Abstract
Aim:
The study aims to report the feasibility and safety of palliative hypofractionated radiotherapy targeting macroscopic bladder tumors in a monocentric cohort of frail and elderly bladder cancer patients not eligible for curative treatments.
Methods:
Patients who underwent hypofractionated radiotherapy to the gross disease or to the tumor bed after transurethral resection of bladder tumor from 2017 to 2021 at the European Institute of Oncology IRCCS, were retrospectively considered. Schedules of treatment were 30 and 25 Gy in 5 fractions (both every other day, and consecutive days). Treatment response was evaluated with radiological investigation and/or cystoscopy. Toxicity assessment was carried out according to RTOG/EORTC v2.0 criteria.
Results:
A total of 16 patients were included in the study, of these 11 received hypofractionated radiotherapy on the macroscopic target volume and five on the tumor bed after transurethral resection of bladder tumor. No grade (G) >2 acute toxicities were described after treatment for both groups. Only one patient in the group receiving radiotherapy on the macroscopic disease reported G4 GU late toxicity. Ten patients had available follow-up status (median FU time 18 months), of them six had complete response, one had stable disease, and three had progression of disease. The overall response rate and disease control rate were 60% and 70%, respectively.
Conclusion:
Our preliminary data demonstrate that palliative hypofractionated radiotherapy for bladder cancer in a frail and elderly population is technically feasible, with an acceptable toxicity profile. These outcomes emphasize the potential of this approach in a non-radical setting and could help to provide more solid indications in this underrepresented setting of patients.
Keywords: Bladder cancer, hypofractionated radiotherapy, palliative radiotherapy, frail patients
Introduction
Bladder cancer accounts for about 3% of global tumor diagnoses and is the tenth most common cancer worldwide. 1 Incidence is higher in males, and age and tabagism are the main risk factors. 2 In this setting, patients are usually diagnosed at an advanced age, therefore comorbidities are often frequent.3,4
The American Joint Committee on Cancer (AJCC) classifies bladder cancer as non-muscle invasive disease (Ta, T1, and Tis), and muscle-invasive one (MIBC, T ⩾2). Usually, around 25% of muscle-invasive bladder cancer (MIBC) is diagnosed at stage III or IV, with a 5-year overall survival (OS) of less than 30%.5,6
To assess the local extent of disease in a patient with suspicious symptoms (e.g., haematuria, urinary frequency, urinary tract infection and/or upper tract obstruction and pain) and positive cystoscopy, transurethral resection of the bladder tumor (TURBT) is mandatory. 4 Then, the curative choices for loco-regional MIBC involve surgery (radical cystectomy with or without neoadjuvant or adjuvant chemotherapy) or bladder-preserving approaches (trimodality treatment). 4 Attempts of a phase III trial to compare surgical and non-surgical approaches have been pursued without considerable results.7,8 Target trial emulations,9 –11 systematic reviews12,13 and meta-analysis12, multicentre cohort analysis, 14 and Markov microsimulation 15 confirmed that survival outcomes are similar between the two approaches, thus clinical decision-making should consider patient age and comorbidities.
As of today, 20–30% of bladder cancer patients present with potentially curable disease but are considered too frail to undergo radical treatments or with a disease stage too advanced to offer curative intent.16,17 In fact, according to a recent systematic review, the 90-day major complication rate (grade 3-5) after cystectomy is 16.9%, with age and comorbidities as the most robust prognostic factors for complications. 18
Usually, this population, too frail to receive a radical approach, often suffers from severe local symptoms (e.g. severe hematuria, dysuria, irritative bladder) which possibly affect their quality of life, and international guidelines 4 recommend best supportive care (BSC) over active treatment in this setting. 19
In the case of MIBC, Stereotactic body radiation therapy (SBRT) is currently rarely used,20,21 although it represents one of the emerging options in treating both primary tumors and (oligo)metastases with either ablative or palliative intent for patients not eligible for curative treatment.22,23,24
SBRT targeting macroscopic bladder tumor is distinct from the conventional palliative treatment of the whole bladder in some critical aspects: shortening of the treatment course, partial target volume and the hypofractionation of the dose. These interventions aim to optimize symptom and tumor control while minimizing toxicities, and reducing overall treatment time, augmenting patients’ compliance to radiotherapy (RT).
The aim of the present study is to report the safety and feasibility of personalized hypofractionated RT to the macroscopic target volume or to the tumor bed after TURBT in a monocentric cohort of frail and elderly bladder cancer patients not eligible for curative treatments.
Patients and methods
Study cohort
Data of patients treated from 2017 to 2021 at the European Institute of Oncology IRCCS, Milan, Italy were retrospectively considered. The study was a part of general SBRT and image-guided radiation therapy (IGRT) research notified to the Ethical Committee of the European Institute of Oncology, Milan, Italy (notifications No. 79/10, 86/11, 87/11, 93/11)
Inclusion criteria were as follows:
- Histological or cytological diagnosis of bladder cancer;
- patients not eligible for cystectomy or trimodal therapy;
- any kind of previous treatment;
- hypofractionated RT treatment to the gross disease or to the tumor bed after TURBT;
- no concomitant systemic therapy during RT;
- written informed consent for the radiation treatment;
- written informed consent for the use of the anonymized data for research and educational purposes.
Patients were divided into two subgroups according to whether they received hypofractionated RT to the macroscopic target volume in the bladder (Group 1 – with evidence of local disease) or to the tumor bed after TURBT (Group 2 – with no evidence of local disease).
