Abstract
Practical relevance:
The health of the cat is a complex interaction between its environment (nurture) and its genetics (nature). Over 70 genetic mutations (variants) have been defined in the cat, many involving diseases, structural abnormalities and clinically relevant health concerns. As more of the cat’s genome is deciphered, less commonly will the term ‘idiopathic’ be used regarding the diagnosis of diseases and unique health conditions. State-of-the-art health care will include DNA profiling of the individual cat, and perhaps its tumor, to establish the best treatment approaches. Genetic testing and eventually whole genome sequencing should become routine diagnostics for feline health care.
Global importance:
Cat breeds have disseminated around the world. Thus, practitioners should be aware of the breeds common to their region and the mutations found in those regional populations. Specific random-bred populations can also have defined genetic characteristics and mutations.
Audience:
This review of ‘the good, the bad and the ugly’ DNA variants provides the current state of knowledge for genetic testing and genetic health management for cats. It is aimed at feline and general practitioners wanting to update and review the basics of genetics, what tests are available for cats and sources for genetic testing. The tables are intended to be used as references in the clinic. Practitioners with a high proportion of cat breeder clientele will especially benefit from the review.
Evidence base:
The data presented is extracted from peer-reviewed publications pertaining to mutation identification, and relevant articles concerning the heritable trait and/or disease. The author also draws upon personal experience and expertise in feline genetics.
Defining mutations
The word ‘mutation’ generally conjures up negative associations, such as Frankenstein’s monster or the X-Men. However, just as Frankenstein’s monster was misunderstood, and the X-Men can use their powers for good or evil, misconceptions surround DNA mutations found in the mammalian genome, including those of the domestic cat. The definition of mutation is: ‘a major change; a significant and basic alteration’ (Webster’s Third New International Dictionary). In the context of heredity, this change implies a difference from the ‘wild type’ – the typical form of an organism as ordinarily encountered in nature. Note that ‘mutation’ and ‘typical’ do not infer good or bad, and ‘ordinarily’ applies to the current space and time. Indeed, to remove negative connotations, the current standard is to use the term ‘variant’ instead of ‘mutation’ when referring to changes in DNA.
The wild type cat is a brown mackerel tabby with moderate body and facial conformation (Figure 1). As species evolve, mutation to DNA occurs, causing DNA variants, and the frequencies of those variants in the population change due to natural and artificial selection, migrations, popular sires and founder individuals. Over time, the extraordinary can become the ordinary. For example, the current ‘ordinary’ Persian cat has a highly brachycephalic head type. However, 100 years ago, Persians were just longhaired cats with a moderately normal face, which would currently be the extraordinary atypical! Although the change in the Persian head structure has been accomplished by artificial selection, natural selection changes the wild type over time as well, especially in relation to genes and alleles that affect fitness (ie, health).
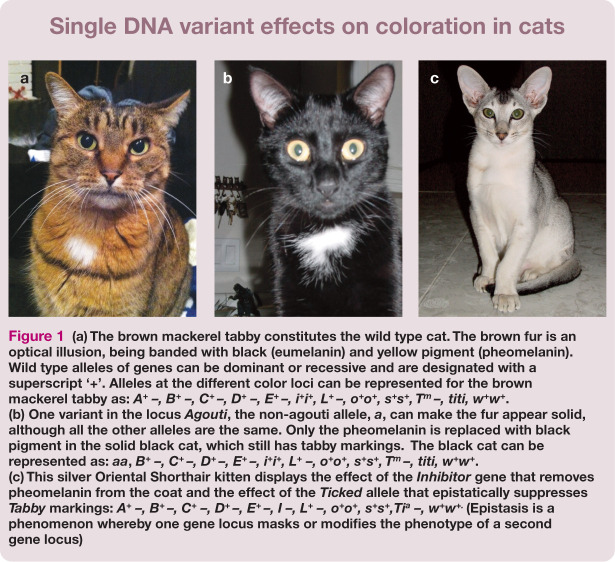
Figure 1.
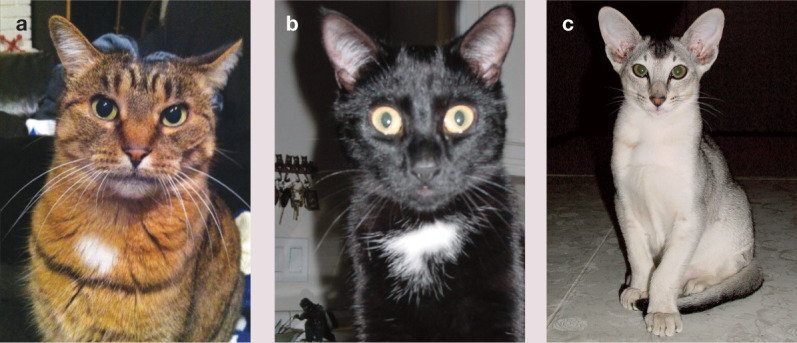
(a) The brown mackerel tabby constitutes the wild type cat. The brown fur is an optical illusion, being banded with black (eumelanin) and yellow pigment (pheomelanin). Wild type alleles of genes can be dominant or recessive and are designated with a superscript ‘+’. Alleles at the different color loci can be represented for the brown mackerel tabby as: A+ -, B+ -, C+ -, D+ -, E+ -, L+ -, o+o+, s+s+, T™ -, titi, ww+w*. (b) One variant in the locus Agouti, the non-agouti allele, a, can make the fur appear solid, although all the other alleles are the same. Only the pheomelanin is replaced with black pigment in the solid black cat, which still has tabby markings. The black cat can be represented as: aa, B+ -, C+ -, D+ -, E+ -, L+ -, o+o+, s+s+, T -, titi, w+w*. (c) This silver Oriental Shorthair kitten displays the effect of the Inhibitor gene that removes pheomelanin from the coat and the effect of the Ticked allele that epistatically suppresses Tabby markings: A+ -, B+-, C+ -, D+ -, E+ -, I -, L+ -, o+o+, s+s+,Tia -, w+w+- (Epistasis is a phenomenon whereby one gene locus masks or modifies the phenotype of a second gene locus)
Fitness is reduced any time a cat cannot reproduce, has lowered fecundity and/or a shortened reproductive lifespan. Barring accidents and traumas (eg, hit-by-car or eaten-by-coyote) the standard non-pedigreed cat, which has access to outdoors, should live at least 12 years and succumb to renal disease, hyperthyroidism or lymphosarcoma. Younger cats with morbidities or mortalities that should generally take the older and the weaker individuals, likely have alleles that are compromising their fitness, making them ‘susceptible’ to disease. Conversely, many cats live well into their teens; their genomes likely possess DNA variants that improve their overall fitness, enhancing their ‘resistance’ to disease.
Feral cats that live in more natural, and often more harsh, environments obtain DNA variants at the same rate as non-pedigreed and pedigreed cats, but often have a shorter lifespan. Besides the cat–cat competition and traumas more likely sustained in the open environment by a feral cat, DNA variants conferring poor fitness will lower the reproductive capabilities of a given cat. Therefore, variants that reduce fitness will more likely be maintained at a lower frequency in the feral population than in our health-managed non-pedigreed and fancy breed cats. In other words, health management allows genes conferring poorer fitness to survive and propagate in the species.
Overall, most variants are neutral and many are good. DNA variants support a species’ evolution by conferring advantages to different selection pressures. A population needs variation so that given individuals can fend off viruses and other infections that are the likely cause of early death in cats, allowing them to propagate and contribute to a species that is slightly more evolved, having better fitness for the current environment. As discussed below, many ‘good’ DNA variants are in fact just aesthetically pleasing and confer beauty and uniqueness to individual cats and their various breeds. Like any species, cats also have some very ‘bad’ and ‘ugly’ DNA variants.
The ‘good’ cat variants – variation humans positively select in cats
To date, over 40 genes with approximately 70 DNA variants have been documented to cause phenotypic, disease or blood type variations in the domestic cat (see reviews)1–3. The clinical descriptions and phenotypes of each of these diseases and traits have been curated at the Online Mendelian Inheritance in Animals (OMIA) website (omia.angis.org.au), which provides an invaluable resource comparison of phenotypes across 216 animal species. 4 The 26 genes in Table 1 are often under positive selection in cats, particularly breeds; however, not all of the variants may be considered ‘good’ by current standards (see later).
Table 1.
