Summary
Background
Few studies have investigated the role of social determinants of health (SDoH) in patients with atrial fibrillation (AF).
Aim
To investigate the relationship between SDoH and adverse events in a large multinational AF cohort.
Design
Retrospective study utilizing a global federated health research network (TriNetX).
Methods
Patients with AF were categorized as socially deprived defined according to ICD codes based on three SDoHs: (i) extreme poverty; (ii) unemployment; and/or (iii) problems related with living alone. The outcomes were the 5-year risk of a composite outcomes of all-cause death, hospitalization, ischemic heart disease (IHD), stroke, heart failure (HF) or severe ventricular arrhythmias. Cox regression was used to compute hazard rate ratios (HRs) and 95% confidence intervals (CIs) following 1:1 propensity score matching (PSM).
Results
The study included 24 631 socially deprived (68.8 ± 16.0 years; females 51.8%) and 2 462 092 non-deprived AF patients (75.5 ± 13.1 years; females 43.8%). Before PSM, socially deprived patients had a higher risk of the composite outcome (HR 1.9, 95% CI 1.87–1.93), all-cause death (HR 1.34, 95% CI 1.28–1.39), hospitalization (HR 2.01, 95% CI 1.98–2.04), IHD (HR 1.67, 95% CI 1.64–1.70), stroke (HR 2.60, 95% CI 2.51–2.64), HF (HR 1.91, 95% CI 1.86–1.96) and severe ventricular arrhythmias (HR 1.83, 95% CI 1.76–1.90) compared to non-deprived AF patients. The PSM-based hazard ratios for the primary composite outcome were 1.54 (95% CI 1.49–1.60) for the unemployed AF patients; 1.39 (95% CI 1.31–1.47) for patients with extreme poverty or with low income; and 1.42 (95% CI 1.37–1.47) for those with problems related with living alone.
Conclusions
In patients with AF, social deprivation is associated with an increased risk of death and adverse cardiac events. The presence of possible unmeasured bias associated with the retrospective design requires confirmation in future prospective studies.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide and is associated with poor clinical outcomes, including higher mortality and morbidity.1–3 More than 33 million people are diagnosed with AF worldwide, and the prevalence is expected to increase with the demographic changes of the general population since the risk of AF is strongly related to age.1,4,5 Patients with AF are at high risk for death, ischemic heart disease (IHD), heart failure (HF) and other cardiovascular events.1,3,5–8
In the last decades, growing evidence suggests that the risk of adverse events in AF patients, behind the presence of classical cardiovascular risk factors, is also related to the social determinants of health (SDoH).1,9–11 The latter, including occupation, cohabitation, income level and poverty are known to represent individual pathways for the disparity in severe health outcomes12–15 and some studies suggest that SDoH affects the risk of poorer clinical outcomes including IHD, HF, stroke and mortality and in patients with AF.1,3,5–7 Despite substantial progress being made in improving health, social disparities between population groups have persisted and remain marked and prominent, especially within cardiovascular diseases and mortality.5,15,16 SDoH may affect the clinical outcomes after AF through multiple pathways, including influencing the ability to change to a healthier lifestyle behavior, persistence to prescribed medications, and undertreatment and underuse of health services among patients with AF who are unemployed, have low income, live in poverty or live alone.5,15
This study aimed to investigate the association between SDoH and clinical outcomes, including all-cause death, hospitalization, IHD, stroke, HF and severe ventricular arrhythmias, in a large AF population from a global federated health research network. The SDoH domains investigated included extreme poverty or low income, unemployment and problems related to living alone.
Methods
This was a retrospective observational study conducted within TriNetX, a global federated health research network with access to electronic medical records (EMRs) from participating healthcare organizations including academic medical centers, specialty physician practices and community hospitals covering ∼69.8 million individuals, mainly in the USA. Within this network, available data include demographics, diagnoses using International Classification of Diseases, Ninth Revision and Tenth Revision, Clinical Modification (ICD-10-CM) codes and medications. More information can be found online (https://trinetx.com/company-overview/).
Cohort
The research on the TriNetX online research platform was performed on 26 November 2022 for patients aged ≥18 years with AF or flutter (ICD-10-CM code I48). As previously described,17 the baseline index event was the first AF diagnosis reported into the TriNetX platform. Any medical diagnosis and cardiovascular procedures or treatment registered into the system within 5 years from the index event were considered as an individual baseline characteristic. At the time of the construction of the cohort, 64 participating healthcare organizations had data available for patients who met the characteristics of interest for the study.
