Abstract
OBJECTIVES:
Low hemoglobin concentration impairs clinical hemostasis across several diseases. It is unclear whether hemoglobin impacts laboratory functional coagulation assessments. We evaluated the relationship of hemoglobin concentration on viscoelastic hemostatic assays in intracerebral hemorrhage (ICH) and perioperative patients admitted to an ICU.
DESIGN:
Observational cohort study and separate in vitro laboratory study.
SETTING:
Multicenter tertiary referral ICUs.
PATIENTS:
Two acute ICH cohorts receiving distinct testing modalities: rotational thromboelastometry (ROTEM) and thromboelastography (TEG), and a third surgical ICU cohort receiving ROTEM were evaluated to assess the generalizability of findings across disease processes and testing platforms. A separate in vitro ROTEM laboratory study was performed utilizing ICH patient blood samples.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Relationships between baseline hemoglobin and ROTEM/TEG results were separately assessed across patient cohorts using Spearman correlations and linear regression models. A separate in vitro study assessed ROTEM tracing changes after serial hemoglobin modifications from ICH patient blood samples. In both our ROTEM (n = 34) and TEG (n = 239) ICH cohorts, hemoglobin concentrations directly correlated with coagulation kinetics (ROTEM r: 0.46; p = 0.01; TEG r: 0.49; p < 0.0001) and inversely correlated with clot strength (ROTEM r: −0.52, p = 0.002; TEG r: −0.40, p < 0.0001). Similar relationships were identified in perioperative ICU admitted patients (n = 121). We continued to identify these relationships in linear regression models. When manipulating ICH patient blood samples to achieve lower hemoglobin concentrations in vitro, we similarly identified that lower hemoglobin concentrations resulted in progressively faster coagulation kinetics and greater clot strength on ROTEM tracings.
CONCLUSIONS:
Lower hemoglobin concentrations have a consistent, measurable impact on ROTEM/TEG testing in ICU admitted patients, which appear to be artifactual. It is possible that patients with low hemoglobin may appear to have normal viscoelastic parameters when, in fact, they have a mild hypocoagulable state. Further work is required to determine if these tests should be corrected for a patient’s hemoglobin concentration.
Keywords: functional coagulation, hemoglobin, intracerebral hemorrhage, rotational thromboelastometry, thromboelastography
It is known that ongoing bleeding can lead to anemia simply through blood loss. However, anemia itself is known to be independently associated with coagulopathy resulting in suboptimal hemostasis and ongoing bleeding risk in several disease processes (1–5). These mechanisms are thought to be related to the impaired platelet adhesion, aggregation, and activation seen with low hemoglobin states, leading to coagulopathy and downstream bleeding complications (6–8). Specifically, in intracerebral hemorrhage (ICH) patients where life threatening bleeding is localized intracranially, yet is too small in volume to directly lower hemoglobin, lower baseline hemoglobin concentrations have been associated with increased ICH volume and prolonged bleeding (4, 5).
Despite clinical evidence for this potential “anemic coagulopathy” in several disease processes, it is unclear whether these physiological effects are accurately reflected in coagulation laboratory testing. Plasma-based conventional coagulation assays, though widely implemented, fail to assess hemoglobin concentration contribution to coagulation given that RBCs are removed prior to testing. Whole blood viscoelastic hemostatic assays: rotational thromboelastometry (ROTEM) and thromboelastography (TEG-5000) in theory provide an opportunity to assess hemoglobin concentration’s contribution to coagulation. However, prior studies have not identified expected relationships between low hemoglobin and impaired, “hypocoagulable” ROTEM/TEG tracings. Rather, in vitro (9, 10), animal based (11, 12), and noncritically ill, nonbleeding human patient studies (13, 14) identified that lower hemoglobin is associated with paradoxical “hypercoagulable” ROTEM/TEG tracings without clinical evidence of thrombosis reflecting a laboratory in vitro testing artifact.
Despite this reported artifact, current clinical viscoelastic hemostatic assay–guided resuscitation paradigms do not account for the impact that hemoglobin concentrations may independently have on the tracing itself. This is largely because it is unknown whether lower hemoglobin will similarly provide hypercoagulable viscoelastic hemostatic assay tracing artifacts in the types of hospitalized, acutely ill patients who are evaluated for treatment purposes with these testing platforms. An assessment of how hemoglobin concentrations impacts these tracings in these patients is necessary and timely given that viscoelastic hemostatic assays are increasingly utilized in goal-directed transfusion therapy paradigms (15–20) despite conflicting evidence supporting its clinical benefit (15, 21). An identification of such a testing artifact in a vulnerable patient population would provide an opportunity to improve current viscoelastic hemostatic assay testing and subsequent treatment paradigms in both clinical and research settings. Subsequently, we sought to investigate whether lower hemoglobin concentrations have a measurable effect on viscoelastic hemostatic assay tracings in ICU admitted patients.
MATERIALS AND METHODS
Data were primarily evaluated from two separate observational cohorts of adult patients admitted to an ICU following a spontaneous primary (normal underlying brain parenchyma) ICH who had measures of baseline hemoglobin as well as ROTEM/TEG laboratory results. Baseline characteristics, medication history, neuroimaging, and other laboratory results were collected for these patients. Each ICH cohort enrolled patients from separate medical centers and utilized separate viscoelastic hemostatic assay platforms. These included: 1) a spontaneous ICH cohort evaluated with admission ROTEM between 2013 and 2017 and 2) a spontaneous ICH cohort evaluated with admission TEG between 2014 and 2021. A third cohort of surgical patients admitted to an ICU and evaluated with baseline ROTEM prior to surgery was analyzed to assess the generalizability of the ICH cohort findings across different patient populations. Although each cohort prospectively enrolled consecutively admitted patients, ROTEM/TEG acquisition and data collection were performed on a sample of convenience based on viscoelastic testing availability.
Given that trauma patients receive empiric transfusion resuscitation prior to laboratory testing, these patients were specifically excluded. Our specific cohorts were chosen to minimize the influence of transfusion products given prior to coagulation testing that could inherently alter both the exposure (hemoglobin level) and the outcome (ROTEM/TEG tracing). Similarly, patients were excluded if ROTEM/TEG testing occurred after any transfusion or hemostatic therapy, if there was a preceding history of anticoagulant (but not antiplatelet) use, or if there was laboratory evidence of coagulopathy using conventional coagulation assays (platelet count <100 × 103/uL, activated partial thromboplastin time [aPTT] >50 s, prothrombin time [PT] >17 s). For the 2 ICH cohorts, secondary ICH etiologies (vascular malformation, aneurysm, malignancy, and ischemic stroke with hemorrhagic transformation) and traumatic ICH were excluded given the heterogeneity of coagulopathy and outcomes in these types of ICH. All ICH patients were managed according to American Heart Association guidelines (22) with treatment protocols described previously (18, 19, 23).
