Abstract
Purpose:
We previously demonstrated the clinical significance of circulating tumor DNA (ctDNA) in patients with HER2-negative breast cancer receiving neoadjuvant chemotherapy (NAC). Here, we compared its predictive and prognostic value with cell-free DNA (cfDNA) concentration measured in the same samples from the same patients.
Experimental Design:
145 hormone receptor (HR)-positive/HER2-negative and 138 triple-negative breast cancer (TNBC) patients with ctDNA data from a previous study were included in the analysis. Associations of serial cfDNA concentration with residual cancer burden (RCB) and distant recurrence-free survival (DRFS) were examined.
Results:
In TNBC, we observed a modest negative correlation between cfDNA concentration 3 weeks after treatment initiation and RCB, but none of the other timepoints showed significant correlation. In contrast, ctDNA was significantly positively correlated with RCB at all timepoints (all R>0.3 and p<0.05). In the HR-positive/HER2-negative group, cfDNA concentration did not associate with response to NAC, but survival analysis showed that high cfDNA-shedders at pretreatment had a significantly worse DRFS than low shedders (hazard ratio 2.12, p=0.037). In TNBC, the difference in survival between high vs. low cfDNA-shedders at all timepoints was not statistically significant. In contrast, as previously reported, ctDNA at all timepoints was significantly correlated with DRFS in both subtypes.
Conclusions:
In TNBC, cfDNA concentrations during therapy were not strongly correlated with response or prognosis. In the HR-positive/HER2-negative group, pretreatment cfDNA concentration was prognostic for DRFS. Overall, the predictive and prognostic value of cfDNA concentration was more limited than that of ctDNA.
Keywords: cell-free DNA, circulating tumor DNA, neoadjuvant chemotherapy, liquid biopsy, breast cancer, HER2-negative
INTRODUCTION
Approximately 25% of high-risk early-stage breast cancers treated with NAC and surgery will have their cancer recur within 5 years (1). The risk of distant recurrence is significantly decreased if the patient achieves a pathologic complete response (pCR) after NAC. Thus, biomarkers that predict response to NAC can improve patient outcomes by aiding treatment selection to increase the probability of a pCR and prevent metastatic recurrence.
CfDNA—which includes all the DNA molecules shed into circulation by dying hematopoietic and tumor cells—is a promising non-invasive biomarker for monitoring disease status during treatment. ctDNA is a subpopulation of cfDNA exclusively released by tumor cells (2). Several studies in early-stage breast cancer receiving NAC have shown that ctDNA levels correlate with clinical outcomes (3). For example, our group recently demonstrated the predictive and prognostic value of ctDNA in patients with high-risk breast cancer enrolled in the neoadjuvant I-SPY2 trial (4,5). Compared to ctDNA, cfDNA concentration is a less well-studied biomarker in breast cancer, particularly in the neoadjuvant setting (6–8).
A key advantage of cfDNA over ctDNA is the lower cost of testing to measure its abundance in the plasma. Measuring cfDNA concentration is about 10–30x and 100x less expensive than next-generation sequencing (NGS)-based tumor-agnostic and tumor-informed ctDNA tests, respectively (9). Thus, if cfDNA concentration can predict response and survival, the cost of liquid biopsy testing for disease assessment during NAC could be significantly reduced.
Previous work from our group examined the association of ctDNA with treatment response and survival in patients with high-risk early-stage HER2-negative breast cancer (4). Here, we compared the clinical significance of ctDNA vs. cfDNA concentration in the same cohort of patients. Both biomarkers were measured in the same samples. Based on previous observations of subtype-specificity in ctDNA associations with clinicopathologic variables (4,5), we hypothesized that the predictive and prognostic value of cfDNA concentration might vary between HR-positive/HER2-negative and TNBC groups.
METHODS
Patients.
283 high-risk (MammaPrint high) early-stage HER2-negative breast cancer patients who had ctDNA data (positive/negative and mean tumor molecules per mL of plasma, MTM/mL) from a previous study (performed in collaboration with Natera) were included in the analysis (4). Of the 283, 145 had HR-positive/HER2-negative breast cancer, and 138 had TNBC (Supplementary Figure 1A). Patients were enrolled in the I-SPY2 trial (NCT01042379) and received taxane and anthracycline/cyclophosphamide (T-AC) regimens with or without an investigational drug (Supplementary Figure 1B). The I-SPY2 trial eligibility criteria include age 18 or over and the ability to give informed consent, a new diagnosis of stage 2 or 3 invasive breast cancer, and a tumor 2.5 cm or larger (10). The representativeness of the patient population is described in Supplementary Table 1.
Institutional Review Boards at all participating institutions approved the I-SPY2 trial protocol. I-SPY2 investigators obtained written informed consent from all participants to allow research on their biospecimen samples. The studies were conducted in accordance with the criteria set by the Declaration of Helsinki.
Specimen characteristics.
Blood was collected at pretreatment (T0), 3 weeks after initiation of treatment (T1), 12 weeks after treatment initiation between paclitaxel-based and AC regimens (T2), and after NAC before surgery (T3) as previously described (4).
Assay methods.
CfDNA was extracted from plasma using a silica-based column that preferentially binds nucleic acids (QIAmp circulating nucleic acid kit, Qiagen). CfDNA concentration was measured using an automated electrophoretic separation assay (cfDNA Screen Tape analysis, Agilent). CfDNA concentration was reported as ng/mL of plasma.
ctDNA analysis was performed on the same isolated cfDNA samples, and the results have been reported elsewhere (4). ctDNA was detected using a tumor-informed assay (Signatera™) that involved whole exome sequencing of pretreatment tumor and the selection of up to 16 patient-specific truncal mutations (high variant allele frequency) in the tumor tissue. Polymerase chain reaction primers were designed to amplify the chromosome region containing the mutation, and the amplicons were subjected to deep sequencing to detect mutant copies (ctDNA) in cfDNA.
