Abstract
Objectives
The objective of this study was to model multiple sclerosis (MS) disease progression and compare disease trajectories by sex, age of onset, and year of diagnosis.
Study Design and Setting
Longitudinal EDSS scores (20,854 observations) were collected for 1,787 relapse-onset MS patients at MS clinics in South Wales and modelled using a multilevel model (MLM). The MLM adjusted for covariates (sex, age of onset, year of diagnosis, and disease-modifying treatments), and included interactions between baseline covariates and time variables.
Results
The optimal model was truncated at 30 years after disease onset and excluded EDSS recorded within 3 months of relapse. As expected, older age of onset was associated with faster disease progression at 15 years (effect size (ES): 0.75; CI: 0.63, 0.86; p: <0.001) and female-sex progressed more slowly at 15 years (ES: −0.43; CI: −0.68, −0.18; p: <0.001). Patients diagnosed more recently (defined as 2007–2011 and >2011) progressed more slowly than those diagnosed historically (<2006); (ES: −0.46; CI: −0.75, −0.16; p: 0.006) and (ES: −0.95; CI: −1.20, −0.70; p: <0.001), respectively.
Conclusion
We present a novel model of MS outcomes, accounting for the non-linear trajectory of MS and effects of baseline covariates, validating well-known risk factors (sex and age of onset) associated with disease progression. Also, patients diagnosed more recently progressed more slowly than those diagnosed historically.
Keywords: Multiple sclerosis, Disability, Multilevel Model, Neurodegeneration, Complex level 1 variation
What Is New
We developed a contemporary multilevel model for multiple sclerosis disease progression with longer follow-up using an ongoing cohort established in 1985 from the same geographical region.
We assessed differences in disease trajectory by sex, age of onset, and year of diagnosis; showing that patients diagnosed in earlier years (before 2006) were found to have faster disability progression, while validating results that male-sex and older age of onset progressed faster than female-sex and younger age of onset, respectively.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disorder affecting the central nervous system and one of the leading causes of neurological disability in young adults [1]. Its aetiology remains unclear, but both genetic and environmental factors are contributors. Onset is most frequently in the third and fourth decades of life and is three times more frequent in females. Pathologically, MS is characterised by focal areas of inflammation and demyelination as well as more diffuse neurodegenerative features, throughout the central nervous system. MS commonly presents as recurrent sub-acute episodes of neurological dysfunction, which may remit to a varying degree [2] in the early stage of the disease but most people with MS (PwMS) develop a secondary progressive phase after a variable interval. In addition, a significant minority of patients present with primary progressive MS (PPMS) [3] with gradual accumulation of fixed disability from disease onset. However, the presentation and subsequent disease course of MS are highly variable and difficult to predict at onset, making decisions on management challenging for clinicians and PwMS [4].
Since the emergence of disease-modifying therapies (DMTs) for MS in the 1990s, an increasing number of DMTs with variable efficacy and safety profile are now available [5, 6]. However, uncertainty remains over selection and sequencing of DMTs; aggressive immune suppression early in MS may affect longer term disease outcomes, but some high-efficacy DMTs have risks of serious adverse events [7, 8]. As a result, there is an urgent need to identify biomarkers that can inform disease prognosis, treatment response, and potential for adverse events to enable a more personalised approach to interventions.
Reliable and contemporary data on MS outcomes are also needed for patient counselling, disease management, exploring the utility of candidate biomarkers, and to establish real-world effectiveness of new treatment or interventions. The expanded disability status scale (EDSS [9]) remains an internationally recognised clinical disability outcome measure for MS and a key element of epidemiological studies, clinical trial design, and treatment approval [10], despite having relatively poor intra- and inter-rater reliability, over-reliance on mobility, and low sensitivity to vision, arm function, or cognition [11].
In previous multilevel modelling (MLM) studies, we were able to highlight the non-linear trajectory of EDSS scores in PwMS eligible for DMTs and demonstrate modest benefits for injectable DMTs [12, 13]. In the current prospective cohort study of both treated and untreated PwMS, we aimed to develop a MLM using contemporary MS data incorporating additional demographic data (sex, age of onset, and year of diagnosis) to investigate differences in disease trajectory.
Methods
Data Source/Measurements
Data have been collected as part of an ongoing prospective observational study since 1985 [14]. Patients are reviewed annually from diagnosis and data on demographics, relapses, disease course, and disability were collected at each encounter. EDSS is routinely assessed and recorded in a standardised web-based form and incorporated into the registry. Inclusion criteria in this study were: a diagnosis of MS, a recorded date of MS onset, and consented for data to be used for research. Individuals were followed up either to October 2021, death, loss to follow-up, or withdrawal from the study. Individuals with PPMS were excluded as this minority progress more rapidly compared to relapse-onset MS (ROMS) patients [15]. This study has been approved by the Research Ethics Committee (REC approval number: 19/WA0289).
