Abstract
Background
Within influenza vaccine effectiveness (VE) studies at primary care level with a laboratory-confirmed outcome, clinical case definitions for recruitment of patients can vary. We used the 2022–23 VEBIS primary care European multicentre study end-of-season data to evaluate whether the clinical case definition affected IVE estimates.
Methods
We estimated VE using a multicentre test-negative case-control design. We measured VE against any influenza and influenza (sub)types, by age group (0–14, 15–64, ≥65 years) and by influenza vaccine target group, using logistic regression. We estimated IVE among patients meeting the European Union (EU) acute respiratory infection (ARI) case definition and among those meeting the EU influenza-like illness (ILI) case definition, including only sites providing information on specific symptoms and recruiting patients using an ARI case definition (as the EU ILI case definition is a subset of the EU ARI one).
Results
We included 24 319 patients meeting the EU ARI case definition, of whom 21 804 patients (90 %) meet the EU ILI case definition, for the overall pooled VE analysis against any influenza. The overall and influenza (sub)type-specific VE varied by ≤2 % between EU ILI and EU ARI populations.
Discussion
Among all analyses, we found similar VE estimates between the EU ILI and EU ARI populations, with few (10%) additional non-ILI ARI patients recruited. These results indicate that VE in the 2022–23 influenza season was not affected by use of a different clinical case definition for recruitment, although we recommend investigating whether this holds true for next seasons.
Keywords: Influenza, Influenza vaccine, Vaccine effectiveness, Clinical case definition, Multicenter study, Test-negative design
1. Introduction
Influenza vaccination is a key measure to protect against infection and severe disease caused by influenza viruses. Because influenza viruses undergo frequent genetic and antigenic changes, vaccines are reformulated each year. Therefore, it is important to monitor influenza vaccine effectiveness (VE) annually. There are many studies globally estimating influenza VE [1], [2], [3], [4], [5], [6], [7], [8], [9]. Clinical case definitions for recruitment of patients vary slightly across studies at primary care level. In the US, acute respiratory illness (ARI) with cough of 7 or fewer days’ duration is used [2], [3]. In Canada [4], Australia [9], Hong Kong [8], South Africa and New Zealand [9], influenza-like illness (ILI) is used, although there is variation between the symptoms used to define ILI.
The clinical case definition used in influenza VE studies without laboratory confirmation may affect VE [10]. VE estimates may also be affected by the case definition for patient inclusion in studies with laboratory-confirmed outcomes, if this case definition affects the case status and vaccination status differentially. There is some evidence that influenza vaccination may attenuate severe disease/outcomes, but also clinical symptoms, notably fever [11], [12]. Given this, if a more sensitive case definition is used, vaccinated influenza cases with milder symptoms may have a higher chance of being selected. This would result in lower influenza VE. Alternatively, the use of a different case definition may affect the distribution of patients with non-influenza aetiologies, which would change the VE if these were also associated with different influenza vaccine coverage.
Since July 2022, the I-MOVE (Influenza – Monitoring Vaccine Effectiveness in Europe) network has been embedded in VEBIS (Vaccine Effectiveness, Burden and Impact Studies), a network to estimate influenza and COVID-19 VE in the European Union (EU) and European Economic Area (EEA). The I-MOVE network has been estimating influenza VE since 2008–9, with up to 12 countries providing data for pooled influenza VE analysis at primary care level. Prior to the COVID-19 pandemic, patients recruited by study sites within the I-MOVE network at primary care level were most often recruited through sentinel ILI surveillance, with a few countries using a mix of ILI or ARI surveillance (the Netherlands, Germany, Sweden). In pooled influenza VE analysis, we would restrict the analysis to those meeting the EU ILI case definition (sudden onset of symptoms AND at least one of fever or feverishness, malaise, headache, myalgia AND at least one of cough, sore throat, shortness of breath) [13].
