Abstract
Overview:
Streptococcus canis is most prevalent in cats, but recently S equi subsp zooepidemicus has been recognised as an emerging feline pathogen.
S canis infection:
S canis is considered part of the commensal mucosal microflora of the oral cavity, upper respiratory tract, genital organs and perianal region in cats. The prevalence of infection is higher in cats housed in groups; and, for example, there may be a high rate of vaginal carriage in young queens in breeding catteries. A wide spectrum of clinical disease is seen, encompassing neonatal septicaemia, upper respiratory tract disease, abscesses, pneumonia, osteomyelitis, polyarthritis, urogenital infections, septicaemia, sinusitis and meningitis.
S equi subsp zooepidemicus infection:
S equi subsp zooepidemicus is found in a wide range of species including cats. It was traditionally assumed that this bacterium played no role in disease of cats, but it is now considered a cause of respiratory disease with bronchopneumonia and pneumonia, as well as meningoencephalitis, often with a fatal course. Close confinement of cats, such as in shelters, appears to be a major risk factor. As horses are common carriers of this bacterium, contact with horses is a potential source of infection. Additionally, the possibility of indirect transmission needs to be considered.
Diagnosis:
Streptococci can be detected by conventional culture techniques from swabs, bronchoalveolar lavage fluid or organ samples. Also real-time PCR can be used, and is more sensitive than culture.
Treatment:
In suspected cases, treatment with broad-spectrum antibiotics should be initiated as soon as possible and, if appropriate, adapted to the results of culture and sensitivity tests.
Introduction
Although different streptococci have been isolated occasionally from cats, including Streptococcus agalactiae, S pneumoniae and S suis, the most prevalent species is S canis. 1 S equi subspecies zooepidemicus has been recognised as an emerging pathogen in dogs, and also recently in cats.2,3
Streptococcus canis
S canis is a beta-haemolytic Lancefield group G gram-positive bacterium that is considered part of the commensal mucosal microflora of the oral cavity, upper respiratory tract, genital organs and perianal region in cats. S canis infection seems to be sporadic in single-cat households, especially in older cats. 1 Young queens (up to 2 years of age) may carry S canis in the vagina, and the prevalence of infection is generally higher in cats housed in groups. Up to 70–100% of young queens in breeding catteries may carry this bacterium in the vagina, resulting in infection of the kittens, but also in the transfer of passive immunity against S canis via colostrum. Various factors, including the level of maternally derived antibodies, immune response, age, infection pressure, stress and probably also the strain virulence, determine whether or not the bacteria cause disease.
Contamination of the umbilical vein may lead to a generalised infection resulting in neonatal septicaemia. 1 In 3- to 7-month-old kittens, a subclinical infection of the pharynx and tonsils may induce cervical lymphadenitis. In older cats, the infection is usually opportunistic, as a result of wounds, surgery, immunosuppression or viral infection (Figure 1). In up to 10% of cats suffering from chronic upper respiratory tract disease, S canis can be isolated from the nasal cavity (Figure 2). 4 Conditions associated with this pathogen include abscesses, pneumonia, discospondylitis, osteomyelitis, polyarthritis, urogenital infections, necrotising fasciitis (toxic shock syndrome), sinusitis and meningitis. Outbreaks of fatal disease in cats have been reported in shelters and breeding colonies, 4 as all of these conditions may result in septicaemia and embolic lesions, especially of the lung and heart. 1
Figure 1.