Time since onset (years) was used as the time-metric. DMTs were time-varying covariate because individuals were administered DMTs at varying time points. Other baseline covariates included sex, age of onset, and year of diagnosis. Time since onset and age of onset were continuous covariates, while sex and year of diagnosis (1986–2006, 2007–2011, >2011) were categorical covariates. The boundaries of year of diagnosis were based on the publication of revised diagnostic criteria [16, 17]. In general, with updated diagnostic criteria, shorter time between onset, diagnosis and initiation of treatment was achieved, which may affect long-term disability [18].
Development of the Statistical Model
Statistical Analysis
Association between EDSS disability outcome and covariates was assessed using MLMs, which are widely used for modelling disease progression [19, 20]. Several MLMs were developed, and interactions were tested (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000536427). To handle relapses, EDSS scores recorded within 1-, 3-, and 6-months post-relapse were removed in a series of MLMs to ascertain the optimal window. Autocorrelation was handled using median EDSS within quarter-year intervals. The best fitting model was identified using Akaike Information Criteria (AIC), root mean square error, proportion of EDSS scores within ± 0.5 predicted EDSS (PWPE), and the proportion outside ± 2 predicted EDSS (POPE). Nonconstant within-person variance was accounted for using a complex level 1 variance model (CLOVM). Statistical analyses and model fitting were performed using R version 4.1.1 [21], R2MLwiN [22], and lme4 [23].
Multilevel Model
MLM is a common statistical model for handling dependent observations, it can summarise the trajectory of the response over time, and account for within and between individual variability, thereby providing better estimates and predictions compared to the classical linear regression model [24]. Since EDSS scores vary regarding presentation and disease trajectories, we initially assumed a random intercept and slope model with time of measurement at level 1 and individuals at level 2:
| (1) |
where yij denotes the EDSS score at time j for individual i, and tij the time for individual i at time j. The β0 is the overall intercept, and β1 is the overall slope. The u0i and u1i are individual-specific random effects assumed to follow a bivariate normal distribution:
| (2) |
Thus, β0+ u0i and β1+ u1i are the intercept and slope for individual i. εij, the observation-level residual, is assumed to follow a normal distribution with mean zero and variance .
Time Metric and Transformations
Time since onset was used as the time variable and due to differences in disease trajectory for pwMS, fractional polynomials were allowed to model non-linear relationships between EDSS scores and time, and a value of 1 was added to the time variable to handle zeros in the data [25]. The fractional polynomial approach was preferred to splines since it is a simple way to choose among different transformations, produces smooth monotonic curves, and avoids selecting location and knots necessary in splines [26].
Relapses and Autocorrelation Structure
Stable recovery from relapse for most patients is achieved within 3 months [27], although some continue to recover beyond 6 months [28]. To avoid modelling EDSS in relapse, all scores within 1-, 3-, and 6-months post-relapse were omitted and the optimal window was selected according to the magnitude of the intercept-variance of the observations.
The observations could be taken at short intervals or remain unchanged for long periods causing autocorrelation, which could be accounted for using autoregressive-moving average models [29] or an integrated Ornstein-Uhlenbeck stochastic process [28]. We reduce autocorrelation by summarising observations within quarter-year intervals using the median score for individuals before model fitting.
Complex Level 1 Variation
The variation within individuals may depend on other covariates such as time, which allows nonconstant variance in residuals [30]. We accounted for nonconstant variance for the time of measurement using the CLOVM, which models level 1 residuals as a function of time [31], and used fractional polynomials to determine the optimal time function. Complex level 1 variation (CLOV) can be caused by measurement error and changes in EDSS scores over time, as disability level ranges considerably [18] and misclassification may be present with more variation than expected at the lower end of the EDSS scale versus the higher end due to intra- and inter-rater differences [11].