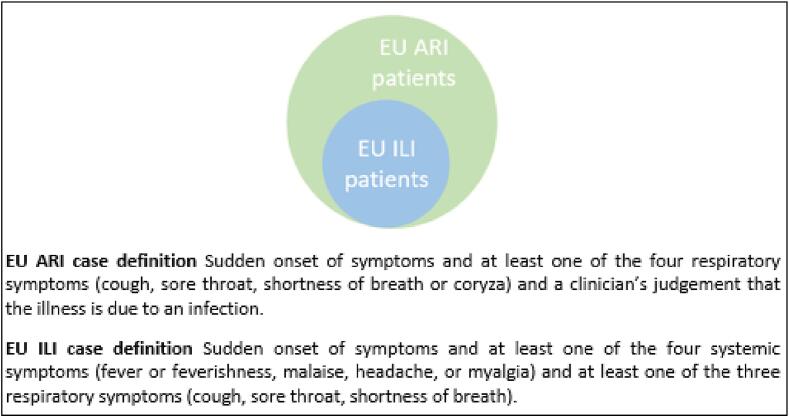
With the COVID-19 pandemic, and WHO and ECDC recommendations for integrated surveillance [14], [15], many countries are moving from recruitment of ILI to ARI patients within sentinel surveillance. An ARI case definition is more sensitive for influenza and COVID-19 than the ILI one (94 % vs. 45–55 % for influenza and 60–96 % vs. 20–55 % for COVID-19, respectively), although with a lower specificity for influenza and COVID-19 (27 % vs. 85–95 % for influenza and 10–45 % vs. 38–90 % for COVID-19, respectively) [14]. The EU ILI case definition is a subset of the EU ARI one; all those meeting the EU ILI case definition should also meet the EU ARI case definition; Fig. 1 [13]. Compared to the EU ILI case definition, the EU ARI case definition does not take into account the systemic symptoms but requires a clinician’s judgement to confirm that the illness is due to an infection. Fever, as a systemic symptom, may be less present among older adults due to a lower febrile response [16], or among disease presentation of other respiratory pathogens, notably RSV [17], [18], [19]. Additionally, certain systemic symptoms (headache, myalgia) may be difficult to collect among very young children. Therefore, excluding the systemic component could have an impact on both cases and controls included in the VE studies.
Fig. 1.
Patients meeting the EU ILI case definition as a subset of patients meeting the EU ARI case definition.
We used the 2022–23 VEBIS primary care multicentre study end-of-season data to evaluate the effect on influenza VE of the clinical case definition used for patient recruitment and for inclusion of patients for the pooled analysis by (sub)type, age group and influenza vaccine target group.
2. Methods
The methods of the multicentre case-control study have been described previously [20], [21]. Briefly, study sites use the ECDC generic case-control study protocol and the I-MOVE+ generic study protocol, recruiting patients using the test-negative design [22], [23], [24].
Study sites in 10 European countries contributed to the 2022–23 multicentre study: Croatia, France, Germany, Hungary, Ireland, the Netherlands, Portugal, Romania, Spain (with two distinct study sites, national and Navarra region) and Sweden. The study period was from September 2022 to May 2023. We restricted this specific analysis to sites collecting information on individual symptoms using the EU ARI case definition to recruit patients, in order to reconstitute the different case definitions. Patients consulting participating general practitioners (GPs) or paediatricians were systematically selected for the study. Physicians either collected specimens from patients themselves or referred them to a medical laboratory or a dedicated testing centre. We restricted our analysis to patients swabbed within 7 days of symptom onset. Patients testing PCR positive for influenza virus were designated as cases and those testing negative as controls. Information on patients was obtained using interviews, questionnaires and/or linkage to electronic health records, depending on study site.
We defined a person as vaccinated if they had received at least one dose of a 2022–23 influenza vaccine 14 or more days before symptom onset. Patients vaccinated fewer than 14 days before symptom onset were excluded. All the others were classified as unvaccinated. The unvaccinated was the reference group for the VE analysis.
For each study site, we included patients presenting symptoms 14 or more days after the start of national influenza vaccination campaigns and controls were excluded if presenting in weeks of onset prior to the first influenza (sub)type positive case for each (sub)type-specific analysis.
We excluded any study site from the pooled analysis that had fewer than 10 influenza type-specific cases. We combined individual patient data and used a one-stage model, with study site as a fixed effect. We estimated influenza VE as 1-(odds of influenza vaccination among cases/odds of influenza vaccination among controls). We conducted a complete case analysis and used logistic regression to estimate VE, including a priori potential confounding factors: age, sex, presence of at least one commonly collected chronic condition (including lung disease, heart disease, immunodeficiency and diabetes) and date of onset. For continuous variables, we used age categorised in narrow groups (0–1, 2, 3–4, 5–9, 10–19, 20–29, …, 60–69, 70+ years), as a linear term or as a restricted cubic spline (with three, four or five knots) and onset date as a restricted cubic spline (with three, four or five knots). We used the Akaike information criterion (AIC) to select the best functional form.
We estimated VE for any influenza and by influenza (sub)type, and where sample size allowed stratified by age group (0–14 years, 15–64 years, 65 years and older). We estimated influenza VE among target groups for influenza vaccination.
For each population, we estimated influenza VE among patients meeting the EU ARI case definition and among patients meeting the EU ILI case definition.