Phenotypic traits of the domestic cat conferred by DNA variants
| Locus (Alleles) OMIA entry link | MOI* | Phenotype | Gene | Gene name | Mutation |
|---|---|---|---|---|---|
|
Agouti (A+, a)
5
000201-9685 |
AR | Banded fur to solid | ASIP | Agouti-signaling protein | c.122_123delCA |
|
Brown (B+, b, ti)6,7
001249-9685 |
AR | Brown, light brown color variants | TYRP1 | Tyrosinase-related protein | b = -5IVS6 b1 = c.298C>T |
|
Color (C+, Cb, Cs, c)7–9
000202-9685 |
AR | Burmese, Siamese color pattern, full albino | TYR | Tyrosinase | cb = c.715G>T cs = c.940G>A c = c.975delC |
|
Dilution (D+, d)
10
000206-9685 |
AR | Black to grey/blue, orange to cream | MLPH | Melanophilin | c.83delT |
|
Dwarfism
000299-9685 |
AD | Shortening of long bones | unknown | unknown | unknown |
|
Extension (E+, e) - Amber
11
001199-9685 |
AR | Brown/red color variant | MC1R | Melanocortin receptor 1 | c.250G>A |
|
Fold (Fd, fd+)
000319-9685 |
AD | Ventral ear fold | unpublished | unpublished | unpublished |
|
Gloves (G+, g)
12
001580-9685 |
AR | White feet | KIT | KIT | c.1035_1036delinsCA |
| Hairless (Hr+, hr) 13 | AR | Atrichia | KRT71 | Keratin 71 | c.816+1G>A |
|
Inhibitor (I, i+)
001584-9685 |
AD | Absence of pheomelanin | unknown | unknown | unknown |
| Japanese Bobtail (J, j+) | AD | Kinked tail | unknown | unknown | unknown |
|
Kurl (K, k+)
000244-9685 |
AD | Rostral curled pinnae | unknown | unknown | unknown |
|
LaPerm
000245-9685 |
AD | Curly hair coat | unknown | unknown | unknown |
|
Longhair (L+, l)14,15
000439-9685 |
AR | Long fur | FGF5 | Fibroblast growth factor 5 | c.356_367insT c.406C>T c.474delT c.475A>C |
|
Tailless (Manx) (M, m+)
16
000975-9685 |
AD | Absent/short tail | TBOX | T-box | c.998delT c.1169delC c.1199delC c.998_1014dup17delGCC |
|
Orange (O, o+)
001201-9685 |
X-linked | Change in pigment hue | unknown | unknown | unknown |
|
Peterbald
001866-9685 |
AD | Hairless, brush coat | unknown | unknown | unknown |
|
Polydactyla (Pd, pd+)
17
000810-9685 |
AD | Extra toes | 5HH | 5onic hedgehog | c.479A>G c.257G>C c.481A>T |
|
Rexing (R+, r)
18
001684-9685 |
AR | Curly hair coat | LPAR6 | Lysophosphatidic acid receptor 6 | c.250_253delTTTG |
|
Rexing (Re+, re)
13
001581-9685 |
AR | Curly hair coat | KRT71 | Keratin 71 | c.1108-4 1184del, c.1184_1185insAGTTGGAG c.1196insT |
|
Rexing (R5, rs+)
19
001712-9685 |
AD | Curly hair coat | KRT71 | Keratin 71 | c.445-1G>C |
|
Spotting (5, s+)
20
000214-9685 |
Co-D | Bicolor/Van white | KIT | KIT | 7125ins FERV1 element |
|
Tabby (T™, tb)
21
001429-9685 |
AR | Blotched/classic pattern | TAQPEP | Transmembrane aminopeptidase Q | c.176C>A c.416C>A c.682C>A c.2522G>A |
|
Ticked (Tia, ti)
001484-9685 |
AD | No tabby pattern | unknown | unknown | unknown |
|
White (W, w+)
20
000209-9685 |
AD | Loss of pigmentation | KIT | FERV1 LTR ins | |
| Wide-band | AR? | Length of pheomelanin band in hair | unknown | unknown | unknown |
Mode of inheritance of the non-wild type variant. ‘+’ implies the wild type allele when known. In reference to the mutant allele, AD = autosomal dominant, AR = autosomal recessive, co-D = co-dominant.
OMIA: Online Mendelian Inheritance in Animals (omia.angis.org.au) entries provide links to citations and clinical descriptions of the phenotypes and the diseases. Listed citations are for the causative variant discovery
Once cats became domesticated, some of the first noticeable genetic alterations conferred phenotypic variations in fur length, fur type, coat colors and coat patterns. Most of the phenotypic genes and loci that affect the appearance of a cat (Table 1) can be remembered simply by referring to letters of the alphabet. Mostly these loci were named after traits that were first discovered in the domestic mouse, before anything was known of the genes and the proteins they produced. 22 A is for Agouti, B is for Brown, C is for Color, D is for Dense, E is for Extension (amber), and so forth. The Color (aka Chinchilla) locus was named after a mouse phenotype that presented similarly to the coloration of the Siamese cat, having color only on its cooler extremities. The Dense (Dilute) locus affects the amount and placement of pigment in the hair shaft, giving the illusion that a cat is grey or blue. Also known as dilution, blue cats only have black pigment, but the refractive qualities of the hair shaft cause the illusion of a lighter pigment.23,24 The coat color variants are common to all cats and are appropriate for genetic typing in all breeds and populations.
Together with I for Inhibitor, L for Longhair (fur), S for Spotting, T for Tabby, O for Orange, and W for White, these loci (A–E, I, L, S, T, O and W) control the major phenotypic traits of cats that appeared before the development of cat breeds.
Many early geneticists studied the coat colors of cats to understand basic inheritance patterns. Orange is defined by a yet unknown gene on the X chromosome in cats.25,26 Because Orange is sex-linked, its inheritance pattern is very different between males and females, males being more likely to be orange since they have only one X chromosome. Orange was one of the first loci to be genetically mapped to a specific chromosome,27–30 for any species! Longhair has four different variants that have likely occurred in different regions of the world and are prevalent in certain breeds to greater or lesser degrees (Figure 2).13,14 A gene for tabby patterns has long been known, but it was recently shown that another gene controls the expression of the Tabby locus and hence the tabby phenotype (Figure 3).21,31 The Tabby locus (TAQPEP gene) controls the blotched/classic tabby pattern (tb). However, a second gene locus, dubbed Ticked, is now considered to turn tabby patterns on and off. The historically known ticked tabby phenotype (tabby Abyssinian, Ta) is at this second locus, the gene for which is not yet identified. The actual pattern is then controlled by TAQPEP (Tabby) and other modifier genes as well.
Figure 2.

A variety of cats have long fur, but different causal variants in the gene fibroblast growth factor 5 (FGF5) exist. Ragdolls (a) have a variant that likely derived in the USA; Persians (b) have the ancient variant that derived from the Near and Middle East and is common to most longhaired breeds; Norwegian Forest Cats (c) have a variant common to Nordic race cats; and Maine Coons (d) may have a fourth variant, likely also derived in the USA. The Ragdoll and Norwegian Forest Cat are also bicolor at the Spotting locus, Ss. Images courtesy of Animal Photography
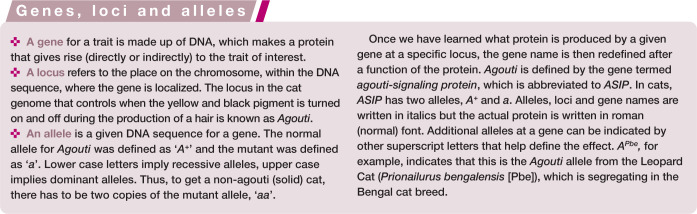
Figure 3.
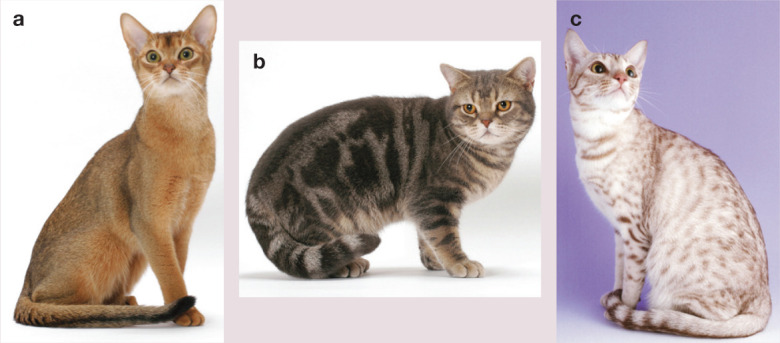
Tabby patterns of cats are complex. The Abyssinian (a) displays a variant at likely the Ticked locus (Tia) that turns off all patterning of the Tabby locus (tabby aby or ticked tabby), no matter what allele is present at Tabby. The American Shorthair (b) has the allele at the Ticked locus (ti) that allows the display of the two recessive blotched (tb) alleles at the Tabby locus to produce a classic or blotched tabby. The Ocicat (c) has the allele at the Ticked locus (ti) that allows the display of the alleles at the Tabbylocus, which are likely modified by other genes to form the spots. The modifier genes are not clearly resolved. Images courtesy of Animal Photography
Spotting and White are two very interesting and complex loci. The gene was recently identified, demonstrating that full white and bicolor white are alleles at the oncogene called KIT. 20 KIT is also responsible for the gloving of the white feet in Birmans, thus comprising at least three alleles that confer white fur. 12 However, white spotting is very complex in most domesticated species; a good example is provided by horse spotting genes and their alleles.32,33 Thus, KIT may not explain all white spotting in cats; nor has the association with deafness been elucidated.34–36 White spotting is also associated with the domestication of foxes and is likely associated with other domesticated species. 37 The first sign of domestication of the wild type cat in Figure 1 is the white spot on the neck.
In cats, the Spotting locus, now known to be KIT, has at least three alleles, wild type and spotting. 38 One copy of the S allele appears to make a bicolor cat (Figure 2), while two copies, SS, appear to produce a high white, ‘Van’, patterned cat. However, other alleles may be present, as white spotting patterns in the Ragdoll breed are difficult to predict, even though they appear similar to those seen in other breeds. For example, the white ‘mittens’ of some Ragdolls are not controlled by the same DNA variants as the white ‘gloves’ in the Birman. 12 The control of white spots on the neck and belly is also unknown, although many of these presentations could be due to random midline closure defects of melanocyte migration.
Thus, many of the ‘good’ DNA variants of cats confer their aesthetical qualities and many more recent DNA variants have been identified since cat breeding became established. Newer variants, such as many of the fur types, are unique and breed-defining. Some of the earliest phenotypes noted for a breed were the curly coats of the Cornish Rex 39 and Devon Rex, 40 which were among the first novelty breeds.41,42 More recent breed-defining fur type variants that are scientifically documented include hairless cats (Sphynx and Peterbalds)43,44 and the highly curled Selkirk Rex. 45 Other rare breeds, such as LaPerm (tica.org/cat-breeds/item/228) and Tennessee Rex (tennesseerex.com) have not been defined but have recognized curly-coated variants.
‘Good’ variants of concern
So which of the DNA variants listed in Table 1 might we not consider ‘good’ by current cat breeding standards?
Many of the coat color variants, such as White and Spotting, may be detrimental in the feral state, especially since they have not been documented in wildcats (Felis silvestris).
Dominant White is associated with deafness and increased risk of melanoma due to depigmentation and ultraviolet exposure.34–36
The Manx DNA alterations are lethal in utero in the homozygous state and many Manx cats have issues with lameness, incontinence and constipation.46–49 The discovery of the Tailless variants has also revealed that Japanese Bobtails 50 do not have DNA changes in the same gene and that the Pixiebob breed has Manx and Japanese Bobtail genetic contributions. 15
Many argue that the hairless phenotypes are ‘too unnatural’ for a cat and they can suffer from potential hypothermia and sunburn.
