Suggested treatment algorithm for patients with AOSD. Severe disease is defined as hospital admission needed, with organ involvement or life-threatening disease. Systemic symptoms: fever, weight loss, sore throat, skin rash, lymphadenopathy, hepatosplenomegaly. In case of predominant arthritis, IL-6 blockade may be preferred over IL-1 blockade. Low-dose glucocorticoid is ≤15 mg prednisone or equivalent. Double arrows indicate equivalent options and a decision should be made upon the physician’s discretion. GC refers to high dose glucocorticoid, i.e. prednisone 0.5–1.0 mg/kg or equivalent. ‘IL-1’ indicates IL-1 blockade; ‘IL-6’ indicates IL-6 blockade. MTX dosing according to EULAR; IL-1 blockade 100–300 mg subcutaneously or off label intravenously. AOSD: Adult onset Still’s Disease; GC: glucocorticoid; TNFi: TNF inhibitors
Diagnostic approach
The statements on the approach to a patient with suspected AOSD are mainly based on expert opinion. The panel’s statements and considerations on diagnostics are presented in Table 1, Supplementary Table S3, available at Rheumatology online and visualized in Fig. 1. AOSD mainly remains a diagnosis of exclusion and its differential diagnosis is broad and includes infections, non-infectious inflammatory diseases, and malignancies [48]. Kontzias et al. have suggested a standard diagnostic approach for AOSD [49]. Scoring of the Yamaguchi criteria [50] is required. If available, measurement of serum soluble IL-2 receptor (sIL-2R) or IL-18 should be considered, preferably before the start of GC. Macrophage activation syndrome (MAS)/haemophagocytic lymphohistiocytosis (HLH) is a major complication of AOSD and present in up to 23% of patients [51].
Treatment of AOSD
The statements that were used as a basis for the treatment algorithm (Fig. 2, Supplementary Figs S1–S3, available at Rheumatology online) are shown in Table 1. Note that for practical purposes AOSD treatment response and disease remission, respectively, were defined by the panel as decrease in serum ferritin, recovery of clinical symptoms, decrease in CRP and/or ESR (consensus 100%); and normalization of serum ferritin, absence of clinical symptoms, normalization of CRP and/or ESR (consensus 94%) [19]. For validity of extrapolation from children to adults two statements on the similarity between sJIA and AOSD were included [3] (Supplementary Table S3, available at Rheumatology online):
The clinical presentation of sJIA is very similar to that of AOSD and therefore these conditions can be considered as expressions of the same disease spectrum [3] (grade C)
Early use of biologics can improve outcomes in adults with AOSD (grade D)
Glucocorticoids
With the literature search (See Supplementary Data S1, available at Rheumatology online), 132 publications were found, of which only two were relevant: one case series of adolescents with a clinical picture fitting AOSD [11] and one review on juvenile chronic arthritis [13]. Broadening the search (see Supplementary Data S1, available at Rheumatology online) yielded 74 results. None specifically examined the effect of systemic GC in AOSD. One observational study (Supplementary Table S5, available at Rheumatology online) including 80 patients [12] compared high-dose GC (prednisone 0.8–1.0 mg/kg/day or equivalent) to low-dose GC (0.2–0.3 mg/kg/day). Monotherapy with high-dose GC led to a statistically significant higher rate of remissions after 6 months of treatment (65% vs 23%; P < 0.01). MAS was seen more often in the low-dose group. A case series including five adolescents, of whom three were treated with prednisone 2 mg/kg, reports rapid response after failure of non-steroidal anti-inflammatory drugs [11] (Supplementary Table S5, available at Rheumatology online).
Based on these results, two main statements (Table 1, statement 1 and 3) were developed.
IL-1 Blockade
Studies on the early/initial use of IL-1 blockade in AOSD have not been performed. As the expert panel considered AOSD and sJIA to be part of the same disease spectrum, evidence from sJIA studies was extrapolated. Six studies were identified [3, 14–17, 52] (Table 2).
Table 2.