The cfDNA and ctDNA assays were performed at a commercial facility (Natera Inc) by technicians blinded to patient outcomes.
Study design.
The study describes an unplanned analysis to test associations of cfDNA concentration—as a continuous and dichotomous variable—with clinicopathologic variables, response, and survival and to compare the predictive and prognostic value of cfDNA concentration vs. ctDNA in the same cohort of patients. The associations of pretreatment (T0) cfDNA concentration with clinical T stage, node-positivity, grade, and MammaPrint scores were examined. Patients within each subtype were dichotomized into the high (≥median) and low (<median) cfDNA-shedders using the median cfDNA concentration at each time point as the cutoff.
The early response endpoint used was RCB, representing the extent of remaining invasive cancer in the breast and axillary lymph nodes following NAC (11). The RCB method yields a continuous score called the RCB index, which can be converted into 4 categorical groups called the RCB classes using empirically derived cutoffs. The RCB classes, RCB-0, -I, -II, and -III, represent pCR, limited, moderate, and extensive residual cancer, respectively (11). pCR or RCB-0 is the absence of invasive cancer in the breast and regional lymph nodes after NAC. RCB is highly predictive of survival (11).
The survival endpoint was DRFS, the time interval between the patient’s consent for treatment and the clinical diagnosis of metastatic recurrence or death by any cause. The participants were enrolled between March 2010 and July 2018. The median follow-up times for the HR-positive/HER2-negative and TNBC groups were 3.10 years (range 0.46–7.6) and 3.12 years (range 0.31–7.91), respectively.
Statistical analyses.
The Wilcoxon rank sum and the Kruskal-Wallis tests were used to compare cfDNA concentrations (ng/mL) between 2 and 3 or more groups, respectively. The Bonferroni correction was used to adjust p-values for multiple comparisons. Fisher’s exact test was used to determine the association between categorical variables. Pearson’s correlation coefficient was used to assess the correlation between 2 continuous variables. Cox proportional hazards regression analysis was used to estimate hazard ratios and 95% confidence intervals, and p values were calculated using the Wald test. In multivariable analysis, we chose RCB-0/pCR and ctDNA as covariates based on previous findings showing a strong prognostic impact of these variables in the neoadjuvant setting (1,4). Kaplan-Meier analysis and the log-rank test were used to visualize and compare survival curves. For Cox proportional hazards model, Kaplan-Meier survival analysis, and log-rank tests, we used the R package “survival”.
Data availability.
The data supporting the findings of this study are available in Supplementary Table 2. Raw data are available upon request to the corresponding author.
RESULTS
Patients, samples, and cfDNA concentration across subtypes
CfDNA concentration was successfully measured in 1,024 serial plasma samples collected from the same 283 patients (145 HR-positive/HER2-negative and 138 TNBC) with ctDNA data previously reported (4). (Supplementary Figure 1A). All plasma samples collected had measurable cfDNA concentrations, and the data were used for the analysis. In patients with missing cfDNA data, the sample was either not collected or available for analysis. CfDNA concentration and ctDNA data were available for all 4 timepoints for 230 and 229 patients, respectively.
There was no significant difference in cfDNA concentration between HR-positive/HER2-negative and TNBC subtypes at pretreatment (T0), on-treatment (T1 and T2), or after NAC before surgery (T3) (Supplementary Figure 2).
Clinical correlates of pretreatment cfDNA concentration
In the TNBC group, patients with larger tumors (stage T3/T4) had significantly higher pretreatment (T0) cfDNA concentration compared to those with smaller tumors (stage T1/T2) (Wilcoxon p=0.023) Supplementary Figure 3). In addition, a significantly higher proportion of patients with grade 3 TNBC were high shedders at pretreatment compared to those with grade 1/2 disease (55.9% vs. 16.7%; Fisher exact p=0.0134, Table 1). In contrast, no significant association was observed between pretreatment cfDNA concentration and clinicopathologic variables in the HR-positive/HER2-negative group (Supplementary Figure 3 and Table 1).
Table 1. Association between cfDNA concentration at pretreatment (T0) and clinicopathologic characteristics.