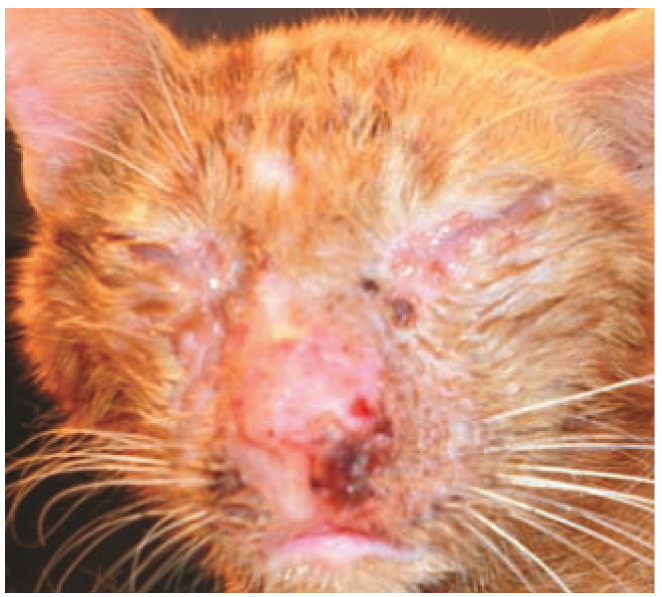
In adult cats, S canis infection is usually opportunistic as a result of wounds, surgery, immunosuppression or viral infection. In this shelter cat, viral infection was severely complicated by secondary bacterial infection due to poor hygienic conditions. Courtesy of Tadeusz Frymus, Faculty of Veterinary Medicine, Warsaw University of Life Sciences, Poland
Figure 2.
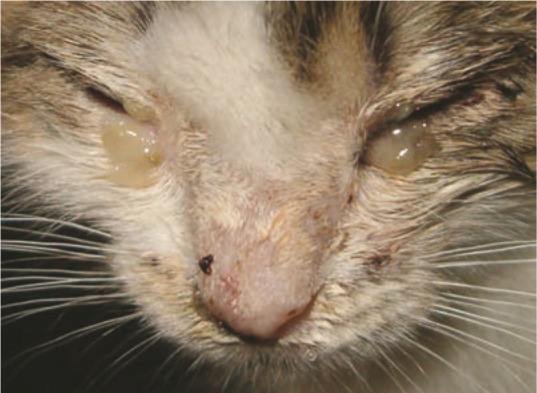
In some cats suffering from upper respiratory tract disease, S canis can be isolated from the nasal cavity. Courtesy of Tadeusz Frymus, Faculty of Veterinary Medicine, Warsaw University of Life Sciences, Poland
Microscopic examination of exudates or tissue reveals gram-positive cocci (usually in chains), and culture can confirm the diagnosis. S canis is generally sensitive to penicillins, and early antibiotic administration is the basis of therapy.
More information can be found in a review by Greene and Prescott. 1
Streptococcus equi subsp zooepidemicus
Agent and host susceptibility
S equi subsp equi (commonly referred to as S equi) and S equi subsp zooepidemicus (S zooepidemicus) are beta-haemolytic gram-positive Lancefield group C bacteria, and the most important equine streptococci worldwide. 5 S equi is an obligate agent causing strangles, the most frequently diagnosed infectious disease of horses, and one which is both devastating and highly contagious. S equi is host-restricted, infecting equids almost exclusively.
S zooepidemicus is regarded as a mucosal commensal, most notably in equids, with a potential to cause serious opportunistic disease secondary to viral infections, heat exposure, transportation or other stressful situations. 6 Believed to be part of the normal microflora of the upper respiratory airways and lower reproductive tract, this bacterium is frequently isolated from suppurative discharge in horses including cases of mixed bacterial/ viral infection of the upper airways.5,6 However, in contrast to S equi, S zooepidemicus strains are highly diverse and are not restricted to causing disease in horses. These strains have been found in a wide range of other species including pigs, cattle, sheep, goats, poultry, dogs, cats, guinea pigs, seals, dolphins, monkeys, llama and farmed red deer.2,7 –17 Occasionally, glomerulonephritis, rheumatic fever, meningitis or purulent arthritis caused by S zooepidemicus have been reported in humans.18 –20 Many of these zoonotic infections have resulted from contact with horses or from the consumption of unpasteurised milk of cows or goats.