Effect of Patient Characteristics on Progression
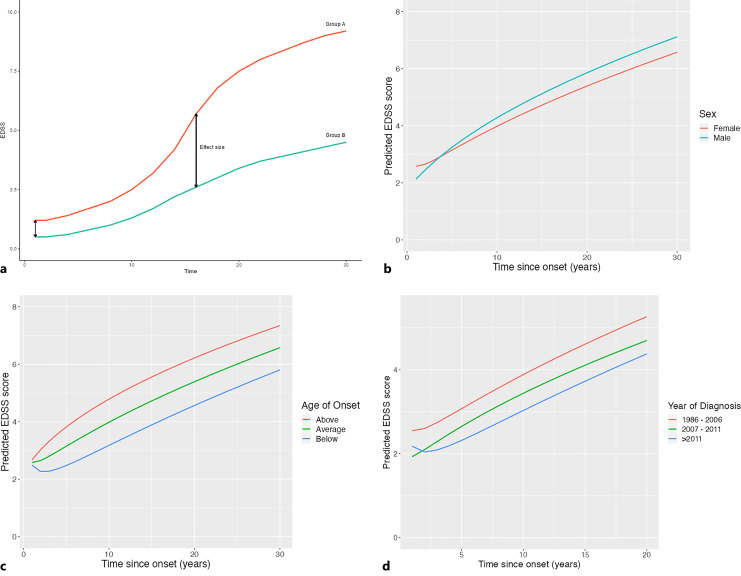
When trajectories are non-linear over time with more than one time term it becomes more difficult to understand the effect a variable (such as sex) has on progression. In a linear time model, the estimate would give the difference in the rate of change between males and females. However, with two non-linear time terms (log of time and square root of time) the effects of individual covariates are complicated and hard to interpret. So, for the important covariates (sex, age at onset and year of diagnosis) we have plotted the trajectories for different groups (Fig. 1a) and derived the effect these covariates would have at different times (initial year and 15 years) post onset (Table 1). The effect these covariates have on EDSS at different time points is a linear combination of the interactions between those covariates with the intercept and transformations of time.
Fig. 1.
a Schematic of progression lines and effect size for categorical covariates; group A (red) and group B (green). b Progression plot of females (red) and males (green). c Progression plot of patients young (blue) and older (red) at onset. d Progression plot for year of diagnosis. 1986–2006 (red), 2007–2011 (green), >2011 (blue).
Table 1.
Summary statistics of patient demographics
| Study characteristics, median (IQR) or N (percent) | |
|---|---|
| All recorded observation | |
| pwMS | 1,787 |
| Females | 1,284 (71.9%) |
| Age of onset | 30.3 (24–38.3) |
| Ever prescribed a DMT | 628 (35.1%) |
| Number of EDSS observation per person | 8 (3–16) |
| Time between EDSS observations, years | 0.38 (0.15–0.84) |
| Follow-up time, years | 6.9 (2.2–12.5) |
| Prospectively followed for ≥5 years | 1,048 (58.7%) |
| Prospectively followed for ≥10 years | 660 (36.9%) |
| First recorded observation | |
| EDSS score: median | 3.5 (2–6) |
| On DMT | 110 (6.2%) |
| Time since onset, years | 7.33 (1.74–17.74) |
| Last recorded observation | |
| EDSS score: median | 5.5 (2.5–6.5) |
| On DMT | 383 (21.4%) |
| Time since onset, years | 16.66 (8.92–27.17) |
SD, standard deviation; EDSS, Expanded Disability Status Scale; DMT, disease modifying therapy; N, number of observations.
Results
A total of 2,293 PwMS were identified with consent to research, of whom 2,167 had a recorded date of onset. After filtering, a total of 20,836 EDSS scores from 1,787 PwMS were included in the analysis.
Patient demographics are summarised in Table 2. A total of 1,787 patients with 20,836 EDSS scores from 1985 to 2021 were included. The mean age of onset was 31.5 years [32], and the majority were females (71.9%) [33]. Median follow-up time was 7.6 years (with a range of 0–24 years), with 58.7% and 36.9% followed-up for over 5 and 10 years, respectively. DMTs were administered to 35.1% of the whole cohort since 1985.
Table 2.
Combined effects of covariates (p value and 95% CI) of complex level 1 variance models with Var(t) = 0 using 30-year truncated data
| Variables | Effect estimate (95% CI) | p value |
|---|---|---|
| Female (onset) | 0.47 (−0.10, 1.0) | 0.11 |
| Female (year 15) | −0.43 (−0.68, −0.18) | 0.001 |
| Age of onset, one SD increase (onset) | 0.07 (−0.19, 0.33) | 0.60 |
| Age of onset, one SD increase (year 15) | 0.73 (0.62, 0.85) | <0.001 |
| Comparing 1986–2006 to other year-categories | ||
| Year of diagnosis (2007–2011) (onset) | −0.64 (−1.41, 0.14) | 0.11 |
| Year of diagnosis (>2011) (onset) | −0.40 (−1.04, 0.25) | 0.23 |
| Year of diagnosis (2007–2011) (year 10) | −0.45 (−0.75, −0.14) | 0.004 |
| Year of diagnosis (>2011) (year 10) | −0.90 (−1.15, −0.65) | <0.001 |
We compared the effect of variables on EDSS at onset and 15 years post onset for sex and age of onset, 10 years post onset for year of diagnosis, using their associations with intercept and time interactions.