Clinical Laboratory Testing
Hemoglobin was the primary exposure of interest and was obtained from a clinical laboratory complete blood count (CBC) blood draw as per local institutional certified hospital clinical laboratory practices. Hemoglobin was utilized as the exposure variable and assessment of a patient’s RBC level given that prior ICH studies have identified relationships of lower hemoglobin concentration, not hematocrit, with larger hemorrhage size, and ongoing bleeding (4, 5).
ROTEM (Instrumentation Laboratory, Bedford, MA) and TEG (TEG5000, Haemonetics, Boston, MA) are separate Food and Drug Administration-approved, point-of-care, viscoelastic hemostatic assay platforms. These tests utilize whole blood to assess multiple components of coagulation including coagulation kinetics (time to clot formation), clot strength, and clot stability/lysis. ROTEM specifically assesses: 1) coagulation kinetics via coagulation time (CT), clot formation time (CFT), and alpha angle; 2) clot strength via maximum clot firmness (MCF), and 3) clot stability/lysis via maximum lysis. TEG assesses: 1) coagulation kinetics via reaction time (R), kinetics time (K), and alpha angle; 2) clot strength via maximum amplitude (MA), and 3) clot stability/lysis via lysis index (LI30) (Fig. 1). Although ROTEM/TEG provides parallel assessments of these coagulation stages, the reagents and operating characteristics are different across platforms. Subsequently, result outputs are not interchangeable between these testing platforms; thus, all cohorts were analyzed separately. For the purposes of our study, ROTEM/TEG assessments of coagulation kinetics and clot strength were assessed as the primary outcome given prior study findings (13, 14). Other ROTEM and TEG parameters were assessed as secondary outcomes.
Figure 1.
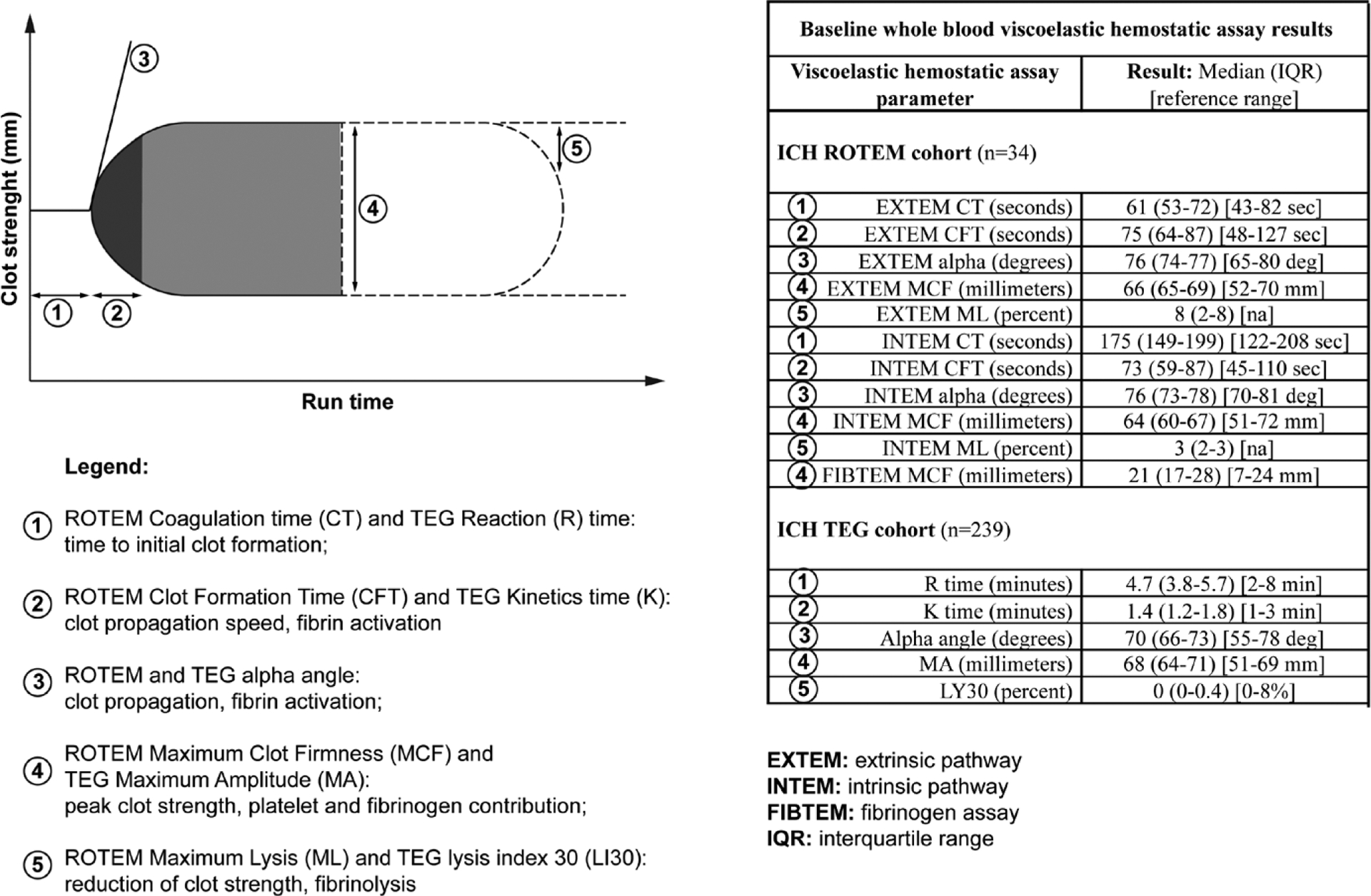
Baseline whole blood viscoelastic hemostatic assay results. (1) Rotational thromboelastometry (ROTEM) coagulation time (CT) and thromboelastography (TEG) reaction (R) time: time to initial clot formation; (2) ROTEM clot formation time (CFT) and TEG kinetics time (K): clot propagation speed, fibrin activation; (3) ROTEM and TEG alpha angle: clot propagation, fibrin activation; (4) ROTEM maximum clot firmness (MCF) and TEG maximum amplitude (MA): peak clot strength, platelet and fibrinogen contribution; (5) ROTEM maximum lysis (ML) and TEG lysis index 30 (LI30): reduction of clot strength, fibrinolysis. EXTEM = extrinsic pathway, FIBTEM = fibrinogen assay, ICH = intracerebral hemorrhage, INTEM = intrinsic pathway, IQR = interquartile range, K = kinetics time, R = reaction time.