Patients with hormone receptor-positive HER2-negative (HR+HER2−) and triple-negative breast cancer (TNBC) were stratified into two groups, cfDNA high shedders vs. cfDNA low shedders, using median cfDNA concentration at pretreatment (T0) as the cutoff. Abbreviations: RCB-residual cancer burden, pCR-pathologic complete response
| cfDNA-high at T0 | cfDNA-low at T0 | Fisher p value | |||||
|---|---|---|---|---|---|---|---|
| Total | N | % | N | % | |||
| HR+HER2− (n=143, cutoff= 6.8297 ng/mL) | |||||||
| Clinical T stage (n=120) | 0.8545 | ||||||
| T1/T2 | 81 | 39 | 48.1 | 42 | 51.9 | ||
| T3/T4 | 39 | 19 | 48.7 | 20 | 51.3 | ||
| Clinical N stage (n=116) | 0.8545 | ||||||
| Node-negative | 55 | 26 | 47.3 | 29 | 52.7 | ||
| Node-positive | 61 | 30 | 49.2 | 31 | 50.8 | ||
| Grade (n=118) | 0.1967 | ||||||
| 1/2 | 56 | 24 | 42.9 | 32 | 57.1 | ||
| 3 | 62 | 35 | 56.5 | 27 | 43.5 | ||
| MammaPrint score (n=143) | 1.0000 | ||||||
| High 1 | 102 | 51 | 50.0 | 51 | 50.0 | ||
| High 2 | 41 | 21 | 51.2 | 20 | 48.8 | ||
| RCB class (n=142) | 0.0993 | ||||||
| RCB-0/pCR | 22 | 9 | 40.9 | 13 | 59.1 | ||
| RCB-I | 10 | 2 | 20.0 | 8 | 80.0 | ||
| RCB-II | 61 | 36 | 59.0 | 25 | 41.0 | ||
| RCB-III | 49 | 25 | 51.0 | 24 | 49.0 | ||
| TNBC (n=137; cutoff=6.2857 ng/mL) | |||||||
| Clinical T stage (n=123) | 0.5532 | ||||||
| T1/T2 | 87 | 42 | 48.3 | 45 | 51.7 | ||
| T3/T4 | 36 | 20 | 55.6 | 16 | 44.4 | ||
| Clinical N stage (n=118) | 0.8538 | ||||||
| Node-negative | 62 | 30 | 48.4 | 32 | 51.6 | ||
| Node-positive | 56 | 29 | 51.8 | 27 | 48.2 | ||
| Grade (n=105) | 0.0134 | ||||||
| 1/2 | 12 | 2 | 16.7 | 10 | 83.3 | ||
| 3 | 93 | 52 | 55.9 | 41 | 44.1 | ||
| MammaPrint score (n=137) | 0.3002 | ||||||
| High 1 | 16 | 6 | 37.5 | 10 | 62.5 | ||
| High 2 | 121 | 63 | 52.1 | 58 | 47.9 | ||
| RCB class (n=132) | 0.7524 | ||||||
| RCB-0/pCR | 34 | 19 | 55.9 | 15 | 44.1 | ||
| RCB-I | 20 | 11 | 55.0 | 9 | 45.0 | ||
| RCB-II | 54 | 28 | 51.9 | 26 | 48.1 | ||
| RCB-III | 24 | 10 | 41.7 | 14 | 58.3 | ||
Predictive value of cfDNA concentration
We assessed the relationship of cfDNA concentration over time with response to NAC. In this biomarker study cohort (4), the RCB-0 (pCR) rates were 15.2% (22/145) in the HR-positive/HER2-negative group and 24.6% (34/138) in the TNBC group. Given the continuous nature of cfDNA concentration, we examined its correlation with RCB index, the continuous measure of RCB. We found no significant correlation between cfDNA concentration and RCB index in the HR-positive/HER2-negative group at any timepoint (Figures 1A and 1B, Supplementary Figures 4 and 5).
Figure 1. Association of cfDNA concentration early during treatment and residual cancer burden (RCB) after neoadjuvant chemotherapy.
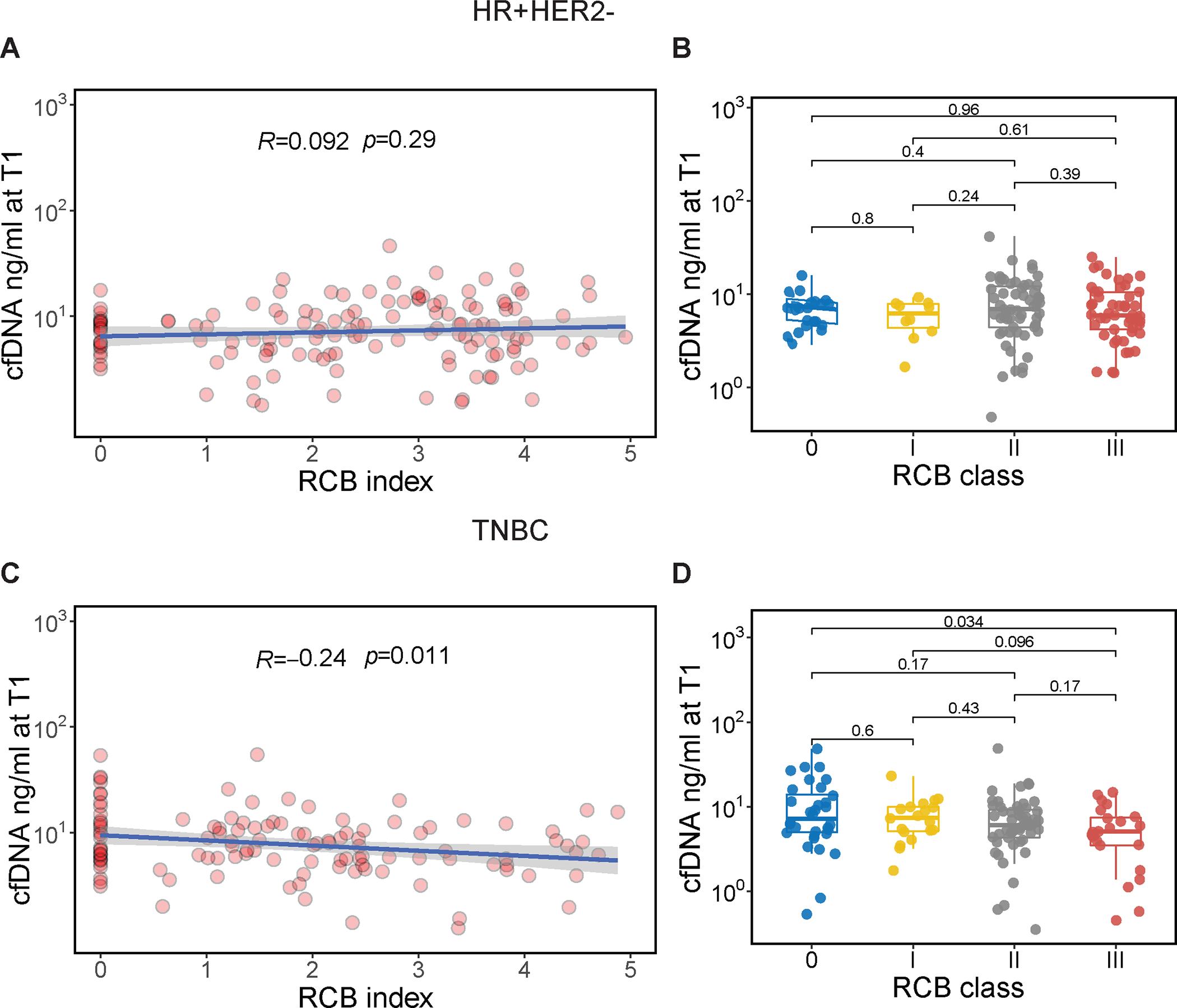
CfDNA concentration was measured in the plasma 3 weeks after treatment initiation (T1) in patients with hormone receptor-positive/HER2-negative (HR+HER2−, top panel) and triple-negative breast cancer (TNBC, lower panel); (A, C) Correlation of cfDNA concentration (ng/ mL, log10-transformed) at T1 and RCB index. The blue line and gray shading represent the regression line and the 95% confidence interval, respectively. Correlation coefficient and p values were calculated using Pearson’s correlation test; (C, D) Distribution of cfDNA concentration at T1 by RCB class. RCB was divided into 4 classes: RCB-0, equivalent to pathologic complete response, and −I, −II, −III, representing limited, moderate, and extensive residual cancer, respectively. For each box plot, the center line represents the median value (50th percentile), while the box contains the 25th to 75th percentiles of the data distribution. The whiskers represent the 5th and 95th percentiles, and the dots beyond the upper and lower bounds are considered outliers. Pairwise P values were calculated using the Wilcoxon rank sum test with Bonferroni correction to adjust for multiple comparisons.
