Key Points
Question
Do children who resolve dyslipidemic non–high-density lipoprotein cholesterol (non–HDL-C) levels by adulthood have attenuated risk of developing cardiovascular disease (CVD) events?
Findings
Among 5121 children pooled from 6 prospective cohort studies in the US and Finland (mean follow-up, 8.9 years after age 40 years), individuals who had dyslipidemic non–HDL-C in childhood that resolved during adulthood did not have a significantly increased risk of fatal or nonfatal CVD events (hazard ratio, 1.13) than those whose non–HDL-C levels were persistently within the guideline-recommended range in childhood and adulthood.
Meaning
Children in whom dyslipidemic non–HDL-C resolves by adulthood have similar risk of CVD to those who never had dyslipidemia.
Abstract
Importance
Elevated non–high-density lipoprotein cholesterol (non–HDL-C; a recommended measure of lipid-related cardiovascular risk) is common in children and increases risk of adult cardiovascular disease (CVD). Whether resolution of elevated childhood non–HDL-C levels by adulthood is associated with reduced risk of clinical CVD events is unknown.
Objective
To examine the associations of non–HDL-C status between childhood and adulthood with incident CVD events.
Design, Setting, and Participants
Individual participant data from 6 prospective cohorts of children (mean age at baseline, 10.7 years) in the US and Finland. Recruitment took place between 1970 and 1996, with a final follow-up in 2019.
Exposures
Child (age 3-19 years) and adult (age 20-40 years) non–HDL-C age- and sex-specific z scores and categories according to clinical guideline–recommended cutoffs for dyslipidemia.
Main Outcomes and Measures
Incident fatal and nonfatal CVD events adjudicated by medical records.
Results
Over a mean length of follow-up of 8.9 years after age 40 years, 147 CVD events occurred among 5121 participants (60% women; 15% Black). Both childhood and adult non–HDL-C levels were associated with increased risk of CVD events (hazard ratio [HR], 1.42 [95% CI, 1.18-1.70] and HR, 1.50 [95% CI, 1.26-1.78] for a 1-unit increase in z score, respectively), but the association for childhood non–HDL-C was reduced when adjusted for adult levels (HR, 1.12 [95% CI, 0.89-1.41]). A complementary analysis showed that both childhood non–HDL-C levels and the change between childhood and adulthood were independently associated with the outcome, suggesting that from a preventive perspective, both childhood non–HDL-C levels and the change into adulthood are informative. Compared with those whose non-HDL-C levels remained within the guideline-recommended range in childhood and adulthood, participants who had incident non–HDL-C dyslipidemia from childhood to adulthood and those with persistent dyslipidemia had increased risks of CVD events (HR, 2.17 [95% CI, 1.00-4.69] and HR, 5.17 [95% CI, 2.80-9.56], respectively). Individuals who had dyslipidemic non–HDL-C in childhood but whose non-HDL-C levels were within the guideline-recommended range in adulthood did not have a significantly increased risk (HR, 1.13 [95% CI, 0.50-2.56]).
Conclusions and Relevance
Individuals with persistent non–HDL-C dyslipidemia from childhood to adulthood had an increased risk of CVD events, but those in whom dyslipidemic non–HDL-C levels resolve by adulthood have similar risk to individuals who were never dyslipidemic. These findings suggest that interventions to prevent and reduce elevated childhood non–HDL-C levels may help prevent premature CVD.
This study of individual participant data from 6 pooled cohorts assesses whether resolution of elevated childhood non–high-density lipoprotein cholesterol (non–HDL-C) by adulthood is associated with reduced risk of clinical cardiovascular disease (CVD) events.
Introduction
Primordial and primary prevention is considered the cornerstone for reducing risk of cardiovascular disease (CVD) later in life.1,2 Dyslipidemia is common in children3 and is well established as a causal risk factor of atherosclerotic CVD.4 In 2011, guidelines sponsored by the National Heart, Lung, and Blood Institute (NHLBI) recommended universal screening of lipid levels in children and adolescents.5 Nevertheless, the value of lipid screening in childhood and early interventions to manage adverse lipid levels has been a point of considerable debate over several decades, due largely to the absence of direct evidence linking childhood lipid levels and their changes into adulthood with CVD events. This debate has been intensified by the most recent US Preventive Services Task Force lipid screening guidelines for children and adolescents, which did not find evidence for or against screening.6
Assessment of non–high-density lipoprotein cholesterol (non–HDL-C) is recommended by the NHLBI-sponsored guidelines and the latest American Heart Association clinical guidelines as a screening tool to identify the pediatric population at increased risk of CVD5,7,8 because it offers a simpler and more accurate measure of lipid-related atherosclerotic CVD risk than low-density lipoprotein cholesterol (LDL-C).9,10 Likewise, data from the Framingham Offspring Study, reinforced by a recent state-of-the-art review, underline the utility of measuring non–HDL-C in young adults for predicting future CVD events.11,12 By using data from the International Childhood Cardiovascular Cohort (i3C) Consortium, we previously reported that elevated non–HDL-C levels in childhood were associated with high carotid intima-media thickness, a subclinical marker of atherosclerosis, in adulthood.13 Moreover, we found that children in whom non–HDL-C dyslipidemia resolved by adulthood have a similar risk of high carotid intima-media thickness than children who never had non–HDL-C dyslipidemia.13 More recently, we have reported that childhood CVD risk factors (total cholesterol, triglycerides, body mass index, smoking, and blood pressure) and the change in risk score based on these factors were associated with adult risk of CVD events.14 Nonetheless, whether childhood non–HDL-C levels contribute to long-term risk of actual CVD events, independent of adult non–HDL-C, has not been previously examined. From a preventive perspective, it is important to determine whether childhood dyslipidemic non–HDL-C increases CVD risk independent of adult non–HDL-C levels and whether resolving dyslipidemia by adulthood reverses the otherwise adverse childhood effects.
Herein, we pooled individual participant data of 6 cohorts from the i3C Consortium to determine (1) whether the association of childhood non–HDL-C with CVD events is independent of adult non–HDL-C levels or independent of the change in non–HDL-C levels from childhood to adulthood and (2) whether resolution of elevated childhood non–HDL-C by adulthood is associated with attenuated risk of developing CVD events in adulthood.