We conducted a sensitivity analysis excluding SARS-CoV-2-positive controls to account for correlation between influenza and COVID-19 vaccination that could bias VE estimates [25].
3. Results
3.1. Main analysis: Among study sites recruiting using the EU ARI case definition
We included eight study sites: Germany, Spain (national, ES), Croatia, Hungary, Ireland, the Netherlands, Portugal and Romania (Table 1). We excluded two study sites (Navarra region and Sweden), where patients were recruited according to respiratory syndromes (ILI and ARI), but individual symptom information was not available. We did not include France as they recruited patients meeting a country-specific ILI case definition.
Table 1.
Symptoms collected by study site, study on effect of case definition on influenza vaccine effectiveness, VEBIS primary care multicentre case-control study, EU/EEA, 2022–23.
| Study site | Sudden onset of symptoms |
Systemic symptoms |
Respiratory symptoms |
Case definition for recruitment of patients for the VE study | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Malaise | Headache | Myalgia | Cough | Sore throat | Shortness of breath | Coryza | |||
| Germany | x | x | x | x | x | x | x | EU ARI | ||
| Spain | x | x | x | x | x | x | x | x | EU ARI | |
| Croatia | x | x | x | x | x | x | x | x | x | EU ARI |
| Hungary | x | x | x | x | x | x | x | x | x | EU ARI |
| Ireland | x | x | x | x | x | x | x | x | EU ARI | |
| The Netherlands | x | x | x | x | x | x | x | x | x | EU ARI |
| Portugal | x | x | x | x | x | x | x | x | x | EU ARI |
| Romania | x | x | x | x | x | x | x | x | x | EU ARI |
The EU ARI case definition was created without taking into account coryza as not all the countries collect information on this symptom. Germany additionally does not collect information on shortness of breath or malaise.
Among the study sites collecting information on symptoms and using the EU ARI case definition for recruitment, there were 27 277 patients meeting the EU ARI case definition, of whom 24 243 patients also met the EU ILI case definition (Table 2). Among influenza cases, only 160/6 715 (2.4 %) met the EU ARI case definition, but not the EU ILI case definition. Among controls, 2 404/20 562 (11.7 %) met the EU ARI case definition, but not the EU ILI case definition. Overall, the proportion of patients meeting both case definitions among the 0–14-year-olds was 88.8 % (7160/8062) compared to 91 % (13926/15319) among the 15–64-year-olds and 81 % (3141/3879) among those aged 65 years and older (Supplementary table 1).
Table 2.
Table of patients meeting the EU ARI and the EU ILI case definitions among study sites using the EU ARI case definition to recruit patients (n = 31 645), study on effect of case definition on influenza vaccine effectiveness, VEBIS primary care multicentre case-control study, EU/EEA, 2022–23.
| Meets ILI case definition |
|||||
|---|---|---|---|---|---|
| Meets ARI case definition | No | Yes | Missingb | Total | |
| All | No | 2 031 | 0 | 0 | 2031 |
| Yes | 2 564 | 24 243 | 470 | 27 277 | |
| Missinga | 23 | 0 | 2 314 | 2337 | |
| Total | 4 618 | 24 243 | 2 784 | 31 645 | |
| Influenza cases | No | 439 | 0 | 0 | 439 |
| Yes | 160 | 6 524 | 31 | 6715 | |
| Missinga | 0 | 0 | 479 | 479 | |
| Total | 599 | 6 524 | 510 | 7633 | |
| Controls | No | 1 592 | 0 | 0 | 1592 |
| Yes | 2 404 | 17 719 | 439 | 20 562 | |
| Missinga | 23 | 0 | 1 835 | 1858 | |
| Total | 4 019 | 17 719 | 2 274 | 24 012 | |
The ARI case definition was coded as “missing” among patients with missing information for “sudden onset” or where at least one of the four respiratory symptoms was coded as missing and none was coded as “yes”.
The ILI case definition was coded as “missing” among patients with missing information for “sudden onset” or where at least one of the four systemic symptoms was coded as missing and none was coded as “yes” or where at least one of the three respiratory symptoms (cough, sore throat, shortness of breath) was coded as missing and none was coded “yes”.
The proportion of influenza positive cases was 24.6 % (6 715/27 277) among patients meeting the EU ARI case definition compared to 26.9 % (6 524/24 243) among those meeting the EU ILI case definition. It was 6.2 % (160/2564) among those not meeting the EU ILI case definition but meeting the EU ARI case definition.