Clinical evidence regarding treatment with IL-1 and IL-6 blockade in AOSD
| Author | Drug | Design | Number of patients | Reference |
|---|---|---|---|---|
| IL-1 blockade | ||||
| Colafrancesco et al. | ANA, RILO | Qualitative | N/A | [3] |
| Jamilloux et al. | ANA | Review | N/A | [14] |
| Vastert et al./Ter Haar et al. | ANA | Prospective | 20 | [15, 16] |
| Junge et al. | ANA, CANA, RILO | Review | N/A | [17] |
| Feist et al. | ANA, CANA, RILO | Review | N/A | [52] |
| IL-6 blockade | ||||
| Vercruysse et al. | TCZ | Retrospective multicentre | 27 | [19] |
| Kaneko et al. | TCZ | RCT | 27 | [20] |
| Li et al. | TCZ | Prospective | 8 | [21] |
| Ma et al. | TCZ | Meta-analysis | 147 | [22] |
| Song et al. | TCZ | Retrospective multicentre | 22 | [23] |
| Bannai et al. | TCZ | Case Series | 7 | [24] |
| Ortiz et al. | TCZ | Retrospective open label | 34 | [25] |
| Cipriani et al. | TCZ | Open label prospective | 11 | [26] |
| Suematsu et al. | TCZ | Retrospective | 11 | [27] |
| Puechal et al. | TCZ | Prospective | 14 | [28] |
| Multicentre | ||||
| Elkayam et al. | TCZ | Retrospective | 15 | [29] |
| Nishina et al. | TCZ | Retrospective | 40 | [30] |
ANA: anakinra; AOSD: adult-onset Still’s disease; CANA: canakinumab; N/A: not available; RILO: rilonacept; TCZ: tocilizumab.
An extensive literature review on efficacy and safety of IL-1 blocking therapy in AOSD is available [3]. Median effectiveness is 83.3% (range 50–100%) with median disease remission rate 70% (range 22.2–100%). Median treatment failure rate was 16.7%. In an international multicentre series 22% of 46 patients received recombinant IL-1 receptor antagonist (eIL-1RA) and 80% of these patients attained complete response without escalation of therapy [53]. In a cohort of 20 sJIA patients treated with rIL-1RA, 75% achieved minimal active or inactive disease after 3 months; and this response was sustained in 13/20 patients [15]. After 1 year without medication, 52% of these still had inactive disease [16], and after 5 years 96% had inactive disease and 75% inactive disease without medication.
Based on these results and personal clinical experience of the core group, three main statements on IL-1 blockade were developed (Table 1, statement 2, 4 and 5). Based on personal experience, a statement on reduced interference with the diagnostic trajectory of IL-1 inhibitors compared with GCs was included (Table 1, statement 5).
IL-6 Blockade
Studies on the effects of IL-6 inhibition are summarized in Table 2. All studies report on the use of tocilizumab (TCZ). TCZ has mainly been studied in refractory AOSD with arthritis. A Japanese trial randomized 27 AOSD patients between biweekly TCZ or placebo [20] for 12 weeks, followed by a 40 week open label study. GC dosages and systemic scores decreased significantly in the TCZ group. In over 160 reported AOSD patients treated with varying dosages of TCZ [19–27], overall clinical response ranged from 64.3% to 100%.
One main statement on the early use of IL-6 blockade was developed (Table 1, statement 6).
MTX
Many recommendations base their advice on MTX on a retrospective cohort of 26 patients [37]. The evaluation of efficacy of MTX in AOSD results from one prospective multicentre cohort study [38], retrospective [37, 39–43] and monocentre studies [44, 45] and several case series [46, 54], predominantly in patients with polyarticular involvement. The reported efficacy is ∼60–83%. In a randomized trial [45] there was no significant improvement compared with placebo. In an open randomized controlled trial (RCT) comparing anakinra to conventional DMARDS, the number of patients on MTX was too small to draw conclusions [44] (Table 3).
Table 3.
Clinical evidence regarding treatment with methotrexate in AOSD
| Author | Drug | Design | No. of patients | Reference |
|---|---|---|---|---|
| Fautrel et al. | MTX | Retrospective, uncontrolled | 26 | [37] |
| Kalyonku et al. | MTX | Prospective multicentre | 202 | [38] |
| Pay et al. | MTX | Retrospective multicentre | 61 | [39] |
| Iliou et al. | MTX | Retrospective, uncontrolled | 11 | [40] |
| Franchini et al. | MTX | Retrospective monocentre | 27 (five combination of DMARDs) | [41] |
| Fuji et al. | MTX | Retrospective monocentre | 13 | [42] |
| Singh et al. | MTX | Retrospective | 10 | [43] |
| Nordström et al. | MTX | RCT | 6 | [44] |
| Woo et al. | MTX | RCT | 45 | [45] |
| Aydintug et al. | MTX | Case series | 6 | [46] |
| Manger et al. | MTX | Review | 71 | [47] |
| Wang et al. | MTX | Prospective | 28 | [54] |
AOSD: adult-onset Still’s disease.