Baseline clinical ROTEM/TEG testing was performed by trained practitioners from three milliliters of whole blood. Per protocols, these samples were collected into a citrated tube (3.2% NaCitrate) and run immediately after collection. Details regarding ROTEM and TEG clinical protocols have been described previously (18, 19, 24). Samples for ROTEM/TEG testing were drawn at the same time as blood taken for the patient’s CBC and traditional plasma coagulation tests (PT, aPTT: STAR automated analyzer [Diagnostia Stago, Parsippany, NJ]).
ICH Measurements
ICH volumes were assessed utilizing baseline diagnostic head computed tomography. The ROTEM ICH cohort had ICH volumes calculated using a semiautomated segmentation measurements using previously described techniques (5). The TEG ICH cohort had ICH volumes calculated using ABC/2 score (17, 25).
In Vitro Study
We performed a separate in vitro study modifying hemoglobin levels from blood samples taken from acute ICH patients. Prior evidence has suggested an in vitro change of viscoelastic hemostatic assay tracings with varying hemoglobin concentration modifications in nonbleeding patients or healthy controls (13, 14). Thus, we sought to assess for the generalizability of these reported in vitro artifacts in ICU admitted patients encountering hemorrhage, specifically ICH, who would be evaluated with viscoelastic hemostatic assays for treatment decisions. Four ICH patients and three healthy control samples were assessed to ensure that hemoglobin modifications would result in the same in vitro ROTEM tracing changes regardless of disease presence. Four separate samples, created from a single blood draw, were created from each patient/control. An initial sample was centrifuged but not otherwise modified. Subsequent samples underwent centrifugation followed by gently removing RBCs from the cellular layer using a pipette. Increasing volumes of RBCs were removed on these subsequent samples to generate a 15–20% sequential decrease in hemoglobin with each sample (the overall volume of the citrated tube used was 2.7 mL; ranges of RBC cellular layer removal were ~250–1,000 uL based on the baseline, nonmodified initial hemoglobin level). Four to five gentle inversions of the tube were performed to remix the samples post centrifugation. Each of these four samples underwent ROTEM and CBC testing. Furthermore, traditional coagulation testing (PT, aPTT, and fibrinogen) was performed to ensure that the manipulations performed did not affect these measures.
Statistical Analysis
All cohorts were analyzed separately. Normality of laboratory data was assessed using Shapiro-Wilk tests. Spearman bivariate correlation was used to assess correlation of hemoglobin concentrations with ROTEM/TEG results. Separate multivariable linear regression models were performed to assess the association of hemoglobin with ROTEM/TEG results among the different cohorts after adjusting for potential covariates that could impact the ROTEM/TEG tracing: age, sex, baseline ICH volume (for the ICH cohorts), and preceding antiplatelet medication use (18, 26, 27). For the in vitro study, Spearman correlations between hemoglobin and ROTEM measurements were similarly performed. We additionally performed a linear mixed model to explore how the ROTEM measures changed within and between subjects with each in vitro modification. Statistical significance was evaluated at p < 0.05. A statistician (AB) analyzed the data with the primary team using SPSS (IBM, Chicago, IL) and SAS (SAS Institute, Cary, NC).
Standard Protocol Approvals and Patient Consents
The Institution’s Committee for the Protection of Human Subjects approved this study (Institutional Review Boards AAAL4106, AAAD4775). Consent was provided by the patient or the family if the patient did not have capacity to consent.
RESULTS
There were 34 (cohort 1) and 239 (cohort 2) ICH patients with baseline hemoglobin concentration and concurrent ROTEM or TEG measurements, respectively. Baseline characteristics are shown in Table 1. Baseline hemoglobin concentrations, conventional coagulation assays, and ICH characteristics were comparable between these ICH cohorts. Baseline ROTEM/TEG results are shown in Figure 1.
TABLE 1.
Baseline Intracerebral Hemorrhage Patient Cohort Characteristics
| Rotational Thromboelastometry ICH Cohort (n = 34) | Thromboelastography ICH Cohort (n = 239) | |
|---|---|---|
| Age: mean (sd) | 63 (16) | 58 (14) |
| Male: n (%) | 19 (56) | 154 (64) |
| Race/ethnicity: n (%) | ||
| White | 9 (26) | 136 (57) |
| Black | 9 (26) | 82 (34) |
| Hispanic | 7 (21) | Not available |
| Other | 9 (26) | 21 (9) |
| Medical history: n (%) | ||
| Hypertension | 23 (68) | 198 (83) |
| Diabetes | 3 (9) | 55 (23) |
| Dyslipidemia | 6 (18) | 65 (27) |
| Prior antiplatelet use: n (%) | 6 (18) | 36 (15) |
| ICH characteristics | ||
| Admit systolic blood pressure: mean (sd) | 194 (34) | 195 (39) |
| ICH volume (mL): median (interquartile range) | 21.7 (7.7–45.8) | 16.2 (7.2–34.3) |
| Deep ICH location: n (%) | 20 (61) | 146 (61) |
| Lobar ICH location: n (%) | 13 (39) | 92 (38) |
| Baseline hemoglobin (g/dL): mean (sd) (reference range: male, 13.3–16.2; female, 12.0–15.8) | 13.1 (2.1) | 13.9 (1.9) |
| Traditional Coagulation Laboratory Testing: mean (sd) | ||
| Prothrombin time (s) (reference range: 11.6–14.6) | 13.5 (0.9) | 13.2 (1.1) |
| Partial thromboplastin time (s) (reference range: 24.8–35.2) | 28.5 (4.0) | 28.2 (4.4) |
| Platelet count (103/uL) (reference range: 165–415) | 232 (66) | 229 (77) |
ICH = intracerebral hemorrhage.
In our ROTEM ICH cohort, hemoglobin concentrations did not correlate with CT. However, hemoglobin had a direct, moderate correlation with other assessments of coagulation kinetics (EXTEM CFT r: 0.46; p = 0.01) and strong, inverse correlations with clot propagation (EXTEM alpha angle r: −0.66; p < 0.0001) and clot strength (EXTEM MCF r: −0.52; p = 0.002) (Fig. 2). In our TEG ICH cohort, hemoglobin similarly did not correlate with R time, but had direct, moderate correlations with other assessments of coagulation kinetics (K time r: 0.49; p < 0.0001) and inverse, moderate correlations with clot propagation (alpha angle r: −0.48; p < 0.0001) and clot strength (MA r: −0.40, p < 0.0001) (Fig. 3). Weak inverse correlations of hemoglobin with patterns of lysis were seen with TEG (LI30 r: −0.20; p = 0.002), but not with ROTEM. Baseline hemoglobin did not correlate with other ROTEM/TEG measurements, conventional coagulation assays, or platelet count. In our adjusted multivariable linear regression models, we confirmed separate relationships between hemoglobin and ROTEM/TEG parameters of coagulation kinetics, clot propagation, and clot strength (Table 2). Specifically, in ICH patients with lower hemoglobin concentrations, there was notably faster coagulation kinetics and greater clot strength.