Well known health concerns surround the ear fold phenotype of the Scottish Fold (see Figure 6a), which is associated with osteochondrodysplasia. 51 While many breeders seem to think that osteochrondrodysplasia occurs only in the homozygous cat, it is likely that some disease in heterozygotes manifests subclinically.
Dwarfism is another controversial phenotype, propagated as the Munchkin cat breed. However, the dwarfism gene and the DNA variant have not been scientifically documented and health concerns not yet identified.
Figure 6.
The Scottish Fold (a) and the Selkirk Rex (b) are derived from random-bred cats that had novel ear and hair coat variants, respectively, and have been molded by crossing with Persians (c) to obtain structure and facial and head morphology. The Selkirk Rex and Scottish Fold are at risk for polycystic kidney disease due to the outcrossing with the Persian (see Figure 5). These cats segregate for longhair variants common to the Persian and have a variety of coloration variants. The Scottish Fold is an orange and white tabby (A-, B-, C-, D-, E-, I-, LI, O-, Ss), the Selkirk is a black smoke (aa, B-, C-, D-, E-, I-, II, oo, ss) and the Persian is cream and high white (aa, B-, C-, dd, E-, I-, II, O-, SS). Images courtesy of Animal Photography
Deciphering the gene alleles associated with Scottish Folds and dominant White cats will likely help us understand the basic biology of the genes and the role of genetic modifiers that influence the undesired and linked health concerns.
The ‘bad’ cat variants – variation humans negatively select in cats
Although current genetics focuses on DNA alterations that affect specific genes, some of the earliest genetic testing for any species involved the examination of the full set of chromosomes (karyotype) of an individual to determine whether the normal number and arrangement of chromosomes were present (Figure 4). Sex chromosome aneuploidies (losses), and trisomies of small acrocentric chromosomes (chromosomes with no short [p] arm), are typically associated with decreased fertility and syndromes that display distinct morphological abnormalities. Turner’s syndrome (XO), Klinefelter’s syndrome (XXY)52,53 and chimerisms have been documented in the domestic cat. Because the cat has a highly recognizable X-linked trait,27–30 Orange, tortoiseshell and calico (tortoiseshell and white) male cats were the first feline suspects of sex chromosomal abnormalities. Karyotypic studies of male tortoiseshell cats have shown that they are often mosaics, or chimeras, being XX/XY in all or some tissues.28,52–59
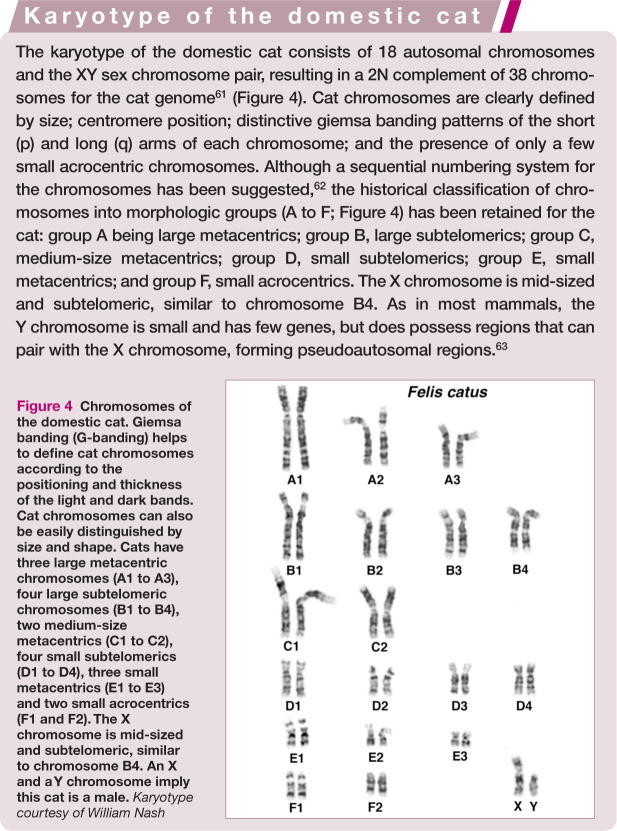
Figure 4.
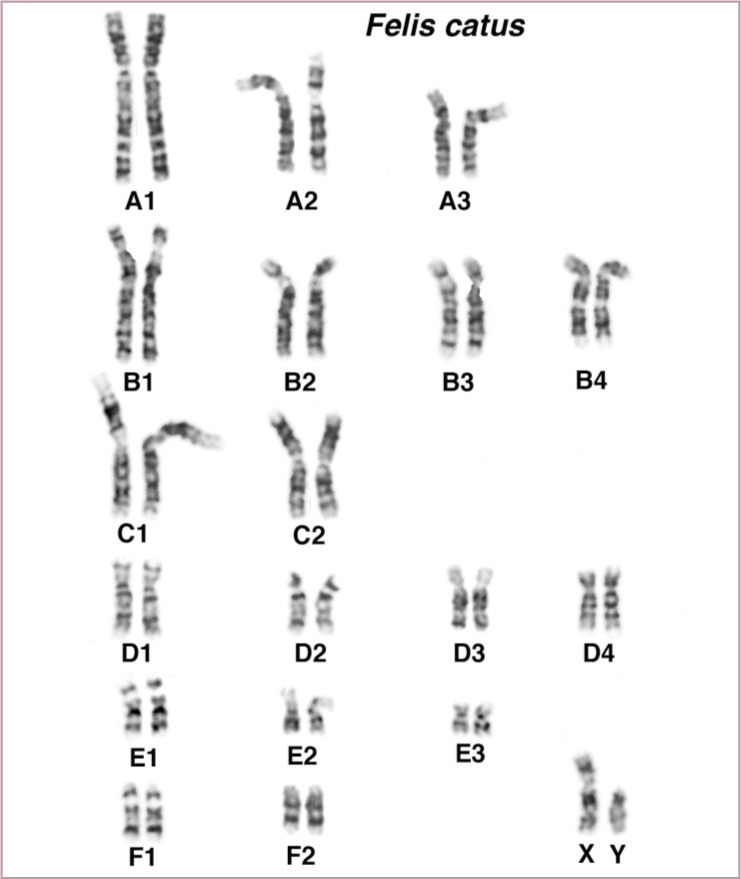
Chromosomes of the domestic cat. Giemsa banding (G-banding) helps to define cat chromosomes according to the positioning and thickness of the light and dark bands. Cat chromosomes can also be easily distinguished by size and shape. Cats have three large metacentric chromosomes (A1 to A3), four large subtelomeric chromosomes (B1 to B4), two medium-size metacentrics (C1 to C2), four small subtelomerics (D1 to D4), three small metacentrics (E1 to E3) and two small acrocentrics (F1 and F2). The X chromosome is mid-sized and subtelomeric, similar to chromosome B4. An X and a Y chromosome imply this cat is a male. Karyotype courtesy of William Nash
Although gene-based assays are common methods to determine if a cat with ambiguous genitalia 60 or a poor reproductive history has a chromosomal abnormality, karyotypes are still often performed. Domestic cats have an easily distinguishable karyotype (see box).
The alignment of genes on chromosomes in cats is very similar to the genomic organization in humans. 64 Humans have their genes distributed onto 22 autosomes; therefore, only a small number of changes is required to rearrange the same genes onto 18 autosomes, as found in cats. However, although the cat genome is conserved to humans, certain well-known chromosomal abnormalities are not found (Figure 4). For example, an analog to Down’s syndrome is not present in the cat since the genes found on human chromosome 21 are represented on the mid-sized metacenteric chromosome C2, which also has genes from human chromosome 3. A trisomy C2 would disrupt more genes than a trisomy 21 in humans; thus, in cats, more likely there is early fetal loss that is never detected. 65 The minor chromosomal differences that are cytogenetically detectable between a domestic cat and a Leopard Cat are likely the cause of fertility problems in the Bengal cat breed,61,66 which is a hybrid between these two species. 67 Other significant chromosomal abnormalities causing common ‘syndromes’ are not well documented in the cat.
Several research and commercial laboratories can perform cat chromosomal analyses when provided with a living tissue, such as a skin biopsy for fibroblast culturing or whole blood for the analysis of white blood cells.
Genetic variants for routine screening
The first gene-specific DNA variants identified in cats were for gangliosidosis and muscular dystrophy; both discovered in 1994,68,69 and both diseases that had well-defined phenotypes and known (candidate) genes with DNA alterations in humans. Most diseases are identified in pedigreed cats, which represent a small percentage of the cat population of the world, perhaps at most 10–15% in the USA. 70 The genetically characterized diseases and health concerns for breeds of domestic cats are presented in Table 2. Most of the identified disease tests in cats that are very specific to breeds and populations are available as commercial genetic tests offered by university and private laboratories (Table 3).
Table 2.