Exposure
The patients were categorized as socially deprived or non-deprived based on presentation of three characteristics: (i) extreme poverty (ICD-10-CM Z59.5) and or with low income (ICD-10-CM Z59.6); (ii) unemployment (ICD-10-CM Z56.0); and (iii) problems related with living alone (ICD-10.CM Z 60.2).18,19 Hence, the patients with AF were categorized as socially deprived if they presented any of these characteristics, and patients without any of these characteristics were categorized as non-deprived. Furthermore, the presentation of the three SDoH characteristics was evaluated individually.
Outcomes
The primary outcome was the occurrence of a composite of major adverse cardiac events including all-cause death, stroke, IHD, HF, severe ventricular arrhythmias and hospitalization within 5 years after AF diagnosis. The choice of the components of this composite outcome was done to confirm in AF patients, the findings from previous studies that showed as SDoH are independent risk factors for such events in the general population.20–22 To better understand the specific weight associated with each indicator of SDoH, the secondary outcomes included each component of the composite outcome within 5 years after the index event. More detailed information about the ICD-10-CM codes utilized to identify the primary and secondary outcomes can be found in the Supplementary Table S1.
Statistical analysis
All statistical analyses were performed on the TriNetX online platform. Continuous variables were expressed as mean (±standard deviation [SD]) and compared by t-test for independent variables, while categorical variables were expressed as counts and percentages and compared by chi-squared test. We performed a balanced 1:1 propensity score matching (PSM) to create balanced cohorts. For the PSM, the following variables were considered: age, sex, ethnicity, comorbidities (hypertension, HF, IHD, cerebrovascular disease, diabetes mellitus, overweight/obesity, chronic kidney disease, neoplasms, dyslipidemia, pulmonary hypertension, chronic rheumatic heart disease and chronic lower respiratory diseases), procedures (cardiac catheterization, echocardiography, electrocardiogram, cardiovascular monitoring services and intracardiac electrophysiological studies) and cardiovascular medications (beta-blockers, diuretics, lipid-lowering agents, angiotensin-converting enzyme inhibitors, calcium channel blockers, antianginals, anticoagulants and platelet aggregation inhibitors). Diagnosis codes are available in the Supplementary Table S2. Standardized mean differences (Std. diff.) were used to show the distribution of demographic and clinical data among the groups and calculated as the difference in the means or proportions of a particular variable divided by the pooled estimate of SD for that variable. After PSM, any baseline characteristic with an Std. diff. <0.1 was considered well matched. We used Cox proportional hazard regression analysis to investigate the 5-year risk for the primary and secondary outcomes both before and after the PSM. The risk was estimated by hazard rate ratio (HR) with 95% confidence intervals (CIs). For the primary outcome between socially deprived and non-socially deprived AF patients, we also reported Kaplan–Meier curves, and survival distribution was compared with log-rank test. For the survival analysis, a two-sided P values <0.05 was considered statistically significant. All the analyses were performed on the TriNetX platform using R software v3.3.
Data availability and ethical approval
TriNetx is a research network utilized for several scientific purposes, compliant with the Health Insurance Portability and Accountability Act and the US federal law which protects the privacy and security of healthcare data, including de-identified data as per the de-identification standard of the HIPAA Privacy Rule (https://trinetx.com/real-world-resources/publications/). To gain access to the data in the TriNetX research network, requests are directed to TriNetX and a data sharing agreement is required. As a federated research network, studies using the TriNetX health research network do not need ethical approval as no patient identifiable identification is received. Further information about the data extraction from TriNetX is reported in the Supplementary Material.
Results
Baseline characteristics before propensity score matching
The initial cohorts consisted of 25 711 socially deprived patients with AF and 2 462 092 non-deprived patients with AF. The socially deprived cohort of patients with AF was composed of 10 510 (41%) unemployed, 3811 (14.7%) with extreme poverty or with a low income and 11 390 (44.3%) with problems related to living alone. A baseline comparison of the characteristics of patients with AF classified as socially deprived or non-deprived is shown in Table 1. The socially deprived patients were younger, more often female, Black or African American, and with a higher prevalence of cardiovascular risk factors than the non-deprived AF patients.
Table 1.