Methods
Study Design and Participants
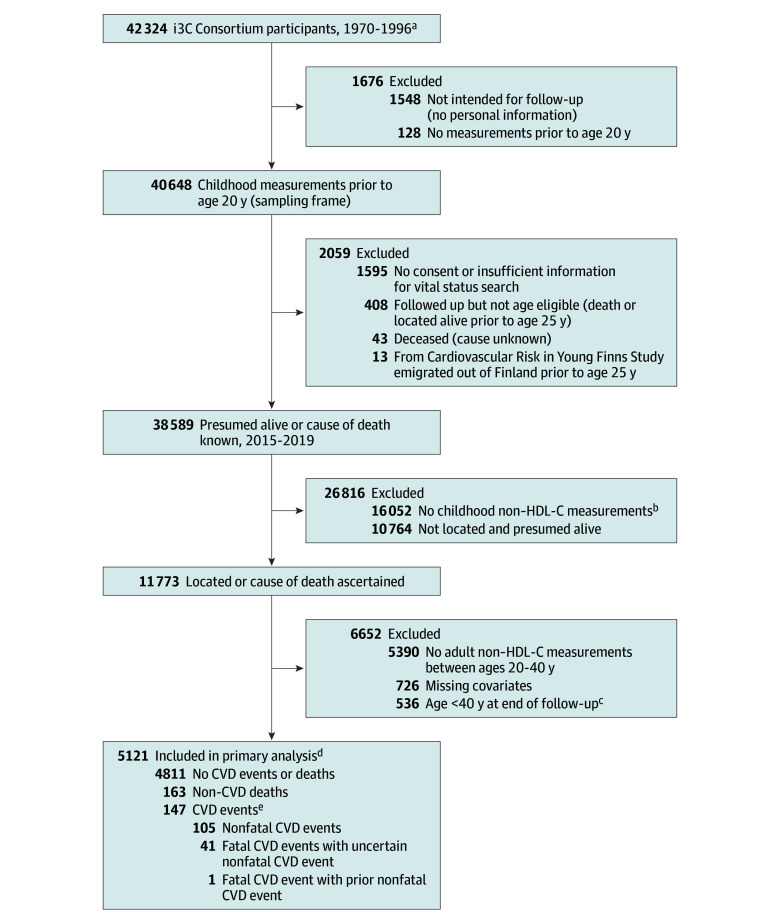
This study was approved by the institutional review boards at each site of the study cohorts. Written informed consent by participants’ legal guardians with oral assent by participants were obtained for childhood visits and by participants for in-person adult visits, and oral consent under waiver of documentation of consent was obtained for the recent follow-up questionnaire. The current study was conducted in accordance with the Declaration of Helsinki15 and is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies. Participants were drawn from 7 prospective cohorts participating in the i3C Consortium.14,16 These included 5 cohorts from the US (Bogalusa Heart Study, Minnesota Childhood Cardiovascular Cohorts, Muscatine Study, NHLBI Growth and Health Study, and Princeton Lipid Research Study), and 1 each from Finland (Cardiovascular Risk in Young Finns Study) and Australia (Childhood Determinants of Adult Health Study). Study characteristics and methods have been described in detail elsewhere.14,16 Of the 42 324 participants enrolled in the 7 cohorts at baseline between 1970 and 1996, 1548 did not have personal information for follow-up and 128 did not have any childhood measurements. Of the remaining 40 648 participants (sampling frame), 38 589 participants were presumed alive or had a known cause of death, of whom 11 773 participants had childhood non–HDL-C measures and were successfully located for ascertainment of nonfatal CVD events or had information on cause of death. Finally, 5390 participants did not have adult non–HDL-C data between ages 20 and 40 years (see Statistical Analysis section), 726 had missing childhood and/or adulthood covariates, and 536 were younger than 40 years at the end of follow-up. As a result, 5121 participants were included in the primary analysis (Figure 1). Final follow-up was conducted in 2019. Notably, the Australian cohort was included in the initial sampling frame but was excluded from the primary analysis because no participants were retained against the exclusion criteria.
Figure 1. Study Cohort Determination.
CVD indicates cardiovascular disease; i3C, International Childhood Cardiovascular Cohort; and non–HDL-C, non–high-density lipoprotein cholesterol.
aDates of first visits ranged from 1970 to 2007 (in the Minnesota Childhood Cardiovascular Cohorts, 1 set of participants was first recruited in 1995-1996, but 438 siblings of participants had their first study visits between 1997 and 2007).
bExclusion of lipid (or HDL-C) testing in the original study protocols of 2 cohorts in the i3C Consortium is the primary reason for no childhood non–HDL-C measurements. Of the 16 052 participants without childhood non–HDL-C measurements, 14 409 (90%) were from the Childhood Determinants of Adult Health Study (CDAH) and the Muscatine Study. In CDAH, due to predetermined criteria based on age and logistical constraints, only 21% of participants (1738/8426) had blood samples collected at the only childhood visit in 1985. The Muscatine cohort began in 1970, and the absence of HDL-C measurement prior to 1974 resulted in a reduced subset of children eligible for inclusion (2121/9842) in the current analysis based on the need for HDL-C to calculate non–HDL-C.
cAge at end of follow-up indicates age at time of event, at time of non–CVD death, or at end of follow-up, whichever occurred first.
dSee Table for participant groups by change in non–HDL-C status from childhood to adulthood. There were 7 combined cohorts, with the Australian cohort (Childhood Determinants of Adult Health) ultimately entirely excluded from analysis.
eIncident CVD included the first occurrence of adjudicated myocardial infarction, stroke, transient ischemic attack, ischemic heart failure, angina, peripheral artery disease, carotid intervention, abdominal aortic aneurysm, or coronary revascularization, as well as CVD deaths. Adjudication of deidentified medical records for CVD events was conducted by 2 physicians blinded to participant identity, with disagreements settled by the adjudication committee (eAppendix in Supplement 1).
Exposure and Covariates
Standardized questionnaires and laboratory protocols were used to collect childhood (age 3-19 years) and adulthood (age ≥20 years) information on the following variables: total cholesterol and HDL-C levels from fasting samples (non–HDL-C was calculated as total cholesterol minus HDL-C) (eAppendix and eTable 1 in Supplement 1), age, sex, smoking habits, lipid-lowering medications (age at the first reported use), type 2 diabetes, blood pressure, and body mass index (calculated as weight in kilograms divided by height in meters squared). Race data were derived from self-reports during the 2015-2019 Heart Health Survey and existing information collected from each cohort, which was subsequently harmonized into standard categories across all cohorts. For analysis, these categories were simplified to Black and non-Black, reflecting the composition of the study population, wherein less than 5% identified with races other than of Black or White. This categorization was chosen as a covariate in our analyses acknowledging race as a social construct and its role in mediating social disadvantages, health care access, and treatment decisions that may affect disease outcomes.17 Given incomplete data on and lack of diversity in Hispanic ethnicity, it was not included in our analyses. These details have been described in the eAppendix in Supplement 1.13,14,18,19,20
Fatal and Nonfatal CVD Events
From 2015 to 2019, we conducted a coordinated study to locate and survey participants of all i3C Consortium cohorts and to search national death indexes for those who were unable to be located. This study did not attempt to systematically obtain or adjudicate records for CVD events that were reported to occur before age 25 years. The classification and adjudication of CVD events are described in detail in the eAppendix in Supplement 1.14 Nonfatal CVD events included the first occurrence of adjudicated myocardial infarction, stroke, transient ischemic attack, ischemic heart failure, angina, peripheral artery disease, carotid intervention, abdominal aortic aneurysm, and coronary revascularization. Fatal and nonfatal CVD events were combined for analysis.
Statistical Analysis
To account for immortal time bias,21 landmark analysis22 was performed wherein the starting follow-up time for events was set at age 40 years (the landmark time) because it is a widely accepted cutoff for young adulthood in clinical guidelines. Non–HDL-C measurements taken between ages 20 and 40 years were used to define adulthood non–HDL-C exposures. As described in our recent publication,14 because of age-related developmental changes, childhood (age 3-19 years) non–HDL-C levels at each visit were standardized as age- and sex-specific z scores (eAppendix and eTable 2 in Supplement 1). The mean of resulting z scores across childhood measurements for each participant was then calculated to obtain a single mean z score for analysis. A single mean z score for adult measurements between ages 20 and 40 years was generated using the same approach (eAppendix in Supplement 1). The Pearson correlation coefficient between childhood and adult non–HDL-C z scores was estimated. To translate the results of the analyses to clinical practice, a single mean of the raw values for each participant, derived from all available measurements in childhood and adulthood, respectively, was used to classify participants into 4 groups for the change in non–HDL-C status between childhood and adulthood. These classifications were based on clinical guideline–recommended cutoffs5: persistent dyslipidemia (dyslipidemia in both childhood and adulthood), incident dyslipidemia (nondyslipidemia in childhood, dyslipidemia in adulthood), resolution (dyslipidemia in childhood, nondyslipidemia in adulthood), and persistent levels within the guideline-recommended range (nondyslipidemia in both childhood and adulthood). We further considered all major combinations of child and adult non–HDL-C status groups (ie, within the guideline-recommended range, elevated, and dyslipidemia at both time points), with participants being categorized into 9 groups. For childhood, the cutoffs for elevated non–HDL-C levels and dyslipidemia were 120 mg/dL (3.10 mmol/L) or greater and 145 mg/dL (3.75 mmol/L) or greater, respectively. In adulthood, the corresponding cutoffs were 150 mg/dL (3.88 mmol/L) or greater and 190 mg/dL (4.91 mmol/L) or greater.5 Incidence rate differences with 95% CIs between groups for incident CVD events were calculated using Poisson regression.