The reported complication rates (e.g. erosive bone disease) are relatively high despite the use of MTX in cohort studies.
One main statement on the early use of MTX in AOSD was developed (Table 1, statement 8).
TNF-blockade
Data on TNF-blockade in AOSD is limited (Table 4). No RCTs are present. Its effect is mainly reported in patients with predominantly arthritis who lack systemic symptoms [27, 31–33, 55]. In the presence of systemic symptoms, most patients only achieve partial response. A few patients with refractory disease (including severe liver diseases) responded to TNF-blockade [34, 35, 56, 57].
Table 4.
Clinical evidence regarding treatment with TNF blockade in AOSD
| Authors | Drug | Design | No. of patients | Reference |
|---|---|---|---|---|
| Suematsu et al. | Infliximab, etanercept | Retrospective multicentre | 13 | [27] |
| Kraetsch et al. | Infliximab | Prospective pilot | 6 | [31] |
| Fautrel et al. | Infliximab, etanercept | Restrospective multicentre | 20 | [32] |
| Dilhuydy et al. | Infliximab | Case report | 1 | [33] |
| Zhou et al. | Infliximab, etanercept, adalimumab | Retrospective multicentre | 293 | [34] |
| Babacan et al. | Infliximab | Case report | 1 | [35] |
| Maeshima et al. | Etanercept | Case report | 1 | [55] |
| Naniwa et al. | Etanercept | Case report | 1 | [56] |
| Singh et al. | Infliximab | Case report | 1 | [57] |
AOSD: adult-onset Still’s disease.
One main statement was developed on the use of TNF-blockade in AOSD (Table 1, statement 7).
Discussion
The aim of this study was to reach consensus on diagnosis and treatment of AOSD with the use of a Delphi process. The 29 out of 33 statements that reached consensus between >80% of 17 panelists form the basis for a treatment guideline to be used in clinical practice (Figs 1 and 2 and Supplementary Figs S1–S3, available at Rheumatology online).
Recommendations for the diagnosis of AOSD
When AOSD is considered in the differential diagnosis, standard lab tests, blood and urine cultures, and serum ferritin should be performed. Scoring the Yamaguchi criteria [50] is required (Fig. 1). Individual clinical features determine whether a patient should be tested for alternative diagnoses (infections, non-infectious inflammatory diseases, malignancy) [48, 49]. Measurement of soluble IL-2 receptor or IL-18 should be considered to support the diagnosis of AOSD, preferably before initiation of GC. Timely start of treatment is important to avoid damage or a more chronic disease course. In case of prolonged fever or inflammation, PET/CT should be considered before starting GC. Upon suspicion of MAS, bone marrow examination should be performed, but this should not delay MAS-targeted treatment, as this is a potentially life-threatening complication. If doubt remains about the diagnosis, a trial with short-acting IL-1 blockade should be considered while waiting for test results (Fig. 1).
Recommendations for the treatment of AOSD
The consent recommendations for treatment of AOSD are the following (Fig. 2):
NSAIDS can be given in any patient suspected of AOSD.
The goal remains to limit the use of GC in AOSD, mainly because of the side effects. IL-1 blockade interferes less during the diagnostic trajectory and can be used for a therapeutic trial.
Mild disease, unresponsive to NSAIDs: short course of GC with or without MTX (dosing and administration see [58]). If after 4–6 weeks GC exceeds 7.5 mg per day, step up to recommendations for more severe disease.
Severe systemic disease: either monotherapy IL-1 blockade, or GC and IL-1 blockade, or monotherapy GC. Step-up to IL-1 blockade should be considered in insufficient response on GC monotherapy or upon GC dependence.
Severe arthritis predominant: MTX and GC combination therapy, GC and IL-6 blockade combination therapy; IL-1 blockade and GC combination therapy; or IL-1 blockade monotherapy. IL-6 blockade may be preferred to IL-1 blockade in arthritis-dominant patients, but comparative studies are lacking.
Severe disease, unresponsive to initial treatment: switch to IL-1 blockade or IL-6 blockade, depending on the initial choice.
TNF blockade may be used in arthritis-predominant AOSD patients without systemic symptoms and upon suboptimal response to both IL-1 and IL-6 blockade.