Interestingly, in the TNBC group, higher cfDNA concentration at 3 weeks after treatment initiation (T1) was significantly correlated with lower RCB index (i.e., less residual cancer) at surgery (Pearson correlation=−0.24, p=0.011, Figure 1C, Supplementary Figure 4). We further examined differences in cfDNA concentration at T1 across RCB classes in the TNBC group. Patients with RCB-0 after NAC had significantly higher cfDNA concentration at T1 than those with extensive residual cancer (RCB-III, Wilcoxon adjusted p=0.034, Figure 1D, Supplementary Figure 5).
Prognostic value of cfDNA concentration
Next, we examined the correlation of cfDNA concentration with survival outcomes. 142 of the 145 and 130 of the 138 patients with HR-positive/HER2-negative and TNBC, respectively, had available DRFS data. DRFS events occurred in 22.5% (32/142) of patients in the HR-positive/HER2-negative group (28 distant recurrences, 4 deaths) and 32.3% (42/130) of patients in the TNBC group (33 distant recurrences, 9 deaths).
We grouped patients as high and low shedders using the median cfDNA concentration as the cutoff. Using this stratification, we investigated whether a high concentration of cfDNA in the blood is associated with poor survival. In the HR-positive/HER2-negative group, we did not observe any significant association except at pretreatment (T0). High cfDNA-shedders had a significantly inferior DRFS compared to low shedders (hazard ratio 2.12, 95% confidence interval 1.05–4.60, Wald p=0.037) (Figure 2A). In multivariable Cox regression analysis adjusting for the effects of pretreatment ctDNA status and response to NAC, high cfDNA shedding remained a significant negative prognostic factor for DRFS (hazard ratio 2.41, 95% confidence interval 1.12–5.18, Wald p=0.02) (Figure 2B). In TNBC, no significant differences in DRFS between groups were observed at any timepoints (Figure 2C, Supplementary Figure 6).
Figure 2. Prognostic significance of pretreatment cfDNA concentration.
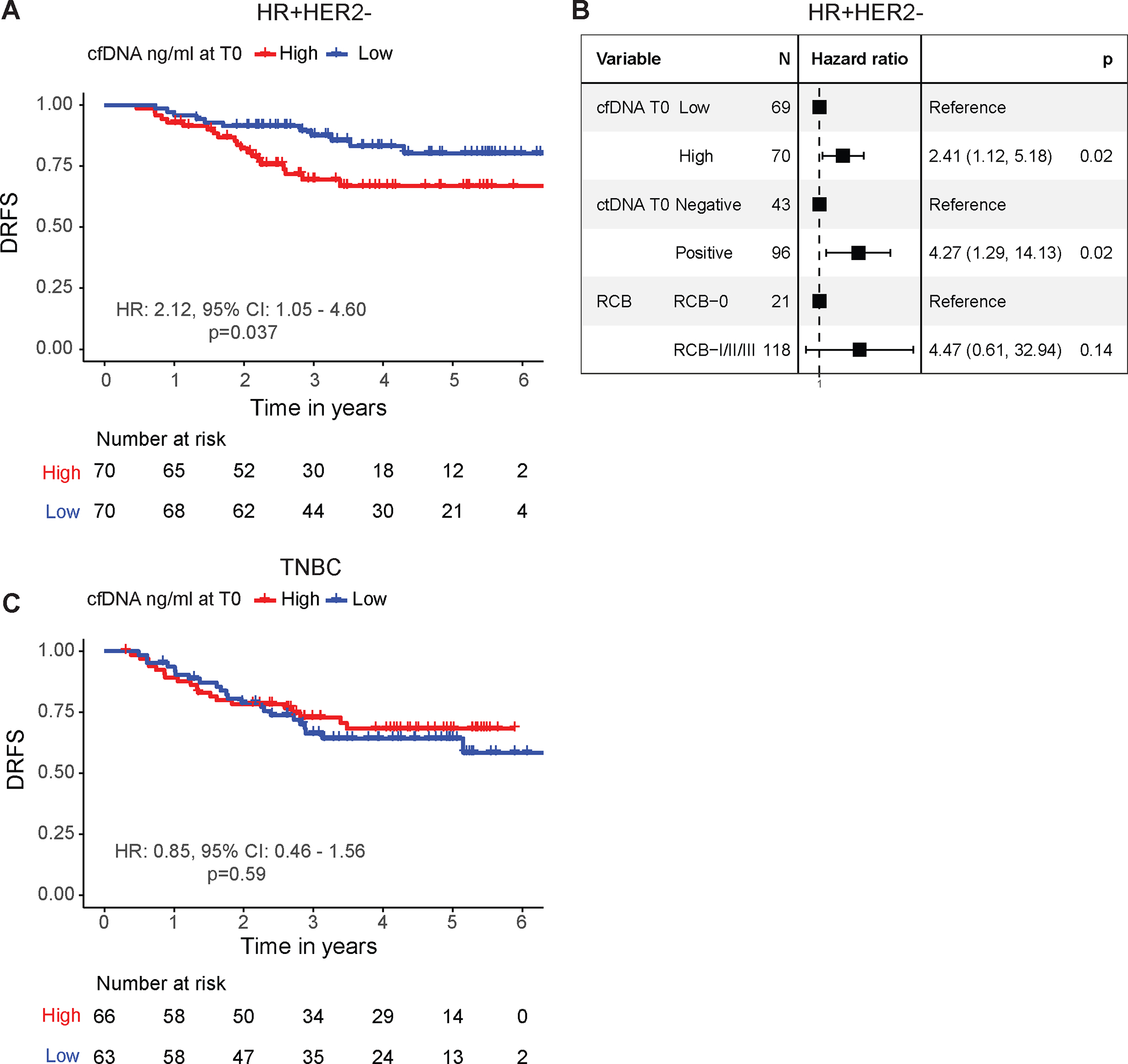
Survival analysis in patients with (A, B) hormone receptor-positive/HER2-negative (HR+HER2−) and (C) triple-negative breast cancer (TNBC) stratified into two groups, high vs. low cfDNA-shedders, using the median cfDNA concentration as the cutoff. The survival endpoint was distant recurrence-free survival (DRFS). Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using univariable Cox regression analysis. P values were calculated using the Wald test. The forest plot in B shows HRs and 95% CIs for patients with HR+HER2− breast cancer, estimated from a multivariable Cox regression model that included cfDNA (cfDNA) concentration at pretreatment (T0), adjusted for circulating tumor DNA (ctDNA) status at pretreatment (T0) and residual cancer burden (RCB).
Clinical significance of cfDNA concentration vs. ctDNA