Figure 2.
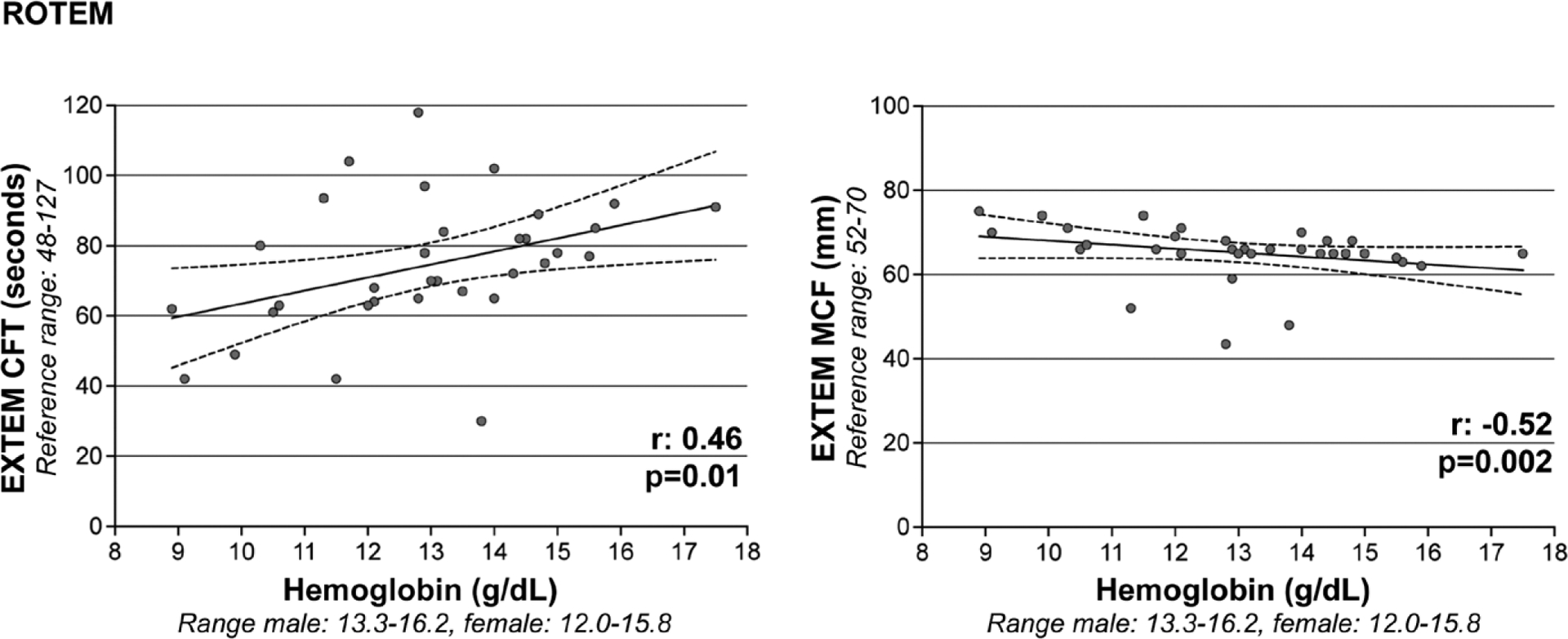
Correlations of baseline hemoglobin with rotational thromboelastometry (ROTEM). Hemoglobin (x axis) is directly correlated with coagulation kinetics (ROTEM extrinsic coagulation pathway [EXTEM] clot formation time [CFT] is depicted on y axis) and inversely correlated with coagulation strength (ROTEM EXTEM maximum clot firmness [MCF] is depicted on y axis).
Figure 3.
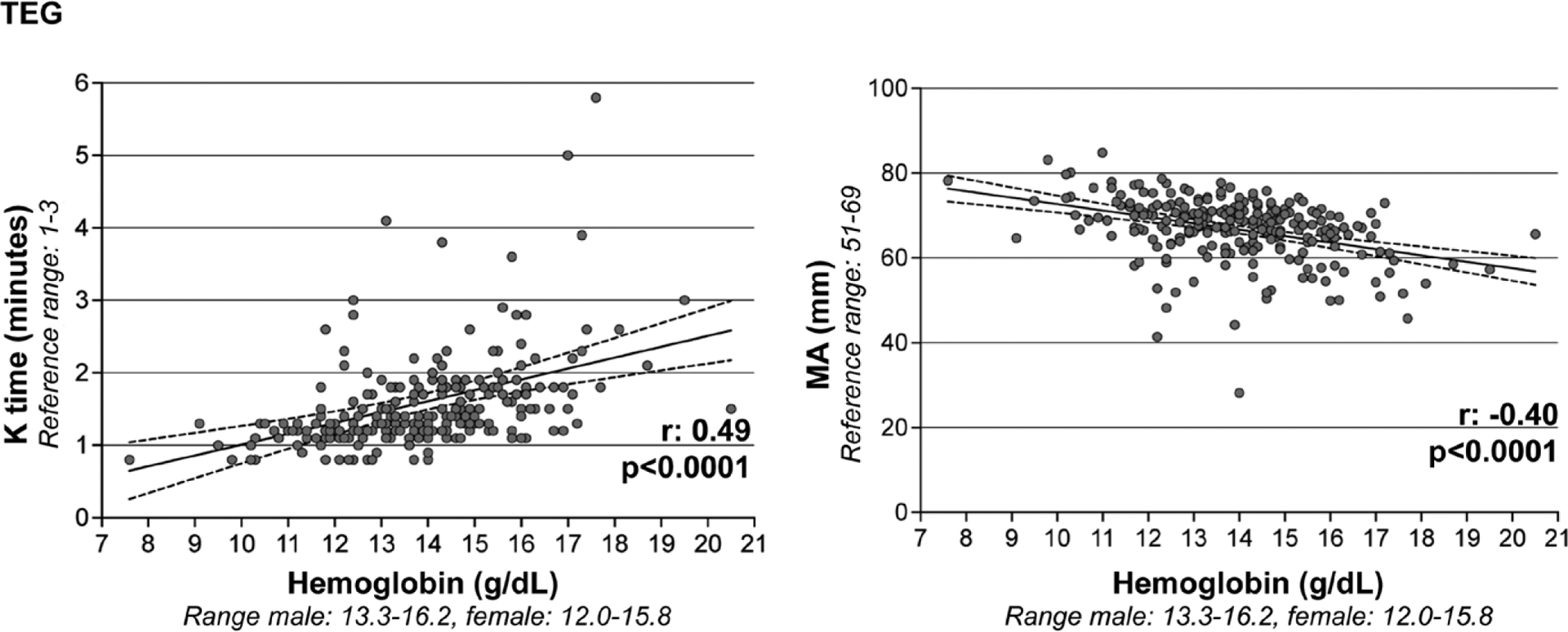
Correlations of baseline hemoglobin with thromboelastography (TEG). Hemoglobin (x axis) is directly correlated with coagulation kinetics (TEG kinetics time [K time], depicted on y axis) and inversely correlated with coagulation strength (TEG maximum amplitude [MA], depicted on y axis).