3.2. Descriptive analysis
Among the patients meeting the EU ARI case definition, the median age was 39 years (IQR: 13–58) among controls and 24 years (IQR: 9–43) among influenza cases (any subtype). Among controls, 15 % were aged 0–4 years old, compared with 10 % and 12 % among influenza A(H1N1)pdm09 and A(H3N2) cases, respectively. Twenty percent (n = 4 093) of controls were vaccinated compared with 7 % (n = 461) of influenza cases. Twelve percent of controls were positive to SARS-CoV-2 vs 2 % of cases (Supplementary table 2).
The results from the descriptive analyses were very similar among the patients meeting the EU ILI case definition for all baseline characteristics (Supplementary table 3).
3.3. VE analysis
We excluded 2 958 patients (11 %) from those meeting the EU ARI case definition and 2 439 patients (10 %) from those meeting the EU ILI case definition from the complete analysis due to missing data for age, sex, influenza vaccination status or chronic condition.
The overall adjusted VE against any influenza was 53 % (95 % CI: 47–59) among the patients meeting the EU ARI case definition and 54 % (95 % CI: 47–59) among the patients meeting the EU ILI case definition (Fig. 2; Supplementary table 4). The adjusted VE among the 0–14-year-olds was 68 % (95 % CI: 57–76) among the patients meeting both case definitions. The adjusted VE among the 15–64-year-olds was 53 % (95 % CI: 44–61) for both populations. Among those aged 65 years and older, the VE was 35 % (95 % CI: 16–49) and 33 % (95 % CI: 12–48) among the patients meeting the EU ARI and those meeting the EU ILI case definitions, respectively.
Fig. 2.
Overall, influenza A, A(H3N2), A(H1N1)pdm09 and B vaccine effectiveness** among study sites collecting information on symptoms and recruiting patients meeting the EU ARI case definition, study on effect of case definition on influenza vaccine effectiveness, VEBIS primary care multicentre case-control study, EU/EEA, 2022–23. **VE estimates were based on a complete case analysis and records with missing data for covariates were dropped. These VE results are a subset of the data and should not be used as the main reference for VEBIS primary care influenza VE within the 2022–23 season.
Against influenza A, the overall adjusted VE was 40 % (95 % CI: 32–48) and 41 % (95 % CI: 32–49) among the patients meeting the EU ARI case definition and the patients meeting the EU ILI case definition, respectively.
Against influenza A(H3N2), the overall adjusted VE was 32 % (95 % CI: 19–43) and 32 % (95 % CI: 18–44) among the patients meeting the EU ARI and those meeting the EU ILI case definitions, respectively.
Against influenza A(H1N1)pdm09, the overall adjusted VE was 54 % (95 % CI: 42–63) for both populations.
Against influenza B, the overall adjusted VE was 76 % (95 % CI: 69–82) and 77 % (95 % CI: 69–83) among the patients meeting the EU ARI and those meeting the EU ILI case definitions, respectively.
Sample size did not allow estimation of VE against influenza B among those aged 65 years and older.
3.4. Sensitivity analysis
The overall adjusted VE against any influenza when excluding SARS-CoV-2 positive patients among controls was 55 % (95 % CI: 49–60) among patients meeting the EU ARI case definition and 55 % (95 % CI: 49–61) among those meeting the EU ILI case definition (Supplementary table 5). Among patients meeting the EU ARI case definition after excluding controls who were SARS-CoV-2-positive, the adjusted VE against any influenza was 69 % (95 % CI: 59–77) among 0–14-year-olds, 54 % (95 % CI: 45–62) among 15–64-year-olds and 38 % (95 % CI: 20–52) among those aged 65 years and older. Among patients meeting the EU ILI case definition without the SARS-CoV-2-positive controls, for the same age groups, adjusted VE against any influenza was 69 % (95 % CI: 58–77), 54 % (95 % CI: 45–62) and 39 % (95 % CI: 20–53), respectively.
When excluding SARS-CoV-2 positive controls, the overall adjusted VE against influenza A(H3N2) was 37 % (95 % CI: 24–47) among patients meeting the EU ARI case definition and 36 % (95 % CI: 23–47) among patients meeting the EU ILI case definition. The overall VE against influenza A(H1N1)pdm09 was 54 % (95 % CI: 42–64) among patients meeting the EU ARI case definition and among patients meeting the EU ILI case definition. The overall VE against influenza B was 77 % (95 % CI: 69–83) among both patients meeting the EU ARI case definition and patients meeting the EU ILI case definition.
4. Discussion
We conducted an analysis of influenza VE among patients meeting the EU ILI vs the EU ARI case definitions, using data from the end-of-season 2022–23 VEBIS multicentre study at primary care level. We performed the study among all study sites collecting individual symptom information and using the EU ARI case definition for patient recruitment. In the analysis, 97 % of influenza cases and 86 % of controls meeting the EU ARI case definition also met the EU ILI case definition.
