Abstract
Ceramides and cardiorespiratory (CR) fitness are both related to cardiovascular diseases. The associations of three blood plasma ceramides (C16:0, C22:0, and C24:0) with CR fitness in the population-based Study of Health in Pomerania (SHIP-START-1; n = 1,102; mean age 50.3 years, 51.5% women) are investigated. In addition, subgroup analysis according to age (</≥54 years) and sex (female/male) is performed. Ceramides are quantified by liquid chromatography/mass spectrometry (LC/MS). CR fitness is assessed by a cardiopulmonary exercise test. Sex and age independent associations are found for higher levels of C24:0 and C24:0/C16:0 ratio with higher maximal oxygen consumption (VO2peak) kg−1 and oxygen consumption at the anaerobic threshold (VO2@AT1) as well as for the relation of C24:0/C16:0 with maximum workload (Wattmax kg−1). In contrast, age/sex subgroup specific inverse associations with Wattmax kg−1 are found in women <54 years for C22:0, while a positive association in men ≥54 years. Higher levels of C24:0 are associated with higher Wattmax kg−1, except for women <54 years, where no significant association can be found. The findings suggest that the use of single ceramides as cardiovascular biomarkers may be inferior, compared to ceramide ratio C24:0/C16:0. Therefore C24:0/C16:0 ratio may be a more suitable and robust cardiovascular biomarker and should be preferred over single ceramides.
Keywords: cardiopulmonary exercise test, cardiorespiratory fitness, cardiovascular risk factors, ceramides
1. Introduction
According to the World Health Organization 17.9 million people die due to cardiovascular diseases (CVD) each year.[1] With that, CVD is the leading cause of death (31%) worldwide.[1] The incidence of cardiovascular events increases markedly, especially after the 5th decade of life. In the last two decades increasing evidence revealed an association between CVD and blood plasma ceramides, a group of sphingophospholipids.[2–11] These lipid molecules play an important role in cellular stress response, atherogenesis, inflammation, apoptosis, and fibrosis.[3,9,10,12] Furthermore, certain ceramides like C16:0 are related with major adverse cardiac events, stroke, and incident heart failure.[3,5,8,9] Therefore, ceramides might be promising new biomarkers to assess cardiovascular risk. In a previous study based on 5776 participants of the Study of Health in Pomerania (SHIP) and the Framingham Heart Study[8] we could demonstrate, that very long-chain ceramides like C24:0 and the ratio to the long-chain ceramide C16:0 (i.e., C24:0/C16:0) are associated with better cardiovascular health, lower cardiovascular event rates as well as lower all-cause mortality. Hence, one may assume that depending on the chain length, some ceramides may be detrimental, whereas others may be beneficial.[3,5,8,10]
Low cardiorespiratory (CR) fitness is an equally important risk factor of higher cardiovascular event rates.[13] CR fitness provides information about pulmonary and cardiac function, the circulatory system, and muscle metabolism.[14–16] In summary, the current literature shows that both low CR fitness[13–17] and specific ceramides,[3,5,8,9] are associated with the incidence of CVD, major adverse cardiac events, sudden cardiac deaths, and all-cause mortality.
So far, however, only limited information is available regarding the link between plasma ceramides and CR fitness. In 443 participants, aged 54 years and older, of the Baltimore Longitudinal Study of Aging (BLSA), ceramides were inversely related with CR fitness with men having stronger associations compared to women.[18] In addition, in a longitudinal study of 100 patients with coronary artery disease, an increase in CR fitness, due to aerobic and resistance training over 6 months, was associated with a decrease of certain ceramides such as C16:0.[19]
To gain further insight into the role of ceramides in the pathogenesis of CVD, we evaluated in a large population-based cohort the association of the most abundant ceramides in blood plasma C16:0, C22:0, C24:0, and their respective ratios, C24:0/C16:0, and C22:0/C16:0, with CR fitness parameters: peak oxygen consumption (VO2peak), oxygen consumption at the anaerobic threshold (VO2@AT1), and maximum exhibited workload (Wattmax). We expected that plasma levels of very long chain ceramides are positively associated with CR fitness, whilst long-chain ceramides are inversely associated. To make our results comparable with BLSA, we stratified our sample cohort by age (< and ≥ 54 years). We also included sex and age interaction analyses to check for potential modifying effects on the association between ceramides and CR fitness.
2. Experimental Section
2.1. Study Design and Participants
Our data and blood plasma samples were based on the first follow-up examination of the Study of Health in Pomerania (SHIP-START-1), a community-based study in the northeast of Germany. The study was approved by the Ethics Committee of the University Medicine of Greifswald and meets the requirements of the Declaration of Helsinki. All participants provided informed written consent. SHIP has the aim of developing an epidemiological health profile based on risk factors, health resources, diseases, and their prevalence. Further details of sampling, recruitment, and cohort profile are published elsewhere.[20–22] Via www.community-medicine.de data usage can be applied for.
SHIP-START-0, the baseline examination, was performed with 4308 participants between 1997 and 2001. A total of 3300 (76,6%) of these subjects attended the first follow-up examination, SHIP-START-1, between 2002 and 2006. Of these participants 1764 subjects were excluded due to non-participation in the voluntary cardiopulmonary exercise test (CPET), non-evaluable measurements, or missing ceramide values. Additionally, 267 participants were excluded due to missing covariables. After exclusion of participants with a history of cancer (n = 62), bronchial asthma and/or chronic lung diseases (n = 22), impaired cardiac and renal function (left ventricular ejection fraction [LVEF] < 40%, n = 29; estimated glomerular filtration rate [eGFR CKD-EPI] < 60 mL per min per 1.73 m2, n = 36), BMI < 18.5 kg m−2 (n = 10), or alcohol abuse (> 68.4 g d−1 [99th P%], n = 8) the final analysis sample size was n = 1102. For a detailed sample flow chart see Figure S1 (Supporting Information).
2.2. Ceramide Quantification and Cardiopulmonary Exercise Test
Non-fasting blood plasma samples were drawn from the cubital vein in supine position. A fully validated liquid chromatography/tandem mass spectrometry assay was performed to quantify C24:0, C22:0, and C16:0 ceramide levels in frozen non-fasting blood plasma samples. For further details see elsewhere.[8,23]
A symptom-limited CPET was performed to assess CR fitness on an electromagnetically-braked cycle ergometer (Ergoselect 100, Ergoline, Blitz, Germany) using a modified Jones protocol.[24,25] Blood pressure, twelve lead electrocardiogram (ECG), and pulse oximetry were monitored during rest, testing, and early recovery period. After a three-minute resting period the first stage started with unloaded cycling, 20 Watt (W). Every minute the workload was increased by 16 W. The test ended either due to exhaustion, chest-pain, or abnormalities during ECG or blood pressure measurement. Heart rate was averaged over 10 s intervals. The highest interval defined the peak heart rate. Maximum exhibited workload (Wattmax) specified the highest workload the participant could withstand. An Oxycon Pro with a Rudolf’s mask (JÄGER/VIASYS Healthcare System, Hoechberg, Germany) was used to determine oxygen consumption (VO2) and carbon dioxide production (VCO2) on a breath-by-breath basis. Each of these parameters were averaged over 10 s intervals. The highest interval of VO2 was specified as the peak oxygen consumption (VO2peak). The oxygen uptake at the first ventilatory threshold (VO2@AT1) was defined by the V-slope method as described elsewhere.[26]
2.3. Covariables
Socio-demographics, medical history, and medication were asked during a computer-assisted personal interview. Education years were divided into three groups based on the general distinctions in the German school system (< 10 years, = 10 years, > 10 years). Physical activity was assessed based on the Baecke score,[27] which is a well-established questionnaire validated with the doubly labeled water method.[28,29] We only used leisure time and sports related physical activity, since work-related physical activity is not associated with CR fitness.[30]
We used the WHO Anatomical Therapeutic Chemical (ATC) classification system (https://www.who.int/tools/atc-ddd-toolkit/atc-classification) to define the use of beta-blockers, lipid-lowering medication, antihypertensive and antidiabetic medication including insulin therapy. The ATC divides drugs in different groups depending on which organ or system they act upon. Beta-blockers were defined as ATC C07. Lipid-lowering medication was defined as ATC C10. Somatometry (height, weight, body mass index) as well as blood pressure were assessed during medical examinations. Blood pressure was measured according to WHO guidelines with three consecutive measurements and taking the mean value of the second/third measurement. Arterial hypertension was defined as self-reported physician diagnosis, or measured systolic blood pressure > 140 mmHg, or measured diastolic blood pressure > 90 mmHg, or the intake of antihypertensive medication (self-report and ATC C02).