There is increasing evidence that the veterinary importance of S zooepidemicus may be underestimated, and concern has been expressed that this bacterium may be ‘potentially more than just an opportunist’. 21 Several outbreaks in species other than horses have been described. In Asia, pandemics have occurred in pigs.14,22 Also in companion animals, the incidence of infections by this agent has apparently increased. Since 2003, several outbreaks of an acute S zooepidemicus-related severe haemorrhagic canine pneumonia have been described in many countries.23 –27 This disease is highly contagious and often fatal. The most prominent signs reported were a sudden-onset fever, dyspnoea, and haemorrhagic nasal discharge. Haemorrhagic pneumonia and pleural effusion were recognised post mortem. Most outbreaks occurred in shelters, where S zooepidemicus infection caused many deaths. Kennels and research facilities were also involved; 28 in addition, individually housed dogs were occasionally affected.20,27
Feline S zooepidemicus-related disease
It was thought that S zooepidemicus played no role in diseases of cats until an outbreak was described in 2010 in a shelter in Israel. 2 Early clinical signs included an effusive purulent nasal discharge and cough (Figure 3), progressing to sinusitis, dyspnoea, pneumonia and death. The vaccination status of the shelter cats was unknown. Between June 2006 and January 2008, 78 dead cats from the shelter, which housed approximately 700 animals, had been submitted for post-mortem examination. In 39 of these, the major necropsy findings were severe, acute and diffuse bronchopneumonia (Figure 4) or bronchoalveolar pneumonia, either suppurative or necrosuppurative. Interstitial multifocal pyogranulomatous pneumonia was present in a few cats, pleuritis in four cases, and pyothorax in one animal. Pyogranulomatous meningoencephalitis was recorded in four cats. Necrosuppurative peritonitis was present in one case. The most common histopathological lesions were a diffuse mixed infiltrate of neutrophils, histiocytes and lymphocytes, thickening of the interalveolar septa and multifocal bacterial colonies with coccoid forms. 2
Figure 3.

Purulent nasal discharge and cough may be early signs of S equi subsp zooepidemicus-related disease in cats. Courtesy of Tadeusz Frymus, Faculty of Veterinary Medicine, Warsaw University of Life Sciences, Poland
Figure 4.
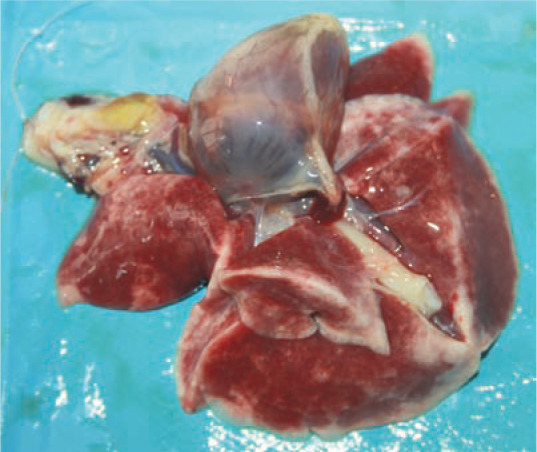
In cats succumbing to fatal S equi subsp zooepidemicus infection, the major necropsy finding is severe, acute and diffuse bronchopneumonia. Courtesy of Karolina Kozlowska, Warsaw, Poland
S zooepidemicus was the main pathogen isolated, both from the dead cats with signs of respiratory disease as well as from nasal and pharyngeal swabs or bronchoalveolar lavage fluid samples obtained from sick animals. 2 In the dead cats, S zooepidemicus was isolated from the lungs in all cases, and additionally from the sinuses in a few. The bacterium was also cultured from the pleura in two of four cases of pleuritis, from the brain in three of four cases of meningoencephalitis and from the peritoneum in one case of peritonitis. Usually S zooepidemicus was isolated alone, or was dominant in mixed cultures. However, the bacterium was not isolated from any of the 29 dead cats without clinical and pathological signs of respiratory disease, and from only two of 10 animals in which respiratory disease was suspected prior to death, but no gross pathological signs were found on necropsy. 2
S zooepidemicus could also be isolated from cats showing vague signs of respiratory disease, which possibly shed the organism long before being detected. 2 This might suggest subclinical carriage. In the few cases with lesions suggesting feline infectious peritonitis, the presence of feline coronavirus (FCoV) was ruled out by immunohistochemistry. Tests for feline herpesvirus (FHV) and feline calicivirus (FCV) were not performed but, based on clinical signs, the authors suspected that the cat population in this shelter was infected with both viruses. They assessed the hygiene and ventilation in this cattery as being adequate and the facilities as not overcrowded. This could mean that S zooepidemicus may become persistent in a cattery in spite of sufficient hygiene practices and treatment. The authors speculated that the transfer to this shelter of a group of cats from another cattery (closed due to poor conditions) prior to the disease outbreak might have induced stress that facilitated this epidemic. However, the source of infection remained unknown. The cats had no contact with horses. 2