Inherited diseases of domestic cats for which a commercial DNA test is available
| Disease/trait (Alleles) OMIA entry link | MOI* | Phenotype | Gene | Gene name | Mutation |
|---|---|---|---|---|---|
|
AB blood type (A+, b)
71
000119-9685 |
AR | Determines type B | CMAH | Cytidine monophospho-N-acetylneuraminic acid hydroxylase | c.1del-53_70 c.139G>A |
| Craniofacial defect | AR | Craniofacial defect | unpublished | unpublished | unpublished |
|
Gangliosidosis 1
72
000402-9685 |
AR | Lipid storage disorder (GM1) | GLB1 | Galactosidase, beta 1 | c.1457G>C |
|
Gangliosidosis 269,73
01462-0985 |
AR | Lipid storage disorder (GM2) | HEXB | Hexominidase B | c.1356del-1_8 c.1356_1362delGTTCTCA c.39delC |
|
Glycogen storage disease IV
74
000420-9685 |
AR | Glycogen storage disorder | GBE1 | Glycogen branching enzyme 1 | IVS11+1552 IVS12-1339 del6.2kb ins334 bp |
|
Hypertrophic cardiomyopathy
75,76
000515-9685 |
AD | Cardiac disease (HCM) | MYBPC | Myosin binding protein C | c.93G>C c.2460C>T |
|
Hypokalemia
77
001759-9685 |
AR | Potassium deficiency (HK) | WNK4 | WNK lysine deficient protein kinase 4 | c.2899C>T |
|
Progressive retinal atrophy
78
001244-9685 |
AR | Late-onset blindness (rdAC) | CEP290 | Centrosomal protein 290kDa | IVS50 + 9T>G |
|
Progressive retinal atrophy
79
000881-9685 |
AD | Early-onset blindness (rdy) | CRX | Cone-rod homeobox | c.546delC |
|
Polycystic kidney disease
80
000807-9685 |
AD | Kidney cysts (PKD) | PKD1 | Polycystin 1 | c.10063C>A |
|
Pyruvate kinase deficiency
81
000844-9685 |
AR | Hemopathy (PK deficiency) | PKLR | Pyruvate kinase, liver, RBC | c.693+304G>A |
|
Spinal muscular atrophy
82
000939-9685 |
AR | Muscular atrophy (SMA) | LIX1-LNPEP | Limb expression 1 homolog -leucyl/cystinyl aminopeptidase | Partial gene deletions |
Mode of inheritance of the non-wild type variant. Not all transcripts for a given gene may have been discovered or well documented in the cat; mutations presented as interpreted from original publication. ‘+’ implies the wild type allele when known. In reference to the mutant allele, AD = autosomal dominant, AR = autosomal recessive. OMIA: Online Mendelian Inheritance in Animals (omia.angis.org.au) entries provide links to citations and clinical descriptions of the phenotypes and the diseases. Listed citations are for the causative variant discovery
Table 3.
Laboratories performing DNA testing of domestic cats
| Laboratory | Region | University research affiliate | ID | Cat tests*/ Disease | Color | Blood | Coat |
|---|---|---|---|---|---|---|---|
|
Animal DNA Laboratory (Orivet Genetic Pet Care)
animalsdna.com |
Australia, New Zealand | Yes | 8 | 4 | Yes | Long | |
|
Animal Health Trust
aht.org.uk |
UK | Animal Health Trust | Yes | PKD | No | No | No |
|
Antagene
antagene.com |
France | Yes | 4 | Color | Yes | No | |
|
BioAxis DNA Research Centre
dnares.in |
India | Yes | PKD | No | No | No | |
|
DNA Diagnostics Center
dnacenter.com |
USA | No | PKD | No | No | No | |
|
Genindexe
genindexe.com |
France | Yes | 7 | 5 | Yes | No | |
|
Genoscoper Laboratories
genoscoper.com |
Finland | Yes | 7 | Yes | Yes | Long | |
|
Gribbles Veterinary
gribblesvets.com |
Australia, New Zealand | No | PKD | No | No | No | |
|
IDEXX Laboratories
idexx.ca |
Canada | No | PK deficiency | No | No | No | |
|
Laboklin
laboklin.de |
Germany | Yes | 9 | 5 | Yes | Long | |
|
Langford Veterinary Services
langfordvets.co.uk |
Bristol | Yes | 10 | 8 | Yes | Long | |
|
PennGen
†
research.vet.upenn.edu/penngen |
USA | Pennsylvania | No | PK deficiency GSD | No | No | No |
|
Progenus
progenus.be |
Belgium | Yes | 7 | 6 | No | Long | |
|
VHL Genetics
vhlgenetics.com |
Netherlands, Belgium, Germany | Yes | 10 | 6 | Yes | Long | |
|
NC State CVM Veterinary Genetics Laboratory
ncstatevets.org/genetics |
USA | North Carolina State | No | HCM | No | No | No |
|
Veterinary Genetics Laboratory
vgl.ucdavis.edu |
USA | California, Davis | Yes | 14 | All | Yes | All |
|
VetGen
vetgen.com |
Michigan | Yes | No | Brown Dilute | No | Long | |
|
VetoGene
vetogene.com |
Italy | Milan | Yes | 5 | No | Yes | Long |
|
Servicio de Genética
ucm.es/genetvet |
Spain | Madrid | Yes | 8 | Yes | No | Yes |
|
Biofocus
biofocus.de |
Germany | Yes | 7 | Yes | Yes | No | |
|
Genefast
genefast.com |
Italy | Yes | No | Yes | No | No |
Tests refer to those listed in Tables 1 and 2. If a laboratory offers only one or two tests, those tests are listed, otherwise the number of tests offered for cats is presented. Polycystic kidney disease (PKD) and the hypertrophic cardiomyopathy (HCM) tests are the most popular cat offerings. †PennGen also offers tests for diseases in Table 5 that are not of concern to the cat breeds or cat population in general. PK = pyruvate kinase, GSD = glycogen storage disease
These are the DNA variants that should be monitored by cat breed registries and that veterinary practitioners should most familiarize themselves with, as they are useful diagnostically. Polycystic kidney disease (PKD), for example, is the most prevalent genetic disease in cats and an important diagnostic for early kidney disease (Figure 5). The prevalence of PKD in Persians was estimated at 30–38% worldwide, prior to the introduction of the genetic test.83–85 Because of crossbreeding with Persians, many other breeds, such as the British Shorthair, American Shorthair, Selkirk Rex and Scottish Fold (Figure 6) also need to be screened for PKD.86–88 The discovery of the hypokalemia DNA variant for Burmese cats reveals that a gene influencing overall potassium levels in cats can also influence blood pressure in humans. 89 By considering the breed relationships in Table 4, genetic health concerns across cat populations and breeds can be inferred due to inheritance (‘identity by descent’) via outcrossing.
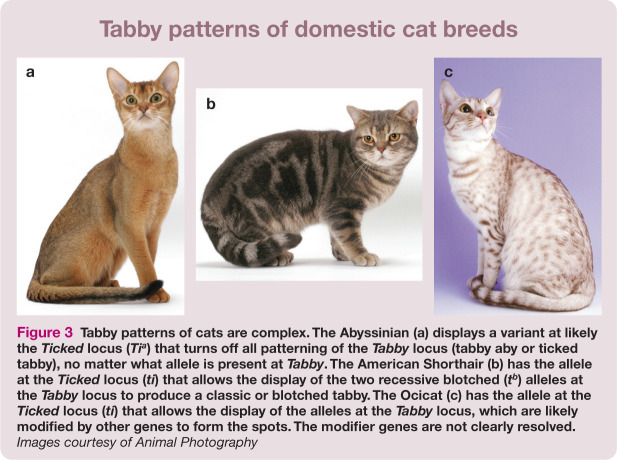
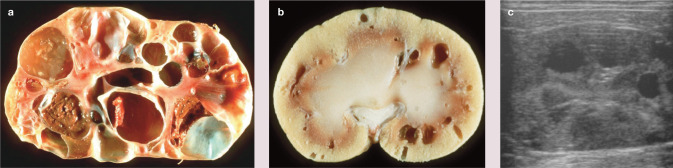
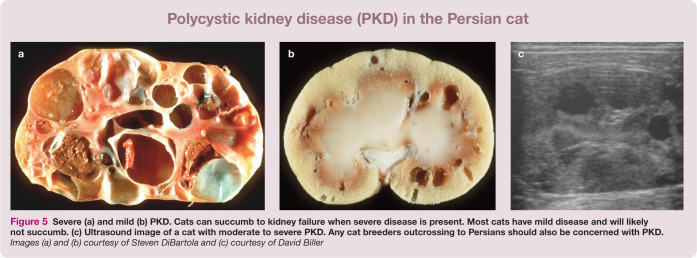
Figure 5.
Severe (a) and mild (b) PKD. Cats can succumb to kidney failure when severe disease is present. Most cats have mild disease and will likely not succumb. (c) Ultrasound image of a cat with moderate to severe PKD. Any cat breeders outcrossing to Persians should also be concerned with PKD. Images (a) and (b) courtesy of Steven DiBartola and (c) courtesy of David Biller
Table 4.
Genetic families of domestic cat breeds
| Breed/family | Origin | Derived breed/grouping* |
|---|---|---|
| Abyssinian | India? | Somali |
| American Bobtail | Natural mutation | United States - random breds |
| American Curl | Natural mutation | United States - random breds |
| American Shorthair | United States | American Wirehair |
| American Wirehair | Natural mutation | American Shorthair |
| Australian Mist | Crossbreed hybrid | Burmese derived |
| Balinese | Variant | Colorpoint, Havana Brown, Javanese, Oriental, Siamese |
| Bengal | Species hybrid | Leopard Cat x Egyptian Mau and Abyssinian |
| Birman | Southeast Asia | |
| Bombay | Variant | Burmese, Singapura, Tonkinese |
| British Shorthair | Europe | Scottish Fold, Selkirk Rex |
| Burmese | Southeast Asia | Bombay, Singapura, Tonkinese |
| Burmilla | Crossbreed hybrid | Burmese, Persian |
| Chartreux | Europe | |
| Colorpoint Shorthair | Variant | Balinese, Havana Brown, Javanese, Oriental, Siamese |
| Cornish Rex | Natural mutation | United Kingdom - random breds |
| Devon Rex | Natural mutation | United Kingdom - random breds, Sphynx |
| Egyptian Mau | Mediterranean | |
| European | Europe | |
| Exotic | Variant | Persian |
| Havana Brown | Variant | Balinese, Colorpoint, Javanese, Oriental, Siamese |
| Japanese Bobtail | Founder | |
| Javanese | Variant | Balinese, Colorpoint, Havana Brown, Oriental, Siamese |
| Korat | Southeast Asia | |
| Kurilean Bobtail | Natural mutation | Eastern Russia, Kuril Islands |
| LaPerm | Natural mutation | United States - random breds |
| Maine Coon | United States | |
| Manx | Natural mutation | United Kingdom - random breds |
| Munchkin | Natural mutation | United States - random breds |
| Norwegian Forest Cat | Europe | |
| Ocicat | Crossbreed hybrid | Siamese x Abyssinian |
| Oriental | Variant | Balinese, Colorpoint, Havana Brown, Javanese, Siamese |
| Persian | Europe | Exotic |
| Peterbald | Mutation | Russian - random breds, Don Sphynx |
| Pixiebob | Crossbreed hybrid | Manx, Japanese Bobtail, United States - random breds |
| Ragdoll | United States | United States - random breds |
| Russian Blue | Europe | |
| Savannah | Species hybrid | Serval x domestic |
| Scottish Fold | Natural mutation | United Kingdom - random breds, British Shorthair, Persian, Exotic |
| Selkirk Rex | Natural mutation | United States - random breds, Persian, British Shorthair, Exotic |
| Siamese | Southeast Asia | Balinese, Havana Brown, Javanese, Colorpoint, Oriental |
| Siberian | Europe | Russian - random breds |
| Singapura | Variant | Bombay, Burmese, Tonkinese |
| Sokoke | Arabian Sea | African - random breds |
| Somali | Variant | Abyssinian |
| Sphynx | Natural mutation | Devon Rex |
| Tonkinese | Variant | Bombay, Burmese, Singapura |
| Turkish Angora | Mediterranean | |
| Turkish Van | Mediterranean |
Other DNA variants for which genetic tests are available
Some DNA variants have been found in individual cats of a certain breed, such as mucopolysaccharidosis type VI in the Siamese, but the variant is not of significant concern in the breed at large.93,94 Table 5 lists DNA alterations identified in random-bred cats and disease-conferring variants that have not propagated within a breed. These genetic variants do not warrant routine screening by cat breeders and registries, but clinicians should know that genetic tests are available for diagnostic purposes, especially from research groups with specialized expertise.