TABLE 2.
Univariable and Multivariable Linear Regression Assessing Association of Hemoglobin With Viscoelastic Hemostatic Assay Results in Intracerebral Hemorrhage Patients
| Rotational Thromboelastometry Outcome | Unadjusted Beta Coefficient (95% CI) p |
Adjusted Beta Coefficient (95% CI) pa |
Thromboelastography Outcome | Unadjusted Beta Coefficient (95% CI) p |
Adjusted Beta Coefficient (95% CI) pa |
|---|---|---|---|---|---|
| EXTEM CT (s) | 0.26 (−0.71 to 4.79) p = 0.14 |
0.17 (−1.95 to 4.28) p = 0.45 |
Reaction time (min) | 0.06 (−0.05 to 0.15) p = 0.34 |
0.03 (−0.08 to 0.12) p = 0.67 |
| EXTEM CFT (s) | 0.40 (0.61–6.85) p = 0.02 |
0.49 (1.03–7.71) p = 0.01 |
Kinetics time (min) | 0.31 (0.09–0.21) p < 0.0001 |
0.30 (0.09–0.21) p < 0.0001 |
| EXTEM alpha (degrees) | −0.56 (−1.69 to −0.49) p = 0.001 |
−0.59 (−1.79 to −0.49) p = 0.001 |
Alpha (degrees) | −0.45 (−2.04 to −1.21) p < 0.0001 |
−0.44 (−2.06 to −1.17) p < 0.0001 |
| EXTEM MCF (millimeters) | −0.29 (−2.09 to 0.21) p = 0.11 |
−0.42 (−2.62 to −0.07) p = 0.04 |
Maximum amplitude (millimeters) | −0.39 (−1.97 to −1.05) p < 0.0001 |
−0.39 (−2.01 to −1.05) p < 0.0001 |
| EXTEM ML (%) | NA | NA | Lysis index (%) | −0.13 (−0.17 to 0.001) p = 0.05 |
−0.10 (−0.16 to 0.02) p = 0.14 |
| INTEM CT (s) | 0.03 (−10.78 to 12.99) p = 0.85 |
−0.02 (−14.95 to 13.90) p = 0.94 |
|||
| INTEM CFT (s) | −0.13 (−9.34 to 4.48) p = 0.48 |
−0.12 (−10.85 to 6.23) p = 0.58 |
|||
| INTEM alpha (degrees) | 0.07 (−0.84 to 1.27) p = 0.68 |
0.07 (−1.11 to 1.52) p = 0.75 |
|||
| INTEM MCF (millimeters) | −0.09 (−1.47 to 0.85) p = 0.59 |
−0.05 (−1.59 to 1.29) p = 0.83 |
|||
| INTEM ML (%) | NA | NA | |||
| Fibrinogen assay MCF (millimeters) | −0.06 (−2.08 to 1.51) p = 0.75 |
0.02 (−2.09 to 2.27) p = 0.94 |
CFT = clot formation time, CT = coagulation time, EXTEM = extrinsic pathway assay, INTEM = intrinsic pathway assay, MCF = maximal clot firmness, ML = maximum lysis, NA = not available.
Adjusted for age, sex, baseline hematoma size, and antiplatelet medication use.
In our third cohort of surgical ICU patients, the average patient age was 64 (± 18) years old, 51% of the cohort was female, and the baseline hemoglobin concentration was notably lower than our ICH cohorts (10.9 ± 1.4 g/dL) (Supplemental Table 1, http://links.lww.com/CCM/H226). Despite these baseline hemoglobin differences, we similarly identified that hemoglobin had direct, moderate correlations with coagulation kinetics (EXTEM CFT r: 0.34; p < 0.0001) and inverse, moderate correlations with clot propagation (EXTEM alpha angle r: −0.41, p < 0.0001) and clot strength (EXTEM MCF r: −0.32; p < 0.0001). Similar correlations were identified in the ROTEM intrinsic pathway assays (data not shown). Unlike our ROTEM ICH cohort, we identified that hemoglobin had moderate, inverse correlations with fibrinogen contribution to clot strength (fibrinogen assay MCF r: −0.37; p < 0.0001). We continued to identify these relationships in linear regression models after adjusting for age, sex, and surgery type (Supplemental Table 2, http://links.lww.com/CCM/H226).
In our in vitro study, seven participant samples (four ICH and three healthy controls) each underwent four serial hemoglobin modifications, resulting in 28 total samples with corresponding hemoglobin and ROTEM results. In this study, we identified that with modifications of blood samples to sequentially lower hemoglobin concentrations, there were progressively faster coagulation kinetics and greater clotting strength (Fig. 4; Supplement Fig. 1, http://links.lww.com/CCM/H226). In repeated measure mixed model analyses, for each 1-g/dL decrease in hemoglobin, there was a 1.3-second decrease in coagulation kinetics (EXTEM CFT) (95% CI, −3.02 to 0.40; p = 0.12) and a 0.55 mm amplitude increase in clot strength (EXTEM MCF) (95% CI, −0.09 to 1.19, p = 0.08). However, these estimates were imprecise and heavily influenced by small sample size comparisons. Notably, although there was a measurable impact of hemoglobin concentration change on viscoelastic testing parameters, these changes primarily fell within normal references ranges. There were no correlations of hemoglobin with PT, aPTT, or platelet count. Although there were moderate correlations seen between hemoglobin and fibrinogen (r: −0.47; p = 0.04), we did not identify relationships of hemoglobin with fibrinogen in our mixed model analyses (effect estimate, 3.62; 95% CI, −25.3 to 32.5; p = 0.79).
Figure 4.