CI, confidence interval; SD, standard deviation.
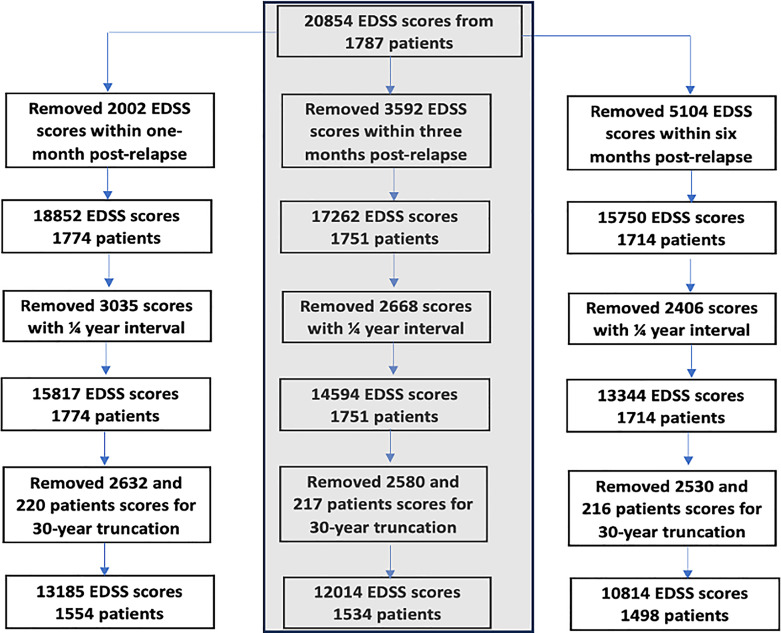
To model progression while ignoring relapses, EDSS scores recorded within 1-month post-relapse were excluded, reducing the number EDSS scores to 18,852. To reduce autocorrelation, EDSS scores within quarter-year intervals were summarised using the median, reducing the number of EDSS scores to 15,817 (Fig. 2).
Fig. 2.
Flowchart of the different models compared within this study (second column was used in the final model).
The CLOV MLM with square root and log of time transformations with interactions was the best model; with better fitted values compared to linear fixed effect model (online suppl. Table 1; online suppl. Fig. 1). However, accounting for CLOV in models with non-linear time proved difficult due to outlying EDSS observations in time. Therefore, observations recorded beyond 30 years for time since onset was excluded in subsequent analyses (online suppl. Table 2; online suppl. Fig. 2).
Because effects of relapse could continue beyond 1 month, EDSS scores within 3- and 6-month post-relapse were removed, and the model refitted. The 3-month post-relapse model was chosen as the optimal model because the CI of the intercept-variance at level 1 for 3- and 6-months are similar (CI: 0.76, 0.89) and (CI: 0.77, 0.84), respectively (online suppl. Table 4). Individuals on DMT showed faster progression than those not exposed to DMT, however, the effect size (ES) is small, and the p value is only borderline significant (ES: 0.06; p: 0.04, CI: 0.001, 0.10). PwMS who never had a DMT were followed up less frequently and had fewer EDSS measurements; their rate of EDSS measurements was ES: 0.57 (p: <0.001; CI: 0.55, 0.58) times that of patients who have had DMT.
The combined effects of covariates and their interactions were tested jointly (Table 1). Sex and sex-time interactions suggested no significant difference between sexes at onset, although, at 15 years females were associated with lower EDSS progression. Similarly, the combined effect of age of onset at 15 years indicates that of age of onset influences progression. At onset, the combined effect of year of diagnosis was not significantly different for patients diagnosed historically (1986–2006) compared to recently diagnosed patients (2007–2011, 2012–2018, and >2018), yet patients diagnosed historically progressed faster than patients diagnosed under the (revised) McDonald criteria. Comparing EDSS scores for patients diagnosed after 2018 to diagnosis made between 2007–2011 and 2012–2018 showed no significant difference at the initial year. However, at 10-year post-onset diagnosis made after 2018 had progressed more slowly compared to 2007–2011, but not 2012–2018 (p: 0.11; CI: −0.72, 0.22). Hence, we combined patients diagnosed >2018 with those diagnosed 2012–2018 in further analysis. The crude effects of these three covariates, when not adjusted for each other or DMT use, were very similar (online suppl. Table 5).
The average trajectories of both sexes (Fig. 1b) indicate that females have higher EDSS scores at onset but progressed more slowly with average EDSS scores of 5.1 and 4.8 for males and females, respectively, at 15 years [33, 34]. Furthermore, individuals with age of onset above the average had higher EDSS at 15 years post onset compared to patients with an age of onset below the average, with average EDSS scores of approximately 3.9 and 5.7, respectively (Fig. 1c) [35–37]. A comparison of the average trajectories for the categorised year of diagnosis showed a faster progression for patients diagnosed historically compared to patients diagnosed more recently (2007–2011 and >2011), with average EDSS scores of approximately 4.0, 3.5, and 3.0, respectively, 10 years post onset (Fig. 1d).