Comparing the clinical significance of cfDNA vs. ctDNA (4) yielded additional observations. Given that ctDNA levels have been shown to differ across subtypes (4,5), we expected the same for cfDNA. However, we found no significant differences in cfDNA concentrations between HR-positive/HER2-negative vs. TNBC subtypes at all timepoints (Supplementary Figure 2).
In the HR-positive/HER2-negative group, pretreatment (T0) cfDNA concentration (as a continuous variable) was not significantly associated with clinical T and N stages, grade, and MammaPrint status (Figure 3A, Supplementary Figure 3). In contrast, pretreatment ctDNA concentration, as previously reported, was significantly associated with all the clinicopathologic variables (4). In the TNBC group, pretreatment cfDNA concentration was significantly higher in patients with larger tumors (stage T3/T4) (Figure 3A, Supplementary Figure 3), and so was pretreatment ctDNA concentration, as well as in node-positive patients, as previously shown (4).
Figure 3. Clinical significance of cell-free DNA (cfDNA) vs. circulating tumor DNA (ctDNA) in hormone receptor-positive HER2-negative (HR+HER2−) and triple-negative breast cancer (TNBC).
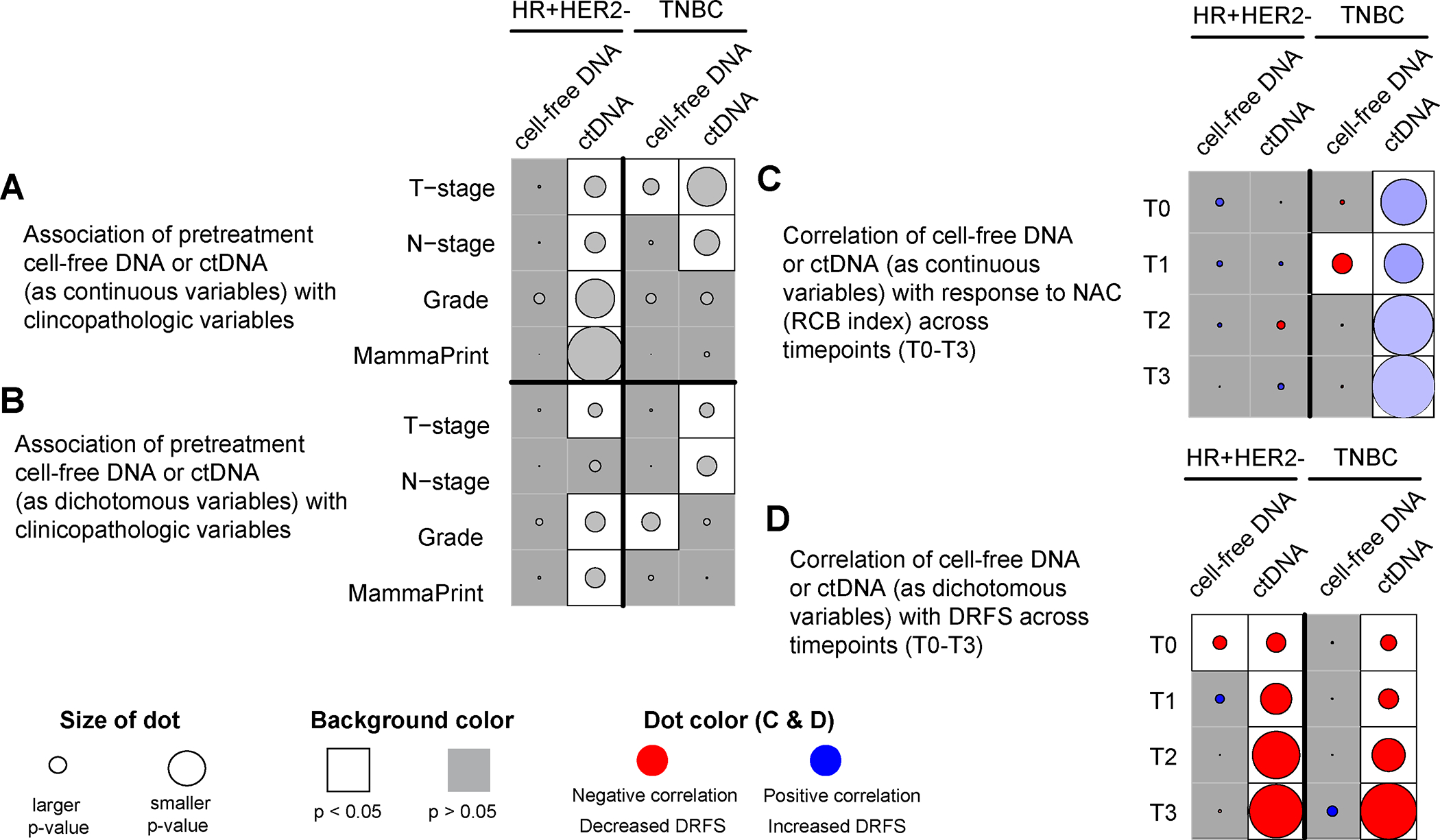
(A) Association of pretreatment (T0) cfDNA (see Supplementary Figure 3) and ctDNA concentration as continuous variables with clinicopathologic variables. The p values were calculated using the Wilcoxon rank-sum test; (B) Association of pretreatment (T0) cfDNA concentration (high vs. low cfDNA shedders using the median as the cutoff, see Table 1) and ctDNA (positive vs. negative) as dichotomous variables with clinicopathologic variables. The p values were calculated using Fisher’s exact test; (C) Correlation of cfDNA and ctDNA concentration (as continuous variables) at different timepoints (T0-T3) vs. residual cancer burden (RCB) index, the continuous measure of residual disease in the breast and regional lymph nodes after NAC. The p values were calculated using Pearson’s correlation test (see Figure 1 and Supplementary Figure 4). The color of the dot represents a negative (red) or positive (blue) correlation; (D) Correlation of cfDNA and ctDNA (as dichotomous variables) at different timepoints (T0-T3) vs. distant recurrence-free survival (DRFS) using Cox regression analysis. The p values were calculated using the Wald test (see Figure 2 and Supplementary Figure 6). The color of the dot represents decreased DRFS (red, hazard ratio>1) or increased DRFS (blue, hazard ratio <1). CfDNA and ctDNA were analyzed in the same plasma sample collected at pretreatment (T0), 3 weeks after treatment initiation (T1), 12 weeks after treatment initiation between paclitaxel-based treatment and anthracycline regimens (T2), and after neoadjuvant chemotherapy before surgery (T3).