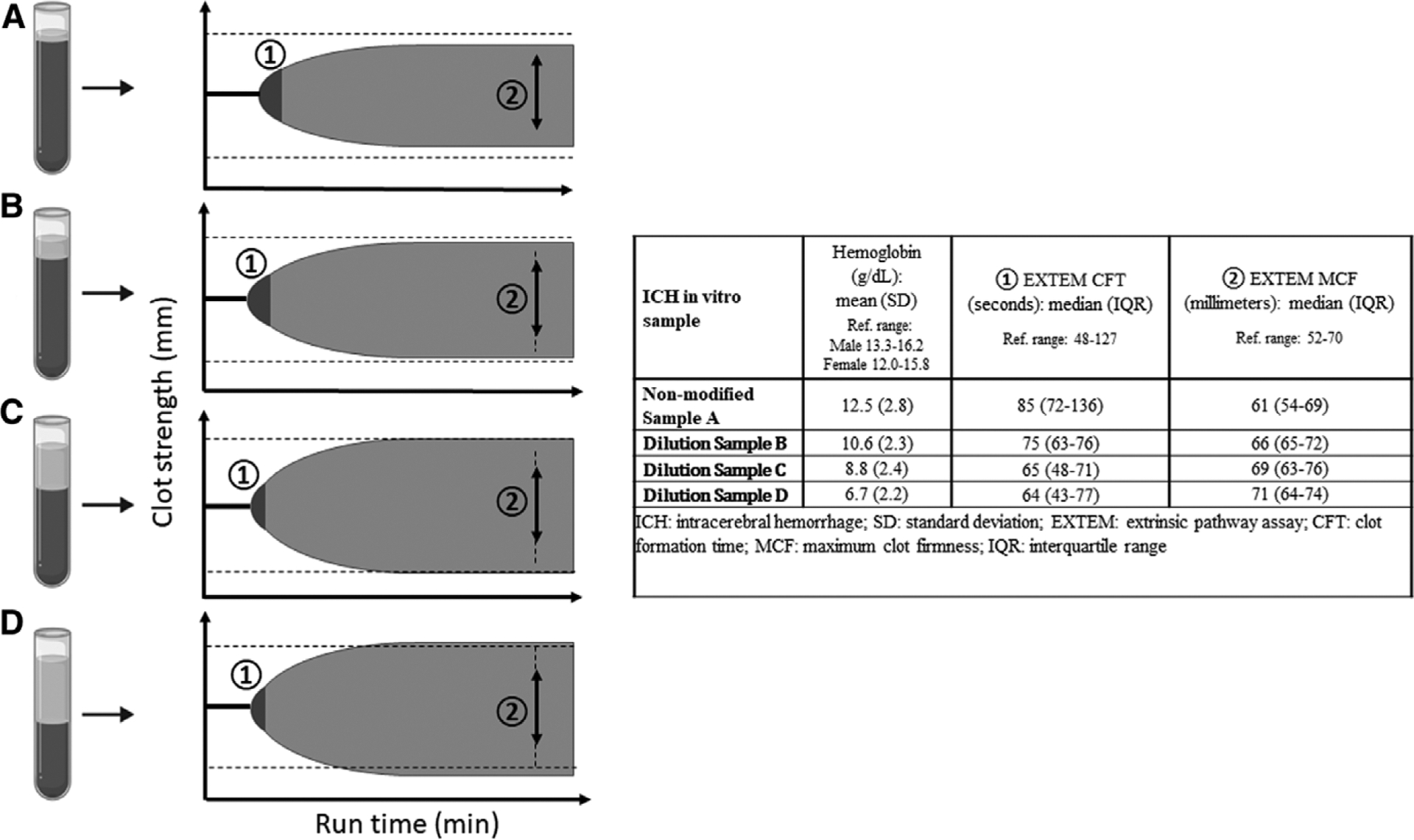
In vitro modification of hemoglobin to lower concentrations results in sequential changes in rotational thromboelastometry (ROTEM) tracings, specifically faster coagulation kinetics and greater clot strength, in intracerebral hemorrhage (ICH) patients and healthy controls. (1) Extrinsic coagulation pathway (EXTEM) clot formation time (CFT): assessment of coagulation kinetics; (2) EXTEM maximum clot firmness (MCF): assessment of clot strength. Initial nonmodified blood sample A, depicting RBC column (dark gray shading) and plasma column (light gray shading). Sequential hemoglobin modifications to lower concentrations by removing greater volumes of RBC from the cell layer (samples B–D) results in faster coagulation kinetics (1) and greater clot strength (2). Dotted lines on ROTEM tracing cartoon depiction indicate reference range of EXTEM MCF.
DISCUSSION
In our three separate patient cohorts who were all admitted to an intensive care unit, we observed that blood samples from patients with lower hemoglobin concentrations had faster ROTEM/TEG coagulation kinetics and stronger clot strength. This finding was consistent across ROTEM/TEG testing platforms, ICH cohorts, and disease state cohorts. Our study confirms prior observations that as hemoglobin decreases (mostly within the normal range), these assays suggest clot propagation becomes more rapid and clot strength increases (9–14). This laboratory relationship runs counter to clinical observations where lower hemoglobin associates with slower bleeding times and increased bleeding complications. Thus, our identified laboratory relationships appear to reflect an artifact of the testing modality itself, rather than a true in vivo relationship. Because our findings were specific to vulnerable patient populations evaluated with viscoelastic hemostatic assay testing for diagnostic and treatment guidance for hemorrhage control, this emphasizes the need to consider the impact of hemoglobin concentrations on the accurate interpretation and treatment decisions made based on these tracings.
Because our hemoglobin-ROTEM/TEG tracing relationships were observed in patients presenting with an acute ICH, it makes it less likely that low hemoglobin states are associated with true conditions of rapid and increased clotting in vivo. There has been evidence to support that lower hemoglobin concentrations are related to larger hemorrhage volumes and bleeding complications, not thrombosis, across multiple disease processes including ICH (1, 2, 4, 5). Yet is notable that ICH itself can impact and upregulate coagulation, resulting in hypercoagulable viscoelastic hemostatic assay tracings in response to the severity of ICH and subsequent inflammatory processes themselves (17, 28). However, it is additionally unlikely that our hemoglobin-ROTEM/TEG tracing relationship was confounded by ICH severity as we adjusted for ICH volume in our models. These separate clinical observations may support that these laboratory relationships represent artifacts of ROTEM/TEG platforms. However, it is worth mentioning the complex relationships that hemoglobin concentrations have at both extremes (i.e., both low and high hemoglobins) with coagulation, hemostasis, and poor clinical outcomes across cardiovascular disease (29, 30). To this extent, it is important to highlight that our data did not contain information on bleeding or thrombotic complications to establish clinical evidence of a discordance of ROTEM/TEG parameters with downstream clinical complications (i.e., ongoing bleeding complications despite hypercoagulable ROTEM/TEG results). Thus, future work will be needed to comprehensively identify relationships of hemoglobin concentrations, viscoelastic tracings, and clinical complications/outcomes to identify better diagnostic treatment paradigms.