Discussion
The purpose of this study was to develop a model for MS disease progression in a contemporary population-based cohort and assess the differences in disability trajectory using baseline covariate (sex, age of onset and year of diagnosis). Overall, we show that patients diagnosed historically appear to have progressed faster compared to those diagnosed more recently and validated previous studies that female-sex progressed more slowly than male-sex [33], and patients with above average age of onset reached disability milestones faster than patients with below average age of onset [36].
Time since onset (years) was the time variable and fractional polynomials were used to allow flexible trajectories of progression in individuals. Models were developed after removing observations within 1-, 3-, and 6-month post-relapse to select the optimal recovery window. The 3-month relapse window was chosen because the CI of the intercept variance at level 1 overlaps with that of 6-month relapse window. Moreover, earlier findings suggest that most individuals recover within 3 months [27, 38, 39]. Autocorrelation was reduced using the median of EDSS scores within a quarter-year interval for individuals before model fitting.
The covariates in the model include time since onset, baseline characteristics, and DMT, including baseline covariates and time since onset interactions. Model comparisons suggest that accounting for CLOV provided a better fit for the data (online suppl. Table 2). However, due to outlying observations in time, the model did not converge for more complex models with many covariates, possibly because the level 1 variance became negative for outlying time observations. Moreover, variance of the residual was nonconstant (online suppl. Fig. 2a), hence, time was truncated at 30 years to remove outlying observations. The AIC for the model with indicates that observational-level variance is a function of time, confirming that EDSS scores have higher variation at lower EDSS scale [40].
Progression in males was faster even though they had lower EDSS scores at onset (time to EDSS 6 was 20 years vs. 25 years for females) in line with previous studies, which show that males progress faster (0.133 vs. 0.112 per year, p < 0.001) [41]. Individuals with below average age of onset took longer to reach EDSS milestones than individuals with above average age of onset, expected time to EDSS 6 was over 30 years versus 17 years, respectively. In a different study, MS progression doubled and tripled when onset occurred at ages 40 (odds ratio = 4.22) and 50 (odds ratio = 6.04), respectively, compared to the risk at age 20 (odds ratio = 2.0) [36]. However, this does not imply more favourable outcome since younger individuals could still accumulate more disabilities at younger age [42, 43]. Individuals diagnosed historically progressed faster than those diagnosed more recently (time to EDSS 6 was 25 years and over 30 years for individuals diagnosed between 2007 and 2012, and >2012–2018, respectively) (Fig. 1). This apparent improvement in outcome coincides with the era of high-efficacy DMTs. In addition, similar direction of effects was observed when combining the main and interaction effects for each baseline covariate (Table 1).
Several reasons may exist for patients diagnosed before 2006 to have a 0.5 higher EDSS score at baseline. Firstly, with the increasing resources, including MRI availability, in the National Health Service (NHS) UK, it is likely that patients with suspected demyelination will be seen sooner after the clinical disease onset. As a result, the lag time to initial assessment for patients with an onset in the 1980s and early 1990s will be longer and could lead to a higher EDSS score at first clinical assessment. Secondly, following the application of the revised 2017 McDonald diagnostic criteria, the time between onset and diagnosis is likely to be shorter, potentially leading to lower EDSS scores in more recently diagnosed patients. Thirdly, some reports suggest that MS has become milder over time leading to lower EDSS scores. Importantly, although the patients diagnosed before 2006 have a slightly higher baseline EDSS, their trajectory over time (the effect size in the MLM model) is similar.
In our cohort, the effect of DMT is uncertain because its effects depend on the model used (online suppl. Table 4). We attribute this effect to several sources of bias. Firstly, PwMS are more likely to be selected for DMT. Secondly, people receiving DMT are often followed up more frequently; this was evident in our cohort where PwMS who had never received DMT had half as many EDSS measurements than those who received DMT. PwMS who did not receive DMT may therefore have reached key disability milestones years before their EDSS scores were measured. Third, the proportion of individuals ever on high-efficacy DMTs (Ocrevus, Mitoxantrone, Natalizumab, and Alemtuzumab) are smaller (0.12) than the proportion of individuals ever on moderate efficacy DMT (0.43) and individuals never on DMT (0.53). In addition, because some individuals receive high, moderate and no DMTs at different times the proportion of observations with high efficacy DMTs is small (0.06) compared to observations on moderate efficacy DMTs (0.19) and observations off DMT (0.75) (online suppl. Fig. 5). In our model, DMT is treated as a time-varying covariate (because individuals are on or off DMTs at different time points), and the estimate represents mean difference in the EDSS of observations when an individual is on versus off DMTs rather than progression rate. Although the direction of the effects seems paradoxical, a mean difference of 0.06 on EDSS is not clinically meaningful. In addition, none of the effect sizes contained within the confidence interval (0, 0.11) for this effect are clinically meaningful.