In 2010, a fatal S zooepidemicus infection in two mature domestic cats housed in separate shelters was also described in Canada. 29 Both animals had been resident for several months in the shelter prior to a sudden onset of a peracute disease with non-specific clinical signs, and blindness in one cat, followed by death within 24 h. Post-mortem examination revealed rhinitis and meningitis, and S zooepidemicus was cultured from the nasal cavity and brain. Both cats had tested negative for feline leukaemia virus (FeLV) antigen and were seronegative for feline immunodeficiency virus (FIV) antibodies. PCR of lung, nasal mucosa and brain, performed post mortem, revealed that both cats were also negative for FCV and FCoV, and one was positive for FHV. Interestingly, other cats in these shelters remained normal. Neither of the cats that succumbed, nor their shelter attendants, had had contact with horses.
The pathogenic role of S zooepidemicus in cat colonies was revealed following a recent investigation of cat hoarding. 3 In this study, about 2000 cats were removed from four sanctuaries following reports consistent with animal hoarding. During intake examination, 27% of the animals (366/1368) showed respiratory disease. A subset of 81 cats with respiratory signs was tested for infectious agents by PCR, and 55% were positive for S zooepidemicus.
A case of acute S zooepidemicus meningoencephalitis was also described in an exclusively indoor cat in the USA in 2011. 30 It was likely secondary to otitis media/interna, as identified by computed tomography. The patient presented with neurological signs of a central vestibular lesion and left Horner’s syndrome. Cerebrospinal fluid analysis revealed marked neutrophilic pleocytosis; S zooepidemicus was isolated in pure culture, while PCR results for Toxoplasma gondii, FCoV and FeLV were negative, as was antigen enzyme immunoassay for Cryptococcus species. A bulla osteotomy and debridement was performed and, in accordance with resistance profile results, the cat was treated with trimethoprim–sulfamethoxazole for 8 weeks. The patient recovered fully.

In addition to the infections of domestic cats reviewed above, a fatal suppurative meningoventriculitis with intralesional S zooepidemicus has been described in an elderly, captive snow leopard in Japan. 31 This animal had had no contact with horses, but defrosted horse meat was fed routinely and was presumed to be the source of infection.
Epidemiology in small animals
It is generally considered that, in contrast to S canis, S zooepidemicus is not part of the normal microflora of dogs and cats.32 –35 Nevertheless, both canine and feline subclinical infections have been observed.2,20,23,36 S zooepidemicus-related diseases secondary to viral infections have been described in dogs, especially in cases of distemper and canine influenza virus (CIV) infection. 37 The bacterium may also act as a primary cause of canine pneumonia, sometimes with a peracute course, although experimental infections have not been performed. 38
Contact with horses, which are common carriers of this bacterium, is a potential source of infection. 36 Dogs experimentally infected with CIV and then kept together with healthy horses acquired S zooepidemicus pulmonary infection. 39 The possibility of indirect transmission should also be taken into consideration, as equine streptococci may survive outdoors for up to several days, and indoors for probably longer. 40 It has been speculated that contact with staff members could explain outbreaks in canine research facilities and urban kennels, where direct contact with horses is excluded. 10 Certainly S zooepidemicus is able to spread between dogs through direct contact, and outbreaks in shelters usually affect large numbers of animals within a short time.