We used cause-specific hazard models to estimate hazard ratios (HRs) for associations of childhood and adult non–HDL-C z scores and change in non–HDL-C status (variables defined above) with incident CVD events. To account for selection bias, inverse probability weighting23 was applied to all analyses. We used age as the time axis, with the origin for time to event set at age 40 years to account for immortal time bias,22 and non-CVD mortality as a competing risk.24 Two different but equivalent parameterizations were made to account for adult non–HDL-C z score when examining the association of childhood z score; one in which the childhood z score was paired with the adult z score, and one in which the childhood z score was paired with the change in z score between childhood and adulthood (eAppendix in Supplement 1). All analyses were stratified by cohort and adjusted for mean age at and calendar year of childhood measurement, childhood mean age- and sex-specific z scores for body mass index, systolic blood pressure, smoking, sex, and Black race. The interaction between childhood and adult non–HDL-C status was tested by a product term that treated the categorical non–HDL-C as continuous variables in the model after assigning a median value for each category. The proportionality assumption (the time dependence of the exposure in the hazard function) was checked by including interactions of the exposure (the 4 groups defined above) and time (log[time]), and the hazards did not vary by participant age during follow-up.
To further examine the incremental value of childhood non–HDL-C levels in predicting incident CVD, the Harrell C index and category-free net reclassification index were estimated for 2 comparisons of models (eAppendix in Supplement 1). From a retrospective perspective, childhood non–HDL-C z score was added to a model with adult non–HDL-C z score (comparison 1). From a prospective/preventive perspective, childhood non–HDL-C z score was added to a model with the change in non–HDL-C z score between childhood and adulthood (comparison 2). All comparisons included the above-mentioned covariates.
Subgroup analysis was performed for the main analysis (ie, the 4 non–HDL-C categories defined above) by baseline age (categorized into 2 groups,14 3-11.99 years and 12-19.99 years), sex, race (Black vs non-Black), and cohort when possible. Sensitivity analyses were performed: (1) adult use of lipid-lowering medications and type 2 diabetes were additionally adjusted; (2) among participants with available data, we additionally adjusted for parental education as a marker of socioeconomic status; (3) a single-measure approach was used instead of mean value to define the change in non–HDL-C status (eAppendix in Supplement 1); (4) individuals who ever used lipid-lowering medications were excluded; and (5) Fine-Gray hazard models were used instead of cause-specific hazard models.24 Analyses were performed in Stata version 17.0 (StataCorp) and R version 4.2.2 (R Foundation). A 2-tailed P < .05 was considered statistically significant.
Results
Childhood characteristics according to the change in non–HDL-C status between childhood and adulthood are presented in the Table. The median age at first childhood visit for non–HDL-C measurements (baseline) was 10.7 years. Over a mean length of follow-up of 8.9 years after age 40 years, 147 fatal or nonfatal CVD events occurred among 5121 participants. From childhood to adulthood, 937 (18%) had resolution of dyslipidemia, 252 (5%) had persistent dyslipidemia, 158 (3%) had incident dyslipidemia, and 3774 (74%) had non–HDL-C levels that were persistently within the guideline-recommended range. Childhood characteristics among those with or without fatal or nonfatal CVD events are presented in eTable 3 in Supplement 1.
Table. Characteristics of Participants by Change in Non–HDL-C Status Between Childhood and Adulthood.
| Characteristics | Non–HDL-C status between childhood and adulthooda | |||
|---|---|---|---|---|
| Resolution (n = 937) | Persistent dyslipidemia (n = 252) | Incident dyslipidemia (n = 158) | Persistently in guideline-recommended range (n = 3774) | |
| Age at first visit for non–HDL-C measurement, median (IQR), y | 9.7 (6.4-15.1) | 12.1 (7.2-15.4) | 9.9 (6.8-12.7) | 10.5 (7.5-13.5) |
| Sex, No. (%) | ||||
| Female | 636 (67.9) | 99 (39.3) | 57 (36.1) | 2271 (60.2) |
| Male | 301 (32.1) | 153 (60.7) | 101 (63.9) | 1503 (39.8) |
| Race, No. (%)b | ||||
| American Indian or Alaska Native | 0 | 0 | 0 | 3 (0.1) |
| Black or African American | 37 (4.0) | 15 (6.0) | 18 (11.4) | 702 (18.6) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 1 (0.03) |
| White | 899 (95.9) | 236 (93.6) | 139 (88.0) | 3053 (80.9) |
| More than 1 race | 1 (0.1) | 1 (0.4) | 1 (0.6) | 15 (0.4) |
| Cohort (country), No. (%) | ||||
| Bogalusa Heart Study (US) | 59 (6.3) | 30 (11.9) | 79 (50.0) | 1605 (42.5) |
| Minnesota Childhood Cardiovascular Cohorts (US) | 5 (0.5) | 0 | 3 (1.9) | 86 (2.3) |
| Muscatine Study (US) | 6 (0.6) | 1 (0.4) | 2 (1.3) | 85 (2.3) |
| NHLBI Growth and Health Study (US) | 19 (2.0) | 3 (1.2) | 4 (2.5) | 230 (6.1) |
| Princeton Lipid Research Study (US) | 31 (3.3) | 18 (7.1) | 13 (8.2) | 190 (5.0) |
| Cardiovascular Risk in Young Finns Study (Finland) | 817 (87.2) | 200 (79.4) | 57 (36.1) | 1578 (41.8) |
| Childhood participant characteristics | ||||
| Mean age at childhood visits, median (IQR), yc | 12.6 (9.5-15.8) | 14.0 (10.9-16.8) | 12.8 (10.9-15.4) | 13.7 (11.3-15.7) |
| Mean calendar year of childhood visits, median (IQR)c | 1983.0 (1980.0-1983.0) | 1981.5 (1980.0-1983.0) | 1979.3 (1976.0-1983.0) | 1980.0 (1976.5-1983.0) |
| Parental education level, No. (%) | n = 867 | n = 230 | n = 136 | n = 3188 |
| Less than high school diploma | 521 (60.1) | 127 (55.2) | 31 (22.8) | 1060 (33.3) |
| High school diploma | 43 (5.0) | 25 (10.9) | 38 (27.9) | 673 (21.1) |
| Higher than high school diploma but no college degree | 199 (22.9) | 52 (22.6) | 35 (25.8) | 776 (24.3) |
| Bachelor-level college degree or higher | 104 (12.0) | 26 (11.3) | 32 (23.5) | 679 (21.3) |
| Cigarette smoking, No. (%) | 246 (36.9) | 111 (44.1) | 70 (44.3) | 1615 (42.8) |
| Body mass index, mean (SD)d | 18.1 (3.6) | 19.1 (3.7) | 17.7 (3.3) | 17.9 (3.3) |
| Systolic blood pressure, mean (SD), mm Hg | 112 (13) | 114 (14) | 106 (12) | 106 (12) |
| Non–HDL-C, mean (SD), mg/dLe | 165.6 (18.3) | 181.5 (28.6) | 124.2 (17.8) | 107.1 (21.9) |
| Adulthood participant characteristics | ||||
| Mean age at adulthood visits, median (IQR), yc | 30.8 (28.7-33.9) | 31.9 (28.9-35.4) | 31.4 (29.0-35.5) | 29.5 (26.6-32.7) |
| Mean calendar year of adulthood visits, median (IQR)c | 2002.0 (1995.0-2006.3) | 2001.0 (1993.5-2004.0) | 1999.1 (1994.2-2002.0) | 1998.0 (1991.3-2002.0) |
| Age at end of follow-up, median (IQR), yf | 46.8 (43.4-52.5) | 49.7 (43.9-52.7) | 49.8 (46.1-53.7) | 49.6 (44.1-53.6) |
| Type 2 diabetes, No./total (%) | 17/867 (2.5) | 20/230 (11.2) | 20/136 (15.2) | 234/3188 (7.5) |
| Lipid medications, No./total (%) | 43/915 (4.7) | 43/249 (17.3) | 26/158 (16.5) | 141/3712 (3.8) |
| Incident CVD events, No. (%)g | 13 (1.4) | 23 (9.1) | 9 (5.7) | 102 (2.7) |
Abbreviations: CVD, cardiovascular disease; NHLBI, National Heart, Lung, and Blood Institute; non–HDL-C, non–high-density lipoprotein cholesterol.
