Abstract
Vectors derived from adeno-associated virus type 2 (AAV2) promote gene transfer and expression in the lung; however, we have found that while gene expression can persist for at least 8 months in mice, it was reduced dramatically in rabbits over a period of 2 months. The efficiency and persistence of AAV2-mediated gene expression in the human lung have yet to be determined, but it seems likely that readministration will be necessary over the lifetime of an individual. Unfortunately, we have found that transduction by a second administration of an AAV2 vector is blocked, presumably due to neutralizing antibodies generated in response to the primary vector exposure. Here, we have explored the use of AAV2 vectors pseudotyped with capsid proteins from AAV serotypes 2, 3, and 6 for readministration in the mouse lung. We found that an AAV6 vector transduced airway epithelial and alveolar cells in the lung at rates that were at least as high as those of AAV2 pseudotype vectors, while transduction rates mediated by AAV3 were much lower. AAV6 pseudotype vector transduction was unaffected by prior administration of an AAV2 or AAV3 vector, and transduction by an AAV2 pseudotype vector was unaffected by prior AAV6 vector administration, showing that cross-reactive neutralizing antibodies against AAV2 and AAV6 are not generated in mice. Interestingly, while prior administration of an AAV2 vector completely blocked transduction by a second AAV2 pseudotype vector, prior administration of an AAV6 vector only partially inhibited transduction by a second administration of an AAV6 pseudotype vector. Analysis of sera obtained from mice and humans showed that AAV6 is less immunogenic than AAV2, which helps explain this finding. These results support the development of AAV6 vectors for lung gene therapy both alone and in combination with AAV2 vectors.
Adeno-associated viruses (AAV) are single-stranded DNA parvoviruses that are dependent on helper viruses, such as adenovirus, for efficient replication and expression. Vectors based on AAV can integrate and promote persistent gene expression in cultured cells and in dividing and nondividing cells in multiple somatic tissues of animals (23). Long-term expression of clinically relevant levels of erythropoietin human clotting factor IX and human granulocyte colony-stimulating factor (G-CSF) have been achieved in mice following AAV-mediated gene transfer to muscle and liver (16–19, 27), indicating the potential of these vectors for human gene therapy.
Transfer of therapeutic genes into the lung epithelium may provide a cure for diseases such as cystic fibrosis (CF). CF affects 1 in 3,000 Caucasian births and is caused by mutations in a chloride ion channel (CF transmembrane conductance regulator [CFTR]) that result in gradual lung destruction, the major cause of morbidity. Current treatments for CF require lifelong therapeutic interventions aimed at alleviating the symptoms. In contrast, the delivery of a functional CFTR gene to the lung would make possible long-term correction of the major defect and prevent progressive fatal lung disease.
AAV vectors can transduce multiple cell types in the lung, but animal data thus far have not shown clinically relevant levels of therapeutic gene expression from AAV vectors in the lung. Bronchoscopic administration of an AAV vector containing the human CFTR cDNA resulted in localized gene transfer and expression in areas of the normal adult rabbit lung at the site of vector delivery (12). Delivery of an AAV vector encoding human placental alkaline phosphatase (AP) to the adult rabbit lung by use of a balloon catheter also showed localized transduction at the site of delivery, which appeared to depend on local tissue damage (14). AAV transduction in the developing neonatal rabbit lung was more efficient and was observed in a variety of airway and alveolar cell types (32). In the adult mouse lung, AAV vector transduction was rare, but the frequency could be increased by addition of adenovirus to provide helper functions (9). These results indicate that the efficiency of AAV transduction in the normal lung epithelium is low but might be enhanced by cell proliferation, tissue injury, or adenovirus helper functions. Alternatively, administration of much higher doses of AAV vector could also increase transduction in the lung epithelium (15).
Although AAV vector expression can persist in the liver and muscle of animals, its persistence in the lung epithelium is more complex. Expression of AAV vectors in neonatal or adult rabbit lungs declined dramatically within the course of the experiments (12, 14, 32), whereas AAV vector expression in the epithelia of the mouse lung persisted for 8 months, the duration of the experiments (15). It is not known whether gene expression in human airways will persist, but it is likely that expression will eventually be lost due to a low but constant turnover rate of the epithelium. Additionally, more than one AAV vector administration may be required to achieve therapeutic levels of vector expression. Therefore, readministration of vector may be required to achieve lifelong therapy in the lung. However, we found that readministration of vector did not result in new transduction events in the rabbit or mouse lung, and this lack of transduction was correlated with the presence of neutralizing antibodies to AAV vector in serum (14, 15). These results are consistent with those obtained with skeletal muscle and liver, where AAV vector readministration resulted in little or no new transduction (10, 29, 30).
Here, we have explored the use of other AAV serotypes to allow repeat transduction. There are six known serotypes of AAV. AAV types 1 (AAV1), 2, and 3 were isolated from humans as contaminants of adenovirus preparations (24). AAV4 was isolated from captive monkeys (4), and AAV5 was obtained from a human genital lesion (1). AAV6 was most recently isolated and cloned and was found as a contaminant of a laboratory adenovirus preparation (25). Serum from rabbits immunized with an AAV2 vector completely neutralized AAV2 and partially neutralized AAV3, but did not neutralize the AAV6 vector in tissue culture assays (25). These results led us to hypothesize that AAV6 and possibly AAV3 could be utilized for readministration purposes. We show here that AAV6 vectors have properties that should be useful for gene therapy, including low immunogenicity and the lack of cross-reactive antibodies generated against AAV2 and AAV6.
MATERIALS AND METHODS
Cell culture.
The 293 human embryonic kidney cells (13), HT-1080 human fibrosarcoma cells (ATCC CCL 121), COS-1 monkey kidney cells (ATCC CRL 1650), and BHK-21 baby hamster kidney cells (20) were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of amphotericin B per ml. Cells were cultured at 37°C in an atmosphere of 10% CO2.
AAV vectors.