Table 5.
Uncommon mutations for inherited diseases of domestic cats*
| Disease | OMIA entry link | Gene | Mutation† |
|---|---|---|---|
| 11 ß-hydroxylase deficiency (congenital adrenal hypoplasia) 95 | 001661-9685 | CYP11B1 | Exon 7 G>A |
| Dihydropyrimidinase deficiency 96 | 001776-9685 | DPY5 | c.1303G>A |
| Factor XII deficiency 97 | 000364-9685 | FXII | c.1321delC |
| Fibrodysplasia ossificans progressiva | 000388-9685 | unpublished | unpublished |
| Gangliosidosis 1 98 | 000402-9685 | GLB1 | c.1448G>C |
| Gangliosidosis 299,100 | 001462-9685 | HEXB | c.1467_1491inv c.667C>T |
| Gangliosidosis 2 74 | 001427-9685 | GM2A | c.390_393GGTC |
| Hemophilia B 101 | 000438-9685 | F9 | c.247G>A c.1014C>T |
| Hyperoxaluria 102 | 000821-9685 | GRHPR | G>A I4 acceptor site |
| Hypothyroidism | 000536-9685 | unpublished | unpublished |
| Lipoprotein lipase deficiency 103 | 001210-9685 | LPL | c.1234G>A |
| Mucolipidosis II 104 | 001248-9685 | GNPTAB | c.2655C>T |
| Mannosidosis, alpha 105 | 000625-9685 | LAMAN | c.1748_1751delCCAG |
| Mucopolysaccharidosis I 106 | 000664-9685 | IDUA | c.1107_1109delCGA c.1108_1110GAC |
| Mucopolysaccharidosis VI 94 | 000666-9685 | AR5B | c.1427T>C |
| Mucopolysaccharidosis VI93,107 | 000666-9685 | AR5B | c.1558G>A |
| Mucopolysaccharidosis VII 108 | 000667-9685 | GU5B | c.1052A>G |
| Muscular dystrophy 68 | 001081-9685 | DMD | 900bp del M promoter - exon 1 |
| Niemann-Pick C 109 | 000725-9685 | NPC | c.2864G>C |
| Polydactyly 17 | 000810-9685 | 5HH | c.479A>G c.257G>C c.481A>T |
| Porphyria (congenital erythropoietic) 110 ‡ | 001175-9685 | URO5 | c.140C>T
c.331G>A |
| Porphyria (acute intermittent) 111 ‡ | 001493-9685 | HMB5 | c.842_844delGAG c.189dupT c.250G>A c.445C>T |
| Vitamin D resistant rickets112,113 | 000837-9685 | CYP27B1 | c.223G>A c.731delG c.637G>T |
The presented conditions are not prevalent in breeds or populations but may have been established into research colonies. †Not all transcripts for a given gene may have been discovered or well documented in the cat; mutations presented as interpreted from original publication. ‡A variety of mutations have been identified, yet unpublished, for porphyrias in domestic cats. Contact PennGen at the University of Pennsylvania for additional information. OMIA: Online Mendelian Inheritance in Animals (omia.angis.org.au) entries provide links to citations and clinical descriptions of the phenotypes and the diseases. Listed citations are for the causative variant discovery
If a newly recognized condition is suspected in cats, based on a number of individuals presenting similarly, researchers will generally consider testing for the known variant as a non-commercial service and may continue analysis of the entire gene to determine if new DNA alterations can be identified that are causative for this particular condition. Other biomarkers are also available at these specialized laboratories to help decipher between specific conditions, such as the lysosomal storage diseases and metabolism disorders.
And the ‘ugly’ cat variants – be careful with selection
Most cat genetic tests developed to date have been for traits that have nearly complete penetrance, little variability in expression and early onset. However, some recognized DNA variants in cats might be considered risk factors, predisposing an individual to a specific health problem. These are not clean and clear, simple genetic traits, but complex risk factors and susceptibilities with ‘ugly’ genetics. Undoubtedly, the majority of the variation in the genome will prove to be modifiers; that is, polygenes that have DNA variants with small heritable effects. Research has thus far focused on just the tip of the iceberg – deciphering ‘ugly’ genetic variation is the future.
The reasons why a condition might not present when a specific DNA variant is present have been predicted and are now being deciphered as we understand more and more about the entire genomic sequence. Environmental interactions certainly play a role in the overall appearance and health of an individual and its organs. There are also several known genetic mechanisms and other factors that can make DNA variants more difficult to interpret and their consequences more difficult to predict (see box below).
HCM variants
An excellent example of a variant that confers a risk is the DNA variant associated with cardiac disease in cats. Hypertrophic cardiomyopathy (HCM) is a recognized genetic condition. 114 In 2005, Drs Meurs, Kittleson and colleagues published findings that a protein alteration, A31P, in the cardiac myosin-binding protein C 3 (MYBPC3) gene was strongly associated with HCM in a research colony of Maine Coon cats at the University of California – Davis. 75 The data clearly showed that not all cats with the variant had HCM and some cats with HCM did not have the DNA variant. Age of onset, variable expression and disease heterogeneity were discussed as confounding factors in this study. This suggested that the identified DNA variant should be considered more of a ‘risk factor’ than a directly causative variant.
Subsequent studies have shown that not all Maine Coon cats with the A31P variant develop HCM,115,116 and one of those papers has interpreted this lack of penetrance as being evidence that the A31P variant is not causal. 116 This interpretation is misleading, and has led to debate as to the validity of the Maine Coon HCM test. As is true in humans with cardiac disease, the finding that not all cats with the A31P variant in MYBPC3 develop HCM is actually usual in the field of HCM genetic testing. The controversy has prompted correspondence within the literature and continued studies to clarify the interpretations. 117 Further, an additional variant in MYBPC3, known as A74T, was suggested at a scientific meeting as being causative for HCM. When some commercial testing laboratories started offering the A74T test to the cat community, it became evident that A74T was a polymorphism occurring in a large number of breeds, and was not significantly correlated to HCM; rather, A31P penetrance was incomplete but highly associated with disease. 118
Other disease variants
Like HCM variants, other disease variants in cats have shown variation in penetrance and expression. The CEP290 PRA variant in Abyssinians has a late age of onset and some cats with subclinical disease have been identified. 119 Some cats with the pyruvate kinase deficiency can have very mild and subclinical presentations. 120 Thus, disease- or trait-causing variants may not be 100% penetrant, and so may not always cause clinically detectable disease.
Blood type variants
Cats are one of the few species to have had the variant for their blood type determined. Blood type incompatibilities can lead to transfusion reactions and neonatal isoerythrolysis for the cat, but inherently this characteristic is not necessarily a disease. A point mutation and an 18 base pair deletion have both been implicated in the gene CMAH as indicative of the B blood type or a B blood type carrier. 71 Because both variants are on the same allele, the true causative variant has not been definitively determined. Thus, at the current time, both variants should be examined in cats to genetically determine blood type. Some laboratories have been identified that are not only typing the incorrect variant, but also not both variants. Breeders generally are the first to recognize when a genetic testing laboratory is making errors. Laboratories associated with research groups are most likely to correct errors and perform further studies to improve their testing accuracy.
Hybrid cats
Several cat breeds have been formed by crossing domestic cats (Felis catus) with different wild species of cat. The Bengal breed is acknowledged worldwide and has become highly popular. To create Bengals, Leopard Cats (P bengalensis) were (and continue to be) bred with Egyptian Mau, Abyssinian and other cats to form a breed that is unique in both color and temperament. 67 The Chausie is developing from crosses with Jungle Cats (Felis chaus), and Savannahs from crosses with Servals (Leptailurus serval). Bobcat (Lynx rufus) hybrids have not been genetically proven to date.
The Leopard Cat had a common ancestor with the domestic cat about 6 million years ago, the Bobcat about 8 million years ago and the Serval about 9.5 million years ago. 121 The Jungle Cat is more closely related to a domestic cat than is the Leopard Cat. In addition, for some of these wild felid species, different subspecies have been incorporated into the breed.