The initial dose of short-acting IL-1 blockade (i.e. anakinra) should depend on disease severity and could range from 100 to 400 mg subcutaneously per day [8]. Dosing over 100 mg is off-label but has proven to be safe in sepsis trials [59] and multiple COVID-19 trials (reviewed in [60]). In children, doses up to 8 mg/kg have been proven safe [15]. In cases with insufficient response within 2 days of monotherapy, the dose can be escalated. For rapid progressive or life-threatening disease, off-label intravenous dosing of anakinra, 200–400 mg per day, in combination with GC or monotherapy GC can be considered. Pulsatile methylprednisolone intravenously is preferred over long-term high-dose oral GC. Intravenous immunoglobulin treatment is optional. If a patient deteriorates despite this in absence of an alternative diagnosis, case reports and case series describe alternative and rescue therapies for AOSD and sJIA, such as cyclophosphamide [61–64], anti-thymocyte globulin [65], intravenous immunoglobulin (IvIg) [66] or etoposide [67, 68]. The human anti-IFN-γ antibody emapalumab showed beneficial effect in 14 patients with sJIA/AOSD-associated macrophage activation syndrome [69]. A new recombinant human IL-18 binding protein (tadekinig alfa) needs to be further evaluated, but showed favourable safety and modest efficacy in a 12-week phase 2 trial [70].
In case of disease remission, GC should be tapered to ≤7.5 mg prednisolone or equivalent daily. If disease remission is obtained for ≥3 months while on short-acting IL-1 blockade 100 mg s.c. daily, the dose can be tapered to every other day for 4–6 weeks and stopped upon persisting remission. If a patient is in a 3-month remission on long-acting IL-1 blockade (canakinumab) monotherapy, the dose of canakinumab can be tapered by increasing the interval of administration.
The current study is the first AOSD consensus study in the Netherlands. In the absence of a strongly evidence-based approach for the diagnosis and treatment of AOSD, this work provides an important milestone in optimizing treatment for patients with AOSD. The Delphi panel was elaborate, the participation rate of clinicians with expertise on AOSD was high, and the panel included an adequate representation of AOSD experts in the Netherlands. Because sJIA and AOSD were considered a spectrum by the panel, study results were to some extent extrapolated from children to adults. Compared with other recent treatment recommendations [71, 72], we advocate the early use of biologics over conventional treatments such as MTX, especially in patients with severe systemic disease. It should be noted that many of the consensus-based statements are based on low level evidence, because of the rarity of AOSD and the scarcity of clinical trials. Comparative studies are needed to validate the recommendations made here. A potential limitation to our Delphi process is that the third and final meeting was held virtually, and because of this anonymity was not preserved, although the Delphi panel method is intended specifically to counteract the effects of any psychological pressure that can influence opinions in a face-to-face, small group discussion environment. The systematic method or rotation for input was used to circumvent psychological pressure.
In conclusion, this paper presents the results of a modified Delphi process that resulted in 29 consensus-based statements on the diagnosis and treatment of AOSD, which form the basis for both a diagnostic and a therapeutic algorithm.
Supplementary Material
Contributor Information
Helen L Leavis, Department of Rheumatology and Clinical Immunology, University Medical Center Utrecht, Utrecht, The Netherlands.
Paul L A van Daele, Department of Immunology, Erasmus Medical Center, Rotterdam, The Netherlands.
Catharina Mulders-Manders, Department of Internal medicine, Radboud University Medical Center, Nijmegen, The Netherlands.
Renée Michels, IQVIA, Amsterdam, The Netherlands.
Abraham Rutgers, Department of Rheumatology and Clinical Immunology, University Medical Center Groningen, Groningen, The Netherlands.
Elizabeth Legger, Department of Pediatric Rheumatology, University Medical Center Groningen, Groningen, The Netherlands.
Marc Bijl, Department of Rheumatology and Clinical Immunology, Martini Hospital, Groningen, The Netherlands.
Elisabeth A Hak, Department of Rheumatology and Clinical Immunology, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Wai-Kwan Lam-Tse, Department Rheumatology, Franciscus Hospital, Rotterdam, The Netherlands.
Femke Bonte-Mineur, Department of Rheumatology and Clinical Immunology, Maasstad Hospital, Rotterdam, The Netherlands.
Peter Fretter, Department of Rheumatology, Treant Hospitals, Emmen/Hoogeveen/Stadskanaal, The Netherlands.