Blood plasma samples were analyzed immediately or stored at −80°C.[31] Triglycerides, total cholesterol, low density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c) levels were measured at the Institute of Clinical Chemistry and Laboratory Medicine of the University Medicine Greifswald. Non-HDL-c was calculated as total cholesterol minus HDL-c. Type 2 diabetes mellitus (T2DM) was defined as self-reported physician diagnosis, or HbA1c > 6.5%, or intake of antidiabetic medication/insulin (ATC A10).
2.4. Statistics
For sample characteristics in Table 1, continuous data were expressed as mean and standard deviation (SD). Nominal data were expressed as numbers and percentages. Differences between sexes were calculated using t-test (continuous variables) or χ2 test (nominal variables), respectively.
Table 1.
Baseline characteristics of the analysis sample.
| Total [n = 1102] | Men [n = 535] | Women [n = 567] | |||||
|---|---|---|---|---|---|---|---|
| Age, years [mean (SD)] | 50.3 | (12.9) | 50.6 | (13.2) | 50.0 | (12.5) | 0.398 |
| Age < 54 years [n (%)] | 644 | (58.4%) | 305 | (57.0%) | 339 | (59.8%) | 0.349 |
| BMI, kg m−2 [mean (SD)] | 27.4 | (4.4) | 28.0 | (4.0) | 26.8 | (4.7) | < 0.001 |
| Smoking [n (%)] | 286 | (25.9%) | 144 | (26.9%) | 142 | (25.0%) | 0.479 |
| SBP, mmHg [mean (SD)] | 129.0 | (17.3) | 133.9 | (15.3) | 124.4 | (17.8) | < 0.001 |
| DBP, mmHg [mean (SD)] | 81.5 | (9.7) | 83.7 | (9.8) | 79.5 | (9.2) | < 0.001 |
| Arterial hypertension [n (%)] | 324 | (29.4%) | 207 | (38.7%) | 117 | (20.6%) | < 0.001 |
| T2DM [n (%)] | 89 | (8.1%) | 59 | (11.0%) | 30 | (5.3%) | < 0.001 |
| Lipids [mean (SD)]: | |||||||
| Triglycerides, mmol L−1 | 1.8 | (1.19) | 2.1 | (1.2) | 1.5 | (0.8) | < 0.001 |
| Total chol., mg dL−1 | 203.0 | (39.4) | 200.6 | (39.5) | 205.3 | (39.2) | 0.042 |
| LDL-c, mg dL−1 | 122.6 | (33.1) | 125.4 | (32.1) | 120.0 | (33.8) | 0.007 |
| HDL-c, mg dL−1 | 49.3 | (14.6) | 43.2 | (11.9) | 55.1 | (14.7) | < 0.001 |
| Non-HDL-c, mg dL−1 | 153.7 | (40.7) | 157.4 | (39.6) | 150.3 | (41.4) | 0.004 |
| Total chol./ LDL-c ratio | 1.7 | (0.3) | 1.6 | (0.2) | 1.8 | (0.3) | < 0.001 |
| Medication [n (%)]: | |||||||
| Beta-blockers | 218 | (19.8%) | 105 | (19.6%) | 113 | (19.9%) | 0.899 |
| Lipid-lowering drugs | 113 | (10.3%) | 67 | (12.5%) | 46 | (8.1%) | < 0.016 |
| Education years [n (%)]: | |||||||
| < 10 years | 341 | (30.9%) | 174 | (32.5%) | 167 | (29.5%) | |
| = 10 years | 558 | (50.6%) | 256 | (47.9%) | 302 | (53.3%) | 0.197 |
| > 10 years | 203 | (18.4%) | 105 | (19.6%) | 98 | (17.2%) | |
| Physical activity [mean (SD)]: | |||||||
| LTPA, Lti | 3.3 | (0.6) | 3.2 | (0.6) | 3.3 | (0.6) | 0.001 |
| SPA, Si | 2.5 | (0.7) | 2.5 | (0.7) | 2.5 | (0.7) | 0.149 |
| Ceramides [mean (SD)]: | |||||||
| C16:0, μg mL−1 | 0.2 | (< 0.1) | 0.2 | (< 0.1) | 0.2 | (< 0.1) | 0.847 |
| C22:0, μg mL−1 | 0.6 | (0.2) | 0.6 | (0.2) | 0.6 | (0.2) | 0.003 |
| C24:0, μg mL−1 | 2.5 | (0.7) | 2.6 | (0.7) | 2.4 | (0.7) | < 0.001 |
| C22:0/16:0 ratio | 3.2 | (0.6) | 3.3 | (0.6) | 3.1 | (0.6) | < 0.001 |
| C24:0/16:0 ratio | 12.2 | (2.6) | 12.7 | (2.7) | 11.7 | (2.3) | < 0.001 |
| CR fitness parameters [mean (SD)]: | |||||||
| VO2peak, mL min−1 | 1998 | (585.4) | 2385 | (543.8) | 1634 | (337.3) | < 0.001 |
| VO2peak kg−1, mL per min per kg | 26 | (6.5) | 28 | (6.7) | 24 | (5.4) | < 0.001 |
| VO2@AT1, mL min−1 | 1111 | (300.0) | 1272 | (301.2) | 960 | (204.8) | < 0.001 |
| VO2@AT1 kg−1, mL per min per kg | 14 | (3.5) | 15 | (3.6) | 14 | (3.2) | < 0.001 |
| Wattmax, W | 157 | (47.5) | 186 | (43.6) | 128 | (31.2) | < 0.001 |
| Wattmax kg−1, W kg−1 | 2 | (0.6) | >2 | (0.6) | <2 | (0.5) | < 0.001 |
Continuous variables are presented as mean (SD), categorial variables are expressed in numbers (percentages %). P-values are presented in italic and were calculated using t-test (continuous variables) or χ2 test (categorical variables). Significant p-values are printed in bold, Abbr. SBP = systolic blood pressure, DBP = diastolic blood pressure, T2DM = type 2 diabetes mellitus, Total chol. = total cholesterol, LDL-c = low density lipoprotein cholesterol, HDL-c = high density lipoprotein cholesterol, LTPA = physical activity during leisure time, SPA = physical activity during sport (Baecke score)
For explorative purposes, we first calculated sex-specific associations of ceramides with age adjusted for smoking, T2DM, intake of lipid-lowering medication, beta-blockers, and education years. The same was done for CR fitness parameters (see Figures S3 and S4, Supporting Information). We used fractional polynomials[32] to check for potential non-linear associations of ceramides with age in our cohort sample.