SI conversion factor: to convert non–HDL-C from mg/dL to mmol/L, divide values by 38.67.
Non–HDL-C cutoffs to define dyslipidemia were 145 mg/dL in childhood and 190 mg/dL in adulthood. Resolution was defined as dyslipidemia in childhood and nondyslipidemia in adulthood; incident dyslipidemia as nondyslipidemia in childhood and dyslipidemia in adulthood; persistent dyslipidemia as dyslipidemia in both childhood and adulthood, and persistent normal levels as nondyslipidemia in both childhood and adulthood.
Collection of race information was based on self-report from various sources and harmonized to the categories shown. See the eAppendix in Supplement 1 for a complete description.
The mean age at childhood and adulthood visits was the mean age of participants across all available childhood (age 3-19 years) or adulthood (age 20-40 years) visits for non–HDL-C measurements. The mean calendar year of childhood and adulthood visits was the mean calendar year across all available childhood or adulthood visits for non–HDL-C measurements. Mean age at childhood visits is larger than age at the first visit for non–HDL-C measurement because most participants had multiple measurements at different ages.
Calculated as weight in kilograms divided by height in meters squared.
Individual mean (if participant had multiple measurements) of the measurements across childhood (age 3-19.99 years).
Age at time of event, non-CVD death, or end of follow-up, whichever occurred first.
Incident CVD events included the first occurrence of adjudicated myocardial infarction, stroke, transient ischemic attack, ischemic heart failure, angina, peripheral artery disease, carotid intervention, abdominal aortic aneurysm, or coronary revascularization, as well as CVD deaths.
Childhood and Adult Non–HDL-C Z Scores and Change in Scores
Childhood and adult non–HDL-C z scores were moderately correlated (r = 0.587; P < .001) (eFigure 1 in Supplement 1). When analyzed separately, both childhood and adult non–HDL-C z scores were associated with increased risk of fatal or nonfatal CVD events (HRs, 1.42 [95% CI, 1.18-1.70] and 1.50 [95% CI, 1.26-1.78] for a 1-unit increase in z score, respectively), adjusting for cohort, sex, Black race, childhood smoking, mean age at and calendar year of childhood measurement, and childhood mean age- and sex-specified z scores for body mass index and systolic blood pressure (Figure 2). When both childhood and adult z scores were included in the same model, the association for childhood non–HDL-C z score was reduced (HR, 1.12 [95% CI, 0.89-1.41]), while the association for adult z score remained significant (HR, 1.41 [95% CI, 1.14-1.74]). However, when both childhood non–HDL-C z score and change in non–HDL-C z scores between childhood and adulthood were included in the same model, childhood non–HDL-C z score remained a significant predictor of the outcome (HR, 1.58 [95% CI, 1.30-1.92]) and the incremental change in the z score was an additional independent predictor (HR, 1.41, [95% CI, 1.14-1.74]). Adding childhood non–HDL-C z score to a model that included adulthood non–HDL-C z score did not improve the C index (0.0013 [95% CI, −0.0005 to 0.0031]), but adding childhood non–HDL-C z score to a model with change in non–HDL-C z score improved the discriminative utility (C-index, 0.0165 [95% CI, 0.0090-0.0239]) (eTable 4 in Supplement 1). Analyses of the net reclassification index showed consistent results.
Figure 2. Associations Between Childhood, Adult, and Childhood Plus Adult Non–HDL-C Z Scores and Adult CVD Events.
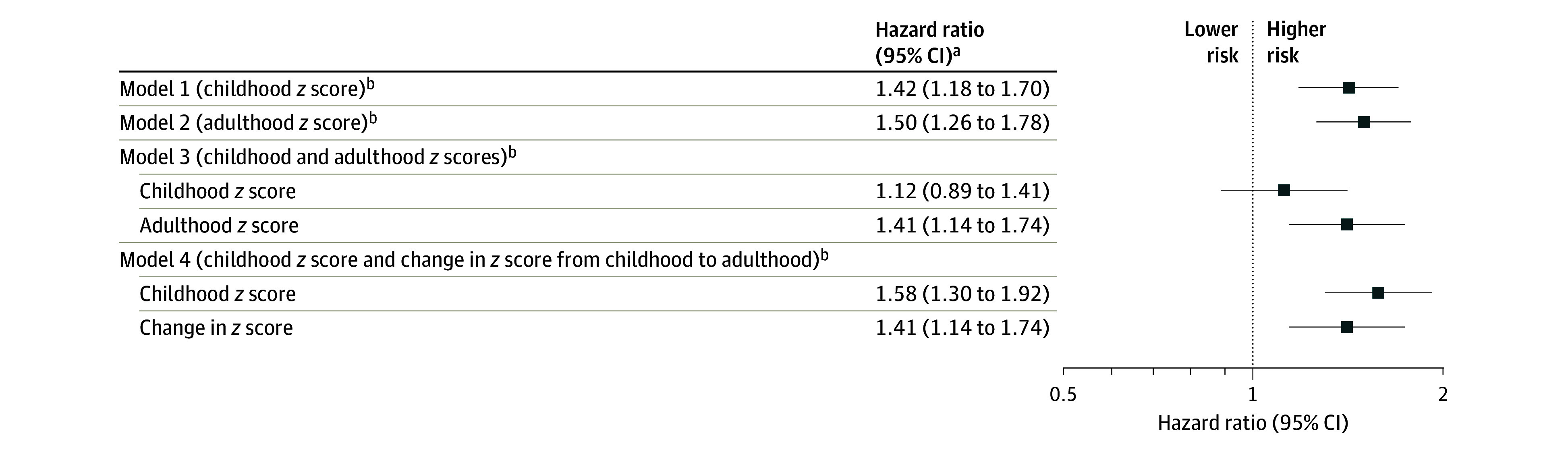
CVD indicates cardiovascular disease; non–HDL-C, non–high-density lipoprotein cholesterol. Incident CVD included the first occurrence of adjudicated myocardial infarction, stroke, transient ischemic attack, ischemic heart failure, angina, peripheral artery disease, carotid intervention, abdominal aortic aneurysm, or coronary revascularization, as well as CVD deaths. There were 147 incident CVD events (5121 participants; incidence rate, 3.23 per 1000 person-years).
aHazard ratios are reported per 1-unit increase in z scores; cohort-stratified cause-specific hazard models were used and analyses were weighted by the inverse of probability of being included in analysis and were adjusted for sex, Black race, childhood smoking, mean age at and calendar year of childhood measurement, childhood mean age- and sex-specific z scores for body mass index and systolic blood pressure, adult smoking, and change in z scores for body mass index and systolic blood pressure between childhood and adulthood.
bVariables in parentheses are exposures of interest in each model. Childhood non–HDL-C levels at each visit were standardized as age- and sex-specific z scores within each age and sex stratum. The mean of resulting z scores across childhood measurements for each participant was then calculated to obtain a single mean z score for analysis (eAppendix in Supplement 1). The same approach was used to obtain adult non–HDL-C z scores. Change in z score was calculated by subtracting childhood mean z score from adult mean z score.