Anna Simon, Department of Internal medicine, Radboud University Medical Center, Nijmegen, The Netherlands.
Pieter van Paassen, Department of Nephrology and Clinical Immunology, Maastricht University Medical Center, Maastricht, The Netherlands.
Marlies C van der Goes, Department of Rheumatology, Meander Medical Center, Amersfoort, The Netherlands.
Marcel Flendrie, Department of Rheumatology, Maartenskliniek, Nijmegen, The Netherlands.
Ward Vercoutere, Department of Rheumatology, Reumazorg Zuid-West Nederland, Goes-Terneuzen-Oostburg, The Netherlands.
Antoine W T van Lieshout, Department of Rheumatology, Jeroen Bosch Ziekenhuis, ’s-Hertogenbosch, The Netherlands.
Arjen Leek, Department of Pediatrics, University Medical Center Utrecht, Utrecht, The Netherlands.
Sebastiaan J Vastert, Department of Pediatrics, University Medical Center Utrecht, Utrecht, The Netherlands.
Sander W Tas, Department of Rheumatology and Clinical Immunology, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Data are available from the corresponding author upon request.
Funding
This work was supported by Novartis pharma (grant ID SP 006.19) and Sobi (HCC-4445).
Disclosure statement: H.L.L reports grants and personal fees from Novartis, grants and personal fees from Sobi, outside the published work. P.L.A.D reports grants from Sobi and Novartis, outside the published work. C.M.-M. reports personal fees from Novartis, outside the published work. E.L. reports grants and personal fees from Sobi and Novartis, outside the published work. P.P. reports personal fees from Sobi and Novartis, outside the published work. S.J.V. reports grants and personal fees from Novartis, outside the published work. The other authors have nothing to disclose.
References
- 1. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun 2018;93:24–36. [DOI] [PubMed] [Google Scholar]
- 2. Yoo DH. Treatment of adult-onset still's disease: up to date. Expert Rev Clin Immunol 2017;13:849–66. [DOI] [PubMed] [Google Scholar]
- 3. Colafrancesco S, Manara M, Bortoluzzi A et al. ; AOSD Consensus Group. Management of adult-onset Still's disease with interleukin-1 inhibitors: evidence- and consensus-based statements by a panel of Italian experts. Arthritis research & therapy 2019;21:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008–15. [PubMed] [Google Scholar]
- 5. Humphrey-Murto S, Varpio L, Wood TJ et al. The use of the Delphi and other consensus group methods in medical education research: a review. Acad Med 2017;92:1491–8. [DOI] [PubMed] [Google Scholar]
- 6. Leek AA, Avcin T, De Benedetti F et al. The SHARE recommendations on diagnosis and treatment of systemic JIA (abstract). Arthritis Reumatol 2020;72(suppl_10):2304–6. [Google Scholar]
- 7. Vastert SJ, Jamilloux Y, Quartier P et al. Anakinra in children and adults with Still's disease. Rheumatology 2019;58:vi9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. NVK Richtlijn. Juveniele idiopathische artritis, medicamenteuze behandeling van kinderen met 2018. https://nvk.nl/themas/kwaliteit/richtlijnen/richtlijn?componentid=7864339&tagtitles=Infectieziekten%2Ben%2BImmunologie,Intensive%2BCare,Maag-Darm-Leverziekten%2B(MDL),Reumatologie,Sociale%2Ben%2BPsychosociale%2Bkindergeneeskunde,Chirurgie (11 April 2018, date last accessed).