In all analyses, the VE among the population meeting the EU ILI and among those meeting the EU ARI case definitions were similar, with absolute differences ranging from 0–2 %, confidence intervals overlapping.
These results indicate that influenza VE in the 2022–23 season in this setting was not affected by use of the EU ILI or EU ARI case definition when restricting to study sites collecting individual information on symptoms and recruiting patients using the EU ARI case definition.
An analysis from the United Kingdom on COVID-19 VE using linked electronic databases, demonstrated that more specific case definitions led to higher VE against the Omicron variant with hospitalization as an outcome [26]. Sullivan and colleagues noted that differences in clinical criteria may be more important in COVID-19 studies of severe vs. mild disease [27]. In our study of mild influenza, we similarly find little effect of specific clinical criteria on influenza VEVE.
Depending on the case definition used, the distribution of patients with non-influenza aetiologies may change, which could lead to a modification of the VE if these patients had different vaccine coverages. This may be the case for the SARS-CoV-2-positive controls, as being vaccinated against COVID-19 and against influenza may be correlated, resulting in a lower influenza vaccination coverage if SARS-CoV-2 controls are included [25]. This was indeed observed in our study, as exclusion of SARS-CoV-2 controls resulted in a higher VE by up to 6 % in absolute value. This was also observed in the main end-of-season 2022–23 influenza VE analysis, where sites recruited patients met the EU ARI or EU ILI case definition [28].
Compared to the main end-of-season 2022–23 influenza VE results, which included all study sites, the VE against any influenza, influenza A (any subtype) and influenza B among all ages differed by ≤ 6 % in absolute value [28]. The differences were higher among the VE against influenza A(H1N1)pdm09. Differences between study results could be due to the properties of the different study populations (different vaccines used, different previous infection/vaccination history, different circulating (sub)clades) or random variation. VEBIS 2022–23 end-of-season influenza VE estimates should be quoted from the main analysis including all study sites [28].
There was considerable overlap between patients meeting the EU ARI and the EU ILI case definition, with 90 % overlap among all ages in the any influenza VE analysis. The overlap was higher among younger adults (92 %) than among children and older adults at 89 % and 82 %, respectively. This indicates that systemic symptoms may be less present, or less easy to measure, in certain age groups [16], [17], [18], [19]. A reason for the overall substantial overlap could be that sentinel GPs were used to using the ILI case definition for recruitment from previous seasons and were less likely to recruit milder ARI patients. However, GPs in Germany and the Netherlands were using a mixed ILI and ARI patient recruitment approach from before the COVID-19 pandemic. Also, in the context of a VE study using primary data collection, the VE may be less affected by case definition used for inclusion in the study than those studies using secondary data sources. GPs, using their clinical experience, may include patients that are part of the source population from which cases arise in a more refined way than if diagnostic codes are used for case selection, resulting in more severe ARI patients. It may also be that in the context of the COVID-19 pandemic, only patients with more severe symptoms consulted the GP, while patients with milder symptoms did not and possibly took SARS-CoV-2 self-tests at home or at pharmacies. For this reason, as well as different proportions of other circulating respiratory pathogens each season, this study may not be generalisable to future years. With an overlap of 82–92 %, the VE among the non-ILI ARI may need to be extreme to affect the overall VE among ARI substantially. However, even if the VE is not extreme, if the vaccination coverage among cases and controls is different among ILI and non-ILI ARI, then confounding by ILI could be introduced, leading to the overall VE among ARI differing substantially from the VE among ILI.
Another reason for lack of generalisability is a potential different proportion of other circulating respiratory pathogens each season. The influenza positivity among ILI in this study is 27 % overall. This positivity among ILI was higher within this network (I-MOVE) before the COVID-19 pandemic: between the 2010–2011 and 2019–2020 seasons, the median influenza positivity was 47 % (IQR: 41–53 %; range 38 to 57 %) (data not shown). This indicates that the EU ILI case definition is now capturing other respiratory virus pathogens, presumably SARS-CoV-2, leading to a lower specificity for influenza. Further research is needed to understand how the circulation of other viruses might drive differences in the specificity of the EU ILI case definition to influenza and therefore potential differences in symptom distribution among those meeting the EU ILI case definition pre- and post-COVID-19 pandemic. While this lower specificity impacts surveillance, it did not affect VE in the 2022–23 season. We aim to repeat the study to understand if this is the case in subsequent seasons. An additional limitation was the lack of individual symptom information from some study sites. The lack of information on coryza from two study sites (Spain and Ireland) may have biased EU ARI patients from milder to more moderate illness. However, among the six study sites collecting information on coryza, 80 % (n = 5 386/6 756) of ARI patients presented with coryza, but none presented with coryza only, indicating that collecting coryza would be unlikely to change the proportion of patients meeting the ARI case definition. Germany did not collect shortness of breath or malaise. Among the seven study sites collecting information on the three respiratory symptoms (cough, sore throat and shortness of breath), 0.4 % (n = 102/23 625) of ARI patients presented with shortness of breath only. Among the seven study sites collecting information on the four systemic symptoms (malaise, fever, headache and myalgia), 7 % (n = 1 621/23 625) of patients presented with malaise only. These two symptoms (shortness of breath and malaise) are part of the EU ILI case definition and it may be that some patients from Germany were misclassified as not meeting the EU-ILI case definition. Due to the low percentages of both systemic symptoms, potential misclassification is unlikely to affect the study, however healthcare-seeking behaviour is likely to differ between countries, and extrapolation of the proportion of patients with given symptoms from one study site to another must be done with caution.