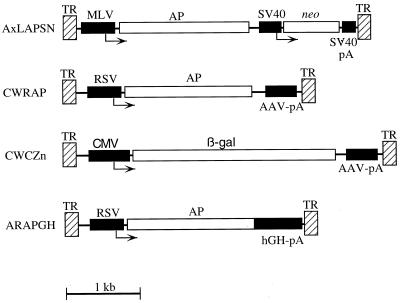
AAV vectors (Fig. 1) and packaging plasmids were propagated in Escherichia coli JC8111 (7) or SURE (Stratagene). The AAV2-based vectors CWRAP (11) and A2LAPSN (25), the AAV3-based vector A3LAPSN (25), and the AAV6-based vector A6LAPSN (25) have been described previously. The AAV2-based vector CWCZn was derived from CWRAP by replacing the Rous sarcoma virus (RSV) promoter and enhancer sequences with an immediate early promoter and enhancer from cytomegalovirus and by replacing the AP cDNA with a cDNA encoding a nuclear-localizing bacterial β-galactosidase (β-Gal) cDNA. The AAV2-based vector ARAPGH contains the human placental AP cDNA driven from an RSV promoter and enhancer sequences and contains the human growth hormone intron and polyadenylation sequences. The serotype of the capsid proteins used to package an AAV vector, or vector pseudotype, is indicated in parentheses after the vector name.
FIG. 1.
AAV vectors. AAV TRs (hatched boxes), coding regions (open boxes), promoters and polyadenylation sequences (solid boxes), and transcriptional start sites (arrows) are indicated. Abbreviations: TR, AAV TR; MLV, Moloney murine leukemia promoter and enhancer; RSV, RSV promoter and enhancer; CMV, cytomegalovirus promoter and enhancer; SV40, simian virus 40 early promoter; SV40-pA, AAV-pA, and hGH-pA, simian virus 40, AAV, and human growth hormone polyadenylation sequences, respectively; AP, human placental AP; β-gal, bacterial β-Gal; neo, neomycin phosphotransferase. There are three related AxLAPSN vectors, where x represents the AAV type from which the vector was derived, that contain either AAV2, AAV3, or AAV6 TRs. All other vectors contain AAV2 TRs.
AAV vector production and characterization.
Vectors with an AAV2 pseudotype were produced with the AAV packaging plasmids pRepCap2 (25) (A2LAPSN and CWRAP) or pACG (31) (ARAPGH and CWCZn). The plasmids pRepCap3 and pRepCap6 were used to produce vectors with the AAV3 and AAV6 pseudotypes, respectively (25). Production of vectors used in cell culture experiments was done as previously described (26). Briefly, 293 cells were infected with wild-type adenovirus 5 and then cotransfected with the vector and AAV packaging plasmids. Clarified crude cell lysates obtained 3 days after transfection were purified by centrifugation through a sucrose cushion, followed by density banding in cesium chloride, dialysis, and heat inactivation (56°C, 1 h). Production of the AAV2, AAV3, and AAV6 pseudotype vectors used in animal studies was done by a similar procedure except that cell lysates were concentrated by ultrafiltration prior to centrifugation through a sucrose cushion, as described previously (14). Southern analysis was done to determine the number of genome-containing particles in the vector preparations. Titers of ARAPGH vector stocks were determined with HT-1080 cells as targets for transduction. The particle-to-focus-forming unit (FFU) ratios were 103 for AAV2, 3 × 104 for AAV3, and 105 for the AAV6 pseudotype vector preparations of ARAPGH. The titers of the CWCZn preparations were determined with BHK-21 cells. The particle-to-transducing unit ratios were 104 for CWCZn(AAV2) and 106 for CWCZn(AAV6). Vector stocks were characterized for the presence of infectious adenovirus by plaque assay (13), and none was detected (<100 infectious units [IU]/ml). Determination of the presence of replication-competent AAV was done by infectious center assay (14). The CWCZn vector preparations did not have detectable replication-competent AAV (<50 IU/ml) while the ARAPGH preparations contained low (500 IU/ml) to undetectable (<50 IU/ml) levels of replication-competent AAV. These levels of replication-competent AAV do not affect transduction by an AAV vector in the lung or in cultured cells (14).
AAV vector delivery to mouse airways.
Animal studies were performed in accordance with the guidelines set forth by the Institutional Review Office of the Fred Hutchinson Cancer Research Center. C57BL/6 and BALB/c mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Animals received vector by nasal aspiration as previously described (15). Mice were given the first AAV vector on day 1 and the second AAV vector at 4 weeks, and they were sacrificed 3 or 4 weeks after the second vector administration. Blood was obtained by the retroorbital or cardiac routes 3 weeks after a primary administration of an AAV vector to assay for AAV-neutralizing activities.
Quantitation of transduction efficiency.
Animals were euthanatized, and AP staining of mouse lungs was done as previously described (15). Stained portions of the lung were cut into 3-mm-thick slices and paraffin embedded. Four ∼5-μm-thick sections were taken from each lung sample. Three of these were counterstained with nuclear-fast red, and the fourth was counterstained with hematoxylin and eosin. Quantitation of transduction efficiency was done by counting the number of AP+ cells per section on the slides stained with nuclear-fast red. AP+ foci in a total area of 1.5 to 3 cm2 were counted for each lung.
Virus neutralization assay.
Mouse blood was collected by retroorbital or cardiac routes. Human blood was obtained from healthy volunteers at the Fred Hutchinson Cancer Research Center. Serum was prepared by incubating blood at 4°C overnight and by removal of the clot by centrifugation, followed by heat inactivation at 56°C for 40 min. Virus neutralization assays were done as previously described (15). Briefly, AAV2, -3, and -6 pseudotype ARAPGH vectors were diluted to 109 genome-containing particles per ml. Serum or diluted serum (1 to 5 μl) was added to 100 μl of diluted virus to achieve the desired final serum dilution. The virus and serum mixtures were incubated for 1 h at 37°C, and then portions of the mixtures (representing 80, 10, 5, and 0.5%) were added to HT-1080 cells plated at 5 × 104 cells per well (six-well plates) the previous day. Two to three days following infection, cells were fixed and stained for AP expression, and AP+ foci were counted. Each assay was performed in duplicate.
RESULTS
Transduction by AAV2, -3, and -6 pseudotype vectors in cultured cells.