In the HR-positive/HER2-negative group, pretreatment cfDNA concentration (as a dichotomous variable, median cutoff) was not associated with any of the clinicopathologic variables examined (Figure 3B, Table 1). In contrast, our previous report showed that pretreatment ctDNA-positivity was significantly associated with larger tumors (stage T3/T4), and higher grade and MammaPrint score (4). In the TNBC group, high pretreatment cfDNA concentration was significantly associated with higher grade (Figure 3B, Table 1), as was pretreatment ctDNA-positivity and node-positivity, as previously reported (4).
In the HR-positive/HER2-negative group, we did not observe significant association between cfDNA and ctDNA concentrations vs. RCB index (Figure 3C, Supplementary Figures 4 and 5). In the TNBC group, a modest negative correlation between cfDNA concentration 3 weeks after initiation of treatment (T1) and RCB index was observed. In contrast, ctDNA concentrations at all timepoints were significantly correlated with RCB index but in the opposite direction (positive correlation).
CfDNA concentration (as a dichotomous variable) at pretreatment but not at other timepoints was significantly correlated with a decreased DRFS only in the HR-positive/HER2-negative group (Figure 3D, Supplementary Figure 6). In contrast, ctDNA-positivity at pretreatment, during and after NAC, as previously shown, was significantly associated with decreased DRFS in both subtypes (4).
DISCUSSION
This study examined the clinical significance of cfDNA concentration as a biomarker of response and survival in patients with high-risk early-stage HER2-negative breast cancer receiving NAC. High pretreatment levels of cfDNA in TNBC were associated with larger tumors and higher-grade disease, suggesting that cfDNA concentration may reflect tumor burden and aggressiveness in this subtype. In addition, patients with TNBC with no residual cancer after NAC (RCB-0) had higher cfDNA concentration 3 weeks after initiation of treatment compared to non-responders, suggesting an early increase in apoptotic rates of tumor and normal cells in responding triple-negative tumors and possibly hematopoietic cells, resulting in increased cfDNA concentrations in the blood. The mechanism involved in the increased cfDNA shedding in responding triple-negative tumors remains unclear. Further clinical studies and pre-clinical experiments using model systems (12) may shed light on the underlying mechanisms that govern cfDNA shedding. Studies suggest that cellular processes [e.g., apoptosis, necrosis, and senescence (2,12)], treatment response (13), and the tumor microenvironment (12,14) play important roles in cfDNA release. Mattox and colleagues showed that the major fraction of cfDNA observed in patients with cancers did not come from tumor cells or epithelial cells surrounding the tumor of origin but from leukocytes (~76%), primarily neutrophils (15). This could explain the lower specificity of cfDNA for predicting patient outcomes compared to ctDNA.