The diagnosis of clinically evident bladder cancer was based on a pelvic computed tomography (CT) scan. The indication of RT for the recurrent disease was discussed in a multidisciplinary tumor board.
In order to classify the comorbidities amongst patients, the age-adjusted Charlson comorbidity index (ACCI) was retrospectively calculated for each patient,25,26 the diagnosis of bladder cancer was included in the ACCI assessment ( Table 1 ).
Table 1.
Age-adjusted Charlson comorbidity index.
| Point | Comorbidity |
|---|---|
| 1 | Myocardial infarction, heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disorder, peptic ulcer disease, mild liver disease, diabetes mellitus without complications |
| 2 | Diabetes mellitus with complications, moderate-to-severe renal disease, hemiplegia, leukemia, lymphoma, tumor without metastases |
| 3 | Moderate-to-severe liver disease |
| 6 | Metastatic solid tumors, acquired immunodeficiency syndrome |
| 1 | For each decade over the age of 40 years |
Radiotherapy treatment
Simulation CT scans were acquired in the supine position with a triangular-shaped knee support, with a full bladder, empty rectum, and 2.5 mm slice thickness. Patients were instructed to maintain the same conditions during CT and RT.
Target volumes and organs at risk were contoured using a RayStation treatment planning system (RaySearch Laboratories, Sweden). For patients of Group 1, gross tumor volume (GTV) was contoured giving the appropriate expansions respectively to generate clinical target volume (CTV) and planning target volume (PTV); in Group 2 only CTV (based on previously identified lesions at CT scan before TURBT) was contoured and the PTV was derived afterward.
Image-guided RT was delivered using three different linear accelerators (Varian Trilogy Linear Accelerator, Accuray TomoTherapy, and BrainLab VERO Systems). Applied constraints were selected and adapted according to available literature.27 –32 Daily positioning verification was achieved by Cone-beam Computed Tomography (CBCT), or by Mega-voltage Computed Tomography (MVCT), otherwise by kilovoltage (kV) imaging depending on which linear accelerator has been used.
Follow-up procedure
Treatment response was evaluated with a CT scan and/or cystoscopy every three months after the end of RT. Toxicity assessment was carried out according to Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) v5.0 criteria. 33 Acute toxicity was analyzed in all patients at the end of RT, while late toxicity was evaluated in the patients with a minimum 6-month follow-up (FU). The local response to treatment (response of the treated lesion) was scored as a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to Response Evaluation Criteria In Solid Tumours (RECIST) criteria. 34 Overall response rate (ORR) was defined as the percentage of patients with CR and PR while disease control rate (DCR) includes CR, PR, and SD.
Results
A total of 16 patients were included in the study, 11 of them received hypofractionated RT on the macroscopic target volume (Group 1) and five on the tumor bed after TURBT (Group 2). Baseline characteristics of the patients are shown in Table 2 .
Table 2.
Summary of the patients’ characteristics.
| Group 1 (n = 11) | Median (IQR) | |
|---|---|---|
| Median age | years | 81.6 (79.8 – 86.4) |
| n | ||
| Gender | male | 8 |
| female | 3 | |
| ACCI | 6 | 1 |
| 7 | 1 | |
| 8 | 1 | |
| 9 | 4 | |
| 10 | 1 | |
| 11 | 2 | |
| 12 | 1 | |
| Histologic type | Urothelial | 10 |
| other | 1 | |
| T stage | ycT1 | 1 |
| cT2 | 3 | |
| ycT2 | 3 | |
| ycT3b | 2 | |
| cT4a | 1 | |
| ycT4a | 1 | |
| N stage | cN0 | 6 |
| ycN0 | 2 | |
| cN1c | 1 | |
| ycN3 | 2 | |
| M stage | cM0 | 5 |
| ycM0 | 1 | |
| ycM1a | 2 | |
| cM1b | 1 | |
| ycM1b | 1 | |
| cMx | 1 | |
| Group 2 (n = 5) | Median (IQR) | |
| Median age | years | 83.0 (73.7 - 85.5) |
| n | ||
| Gender | male | 3 |
| female | 2 | |
| ACCI | 7 | 1 |
| 8 | 2 | |
| 11 | 1 | |
| 12 | 1 | |
| Histologic type | Urothelial | 5 |
| T stage | ycT2 | 2 |
| ycTx | 3 | |
| N stage | cN0 | 4 |
| cNx | 1 | |
| M stage | cM0 | 4 |
| cM1b | 1 |
List of abbreviations: ACCI, age-adjusted Charlson comorbidity index; GT, gross tumour; IQR, interquartile range; N, lymph node; ND, not available; M, metastasis; T, tumour; TURBT, transurethral resection of bladder tumour; UC, urothelial carcinoma.
The majority of patients (94%) had a histological diagnosis of urothelial bladder cancer and all of them had ACCI greater than 6. Nine patients (56.6%) had a clinical history of at least one additional cancer at the diagnosis of bladder cancer, among them, five out of nine had two additional diagnosed malignancies (e.g. prostate adenocarcinoma, breast cancer, lymphoma). For all the included patients all the other malignancies resulted in control at the time of RT treatment.
Three patients underwent previous systemic treatments: two patients received chemotherapy (platinum-based and/or gemcitabine) and one pembrolizumab and paclitaxel. One oligometastatic patient underwent concomitant SBRT on the left internal iliac lymph node (35 Gy in 5 daily fractions every other day).