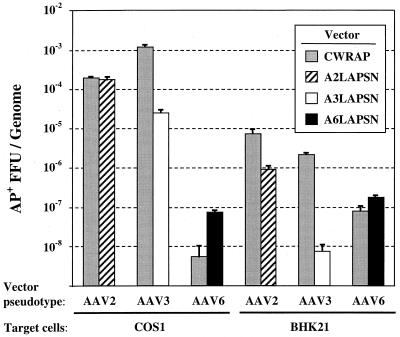
We determined the transduction rates in cultured cells of AAV vectors having AAV2, -3, or -6 capsid proteins (Fig. 2). In one experiment, the DNA genome in the virions was based on AAV2 (CWRAP) and the capsid proteins were varied, and in another experiment the vector genomes were derived from the same AAV serotypes as the viral proteins (A2LAPSN, A3LAPSN, and A6LAPSN). All of the vectors encoded AP, and the number of AP+ foci induced by each virus was normalized to the number of vector genomes added to the cells to obtain the transduction rates. The data show that the AAV2-based vector CWRAP could be packaged into infectious virions by the capsid proteins from AAV2, -3, and -6, although the transduction rate of CWRAP with an AAV6 pseudotype was particularly low in COS-1 cells. In the case of AAV3 pseudotype vectors, the transduction rate for both COS-1 and BHK-21 cells was much higher with the AAV2-based vector CWRAP(AAV3) in comparison to that of the AAV3-based vector A3LAPSN. The opposite was true for the AAV6 pseudotype vectors where the presence of AAV6 sequences in the A6LAPSN vector resulted in improved transduction compared with that of the AAV2-based vector CWRAP(AAV6).
FIG. 2.
Transduction by AAV vectors in cultured cells. Transduction rates in COS-1 and BHK-21 cells are expressed as the number of AP+ FFU per vector genome. The CWRAP vector contains AAV2 TRs and was pseudotyped with AAV2, -3, or -6 Rep and Cap proteins. The AxLAPSN vectors contain TRs from the same AAV serotype as that of the capsid proteins.
Transduction by AAV2, -3, and -6 pseudotype vectors in mouse lung.
We next examined transduction by AAV2, -3, or -6 pseudotype vectors in mouse lung to establish the relative transduction rates and the cell-type specificity of the vectors. For these experiments, the same AAV2-based vector genome was packaged into the different capsid proteins to eliminate effects of vector sequences on transduction. We used the AAV2-based vector ARAPGH, which is similar to the CWRAP vector. These experiments were part of larger experiments designed to study repeat transduction, and the animals described here received saline 1 month before receiving the test vector. Naive animals exhibited no AP+ cells in the lung (Table 1, row 1). Animals that were given 1010 genome-containing particles of the ARAPGH(AAV2) vector showed staining in alveolar cells, smooth muscle cells, and cells of the airway epithelium (Table 1, row 2). Animals given the same amount of ARAPGH(AAV6) showed a twofold-higher level of transduction in alveolar cells compared to those given ARAPGH(AAV2) (Table 1, compare rows 2 and 6 [P < 0.005]). The AAV3 vector ARAPGH(AAV3) yielded a lower transduction rate in alveolar cells (P < 0.005), but the proportion of transduction events in smooth muscle was higher than that of the AAV2 or -6 pseudotype vectors (P < 0.05). The comparison of the three vectors shows that AAV2 and -6 pseudotype vectors transduce airway epithelia much more efficiently than the AAV3 pseudotype vector.
TABLE 1.
Transduction in the mouse lung by AAV2, -3, and -6 pseudotype vectors after primary administration of an AAV2 vectora
| Vector pseudotype
|
No. of animals | No. of AP-positive cells per cm2 in:
|
|||||
|---|---|---|---|---|---|---|---|
| Primary (β-Gal vector) | Secondary (AP vector) | Alv | DAE | BE | ASM | VSM | |
| None | None | 2 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Saline | AAV2 | 4 | 206 ± 26 | 4.5 ± 1.5 | 0.08 ± 0.08 | 1.0 ± 0.2 | 17 ± 14 |
| AAV2 | AAV2 | 3 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Saline | AAV3 | 4 | 5.7 ± 0.3 | <0.08 | <0.08 | 2.6 ± 0.7 | 0.5 ± 0.5 |
| AAV2 | AAV3 | 4 | 1.9 ± 1.3 | <0.08 | <0.08 | <0.08 | <0.08 |
| Saline | AAV6 | 4 | 408 ± 73 | 12 ± 4 | 0.5 ± 0.1 | 0.4 ± 0.4 | <0.08 |
| AAV2 | AAV6 | 4 | 383 ± 71 | 18 ± 4 | 0.7 ± 0.6 | 1.2 ± 0.4 | 0.08 ± 0.08 |
Vector stocks were generated as described in Materials and Methods except that a 293 cell line containing the integrated ARAPGH vector was used. Mice were given CWCZn(AAV2) (5 × 109 genome-containing particles) and 4 weeks later were given AAV2, -3, or -6 pseudotype ARAPGH vectors (1010 genome-containing particles each). The animals were euthanatized 4 weeks after the second vector administration, and the number of AP-positive alveolar cells (Alv), distal airway epithelial cells (DAE), bronchial airway epithelial cells having underlying cartilage (BE), airway smooth muscle cells underlying the epithelium (ASM), and vasculature smooth muscle cells (VSM) were quantitated. The means and standard errors of the means are shown.