Survival analysis revealed that high cfDNA-shedders at pretreatment in the HR-positive/HER2-negative group, but not in TNBC, had a significantly increased risk of metastatic recurrence and death compared to low shedders. Our previous study in the same cohort revealed a strong association between the clearance of ctDNA and pCR in the TNBC group and DRFS in both HER2-negative subtypes (4). In this study, we observed that none of the patients cleared their cfDNA, i.e., all had measurable levels of cfDNA at all timepoints, regardless of tumor burden. This would require a complex process of setting cut-offs if cfDNA concentration is to be a useful predictor.
The study has several limitations. First, patients received different investigational agents with the T-AC backbone. Also, our study was limited to patients with HER2-negative disease due to the small sample size of the HER2-positive group. Moreover, the median split of cfDNA concentration to group patients into high and low cfDNA-shedders was arbitrarily chosen. Survival analyses using other cutoffs (e.g., 75th percentile) did not improve the prognostic signal. The median follow-up time of ~3 years was not long enough to observe late recurrences, especially in the HR-positive/HER2-negative group, where there is a persistent risk of recurrence for at least 20 years after the original breast cancer diagnosis (16). Finally, we analyzed the correlation of ctDNA concentration with patient outcomes at each timepoint separately; however, longitudinal analyses in larger cohorts that consider cfDNA concentration dynamics (increase or decrease) during treatment may provide further insights into the predictive value of cfDNA.
Studies from our group (4,5,17) and others [reviewed in (3)] have consistently shown the negative prognostic impact of ctDNA detection in patients with breast cancer receiving neoadjuvant therapy. Analysis of 6 studies in early-stage breast cancer receiving neoadjuvant therapy showed no association between ctDNA detection and pCR (3). In contrast, a recent report from our group showed that early ctDNA clearance in the TNBC but not in the HR-positive/HER2-negative group was significantly associated with an increased probability of achieving a pCR (4). The disparate findings indicate that further research is needed to better understand the predictive value of ctDNA.
The limited clinical significance of cfDNA concentration relative to ctDNA may be due to the inter- and intra-individual variations (18), which can hinder the elucidation of clinically relevant changes during treatment. Also, technical [e.g., quantification method (19)] and biological factors [e.g., age, body mass index, and chronic diseases (20)] not directly related to tumor cell apoptosis, necrosis or senescence can affect cfDNA levels in the blood and lead to spurious findings.
In summary, cfDNA concentration in TNBC reflected initial tumor burden and aggressiveness but was not strongly correlated with response or prognosis. Pretreatment cfDNA concentration in HR-positive/HER2-negative breast cancer was prognostic for DRFS. Overall, the clinical significance of cfDNA concentration was more limited than that of ctDNA. Our findings and those of others (3) support further investigation of ctDNA as a read-out of tumor response for predicting clinical outcomes in patients with breast cancer receiving NAC.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Measuring cell-free DNA (cfDNA) concentration in the blood and its subset, circulating tumor DNA (ctDNA) exclusively shed by tumor cells, offers a minimally invasive approach to monitoring tumor response and predicting patient survival. The clinical significance of cfDNA concentration is less studied than that of ctDNA. A key advantage of cfDNA over ctDNA is the lower cost of testing to measure its abundance in the plasma. This study compared the predictive and prognostic value of cfDNA concentration vs. ctDNA in patients with high-risk early-stage HER2-negative breast cancer (hormone receptor-positive/HER2-negative and triple-negative) receiving neoadjuvant chemotherapy in the I-SPY2 trial. Overall, the clinical significance of cfDNA concentration was more limited than that of ctDNA. cfDNA concentration had lower specificity for predicting response and survival compared to ctDNA. Our data and accumulating evidence from other studies support further investigation of ctDNA as a read-out of tumor response in the neoadjuvant setting.
ACKNOWLEDGMENTS
The authors thank the I-SPY2 Biomarker Working Group, patients, advocates, and investigators. The work reported in this paper is funded in part by NIH/NCI (grant R01CA255442), NIH/NCI I-SPY2+ (Grant PO1-CA210961), NIH/NCI Imaging (Grant 28XS197 P-0518835), NIH/NCI CCMI (Grant U54CA209891), NIH/NCI CCSG (Grant P30-CA82103), NIH/NHGRI Big Data (Grant U54-HG007990), Breast Cancer Research Foundation (Grant BCRF-20-142), Breast Cancer Research Foundation (Grant BCRF-20-165), Breast Cancer Research – Atwater Trust, Stand up to Cancer, California Breast Cancer Research Program, and Give Breast Cancer the Boot. Natera provided in-kind whole exome sequencing of tumor biopsies and Signatera testing of blood specimens while blinded to patient outcome data and contracted through the non-profit Quantum Leap Healthcare Collaborative – the I-SPY sponsor.