Baseline characteristics of patients with atrial fibrillation
| Before propensity score match |
After propensity score match |
|||||
|---|---|---|---|---|---|---|
| Socially deprived (n = 24 631) | Non-socially deprived (n = 2 462 092) | Std. diff. | Socially deprived (n = 24 627) | Non-socially deprived (n = 24 627) | Std. diff. | |
| Age, years (±SD) | 68.8 ± 16.0 | 75.5 ± 13.1 | 0.463 | 68.8 ± 15.9 | 69.1 ± 16.0 | 0.022 |
| Female | 12 760 (51.8) | 1 079 576 (43.8) | 0.160 | 12 756 (51.8) | 13 169 (53.5) | 0.034 |
| White | 17 837 (72.4) | 1 878 987 (76.3) | 0.089 | 17 835 (72.4) | 17 755 (72.1) | 0.007 |
| Black or African American | 3945 (16.0) | 227 991 (9.3) | 0.204 | 3943 (16.0) | 4051 (16.4) | 0.012 |
| Hypertension | 13 636 (55.4) | 806 136 (12.9) | 0.468 | 13 632 (55.4) | 13 435 (54.6) | 0.016 |
| Obesity | 6446 (26.2) | 210 325 (8.5) | 0.479 | 6446 (26.2) | 6637 (27.0) | 0.018 |
| Diabetes mellitus | 7675 (31.2) | 354 729 (14.4) | 0.408 | 7672 (31.2) | 7700 (31.3) | 0.002 |
| Chronic kidney disease | 4640 (18.8) | 208 390 (8.5) | 0.306 | 4638 (18.8) | 4622 (18.8) | 0.002 |
| Pulmonary hypertension | 2956 (40.0) | 604 055 (24.5) | 0.281 | 2953 (12.0) | 3034 (12.3) | 0.010 |
| Ischemic heart disease | 7617 (30.9) | 417 021 (16.9) | 0.332 | 7613 (24.2) | 7457 (30.3) | 0.014 |
| Heart failure | 5465 (22.2) | 256 172 (10.4) | 0.323 | 5463 (22.2) | 5470 (22.2) | 0.001 |
| Cerebrovascular diseases | 4978 (20.2) | 220 919 (9.0) | 0.322 | 4975 (20.2) | 4965 (20.2) | 0.001 |
| Chronic lower respiratory diseases | 6847 (27.8%) | 282 923 (11.5%) | 0.419 | 6843 (27.8) | 6965 (28.3) | 0.011 |
| Neoplasms | 8257 (33.5%) | 422 349 (17.2%) | 0.383 | 8253 (33.5) | 8281 (33.6) | 0.002 |
| Cardiac catheterization procedures | 2214 (9.0) | 104 810 (4.3) | 0.191 | 2213 (9.0) | 2202 (8.9) | 0.002 |
| Echocardiography procedures | 8007 (32.5) | 408 553 (16.6) | 0.376 | 8003 (32.5) | 7914 (32.1) | 0.008 |
| Electrocardiogram, routine ECG with at least 12 leads | 12 608 (51.2) | 650 076 (26.4) | 0.526 | 12 604 (51.2) | 12 476 (50.7) | 0.008 |
| Cardiovascular monitoring services | 1481 (6.0) | 74 781 (3.0) | 0.144 | 1480 (6.0) | 1441 (5.9) | 0.007 |
| Intracardiac electrophysiological procedures/studies | 376 (1.5) | 20 555 (0.8) | 0.064 | 376 (1.5) | 378 (1.5) | 0.001 |
| Lipid-lowering drugs | 9060 (42.5) | 672 266 (27.3) | 0.204 | 9059 (36.8) | 8693 (35.3) | 0.031 |
| Beta-blockers | 10 474 (47.6) | 743 364 (30.2) | 0.258 | 10 471 (42.5) | 10 159 (41.3) | 0.026 |
| Diuretics | 9330 (37.9) | 606 735 (24.6) | 0.288 | 9328 (37.9) | 9176 (37.3) | 0.013 |
| Calcium channel blockers | 6829 (27.7) | 444 972 (18.1) | 0.231 | 6826 (27.7) | 6682 (27.1) | 0.013 |
| ACE inhibitors | 6938 (25.4) | 397 944 (16.2) | 0.292 | 6936 (28.2) | 6843 (27.8) | 0.008 |
| Angiotensin II inhibitors | 3351 (13.6) | 259 172 (10.5) | 0.095 | 3351 (13.6) | 3237 (13.1) | 0.014 |
| Anticoagulants | 10 915 (44.3) | 664 504 (27.0) | 0.368 | 10 911 (44.3) | 10 622 (43.1) | 0.024 |
| Platelet aggregation inhibitors | 9833 (39.9) | 610 742 (24.8) | 0.327 | 9829 (39.9) | 9620 (39.1) | 0.017 |
ACE: angiotensin-converting enzyme; ECG: electrocardiogram; SD: standard deviation; Std. diff.: standardized mean difference.