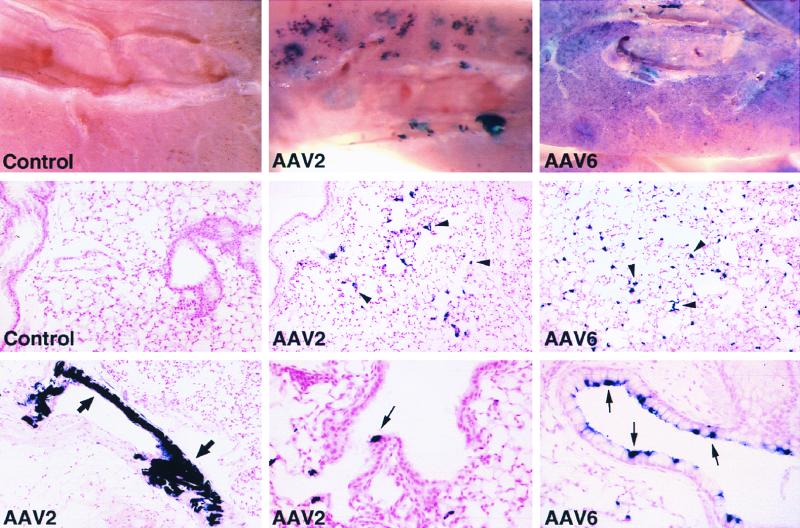
Higher doses of the vectors were administered in an attempt to increase transduction in all cell types. Animals given 1011 genome-containing particles of the AAV2 or AAV6 pseudotype vectors showed differences in the distribution of AP+ cells and transduction rates (Fig. 3). All lungs were taken for analysis 1 month after vector delivery. Naive animals (Fig. 3, rows 1 and 2, left panels) and saline-treated animals (data not shown) exhibited no AP+ cells in the lung. The staining in the lung parenchyma appeared clustered for AAV2 while it was more evenly distributed for AAV6 (Fig. 3, row 1, center and right panels). Histological analysis showed that the majority of AP+ cells were alveolar cells (Fig. 3, row 2, center and right panels), and the number was significantly higher for the AAV6 vector than for the AAV2 vector (P < 0.05) (5,800 ± 2,000 [mean ± standard error] versus 565 ± 164 AP+ alveolar cells per cm2, respectively; n = 4 per group). Transduction of distal airway epithelial cells was also higher for the AAV6 vector than for the AAV2 vector (P = 0.06) (97 ± 43 versus 35 ± 15 AP+ distal airway epithelial cells per cm2) (Fig. 3, row 3, compare center and right panels). This level of transduction by the AAV6 vector represents 5% of the alveolar cells and 0.5% of the distal airway epithelial cells. Transduction of smooth muscle cells was also found in animals given AAV2 vector (Fig. 3, row 3, left panel) and AAV6 vector (not shown). Airway epithelia exhibiting a high level of transduction (up to 30%) could be found in animals administered the AAV6 vector but not in those given the AAV2 vector (Fig. 3, row 3, compare center and right panels). Although airway epithelia exhibiting such a high percentage of AP+ cells were still seen infrequently, even in the AAV6-treated animals, the results show a trend towards higher transduction rates in airway epithelial cells by the AAV6 pseudotype vector in comparison to the AAV2 pseudotype vector.
FIG. 3.
Histochemical detection of AP expression in mouse lungs 1 month after vector exposure. Naive mouse lungs (control) did not exhibit AP+ cells, while AAV vector-treated lungs exhibited AP+ alveolar cells (arrowheads), airway epithelial cells (small arrows), and smooth muscle cells (large arrows). Lungs given AAV2 or -6 pseudotype vectors (1011 genome-containing particles) are indicated. Original magnifications: top row, ×80; middle row and bottom row (left panel), ×100; bottom row (middle and right panels), ×200.
Transduction by AAV2, -3, and -6 pseudotype vectors in mouse lung following primary exposure to an AAV2 pseudotype vector.
We tested the abilities of AAV2, -3, and -6 pseudotype vectors to transduce the lung after primary exposure to an AAV2 pseudotype vector. In all cases, we used AAV2-based vector genomes to eliminate effects due to vector sequences. Mice were given 5 × 109 genome-containing particles of CWCZn(AAV2), and blood was obtained to analyze AAV2 neutralization activity on day 21. All animals exhibited a robust neutralizing immune response against this AAV2 pseudotype vector (data not shown). Four weeks after the initial vector exposure, the mice were given an ARAPGH vector with an AAV2, -3, or -6 pseudotype (1010 genome-containing particles each). The animals were euthanatized 3 weeks later, and the lungs were stained for AP to assess transduction by the second vector (Table 1). The positive control for AAV2 transduction, i.e., mice given saline followed by ARAPGH(AAV2), displayed a modest transduction rate in alveolar cells and much lower rates in the smooth muscle cells and distal airway epithelial cells (Table 1, row 2). Transduction in bronchial epithelial cells was barely detectable (0.08 per cm2). When ARAPGH(AAV2) was given after one prior exposure to an AAV2 vector, no AP+ cells were observed in three animals, indicating that transduction was abrogated or at least reduced to undetectable levels (<0.1 AP+ cell per cm2) (Table 1, row 3). When ARAPGH(AAV3) was given after prior exposure to CWCZn(AAV2), we observed transduction of alveolar cells that was one-third of the value obtained when the AAV3 vector was administered to naive animals (P < 0.05) (Table 1, rows 4 and 5). Additionally, transduction of smooth muscle cells was reduced (P < 0.01) to undetectable levels. When ARAPGH(AAV6) was given after primary exposure to the AAV2 vector, transduction was observed in all cell categories at values that were similar to those of naive animals (P > 0.3 [no statistically significant difference]) (Table 1, rows 6 and 7).
The result showing that one exposure to an AAV2 vector can prevent later transduction by another AAV2 vector has been observed in previous experiments with rabbits and mice (13, 15). The results with the other serotypes show that both AAV3 and AAV6 pseudotype vectors can be effectively readministered following AAV2 vector exposure. However, AAV3 vector transduction was reduced by prior AAV2 vector exposure, while AAV6 vector transduction was unaffected under the same conditions. Additionally, given approximately similar doses of the two vectors as measured with genome-containing particles, the AAV6 vector transduced airway epithelial and alveolar cells at a much higher rate than did the AAV3 vector. This result is remarkable given the poor transduction of AAV6 pseudotype vectors in cultured cells.
Virus-neutralizing activity and cross-reactivity of antibodies directed against AAV2, -3, and -6 pseudotype vectors in mice.