CONFLICT OF INTEREST
GLH reports grants from National Institutes of Health during the conduct of the study. CY reports grants from National Institutes of Health/National Cancer Institute; support from Quantum Leap Healthcare Collaborative during the conduct of the study; and has a patent pending for US Application No. 18/174,191. PRP reports personal fees from Frontiers and Pfizer; grants from Pfizer, Carisma Therapeutics and Orum Therapeutics outside the submitted work; and other support from Seagen. WFS reports a patent for Method to measure residual cancer burden after neoadjuvant chemotherapy issued and licensed to Delphi Diagnostics and a patent for Method to predict sensitivity to endocrine therapy of breast cancer issued and licensed to Delphi Diagnostics. DY reports support from Quantum Leap Health Care Collaborative during the conduct of the study. NMH reports grants from National Institutes of Health during the conduct of the study. LJE reports grants from Quantum Leap Healthcare Collaborative during the conduct of the study; reports participation on the Blue Cross Medical Advisory Panel; reports participation as an uncompensated board member of Quantum Leap Healthcare Collaborative; and serves as principal investigator for an investigator initiated Phase1 trial for high-risk DCIS funded by Moderna. AMD reports grants from Quantum Leap Healthcare Collaborative during the conduct of the study; reports grants from Neogenomics, Novartis, Genentech, and Pfizer outside the submitted work. HSR reports grants from OBI Pharma, AstraZeneca, Pfizer, Novartis, Eli Lilly, Hoffmann-La Roche AG/Genentech, Merck and Daiichi Sankyo, Inc.; reports personal fees from NAPO, Daiichi Sankyo, inc. and Eisai; reports grants from Gilead Sciences, Inc., Stemline Therapeutics, Ambrx; and reports personal fees from Mylan/Viatris during the conduct of the study. LJvV reports personal fees and other support from Agendia during the conduct of the study; and reports other support from ExaiBio outside the submitted work. All other authors declare no competing interests.
REFERENCES
- 1.I-SPY Trial Consortium, Yee D, DeMichele AM, Yau C, Isaacs C, Symmans WF, et al. Association of Event-Free and Distant Recurrence-Free Survival With Individual-Level Pathologic Complete Response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: Three-Year Follow-up Analysis for the I-SPY2 Adaptively Randomized Clinical Trial. JAMA Oncol 2020;6:1355–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stejskal P, Goodarzi H, Srovnal J, Hajduch M, van ‘t Veer LJ, Magbanua MJM. Circulating tumor nucleic acids: biology, release mechanisms, and clinical relevance. Mol Cancer 2023;22:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papakonstantinou A, Gonzalez NS, Pimentel I, Sunol A, Zamora E, Ortiz C, et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: A systematic review and meta-analysis. Cancer Treat Rev 2022;104:102362. [DOI] [PubMed] [Google Scholar]
- 4.Magbanua MJM, Brown Swigart L, Ahmed Z, Sayaman RW, Renner D, Kalashnikova E, et al. Clinical significance and biology of circulating tumor DNA in high-risk early-stage HER2-negative breast cancer receiving neoadjuvant chemotherapy. Cancer Cell 2023;41:1091–102 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol 2021;32:229–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Garcia D, Hills A, Page K, Hastings RK, Toghill B, Goddard KS, et al. Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res 2019;21:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan F, Wang JH, Cullinane C, Ita M, Corrigan M, O’Leary DP, et al. Assessment of cell-free DNA (cfDNA) concentrations in the perioperative period can predict risk of recurrence in patients with non-metastatic breast cancer. Surg Oncol 2022;42:101753. [DOI] [PubMed] [Google Scholar]
- 8.Peled M, Agassi R, Czeiger D, Ariad S, Riff R, Rosenthal M, et al. Cell-free DNA concentration in patients with clinical or mammographic suspicion of breast cancer. Sci Rep 2020;10:14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gobbini E, Swalduz A, Levra MG, Ortiz-Cuaran S, Toffart AC, Perol M, et al. Implementing ctDNA Analysis in the Clinic: Challenges and Opportunities in Non-Small Cell Lung Cancer. Cancers (Basel) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rugo HS, Olopade OI, DeMichele A, Yau C, van ‘t Veer LJ, Buxton MB, et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N Engl J Med 2016;375:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symmans WF, Yau C, Chen YY, Balassanian R, Klein ME, Pusztai L, et al. Assessment of Residual Cancer Burden and Event-Free Survival in Neoadjuvant Treatment for High-risk Breast Cancer: An Analysis of Data From the I-SPY2 Randomized Clinical Trial. JAMA Oncol 2021;7:1654–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, Necrosis, and Apoptosis Govern Circulating Cell-free DNA Release Kinetics. Cell Rep 2020;31:107830. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Cai GX, Han BW, Guo ZW, Wu YS, Lyu X, et al. Association between the nucleosome footprint of plasma DNA and neoadjuvant chemotherapy response for breast cancer. NPJ Breast Cancer 2021;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouliere F, Thierry AR. The importance of examining the proportion of circulating DNA originating from tumor, microenvironment and normal cells in colorectal cancer patients. Expert Opin Biol Ther 2012;12 Suppl 1:S209–15 [DOI] [PubMed] [Google Scholar]
- 15.Mattox AK, Douville C, Wang Y, Popoli M, Ptak J, Silliman N, et al. The origin of highly elevated cell-free DNA in healthy individuals and patients with pancreatic, colorectal, lung, or ovarian cancer. Cancer Discov 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 2017;377:1836–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magbanua MJM, Li W, Wolf DM, Yau C, Hirst GL, Swigart LB, et al. Circulating tumor DNA and magnetic resonance imaging to predict neoadjuvant chemotherapy response and recurrence risk. NPJ Breast Cancer 2021;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madsen AT, Hojbjerg JA, Sorensen BS, Winther-Larsen A. Day-to-day and within-day biological variation of cell-free DNA. EBioMedicine 2019;49:284–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Pol Y, Mouliere F. Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA. Cancer Cell 2019;36:350–68 [DOI] [PubMed] [Google Scholar]
- 20.Orntoft MW, Jensen SO, Ogaard N, Henriksen TV, Ferm L, Christensen IJ, et al. Age-stratified reference intervals unlock the clinical potential of circulating cell-free DNA as a biomarker of poor outcome for healthy individuals and patients with colorectal cancer. Int J Cancer 2021;148:1665–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in Supplementary Table 2. Raw data are available upon request to the corresponding author.