However, our in vitro study appeared to provide additive evidence that the hemoglobin-ROTEM/TEG tracing relationships were driven by laboratory-based artifacts. We identified sequentially faster coagulation kinetics and greater clot strength as blood samples were modified to lower hemoglobin concentrations. These in vitro findings were seen across and within ICH patient and control subjects’ blood sample modifications. Importantly, this appears to confirm the relevance and generalizability of similar relationships identified from prior in vitro studies (9, 10, 13) to hospitalized, bleeding patients whose coagulation response to injury is different compared to noninjured controls. The mechanism for these in vitro artifacts has been posited to be driven by increased plasma content in the testing cuvette. Given the higher plasma to RBC content ratio seen in the cuvette from patient samples with lower hemoglobin states, it is feasible that these testing platforms will detect faster coagulation kinetics and clotting strength due to the higher concentration of plasma coagulation factors and fibrinogen generation and decreased interference of this process from RBCs.
Collectively, these findings emphasize the clinical importance of considering the impact that hemoglobin has on ROTEM/TEG tracings and a potential need to develop a “correction factor” for these tracings based on hemoglobin concentrations. Because this testing has become an increasingly utilized clinical diagnostic tool in identifying coagulopathy and treatment targets for bleeding patients, it is easy to envision a scenario where a patient-specific variable (i.e., low hemoglobin) that results in artifactually faster and stronger clotting test results could lead to undertreatment of relevant coagulopathy. Notably, most of our patients had “normal,” nonanemic hemoglobin levels as well as viscoelastic parameters falling within “normal” references ranges. Yet, our hemoglobin-ROTEM/TEG relationship was consistent across a broad range of these concentrations. We did not note a threshold effect of our data, thus speaking to the large influence and measurable effect that hemoglobin concentrations had on our ROTEM/TEG data.
Subsequently, this hemoglobin-ROTEM/TEG relationship becomes even more relevant to consider in scenarios outside of the ICH and perioperative patients that we studied. In settings of trauma where blood loss can be significant, dynamic changes in viscoelastic hemostatic assay tracings may occur in response to a patient’s changing hemoglobin concentration, thus masking the patient’s true clinical/in vivo coagulation status. Although there are no studies that have yet investigated whether this hemoglobin-ROTEM/TEG relationship exists in samples from trauma patients, this needs to be explored as they may be the ones most vulnerable to the effects of the testing artifact that we identified. The clinical efficacy of viscoelastic hemostatic assay–guided resuscitation approaches in trauma is currently uncertain. Although one trial identified a clinical benefit of viscoelastic hemostatic assay–guided resuscitation in trauma, the follow-up Implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy (ITACTIC) study was unable to replicate these results (15, 21). Though baseline hemoglobin concentrations were not available to compare between these two trials, the prerandomization RBC transfusion strategies differed between the two studies. It could be posited that a patient cohort with a “less aggressive” prerandomization RBC transfusion strategy would have patients that had lower baseline hemoglobin subject to potential viscoelastic hemostatic assay in vitro results that do not reflect the in vivo coagulation status, leading to potential undertreatment in the viscoelastic hemostatic assay-guided transfusion arm.
Although there were inherent strengths to our study, there are several study limitations worth noting. First, our observational data did not have available baseline assessments of critical illness severity to assess how this could impact the observations noted between hemoglobin and ROTEM/TEG tracings. Though we accounted for ICH volume, a surrogate marker of ICH severity, it is unclear whether other ICH specific severity factors (i.e., ICH location) or concurrent systemic critical illness could modulate hemoglobin concentrations and ROTEM/TEG tracing relationships over time. Second, our observational study did not have data on fibrinogen or more granular assessments of functional coagulation to provide additional mechanistic insights into the relationship between ROTEM/TEG parameters and hemoglobin levels observed. Our in vitro study provided evidence that the observed relationships appear to be driven by changes in hemoglobin to plasma concentration ratios. However, future work is required to assess whether this is driven by augmented fibrinogen, platelet, coagulation factor, or some other activity. In parallel, we did not have complete CBC data to differentiate any potential impacts that hemoglobin, hematocrit, or RBC number could separately have on these viscoelastic testing results. Thus, it remains to be seen whether our hemoglobin-viscoelastic hemostatic assay relationships were driven by hemoglobin itself, or the number and/or size of the RBCs. Additionally, all samples analyzed from this study were collected using citrate and then recalcified prior to testing. Given described relationships of this processing technique with hypercoagulable tracing changes (31), it is unclear whether our relationships would similarly be identified in whole blood point-of-care processing techniques (those that do not utilize citrate and recalcification). Finally and perhaps most importantly, we did not have the ability to test the sequential changes in ROTEM/TEG tracings across a comprehensive span of hemoglobin concentrations to create a reliable “correction factor” for the relationship observed. These limitations highlight a growing need for clinicians and researchers to understand preanalytic variables that impact clinical laboratory testing results. A better case-by-case and systematic understanding of these variables will allow for improved clinical implementation and treatment decisions based on a patient’s true in vivo coagulation status to improve outcomes for these types of bleeding patients.
CONCLUSIONS
Hemoglobin concentration appears to have a measurable effect on viscoelastic hemostatic assay parameters in ICU admitted patients who receive these tests for diagnostic and treatment guiding purposes. These effects need to be accounted for in the interpretation and subsequent clinical treatment decisions made upon these results.
Supplementary Material
KEY POINTS.
Question:
Does hemoglobin concentration impact viscoelastic hemostatic assay parameters?
Findings:
ICU admitted patients with lower hemoglobin concentrations had faster and stronger viscoelastic clotting characteristics. Rather than a true in vivo relationship, an in vitro study demonstrated that blood samples sequentially modified to lower hemoglobin concentrations resulted in artificially faster and stronger viscoelastic clotting characteristics.
Meaning:
As hemoglobin concentration drops, viscoelastic assay parameters appear to show faster and stronger clotting. This runs counter to what is seen clinically and reflects an artifact of the test. Further work should be undertaken to determine the clinical implications of this phenomenon.
Acknowledgments
Dr. Roh is supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute K23HL151901, NIH NHLBI R01HL148151, the National Blood Foundation Science Research Grant, and The Department of Defense W81XWH-20-PRMRP-IIRA-COV. Dr. Roh’s institution received funding from the NIH and the National Blood Foundation; he received funding from Portola Pharmaceuticals; he received support for article research from the NIH. Dr. Hansen received funding from Omix Technologies. Drs. Velazquez and Vrosgou disclosed work for hire. Dr. Claassen received funding from Marinus and disclosed that he is a minority shareholder in iCE Neurosystems. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
For information regarding this article, dr2753@cumc.columbia.edu
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
All authors have read and approved the submitted article. The article has not been submitted elsewhere nor published elsewhere in whole or in part.