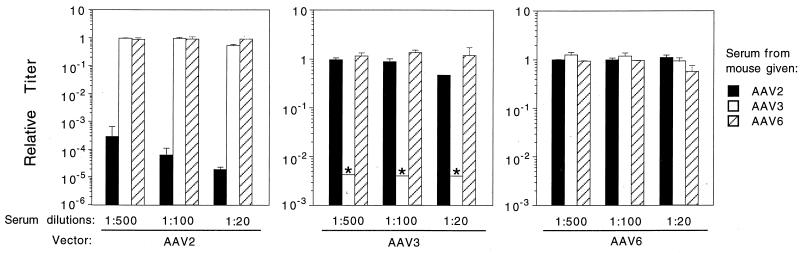
Blood was obtained at the time the lungs were removed for AP staining to analyze AAV neutralization activity in animals given only one dose of an AAV vector. Similar numbers of genome-containing particles of ARAPGH pseudotyped in AAV2, -3, or -6 capsids were exposed in 1:20, 1:100, and 1:500 dilutions of pooled serum, and the remaining transduction activity was determined in cultured cells (Fig. 4). The pooled serum from three mice given one dose of AAV2 vector almost completely neutralized the AAV2 vector, partially neutralized the AAV3 pseudotype vector, and did not neutralize the AAV6 pseudotype vector. The pooled serum from four animals given the AAV3 pseudotype vector completely neutralized the AAV3 vector, partially neutralized the AAV2 vector, and did not exhibit any neutralizing activity against the AAV6 pseudotype vector. The pooled serum sample from animals given one dose of AAV6 vector did not show cross-reactive neutralizing antibodies to AAV2 or AAV3. This pooled serum sample partially neutralized the AAV6 vector, but only at the lowest dilution of serum (1:20). The titer (dilution at which transduction is decreased 50%) of the AAV6 serum against AAV6 was 1:20, whereas the AAV3 and AAV2 serum still exhibited nearly complete neutralization of the cognate vectors at the 1:500 dilution.
FIG. 4.
AAV-neutralizing antibody titers in serum from mice after administration of AAV vectors. AAV2-, -3, or -6 pseudotype vectors were incubated with dilutions of pooled sera from mice given the same pseudotype vectors 3 weeks previously. The relative titer was determined by comparison to the titer of the vector incubated without mouse serum. The asterisks indicate that no stained cells were observed, and the underlying bars represent the limit of sensitivity of the assay in these cases. Duplicate wells in three repeat assays were scored. Mean values ± standard deviation are given.
The results show that the cross-reactivity of serum from mice exposed to an AAV2 vector is similar to that found with rabbits (25). The new data concerning the cross-reactivities of serum from animals exposed to AAV3 or AAV6 vectors were not unexpected and showed a reciprocal cross-reactivity between AAV3 and AAV2 and no cross-reactivity between AAV6 and the other two serotypes. The surprising result was that seen with the reactivity of AAV6 serotype to itself. Although all animals received comparable inocula of the corresponding AAV vectors, one dose of AAV6 did not elicit a strong neutralizing immune response against itself, whereas similar doses of the other two serotypes did elicit strong neutralizing immune responses against vectors pseudotyped with the same capsid proteins.
Successful readministration of AAV2 and AAV6 pseudotype vectors following primary exposure to an AAV6 pseudotype vector.
Because the AAV6 vector elicited only low titers of neutralizing antibodies in mice, there was a possibility that AAV6 vectors could be utilized for repeat transduction. We tested this hypothesis in the next experiment and also tested the possibility of using an AAV2 vector after an AAV6 vector. Animals were inoculated with saline, CWCZn(AAV2), or CWCZn(AAV6). One month after administration of the first vector or saline, the animals were given ARAPGH(AAV2) or ARAPGH(AAV6). One month later, the animals were euthanatized, and lungs were stained for AP expression (Table 2). Saline-treated animals did not exhibit any AP+ cells in the lung (Table 2, row 1). Transduction by the AAV2 vector in naive animals was low in all cell categories and was undetectable in the bronchial epithelium. Transduction by AAV2 in the group of mice that had been previously exposed to an AAV6 vector was not significantly different from the AAV2 control group, indicating that the AAV2 vector transduction was unaffected by prior exposure of mice to an AAV6 vector (P > 0.05). The AAV6 vector transduced the lung after prior exposure to an AAV6 vector; however, the efficiency of transduction was reduced compared to AAV6 transduction in control animals (P < 0.05). Administration of AAV6 after AAV2 resulted in transduction at a level comparable to that obtained with naive animals (Table 2), consistent with results from the previous experiment (Table 1).
TABLE 2.
Transduction in the mouse lung by AAV2, -3, and -6 pseudotype vectors after primary administration of AAV2 or AAV6 pseudotype vectorsa
| Vector pseudotype
|
No. of animals | No. of AP-positive cells per cm2 in:
|
|||||
|---|---|---|---|---|---|---|---|
| Primary (β-Gal vector) | Secondary (AP vector) | Alv | DAE | BE | ASM | VSM | |
| None | Saline | 3 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Saline | AAV2 | 3 | 22 ± 10 | 1.3 ± 1.2 | <0.1 | 2.5 ± 1.9 | 1.9 ± 1.3 |
| AAV6 | AAV2 | 4 | 19 ± 5 | 0.1 ± 0.1 | <0.1 | 1.1 ± 0.6 | 0.5 ± 0.5 |
| Saline | AAV6 | 4 | 5,800 ± 2,000 | 97 ± 43 | 43 ± 41 | 50 ± 14 | 12 ± 8 |
| AAV6 | AAV6 | 5 | 1,210 ± 450 | 4.5 ± 2.1 | <0.1 | 0.7 ± 0.3 | <0.1 |
| AAV2 | AAV6 | 5 | 4,300 ± 2,200 | 30 ± 14 | <0.1 | 14 ± 6 | 8 ± 6 |
Mice were given 1010 genome-containing particles of CWCZn(AAV2) or CWCZn(AAV6) and 4 weeks later were given ARAPGH(AAV2) or ARAPGH(AAV6) (5 × 109 or 1011 genome-containing particles, respectively). The animals were euthanatized 4 weeks after the second vector administration, and the AP-positive alveolar cells (Alv), distal airway epithelial cells (DAE), bronchial airway epithelial cells having underlying cartilage (BE), airway smooth muscle cells underlying the epithelium (ASM), and vasculature smooth muscle cells (VSM) were quantitated. The means and standard errors of the means are shown.