Risk of adverse cardiovascular outcomes before propensity score matching
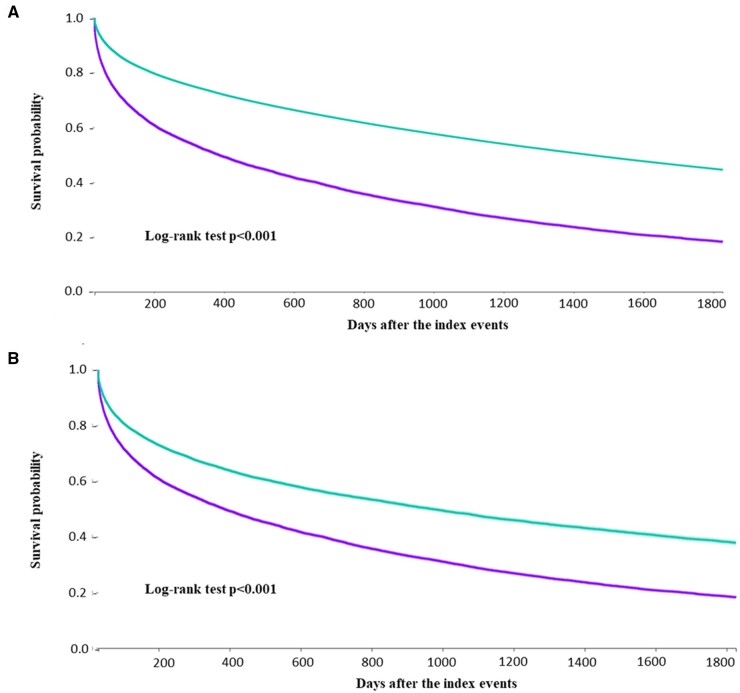
The number of outcome events reported within 5 years from the index event for patients with AF socially deprived when compared to those non-deprived, as shown in Table 2 before PSM. Cox regression analysis revealed that socially deprived patients with AF showed a higher 5-year risk of composite outcome (Figure 1, Panel A), all-cause death, hospitalization, IHD, stroke, HF and severe ventricular arrhythmias, as shown in Table 2.
Table 2.
Number and risk of adverse events before and after propensity score matching in socially deprived and non-deprived patients with atrial fibrillation
| Outcome | Before PSM |
After PSM |
||||
|---|---|---|---|---|---|---|
| Socially deprived (n = 24 631) | Non-deprived (n = 2 462 092) | HR (95% CI) | Socially deprived (n = 24 627) | Non-deprived (n = 24 627) | HR (95% CI) | |
| Composite outcome, n (%) | 18 163 (73.8%) | 1 145 707 (46.5%) | 1.90 (1.87–1.93) | 18 163 (73.8%) | 13 473 (54.7%) | 1.47 (1.44–1.50) |
| All-cause death, n (%) | 2893 (11.75%) | 183 310 (7.4%) | 1.34 (1.28–1.39) | 2893 (11.7%) | 1918 (7.8%) | 1.27 (1.20–1.34) |
| Hospitalization, n (%) | 14 402 (58.5%) | 794 359 (32.3%) | 2.01 (1.98–2.04) | 14 398 (58.5%) | 9939 (40.4%) | 1.51 (1.47–1.55) |
| Ischemic heart disease, n (%) | 12 728 (51.7%) | 778 981 (31.6%) | 1.67 (1.64–1.70) | 12 725 (35.6%) | 8800 (51.2%) | 1.44 (1.40–1.47) |
| Stroke, n (%) | 12 728 (51.7%) | 214 110 (8.7%) | 2.60 (2.51–2.64) | 5778 (23.5%) | 2886 (11.7%) | 1.88 (1.80–1.97) |
| Heart failure, n (%) | 5775 (23.4%) | 280 264 (11.4%) | 1.91 (1.86–1.96) | 5773 (23.4%) | 3653 (14.8%) | 1.44 (1.38–1.50) |
| Ventricular arrythmias, n (%) | 2875 (11.7%) | 140 045 (5.7%) | 1.83 (1.76–1.90) | 2875 (11.7%) | 1786 (7.3%) | 1.42 (1.34–1.51) |
PSM: propensity score matching; HR: hazard ratio; CI: confidence interval.