The limitations of the study include potential measurement bias which may be present in the EDSS scores, especially because of inter-rater variability is higher at lower scores [18]. This could affect the true disability score and trajectory of individuals. We did not have data to incorporate comorbidities such as depression, and smoking, which could be associated with MS outcome [44–48]. These data were gathered from a population-based cohort in the UK, so may not reflect rates of disability progression elsewhere since rates of progression are affected to some extent by geographical location [49]. Due to the problem of extrapolation, we have compared expected EDSS at 10 years with year of diagnosis because the group of MS patients diagnosed after 2011 does not have a follow-up data of 15 years post diagnosis. There may be confounders of the relationships between age, sex, year of diagnosis and progression which we have not attempted to adjust for. We have not adjusted for covariates that may act as potential confounders for age, sex, year of diagnosis, and disease progression.
Compared to the earlier model [12], both models confirm that the progression of PwMS is non-linear, even in a much larger contemporary sample. However, the current model adjusts for baseline covariates, and interactions with time. We found that males, older patients, and historical patients progress faster.
The modelling approach could be applied to other cohorts to estimate EDSS scores at certain time points after adjusting for baseline covariates. The ability to produce long-term estimations gives this model great potential as an outcome measure to identify meaningful biomedical, genetic, and MRI biomarkers for clinical studies. For instance, estimated EDSS scores at latter years can be used as a phenotype in a genome-wide association studies of MS progression.
Acknowledgments
We would also like to thank the late Professor Lesley Jones for her valuable contributions that made this work possible.
Statement of Ethics
PwMS were selected from a regional disease registry according to accepted diagnostic criteria and were under the care of regional specialists in an MS centre in Cardiff, UK A minority from neighbouring health boards (Aneurin Bevan, Swansea Bay, Powys) who had received some care or were previously resident in Cardiff were also included. Patients in the registry with a diagnosis of MS, a recorded date of MS onset, and who had consented to research were included. This study was approved by the Research Ethics Committee of Health and Care Research Wales, reference number 19/WA/0289, and all participants provided written informed consent before inclusion.
Conflict of Interest Statement
K.E.H. reports speaker and personal fees from Roche, Merck, and Biogen, and travel grants to attend educational meetings from Roche, Novartis, Merck, and Biogen. E.C.T. has received honoraria for consulting work from Novartis, Merck, Biogen and Roche, and funding to attend or speak at educational meetings from Biogen, Janssen, Merck, Roche, Takeda, and Novartis. N.P.R. reports honoraria from Roche, Sanofi Genzyme and Novartis, and research grants from Novartis, Sanofi Genzyme, and Biogen. M.L. received fees for advising on a secondary analysis of an R.C.T. sponsored by North Bristol NHS Trust. E.C.U. declares no financial interests. P.H. is a member of the Scientific Review Committee of Enroll-HD, for which he receives a small Honorarium. K.L.K. received consultancy fees from Biogen, speaker fees from Biogen and Roche, and travel grants from Merck, Janssen and Novartis.
Funding Sources
ECU received salary from Cardiff University Wellcome Institution Strategic Funding award, Award Number: 204824/Z/16/Z. ML employed by University of Bristol, core-funded. PH is supported by the Medical Research Council (MRC) Centre for Neuropsychiatric Genetics and Genomics, Award Number: MR/K013041/1.
Author Contributions
P.H., L.J., N.P.R., and E.C.T. contributed to conceptualization of the project; E.C.T., N.P.R., J.H., M.L., L.J., and PH contributed to funding acquisition; E.C.U., K.E.H., and E.C.T. contributed to Data curation; E.C.U., M.L., P.H., and K.L.K. contributed to methodology and formal analysis; M.L., P.H., and E.C.T. contributed to supervision; E.C.U. and E.C.T. contributed to original draft; K.E.H., J.H., K.L.K., P.H., N.P.R., and M.L. contributed to reviewing and editing.
Funding Statement
ECU received salary from Cardiff University Wellcome Institution Strategic Funding award, Award Number: 204824/Z/16/Z. ML employed by University of Bristol, core-funded. PH is supported by the Medical Research Council (MRC) Centre for Neuropsychiatric Genetics and Genomics, Award Number: MR/K013041/1.