Comparison of the DNA sequence between a domestic cat and one of these wild felid species will reveal many (possibly several percentage) genetic differences – fewer for the Jungle Cat, more for the Serval. The genetic differences are most likely silent (neutral) variants, but the variation will interplay with genetic assays and may cause more test failures or inaccuracies than would normally be anticipated. No genetic tests are validated in the hybrid cat breeds, although the tests are typically used very frequently in Bengal cats. Thus, the accuracy of any genetic test is not known for hybrid cat breeds. Consider, for example, the charcoal coloration in the Bengal cat. 122 This is due to the cats having one allele from the Leopard Cat, APbe, for Agouti and their second allele from the domestic cat for non-agouti, a. If Bengal cats can have alleles from different species for color, there is the possibility of different alleles at all other genes in their genome as well.
Whole-genome sequencing for feline health care
DNA testing for diseases and phenotypic traits of domestic cats is a rapidly growing asset for veterinary medicine. A number of commercial laboratories around the world can now perform cat genetic diagnostics (Table 3), allowing both the veterinary clinician and the private owner to obtain DNA test results. DNA is easily obtained from a cat via a buccal swab using a standard cotton bud or cytological brush, and samples may be sent to any of these laboratories. Test results identify carriers of traits, predict the incidence of traits for breeding programs, and influence medical prognoses and treatments. Besides controlling the occurrence of disease, one long-term goal of identifying these genetic variants is the correction of the defect via gene therapies and designer drug therapies. Thus, genetic testing is an effective preventative medicine tool and a potential ultimate cure through selective breeding. However, genetic diagnostic tests may still be novel for many veterinary practitioners and their application in the clinical setting needs to be subject to the same scrutiny as any other diagnostic procedure.
Genetic testing has been a part of modern human health care in the USA since the implementation of the DNA mutation test for phenylketonuria (PKU) in the 1960s. 124 Most individuals in the USA do not realize that, at birth, after a heal prick blood collection, they were tested for a battery of genetic diseases. Similar testing is now available to the public as an elective evaluation of a person’s genome. Companies, such as 23andMe (23andme.com), offer a personal genetics service that presents an individual with their own variants for known single gene traits and also variants that confer risk. Similar offerings are available for domestic cats. Clearly, these genetic services must not replace the role of the clinician or veterinarian, since the interpretation of the genetic results should be made by a professional as part of an overall health care plan. Many laboratories, such as the University of California – Davis (vgl.ucdavis.edu), offer both the majority of the genetic tests important for cat breeds and also a Cat Ancestry panel that can determine a cat’s race and potential breed.
However, in humans, and hopefully cats, too, the future of individual health care will rely on the individual’s personal genome. A result of the Human Genome Project has been the development of rapid and cost-effective means to sequence an entire genome of an individual in less than a month. Currently, whole-genome sequencing is becoming the standard of health care for genetic profiling of cancers, with ‘cancer genomics’ dictating the selection of chemotherapies based on DNA mutations of the tumor. 125 At specialized centers around the world, newborns with sporadic, congenital abnormalities can be whole-genome sequenced, which often, but not always, detects the cause of their maladies. 126 Since over 100,000 people have now had their genomes sequenced, the database of normal and detrimental genetic variants is fairly well defined in some human populations, though requires substantially better definition in others. 127 Likely, whole-genome sequencing will become part of the health care package for human health. 128 Recently, the $1000 genome has been achieved for humans, and shortly this technology will be adapted for other species. 129
For cats, currently whole-genome sequencing is being used to investigate diseases and traits that are known to be heritable, and also when sufficient individuals are not available for a different means of genetic analysis, such as family studies or case-control association studies. Like humans, eventually the genetic variant databases will be sufficient for the analysis of an individual cat with an unusual health presentation. The 99 Lives Cat Genome Sequencing Initiative (felinegenetics.missouri. edu/ninety-nine-lives) has been launched to meet the same standard in health care for cats as for humans. What are currently recognised as sporadic or idiopathic conditions will slowly be determined to have individual specific genetic causes, leading to highly specific personalized medicine for our companion animals.
Key Points
Genetic testing is an important diagnostic tool for the veterinarian, breeder and owner.
Genetic tests are not 100% foolproof and the accuracy of the test procedure and the reputation and customer service of the genetic testing laboratory need to be considered.
Understanding the relationship of cats to the race of origin and their breed families can be predictive for healthcare issues.
Some traits are highly desired and genetic testing can help breeders to more accurately determine appropriate breedings; thus, potentially becoming more efficient breeders, and lowering costs and helping to address overpopulation.
Other traits or diseases are undesired. In this context, genetic testing can be used to prevent disease and potentially eradicate the concern from the population.
Genetic tests for simple genetic traits are more consistent with predicting the trait or disease presentation, but, as genomic research progresses for the cat, tests for variation that confers risk will become more commonplace.
Veterinarians will have to consider the significance of a risk-conferring disease variant as part of their differentials and treatment plans, and breeders will have to consider these same risk factors, along with the other important attributes of a cat, in their breeding decisions.
Supplemental Material
Linked version of Table 1
Linked version of Table 2
Linked version of Table 5
Footnotes
Funding: This work was supported in part by funding from the National Center for Research Resources R24 RR016094 and is currently supported by the Office of Research Infrastructure Programs/OD R24OD010928.
The author declares that there is no conflict of interest.
References
- 1. Lyons LA. Feline genetics: clinical applications and genetic testing. Top Companion Anim Med 2010; 25: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyons LA. Genetic testing in domestic cats. In: August JR. (ed). Consultations on feline internal medicine. St Louis, MO: Saunders Elsevier, 2010, 793–799. [Google Scholar]
- 3. Lyons LA. Genetic testing in domestic cats. Mol Cell Probes 2012; 26: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. online Mendelian inheritance in Animals, oMiA. Faculty of Veterinary Science, University of Sydney, 2014. [Google Scholar]
- 5. Eizirik E, Yuhki N, Johnson WE, et al. Molecular genetics and evolution of melanism in the cat family. Curr Biol 2003; 13: 448–453. [DOI] [PubMed] [Google Scholar]
- 6. Lyons LA, Foe IT, Rah HC, et al. Chocolate coated cats: TYRP1 mutations for brown color in domestic cats. Mamm Genome 2005; 16: 356–366. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt-Kuntzel A, Eizirik E, O’Brien SJ, et al. Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the Albino and Brown loci. J Hered 2005; 96: 289–301. [DOI] [PubMed] [Google Scholar]
- 8. Imes DL, Geary LA, Grahn RA, et al. Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation. Anim Genet 2006; 37: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyons LA, Imes DL, Rah HC, et al. Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus). Anim Genet 2005; 36: 119–126. [DOI] [PubMed] [Google Scholar]
- 10. Ishida Y, David VA, Eizirik E, et al. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics 2006; 88: 698–705. [DOI] [PubMed] [Google Scholar]
- 11. Peterschmitt M, Grain F, Arnaud B, et al. Mutation in the melanocortin 1 receptor is associated with amber colour in the Norwegian Forest Cat. Anim Genet 2009; 40: 547–552. [DOI] [PubMed] [Google Scholar]
- 12. Montague MJ, Li G, Gandolfi B, et al. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc Natl Acad Sci USA 2014; 111: 17230–17235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandolfi B, Outerbridge C, Beresford L, et al. The naked truth: Sphynx and Devon Rex cat breed mutations in KRT71. Mamm Genome 2010; 21; 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kehler JS, David VA, Schaffer AA, et al. Four independent mutations in the feline fibroblast growth factor 5 gene determine the long-haired phenotype in domestic cats. J Hered 2007; 98: 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drogemuller C, Rufenacht S, Wichert B, et al. Mutations within the FGF5 gene are associated with hair length in cats. Anim Genet 2007; 38: 218–221. [DOI] [PubMed] [Google Scholar]
- 16. Buckingham KJ, McMillin MJ, Brassil MM, et al. Multiple mutant T alleles cause haploinsufficiency of Brachyury and short tails in Manx cats. Mamm Genome 2013; 24: 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lettice LA, Hill AE, Devenney PS, et al. Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. Hum Mol Genet 2008; 17: 978–985. [DOI] [PubMed] [Google Scholar]