In Group 1 nine out of 11 patients received hypofractionated RT every other day with a schedule of 30 Gy in 5 and two out of 11 patients received 25 Gy in 5 fractions (one on consecutive days and one every other day). In Group 2, three out of five patients received RT treatment with a schedule of 25 Gy in 5 daily fractions every other day, and two out of five patients with a schedule of 30 Gy in 5 fractions every other day. All patients completed the scheduled RT course. An example of dose distribution for both groups is shown in Figure 1 .
Figure 1.
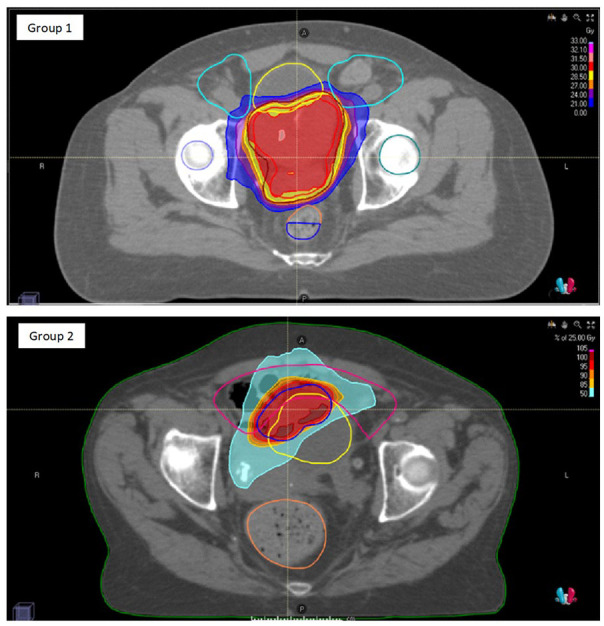
Example of dose distribution for a patient of Group 1 (with evidence of local disease) and Group 2 (with no evidence of local disease).
GI and GU toxicities
Reported acute and chronic gastrointestinal (GI) and genitourinary (GU) toxicities are summarized in Table 3 .
Table 3.
Reported acute and chronic gastrointestinal (GI) and genitourinary (GU) toxicities.
| Group 1 (n = 11) | Type | n | |
|---|---|---|---|
| acute toxicity | GI | G0 | 9 |
| G1 | 1 | ||
| G2 | 1 | ||
| GU | G0 | 7 | |
| G1 | 1 | ||
| G2 | 3 | ||
| late toxicity* | GI | G0 | 6 |
| GU | G0 | 4 | |
| G1 | 1 | ||
| G4 | 1 | ||
| Group 2 (n = 5) | |||
| acute toxicity | GI | G0 | 5 |
| GU | G0 | 4 | |
| G1 | 1 | ||
| late toxicity § | GI | G0 | 2 |
| GU | G0 | 1 | |
| G1 | 1 | ||
Data available for five out of ten patients; § data available for three out of six patients.
List of abbreviations: G, grade; GI, gastrointestinal; GT, gross tumor; GU, genitourinary; TURBT, transurethral resection of bladder tumor.
Regarding acute toxicities, no grade (G) ⩾2 was described. Among Group 1, two patients developed acute G1 and 2 GI toxicities (diarrhea); no GI acute toxicities were reported in Group 2. No > G2 and no > G1 acute GU toxicities were reported among patients in Groups 1 and 2, respectively.
Data about late toxicities were available for five out of 11 patients in Group 1 and for two out of five patients in Group 2. One patient in Group 1 reported G4 GU toxicity (urinary incontinence with the need for permanent catheterization), however this patient had already undergone two previous courses of pelvic RT. In Group 2, no G > 1 late toxicities were reported.
Oncological outcomes
Ten patients had available follow-up status, with a median follow-up of 18 months (range 5 – 49 months). Of them, six had complete response (three in Group 1 and three patients maintained complete response in Group 2), one had stable disease (Group 1), and three had progression of disease (two progressions – one distant and one local – in Group 1 and one local progression in Group 2). The overall response rate (ORR) was 60% and the disease control rate (DCR) was 70%. A total of seven patients was died at the time of the analysis, among them six had available FU and were followed for a median time of seven months.
Discussion
To the best of our knowledge, this is the first report regarding the feasibility and safety of palliative hypofractionated RT in elderly and heavily pre-treated patients with no curative treatment options. Our results show that this approach is feasible with an acceptable toxicity profile and, even if oncological outcomes were not the main focus of the study due to the heterogeneous populations, reported ORR and DCR were encouraging. In the curative setting, hypofractionated schedule on the whole bladder +/- boost has been tested in the RAIDER trial, 35 reporting no significant differences in grade >2 toxicities with respect to normofractionated schedules. In the palliative setting as of today, the use of hypofractionated treatments in muscle-invasive bladder cancer (MIBC) has been quite limited, with less than 4% of patients receiving any kind of supportive treatment in the USA, regardless of solid proof for integrating its utilization into standard oncological care. 36 This could be explained by the lack of dedicated guidelines and the presence of only general palliative care indications from the European Association of Urology. 21 This topic is even more important because untreated MIBC cancer patients have unfavorable cancer-related outcomes, 37 and potentially curative treatments as well as palliative therapies to improve quality of life (QoL) should also be offered even to older patients. 38 For a subset of patients who are too frail to undergo curative treatments and may benefit from a minimally invasive approach, intensification of non-radical strategies may be the last available approach.