Immunity acquired from previous infections can also influence the clinical outcome. For example, patients in the unvaccinated group may also have some immunity to the pathogen, which may prevent a stronger expression of systemic symptoms. In this case, a stricter case definition for VE would be beneficial. However, our study shows that most patients who meet the EU ARI definition also meet the EU ILI definition. Consequently, there was not much difference in VE between the ARI and ILI definitions. Globally, case definitions for recruitment for VE studies at primary care vary, although many studies include fever or history of fever and cough as a requirement in the case definitions [29], [30], making them more similar to our ILI case definition that requires a systemic and respiratory component. This makes our analysis and future analyses more important to ensure comparability of our results to those studies. We recommend that VE study publications include both the case definition for recruitment and the case definition used for analysis (if different), in order to better compare results.
5. Conclusion
Results from the end-of-season VEBIS 2022–23 influenza season clearly indicate that influenza VE estimates are not affected by the use of the EU ILI vs the EU ARI clinical case definition for patient recruitment. This implies that the change to recruitment of ARI patients in sentinel surveillance in some EU/EEA countries is unlikely to affect influenza VE and broadening to an ARI case definition could be considered. However, as seasons have differing proportions of pathogens causing ARI and as primary care practitioners get used to changes in clinical case definitions for recruitment, we recommend replication of this study in other seasons.
6. Ethics statement
The planning, conduct and reporting of the studies was in line with the Declaration of Helsinki [31]. Some studies did not require official ethical approval or patient consent as they are part of routine care/surveillance: Spain. In the Netherlands, as the data are initially collected through surveillance, no formal ethical approval was necessary. Verbal informed consent from patients for participation in the national respiratory surveillance is required. In addition, patients have the option to opt out for participation in any further research (including VE studies). Other study sites received local ethical approval from a national or regional review board: Croatia: approved by the Ethics Committee of the Croatian Institute of Public Health (class 030-02/22-01/4 and class 030-02/23-01/1); France: 471393; Germany: EA2/126/11; Hungary: approved by the National Scientific and Ethical Committee (IV/1885-5/2021/EKU); Ireland: ICGP2019.4.04; Portugal: approved 18 January 2012 by the Ethics Committee of Instituto Nacional de Saúde Doutor Ricardo Jorge, no registration number given; Romania: CE199/2022; Sweden: 2006/1040–31/2.
All authors attest they meet the ICMJE criteria for authorship.