Figure 1.
Kaplan–Meier curves for the risk of primary outcome in socially deprived and non-socially deprived patients, before (A) and after (B) propensity score matching.
Propensity score-matched analyses
After PSM on a 1:1 ratio for the comparison between socially deprived and non-deprived patients 24 627 AF patients were included in each group. The standardized mean difference for all the variables assessed showed no substantial difference between the two groups (Table 1). The numbers of outcome events reported within 5 years from the index event for patients with AF socially deprived when compared to those non-deprived are shown in Table 2.
Cox regression analysis on the PSM cohort also found that socially deprived patients with AF had a higher 5-year risk of the composite outcome (Figure 1, Panel B), all-cause death, hospitalization, IHD, stroke, HF and severe ventricular arrhythmias.
The 5-year risk of adverse events was evaluated according to each component of the socially deprived AF groups compared to AF patients non-deprived after PSM 1:1. The number of patients considered were: 10 488 unemployed, 3791 with extreme poverty or with low income and 11 390 with problems related to living alone. The number of composite events in each subgroup of socially deprived patients and controls was: 8014 (76.4%) and 5658 (53.9%) in the unemployed, 8030 (70.8%) and (55.2%) in those with problems related to living alone.
The PSM HRs for the primary composite outcome were 1.54 (95% CI 1.49–1.60) for the unemployed AF patients, 1.39 (95% CI 1.31–1.47) for patients with extreme poverty or with low income and 1.42 (95% CI 1.37–1.47) for those with problems related with living alone.
Discussion
The present study found that socially deprived patients with AF have a higher 5-year risk of all-cause death, hospitalization and cardiovascular events compared to those non-socially deprived, even after PSM. These findings were robust across all investigated domains of SDoH, including unemployment, extreme poverty, low income and having problems related to living alone.
The 34% higher risk of all-cause death we found in socially deprived AF patients from our population confirms the findings of previous studies performed in different AF cohorts. Indeed, a retrospective study performed on 4503 AF patients from the USA with a mean follow-up of 4.5 years, found that low socio-economic status was associated with a 30% increased risk of all-cause death (odds ratio 1.30, 95% CI 1.1–1.5).23 In a study population of 12 283 Swedish AF patients followed for 3.5 years, patients living in low socio-economic status neighborhoods showed a 49% higher risk of all-cause death compared to patients living in high socio-economic status neighborhoods (HR 1.49, 95% CI 1.13–1.96)24; whereas a nationwide register-based follow-up study performed in Denmark on 150 544 patients showed that after 1 year of follow-up, the risk of all-cause death in AF patients with low socio-economic status was 64% higher than those with better socio-economic conditions (HR 1.64, 0.61–0.68).25 Thus, compared to these results, our study suggests the presence of a wide heterogeneity of the impact of low socio-economic status in determining the risk of death in different geographical areas, which could be related to several factors such as the presence of health insurance-based health systems, different welfare policies and the presence of economic and social supports to the poorest of the populations.