Data Availability Statement
Access to the data used in this study can be provided by the Division of Psychological Medicine and Clinical Neuroscience, School of Medicine, Cardiff University, UK upon reasonable request.
Supplementary Material
References
- 1. McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–79. [DOI] [PubMed] [Google Scholar]
- 2. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17. [DOI] [PubMed] [Google Scholar]
- 3. Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu J, Kelly E, Bielekova B. “Current status and future opportunities in modeling clinical characteristics of multiple sclerosis”. Front Neurol. 2022;13:884089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jokubaitis VG, Spelman T, Kalincik T, Lorscheider J, Havrdova E, Horakova D, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol. 2016;80(1):89–100. [DOI] [PubMed] [Google Scholar]
- 6. Kavaliunas A, Manouchehrinia A, Stawiarz L, Ramanujam R, Agholme J, Hedström AK, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler. 2017;23(9):1233–40. [DOI] [PubMed] [Google Scholar]
- 7. Binzer S, McKay KA, Brenner P, Hillert J, Manouchehrinia A. Disability worsening among persons with multiple sclerosis and depression: a Swedish cohort study. Neurology. 2019;93(24):e2216–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kopp TI, Blinkenberg M, Petersen T, Sorensen PS, Magyari M. Long term effect of delayed treatment on disability in patients with paediatric onset multiple sclerosis: a prospective Danish cohort study. Mult Scler Relat Disord. 2020;40:101956. [DOI] [PubMed] [Google Scholar]
- 9. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. [DOI] [PubMed] [Google Scholar]
- 10. D’Souza M, Kappos L, Czaplinski A. Reconsidering clinical outcomes in Multiple Sclerosis: relapses, impairment, disability and beyond. J Neurol Sci. 2008;274(1–2):76–9. [DOI] [PubMed] [Google Scholar]
- 11. Meyer-Moock S, Feng Y-S, Maeurer M, Dippel F-W, Kohlmann T. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawton M, Tilling K, Robertson N, Tremlett H, Zhu F, Harding K, et al. A longitudinal model for disease progression was developed and applied to multiple sclerosis. J Clin Epidemiol. 2015;68(11):1355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tilling K, Lawton M, Robertson N, Tremlett H, Zhu F, Harding K, et al. Modelling disease progression in relapsing-remitting onset multiple sclerosis using multilevel models applied to longitudinal data from two natural history cohorts and one treated cohort. Health Technol Assess. 2016;20(81):1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swingler RJ, Compston DA. The prevalence of multiple sclerosis in south east Wales. J Neurol Neurosurg Psychiatry. 1988;51(12):1520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harding KE, Wardle M, Moore P, Tomassini V, Pickersgill T, Ben-Shlomo Y, et al. Modelling the natural history of primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(1):13–9. [DOI] [PubMed] [Google Scholar]
- 16. Chataway J. Evolving diagnostic criteria for multiple sclerosis. Lancet Neurol. 2018;17(2):118. [DOI] [PubMed] [Google Scholar]
- 17. Solomon AJ, Naismith RT, Cross AH. Misdiagnosis of multiple sclerosis: impact of the 2017 McDonald criteria on clinical practice. Neurology. 2019;92(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tallantyre EC, Major PC, Atherton MJ, Davies WA, Joseph F, Tomassini V, et al. How common is truly benign MS in a UK population? J Neurol Neurosurg Psychiatry. 2019;90(5):522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iddi S, Li D, Aisen PS, Rafii MS, Litvan I, Thompson WK, et al. Estimating the evolution of disease in the Parkinson’s progression markers initiative. Neurodegener Dis. 2018;18(4):173–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raket LL. “Statistical disease progression modeling in alzheimer disease”. Front Big Data. 2020;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team ‘R . A language and environment for statistical computing. 2020. [Online]. Available from: https://www.semanticscholar.org/paper/R%3A-A-language-and-environment-for-statistical-Team/659408b243cec55de8d0a3bc51b81173007aa89b (accessed Mar 30, 2022). [Google Scholar]
- 22. Zhang Z, Parker R, Charlton CMJ, Leckie G, Browne W. “R2MLwiN. A package to run MLwiN from within R”. 2016. [Google Scholar]
- 23. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 24. Hunter MD. Multilevel modeling in classical twin and modern molecular behavior genetics. Behav Genet. 2021;51(3):301–18. [DOI] [PubMed] [Google Scholar]