Recent evidence from three trials presented at ASCO 2023 (EV-302/KEYNOTE-A39, 39 CheckMate 901, 40 and THOR 41 ) highlights how, with the availability of new drugs (enfortumab vedotin plus pembrolizumab, nivolumab, erdafitinib, respectively) combined or as an alternative to the standard chemotherapy, the range of new lines of therapy is expanding, improving the life expectancy of these patients, who may result in long survival. Therefore, the need for palliative treatments aimed at improving the quality of life and controlling local symptoms of these patients during the natural history of their disease is likely going to increase.
As reported by the AIRO Uro-GROUP on the role of palliative RT in the management of elderly and frail patients with advanced bladder cancer, most respondents believe that radiation oncologists are poorly involved in the palliative care of MIBC, and in addition, these cases are often not discussed in the multidisciplinary board. 21
In this context, the present study reported an encouraging toxicity outcome, improving symptoms and consequently the QoL of treated patients. Only one among our patients, who already had a right ureteral stent, underwent both permanent left ureteral stenting for left hydronephrosis and bladder catheterization for chronic G4 toxicity. However, this patient had already undergone two previous courses of pelvic RT (radical RT for prostate cancer - 76 Gy/38fr and adjuvant RT for right colon adenocarcinoma - 50.4 Gy/28 fr, 14 and 13 years before our treatment, respectively).
One of the most numerous series on palliative setting in bladder cancer was recently published by Ali and Colleagues. 42 They retrospectively included 241 MIBC patients treated with 3D conformal RT investigating the effectiveness of palliative RT in bladder cancer and identifying eventual factors associated with treatment outcome. Regarding RT schemes, a rather wide dose range was used, from 8 Gy/1 Fr to 30 Gy/10 Fr. Hypofractionated schemes, such as 21 Gy/3 Fr or 20 Gy/5 Fr, have also been used. Interestingly, no correlation between the RT scheme and clinical outcomes has been reported. Authors concluded that patients with good performance status, early stages of disease, and fewer comorbidities, survived longer and palliative RT was an effective and well-tolerated treatment. Finally, they defined a futile treatment (defined as a patient who died within 30 days of completing RT or was unable to finish the course of treatment) highlighting how comprehensive evaluation and patient selection are essential to avoid this kind of treatment. An important focus should be given to symptom control: at the end of RT, 53% of alive patients experienced improvement after treatment in hematuria, local symptoms (i.e., urinary frequency and dysuria), and pain score (54.1%, 56.8%, and 47.6%, respectively).
According to Ali et al.’s definition of futile treatment, none of our RT treatment resulted futile with a 100% three-month survival for the patients and all patients were able to conclude the RT treatment course. It is to be noted that, almost 50% of responders from the AIRO Uro-Group above mentioned 21 will offer a palliative RT treatment even with a life expectancy <3 months.
A summary of the main studies of non-radical RT for bladder cancer from 2000 to 202243 –53 is reported in Table 4 . The schedules and the range of doses delivered as reported in the literature are numerous and it should be noted that in the majority of these trials, the clinical target volume (CTV), which corresponds to the whole bladder, was expanded to yield the planned target volume (PTV), with a margin of 1-2 cm. Because bladder cancer is typically multifocal, it can be challenging to accurately localize the tumor and give treatment. For these reasons, the entire bladder is typically considered the target volume. However, as demonstrated by brachytherapy data, treating the tumor directly may provide comparable local control. 54 In addition, data from the BC2001 study (CRUK/01/004), a randomized noninferiority trial, demonstrated comparable results with partial and total bladder irradiation. Indeed, there were even indications of improved local control and survival with partial bladder treatment. 55
Table 4.
Characteristics of studies of non-radical and palliative radiotherapy for bladder cancer.
| Author/Year | Study design | Treatment period | Median age (years; IQR) | Total patients | Stage | RT technique | RT schedule | Acute toxicity (scale) | Median follow-up (months; IQR) |
|---|---|---|---|---|---|---|---|---|---|
| Duchesne 2000 | Randomized | 1992-1997 | 79–80 | 500 | Mixed | 3DCRT | 35 Gy / 10 fx; 21 Gy / 3 fx | (3 mo) GI: 20% G2; Rectal <6% G2, <1% G3. GU: urinary frequency 18% G⩾1; nocturia 36% G⩾1; haematuria 12% G⩾1; dysuria 28% G⩾1. (NR) |
NR |
| Zygogianni 2013 | Retrospective | 2005 – 2009 | 75 (68 – 90) | 43 | II – IIIA | NR | 36 Gy / 6 fx; weekly | GI: Rectal 67.4% G1, 30.2% G2, 2.3% G3. GU: NR. (RTOG/EORTC). |
NR |
| Kouloulias 2013 | Prospective Phase 2 | 2005-2011 | 77 (70–91) | 58 | Locally advanced | 3DCRT | 36 Gy / 6 fx; weekly | GI: 22.4% G1, 5.6% G2. GU: 32.7% G1, 17.2% G2 (RTOG/EORTC). |