For the main analyses, we used multivariable linear regression models to assess the association of C16:0, C22:0, and C24:0 ceramide levels as well as their ratios, C24:0/C16:0 and C22:0/C16:0, with CR fitness parameters. The former were defined as exposures, the latter as outcomes. Recent studies could show that ceramide ratios, especially C24:0/C16:0, have a better predictive value for CVD events than specific single ceramide levels.[3,10] In a previous cooperation between SHIP and the Framingham heart study we were already able to demonstrate similar findings.[8] Additionally, C16:0/C24:0 ratio is an important component of the CERT1 CVD risk score.[5] However, in order to be consistent with our previous work, we used the C24:0/C16:0 ratio as well in the present analysis instead of the inversed ratio used by Hilvo and colleagues in the CERT1 score. VO2peak, VO2@AT1, and Wattmax were used as crude CR fitness parameters, and respectively indexed per kg total body weight as normalized parameters. Furthermore, we generated a directed acyclic graph (see supplementary material, Figure S2, Supporting Information) to identify potential confounding on the association between ceramides and CR fitness. The DAG was used to determine then the minimal sufficient adjustment set. Based on that, three adjustment sets: simple, full1, and full2 were created. The simple set included: continuous age (linear spline with knot at 54 years), sex, BMI (except for normalized CR fitness parameters), physical activity (leisure time index, sports index), and current smoking status. In full1 we additionally added arterial hypertension, T2DM, blood lipids (i.e., triglycerides, and total cholesterol/LDL-c ratio), intake of beta-blockers and lipid-lowering medication, as well as education years. So far, there is no agreed-upon way to adjust for different lipid levels in the context of ceramide-outcome associations. Results are still inconclusive at the moment how different lipids and lipid levels influence those ceramide-outcome associations.[6–8,18] Therefore, we generated an alternative model: full2, where we used non-HDL-c instead of total cholesterol/LDL-c ratio. We calculated all three regression models (simple, full1, full2) for each ceramide or ceramide ratio separately without using any combinations of our ceramides in the same model to avoid collinearity issues.
In a second step, we categorized age (</≥ 54 years) to investigate subgroups reflecting the BLSA cohort analysis by Fabbri et al. Categorized age (AGEcat.[</≥54]) was only used in combination with sex to generate the according four subgroups, we wanted to investigate: men or women, younger or older than 54 years, respectively. However, to avoid reduction of power we did not do this subgroup stratification by creating a priori subsamples and running all models separately within each subsample again, but instead introduced the interaction term SEX*AGEcat.[</≥54]*’ceramide’ into our set of models. By doing this, it was possible to simultaneously calculate within one model four subgroup-specific slope estimates for the particular ceramide-CR fitness association utilizing the whole sample of 1102 participants (instead of the particular subgroup sample only). We report only subgroup results where the interaction term SEX*AGEcat.[</≥54]*’ceramide’ was statistically significant. Additionally, this approach enabled us to search post-hoc for possible subgroup differences as well. To address potential confounding by age and sex in that second set of models as well, again main effects of age and sex were also included in all interactive models. Main effect of age was included again in a continuous fashion using a linear spline with one knot at 54 years. Detailed results regarding effect estimates etc. are provided in Tables S1 and S2 (Supporting Information). For potential sole age-only or sex-only interactions please refer to those tables as well.
Continuous variables included in linear regression models (ceramides, BMI, physical activity, blood lipids) were z-transformed separately for men and women (sex-specific: mean = 0, and SD = 1). Hence, reported effect estimates (β-estimates [95% confidence limits]) reflect the change in CR fitness parameters corresponding to a sex-specific 1-SD change in ceramide levels.
Moreover, in sensitivity analyses we investigated whether the intake of beta-blockers, or lipid-lowering medication modify the association of ceramides with CR fitness by including appropriate interaction terms into our initial global model set as well.
Normality and homoscedasticity of residuals were ensured by visually checking QQ-plots, kernel-density plots as well as residuals-versus-fitted plots for each regression model. Significance level was set to alpha = 0.05. No adjustments for multiplicity were done in accordance with Rothman[33] as the study is rather exploratory in nature than confirmatory. All statistical analyses were done with Stata statistical software (17.0, StataCorp LLC, College Station, TX).
2.5. Ethics Approval Statement
The study was reviewed and approved by the Ethics Committee of the University Medicine of Greifswald and meets the requirements of the Declaration of Helsinki (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/) and Taipei (https://www.wma.net/what-we-do/medical-ethics/declaration-of-taipei/) in its latest form.
3. Results
3.1. Sample Cohort Characteristics
Table 1 summarizes the main characteristics of the study population in total and sex-stratified groups (51.5% women). The age of the total sample ranged from 25 to 84 years (mean age (SD) = 50.3 (12.9) years). Approximately 29% of the study participants had arterial hypertension, 26% were current smokers, and 8% had T2DM. Men and women differed significantly in BMI, systolic and diastolic blood pressure, the presence of arterial hypertension and TD2M, blood lipids, the intake of lipid-lowering medication, as well as in leisure time related physical activity. Furthermore, women had significantly lower levels of ceramide C22:0, C24:0, C22:0/16:0 ratio, and C24:0/C16:0 ratio as well as lower values of all CR fitness parameters.
3.2. Sex-Specific Associations of Ceramides with Age
The association between ceramides and age varied greatly depending on the ceramide species and sex (for further details see Figure S3, Supporting Information). In men, we found always significant (p < 0.01) non-linear associations with age for all three single ceramide species (i.e., C16:0, C22:0, and C24:0) with a maximum ceramide plasma level for all of them at ≈40 years. In women, a significant (p < 0.001) positive linear association for C16:0 was found, while even more pronounced non-linear associations (p < 0.001) for C22:0 and C24:0, with a maximum ceramide plasma level at an age of 60 – 70 years for the latter two ceramide species. The two ceramide ratios C22:0/C16:0, and C24:0/C16:0 revealed only non-significant (p > 0.2) linear associations with age in both sexes.
3.3. Sex-Specific Association of CR Fitness Parameters with Age
Apart from Wattmax in men all assessed crude CR fitness parameter and VO2peak, VO2@AT1, and Wattmax in women, showed an inverse linear association with age (p < 0.001). Wattmax showed a non-linear inverse association in men (p < 0.001, see Figure S4 A, Supporting Information).
As with the crude CR fitness parameters, normalized CR fitness parameters were inversely and linear associated with age (p < 0.001), too (see Figure S4B, Supporting Information). Only VO2@AT1 kg−1 in women showed a non-linear inverse association with age (p < 0.001).
3.4. Association Between Ceramides and Crude CR Fitness Parameters
Only the ceramide C16:0 was significantly inversely associated with crude CR fitness parameter maximum oxygen consumption (VO2peak) in younger men ≤ 54 years (per 1-SD ceramide increase: simple: pinteraction = 0.012; −72.89 mL min−1 [−118.53; −27.26], full1: pinteraction = 0.034, −72.80 mL min−1 [−117.41; −28.19], full2: pinteraction = 0.029, −70.81 mL min−1 [−118.38; −23.24]. No other significant associations of ceramides with crude CR parameters could be detected (see Figure 1 and see further details in Table S1, Supporting Information).
Figure 1.