Change in Non–HDL-C Status
The medians of childhood and adulthood non–HDL-C levels according to change in non–HDL-C status between childhood and adulthood are shown in eFigure 2 in Supplement 1. Compared with those who had persistently normal non–HDL-C in childhood and adulthood, participants who had incident dyslipidemia had a significantly increased risk of fatal or nonfatal CVD events (HR, 2.17 [95% CI, 1.00-4.69]) and those with persistent non–HDL-C dyslipidemia from childhood to adulthood had double that risk (HR, 5.17 [95% CI, 2.80-9.56]) (Figure 3). Individuals who had dyslipidemic non–HDL-C in childhood but nondyslipidemia as adults had a similar risk as those with both childhood and adulthood nondyslipidemia (HR, 1.13 [95% CI, 0.50-2.56]). The interactions between childhood and adult non–HDL-C status were not significant (P > .05 for both 2-category and 3-category variables) (Figure 3 and Figure 4) (eTable 5 in Supplement 1).
Figure 3. Hazard Ratios and Cumulative Hazard of CVD Events According to Change in Non–HDL-C Status Between Childhood and Adulthood.
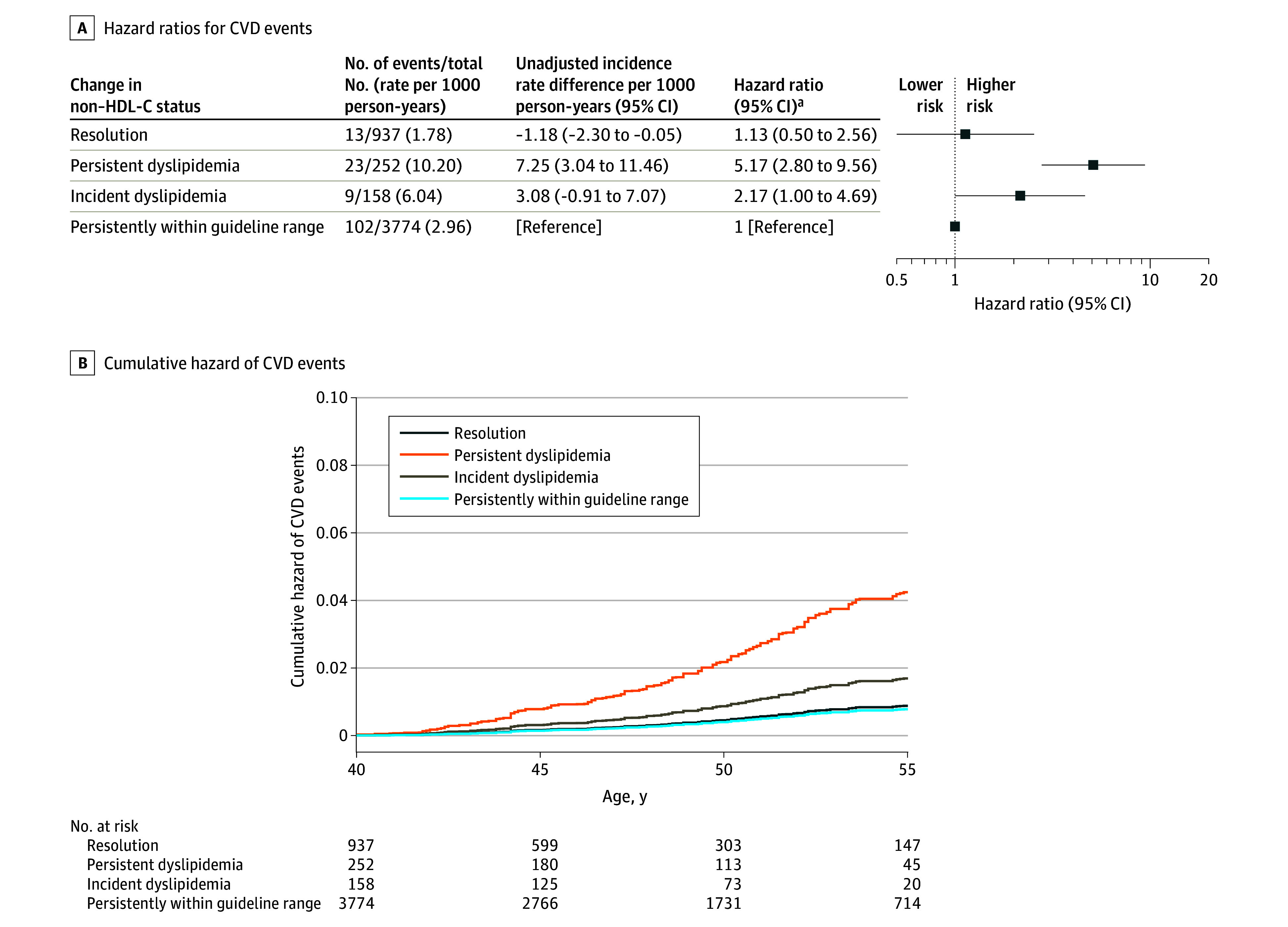
CVD indicates cardiovascular disease; non–HDL-C, non–high-density lipoprotein cholesterol. See Figure 2 legend for conditions included in incident CVD events. There were 147 incident CVD events (5121 participants; incidence rate, 3.23 per 1000 person-years). The graph is truncated at age 55 years, after which only 5% of participants were followed up.
aCohort-stratified cause-specific hazard models were used and analyses were weighted by the inverse of probability of being included in analysis and were adjusted for sex, Black race, mean age at and calendar year of childhood measurement, childhood smoking, mean age- and sex-specific z scores for body mass index and systolic blood pressure, adult smoking, and change in z scores for body mass index and systolic blood pressure between childhood and adulthood. Childhood/adult individual means of non–HDL-C for each participant were used to classify risk status. Non–HDL-C cutoffs to define dyslipidemia were 145 mg/dL in childhood and 190 mg/dL in adulthood (to convert non–HDL-C from mg/dL to mmol/L, divide values by 38.67). The 4 groups were defined as resolution (dyslipidemia in childhood, nondyslipidemia in adulthood), persistent dyslipidemia (dyslipidemia in both childhood and adulthood), incident dyslipidemia (nondyslipidemia in childhood, dyslipidemia in adulthood), and persistent normal non–HDL-C levels (nondyslipidemia in both childhood and adulthood).
Figure 4. Hazard Ratios for Adult CVD Events According to 9 Categories of Change in Non–HDL-C Status Between Childhood and Adulthood.