Similar probably applies in cats. It has been postulated that close confinement of animals, such as in shelters, research laboratories and other facilities, appears to be the major risk factor for the development of S zooepidemicus-associated disease in dogs and cats.23,29 Co-infection with other respiratory pathogens, as well as the age and health of the animal on entry to the facility, has been shown to be unrelated to later colonisation of the respiratory tract by S zooepidemicus in dogs.23,28 The role of infected dogs as a source for feline infections is not known; however, in one shelter, canine haemorrhagic pneumonia caused by this bacterium did not spread to cats located in an adjacent building of the same facility. 26
Pathogenesis in small animals
The pathogenesis of S zooepidemicus infection in small animals is poorly understood. The existence in dogs of both subclinical and clinical infections of different severity suggests that some isolates might be more pathogenic than others.
In many dogs, the rapid onset of disease and progression of clinical signs are similar to human toxic shock syndrome caused by Streptococcus pyogenes. 41 Toxic shock is characterised by a hyperreactive inflammatory response, resulting in increased vascular permeability, vasodilation, increased coagulation and migration of inflammatory cells to the site of infection. 42 Pyrogenic exotoxins produced by some streptococci, including S pyogenes, act as superantigens by binding simultaneously to major histocompatibility complex class II receptors on macrophages and T-cell receptors, bypassing conventional antigen presentation, and leading to the activation of a large proportion of T lymphocytes. 43 The resulting hyperproduction of proinflammatory cytokines has been linked to increased virulence and has also been suggested to contribute to the pathogenesis of some streptococcal diseases. Marked elevation of the mRNA of some proinflammatory cytokines was also observed in dogs with S zooepidemicus-induced pneumonia, and three superantigen genes were prevalent among canine isolates of the bacterium. 41
So far, no clinical signs similar to the toxic shock syndrome have been described in cats. Various typing methods have been used to determine the virulence factors and genetic relationships among different S zooepidemicus isolates; M-like protein, IgG-binding proteins and fibronectin-binding protein appear to be the main virulence factors for this bacterium.44 –46 To date, the factors underlying the differences in pathogenicity of some isolates/genotypes in cats and dogs remain unknown.
Diagnosis
A tentative diagnosis of a streptococcal infection can be made based on the history, clinical signs, lesions and the presence of gram-positive coccus chains in the lesions. S zooepidemicus can be isolated from nasal and pharyngeal swabs, as well as from bronchoalveolar lavage fluid samples, from cats with respiratory disease, and from lung samples or other lesions in fatal cases. 2 Selective media for gram-positive organisms, such as Columbia agar with 5% sheep or horse blood containing colistin and nalidixic acid, should be used. If Lancefield group C streptococci grow, the presence of S zooepidemicus can be confirmed by biochemical methods (eg, API 20 Strep; bioMérieux). In contrast to S equi, S zooepidemicus has the ability to ferment ribose, sorbitol and lactose, properties that are commonly used to discriminate the subspecies. 47 Real-time PCR was found to be more sensitive than conventional culture for diagnosis and differentiation of S equi and S zooepidemicus. 48
Treatment
There is only one report of effective treatment in cats, involving a case of acute S zooepidemicus meningoencephalitis. 30 Trimethoprim–sulfamethoxazole administered over several weeks was the main antibiotic [EBM grade IV]. In suspected cases, treatment with broad-spectrum antibiotics should be initiated as soon as possible, and then adapted, if required, in the light of the results of culture and sensitivity tests. S zooepidemicus isolates from dogs were found to be susceptible to penicillin, ampicillin, amoxicillin and enrofloxacin [EBM grade IV]. 26 Some isolates were found to be resistant to tetracycline and doxycycline [EBM grade IV].25,28
Prevention
There is little data about the management of S zooepidemicus infections in feline shelters. However, sick cats should be isolated and staff should wear protective clothing when caring for them. Hands, premises and all contaminated equipment should be thoroughly cleaned and disinfected. Quaternary ammonium disinfectants, phenol-based solutions or oxidising agents are generally recommended.