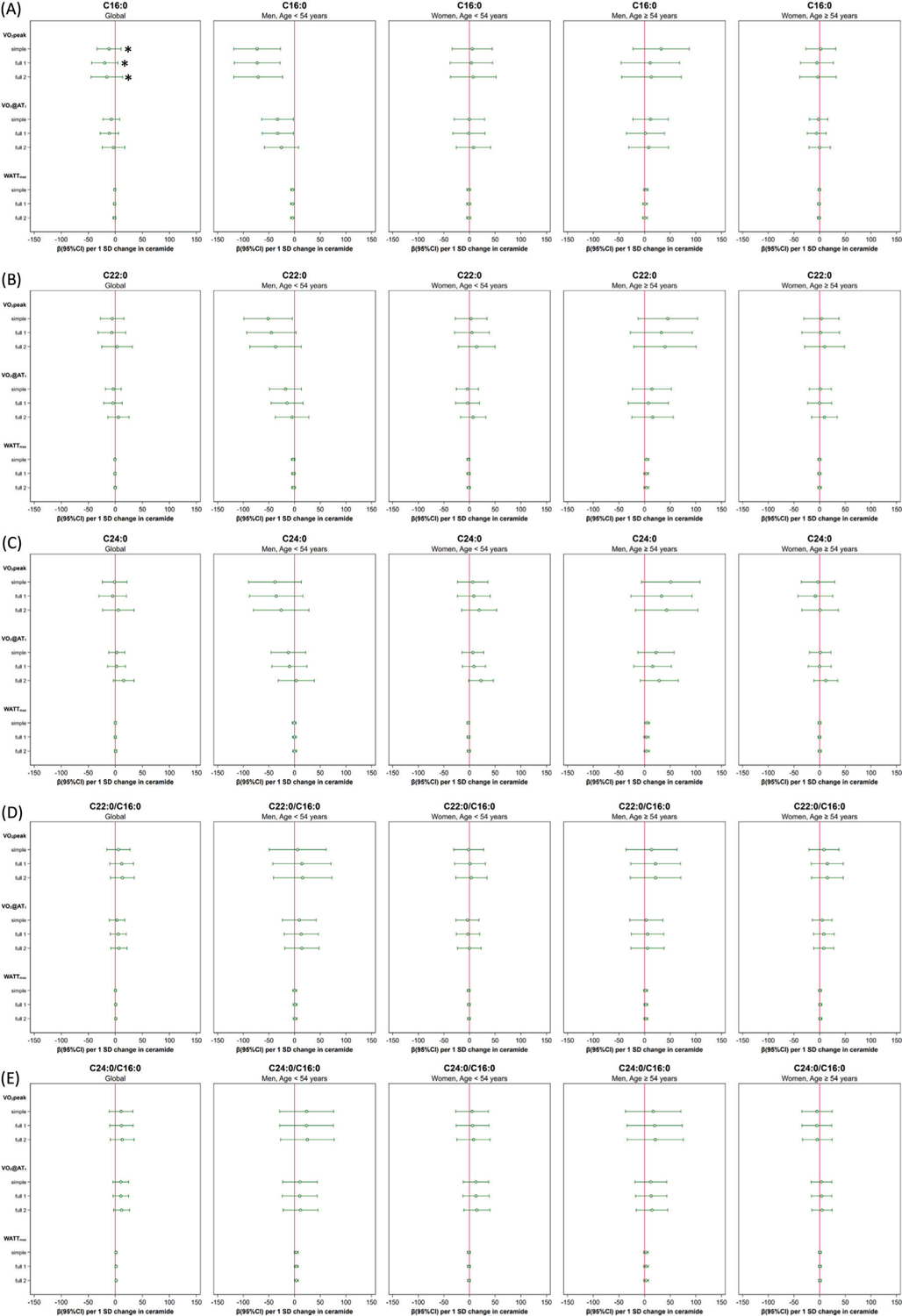
Forest plots: effects (95% confidence interval) of each ceramide species on crude cardiorespiratory fitness (CR fitness) parameters (VO2peak, VO2@AT1, Wattmax) stratified by sex and age (</≥ 54 years): A) ceramide C16:0, B) ceramide C22:0, C) ceramide C24:0, D) ceramide ratio C22:0/C16:0, E) ceramide ratio C24:0/C16:0. Significant age/sex three-way interactions are marked with an asterisk. Adjustment models: simple included age, sex, BMI, physical activity, and smoking; full1 additionally included arterial hypertension, T2DM, total cholesterol/LDL-c ratio, beta-blockers, lipid-lowering medication, and education years, full2 included non-HDL-c instead of total cholesterol/LDL-c ratio.
3.5. Association Between Ceramides and Normalized CR Fitness Parameters
In the whole sample higher levels of ceramide C24:0, and C24:0/C16:0 ratio were in general associated with greater CR fitness, even after extended adjustments to address potential confounding (see Figure 2C,E and see further details in Table S2, Supporting Information). For ceramide C24:0 those significant associations were visible only in the two fully adjusted models (per 1-SD ceramide increase: VO2peak kg-: simple: 0.15 mL per min per kg [−0.15;0.45], full1: 0.49 mL per min per kg [0.16;0.82], full2: 0.67 mL per min per kg [0.29;1.05]; VO2@AT1 kg−1: simple: 0.16 mL per min per kg [−0.03;0.35], full1: 0.35 mL per min per kg [0.14;0.56], full2: 0.55 mL per min per kg [0.30;0.79]; and Wattmax kg−1: simple: 0.01 W kg−1 [−0.02;0.03], full1: 0.05 W kg−1 [0.02; 0.07], full2: 0.06 W kg−1 [0.03; 0.09]; see further details Table S2, Supporting Information). Furthermore, one can see that for oxygen consumption parameters those associations were more pronounced in models adjusting for non-HDL-c (full2) instead of total cholesterol/LDL-c ratio (full1).
Figure 2.