NR |
| Lacarrière 2013 | Retrospective | 1993-2009 | 81 (65–93) | 32 | Mixed | 3DCRT | 30 Gy / 10 fr; 20 Gy / 5 fx | (2 weeks) GU: 68.75% G1. (CTCAE v4.02). |
25 (7–42) |
| Méry 2015 | Retrospective | 2003-2013 | 92.7 mean (SD 90.4 –101.5) | 14 | Mixed | NR | Median 34.01 Gy (8–60 Gy) | GI, GU: 0% G3, 0% G4. (CTCAE v3.0). |
3.5 (0–61) |
| Dirix 2016 | Retrospective | 2004-2013 | 78.5 | 44 | Mixed | 3DCRT | 34.5 Gy / 6 fx weekly | GU: 46% <G3, 9% G⩾3. (CTCAE v3.0). |
9.7 (0.5–57.5) |
| Hafeez 2017 | Prospective Phase 2 | 2009 – 2014 | 86 (68 – 97) | 55 | II - IV | 3DCRT | 36 Gy / 6 fx; weekly | GI: 38% G2; 4% G3. (3 mo) GU: 27% G2, 4.5% G3. Other (fatigue or anemia: 42% G2; hyponatremia and syncope 4%). (CTCAE v3.0). |
24 |
| Aljabab 2017 | Retrospective | 2002-2013 | 78 | 67 | Mixed | 2DRT (Co-60), 3DCRT | 4–40 Gy / 1–15 fx | NR | NR |
| Hasan 2018 | Retrospective | 2005-2015 | 73 | 15 | Mixed | 3DCRT, IMRT | Median 30 Gy / 10 fx (range: 6-40 Gy) | GI, GU: < G3. (CTCAE v4). |
NR |
| Ali 2019 | Retrospective | 2014-2017 | 80 (41 –97) | 241 | Mixed | 3DCRT | 8–36 Gy / 1–10 fx | NR | NR |
| Varughese 2019 | Randomized | 05.12.2016–27.03.2017 | 80 | 228 | II - III | 3DCRT, IMRT/VMAT | 6 – 8 Gy/1 fx, 30 – 36 Gy/5 – 6 fx or 21 Gy/ 3 fx (42% received one of 11 non-RCR guidelines palliative radiotherapy prescriptions or an unknown prescription) | NR | NR |
| Tey 2021 | Retrospective | 2001-2016 | 82 | 58 | Mixed | 3DCRT | 8–40 Gy / 1–16 fx | GI: 3.5%G1-G2; nausea and vomit: 1.7% G3. Pelvic pain: 1.7% G1. (CTCAE v4.0). |
24.3 (0–47.6) |
| Huddart 2021 | Prospective | 2014 – 2016 | 85 (81 – 89) | 63 (SP group: 30; AP group: 33) | Mixed | 3DCRT, IMRT, IMRT/VMAT | 36 Gy / 6 fx; weekly | AP group: non-GU 6% ⩾ G3; GU 9% ⩾ G3. SP group: non-GU 13% ⩾ G3; GU 17% ⩾ G3. (CTCAE v4.0). |
38.8 (36.8-51.3) |
| Current study | Retrospective | 2017-2021 | Group 1 : 80.9 (79.6 –83.8); Group 2: 84.3 (76.1–85.5) |
16 (Group 1:10; Group 2: 6) |
Mixed | SBRT | 25 – 30 Gy / 5 fx every day or every other day | Group 1: GI (diarrhea) 10% G1, 10% G2; GU 10% G1 (dysuria); 30% G2 (dysuria 10%; haematuria and dysuria 10%; strangury 10%). Group 2: GI 0%; GU (urinary frequency) 16.7% G1. (EORTC/RTOG). |
Group 1: 13.3 (9.9 – 27.8); Group 2: 10.3 (9.7–18.8) |
List of abbreviations: 2DRT, Two-dimensional radiation therapy; 3DCRT, Three-dimensional conformal radiation therapy; AP, adaptive planning; Co-60, Cobalt-60; CTCAE, Common Terminology Criteria for Adverse Events; fx, fractions; G, grade; GT, gross tumor; IMRT, intensity-modulated radiation therapy; IQR, interquartile range; mo, months; NR, not reported; RT, radiotherapy, RCR, The Royal College of Radiologists; SBRT, stereotactic body radiation therapy; SP: standard planning; TURBT, transurethral resection of bladder tumor; VMAT, volumetric modulated arc therapy.
As shown, our study stands out from the rest in that it has a higher effective dose in fewer fractions, and in studies where ultra-hypofractionated (daily RT dose ⩾5Gy) was used, the dose was delivered once weekly. Our approach, which administered a higher effective dose than many prior studies, demonstrated an acceptable toxicity profile, likely benefitting from modern treatment planning and delivery techniques. Indeed, in similar studies the techniques varied from Cobalt 60 - Two-Dimensional RT (2DRT), 3DCRT to Intensity-Modulated RT/Volumetric Modulated Arc Therapy (IMRT/VMAT). To be noted is that techniques for imaging and radiation delivery have drastically changed over the years since these reported initial investigations. With the advent of IMRT and image-guided radiation therapy (IGRT), there has been a decrease in gastrointestinal and genitourinary toxicities as well as an improvement in radiation dose compliance to target volume. 56
As of today, SBRT in bladder cancer is mainly used in the oligometastatic setting, 57 nevertheless, the low alpha-beta ratio of bladder cancer could provide a radiobiological explanation for the efficacy of hypofractionation and the use of SBRT even in other settings. 58 Additionally, in the SBRT treatment, the target volume is reduced and the daily dose is typically hypofractionated, allowing the optimization of symptoms and tumor control while reducing bowel and urinary toxicities. In a highly palliative setting, like the one represented by our cohort, the possibility to reduce overall time treatment is crucial and hypofractionation seems a reasonable option in order to reduce time traveling to the hospital and to augment patient compliance to RT.
Our study, based on a retrospective design with a small and heterogeneous sample, may have inherent biases. There were noticeable inconsistencies in RT techniques and schemes across patients, and 25% were lost to follow-up. A more proactive scheduling system with all the specialists involved in the multidisciplinary team (radiation oncologists, medical oncologists, and urologists) would have been beneficial. The absence of standardized quality-of-life questionnaires and oncogeriatric assessments, along with clear guidelines for GTV contouring and protocols for bladder and rectum preparation, were also limitations we recognized.