CRediT authorship contribution statement
Marine Maurel: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Formal analysis, Data curation. Clara Mazagatos: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Luise Goerlitz: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Beatrix Oroszi: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Mariette Hooiveld: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Ausenda Machado: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Lisa Domegan: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Maja Ilić: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Rodica Popescu: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Noémie Sève: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Iván Martínez-Baz: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Amparo Larrauri: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Silke Buda: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Gergő Túri: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Adam Meijer: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Verónica Gomez: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Joan O'Donnell: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Ivan Mlinarić: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Olivia Timnea: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Ana Ordax Diez: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Ralf Dürrwald: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Judit Krisztina Horváth: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Frederika Dijkstra: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Ana Paula Rodrigues: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Adele McKenna: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Sanja Kurečić Filipović: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Mihaela Lazar: Writing – review & editing, Resources, Methodology, Investigation, Data curation. Marlena Kaczmarek: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Sabrina Bacci: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Esther Kissling: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Study teams are very grateful to all patients, general practitioners, paediatricians, laboratory teams, and regional epidemiologists who have contributed to the studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2024.04.060.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- 1.Rose A., Kissling E., Emborg H.D., Larrauri A., McMenamin J., Pozo F., et al. Interim 2019/20 influenza vaccine effectiveness: six European studies, September 2019 to January 2020. Eurosurveillance. 2020 Mar 12;25(10):2000153. doi: 10.2807/1560-7917.ES.2020.25.10.2000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flannery B., Chung J.R., Monto A.S., Martin E.T., Belongia E.A., McLean H.Q., et al. Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019 Jun 1;68(11):1798–1806. doi: 10.1093/cid/ciy775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flannery B., Chung J.R., Belongia E.A., McLean H.Q., Gaglani M., Murthy K., et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness — United States, February 2018. Am J Transplant. 2018;18(4):1020–1025. [Google Scholar]
- 4.Skowronski D.M., Zou M., Sabaiduc S., Murti M., Olsha R., Dickinson J.A., et al. Interim estimates of 2019/20 vaccine effectiveness during early-season co-circulation of influenza A and B viruses, Canada, February 2020. Eurosurveillance. 2020 Feb 20;25(7):2000103. doi: 10.2807/1560-7917.ES.2020.25.7.2000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowronski D.M., Leir S., Sabaiduc S., Chambers C., Zou M., Rose C., et al. Influenza vaccine effectiveness by A(H3N2) phylogenetic subcluster and prior vaccination history: 2016–2017 and 2017–2018 epidemics in Canada. J Infect Dis. 2020 Mar 26;225(8):1387–1398. doi: 10.1093/infdis/jiaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kissling E., Rose A., Emborg H.D., Gherasim A., Pebody R., Pozo F., et al. Interim 2018/19 influenza vaccine effectiveness: six European studies, October 2018 to January 2019. Eurosurveillance. 2019 Feb 21;24(8):1900121. doi: 10.2807/1560-7917.ES.2019.24.1900121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S., Chiu S.S., Chan E.L.Y., Kwan M.Y.W., Wong J.S.C., Leung C.W., et al. Effectiveness of influenza vaccination on influenza-associated hospitalisations over time among children in Hong Kong: a test-negative case-control study. Lancet Respir Med. 2018 Dec;6(12):925–934. doi: 10.1016/S2213-2600(18)30419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan Y wai D, Wong M ling, Au K wing, Chuang S kwan. Seasonal influenza vaccine effectiveness at primary care level, Hong Kong SAR, 2017/2018 winter. Hum Vaccines Immunother. 2019 Jan 2;15(1):97–101. [DOI] [PMC free article] [PubMed]
- 9.Sullivan S.G., Arriola C.S., Bocacao J., Burgos P., Bustos P., Carville K.S., et al. Heterogeneity in influenza seasonality and vaccine effectiveness in Australia, Chile, New Zealand and South Africa: early estimates of the 2019 influenza season. Eurosurveillance. 2019 Nov 7;24(45):1900645. doi: 10.2807/1560-7917.ES.2019.24.45.1900645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichol K.L. Heterogeneity of influenza case definitions and implications for interpreting and comparing study results. Vaccine. 2006 Nov 10;24(44–46):6726–6728. doi: 10.1016/j.vaccine.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 11.Ferdinands J.M., Thompson M.G., Blanton L., Spencer S., Grant L., Fry A.M. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine. 2021 Jun 23;39(28):3678–3695. doi: 10.1016/j.vaccine.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Regan A.K., Arriola C.S., Couto P., Duca L., Loayza S., Nogareda F., et al. Severity of influenza illness by seasonal influenza vaccination status among hospitalised patients in four South American countries, 2013–19: a surveillance-based cohort study. Lancet Infect Dis. 2023 Feb;23(2):222–232. doi: 10.1016/S1473-3099(22)00493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Commission. COMMISSION IMPLEMENTING DECISION (EU) 2018/ 945 - of 22 June 2018 - on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. 2009 Jan; Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945.
- 14.Copenhagen: WHO Regional Office for Europe and Stockholm: European Centre for Disease Prevention and Control. Operational considerations for respiratory virus surveillance in Europe [Internet]. 2022 [cited 2022 Sep 16]. Available from: https://www.ecdc.europa.eu/en/publications-data/operational-considerations-respiratory-virus-surveillance-europe.
- 15.World Health Organization. End-to-end integration of SARS-CoV-2 and influenza sentinel surveillance: Revised interim guidance [Internet]. World Health Organization; 2022. Available from: https://iris.who.int/bitstream/handle/10665/351409/WHO-2019-nCoV-Integrated-sentinel-surveillance-2022.1-eng.pdf?sequence=1.