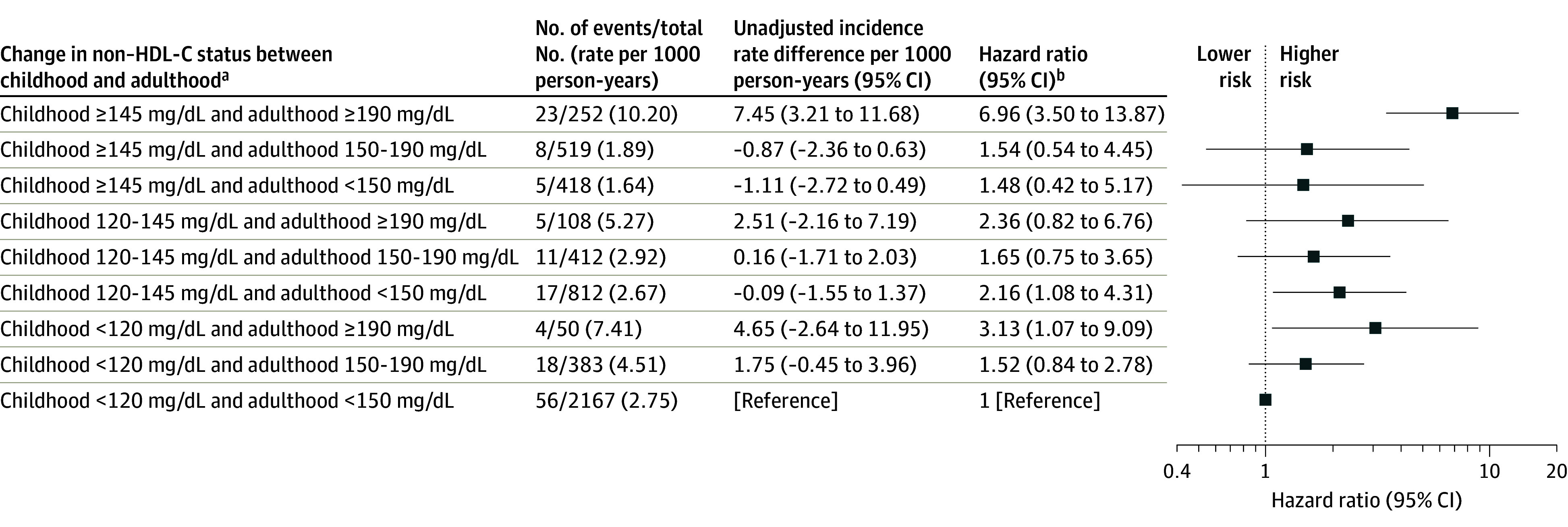
CVD indicates cardiovascular disease; non–HDL-C, non–high-density lipoprotein cholesterol. To convert non–HDL-C from mg/dL to mmol/L, divide values by 38.67. See Figure 2 legend for conditions included in incident CVD events. There were 147 incident CVD events (5121 participants; incidence rate, 3.23 per 1000 person-years).
aThese groupings considered all major combinations of child and adult non–HDL-C status groups (ie, normal, elevated, and dyslipidemia at both time points). From the groupings in the Table and Figure 3, the nondyslipidemia group in childhood or adulthood was further split into normal lipid level and elevated lipid level groups, creating 3 categories for non–HDL-C status at each age period and 9 categories for change in non–HDL-C status between childhood and adulthood. Childhood and adulthood individual means of non–HDL-C levels for each participant were used to classify risk status. Non–HDL-C cutoffs of ≥120 mg/dL and ≥150 mg/dL, respectively, were used to define childhood and adulthood elevated levels, and 145 mg/dL and 190 mg/dL, respectively, to define childhood and adulthood dyslipidemia.
bCohort-stratified cause-specific hazard models were used, and analyses were weighted by the inverse of probability of being included in analysis and adjusted for sex, Black race, mean age at and calendar year of childhood measurement, childhood smoking, mean age- and sex-specified z scores for body mass index and systolic blood pressure, adult smoking, and change in z scores for body mass index and systolic blood pressure between childhood and adulthood.
Subgroup Analysis
There were no significant interactions between change in non–HDL-C status with sex or race (P > .05 for interaction for all) (eTable 6 in Supplement 1), but the association of incident dyslipidemia with CVD events was stronger in the younger age group (P = .001 for interaction). Cohort-specific analysis for studies with meaningful number of outcomes showed similar results from the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study (eTable 7 in Supplement 1).
Sensitivity Analysis
Additional adjustment of adult risk factors or excluding those who ever used lipid-lowering medications did not materially change associations between the change in non–HDL-C status with CVD events (eFigure 3 in Supplement 1). Compared with the included participants, those who were excluded from analysis were younger, were more likely to be Black, were more likely to be male, were less likely to smoke cigarettes, and had lower systolic blood pressure in childhood (eTable 8 in Supplement 1). The associations remained essentially unchanged when adjusted for childhood parental education among participants with available data on this factor (eTable 9 in Supplement 1). Using a single value of childhood and adulthood non–HDL-C measurements to define the change in non–HDL-C status did not considerably alter the associations (eTable 10 in Supplement 1). Using Fine-Gray hazard models showed associations similar to those using cause-specific hazard models (eTable 11 in Supplement 1).
Discussion
These data from 6 prospective cohorts suggest that elevated childhood non–HDL-C levels are associated with increased risk of CVD events in adulthood, but those in whom dyslipidemic non–HDL-C resolves by adulthood have risk similar to those with persistently normal non–HDL-C in both childhood and adulthood. These findings suggest that from a preventive perspective (1) assessment of non–HDL-C should begin in childhood and (2) efforts to reduce non–HDL-C levels from childhood to adulthood may help prevent premature CVD.
The public health importance of lipid screening in childhood and early interventions to reduce adverse lipid levels has been questioned due to the low incidence of CVD in childhood and the lack of direct evidence linking childhood lipid levels and their changes into adulthood with CVD events.1,5,6,25,26 In prior publications, we have reported that elevated childhood total cholesterol was associated with increased risk of fatal and fatal or nonfatal CVD events in adulthood,14 and elevated childhood non–HDL-C was associated with high carotid intima-media thickness.13 For non–HDL-C, the current study using clinical events extends our previous observation that those who had resolution of childhood dyslipidemia in adulthood did not have an increased risk of high carotid intima-media thickness.13 The current study found that the benefits of reduction in non–HDL-C as shown in carotid intima-media thickness are maintained into later adulthood, when CVD events occur. These data indicate that there is a window of opportunity prior to or during young adulthood when effective interventions may reverse the detrimental effect of childhood dyslipidemia regarding CVD later in life. Consistent with this explanation, in the model including both childhood and adult non–HDL-C z scores, the adult z score was found to be a strong predictor of adult CVD events, but the association for childhood z score was largely attenuated. This suggests that childhood non–HDL-C is associated with adult events mainly through its strong tracking from childhood to adulthood.27 In comparison, a complementary analysis28 further showed that both childhood non–HDL-C z score and change in z score from childhood to adulthood were independently associated with risk of CVD events in adulthood. This provides a forward-looking assessment of lipid-related CVD risk and points to a life-course approach to the prevention of early-onset CVD in which monitoring non–HDL-C levels from childhood could help early identification of individuals at high lipid-related risk of developing future CVD events, and the risk could be offset by improving non–HDL-C levels through to adulthood.