- 9. Guyatt GH, Oxman AD, Vist GE et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diamond IR, Grant RC, Feldman BM et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014;67:401–9. [DOI] [PubMed] [Google Scholar]
- 11. Prendiville JS, Tucker LB, Cabral DA, Crawford RI. A pruritic linear urticarial rash, fever, and systemic inflammatory disease in five adolescents: adult-onset still disease or systemic juvenile idiopathic arthritis sine arthritis? Pediatr Dermatol 2004;21:580–8. [DOI] [PubMed] [Google Scholar]
- 12. Ruscitti P, Cipriani P, Liakouli V et al. Managing Adult-onset Still's disease: the effectiveness of high-dosage of corticosteroids as first-line treatment in inducing the clinical remission. Results from an observational study. Medicine (Baltimore) 2019;98:e15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cassidy JT. Medical management of children with juvenile rheumatoid arthritis. Drugs 1999;58:831–50. [DOI] [PubMed] [Google Scholar]
- 14. Jamilloux Y, Gerfaud-Valentin M, Henry T, Seve P. Treatment of adult-onset Still's disease: a review. Ther Clin Risk Manag 2015;11:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vastert SJ, de Jager W, Noordman BJ et al. Effectiveness of first-line treatment with recombinant interleukin-1 receptor antagonist in steroid-naive patients with new-onset systemic juvenile idiopathic arthritis: results of a prospective cohort study. Arthritis Rheumatol 2014;66:1034–43. [DOI] [PubMed] [Google Scholar]
- 16. Ter Haar NM, van Dijkhuizen EHP, Swart JF et al. Treatment to target using recombinant interleukin-1 receptor antagonist as first-line monotherapy in new-onset systemic juvenile idiopathic arthritis: results from a five-year follow-up study. Arthritis Rheumatol 2019;71:1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Junge G, Mason J, Feist E. Adult onset Still's disease-The evidence that anti-interleukin-1 treatment is effective and well-tolerated (a comprehensive literature review). Semin Arthritis Rheum 2017;47:295–302. [DOI] [PubMed] [Google Scholar]
- 18. De Bandt M, Saint-Marcoux B. Tocilizumab for multirefractory adult-onset Still's disease. Ann Rheum Dis 2009;68:153–4. [DOI] [PubMed] [Google Scholar]
- 19. Vercruysse F, Barnetche T, Lazaro E et al. Adult-onset Still's disease biological treatment strategy may depend on the phenotypic dichotomy. Arthritis Res Ther 2019;21:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaneko Y, Kameda H, Ikeda K et al. Tocilizumab in patients with adult-onset still's disease refractory to glucocorticoid treatment: a randomised, double-blind, placebo-controlled phase III trial. Ann Rheum Dis 2018;77:1720–9. [DOI] [PubMed] [Google Scholar]
- 21. Li T, Gu L, Wang X et al. A pilot study on tocilizumab for treating refractory adult-onset Still's disease. Sci Rep 2017;7:13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma Y, Wu M, Zhang X et al. Efficacy and safety of tocilizumab with inhibition of interleukin-6 in adult-onset Still's disease: a meta-analysis. Mod Rheumatol 2018;28:849–57. [DOI] [PubMed] [Google Scholar]
- 23. Song ST, Kim JJ, Lee S et al. Efficacy of tocilizumab therapy in Korean patients with adult-onset Still's disease: a multicentre retrospective study of 22 cases. Clin Exp Rheumatol 2016;34(6 Suppl 102):S64–S71. [PubMed] [Google Scholar]
- 24. Bannai E, Yamashita H, Kaneko S et al. Successful tocilizumab therapy in seven patients with refractory adult-onset Still's disease. Mod Rheumatol 2016;26:297–301. [DOI] [PubMed] [Google Scholar]
- 25. Ortiz-Sanjuan F, Blanco R, Calvo-Rio V et al. Efficacy of tocilizumab in conventional treatment-refractory adult-onset Still's disease: multicenter retrospective open-label study of thirty-four patients. Arthritis Rheumatol 2014;66:1659–65. [DOI] [PubMed] [Google Scholar]
- 26. Cipriani P, Ruscitti P, Carubbi F et al. Tocilizumab for the treatment of adult-onset Still's disease: results from a case series. Clin Rheumatol 2014;33:49–55. [DOI] [PubMed] [Google Scholar]
- 27. Suematsu R, Ohta A, Matsuura E et al. Therapeutic response of patients with adult Still's disease to biologic agents: multicenter results in Japan. Mod Rheumatol 2012;22:712–9. [DOI] [PubMed] [Google Scholar]
- 28. Puechal X, DeBandt M, Berthelot JM et al. ; Club Rhumatismes Et Inflammation. Tocilizumab in refractory adult Still's disease. Arthritis Care Res 2011;63:155–9. [DOI] [PubMed] [Google Scholar]