The reasons behind the higher risk of mortality in socially deprived patients with AF are complex and involve the interaction among social, political and traditional risk factors. Patients with low socio-economic status have a higher chance of having health illiteracy, and thus to do not understand the importance of healthy lifestyles, including healthy diet, regular exercise and avoiding smoking; and to be less compliant with the recommended pharmacotherapies, and the required medical follow-up needed to early recognize the onset of symptoms and signs associated with cardiovascular diseases.13 The close relationship between SDoH and the risk of cardiovascular events we found in our study seems to confirm these hypotheses. Indeed, we identified a social gradient in cardiovascular morbidity in AF patients, where unemployment, extreme poverty, low income and having problems related to living alone were associated with a higher 5-year risk of stroke, HF, IHD and hospitalization. This confirms the findings of a Nationwide Population-Based Cohort Study performed on 317 017 Korean AF patients, where those with low socio-economic status were associated with a higher risk of emergency department visit, 30- and 90-day mortality and rehospitalization after the demission,26 and the higher risk of stroke (HR 1.17, 95% CI 1.05–1.30) and myocardial infarction (HR 1.18, 95% CI 0.98–1.41) described in AF patients with low income compare to those with high income, as shown in a large insurance database study on more than 300 000 AF patients from the USA.27
In our cohort, each SDoH component (unemployment, extreme poverty, low income and having problems related to living alone) was associated with a different effect size in determining the risk of composite outcome in AF patients, suggesting that the underlying mechanisms behind the high risk of adverse events in socially deprived patients may be heterogeneous and require future detailed evaluation.28 This is even more important considering the Atrial fibrillation Better Care (ABC) pathway advocated by the last international guidelines29,30 and the compliance which was associated with a significative reduction of the risk of hospitalization, cardiovascular events, and all-cause death in different AF populations.31,32 The ABC pathway is an integrated clinical approach based on three main pillars: ‘A’ avoid stroke with oral anticoagulation; ‘B’ better management of the symptoms with a patient-centered symptom-directed decisions on rate or rhythm control; and ‘C’ Cardiovascular risk factor optimization and lifestyle changes.33 One of the pivotal concepts of this holistic approach is that the risk of adverse events in AF patients already anticoagulated is due to the coexistence of other cardiovascular risk factors that often coexist in those patients. In light of the results of this study, SDoHs should be considered in the same way as a traditional risk factor and thus utilized for a more accurate risk stratification. Furthermore, targeted patient education and information to those who are socially deprived may influence other patient-related factors related to the health outcome (e.g. lifestyle behavior, persistence to prescribed medications and underuse of health services). Hence, SDoH could represent a future possible target for such integrated care approaches to be considered together with the classical cardiovascular risk factors, with the aim of reducing the risk of morbidity and mortality in AF patients.
Limitations
There are several limitations to consider while interpreting the results. Healthcare organization EMR data are subject to entry errors and data gaps, and some diagnoses may be underreported, while outcomes that occurred outside the network may have not been well captured and only those with an obvious social deprivation at the hospitalization may be registered as such. This resulted in only 1% of the cohort being registered as socially deprived according to any of the three exposures and could imply some under-detection, misclassification or selection bias. Moreover, administrative data may fail to identify a relatively significant proportion of patients with AF and thus may bias estimates between SDoH and prognosis. However, it would be expected that the patients not captured by the database would be even worse off regarding both SDoH and health outcomes, meaning that what is presented in this study may just be the tip of the iceberg. The fact that low income and extreme poverty are related to an increased risk of hospitalization implies that the costs do not affect the willingness to seek hospitalization, or that the actual disparity in the need for hospitalization is even bigger than identified in the present study. Furthermore, the study is limited by the inability to stratify the analysis according to sex or ethnicity to identify possible different patterns in the social disparities in the clinical outcomes after AF among men and women as well as in different ethnic groups.
Another possible limitation of this study is the lack of statistical analysis aimed at assessing the changes in SDoH over time. SDoH are dynamic entities that should be confirmed in different time frames. We considered as baseline characteristics the information reported before the index event and cannot exclude that some of the patients considered in the socially deprived groups ameliorated their condition over time and vice versa.
Conclusion
In patients with AF, social deprivation is associated with an increased risk of death and adverse cardiac events. There is a need for the implementation of strategies to eliminate health inequalities among AF patients.
Supplementary Material
Contributor Information
A H Simoni, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Clinical Medicine, Danish Center for Health Services Research, Aalborg University, Aalborg, Denmark.
T Bucci, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of General and Specialized Surgery, Sapienza University of Rome, Rome, Italy.
G F Romiti, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy.
J Frydenlund, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Clinical Medicine, Danish Center for Health Services Research, Aalborg University, Aalborg, Denmark.
S P Johnsen, Department of Clinical Medicine, Danish Center for Health Services Research, Aalborg University, Aalborg, Denmark.
A H Abdul-Rahim, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Stroke Division, Department of Medicine for Older People, Whiston Hospital, St Helens and Knowsley Teaching Hospitals NHS Trust, Prescot, UK.
G Y H Lip, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Clinical Medicine, Danish Center for Health Services Research, Aalborg University, Aalborg, Denmark.
Supplementary material
Supplementary material is available at QJMED online.