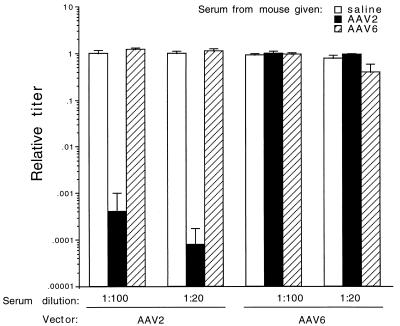
Serum was obtained 3 weeks after the first dose of AAV vector and tested for neutralizing activity against AAV2- and AAV6-pseudotyped ARAPGH vectors prior to readministration. The sera were individually analyzed, and the average neutralizing values are shown (Fig. 5). AAV2 sera neutralized ARAPGH(AAV2) at all dilutions of serum tested, but had no effect against ARAPGH(AAV6). AAV6 sera partially neutralized ARAPGH(AAV6) at the lower serum dilution and did not neutralize ARAPGH(AAV2). The neutralization results of this experiment were consistent with the results of the previous experiment, showing that AAV2-encapsidated vectors elicit a more robust humoral immune response than AAV6-encapsidated vectors, and there is no cross-reactivity between these AAV serotypes.
FIG. 5.
AAV-neutralizing antibody titers in mouse serum after administration of AAV2- or AAV6-pseudotyped vector. AAV2- or AAV6-pseudotyped ARAPGH vectors were incubated with dilutions of sera from mice given the AAV2- or AAV6-pseudotyped CWCZn vectors 3 weeks previously (n = 4 per group; see Table 2). The relative titer was determined by comparison to the number of AP+ FFU/ml of sample, in which vector was incubated with serum from a saline-treated mouse (n = 4). Duplicate wells for each animal serum were scored. Mean values ± standard deviation are given.
It is known that different strains of mice mount different humoral and cellular immune responses to viral vectors and other immunogens. For example, BALB/c and C3H/HeJ mouse strains clear adenovirus vector-transduced cells more rapidly than other strains of mice, such as C57BL/6 (2). To compare the immunogenicity of AAV2- and AAV6-pseudotyped vectors in another strain of mice, BALB/c mice were given either the AAV2- or AAV6-pseudotyped CWCZn vectors (1010 genome-containing particles), and serum was obtained 21 days later. Pooled serum samples from each group of mice (n = 4 in each group) were tested for neutralizing activity against AAV2- and AAV6-pseudotyped ARAPGH. The serum from animals exposed to the AAV6 vector exhibited 50% neutralization of the AAV6 cognate vector at a serum dilution of 1:20, whereas the serum from animals exposed to the AAV2 vector demonstrated a 50% inhibition of the AAV2 vector at a 1:1,000 dilution (data not shown). Neither group exhibited cross-reactive neutralizing antibodies (data not shown). These results show that in another strain of mice, the AAV6 and AAV2 vectors are serologically distinct and that AAV6 vector is less immunogenic.
Human serum neutralizing activity against an AAV6 vector is less potent than that against an AAV2 vector.
To determine the prevalence and level of neutralizing activity against AAV2, -3, and -6 in humans, serum samples from several volunteers were tested in an initial screen (Table 3). Four out of seven serum samples showed high neutralizing activity against an AAV2 vector. One individual showed a low activity, and two had no neutralizing activity against the AAV2 vector. These serum samples showed a similar profile of reactivity to an AAV3 vector. The same serum samples that exhibited reactivity to the AAV2 vector also exhibited reactivity to the AAV6 vector; however, the titer of the neutralizing antibody was significantly lower. The highest titers of antibodies against AAV2 ranged from 3,200 to 12,800, whereas those against AAV6 ranged from 200 to 800. The reactivity to AAV3 was intermediate.
TABLE 3.
Neutralization of AAV2, AAV3, and AAV6 pseudotype vectors by human seruma
| Human serum sample no. | Titer of neutralizing serum with antibody against:
|
||
|---|---|---|---|
| ARAPGH(AAV2) | ARAPGH(AAV3) | ARAPGH(AAV6) | |
| 1 | 3,200 | 1,600 | 400 |
| 2 | 6,400 | 800 | 200 |
| 3 | 12,800 | 12,800 | 800 |
| 4 | 20 | <1:20 | 20 |
| 5 | <20 | <20 | <20 |
| 6 | <20 | <20 | <20 |
| 7 | 12,800 | 1,600 | 800 |
Dilutions of serum (1:20 and twofold dilutions starting from 1:100) were incubated with 108 genome-containing particles of AAV2-, AAV3-, and AAV6-pseudotyped ARAPGH vector. Titer was defined as the reciprocal of the highest dilution of serum that neutralized at least 50% of the vector.
DISCUSSION
We previously constructed vector and helper plasmids for the production of vector stocks based entirely on either AAV2, -3, or -6 (25). In this study, we showed that an AAV2-based vector genome (containing AAV2 terminal repeats [TRs]) can be pseudotyped into AAV3 or AAV6 capsids by using helper plasmids containing the rep and cap genes from these AAV serotypes. Experiments performed with different pseudotypes of the AAV2 vector allowed us to eliminate effects of different vector TR sequences on the results.
Our results show that AAV2, -3, and -6 pseudotype vectors were able to transduce various cell types in the mouse lung. A comparison of transduction efficiencies between vectors with the three pseudotypes indicates that vectors with an AAV3 pseudotype are the least efficient of the three, and those with an AAV6 pseudotype are at least as efficient as AAV2 vectors. This comparison is based on inoculating the animals with similar numbers of genome-containing vector particles rather than on similar functional vector titers as measured in cell culture because of the dependence of vector titer on the particular cells used for assaying. Indeed, we showed previously (25) and in Fig. 2 that similar numbers of genome-containing particles of AAV2, -3, or -6 vectors yielded different transduction efficiencies in different target cell lines, and we hypothesize that this is due to differences in receptor utilization by the three AAV serotypes. Similar observations have been made with vectors based on AAV3 and AAV4 (8, 21, 22). In vivo, transduction by an AAV2 vector was higher in alveolar cells than in any other cell type of the mouse lung (15). Here, we show that AAV3 and AAV6 pseudotype vectors also transduce alveolar cells more efficiently than other cells in the mouse lung and that transduction of smooth muscle cells and airway epithelial cells also occur, as has been observed for AAV2 vectors. Members of our group have observed transduction of submucosal smooth muscle in rabbits after delivery of AAV vector by use of a balloon catheter (14). In the mouse lung, transduction of submucosal and vascular smooth muscle was also observed, independent of the method of vector deliver (by intratracheal injection with a syringe needle or by nasal aspiration) (15) and independent of vector preparation quality (data not shown). It is not immediately apparent how a vector delivered by nasal aspiration can circumvent physical barriers imposed by the basement membrane of the epithelium.