Conclusions
In our pioneering study examining personalized hypofractionated RT treatment on part of the bladder, we achieved promising safety results for elderly and frail patients. Our approach, which administered a higher effective dose than many prior studies, demonstrated an acceptable toxicity profile, likely benefitting from modern treatment planning techniques. These outcomes emphasize the potential of our intensified non-radical strategy, underscoring the importance of tailored treatments for the elderly demographic.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: IEO, the European Institute of Oncology, is partially supported by the Italian Ministry of Health (with “Ricerca Corrente” and “5x1000” funds) and by Institutional grants from Accuray Inc. MZ, and MGV were supported by a research fellowship from the Associazione Italiana per la Ricerca sul Cancro (AIRC) entitled “Radioablation ± hormonotherapy for prostate cancer oligorecurrences (RADIOSA trial): potential of imaging and biology” registered at ClinicalTrials.gov NCT03940235, approved by the Ethics Committee of IRCCS Istituto Europeo di Oncologia and Centro Cardiologico Monzino (IEO-997).
ORCID iDs: Maria Giulia Vincini  https://orcid.org/0000-0001-7830-3149
https://orcid.org/0000-0001-7830-3149
Mattia Zaffaroni  https://orcid.org/0000-0003-4655-4634
https://orcid.org/0000-0003-4655-4634
Dario Zerini  https://orcid.org/0000-0002-9990-694X
https://orcid.org/0000-0002-9990-694X
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6 Suppl 1):4-34. doi: 10.1016/j.urology.2005.07.062 [DOI] [PubMed] [Google Scholar]
- 3. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of Bladder Cancer. Med Sci. 2020;8(1):15. doi: 10.3390/medsci8010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network. Bladder Cancer (Version 1.2023), https://www.nccn.org/professionals/physician_gls/pdf/bladder_blocks.pdf (accessed 16 April 2023).
- 5. Amin MB, Edge, Stephen B, Greene FL, et al. AJCC Cancer Staging Manual. 8 th. Springer Cham; 2017. [Google Scholar]
- 6. Babjuk M, Burger M, Capoun O, et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75-94. doi: 10.1016/j.eururo.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 7. Huddart RA, Birtle A, Maynard L, et al. Clinical and patient-reported outcomes of SPARE - a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. 2017;120(5):639-650. doi: 10.1111/bju.13900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaushik D, Shi Z, Liss MA, et al. Screening logs from a pilot randomized controlled trial of radical cystectomy versus chemoradiation therapy for muscle-invasive bladder cancer. Urol Oncol Semin Orig Investig. 2020;38(1):4.e1-4.e6. doi: 10.1016/j.urolonc.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Softness K, Kaul S, Fleishman A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive urothelial carcinoma of the bladder. Urol Oncol Semin Orig Investig. 2022;40(6):272.e1-272.e9. doi: 10.1016/j.urolonc.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 10. Bharadwaj M, Phil M, Kaul S, Korets R, Kim S, Olumi AF. Adjuvant chemotherapy versus observation following radical cystectomy for locally advanced urothelial carcinoma of the bladder. Urol Oncol 2022: 9. Published online. [DOI] [PubMed] [Google Scholar]
- 11. Zlotta AR. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. 2023; 24(6): 669-681. [DOI] [PubMed] [Google Scholar]
- 12. Fahmy O, Khairul-Asri MG, Schubert T, et al. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol Semin Orig Investig. 2018;36(2):43-53. doi: 10.1016/j.urolonc.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 13. Burdett S, Fisher DJ, Vale CL, et al. Adjuvant Chemotherapy for Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis of Individual Participant Data from Randomised Controlled Trials. Eur Urol. 2022;81(1):50-61. doi: 10.1016/j.eururo.2021.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu J, Zhang H, Xu D, et al. Comparing Long-Term Survival Outcomes for Muscle-Invasive Bladder Cancer Patients Who Underwent with Radical Cystectomy and Bladder-Sparing Trimodality Therapy: A Multicentre Cohort Analysis. P Franco, ed. J Oncol. 2022;2022:1-11. doi: 10.1155/2022/7306198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magee D, Cheung D, Hird A, et al. Trimodal therapy vs. radical cystectomy for muscle-invasive bladder cancer: A Markov microsimulation model. Can Urol Assoc J. 2021;16(4). doi: 10.5489/cuaj.7453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Novara G, Catto JWF, Wilson T, et al. Systematic Review and Cumulative Analysis of Perioperative Outcomes and Complications After Robot-assisted Radical Cystectomy. Eur Urol. 2015;67(3):376-401. doi: 10.1016/j.eururo.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 17. Verschoor N, Heemsbergen WD, Boormans JL, Franckena M. Bladder-sparing (chemo)radiotherapy in elderly patients with muscle-invasive bladder cancer: a retrospective cohort study. Acta Oncol. Published online July 26, 2022:1-7. doi: 10.1080/0284186X.2022.2101381 [DOI] [PubMed] [Google Scholar]