Author contributions
Amalie H. Simoni (Conceptualization [equal], Writing—original draft [equal]), Tommaso Bucci (Conceptualization [equal], Formal analysis [equal], Investigation [equal], Writing—original draft [equal]), Giulio Francesco Romiti (Writing—review & editing [equal]), Juliane Frydenlund (Writing—review & editing [equal]), Søren Paaske Johnsen (Writing—review & editing [equal]), Azmil H. Abdul-Rahim (Supervision [equal], Validation [equal], Writing—original draft [equal]) and Gregory Y.H. Lip (Supervision [equal], Validation [equal], Writing—original draft [equal])
Conflict of interest
G.F.R. reports consultancy for Boehringer Ingelheim and an educational grant from Anthos, outside the submitted work. No fees are directly received personally. G.Y.H.L. is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Anthos and Daiichi-Sankyo. No fees are received personally. G.Y.H.L. and S.P.J. are co-principal investigators of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 899871. S.P.J. has been consultant for BMS/Pfizer. All other authors report no disclosures.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Lunde ED, Nielsen PB, Riahi S, Larsen TB, Lip GYH, Fonager K, et al Associations between socioeconomic status, atrial fibrillation, and outcomes: a systematic review. Expert Rev Cardiovasc Ther 2018; 16:857–73. [DOI] [PubMed] [Google Scholar]
- 2. Lunde ED, Joensen AM, Fonager K, Lundbye-Christensen S, Johnsen SP, Larsen ML, et al. Socioeconomic inequality in oral anticoagulation therapy initiation in patients with atrial fibrillation with high risk of stroke: a register-based observational study. BMJ Open 2021; 11:e048839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akerkar R, Ebbing M, Sulo G, Ariansen I, Igland J, Tell GS, et al. Educational inequalities in mortality of patients with atrial fibrillation in Norway. Scand Cardiovasc J 2017; 51:82–7. [DOI] [PubMed] [Google Scholar]
- 4. Johnsen SP, Dalby LW, Tackstrom T, Olsen J, Fraschke A.. Cost of illness of atrial fibrillation: a nationwide study of societal impact. BMC Health Serv Res 2017; 17:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, et al National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation 2014; 129:1407–14. [DOI] [PubMed] [Google Scholar]
- 6. Cressman AM, Macdonald EM, Yao Z, Austin PC, Gomes T, Paterson JM, et al. ; Canadian Drug Safety and Effectiveness Research Network (CDSERN). Socioeconomic status and risk of hemorrhage during warfarin therapy for atrial fibrillation: a population-based study. Am Heart J 2015; 170:133–40, 140.e1–3. [DOI] [PubMed] [Google Scholar]
- 7. Christesen AMS, Vinter N, Mortensen LS, Fenger-Gron M, Johnsen SP, Frost L.. Inequality in oral anticoagulation use and clinical outcomes in atrial fibrillation: a Danish nationwide perspective. Eur Heart J Qual Care Clin Outcomes 2018; 4:189–99. [DOI] [PubMed] [Google Scholar]
- 8. Vinter N, Huang Q, Fenger-Gron M, Frost L, Benjamin EJ, Trinquart L.. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): community based cohort study. BMJ 2020; 370:m2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vinter N, Fawzy AM, Gent D, Ding WY, Johnsen SP, Frost L, et al. Social determinants of health and cardiovascular outcomes in patients with heart failure. Eur J Clin Invest 2022; 52:e13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lunde ED, Joensen AM, Lundbye-Christensen S, Fonager K, Paaske Johnsen S, Larsen ML, et al. Socioeconomic position and risk of atrial fibrillation: a nationwide Danish cohort study. J Epidemiol Community Health 2020; 74:7–13. [DOI] [PubMed] [Google Scholar]
- 11. Christensen AV, Koch MB, Davidsen M, Jensen GB, Andersen LV, Juel K.. Educational inequality in cardiovascular disease depends on diagnosis: a nationwide register based study from Denmark. Eur J Prev Cardiol 2016; 23:826–33. [DOI] [PubMed] [Google Scholar]
- 12. Giesinger I, Goldblatt P, Howden-Chapman P, Marmot M, Kuh D, Brunner E.. Association of socioeconomic position with smoking and mortality: the contribution of early life circumstances in the 1946 birth cohort. J Epidemiol Community Health 2014; 68:275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galobardes B, Lynch J, Smith GD.. Measuring socioeconomic position in health research. Br Med Bull 2007; 81–82:21–37. [DOI] [PubMed] [Google Scholar]
- 14. Parnia A, Siddiqi A.. Socioeconomic disparities in smoking are partially explained by chronic financial stress: marginal structural model of older US adults. J Epidemiol Community Health 2020; 74:248–54. [DOI] [PubMed] [Google Scholar]