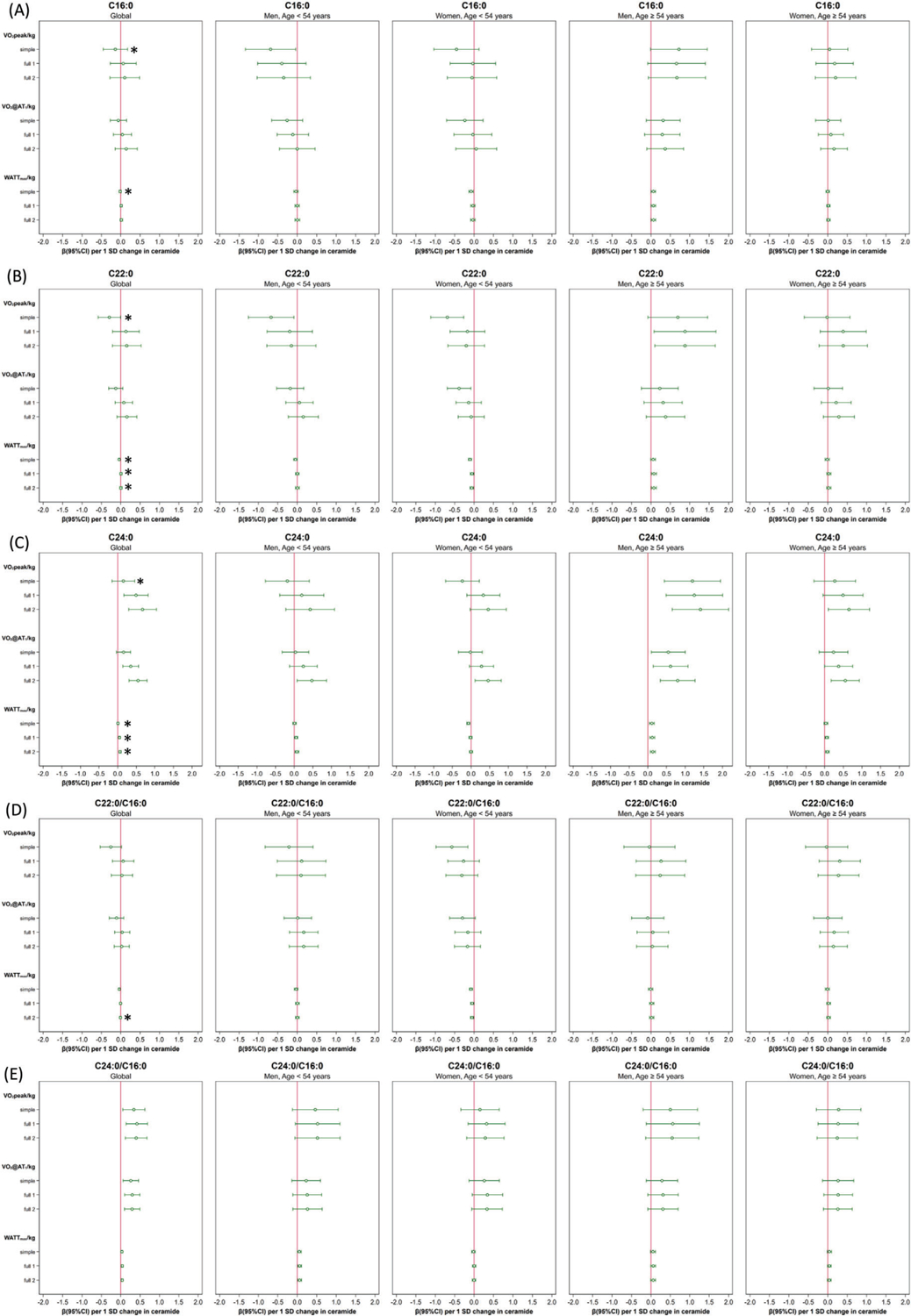
Forest plots: effects (95% confidence interval) of each ceramide species on normalized cardiorespiratory fitness (CR fitness) parameters (VO2peak/kg, VO2AT1/kg, Wattmax/kg) stratified by sex and age (</≥ 54 years): (A) ceramide C16:0, (B) ceramide C22:0, (C) ceramide C24:0, (D) ceramide ratio C22:0/C16:0, (E) ceramide ratio C24:0/C16:0. Significant age/sex three-way interactions are marked with an asterisk. Normalized CR fitness parameters = indexed per kg total body weight, Adjustment models: simple included age, sex, physical activity, and smoking; full1 additionally included arterial hypertension, T2DM, total cholesterol/LDL-c ratio, beta-blockers, lipid-lowering medication, and education years, full2 included non-HDL-c instead of total cholesterol/LDL-c ratio.
A larger C24:0/C16:0 ceramide ratio was always related to greater values of every measured normalized CR fitness parameter independent of the adjustment set applied (per 1-SD ratio increase: VO2peak kg−1: simple: 0.34 mL per min per kg [0.05;0.62], full1: 0.42 mL per min per kg [0.14;0.69], full2: 0.40 mL per min per kg [0.12;0.68]; VO2@AT1 kg−1: simple: 0.26 mL per min per kg [0.06;0.46], full1: 0.30 mL per min per kg [0.10;0.49], full2: 0.29 mL per min per kg [0.09;0.49]; and Wattmax kg−1: simple: 0.03 W kg−1 [0.00;0.06], full1: 0.04 W kg−1 [0.01; 0.06], full2: 0.04 W kg−1 [0.01; 0.06]; see further details in Table S2, Supporting Information).
Higher levels of ceramide C16:0 were only significantly associated with lower VO2peak/kg in the simple adjustment set, whereas this association was no longer significant after extended adjustments (per 1-SD ceramide increase: VO2peak kg−1: simple: −0.14 mL per min per kg [−0.45;−0.17], full1: 0.06 mL per min per kg [−0.27;0.40], full2: 0.10 mL per min per kg [−0.28;0.48]; see further details in Table S2, Supporting Information). The same was true for C22:0 (per 1-SD ceramide increase: VO2peak kg−1: simple: −0.29 mL per min per kg [−0.58; −0.01], full1: 0.13 mL per min per kg [−0.21;0.48], full2: 0.15 mL per min per kg [−0.22;0.52]; see further details in Table S2, Supporting Information). The ceramide ratio C22:0/C16:0 was significantly inversely associated with Wattmax kg−1 only in the simple adjustment set as well (per 1-SD ceramide increase: Wattmax kg−1: simple: −0.04 W kg−1 [−0.06; −0.01], full1: −0.01 W kg−1 [−0.03;0.02], full2: −0.01 W kg−1 [−0.03;0.01]; see further details in Table S2, Supporting Information).
With regard to the age/sex dependent effects, we refer in the following to the fully adjusted models full1 and full2. We found age/sex modifying effects primarily for the association of single ceramides C22:0 (full1: pinteraction = 0.003, full2: pinteraction = 0.001) and C24:0 (full1: pinteraction = 0.002, full2: pinteraction = 0.001) with Wattmax/kg (see Figure 2B,C and further details in Table S2, Supporting Information): namely for C22:0, an inverse association in younger women < 54 years (full1: −0.05 W kg−1 [−0.10;−0.01]; full2: −0.06 W kg−1 [−0.10;−0.02]), while a positive association in older men ≥ 54 years full1: 0.08 W kg−1 [0.01;0.14]; full2: 0.08 W kg−1 [0.01;014]). In contrast, higher levels of C24:0 were instead always associated with higher Wattmax kg−1, except for younger women < 54 years, where no association at all could be found (full1: <54/men: 0.05 W kg−1 [0.01;0.10], <54/women: −0.01 [−0.05;0.03], ≥54/men: 0.12 W kg−1 [0.05;0.18], ≥54/women: 0.06 W kg−1 [0.01;0.10]; full2: <54/men: 0.07 W kg−1 [0.02;0.12], <54/women: 0.00 [−0.04;0.04], ≥54/men: 0.13 W kg−1 [0.07;0.20], ≥54/women: 0.07 W kg−1 [0.02;0.12]; see further details in Table S2, Supporting Information)
There were no significant interactions (p > 0.05) with the intake of beta-blockers, or lipid-lowering medication detectable.
4. Discussion
CVD is one of the leading global causes of death and also a major financial burden.[1] As described in literature, blood plasma ceramides[2–10] as well as low CR fitness[13–17] are closely related to the development of CVD. However, whether ceramides are related to CR fitness was heretofore unclear.
Based on a large sample from a population-based cohort, this study assessed the association of three of the most abundant blood plasma ceramides and their respective ratios with CR fitness. We found that in general higher levels of ceramide C24:0, and C24:0/C16:0 ratio were associated with greater CR fitness independent of age or sex. Higher levels of ceramide C16:0 were inversely associated with CR fitness only in men below 54 years. These findings confirm previously published data showing ceramides might be beneficial or detrimental with respect to cardiovascular health depending on their chain length.[3–5,8,9] In addition, we found sex and age modifying effects on the association of single ceramides with CR fitness parameters. Ceramides C22:0 and C24:0 showed age/sex subgroup specific associations with body weight normalized maximum exhibited workload: an inverse association in younger women below 54 years for C22:0, while a positive association in men greater or equal 54 years. Higher levels of C24:0 were always associated with higher maximum exhibited workload, except for younger women below 54 years, where no association could be found. As already mentioned, the ceramide C16:0 was only (inversely) associated with maximum oxygen consumption in younger men below 54 years. Thus, our results show that, in addition to the chain length, sex and age of participants may be important in determining whether a single ceramide species are associated with good or poor CR fitness. This means that while a particular ceramide could be associated with poor CR fitness, e.g., in the younger population, it could also be associated with greater CR fitness in the older population. On the other hand, beta estimates describing the association of ceramide ratio C24:0/C16:0 with CR fitness were robust against any modifying effects of age or sex. In addition, this ratio was found to have the most significant positive associations with all measured CR fitness parameters.