- 18. Gandolfi B, Alhaddad H, Affolter VK, et al. To the root of the curl: a signature of a recent selective sweep identifies a mutation that defines the Cornish Rex cat breed. PloS One 2013; 8: e67105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gandolfi B, Alhaddad H, Joslin SE, et al. A splice variant in KRT71 is associated with curly coat phenotype of Selkirk Rex cats. Sci Rep 2013; 3: 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. David VA, Menotti-Raymond M, Wallace AC, et al. Endogenous retrovirus insertion in the KIT oncogene determines white and white spotting in domestic cats. G3 (Bethesda) 2014; 4: 1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaelin CB, Xu X, Hong LZ, et al. Specifying and sustaining pigmentation patterns in domestic and wild cats. Science 2012; 337: 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silvers WK. The coat colors of mice. New York: Springer-Verlag, 1979. [Google Scholar]
- 23. Prieur DJ, Collier LL. Morphologic basis of inherited coat-color dilutions of cats. J Hered 1981; 72: 178–182. [DOI] [PubMed] [Google Scholar]
- 24. Prieur DJ, Collier LL. Maltese dilution of domestic cats. A generalized cutaneous albinism lacking ocular involvement. J Hered 1984; 75: 41–44. [DOI] [PubMed] [Google Scholar]
- 25. Grahn RA, Lemesch BM, Millon LV, et al. Localizing the X-linked orange colour phenotype using feline resource families. Anim Genet 2005; 36: 67–70. [DOI] [PubMed] [Google Scholar]
- 26. Schmidt-Kuntzel A, Nelson G, David VA, et al. Linkage map and the sex-linked Orange locus-mapping of Orange, multiple origins, and epistasis over non-agouti. Genetics 2009; 181: 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bamber RC, Herdman EC. The inheritance of black, yellow and tortoiseshell coat colour in cats. J Genetics 1927; 18: 87–97. [Google Scholar]
- 28. Doncaster L. On the inheritance of tortoiseshell and related colours in cats. Proc Cambridge Philosophical Soc 1904; 13: 35–38. [Google Scholar]
- 29. Ibsen HL. Tricolor inheritance. III. Tortoiseshell cats. Genetics 1916; 1: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Little CC. Colour inheritance in cats, with special reference to colours, black, yellow and tortoiseshell. J Genetics 1919; 8: 279–290. [Google Scholar]
- 31. Lyons LA, Bailey SJ, Baysac KC, et al. The Tabby cat locus maps to feline chromosome B1. Anim Genet 2006; 37: 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haase B, Jude R, Brooks SA, et al. An equine chromosome 3 inversion is associated with the tobiano spotting pattern in German horse breeds. Anim Genet 2008; 39: 306–309. [DOI] [PubMed] [Google Scholar]
- 33. Rieder S, Hagger C, Obexer-Ruff G, et al. Genetic analysis of white facial and leg markings in the Swiss Franches-Montagnes Horse Breed. J Hered 2008; 99: 130–136. [DOI] [PubMed] [Google Scholar]
- 34. Bamber RC. Correlation between white coat colour, blue eyes, and deafness in cats. J Genetics 1933; 27: 407–413. [Google Scholar]
- 35. Bergsma DR, Brown KS. White fur, blue eyes, and deafness in the domestic cat. J Hered 1971; 62: 171–185. [DOI] [PubMed] [Google Scholar]
- 36. Wilson TG, Kane F. Congenital deafness in white cats. Acta Otolaryngol 1959; 50: 269–275; discussion 75–77. [DOI] [PubMed] [Google Scholar]
- 37. Kukekova AV, Acland GM, Oskina IN, et al. The genetics of domesticated behavior in canids: what can dogs and silver foxes tell us about each other? In: Ostrander EA, Giger U, Lindblad-Tioh K. (eds). The dog and its genome. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 2006, pp 515–537. [Google Scholar]
- 38. Cooper MP, Fretwell N, Bailey SJ, et al. White spotting in the domestic cat (Felis catus) maps near KIT on feline chromosome B1. Anim Genet 2006; 37: 163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Searle A, Jude A. The ‘rex’ type of goat in the domestic cat. J Genetics 1956; 54: 506–512. [Google Scholar]
- 40. Robinson R. Devon rex – a third rexoid coat mutant in the cat. Genetica 1969; 40: 597–599. [Google Scholar]
- 41. Morris D. Cat breeds of the world. New York: Penguin Books, 1999. [Google Scholar]
- 42. Morris D. Cat breeds of the world: a complete illustrated encyclopedia. New York: Viking Penquin, 1999, pp 10–12. [Google Scholar]
- 43. Robinson R. The Canadian hairless of Sphinx cat. J Hered 1973; 64: 47–49. [DOI] [PubMed] [Google Scholar]
- 44. Filler S, Alhaddad H, Gandolfi B, et al. Selkirk Rex: morphological and genetic characterization of a new cat breed. J Hered 2012; 103: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhigachev AL, Vladimirova MV, Kaster I. Phenotypic and genotypic characteristics of russian hairless cats [article in Russian]. Genetika 2000; 36: 538–544. [PubMed] [Google Scholar]
- 46. Howell JM, Siegel PB. Morphological effects of the Manx factor in cats. J Hered 1966; 57: 100–104. [DOI] [PubMed] [Google Scholar]
- 47. Howell JM, Siegel PB. Phenotypic variability of taillessness in Manx cats. J Hered 1963; 54: 167–169. [DOI] [PubMed] [Google Scholar]
- 48. James CC, Lassman LP, Tomlinson BE. Congenital anomalies of the lower spine and spinal cord in Manx cats. J Pathol 1969; 97: 269–276. [DOI] [PubMed] [Google Scholar]
- 49. Todd NB. The manx factor in domestic cats: a possible genetic basis for expressivity of taillessness and other associated anomalies. J Hered 1964; 55: 225–230. [PubMed] [Google Scholar]
- 50. Pollard RE, Koehne AL, Peterson CB, et al. Japanese Bobtail: vertebral morphology and genetic characterization of an established cat breed. J Feline Med Surg. Epub ahead of print 8 December 2014. DOI: 10.1177/1098612X14558147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malik R, Allan GS, Howlett CR, et al. Osteochondrodysplasia in Scottish Fold cats. Aust Vet J 1999; 77: 85–92. [DOI] [PubMed] [Google Scholar]
- 52. Centerwall WR, Benirschke K. Animal model for the XXY Klinefelter’s syndrome in man: tortoiseshell and calico male cats. Am J Vet Res 1975; 36: 1275–1280. [PubMed] [Google Scholar]
- 53. Pyle RL, Patterson DF, Hare WC, et al. XXY sex chromosome constitution in a Himalayan cat with tortoise-shell points. J Hered 1971; 62: 220–222. [DOI] [PubMed] [Google Scholar]
- 54. Ishihara T. Cytological studies on tortoiseshell male cats. Cytologia 1956; 21: 391–398. [Google Scholar]
- 55. Chu EHY, Thuline HC, Norby DE. Triploid-diploid chimerism in a male tortoiseshell cat. Cytogenetics 1964; 3: 1–18. [DOI] [PubMed] [Google Scholar]
- 56. Thuline HC. Male tortoiseshell, chimerism and true hermaphroditism. J Cat Genet 1964; 4: 2–3. [Google Scholar]
- 57. Gregson NM, Ishmael J. Diploid triploid chimerism in three tortoiseshell cats. Res Vet Sci 1971; 12: 275–279. [PubMed] [Google Scholar]
- 58. Kosowska B, Januszewski A, Tokarska M, et al. Cytogenetic and histologic studies of tortoiseshell cats. Med Weter 2001; 57: 475–479. [Google Scholar]
- 59. Kuiper H, Hewicker-Trautwein M, Distl O. Cytogenetic and histologic examination of four tortoiseshell cats [article in German]. Dtsch Tierarztl Wochenschr 2003; 110: 457–461. [PubMed] [Google Scholar]
- 60. Schlafer DH, Valentine B, Fahnestock G, et al. A case of SRY-positive 38,XY true hermaphroditism (XY sex reversal) in a cat. Vet Pathol 2011; 48: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wurster-Hill DH, Gray CW. Giemsa banding patterns in the chromosomes of twelve species of cats (Felidae). Cytogenet Cell Genet 1973; 12: 388–397. [PubMed] [Google Scholar]
- 62. Cho KW, Youn HY, Watari T, et al. A proposed nomenclature of the domestic cat karyotype. Cytogenet Cell Genet 1997; 79: 71–78. [DOI] [PubMed] [Google Scholar]
- 63. Li G, Davis BW, Raudsepp T, et al. Comparative analysis of mammalian Y chromosomes illuminates ancestral structure and lineage-specific evolution. Genome Res 2013; 23: 1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O’Brien SJ, Wienberg J, Lyons LA. Comparative genomics: lessons from cats. Trends Genet 1997; 13: 393–399. [DOI] [PubMed] [Google Scholar]
- 65. Lejeune J, Turpin R, Gautier M. Chromosomic diagnosis of mongolism. Arch Fr Pediatr 1959; 16: 962–963. [PubMed] [Google Scholar]
- 66. Modi WS, Fanning TG, Wayne RK, et al. Chromosomal localization of satellite DNA sequences among 22 species of felids and canids (Carnivora). Cytogenet Cell Genet 1988; 48: 208–213. [DOI] [PubMed] [Google Scholar]
- 67. Johnson G. The bengal cat. Greenwell Springs, LA: Gogees Cattery, 1991. [Google Scholar]
- 68. Winand NJ, Edwards M, Pradhan D, et al. Deletion of the dystrophin muscle promoter in feline muscular dystrophy. Neuromuscul Disord 1994; 4: 433–445. [DOI] [PubMed] [Google Scholar]
- 69. Muldoon LL, Neuwelt EA, Pagel MA, et al. Characterization of the molecular defect in a feline model for type II GM2-gangliosidosis (Sandhoff disease). Am J Pathol 1994; 144: 1109–1118. [PMC free article] [PubMed] [Google Scholar]
- 70. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med 2005; 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 71. Bighignoli B, Niini T, Grahn RA, et al. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genet 2007; 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. De Maria R, Divari S, Bo S, et al. Beta-galactosidase deficiency in a Korat cat: a new form of feline GM1-gangliosidosis. Acta Neuropathol 1998; 96: 307–314. [DOI] [PubMed] [Google Scholar]
- 73. Bradbury AM, Morrison NE, Hwang M, et al. Neurodegenerative lysosomal storage disease in European Burmese cats with hexosaminidase beta-subunit deficiency. Mol Genet Metab 2009; 97: 53–59. [DOI] [PubMed] [Google Scholar]
- 74. Martin DR, Cox NR, Morrison NE, et al. Mutation of the GM2 activator protein in a feline model of GM2 gangliosidosis. Acta Neuropathol 2005; 110: 443–450. [DOI] [PubMed] [Google Scholar]
- 75. Meurs KM, Sanchez X, David RM, et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet 2005; 14: 3587–3593. [DOI] [PubMed] [Google Scholar]
- 76. Meurs KM, Norgard MM, Ederer MM, et al. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics 2007; 90: 261–264. [DOI] [PubMed] [Google Scholar]
- 77. Gandolfi B, Gruffydd-Jones TJ, Malik R, et al. First WNK4-hypokalemia animal model identified by genome-wide association in Burmese cats. PloS One 2012; 7: e53173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Menotti-Raymond M, David VA, Schaffer AA, et al. Mutation in CEP290 discovered for cat model of human retinal degeneration. J Hered 2007; 98: 211–220. [DOI] [PubMed] [Google Scholar]
- 79. Menotti-Raymond M, Deckman K, David V, et al. Mutation discovered in a feline model of human congenital retinal blinding disease. Invest Ophthalmol Vis Sci 2010; 51: 2852–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lyons LA, Biller DS, Erdman CA, et al. Feline polycystic kidney disease mutation identified in PKD1. J Am Soc Nephrol 2004; 15: 2548–2555. [DOI] [PubMed] [Google Scholar]