In one experiment, administration of 1010 genome-containing particles resulted in a twofold-greater transduction of alveolar cells and distal airway cells for AAV6 than for AAV2 (Table 1). In a second experiment, doses of AAV2 and AAV6 equivalent to 5 × 109 and 1011 genome-containing particles, respectively (a 20-fold difference in genomes), resulted in dramatically higher transducing values for the AAV6 vector, particularly in alveolar cells (200-fold greater than for AAV2) (Table 2). In a third experiment, where 1011 genome-containing particles of ARAPGH(AAV2) were administered (Fig. 3), the transduction rate of the AAV2 vector was still 10-fold lower than that of a similar dose of the AAV6 vector that was determined in the second experiment. These results suggest that the AAV6 pseudotype results in more efficient transduction in the lung than the AAV2 pseudotype vector. It is apparent that the vector titers obtained in tissue culture cell lines were not predictive of AAV6 pseudotype vector performance in vivo, since titers of AAV6 pseudotypes of the ARAPGH and CWRAP vectors were at least 100- to 10,000-fold lower than those of the AAV2 pseudotypes of the same vectors (Fig. 2; see Materials and Methods). Additionally, the results from Fig. 2 indicate that an AAV2 vector pseudotyped in an AAV6 capsid may be less efficient than an AAV6-based vector (containing AAV6 TRs). Generation of helper constructs that separate the rep and cap functions, as well as encapsidation of a vector with the cognate AAV vector sequences, will help delineate the optimal combination of vector, rep, and capsid serotypes for gene transfer to the lung and other tissues.
The fact that AAV vector transduction correlated inversely with the presence of neutralizing antibodies suggests that the use of AAV vectors in humans may be limited to those individuals who have not previously been infected by AAV. Results obtained from earlier studies showed that serum reactivity to AAV2 and -3 occurred in approximately 60% of adults (4). Seropositivity was minimal in children under the age of 2 but rapidly increased to 60% thereafter (4, 6). The results of these studies were based on the use of assays for complement fixation as well as neutralizing activity. In a preliminary screen of adult subjects, we found that the prevalence of AAV2 and -3 reactivity was similar to that in the earlier reported study. A robust neutralizing reactivity to AAV2 and AAV3 serotypes was detected in four of seven serum samples, another one had a low reactivity, and two others did not exhibit any neutralizing activities. Neutralizing activities against AAV6 capsids also were detected in the serum samples that were reactive against AAV2 and AAV3 capsid proteins. However, the neutralizing activity to AAV2 and AAV3 was more robust than the activity to AAV6. This result is consistent with the lower neutralizing titers of antibody to AAV6 vectors observed in the mouse. It is tantalizing to speculate that AAV6 is also less immunogenic in humans and that the lower titers of neutralizing antibody detected in our screen occurred even after a bona fide infection with the wild-type AAV6. Alternatively, the low antibody titers may reflect the ability of human neutralizing antibodies generated against AAV2 or -3 to cross-react with AAV6, although this seems unlikely due to the distinct serotypic characteristics of AAV6 in the mouse and rabbit.
Recently, Xiao et al. examined the use of vectors based on AAV1 for gene therapy (29). Nucleotide and amino acid sequence analyses show that the AAV1 and AAV6 capsid proteins are closely related (25, 29). Analysis of sera from healthy human subjects showed a remarkable lack of neutralizing activity against AAV1 and AAV2 in contrast to our results and previous reports in the literature (4–6). Their low percentage of seropositivity may be due to the sensitivity of the neutralization assay. Our assay utilized 108 genome-containing particles of vector in incubations with serum, and the titer of neutralizing serum was determined on a 50% reduction in AP FFU. Their neutralizing titers were calculated as the highest dilution at which 50% of a monolayer of cells still stained positive for the green fluorescent protein encoded by the vector. Since the amount of vector particles used in incubation with serum was not given, we cannot directly compare our results. Alternatively, their low percentage of seropositivity may reflect the population analyzed. We have initiated a study to screen a larger human population which would include CF and healthy individuals. Preliminary results showed that only 1 of 22 CF individuals under the age of 18 showed seropositivity to AAV (data not shown). In this individual, the neutralizing titer of antibody to AAV2 was 10-fold higher than that to AAV6. We used the same assay for detection of AAV-neutralizing antibodies in CF individuals and in healthy individuals, shown in Table 3; thus, we are unable to explain the difference in incidence of AAV immunoreactivity based on methodological grounds.
In animal experiments, Xiao and coworkers reported differences in the ability of the vector to transduce in primary and secondary administrations in liver and muscle (29). Previous exposure to an AAV1 or AAV2 vector abrogated subsequent transduction by the same vector and had a variable effect on secondary transduction by the other vector, which was dependent on the organ studied (liver or muscle). Their results and ours suggest that initial transduction and readministration results may be dependent on the route of delivery and the target tissue being analyzed. In addition, although the AAV1 and AAV6 capsid proteins are 99% identical, there are six amino acid differences between AAV1 and AAV6 capsid proteins that could lead to differences in the immunogenicity and cross-neutralization activities of AAV1 and AAV6. By comparison to the crystal structure determined for canine parvovirus (28), a major difference (lysine in AAV6 compared to glutamate in AAV1) appears to be on the surface of the virion, and two other changes could alter an exposed loop. Admittedly, this analysis is only approximate, given the large divergence in sequence between AAV and canine parvovirus capsid proteins, but these differences could result in different host immune responses to vectors with AAV1 or AAV6 pseudotypes.
It appears that AAV2 and -3 can elicit the generation of cross-reactive neutralizing antibodies in several species, including humans (4–6), rabbits (26), and mice (present data). Our data show that exposure to AAV2 or AAV3 vectors elicited a robust immune response against a vector with the same serotype and a weaker response to the cross-reacting serotype. This weak immune response resulted in reduced transduction rates when animals were given a vector with the cross-reactive serotype. The mouse data also show that AAV6 is immunologically distinct from AAV2 and AAV3 since exposure to these serotypes did not elicit detectable neutralizing antibody to AAV6. Indeed, AAV6 vector transduction rates were not significantly affected by prior AAV2 vector exposure. The same was true for AAV2 vector transduction following an exposure to AAV6. In addition, the AAV6 vector generated only a weak neutralizing immune response, and this was correlated with its ability to transduce the lung after a prior exposure to AAV6.