REFERENCES
- 1.Valeri CR, Cassidy G, Pivacek LE, et al. : Anemia-induced increase in the bleeding time: Implications for treatment of non-surgical blood loss. Transfusion (Paris) 2001; 41:977–983 [DOI] [PubMed] [Google Scholar]
- 2.Dauerman HL, Lessard D, Yarzebski J, et al. : Bleeding complications in patients with anemia and acute myocardial infarction. Am J Cardiol 2005; 96:1379–1383 [DOI] [PubMed] [Google Scholar]
- 3.Livio M, Gotti E, Marchesi D, et al. : Uraemic bleeding: role of anaemia and beneficial effect of red cell transfusions. Lancet Lond Engl 1982; 2:1013–1015 [DOI] [PubMed] [Google Scholar]
- 4.Kumar MA, Rost NS, Snider RW, et al. : Anemia and hematoma volume in acute intracerebral hemorrhage. Crit Care Med 2009; 37:1442–1447 [DOI] [PubMed] [Google Scholar]
- 5.Roh DJ, Albers DJ, Magid-Bernstein J, et al. : Low hemoglobin and hematoma expansion after intracerebral hemorrhage. Neurology 2019; 93:e372–e380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkhamis TM, Beissinger RL, Chediak JR: Red blood cell effect on platelet adhesion and aggregation in low-stress shear flow. Myth or fact? ASAIO Trans 1988; 34:868–873 [PubMed] [Google Scholar]
- 7.Baskurt OK, Meiselman HJ: Blood rheology and hemodynamics. Semin Thromb Hemost 2003; 29:435–450 [DOI] [PubMed] [Google Scholar]
- 8.Reimers RC, Sutera SP, Joist JH: Potentiation by red blood cells of shear-induced platelet aggregation: Relative importance of chemical and physical mechanisms. Blood 1984; 64:1200–1206 [PubMed] [Google Scholar]
- 9.Iselin BM, Willimann PF, Seifert B, et al. : Isolated reduction of haematocrit does not compromise in vitro blood coagulation. Br J Anaesth 2001; 87:246–249 [DOI] [PubMed] [Google Scholar]
- 10.Bochsen L, Johansson PI, Kristensen AT, et al. : The influence of platelets, plasma and red blood cells on functional haemostatic assays. Blood Coagul Fibrinolysis Int J Haemost Thromb 2011; 22:167–175 [DOI] [PubMed] [Google Scholar]
- 11.Brooks AC, Guillaumin J, Cooper ES, et al. : Effects of hematocrit and red blood cell-independent viscosity on canine thromboelastographic tracings. Transfusion (Paris) 2014; 54:727–734 [DOI] [PubMed] [Google Scholar]
- 12.Shibata J, Hasegawa J, Siemens H-J, et al. : Hemostasis and coagulation at a hematocrit level of 0.85: Functional consequences of erythrocytosis. Blood 2003; 101:4416–4422 [DOI] [PubMed] [Google Scholar]
- 13.Spiezia L, Radu C, Marchioro P, et al. : Peculiar whole blood rotation thromboelastometry (Rotem) profile in 40 sideropenic anaemia patients. Thromb Haemost 2008; 100:1106–1110 [PubMed] [Google Scholar]
- 14.Özdemir ZC, Düzenli Kar Y, Gündüz E, et al. : Evaluation of hypercoagulability with rotational thromboelastometry in children with iron deficiency anemia. Hematology 2018; 23:664–668 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez E, Moore EE, Moore HB, et al. : Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: A pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg 2016; 263:1051–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baksaas-Aasen K, Van Dieren S, Balvers K, et al. : Data-driven development of ROTEM and TEG algorithms for the management of trauma hemorrhage: A prospective observational multicenter study. Ann Surg 2019; 270:1178–1185 [DOI] [PubMed] [Google Scholar]
- 17.Kawano-Castillo J, Ward E, Elliott A, et al. : Thrombelastography detects possible coagulation disturbance in patients with intracerebral hemorrhage with hematoma enlargement. Stroke J Cereb Circ 2014; 45:683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roh D, Torres GL, Cai C, et al. : Coagulation differences detectable in deep and lobar primary intracerebral hemorrhage using thromboelastography. Neurosurgery 2020; 87:918–924 [DOI] [PubMed] [Google Scholar]
- 19.Roh D, Chang T, Zammit C, et al. : Functional coagulation differences between lobar and deep intracerebral hemorrhage detected by rotational thromboelastometry: A pilot study. Neurocrit Care 2019; 31: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baksaas-Aasen K, Van Dieren S, Balvers K, et al. : Data-driven development of ROTEM and TEG algorithms for the management of trauma hemorrhage: A prospective observational multicenter study. Ann Surg 2019; 270:1178–1185 [DOI] [PubMed] [Google Scholar]
- 21.Baksaas-Aasen K, Gall LS, Stensballe J, et al. : Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): A randomized, controlled trial. Intensive Care Med 2021; 47:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemphill JC, Greenberg SM, Anderson CS, et al. : Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015: 46:2032–2060 [DOI] [PubMed] [Google Scholar]
- 23.Witsch J, Bruce E, Meyers E, et al. : Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology 2015; 84:989–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hincker A, Feit J, Sladen RN, et al. : Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care Lond Engl 2014; 18:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kothari RU, Brott T, Broderick JP, et al. : The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27:1304–1305 [DOI] [PubMed] [Google Scholar]
- 26.McDonald MM, Almaghrabi TS, Saenz DM, et al. : Dual anti-platelet therapy is associated with coagulopathy detectable by thrombelastography in acute stroke. J Intensive Care Med 2020; 35:68–73 [DOI] [PubMed] [Google Scholar]
- 27.Lang T, Bauters A, Braun SL, et al. : Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis Int J Haemost Thromb 2005; 16:301–310 [DOI] [PubMed] [Google Scholar]
- 28.Meier K, Saenz DM, Torres GL, et al. : Thrombelastography suggests hypercoagulability in patients with renal dysfunction and intracerebral hemorrhage. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2018; 27:1350–1356 [DOI] [PubMed] [Google Scholar]
- 29.Kishimoto S, Maruhashi T, Kajikawa M, et al. : Hematocrit, hemoglobin and red blood cells are associated with vascular function and vascular structure in men. Sci Rep 2020; 10:11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paquette M, Bernard S, Baass A: Hemoglobin concentration, hematocrit and red blood cell count predict major adverse cardiovascular events in patients with familial hypercholesterolemia. Atherosclerosis 2021; 335:41–46 [DOI] [PubMed] [Google Scholar]
- 31.Gilman EA, Koch CD, Santrach PJ, et al. : Fresh and citrated whole-blood specimens can produce different thromboelastography results in patients on extracorporeal membrane oxygenation. Am J Clin Pathol 2013; 140:165–169 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