- 29. Elkayam O, Jiries N, Dranitzki Z et al. Tocilizumab in adult-onset Still's disease: the Israeli experience. J Rheumatol 2014;41:244–7. [DOI] [PubMed] [Google Scholar]
- 30. Nishina N, Kaneko Y, Kameda H, Takeuchi T. The effect of tocilizumab on preventing relapses in adult-onset Still's disease: a retrospective, single-center study. Mod Rheumatol 2015;25:401–4. [DOI] [PubMed] [Google Scholar]
- 31. Kraetsch HG, Antoni C, Kalden JR, Manger B. Successful treatment of a small cohort of patients with adult onset of Still's disease with infliximab: first experiences. Ann Rheum Dis 2001;60(Suppl 3):iii55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fautrel B, Sibilia J, Mariette X, Combe B, Club Rhumatismes et I. Tumour necrosis factor alpha blocking agents in refractory adult Still's disease: an observational study of 20 cases. Ann Rheum Dis 2005;64:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dilhuydy MS, Vatan R, Etienne G, Longy-Boursier M, Mercie P. Prolonged efficacy of infliximab for refractory adult-onset Still's disease. Clin Exp Rheumatol 2005;23:121–2. [PubMed] [Google Scholar]
- 34. Zhou S, Qiao J, Bai J, Wu Y, Fang H. Biological therapy of traditional therapy-resistant adult-onset Still's disease: an evidence-based review. Ther Clin Risk Manag 2018;14:167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babacan T, Onat AM, Pehlivan Y, Comez G, Karakök M. Successful treatment of refractory adult Still's disease and membranous glomerulonephritis with infliximab. Clin Rheumatol 2010;29:423–6. [DOI] [PubMed] [Google Scholar]
- 36. Rech J, Ronneberger M, Englbrecht M et al. Successful treatment of adult-onset Still's disease refractory to TNF and IL-1 blockade by IL-6 receptor blockade. Ann Rheum Dis 2011;70:390–2. [DOI] [PubMed] [Google Scholar]
- 37. Fautrel B, Borget C, Rozenberg S et al. Corticosteroid sparing effect of low dose methotrexate treatment in adult Still's disease. J Rheumatol 1999;26:373–8. [PubMed] [Google Scholar]
- 38. Kalyoncu U, Solmaz D, Emmungil H et al. Response rate of initial conventional treatments, disease course, and related factors of patients with adult-onset Still's disease: data from a large multicenter cohort. J Autoimmun 2016;69:59–63. [DOI] [PubMed] [Google Scholar]
- 39. Pay S, Turkcapar N, Kalyoncu M et al. ; Ankara Rheumatology Study Group. A multicenter study of patients with adult-onset Still's disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol 2006;25:639–44. [DOI] [PubMed] [Google Scholar]
- 40. Iliou C, Papagoras C, Tsifetaki N, Voulgari PV, Drosos AA. Adult-onset Still's disease: clinical, serological and therapeutic considerations. Clin Exp Rheumatol 2013;31:47–52. [PubMed] [Google Scholar]
- 41. Franchini S, Dagna L, Salvo F et al. Efficacy of traditional and biologic agents in different clinical phenotypes of adult-onset Still's disease. Arthritis Rheum 2010;62:2530–5. [DOI] [PubMed] [Google Scholar]
- 42. Fujii T, Akizuki M, Kameda H et al. Methotrexate treatment in patients with adult onset Still's disease–retrospective study of 13 Japanese cases. Ann Rheum Dis 1997;56:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh S, Samant R, Joshi VR. Adult onset Still's disease: a study of 14 cases. Clin Rheumatol 2008;27:35–9. [DOI] [PubMed] [Google Scholar]
- 44. Nordstrom D, Knight A, Luukkainen R et al. Beneficial effect of interleukin 1 inhibition with anakinra in adult-onset Still's disease. An open, randomized, multicenter study. J Rheumatol 2012;39:2008–11. [DOI] [PubMed] [Google Scholar]
- 45. Woo P, Southwood TR, Prieur AM et al. Randomized, placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum 2000;43:1849–57. [DOI] [PubMed] [Google Scholar]
- 46. Aydintug AO, D'Cruz D, Cervera R, Khamashta MA, Hughes GR. Low dose methotrexate treatment in adult Still's disease. J Rheumatol 1992;19:431–5. [PubMed] [Google Scholar]
- 47. Manger B, Rech J, Schett G. Use of methotrexate in adult-onset Still's disease. Clin Exp Rheumatol 2010;28(5 Suppl 61):S168–71. [PubMed] [Google Scholar]
- 48. Fautrel B. Adult-onset Still disease. Best Pract Res Clin Rheumatol 2008;22:773–92. [DOI] [PubMed] [Google Scholar]
- 49. Kontzias A, Efthimiou P. Adult-onset Still's disease: pathogenesis, clinical manifestations and therapeutic advances. Drugs 2008;68:319–37. [DOI] [PubMed] [Google Scholar]
- 50. Yamaguchi M, Ohta A, Tsunematsu T et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol 1992;19:424–30. [PubMed] [Google Scholar]