Similar to our findings men also had higher blood plasma levels of ceramides compared to women in 443 participants of BLSA.[18] Additionally, like in BLSA, in SHIP men reached greater CR fitness than women. In BLSA ceramides were inversely associated with CR fitness. In addition, to assessing the relationship between specific single ceramides and CR fitness, we also explored in the present study the relation between ceramide ratios and CR fitness. Here, we found that a higher C24:0/C16:0 ceramide ratio was associated with greater CR fitness. Moreover, in BLSA the effect estimates were stronger for men compared to women. We found significant age/sex differences only for single ceramides and their relation with CR fitness. That supporting, in an exploratory cross-sectional age analysis, we were able to show that ceramides differ not only in their plasma concentration between the sexes, but over the age range of the same sex (see Figure S3, Supporting Information).
A biomarker for cardiovascular risk should be preferably widely applicable and robust against confounders. We found that the association of single ceramides may be significantly influenced by sex and age. Furthermore, as shown sex and age affected not only the strength of the association but also its direction. Age and sex modifying effects could also be relevant to the association of ceramides with cardiovascular events. Thus, this could limit the use of ceramides as cardiovascular biomarkers. To use single ceramides in the clinical practice age/sex-specific reference values may then be needed. On the other hand, we found that the associations of ceramide ratio C24:0/C16:0 with CR fitness were robust against many confounders, including effect modifications by age and sex. Thus, C24:0/C16:0 ceramide ratio may be a better candidate as biomarker for the cardiovascular risk prediction than single ceramides and should be favored in our opinion.
Consistent with our findings many other studies also found age and sex differences in the distribution of ceramides or lipid profiles.[7,8,34–37] The exact mechanisms leading to sex and age differences are currently unclear. One may speculate that endocrinological influences play a role. Hormone levels change with increasing age and differ between the sexes. A previous study demonstrated that the supply of estradiol to cells with estrogen receptors reduces the synthesis of individual ceramides in vitro.[38] It is tempting to speculate that this may be related to the finding that premenopausal women have less cardiovascular events than postmenopausal women.[39–41] Supporting this idea, we found differences between younger and older women in the association of ceramides C22:0, C24:0, and C22:0/C16:0 ratio with maximum exhibited workload. However, unlike the other ceramides, C24:0/C16:0 ceramide ratio was robust to any modifying effects of sex or age.
Ceramides are known to be closely associated with a wide range of CVD such as coronary heart disease, stroke, or heart failure.[2–10] Low CR fitness also increases the risk of CVD.[13,14,16,17] More importantly, CR fitness increases the predictive value of a cardiovascular risk profile if added to established risk parameters like arterial hypertension, T2DM, or obesity.[16,42] To date, there is no mechanistic pre-clinical study that specifically links ceramides and CR fitness in the general population. But there is a study based on 100 CAD patients (mean age (SD) = 64 (6) years, 85% male), which shows that after a six month cardiac rehabilitation program an increase in CR Fitness was associated with a decrease in ceramides like C16:0.[19] Furthermore, recent research shows that ceramides have a strong influence on the vascular system, cardiac function, and muscle metabolism, being important determinants of CR fitness.[2,3,9] Therefore, a causal relationship between ceramides and CR fitness seems likely. Ceramides inhibit the production of nitric oxide by promoting the formation of reactive oxygen species and dephosphorylation of the endothelial nitric oxide synthase.[3,43] Thus, they promote vasoconstriction and inhibit vasorelaxation, thereby inhibiting muscle blood perfusion. Furthermore, high levels of circulating ceramides damage the cardiac endothelium and induce fibrosis which may limit CR fitness.[2,3,9,12,44] On the cellular level, ceramides inhibit the utilization of glucose as a quick energy supply during high intensity physical activity.[2,3,9] In addition, C16:0 ceramide inhibits the mitochondrial respiratory chain, which is an important component of CR fitness and could lower the performance during CPET.[2,3,9,45] New findings suggest very-long chain ceramides could be involved in cellular respiration blockade, too.[46] The latter might be a possible explanation (besides residual confounding) for ceramides C22:0 and C22:0/C16:0 being associated with poor CR fitness in our analysis, though only in the simple adjustment set.
Due to the observational nature of the study, we cannot definitively establish a causal link between ceramides and CR fitness. However, as stated above, it can be assumed that there might be a causal relation. Future (longitudinal and prospective) investigations are needed to find out whether a causality exists and, if so, whether it can be influenced, e.g., by exercise training or medical treatment. Longitudinal intervention studies with a control group are needed to determine whether changes in ceramide concentrations, e.g., caused by medication, have an effect on CR fitness. Based on our results, we have shown that statins or other common lipid-lowering drugs (ATC C10) have no effect on the association between ceramides and CR fitness. On the other hand, recent literature shows that an increase in CR fitness based on physical activity in CAD patients[19] is associated with a decrease in specific ceramides, which are associated with higher cardiovascular risk. In addition, molecular research[47,48] based on measuring ceramide levels immediately before and after physical activity has shown that specific ceramide levels such as C16:0 increase and then quickly return to baseline. This suggests that in addition to CR fitness, ceramides are also associated with physical activity. So, in total, further work needs to be done to investigate, if physical activity is another target to influence circulating ceramide levels in addition to medication.
Taken together, our findings indicate that specific ceramides could influence CR fitness and thereby the related cardiovascular risk as well. Sex and age modifying effects could limit the use of single ceramides as cardiovascular biomarkers. C24:0/C16:0 ceramide ratio was robust against those effects and should be preferred. Future longitudinal studies need to investigate the potential causal relationship between ceramides and CR fitness and its effects on CVD prognosis as well as should look deeper into potential modifying effects of age and sex.
4.1. Strengths and Limitations
Our study has several strengths but also limitations. One of the strengths of our study is the large number of study participants (n = 1102) from the population-based Study of Health in Pomerania (SHIP-START-1). The sample was well and deeply phenotyped, so we could address many covariables and use multivariable adjustment sets to address confounding issues and assess the association between ceramides and CR fitness. However, residual confounding could not be fully ruled out. Furthermore, we used objectively measurable exposure-, outcome-, and covariables. To assess CR fitness, we used the gold standard, a symptom limited maximal CPET. Ceramides were quantified by a fully validated, robust, and reproducible targeted liquid chromatography/tandem mass spectrometry assay.