Though significant attempts have been made, there are no S zooepidemicus vaccines available for any species.

Key Points
Streptococcus equi subsp zooepidemicus is an emerging pathogen in cats.
Infection is highly contagious and often fatal.
In cats, the pathogen mainly affects the respiratory tract, and clinical signs include purulent nasal discharge, coughing, sinusitis, dyspnoea, pneumonia and death.
Meningoencephalitis has also been described.
Horses are common carriers of this bacterium, and contact with these animals is a potential source of infection.
Close confinement of cats, such as in shelters, research laboratories and other facilities, appears to be the major risk factor for infection.
In the case of respiratory disease, S zooepidemicus can be isolated from nasal and pharyngeal swabs as well as from bronchoalveolar lavage fluid; in fatal cases, these bacteria can be isolated from lung samples or other lesions.
In suspected cases, treatment with broad-spectrum antibiotics should be initiated as soon as possible and then adapted according to the results of culture and sensitivity tests, where required.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCD is supported by Merial, but is a scientifically independent body and its members receive no stipends from Merial.
The authors do not have any potential conflicts of interest to declare.
References
- 1. Greene CE, Prescott JF. Streptococcal infections. In: Greene CE. (ed). Infectious diseases of the dog and cat. 4th ed. Elsevier, 2012, pp 325–333. [Google Scholar]
- 2. Blum S, Elad D, Zukin N, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infections in cats. Vet Microbiol 2010; 144: 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polak KC, Levy JK, Crawford PC, et al. Infectious diseases in large-scale cat hoarding investigations. Vet J 2014; 20: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pesavento PA, Murphy BG. Common and emerging infectious diseases in the animal shelter. Vet Pathol 2014; 51: 478–491. [DOI] [PubMed] [Google Scholar]
- 5. Timoney JF, Gillespie JH, Scott FW, et al. The genus streptococcus. In: Timoney JF. (ed). Hagan and Bruner’s microbiology and infectious diseases of domestic animals. 8th ed. Ithaca: Comstock, 1998, pp 181–196. [Google Scholar]
- 6. Hoffman A, Viel L, Prescott JF, et al. Association of microbiologic flora with clinical endoscopic and pulmonary cytologic findings in foals with distal respiratory tract infection. Am J Vet Res 1993; 54: 1615–1622. [PubMed] [Google Scholar]
- 7. Lamm CG, Ferguson AC, Lehenbauer TW, et al. Streptococcal infection in dogs: a retrospective study of 393 cases. Vet Pathol 2010; 47: 387–395. [DOI] [PubMed] [Google Scholar]
- 8. Las Heras A, Vela AI, Fernandez E, et al. Unusual outbreak of clinical mastitis in dairy sheep caused by Streptococcus equi subsp. zooepidemicus. J Clin Microbiol 2002; 40: 1106–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pisoni G, Zadoks RN, Vimercati C, et al. Epidemiological investigation of Streptococcus equi subspecies zooepidemicus involved in clinical mastitis in dairy goats. J Dairy Sci 2009; 92: 943–951. [DOI] [PubMed] [Google Scholar]
- 10. Priestnall S, Erles K. Streptococcus zooepidemicus: an emerging canine pathogen. Vet J 2011; 188: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharp MW, Prince MJ, Gibbens J. S. zooepidemicus infection and bovine mastitis. Vet Rec 1995; 137: 128. [DOI] [PubMed] [Google Scholar]
- 12. Venn-Watson S, Daniels R, Smith C. Thirty year retrospective evaluation of pneumonia in a bottlenose dolphin Tursiops truncatus population. Dis Aquat Org 2012; 99: 237–242. [DOI] [PubMed] [Google Scholar]