Data on the long-term effectiveness of early-life interventions to prevent cardiovascular risk factors or events are scarce. A randomized clinical trial, the Special Turku Coronary Risk Factor Intervention Project, has shown that a 20-year dietary counseling intervention starting in infancy and focusing on a heart-healthy diet (low proportional intake of saturated fat and cholesterol) improved adherence to dietary guidelines and reduced lipid levels,29,30 and these effects were largely maintained into adulthood 6 years after the end of the intervention.2 While these findings are encouraging, the clinical benefit of the improvements in cardiovascular risk factors on CVD morbidity and mortality and whether a greater effect could be seen among those at high risk (eg, non–HDL-C dyslipidemia) in early life remain to be determined.
The main strengths of this study are the large sample size and the long follow-up of 6 international longitudinal cohorts, allowing examination of childhood through adult non–HDL-C levels associated with definite CVD events. In particular, the high proportion of children with dyslipidemia during the 1970-1990s and the overall favorable trends thereafter3,31,32 provides a unique opportunity to examine the potential implications for CVD events of resolving high non–HDL-C.
Limitations
This study also has limitations. First, given that the study was not specifically powered to detect subgroup differences by age, sex, or race and ethnicity, the reliability of the conclusions drawn from these analyses could be limited. Notably, participants in this study were predominantly White and non-Hispanic, which may not generalize to other races and ethnicities. Nonetheless, we observed that the main findings regarding the associations between change in non–HDL-C status and CVD events were consistent between Black and non-Black participants, suggesting no heterogeneity between these groups. However, as the subgroup differences were not guided by prior hypotheses, we recommend cautious interpretation and suggest that these findings be confirmed. Similarly, caution is also needed in the interpretation of the precision of the effect estimates in exposure groups with relatively small sample size, particularly in the incident dyslipidemia group. Second, observational studies cannot be used to infer causality. However, mendelian randomization studies33,34 have confirmed the causal relationship between non–HDL-C and CVD, and therapeutic interventions lowering LDL-C, a major component of non–HDL-C, show compelling evidence of benefits in reducing the risk of CVD.35 Third, we were not able to determine an optimal adult age when elevated childhood dyslipidemia should be resolved because only about one-third of participants had non–HDL-C measured in childhood, between ages 20 to 30 years, and at age 30 years or older, although most participants with resolving high non–HDL-C showed resolution by their 20s. Nonetheless, a large study of 6 US cohorts suggested that LDL-C in young adulthood (age 18-39 years) was associated with risk of coronary heart disease independent of LDL-C during later adulthood (≥40 years)36; thus, we speculate that interventions to reduce elevated childhood non–HDL-C may need to begin prior to age 40 years. Fourth, we did not have complete information on the use of lipid-lowering medications. However, the results remained unchanged after excluding participants who used lipid-lowering medications (only 4.8%, all in adulthood). The absence of childhood non–HDL-C measurements for a large subset of participants and loss to follow-up may have introduced selection bias. This primarily stemmed from the original study protocols of 2 cohorts, which did not include lipid or HDL-C testing for some childhood visits, rather than systematic factors likely to influence non–HDL-C levels. Therefore, the missingness of childhood non–HDL-C data is presumed to be nondifferential. Despite these challenges, the application of inverse probability weighting in the primary analyses yielded slightly stronger associations compared with the complete case analysis, suggesting that any bias introduced by missing data may be toward the null. Fifth, most cohorts did not have data available on some possible childhood confounders, such as socioeconomic factors. The main findings remained essentially unchanged in sensitivity analysis with further adjustment of childhood parental education among participants with available data on this factor.
Conclusions
In conclusion, individuals with persistent non–HDL-C dyslipidemia from childhood to adulthood had an increased risk of CVD events, but those in whom non–HDL-C dyslipidemia resolved by adulthood had a similar risk as those with normal HDL-C in both childhood and adulthood. These findings suggest that primordial and primary interventions to prevent and reduce elevated childhood non–HDL-C levels may help prevent premature CVD.
Educational Objective: To identify the key insights or developments described in this article.
-
Why did the authors undertake this study?
Because non–high-density lipoprotein cholesterol (non–HDL-C) is indirectly measured (as total cholesterol minus HDL-C), it has not been demonstrated that measurements are sufficiently consistent and reliable to be used for risk assessment.
Recent US Preventive Services Task Force guidelines recommend screening despite debate over the value of lipid screening in childhood.
Whether childhood non–HDL-C levels contribute to long-term risk of actual cardiovascular disease (CVD) events had not been previously examined.
-
Among individuals with dyslipidemic non–HDL-C in childhood and subsequent resolution of dyslipidemia in adulthood, what was the risk of CVD events?
Elevated and similar to the risk among those with elevated non–HDL-C in childhood that persisted into adulthood
Similar risk as those who had unremarkable non–HDL-C in both childhood and adulthood
Too few children with elevated non–HDL-C had resolution of dyslipidemia in adulthood to allow for clinically meaningful risk assessment.
-
The authors note that lack of childhood non–HDL-C measurements in the initially available cohorts may have introduced selection bias. How did they address this limitation?
Alternative sensitivity analyses placed all children with missing data in the “persistent dyslipidemia” category and imputed presence of CVD events.
Inverse probability weighting was applied to all analyses.
The authors conducted a sensitivity analysis omitting 2 original study cohorts that used risk assessment to identify children to test for non–HDL-C.
eAppendix. Measurement of Childhood and Adulthood Factors and Analytic Details
eTable 1. Summary for the Methods of Total Cholesterol and HDL-C in Each Cohort
eTable 2. The Distribution of the Number of Non-HDL-C Measures in Childhood and Adulthood
eFigure 1. The Correlation Between Childhood and Adulthood Non-HDL-C Z Score
eFigure 2. Medians of Childhood and Adulthood Non-HDL-C Levels According to Change in Non-HDL-C Status Between Childhood and Adulthood
eTable 3. Characteristics of Participants According to Adult Cardiovascular Outcomes
eTable 4. C-Index and Category-Free Net Reclassification Index for the Additive Predictive Value of Childhood Non-HDL-C in Addition to Adult Non-HDL-C or the Change in Non-HDL-C From Childhood to Adulthood
eTable 5. Hazard Ratios for Adult Cardiovascular Events According to Adult Non-HDL-C Category Within Each Child Risk Category
eTable 6. Hazard Ratios for Adult Cardiovascular Events According to Change in Non-HDL-C Status Between Childhood and Adulthood by Sex, Age, or Race
eTable 7. Cohort-Specific Hazard Ratios for Adult Cardiovascular Events According to Change in Non-HDL-C Status Between Childhood and Adulthood
eFigure 3. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood – Sensitivity Analyses
eTable 8. Characteristics of Participants Included vs. Excluded From the Analysis
eTable 9. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood – With or Without Further Adjustment of Parental Education
eTable 10. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood – Based on Different Choices of Single Value of Childhood and Adulthood Non-HDL-C Measurements
eTable 11. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood Using Cohort-Stratified Fine-Gray Subdistribution Hazard Models
eReferences