Our study also has some limitations. The study sample was primarily of Caucasian descent. Both, ceramide levels and CR fitness differ between distinct ethnic groups.[35,49–52] Thus, results cannot be readily applied to other ethnicities. In SHIP-START-1 non-fasting blood plasma samples were drawn. The European Heart Journal published 2016 a statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine,[53] which proclaimed that fasting blood plasma levels are not required for assessing cardiovascular risk. Because it is not known whether fasting samples have an advantage over non-fasting samples, we consider the use of non-fasting samples is at most a minor limitation. Moreover, we were unable to include dietary information in our analyses or a comprehensive lipid profile, since only C16:0, C22:0, and C24:0 ceramides were quantified in SHIP-START-1 so far. Finally, this study is based on cross-sectional epidemiological data. Therefore, we cannot clearly derive a causal relationship between ceramides and CR fitness parameters. Furthermore, no adjustments for multiplicity were done. This was done primarily as our study is exploratory mainly in nature instead of confirmatory. We did not want to reduce the power of our study to detect potential new effects between ceramides and CR fitness parameters.[33]
5. Conclusion
Summarizing, higher C24:0 and C24:0/C16:0 ratio were associated with greater values of CR fitness in a large population-based study. Age and sex significantly influenced the direction and strength of the associations of single ceramides with CR fitness, whereas C24:0/C16:0 ceramide ratio was robust against these effect modifications. Based on our results we were able to show that not solely the chain length, but also sex and age of a subject may be important for potential beneficial or detrimental effects of single ceramides. These findings suggest that the use of single ceramides as cardiovascular biomarkers may be limited. In order to use single ceramides in the clinical practice age- and sex-specific reference values may be needed. In contrast, ceramide ratio C24:0/C16:0 was stable against any sex or age modifying effects. Therefore C24:0/C16:0 ratio may be a better cardiovascular biomarker und should be preferred over single ceramides. Furthermore, the strongest associations with CR fitness were found with this ratio.
Supplementary Material
Acknowledgements
The present study was supported by a grant of the Federal Ministry of Education, Germany, grant no. BMBF/DZHK FKZ 81Z0400101 to M.D., M.B., and S.B.F. Measurement of ceramides was supported by the National Institutes of Health, grant no. P20 HL113444, P30 DK020579 to J.E.S.
Open access funding enabled and organized by Projekt DEAL.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Jule Zatloukal, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany; German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany.
Stephanie Zylla, German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany; Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, 17475 Greifswald, Germany.
Marcello R.P. Markus, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany.
Ralf Ewert, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany.
Sven Gläser, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany; Clinic for Internal Medicine, Vivantes Klinikum Spandau/Neukölln, 12351 Berlin, Germany.
Henry Völzke, German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany; Institute of Community Medicine, University Medicine Greifswald, 17475 Greifswald, Germany.
Diana Albrecht, Institute of Community Medicine, University Medicine Greifswald, 17475 Greifswald, Germany; Leibniz Institute for Plasma Science and Technology, 17489 Greifswald, Germany.
Nele Friedrich, German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany; Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, 17475 Greifswald, Germany.
Matthias Nauck, German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany; Institute of Clinical Chemistry and Laboratory Medicine, University Medicine Greifswald, 17475 Greifswald, Germany.
Linda R. Peterson, Division of Cardiology, Department of Medicine, Washington University, St Louis, MO 63110, USA
Xuntian Jiang, Division of Cardiology, Department of Medicine, Washington University, St Louis, MO 63110, USA.
Jean E. Schaffer, Joslin Diabetes Center, Harvard Medical School, Boston, MA 02215, USA
Stephan B. Felix, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany.
Marcus Dörr, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany; German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany.
Martin Bahls, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany; German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany.
Stefan Gross, Dept. of Internal Medicine B, University Medicine Greifswald, 17475 Greifswald, Germany; German Centre for Cardiovascular Research (DZHK), 17475 Partner-site Greifswald, Germany.
Data Availability Statement
Data are available at the Study of Health in Pomerania and can be applied for at https://www.fvcm.med.uni-greifswald.de/dd_service/data_use_intro.php. Data and analysis code of the current study is available upon reasonable request from the corresponding author.
References
- [1].WHO, World Health Statistics 2020 – Monitoring Health for the SDGs, Geneva, https://creativecommons.org/licenses/by-nc-sa/3.0/igo 2020 [Google Scholar]
- [2].Chaurasia B, Summers SA, Trends Endocrinol. Meta 2015, 26, 538. [DOI] [PubMed] [Google Scholar]
- [3].Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL, Nat. Rev. Cardiol 2021, 18, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, Jylhä A, Huynh K, Mellett NA, Tonkin AM, Sullivan DR, Simes J, Nestel P, Koenig W, Rothenbacher D, Nygård O, Laaksonen R, Eur. Heart J 2020, 41, 371. [DOI] [PubMed] [Google Scholar]
- [5].Hilvo M, Vasile VC, Donato LJ, Hurme R, Laaksonen R, Front. Endocrinol. (Lausanne) 2020, 11, 570628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hilvo M, Wallentin L, Ghukasyan Lakic T, Held C, Kauhanen D, Jylhä A, Lindbäck J, Siegbahn A, Granger CB, Koenig W, Stewart RAH, White H, Laaksonen R, Am J. Heart Assoc. 2020, 9, e015258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lemaitre RN, Jensen PN, Hoofnagle A, Mcknight B, Fretts AM, King IB, Siscovick DS, Psaty BM, Heckbert SR, Mozaffarian D, Sotoodehnia N, AHA/ASA Journals 2019, 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Völzke H, Felix SB, Jiang H, Sidhu R, Nauck M, Jiang X, Ory DS, Dörr M, Vasan RS, Schaffer JE, Am J. Heart Assoc. 2018, 7, 7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Summers SA, Chaurasia B, Holland WL, Nat. Metab 2019, 1, 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zelnik ID, Kim JL, Futerman AH, J. Lipid Atheroscler. 2021, 10, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zietzer A, Düsing P, Reese L, Nickenig G, Jansen F, Arterioscler. Thromb. Vasc. Biol 2022, 42, 1220. [DOI] [PubMed] [Google Scholar]
- [12].Ji R, Akashi H, Drosatos K, Liao X, Jiang H, Kennel PJ, Brunjes DL, Castillero E, Zhang X, Deng LY, Homma S, George IJ, Takayama H, Naka Y, Goldberg IJ, Schulze PC, JCI Insight 2017, 2, 82922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bahls M, Groß S, Baumeister SE, Völzke H, Gläser S, Ewert R, Markus MRP, Medenwald D, Kluttig A, Felix SB, Dörr M, Sci. Rep 2018, 8, 16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaminsky LA, Arena R, Ellingsen Ø, Harber MP, Myers J, Ozemek C, Ross R, Prog. Cardiovasc. Dis 2019, 62, 86. [DOI] [PubMed] [Google Scholar]