- 51. Efthimiou P, Kontzias A, Hur P et al. Adult-onset Still's disease in focus: clinical manifestations, diagnosis, treatment, and unmet needs in the era of targeted therapies. Semin Arthritis Rheum 2021;51:858–74. [DOI] [PubMed] [Google Scholar]
- 52. Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still's disease. Nat Rev Rheumatol 2018;14:603–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nigrovic PA, Mannion M, Prince FH et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum 2011;63:545–55. [DOI] [PubMed] [Google Scholar]
- 54. Wang CY, Guo SH, Wang LP, Shen HL. Refractory adult-onset Still disease treated by tocilizumab combined with methotrexate: a STROBE-compliant article. Medicine 2019;98:e16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maeshima K, Ishii K, Iwakura M et al. Adult-onset Still's disease with macrophage activation syndrome successfully treated with a combination of methotrexate and etanercept. Mod Rheumatol 2012;22:137–41. [DOI] [PubMed] [Google Scholar]
- 56. Naniwa T, Tamechika S, Iwagaitsu S, Maeda S, Togawa H. Successful use of higher-dose etanercept for multirefractory systemic flare of adult-onset Still's disease with liver failure with no response to tocilizumab therapy. Case Rep Rheumatol 2013;2013:923497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Singh B, Biboa J, Musuku S et al. Reversal of severe hepatitis with infliximab in adult-onset Still's disease. Am J Med 2013;126:e3–4. [DOI] [PubMed] [Google Scholar]
- 58. Smolen JS, Landewe RBM, Bijlsma JWJ et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 59. Shakoory B, Carcillo JA, Chatham WW et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016;44:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kyriazopoulou E, Huet T, Cavalli G et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol 2021;3:e690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsuji Y, Iwanaga N, Adachi A et al. Successful treatment with intravenous cyclophosphamide for refractory adult-onset Still's disease. Case Rep Rheumatol 2015;2015:163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Castro TC, Terreri MT, Len C, Hilario MO. Treatment of refractory juvenile idiopathic arthritis via pulse therapy using methylprednisolone and cyclophosphamide. Sao Paulo Med J 2003;121:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Falkenbach A, Lembcke B, Schneider M et al. Polyserositis in adult Still's disease with onset during pregnancy [corrected]. Clin Rheumatol 1994;13:513–7. [DOI] [PubMed] [Google Scholar]
- 64. Han SA, Yoon YM, Lee WS et al. Cyclophosphamide therapy for secondary amyloidosis in a patient with juvenile idiopathic arthritis unresponsive to tumor necrosis factor alpha inhibitor therapy. Korean J Intern Med 2016;31:601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lanza F, Dominici M, Govoni M et al. Prolonged remission state of refractory adult onset Still's disease following CD34-selected autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 2000;25:1307–10. [DOI] [PubMed] [Google Scholar]
- 66. Vignes S, Wechsler B, Amoura Z et al. Intravenous immunoglobulin in adult Still's disease refractory to non-steroidal anti-inflammatory drugs. Clin Exp Rheumatol 1998;16:295–8. [PubMed] [Google Scholar]
- 67. Wang R, Li T, Ye S et al. Short-term, low-dose etoposide in refractory adult-onset Still's disease-associated macrophage activation syndrome. Clin Rheumatol 2022;41:2817–23. [DOI] [PubMed] [Google Scholar]
- 68. Minoia F, Davi S, Horne A et al. ; Histiocyte Society. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol 2014;66:3160–9. [DOI] [PubMed] [Google Scholar]
- 69. De Benedetti F, Grom AA, Brogan PA et al. Efficacy and safety of emapalumab in macrophage activation syndrome. Ann Rheum Dis 2023;82:857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gabay C, Fautrel B, Rech J et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease. Annals of the rheumatic diseases 2018;77:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. PNDS. Protocole National de Diagnostic et de Soins (PNDS). http://www.snfmi.org/sites/default/files/uploads/Maladie_de_Still_FR_fr_PNDS.pdf.
- 72. Mimura T, Kondo Y, Ohta A et al. Evidence-based clinical practice guideline for adult Still's disease. Mod Rheumatol 2018;28:736–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon request.