While this study was under review, another report appeared; it suggests that neither AAV2 nor AAV3 vector transduction in the rabbit airway is affected by two prior administrations of an AAV2 vector, even though neutralizing antibodies against AAV2 and -3 were detected in serum (3). This is in contrast to the current and previously published results obtained with mice (15) and our prior results obtained with rabbits (14), which showed a large reduction in transduction by an AAV2 vector following prior administration of an AAV2 vector. Perhaps the reason for the discrepancy in this recent study is the delivery of a large amount of vector to a very small area of the airway with a fiber optic bronchoscope. Prior to vector delivery, bronchoalveolar lavage was performed and may have removed surface antibodies. In addition, both the lavage procedure and the use of a bronchoscope for vector delivery likely resulted in significant damage to the bronchus in the area of vector administration, and this may have aided transduction. It appears that transduction was very low elsewhere in the lung, and thus it is unclear what utility this localized delivery approach will have for treatment of diseases such as CF. In contrast, our results (especially with AAV6) showed significant transduction as measured by transgene expression (rather than PCR for vector genomes) throughout the lung.
In summary, we have shown that vectors based on AAV2 pseudotyped into AAV2, AAV3, and AAV6 capsids can transduce the lung and are useful reagents for successful repeat transduction by AAV vectors. Of the three AAV types examined, AAV6 appears to be the most versatile for readministration because it is immunologically distinct and is also less immunogenic. The results presented here indicate that vectors based on AAV6 show promise for gene therapy to the lung and support the development of gene therapy based on multiple AAV serotypes.
ACKNOWLEDGMENTS
We thank J. M. Alfano for excellent technical assistance.
This work was supported by grant DK47754 from the National Institutes of Health, grants from the Cystic Fibrosis Foundation (C.L.H., A.D.M., J.M.A.), and grants from the American Society of Hematology, the March of Dimes Birth Defects Foundation, and the Heart, Lung, and Blood Institute of the U.S. National Institutes of Health (E.A.R., D.W.R.).
REFERENCES
- 1.Bantel-Schaal U, zur Hausen H. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology. 1984;134:52–63. doi: 10.1016/0042-6822(84)90271-x. [DOI] [PubMed] [Google Scholar]
- 2.Bar D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variation in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 3.Beck S E, Jones L A, Chesnut K, Walsh S M, Reynolds T C, Carter B J, Askin F B, Flotte T R, Guggino W B. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J Virol. 1999;73:9446–9455. doi: 10.1128/jvi.73.11.9446-9455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blacklow N R, Hoggan M D, Kapikian A Z, Austin J B, Rowe W P. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968;88:363–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 5.Blacklow N R, Hoggan M D, Rowe W P. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 6.Blacklow N R, Hoggan M D, Sereno M S, Brandt C D, Kim H W, Parrott R H, Chanock R M. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am J Epidemiol. 1971;94:359–366. doi: 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- 7.Boissy R, Astell C R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5′-terminal palindrome of minute virus of mice. Gene. 1985;35:179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- 8.Chiorini J A, Yang L, Liu Y, Safer B, Kotin R M. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher K J, Gao G, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 11.Fisher-Adams G, Wong K K, Jr, Podsakoff G, Forman S J, Chatterjee S. Integration of adeno-associated virus vectors in CD34+ human hematopoietic progenitor cells after transduction. Blood. 1996;88:492–504. [PubMed] [Google Scholar]
- 12.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P Z, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham F L, Smiley J. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 14.Halbert C L, Standaert T A, Aitken M L, Alexander I E, Russell D W, Miller A D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halbert C L, Standaert T A, Wilson C B, Miller A D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog R W, Yang E Y, Couto L B, Hagstrom J N, Elwell D, Fields P A, Burton M, Bellinger D A, Read M S, Brinkhous K M, Podsakoff G M, Nichols T C, Kurtzman G J, High K A. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 17.Herzog R W, Hagstrom J N, Kung S, Tai S J, Wilson J M, Fisher K J, High K A. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koeberl D D, Bonham L, Halbert C L, Allen J M, Birkebak T, Miller A D. Persistent, therapeutically relevant levels of human granulocyte colony-stimulating factor in mice after systemic delivery of adeno-associated virus vectors. Hum Gene Ther. 1999;10:2133–2140. doi: 10.1089/10430349950017121. [DOI] [PubMed] [Google Scholar]
- 20.Macpherson I, Stoker M. Polyoma transformation of hamster cell clones—an investigation of genetic factors affecting cell competence. Virology. 1962;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- 21.Mizukami H, Young N S, Brown K E. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217:124–130. doi: 10.1006/viro.1996.0099. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu S, Mizukami H, Young N S, Brown K E. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 23.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 24.Parks W P, Boucher D W, Melnick J L, Taber L H, Yow M D. Seroepidemiological and ecological studies of the adenovirus-associated satellite viruses. Infect Immun. 1970;2:716–722. doi: 10.1128/iai.2.6.716-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutledge E A, Halbert C L, Russell D W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutledge E A, Russell D W. Adeno-associated virus vector integration junctions. J Virol. 1997;71:8429–8436. doi: 10.1128/jvi.71.11.8429-8436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 28.Tsao J, Chapman M S, Agbandje M, Keller W, Smith K, Wu H, Luo M, Smith T J, Rossman M G, Compans R W, Parrish C R. The three-dimensional structure of canine parvovirus and its functional implications. Science. 1991;251:1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- 29.Xiao W, Chirmule N, Berta S C, McCullough B, Gao G, Wilson J M. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao X, Li J, Samulski R J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeitlin P L, Chu S, Conrad C, McVeigh U, Ferguson K, Flotte T R, Guggino W B. Alveolar stem cell transduction by an adeno-associated viral vector. Gene Ther. 1995;2:623–631. [PubMed] [Google Scholar]