- 16.Norman D.C. Fever in the elderly. Clin Infect Dis Off Publ Infect Dis Soc Am. 2000 Jul;31(1):148–151. doi: 10.1086/313896. [DOI] [PubMed] [Google Scholar]
- 17.Korsten K., Adriaenssens N., Coenen S., Butler C.C., Verheij T.J.M., Bont L.J., et al. World Health Organization Influenza-Like Illness Underestimates the Burden of Respiratory Syncytial Virus Infection in Community-Dwelling Older Adults. J Infect Dis. 2022 Aug 12;226(Suppl 1):S71–S78. doi: 10.1093/infdis/jiab452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rha B., Dahl R.M., Moyes J., Binder A.M., Tempia S., Walaza S., et al. Performance of surveillance case definitions in detecting respiratory syncytial virus infection among young children hospitalized with severe respiratory illness-South Africa, 2009–2014. J Pediatr Infect Dis Soc. 2019 Sep 25;8(4):325–333. doi: 10.1093/jpids/piy055. [DOI] [PubMed] [Google Scholar]
- 19.Saha S., Pandey B.G., Choudekar A., Krishnan A., Gerber S.I., Rai S.K., et al. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalizations among children in a rural community of northern India. J Glob Health. 2015 Dec;5(2) doi: 10.7189/jogh.05.020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenciano M, Ciancio BC, Team C on behalf of the IM study. I-MOVE: a European network to measure the effectiveness of influenza vaccines. Eurosurveillance. 2012 Sep 27;17(39):20281. [DOI] [PubMed]
- 21.Valenciano M., Kissling E., Cohen J.M., Oroszi B., Barret A.S., Rizzo C., et al. Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study. PLOS Med. 2011 Jan 11;8(1):e1000388. doi: 10.1371/journal.pmed.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Centre for Disease Prevention and Control. Core protocol for ECDC studies of vaccine effectiveness against symptomatic laboratory-confirmed influenza or SARS-CoV-2 infection at primary care level. Stockholm: ECDC; 2023. [Internet]. Available from: https://data.europa.eu/doi/10.2900/25966.
- 23.European Union. Google Docs. 2015 [cited 2024 Jan 12]. I-MOVE+ generic study protocol: generic protocol for the test negative design case control studies to measure pandemic and seasonal influenza vaccine effectiveness in the European Union and European Economic Area Member States. Available from: https://drive.google.com/file/d/0Byv9pYYPpY4PM25qSXczQ3g4T0E/view?usp=embed_facebook.
- 24.De Serres G., Skowronski D.M., Wu X.W., Ambrose C.S. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2013 Sep 12;18(37):20585. doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 25.Doll M.K., Pettigrew S.M., Ma J., Verma A. Effects of Confounding Bias in Coronavirus Disease 2019 (COVID-19) and Influenza Vaccine Effectiveness Test-Negative Designs Due to Correlated Influenza and COVID-19 Vaccination Behaviors. Clin Infect Dis Off Publ Infect Dis Soc Am. 2022 Aug 24;75(1):e564–e571. doi: 10.1093/cid/ciac234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stowe J., Andrews N., Kirsebom F., Ramsay M., Bernal J.L. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study. Nat Commun. 2022 Sep 30;13(1):5736. doi: 10.1038/s41467-022-33378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan S.G., Khvorov A., Huang X., Wang C., Ainslie K.E.C., Nealon J., et al. The need for a clinical case definition in test-negative design studies estimating vaccine effectiveness. npj Vaccines. 2023 Aug;12(8):118. doi: 10.1038/s41541-023-00716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurel M., Pozo F., Pérez-Gimeno G., Buda S., Sève N., Oroszi B., et al. Influenza vaccine effectiveness in Europe: Results from the 2022–2023 VEBIS (Vaccine Effectiveness, Burden and Impact Studies) primary care multicentre study. Influenza Other Respir Viruses. 2024 Jan 10;18(1):e13243. doi: 10.1111/irv.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean H.Q., Petrie J.G., Hanson K.E., Meece J.K., Rolfes M.A., Sylvester G.C., et al. Interim Estimates of 2022–23 Seasonal Influenza Vaccine Effectiveness — Wisconsin, October 2022–February 2023. Morb Mortal Wkly Rep. 2023 Feb 24;72(8):201–205. doi: 10.15585/mmwr.mm7208a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skowronski D.M., Chuang E.S., Sabaiduc S., Kaweski S.E., Kim S., Dickinson J.A., et al. Vaccine effectiveness estimates from an early-season influenza A(H3N2) epidemic, including unique genetic diversity with reassortment, Canada, 2022/23. Eurosurveillance. 2023 Feb 2;28(5):2300043. doi: 10.2807/1560-7917.ES.2023.28.5.2300043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.