- 15. Singh GK, Daus GP, Allender M, Ramey CT, Martin EK, Perry C, et al Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS 2017; 6:139–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019; 74:1376–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ntaios G, Sagris D, Buckley BJR, Harrison SL, Abdul-Rahim A, Austin P, et al. Risk of myocardial infarction and ischemic stroke in individuals with first-diagnosed paroxysmal vs. non-paroxysmal atrial fibrillation under anticoagulation. Europace 2023; 25:euad143. 10.1093/europace/euad143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo Y, Chen Z, Xu K, George TJ, Wu Y, Hogan W, et al. International Classification of Diseases, Tenth Revision, Clinical Modification social determinants of health codes are poorly used in electronic health records. Medicine (Baltimore) 2020; 99:e23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis JH, Whelihan K, Navarro I, Boyle KR; SDH Card Study Implementation Team. Community health center provider ability to identify, treat and account for the social determinants of health: a card study. BMC Fam Pract 2016; 17:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zulman DM, Maciejewski ML, Grubber JM, Weidenbacher HJ, Blalock DV, Zullig LL, et al. Patient-reported social and behavioral determinants of health and estimated risk of hospitalization in high-risk veterans affairs patients. JAMA Netw Open 2020; 3:e2021457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reshetnyak E, Ntamatungiro M, Pinheiro LC, Howard VJ, Carson AP, Martin KD, et al. Impact of multiple social determinants of health on incident stroke. Stroke 2020; 51:2445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Acquah I, Hagan K, Javed Z, Taha MB, Valero-Elizondo J, Nwana N, et al. Social determinants of cardiovascular risk, subclinical cardiovascular disease, and cardiovascular events. J Am Heart Assoc 2023; 12:e025581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kargoli F, Shulman E, Aagaard P, Briceno DF, Hoch E, Di Biase L, et al. Socioeconomic status as a predictor of mortality in patients admitted With atrial fibrillation. Am J Cardiol 2017; 119:1378–81. [DOI] [PubMed] [Google Scholar]
- 24. Wandell P, Carlsson AC, Gasevic D, Sundquist J, Sundquist K.. Neighbourhood socio-economic status and all-cause mortality in adults with atrial fibrillation: a cohort study of patients treated in primary care in Sweden. Int J Cardiol 2016; 202:776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagengaard L, Andersen MP, Polcwiartek C, Larsen JM, Larsen ML, Skals RK, et al. Socioeconomic differences in outcomes after hospital admission for atrial fibrillation or flutter. Eur Heart J Qual Care Clin Outcomes 2021; 7:295–303. [DOI] [PubMed] [Google Scholar]
- 26. Lee SY, Lee SR, Choi EK, Han KD, Oh S, Lip GYH.. Impact of socioeconomic status on emergency department visits in patients With atrial fibrillation: a nationwide population-based cohort study. J Am Heart Assoc 2022; 11:e027192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LaRosa AR, Claxton J, O'Neal WT, Lutsey PL, Chen LY, Bengtson L, et al. Association of household income and adverse outcomes in patients with atrial fibrillation. Heart 2020; 106:1679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Essien UR, Kornej J, Johnson AE, Schulson LB, Benjamin EJ, Magnani JW.. Social determinants of atrial fibrillation. Nat Rev Cardiol 2021; 18:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42:373–498. [DOI] [PubMed] [Google Scholar]
- 30. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH, et al 2021 Focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost 2022; 122:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, et al. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: impact on clinical outcomes—a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 2022; 122:406–14. [DOI] [PubMed] [Google Scholar]
- 32. Bucci T, Proietti M, Shantsila A, Romiti GF, Teo WS, Park HW, et al; APHRS-AF Registry Investigators. Integrated care for atrial fibrillation using the ABC pathway in the prospective APHRS-AF registry. JACC Asia 2023; 3:580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017; 14:627–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
TriNetx is a research network utilized for several scientific purposes, compliant with the Health Insurance Portability and Accountability Act and the US federal law which protects the privacy and security of healthcare data, including de-identified data as per the de-identification standard of the HIPAA Privacy Rule (https://trinetx.com/real-world-resources/publications/). To gain access to the data in the TriNetX research network, requests are directed to TriNetX and a data sharing agreement is required. As a federated research network, studies using the TriNetX health research network do not need ethical approval as no patient identifiable identification is received. Further information about the data extraction from TriNetX is reported in the Supplementary Material.
The data underlying this article will be shared upon reasonable request to the corresponding author.