- 18. Maibom SL, Joensen UN, Poulsen AM, Kehlet H, Brasso K, Røder MA. Short-term morbidity and mortality following radical cystectomy: a systematic review. BMJ Open. 2021;11(4):e043266. doi: 10.1136/bmjopen-2020-043266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tey J, Ho F, Koh WY, et al. Palliative radiotherapy for bladder cancer: a systematic review and meta-analysis. Acta Oncol. 2021;60(5):635-644. doi: 10.1080/0284186X.2021.1880025 [DOI] [PubMed] [Google Scholar]
- 20. Jereczek-Fossa BA, Marvaso G. Palliative radiation therapy in bladder cancer: a matter of dose, techniques and patients’ selection. Ann Palliat Med. 2019;8(5):786-789. doi: 10.21037/apm.2019.11.02 [DOI] [PubMed] [Google Scholar]
- 21. Marvaso G, Nicosia L, Vinciguerra A, et al. The role of palliative radiotherapy in the management of elderly and frail patients with advanced bladder cancer: A survey by the AIRO uro-group. Med Oncol. 2021;38(2):14. doi: 10.1007/s12032-021-01455-4 [DOI] [PubMed] [Google Scholar]
- 22. Parisi S, Lillo S, Cacciola A, et al. Non-stereotactic radiotherapy in older cancer patients. Heliyon. 2022;8(6):e09593. doi: 10.1016/j.heliyon.2022.e09593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Augugliaro M, Marvaso G, Ciardo D, et al. Recurrent oligometastatic transitional cell bladder carcinoma: is there room for radiotherapy? Neoplasma. 2019;66(01):160-165. doi: 10.4149/neo_2018_180522N333 [DOI] [PubMed] [Google Scholar]
- 24. Longo N, Celentano G, Napolitano L, et al. Metastasis-Directed Radiation Therapy with Consolidative Intent for Oligometastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2022;14(10):2373. doi: 10.3390/cancers14102373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 26. Zhou S, Zhang XH, Zhang Y, Gong G, Yang X, Wan WH. The Age-Adjusted Charlson Comorbidity Index Predicts Prognosis in Elderly Cancer Patients. Cancer Manag Res. 2022; 14:1683-1691. doi: 10.2147/CMAR.S361495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18(4):215-222. doi: 10.1016/j.semradonc.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 28. Folkert MR, Timmerman RD. Stereotactic ablative body radiosurgery (SABR) or Stereotactic body radiation therapy (SBRT). Adv Drug Deliv Rev. Published online 2017:12. [DOI] [PubMed] [Google Scholar]
- 29. King CR, Brooks JD, Gill H, Presti JC. Long-Term Outcomes From a Prospective Trial of Stereotactic Body Radiotherapy for Low-Risk Prostate Cancer. Int J Radiat Oncol. 2012;82(2):877-882. doi: 10.1016/j.ijrobp.2010.11.054 [DOI] [PubMed] [Google Scholar]
- 30. Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S101-107. doi: 10.1016/j.ijrobp.2009.05.071 [DOI] [PubMed] [Google Scholar]
- 31. Chen LN, Suy S, Uhm S, et al. Stereotactic Body Radiation Therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Published online 2013:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alsadius D, Hedelin M, Lundstedt D, Pettersson N, WilderAng U, Steineck G. Mean Absorbed Dose to the Anal-Sphincter Region and Fecal Leakage among Irradiated Prostate Cancer Survivors. 2012;84(2):5. [DOI] [PubMed] [Google Scholar]
- 33. Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol. 2000;47(1):13-47. doi: 10.1016/S0360-3016(99)00559-3 [DOI] [PubMed] [Google Scholar]
- 34. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 35. Huddart R, et al. Acute toxicity of hypofractionated and conventionally fractionated (chemo)radiotherapy regimens for bladder cancer: an exploratory analysis from the RAIDER trial. Clin Oncol R Coll Radiol GB 2023;35: 586–597. [DOI] [PubMed] [Google Scholar]
- 36. Hugar LA, Lopa SH, Yabes JG, et al. Palliative care use amongst patients with bladder cancer. BJU Int. 2019;123(6):968-975. doi: 10.1111/bju.14708 [DOI] [PubMed] [Google Scholar]
- 37. Westergren DO, Gårdmark T, Lindhagen L, Chau A, Malmström PU. A Nationwide, Population Based Analysis of Patients with Organ Confined, Muscle Invasive Bladder Cancer Not Receiving Curative Intent Therapy in Sweden from 1997 to 2014. J Urol. 2019;202(5):905-912. doi: 10.1097/JU.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 38. Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JWF. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013;108(7):1534-1540. doi: 10.1038/bjc.2013.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ESMO 2023: EV-302/KEYNOTE-A39: Enfortumab Vedotin in Combination with Pembrolizumab (EV+P) Vs Chemotherapy in Previously Untreated Locally Advanced Metastatic Urothelial Carcinoma. Accessed January 4, 2024. https://www.urotoday.com/conference-highlights/esmo-2023/esmo-2023-bladder-cancer/147538-esmo-2023-ev-302-keynote-a39-enfortumab-vedotin-in-combination-with-pembrolizumab-ev-p-vs-chemotherapy-in-previously-untreated-locally-advanced-metastatic-urothelial-carcinoma.html
- 40. van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023;389(19):1778-1789. doi: 10.1056/NEJMoa2309863 [DOI] [PubMed] [Google Scholar]