- 81. Grahn RA, Grahn JC, Penedo MC, et al. Erythrocyte pyruvate kinase deficiency mutation identified in multiple breeds of domestic cats. BMC Vet Res 2012; 8: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fyfe JC, Menotti-Raymond M, David VA, et al. An approximately 140-kb deletion associated with feline spinal muscular atrophy implies an essential LIX1 function for motor neuron survival. Genome Res 2006; 16: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cannon MJ, MacKay AD, Barr FJ, et al. Prevalence of polycystic kidney disease in Persian cats in the United Kingdom. Vet Rec 2001; 149: 409–411. [DOI] [PubMed] [Google Scholar]
- 84. Barrs VR, Gunew M, Foster SF, et al. Prevalence of autosomal dominant polycystic kidney disease in Persian cats and related-breeds in Sydney and Brisbane. Aust Vet J 2001; 79: 257–259. [DOI] [PubMed] [Google Scholar]
- 85. Barthez PY, Rivier P, Begon D. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. J Feline Med Surg 2003; 5: 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Biller DS, Chew DJ, DiBartola SP. Polycystic kidney disease in a family of Persian cats. J Am Vet Med Assoc 1990; 196: 1288–1290. [PubMed] [Google Scholar]
- 87. Eaton KA, Biller DS, DiBartola SP, et al. Autosomal dominant polycystic kidney disease in Persian and Persian-cross cats. Vet Pathol 1997; 34: 117–126. [DOI] [PubMed] [Google Scholar]
- 88. Grahn RA, Biller DS, Young AE, et al. Genetic testing for feline polycystic kidney disease. Anim Genet 2004; 35: 503–504. [DOI] [PubMed] [Google Scholar]
- 89. Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science 2001; 293: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 90. Lipinski MJ, Froenicke L, Baysac KC, et al. The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics 2008; 91: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kurushima JD, Lipinski MJ, Gandolfi B, et al. Variation of cats under domestication: genetic assignment of domestic cats to breeds and worldwide random-bred populations. Anim Genet 2013; 44: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Menotti-Raymond M, David VA, Weir BS, et al. A population genetic database of cat breeds developed in coordination with a domestic cat STR multiplex. J Forensic Sci 2012; 57: 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yogalingam G, Hopwood JJ, Crawley A, et al. Mild feline mucopolysaccharidosis type VI. Identification of an N-acetylgalactosamine-4-sulfatase mutation causing instability and increased specific activity. J Biol Chem 1998; 273: 13421–13429. [DOI] [PubMed] [Google Scholar]
- 94. Yogalingam G, Litjens T, Bielicki J, et al. Feline mucopolysaccharidosis type VI. Characterization of recombinant N-acetylgalactosamine 4-sulfatase and identification of a mutation causing the disease. J Biol Chem 1996; 271: 27259–27265. [DOI] [PubMed] [Google Scholar]
- 95. Owens SL, Downey ME, Pressler BM, et al. Congenital adrenal hyperplasia associated with mutation in an 11beta-hydroxylase-like gene in a cat. J Vet Intern Med 2012; 26: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 96. Chang HS, Shibata T, Arai S, et al. Dihydropyrimidinase deficiency: the first feline case of dihydropyrimidinuria with clinical and molecular findings. JIMD Rep 2012; 6: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bender DE, Kloos MT, Pontius JU, et al. Molecular characterization of cat factor XII gene and identification of a mutation causing factor XII deficiency in a domestic shorthair cat colony. Vet Pathol 2014. Epub ahead of print 2 May 2014. DOI: 10.1177/0300985814532821. [DOI] [PubMed] [Google Scholar]
- 98. Uddin MM, Hossain MA, Rahman MM, et al. Identification of Bangladeshi domestic cats with GM1 gangliosidosis caused by the c.1448G>C mutation of the feline GLB1 gene: case study. J Vet Med Sci 2013; 75: 395–397. [DOI] [PubMed] [Google Scholar]
- 99. Martin DR, Krum BK, Varadarajan GS, et al. An inversion of 25 base pairs causes feline GM2 gangliosidosis variant. Exp Neurol 2004; 187: 30–37. [DOI] [PubMed] [Google Scholar]
- 100. Kanae Y, Endoh D, Yamato O, et al. Nonsense mutation of feline beta-hexosaminidase beta-subunit (HEXB) gene causing Sandhoff disease in a family of Japanese domestic cats. Res Vet Sci 2007; 82: 54–60. [DOI] [PubMed] [Google Scholar]
- 101. Goree M, Catalfamo JL, Aber S, et al. Characterization of the mutations causing hemophilia B in 2 domestic cats. J Vet Intern Med 2005; 19: 200–204. [DOI] [PubMed] [Google Scholar]
- 102. Goldstein R, Narala S, Sabet N, et al. Primary hyperoxaluria in cats caused by a mutation in the feline GRHPR gene. J Hered 2009; 100: S2–S7. [Google Scholar]
- 103. Ginzinger DG, Lewis ME, Ma Y, et al. A mutation in the lipoprotein lipase gene is the molecular basis of chylomicronemia in a colony of domestic cats. J Clin Invest 1996; 97: 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mazrier H, Van Hoeven M, Wang P, et al. Inheritance, biochemical abnormalities, and clinical features of feline mucolipidosis II: the first animal model of human I-cell disease. J Hered 2003; 94: 363–373. [DOI] [PubMed] [Google Scholar]
- 105. Berg T, Tollersrud OK, Walkley SU, et al. Purification of feline lysosomal alpha-mannosidase, determination of its cDNA sequence and identification of a mutation causing alpha-mannosidosis in Persian cats. Biochem J 1997; 328: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. He X, Li CM, Simonaro CM, et al. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol Genet Metab 1999; 67: 106–112. [DOI] [PubMed] [Google Scholar]
- 107. Crawley AC, Yogalingam G, Muller VJ, et al. Two mutations within a feline mucopolysaccharidosis type VI colony cause three different clinical phenotypes. J Clin Invest 1998; 101: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fyfe JC, Kurzhals RL, Lassaline ME, et al. Molecular basis of feline beta-glucuronidase deficiency: an animal model of mucopolysaccharidosis VII. Genomics 1999; 58: 121–128. [DOI] [PubMed] [Google Scholar]
- 109. Somers K, Royals M, Carstea E, et al. Mutation analysis of feline Niemann-Pick C1 disease. Mol Genet Metab 2003; 79: 99–103. [DOI] [PubMed] [Google Scholar]
- 110. Clavero S, Bishop DF, Giger U, et al. Feline congenital erythropoietic porphyria: two homozygous UROS missense mutations cause the enzyme deficiency and porphyrin accumulation. Mol Med 2010; 16: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Clavero S, Bishop DF, Haskins ME, et al. Feline acute intermittent porphyria: a phenocopy masquerading as an erythropoietic porphyria due to dominant and recessive hydroxymethylbilane synthase mutations. Hum Mol Genet 2010; 19: 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Geisen V, Weber K, Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med 2009; 23: 196–199. [DOI] [PubMed] [Google Scholar]
- 113. Grahn RA, Ellis MR, Grahn JC, et al. A novel CYP27B1 mutation causes a feline vitamin D-dependent rickets type 1A. J Feline Med Surg 2012; 14: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kittleson MD, Meurs KM, Munro MJ, et al. Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease. Circulation 1999; 99: 3172–3180. [DOI] [PubMed] [Google Scholar]
- 115. Sampedrano C, Chetboul V, Mary J, et al. Prospective echocardiographic and tissue doppler imaging screening of a population of Maine Coon cats tested for the A31P mutation in the myosin-binding protein C gene: a specific analysis of the heterozygous status. J Vet Intern Med 2009; 23: 91–99. [DOI] [PubMed] [Google Scholar]
- 116. Wess G, Schinner C, Weber K, et al. Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in Maine Coon and other breed cats. J Vet Intern Med 2010; 24: 527–532. [DOI] [PubMed] [Google Scholar]
- 117. Kittleson MD, Meurs K, Munro M. Re: Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in Maine Coon and other breed cats. J Vet Intern Med 2010; 24: 1242–1243. author reply 1244. [DOI] [PubMed] [Google Scholar]
- 118. Longeri M, Ferrari P, Knafelz P, et al. Myosin-binding protein C DNA variants in domestic cats (A31P, A74T, R820W) and their association with hypertrophic cardiomyopathy. J Vet Intern Med 2013; 27: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Menotti-Raymond M, David VA, Pflueger S, et al. Widespread retinal degenerative disease mutation (rdAc) discovered among a large number of popular cat breeds. Vet J 2009; 186: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kohn B, Fumi C. Clinical course of pyruvate kinase deficiency in Abyssinian and Somali cats. J Feline Med Surg 2008; 10: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Johnson WE, Eizirik E, Pecon-Slattery J, et al. The late Miocene radiation of modern Felidae: a genetic assessment. Science 2006; 311: 73–77. [DOI] [PubMed] [Google Scholar]
- 122. Gershony LC, Penedo MC, Davis BW, et al. Who’s behind that mask and cape? The Asian leopard cat’s Agouti (ASIP) allele likely affects coat colour phenotype in the Bengal cat breed. Anim Genet 2014; 45: 893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lipinski MJ, Amigues Y, Blasi M, et al. An international parentage and identification panel for the domestic cat (Felis catus). Anim Genet 2007; 38: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Brosco JP, Paul DB. The political history of PKU: reflections on 50 years of newborn screening. Pediatrics 2013; 132: 987–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Stadler ZK, Schrader KA, Vijai J, et al. Cancer genomics and inherited risk. J Clin Oncol 2014; 32: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Roach JC, Glusman G, Smit AF, et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 2010; 328: 636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Torjesen I. Genomes of 100,000 people will be sequenced to create an open access research resource. BMJ 2013; 347: f6690. [DOI] [PubMed] [Google Scholar]
- 128. Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Human Genet 2014; 59: 5–15. [DOI] [PubMed] [Google Scholar]
- 129. Hayden EC. Technology: the $1,000 genome. Nature 2014; 507: 294–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linked version of Table 1
Linked version of Table 2
Linked version of Table 5