- 13. Akineden Ö, Alber J, Lämmler C, et al. Relatedness of Streptococcus equi subsp. zooepidemicus strains isolated from harbour seals (Phoca vitulina) and grey seals (Halichoerus grypus) of various origins of the North Sea during 1988–2005. Vet Microbiol 2007; 121: 158–162. [DOI] [PubMed] [Google Scholar]
- 14. Soedarmanto I, Pasaribu FH, Wibawan IW, et al. Identification and molecular characterization of serological group C streptococci isolated from diseased pigs and monkeys in Indonesia. J Clin Microbiol 1996; 34: 2201–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Lisle GW, Anderson CD, Southern AL, et al. Meningoencephalitis in farmed red deer (Cervus elaphus) caused by Streptococcus zooepidemicus. Vet Rec 1988; 122: 186–187. [DOI] [PubMed] [Google Scholar]
- 16. Hewson J, Cebra CK. Peritonitis in a llama caused by Streptococcus equi subsp. zooepidemicus. Can Vet J 2001; 42: 465–467. [PMC free article] [PubMed] [Google Scholar]
- 17. Timoney JF. Streptococcus zooepidemicus. In: Gyles CL, Prescott JF, Songer JG, et al. (eds). Pathogenesis of bacterial infections in animals. 3rd ed. Ames: Blackwell Publishing, 2008, pp 31–32. [Google Scholar]
- 18. Pelkonen S, Lindahl SB, Suomala P, et al. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg Infect Dis 2013; 19: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuusi M, Lahti E, Virolainen A, et al. An outbreak of Streptococcus equi subspecies zooepidemicus associated with consumption of fresh goat cheese. BMC Infect Dis 2006; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abbott Y, Acke E, Khan S, et al. Zoonotic transmission of Streptococcus equi subsp. zooepidemicus from a dog to a handler. J Med Microbiol 2010; 59: 120–123. [DOI] [PubMed] [Google Scholar]
- 21. Björnsdóttir S, Holden MTG, Svansson V, et al. Streptococcus zooepidemicus: more than just an opportunist? J Equine Vet Sci 2012; 32 Suppl: S8. [Google Scholar]
- 22. Feng ZG, Hu JS. Outbreak of swine streptococcosis in Sichuan province and identification of pathogen. Anim Husbandry Vet Med Lett 1977; 2: 7–12. [Google Scholar]
- 23. Chalker VJ, Brooks HW, Brownlie J. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet Microbiol 2003; 95: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim MK, Jee H, Shin SW, et al. Outbreak and control of haemorrhagic pneumonia due to Streptococcus equi subspecies zooepidemicus in dogs. Vet Rec 2007; 161: 528–529. [DOI] [PubMed] [Google Scholar]
- 25. Pesavento PA, Hurley KF, Bannasch MJ, et al. A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs caused by Streptococcus equi subsp. zooepidemicus. Vet Pathol 2008; 45: 51–53. [DOI] [PubMed] [Google Scholar]
- 26. Byun JW, Yoon SS, Woo GH, et al. An outbreak of fatal hemorrhagic pneumonia caused by Streptococcus equi subsp. zooepidemicus in shelter dogs. J Vet Sci 2009; 10: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gibson D, Richardson G. Haemorrhagic streptococcal pneumonia in a dog. Vet Rec 2008; 162: 423–424. [DOI] [PubMed] [Google Scholar]
- 28. Garnett NL, Eydelloth RS, Swindle MM, et al. Hemorrhagic streptococcal pneumonia in newly procured research dogs. J Am Vet Med Assoc 1982; 181: 1371–1374. [PubMed] [Google Scholar]