- 25. Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28(5):964–74. [DOI] [PubMed] [Google Scholar]
- 26. Austin PC, Fang J, Lee DS. Using fractional polynomials and restricted cubic splines to model non-proportional hazards or time-varying covariate effects in the Cox regression model. Stat Med. 2022;41(3):612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kantarci OH, Zeydan B, Atkinson EJ, Conway BL, Castrillo-Viguera C, Rodriguez M. Relapse recovery: the forgotten variable in multiple sclerosis clinical trials. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hughes RA, Kenward MG, Sterne JAC, Tilling K. Analyzing repeated measurements while accounting for derivative tracking, varying within-subject variance, and autocorrelation: the xtmixediou command. The Stata J. 2017;17(3):573–99. [Google Scholar]
- 29. Mikkonen S, Rahikainen M, Virtanen J, Lehtonen R, Kuikka S, Ahvonen A. A linear mixed model with temporal covariance structures in modelling catch per unit effort of Baltic herring. ICES J Mar Sci. 2008;65(9):1645–54. [Google Scholar]
- 30. Goldstein H. An introduction to multilevel models. Multilevel statistical models. 2010; p. 1–14. [Google Scholar]
- 31. Steele F. Module 5: introduction to multilevel modelling Concepts. 2008. [Google Scholar]
- 32. “Gender issues in multiple sclerosis: an update: céline jobin, catherine larochelle, hélène parpal, patricia K coyle, pierre duquette. 2010’. [Online]. Available from: https://journals.sagepub.com/doi/10.2217/WHE.10.69 (accessed Nov 01, 2023). [DOI] [PubMed]
- 33. Coyle PK. What can we learn from sex differences in MS? J Pers Med. 2021;11(10):1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voskuhl RR, Patel K, Paul F, Gold SM, Scheel M, Kuchling J, et al. Sex differences in brain atrophy in multiple sclerosis. Biol Sex Differ. 2020;11(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cierny D, Lehotsky J, Hanysova S, Michalik J, Kantorova E, Sivak S, et al. The age at onset in Multiple Sclerosis is associated with patient’s prognosis. Bratisl Lek Listy. 2017;118(6):374–7. [DOI] [PubMed] [Google Scholar]
- 36. Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77(13):1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ow N, Kuspinar A, Mayo NE; for MSOAC . Age differences in trajectories of self-rated health of young people with Multiple Sclerosis. Mult Scler Relat Disord. 2022;57:103322. [DOI] [PubMed] [Google Scholar]
- 38. Benedict RH, Pol J, Yasin F, Hojnacki D, Kolb C, Eckert S, et al. Recovery of cognitive function after relapse in multiple sclerosis. Mult Scler. 2021;27(1):71–8. [DOI] [PubMed] [Google Scholar]
- 39. Hirst C, Ingram G, Pickersgill T, Swingler R, Compston Da. S, Robertson NP. Increasing prevalence and incidence of multiple sclerosis in South East Wales. J Neurol Neurosurg Psychiatry. 2009;80(4):386–91. [DOI] [PubMed] [Google Scholar]
- 40. Hughes S, Spelman T, Trojano M, Lugaresi A, Izquierdo G, Grand’maison F, et al. The Kurtzke EDSS rank stability increases 4 years after the onset of multiple sclerosis: results from the MSBase Registry. J Neurol Neurosurg Psychiatry. 2012;83(3):305–10. [DOI] [PubMed] [Google Scholar]
- 41. Ribbons KA, McElduff P, Boz C, Trojano M, Izquierdo G, Duquette P, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One. 2015;10(6):e0122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595–605. [DOI] [PubMed] [Google Scholar]
- 43. Vukusic S, Confavreux C. Natural history of multiple sclerosis: risk factors and prognostic indicators. Curr Opin Neurol. 2007;20(3):269–74. [DOI] [PubMed] [Google Scholar]
- 44. Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McKay KA, Tremlett H, Fisk JD, Zhang T, Patten SB, Kastrukoff L, et al. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology. 2018;90(15):e1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moccia M, Lanzillo R, Palladino R, Maniscalco GT, De Rosa A, Russo C, et al. The Framingham cardiovascular risk score in multiple sclerosis. Eur J Neurol. 2015;22(8):1176–83. [DOI] [PubMed] [Google Scholar]
- 48. Panda SP, Das RC, Srivastava K, Ratnam A, Sharma N. Psychiatric comorbidity in multiple sclerosis. Neurologia i Neurochirurgia Polska. 2018;52(6):704–9. [DOI] [PubMed] [Google Scholar]
- 49. Sharmin S, Roos I, Simpson-Yap S, Malpas C, Sánchez MM, Ozakbas S, et al. The risk of secondary progressive multiple sclerosis is geographically determined but modifiable. Brain. 2023;146(11):4633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the data used in this study can be provided by the Division of Psychological Medicine and Clinical Neuroscience, School of Medicine, Cardiff University, UK upon reasonable request.