- 41. Catto JWF, B Tran, Rouprêt M, et al. Erdafitinib in BCG-treated high-risk non-muscle invasive bladder cancer. Ann Oncol Off J Eur Soc Med Oncol. Published online October 5, 2023:S0923-7534(23)04015-2. doi: 10.1016/j.annonc.2023.09.3116 [DOI] [Google Scholar]
- 42. Ali A, Song YP, Mehta S, et al. Palliative Radiation Therapy in Bladder Cancer-Importance of Patient Selection: A Retrospective Multicenter Study. Int J Radiat Oncol Biol Phys. 2019;105(2):389-393. doi: 10.1016/j.ijrobp.2019.06.2541. [DOI] [PubMed] [Google Scholar]
- 43. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol. 2000;47(2):379-388. doi: 10.1016/S0360-3016(00)00430-2 [DOI] [PubMed] [Google Scholar]
- 44. Zygogianni A, Kouloulias V, Armpilia C, et al. A weekly hypofractionated radiotherapeutic schedule for bladder carcinoma in elderly patients: local response, acute and late toxicity, dosimetric parameters and pain relief. J BUON Off J Balk Union Oncol. 2013;18(2):407-412. [PubMed] [Google Scholar]
- 45. Kouloulias V, Tolia M, Kolliarakis N, Siatelis A, Kelekis N. Evaluation of acute toxicity and symptoms palliation in a hypofractionated weekly schedule of external radiotherapy for elderly patients with muscular invasive bladder cancer. Int Braz J Urol Off J Braz Soc Urol. 2013;39(1):77-82. doi: 10.1590/S1677-5538.IBJU.2013.01.10 [DOI] [PubMed] [Google Scholar]
- 46. Lacarrière E, Smaali C, Benyoucef A, Pfister C, Grise P. The efficacy of hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery. Int Braz J Urol Off J Braz Soc Urol. 2013;39(6):808-816. doi: 10.1590/S1677-5538.IBJU.2013.06.06 [DOI] [PubMed] [Google Scholar]
- 47. Méry B, Falk AT, Assouline A, et al. Hypofractionated radiation therapy for treatment of bladder carcinoma in patients aged 90 years and more: A new paradigm to be explored? Int Urol Nephrol. 2015;47(7):1129-1134. doi: 10.1007/s11255-015-0999-8 [DOI] [PubMed] [Google Scholar]
- 48. Dirix P, Vingerhoedt S, Joniau S, Van Cleynenbreugel B, Haustermans K. Hypofractionated palliative radiotherapy for bladder cancer. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2016;24(1):181-186. doi: 10.1007/s00520-015-2765-y [DOI] [PubMed] [Google Scholar]
- 49. Hafeez S, McDonald F, Lalondrelle S, et al. Clinical Outcomes of Image Guided Adaptive Hypofractionated Weekly Radiation Therapy for Bladder Cancer in Patients Unsuitable for Radical Treatment. Int J Radiat Oncol Biol Phys. 2017;98(1):115-122. doi: 10.1016/j.ijrobp.2017.01.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aljabab S, Cheung P, Dennis K, Morgan SC. Hemostatic radiotherapy in advanced bladder cancer: a single-institution experience. J Radiat Oncol. 2017;6(4):379-385. doi: 10.1007/s13566-017-0318-3 [DOI] [Google Scholar]
- 51. Hasan S, Galvan EM, Shaver C, Hermans M, Ha CS, Swanson GP. Outcomes of patients undergoing radiation therapy for bladder cancer. Bladder San Franc Calif. 2018;5(4):e37. doi: 10.14440/bladder.2018.785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Varughese M, Treece S, Drinkwater KJ. Radiotherapy Management of Muscle Invasive Bladder Cancer: Evaluation of a National Cohort. Clin Oncol R Coll Radiol G B. 2019;31(9):637-645. doi: 10.1016/j.clon.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 53. Huddart R, Hafeez S, Lewis R, et al. Clinical Outcomes of a Randomized Trial of Adaptive Plan-of-the-Day Treatment in Patients Receiving Ultra-hypofractionated Weekly Radiation Therapy for Bladder Cancer. Int J Radiat Oncol Biol Phys. 2021;110(2):412-424. doi: 10.1016/j.ijrobp.2020.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109-122. doi: 10.1016/0360-3016(91)90171-y [DOI] [PubMed] [Google Scholar]
- 55. Hall E, Hussain SA, Porta N, et al. Chemoradiotherapy in Muscle-invasive Bladder Cancer: 10-yr Follow-up of the Phase 3 Randomised Controlled BC2001 Trial. Eur Urol. 2022;82(3):273-279. doi: 10.1016/j.eururo.2022.04.017 [DOI] [PubMed] [Google Scholar]
- 56. Stuk J, Vanasek J, Odrazka K, et al. Image-guided radiation therapy produces lower acute and chronic gastrointestinal and genitourinary toxicity in prostate cancer patients. J BUON Off J Balk Union Oncol. 2021;26(3):940-948. [PubMed] [Google Scholar]
- 57. Bamias A. Definition and Diagnosis of Oligometastatic Bladder Cancer: A Delphi Consensus Study Endorsed by the European Association of Urology, European Society for Radiotherapy and Oncology, and European Society of Medical Oncology Genitourinary Faculty. Eur Urol. Published online 2023. [DOI] [PubMed] [Google Scholar]
- 58. Kang JJ, Iwamoto KS, Peek EM, Chin AC, King CR. The Low Alpha-Beta Ratio of Bladder Cancer: A Rationale for Hypofractionation. In: Proceedings of the 96th Annual Meeting of the American Radium Society 2014. Vol 28. Oncology Vol 28 No 4_Suppl_1. MJH Life Sciences; 2014. Accessed December 18, 2022. https://www.cancernetwork.com/view/s036-low-alpha-beta-ratio-bladder-cancer-rationale-hypofractionation [Google Scholar]