- 29. Britton AP, Davies JL. Rhinitis and meningitis in two shelter cats caused by Streptococcus equi subspecies zooepidemicus. J Comp Pathol 2010; 143: 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin-Vaquero P, da Costa RC, Daniels JB. Presumptive meningoencephalitis secondary to extension of otitis media/interna caused by Streptococcus equi subspecies zooepidemicus in a cat. J Feline Med Surg 2011; 13: 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamaguchi R, Nakamura S, Hori H, et al. Purulent meningoventriculitis caused by Streptococcus equi subspecies zooepidemicus in a snow leopard (Panthera uncia). J Comp Pathol 2012; 147: 397–400. [DOI] [PubMed] [Google Scholar]
- 32. Biberstein EL, Brown C, Smith T. Serogroups and biotypes among betahemolytic streptococci of canine origin. J Clin Microbiol 1980; 11: 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith JE. The aerobic bacteria of the nose and tonsils of healthy dogs. J Comp Pathol 1961; 71: 428–433. [DOI] [PubMed] [Google Scholar]
- 34. Bailie WE, Stowe EC, Schmitt AM. Aerobic bacterial flora of oral and nasal fluids of canines with reference to bacteria associated with bites. J Clin Microbiol 1978; 7: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Devriese LA, Cruz Colque JI, de Herdt P, et al. Identification and composition of the tonsillar and anal enterococcal and streptococcal flora of dogs and cats. J Appl Bacteriol 1992; 73: 421–425. [DOI] [PubMed] [Google Scholar]
- 36. Acke E, Abbott Y, Pinilla M, et al. Isolation of Streptococcus zooepidemicus from three dogs in close contact with horses. Vet Rec 2010; 167: 102–103. [DOI] [PubMed] [Google Scholar]
- 37. Yoon KJ, Cooper VL, Schwartz KJ, et al. Influenza virus infection in racing greyhounds. Emerg Infect Dis 2005, 11: 1974–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gower S, Payne R. Sudden deaths in greyhounds due to canine haemorrhagic pneumonia. Vet Rec 2012; 170: 630. [DOI] [PubMed] [Google Scholar]
- 39. Yamanaka T, Nemoto M, Bannai H, et al. No evidence of horizontal infection in horses kept in close contact with dogs experimentally infected with canine influenza A virus (H3N8). Acta Vet Scand 2012, 54: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weese JS, Jarlot C, Morley PS. Survival of Streptococcus equi on surfaces in an outdoor environment. Can Vet J 2009; 50: 968–970. [PMC free article] [PubMed] [Google Scholar]
- 41. Priestnall SL, Erles K, Brooks HW, et al. Characterization of pneumonia due to Streptococcus equi subsp. zooepidemicus in dogs. Clin Vaccine Immunol 2010; 17: 1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis 2009; 9: 281–290. [DOI] [PubMed] [Google Scholar]
- 43. Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev 2008; 225: 226–243. [DOI] [PubMed] [Google Scholar]
- 44. Timoney JF, Walker J, Zhou M, et al. Cloning and sequence analysis of a protective M-like protein gene from Streptococcus equi subsp. zooepidemicus. Infect Immun 1995; 63: 1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jonsson H, Lindmark H, Guss B. A protein G-related cell surface protein in Streptococcus zooepidemicus. Infect Immun 1995; 63: 2968–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hong K. Identification and characterization of a novel fibronectin-binding protein gene from Streptococcus equi subspecies zooepidemicus strain VTU211. FEMS Immunol Med Microbiol 2005; 45: 231–237. [DOI] [PubMed] [Google Scholar]
- 47. Bannister MF, Benson CE, Sweeney CR. Rapid species identification of group C streptococci isolated from horses. J Clin Microbiol 1985; 21: 524–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baverud V, Johansson SK, Aspan A. Real-time PCR for detection and differentiation of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus. Vet Microbiol 2007; 124: 219–229. [DOI] [PubMed] [Google Scholar]