Data Sharing Statement
References
- 1.Raitakari O, Pahkala K, Magnussen CG. Prevention of atherosclerosis from childhood. Nat Rev Cardiol. 2022;19(8):543-554. doi: 10.1038/s41569-021-00647-9 [DOI] [PubMed] [Google Scholar]
- 2.Pahkala K, Laitinen TT, Niinikoski H, et al. Effects of 20-year infancy-onset dietary counselling on cardiometabolic risk factors in the Special Turku Coronary Risk Factor Intervention Project (STRIP): 6-year post-intervention follow-up. Lancet Child Adolesc Health. 2020;4(5):359-369. doi: 10.1016/S2352-4642(20)30059-6 [DOI] [PubMed] [Google Scholar]
- 3.Perak AM, Ning H, Kit BK, et al. Trends in levels of lipids and apolipoprotein B in US youths aged 6 to 19 years, 1999-2016. JAMA. 2019;321(19):1895-1905. doi: 10.1001/jama.2019.4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18(10):689-700. doi: 10.1038/s41569-021-00541-4 [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents . Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force . Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2023;330(3):253-260. doi: 10.1001/jama.2023.11330 [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. ; American Heart Association . Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146(5):e18-e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4(3):337-345. doi: 10.1161/CIRCOUTCOMES.110.959247 [DOI] [PubMed] [Google Scholar]
- 10.Frost PH, Havel RJ. Rationale for use of non-high-density lipoprotein cholesterol rather than low-density lipoprotein cholesterol as a tool for lipoprotein cholesterol screening and assessment of risk and therapy. Am J Cardiol. 1998;81(4A):26B-31B. doi: 10.1016/S0002-9149(98)00034-4 [DOI] [PubMed] [Google Scholar]
- 11.Stone NJ, Smith SC Jr, Orringer CE, et al. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(8):819-836. doi: 10.1016/j.jacc.2021.12.016 [DOI] [PubMed] [Google Scholar]
- 12.Pencina KM, Thanassoulis G, Wilkins JT, et al. Trajectories of non-HDL cholesterol across midlife: implications for cardiovascular prevention. J Am Coll Cardiol. 2019;74(1):70-79. doi: 10.1016/j.jacc.2019.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juonala M, Wu F, Sinaiko A, et al. Non-HDL cholesterol levels in childhood and carotid intima-media thickness in adulthood. Pediatrics. 2020;145(4):e20192114. doi: 10.1542/peds.2019-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs DR Jr, Woo JG, Sinaiko AR, et al. Childhood cardiovascular risk factors and adult cardiovascular events. N Engl J Med. 2022;386(20):1877-1888. doi: 10.1056/NEJMoa2109191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) Consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: design and recruitment. Contemp Clin Trials. 2018;69:55-64. doi: 10.1016/j.cct.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagin A, Frey T, Christiansen SL; AMA Manual of Style Committee . Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326(7):621-627. doi: 10.1001/jama.2021.13304 [DOI] [PubMed] [Google Scholar]
- 18.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876-1885. doi: 10.1056/NEJMoa1010112 [DOI] [PubMed] [Google Scholar]
- 19.Koskinen J, Juonala M, Dwyer T, et al. Utility of Different blood pressure measurement components in childhood to predict adult carotid intima-media thickness. Hypertension. 2019;73(2):335-341. doi: 10.1161/HYPERTENSIONAHA.118.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu T, Gall SL, Widome R, et al. Childhood/adolescent smoking and adult smoking and cessation: the International Childhood Cardiovascular Cohort (i3C) Consortium. J Am Heart Assoc. 2020;9(7):e014381. doi: 10.1161/JAHA.119.014381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492-499. doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 22.Zabor EC, Assel M. On the need for landmark analysis or time-dependent covariates. J Urol. 2023;209(6):1060-1062. doi: 10.1097/JU.0000000000003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels SR, Greer FR; Committee on Nutrition . Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198-208. doi: 10.1542/peds.2008-1349 [DOI] [PubMed] [Google Scholar]
- 26.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force . Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316(6):625-633. doi: 10.1001/jama.2016.9852 [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Kim HC, Kang DR, Suh I. The 23-year tracking of blood lipids from adolescence to adulthood in Korea: the Kangwha study. Lipids Health Dis. 2017;16(1):221. doi: 10.1186/s12944-017-0615-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Stavola BL, Nitsch D, dos Santos Silva I, et al. Statistical issues in life course epidemiology. Am J Epidemiol. 2006;163(1):84-96. doi: 10.1093/aje/kwj003 [DOI] [PubMed] [Google Scholar]
- 29.Laitinen TT, Nuotio J, Juonala M, et al. Success in achieving the targets of the 20-year Infancy-Onset Dietary Intervention: association with insulin sensitivity and serum lipids. Diabetes Care. 2018;41(10):2236-2244. doi: 10.2337/dc18-0869 [DOI] [PubMed] [Google Scholar]
- 30.Niinikoski H, Lagström H, Jokinen E, et al. Impact of repeated dietary counseling between infancy and 14 years of age on dietary intakes and serum lipids and lipoproteins: the STRIP study. Circulation. 2007;116(9):1032-1040. doi: 10.1161/CIRCULATIONAHA.107.699447 [DOI] [PubMed] [Google Scholar]
- 31.Jousilahti P, Laatikainen T, Salomaa V, Pietilä A, Vartiainen E, Puska P. 40-year CHD mortality trends and the role of risk factors in mortality decline: the North Karelia Project Experience. Glob Heart. 2016;11(2):207-212. doi: 10.1016/j.gheart.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME. Trends in lipids and lipoproteins in US adults, 1988-2010. JAMA. 2012;308(15):1545-1554. doi: 10.1001/jama.2012.13260 [DOI] [PubMed] [Google Scholar]
- 33.Helgadottir A, Gretarsdottir S, Thorleifsson G, et al. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat Genet. 2016;48(6):634-639. doi: 10.1038/ng.3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helgadottir A, Thorleifsson G, Snaebjarnarson A, et al. ; DBDS Genomic Consortium . Cholesterol not particle concentration mediates the atherogenic risk conferred by apolipoprotein B particles: a mendelian randomization analysis. Eur J Prev Cardiol. 2022;29(18):2374-2385. doi: 10.1093/eurjpc/zwac219 [DOI] [PubMed] [Google Scholar]
- 35.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289-1297. doi: 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74(3):330-341. doi: 10.1016/j.jacc.2019.03.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Measurement of Childhood and Adulthood Factors and Analytic Details
eTable 1. Summary for the Methods of Total Cholesterol and HDL-C in Each Cohort
eTable 2. The Distribution of the Number of Non-HDL-C Measures in Childhood and Adulthood
eFigure 1. The Correlation Between Childhood and Adulthood Non-HDL-C Z Score
eFigure 2. Medians of Childhood and Adulthood Non-HDL-C Levels According to Change in Non-HDL-C Status Between Childhood and Adulthood
eTable 3. Characteristics of Participants According to Adult Cardiovascular Outcomes
eTable 4. C-Index and Category-Free Net Reclassification Index for the Additive Predictive Value of Childhood Non-HDL-C in Addition to Adult Non-HDL-C or the Change in Non-HDL-C From Childhood to Adulthood
eTable 5. Hazard Ratios for Adult Cardiovascular Events According to Adult Non-HDL-C Category Within Each Child Risk Category
eTable 6. Hazard Ratios for Adult Cardiovascular Events According to Change in Non-HDL-C Status Between Childhood and Adulthood by Sex, Age, or Race
eTable 7. Cohort-Specific Hazard Ratios for Adult Cardiovascular Events According to Change in Non-HDL-C Status Between Childhood and Adulthood
eFigure 3. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood – Sensitivity Analyses
eTable 8. Characteristics of Participants Included vs. Excluded From the Analysis
eTable 9. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood – With or Without Further Adjustment of Parental Education
eTable 10. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood – Based on Different Choices of Single Value of Childhood and Adulthood Non-HDL-C Measurements
eTable 11. Hazard Ratios for Adult Cardiovascular Events According to Change in non-HDL-C Status Between Childhood and Adulthood Using Cohort-Stratified Fine-Gray Subdistribution Hazard Models
eReferences
Data Sharing Statement