- [15].Kondamudi N, Mehta A, Thangada ND, Pandey A, Curr. Cardiol. Rep 2021, 23, 172. [DOI] [PubMed] [Google Scholar]
- [16].Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U, Circulation 2016, 134, e653. [DOI] [PubMed] [Google Scholar]
- [17].Steell L, Ho FK, Sillars A, Petermann-Rocha F, Li H, Lyall DM, Iliodromiti S, Welsh P, Anderson J, MacKay DF, Pell JP, Sattar N, Gill JMR, Gray SR, Celis-Morales CA, Br. J. Sports Med 2019, 53, 1371. [DOI] [PubMed] [Google Scholar]
- [18].Fabbri E, Yang A, Simonsick EM, Chia CW, Zoli M, Haughey NJ, Mielke MM, Ferrucci L, Coen PM, Aging Cell 2016, 15, 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saleem M, Herrmann N, Dinoff A, Marzolini S, Mielke MM, Andreazza A, Oh PI, Vattem Venkata SL, Haughey NJ, Lanctôt KL, Journals Gerontol. – Ser. A Biol. Sci. Med. Sci 2020, 75, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].John U, Hensel E, Lüdemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, Haertel U, Hense HW, Haerting J, Willich S, Kessler C, Soz. Praventivmed 2001, 46, 186. [DOI] [PubMed] [Google Scholar]
- [21].Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, Havemann C, Ittermann T, Schipf S, Haring R, Baumeister SE, Wallaschofski H, Nauck M, Frick S, Arnold A, Jünger M, Mayerle J, Kraft M, Lerch MM, Dörr M, Reffelmann T, Empen K, Felix SB, Obst A, Koch B, Gläser S, et al. , Int. J. Epidemiol 2011, 40, 294. [DOI] [PubMed] [Google Scholar]
- [22].Völzke H, Schössow J, Schmidt CO, Jürgens C, Richter A, Werner A, Werner N, Radke D, Teumer A, Ittermann T, Schauer B, Henck V, Friedrich N, Hannemann A, Winter T, Nauck M, Dörr M, Bahls M, Felix SB, Stubbe B, Ewert R, Frost F, Lerch MM, Grabe HJ, Bülow R, Otto M, Hosten N, Rathmann W, Schminke U, Großjohann R, et al. , Int J Epidemiol 2022, 51, e372. [DOI] [PubMed] [Google Scholar]
- [23].Jiang H, Hsu F-F, Farmer MS, Peterson LR, Schaffer JE, Ory DS, Jiang X, Anal. Bioanal. Chem 2013, 405, 7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jones LN, Makrides L, Hitchcock C, Chypchar T, McCartney N, Am. Rev. Respir. Dis 1985, 131, 700. [DOI] [PubMed] [Google Scholar]
- [25].Koch B, Schäper C, Ittermann T, Spielhagen T, Dörr M, Völzke H, Opitz CF, Ewert R, Gläser S, Eur. Respir. J 2009, 33, 389. [DOI] [PubMed] [Google Scholar]
- [26].Wasserman K, Hansen J, Sue D, Stringer W, Whipp B, Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 4th ed., Lippincott Williams & Wilkins, Philadelphia: 2005. [Google Scholar]
- [27].Baecke JAH, Burema J, Frijters JER, Am. J. Clin. Nutr 1982, 36, 936. [DOI] [PubMed] [Google Scholar]
- [28].Hertogh EM, Monninkhof EM, Schouten EG, Peeters PH, Schuit AJ, Int. J. Behav. Nutr. Phys. Act 2008, 5, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Philippaerts RM, Westerterp KR, Lefevre J, Int. J. Sports Med 1999, 20, 284. [DOI] [PubMed] [Google Scholar]
- [30].Bahls M, Ittermann T, Ewert R, Stubbe B, Völzke H, Friedrich N, Felix SB, Dörr M, J. Med. Sci. Sport 2020, 31, 742. [DOI] [PubMed] [Google Scholar]
- [31].Winter T, Friedrich N, Lamp S, Schäfer C, Schattschneider M, Bollmann S, Brümmer D, Riemann K, Petersmann A, Nauck M, Open J Bioresour. 2020, 7, 2. [Google Scholar]
- [32].Royston P, Sauerbrei W, Multivariable Model – Building: A Pragmatic Approach to Regression Anaylsis based on Fractional Polynomials for Modelling Continuous Variables, John Wiley & Sons, Chichester, West Sussex, England: 2008. [Google Scholar]
- [33].Rothman K, Epidemiology 1990, 1, 43. [PubMed] [Google Scholar]
- [34].Beyene HB, Olshansky G, Smith AAT, Giles C, Huynh K, Cinel M, Mellett NA, Cadby G, Hung J, Hui J, Beilby J, Watts GF, Shaw JS, Moses EK, Magliano DJ, Meikle PJ, PLoS Biol. 2020, 18, e3001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mielke MM, Bandaru VVR, Han D, An Y, Resnick SM, Ferrucci L, Haughey NJ, Aging Cell 2015, 14, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Slade E, Irvin MR, Xie K, Arnett DK, Claas SA, Kind T, Fardo DW, Graf GA, Lipids Health Dis. 2021, 20, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wong MWK, Braidy N, Pickford R, Vafaee F, Crawford J, Muenchhoff J, Schofield P, Attia J, Brodaty H, Sachdev P, Poljak A, PLoS One 2019, 14, e0214141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vozella V, Basit A, Piras F, Realini N, Armirotti A, Bossù P, Assogna F, Sensi SL, Spalletta G, Piomelli D, Aging 2019, 11, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Agrinier N, Cournot M, Dallongeville J, Arveiler D, Ducimetière P, Ruidavets JB, Ferrières J, Maturitas 2010, 65, 237. [DOI] [PubMed] [Google Scholar]
- [40].Van Der Graaf Y, De Kleijn MJJ, Van Der Schouw YT, J. Psychosom. Obstet. Gynaecol 1997, 18, 113. [DOI] [PubMed] [Google Scholar]
- [41].Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K, J. Am. Coll. Cardiol 2009, 54, 2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lamoureux NR, Fitzgerald JS, Norton KI, Sabato T, Tremblay MS, Tomkinson GR, Sport. Med 2019, 49, 41. [DOI] [PubMed] [Google Scholar]
- [43].Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PVA, Holland WL, Zhang QJ, Abel ED, Symons JD, Diabetes 2015, 64, 3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shalaby YM, Al Aidaros A, Valappil A, Ali BR, Akawi N, Front. Cell Dev. Biol 2022, 9, 816301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Funai K, A. S &. Summers, Rutter J, Curr. Opin. Cell Biol 2020, 63, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Law BA, Liao X, Moore KS, Southard A, Roddy P, Ji R, Szulc Z, Bielawska A, Schulze PC, Cowart LA, FASEB J. 2018, 32, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Contrepois K, Wu S, Moneghetti KJ, Hornburg D, Ahadi S, Tsai MS, Metwally AA, Wei E, Lee-McMullen B, Quijada JV, Chen S, Christle JW, Ellenberger M, Balliu B, Taylor S, Durrant MG, Knowles DA, Choudhry H, Ashland M, Bahmani A, Enslen B, Amsallem M, Kobayashi Y, Avina M, Perelman D, Schüssler-Fiorenza Rose SM, Zhou W, Ashley EA, Montgomery SB, Chaib H, et al. , Cell 2020, 181, 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nayor M, Shah RV, Miller PE, Blodgett JB, Tanguay M, Pico AR, Murthy VL, Malhotra R, Houstis NE, Deik A, Pierce KA, Bullock K, Dailey L, Velagaleti RS, Moore SA, Ho JE, Baggish AL, Clish CB, Larson MG, S. R &. Vasan, Lewis GD, Circulation 2020, 142, 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Eikelis N, Lambert EA, Phillips S, Sari CI, Mundra PA, Weir JM, Huynh K, Grima MT, Straznicky NE, Dixon JB, Schlaich MP, Meikle PJ, Lambert GW, J. Clin. Endocrinol. Metab 2017, 102, 2059. [DOI] [PubMed] [Google Scholar]
- [50].Jang WY, Kim W, Kang DO, Park Y, Lee J, Choi JY, Roh S-Y, Na JO, Choi CU, Rha S-W, Park CG, Seo HS, Park SH, S. &. Park, Kim EJ, J. Clin. Med 2019, 8, 2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lavie CJ, Kuruvanka T, Milani RV, Prasad A, Ventura HO, Chest J 2004, 126, 1962. [DOI] [PubMed] [Google Scholar]
- [52].McDonald SM, Ortaglia A, Supino C, Bottai M, Racial Ethn J. Heal. Disparities 2019, 6, 292. [DOI] [PubMed] [Google Scholar]
- [53].Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Borén J, Chapman MJ, Cobbaert C, Descamps OS, Von Eckardstein A, Kamstrup PR, Pulkki K, Kronenberg F, Remaley AT, Rifai N, Ros E, Langlois M, Eur. Heart J 2016, 37, 1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at the Study of Health in Pomerania and can be applied for at https://www.fvcm.med.uni-greifswald.de/dd_service/data_use_intro.php. Data and analysis code of the current study is available upon reasonable request from the corresponding author.


