Abstract
In targeted radionuclide therapy, determining the absorbed dose of the ligand distributed to the whole body is vital due to its direct influence on therapeutic and adverse effects. However, many targeted alpha therapy drugs present challenges for in vivo quantitative imaging. To address this issue, we developed a planar imaging system equipped with a cadmium telluride semiconductor detector that offers high energy resolution. This system also comprised a 3D-printed tungsten collimator optimized for high sensitivity to astatine-211, an alpha-emitting radionuclide, and adequate spatial resolution for mouse imaging. The imager revealed a spectrum with a distinct peak for X-rays from astatine-211 owing to the high energy resolution, clearly distinguishing these X-rays from the fluorescent X-rays of tungsten. High collimator efficiency (4.5 × 10−4) was achieved, with the maintenance of the spatial resolution required for discerning mouse tissues. Using this system, the activity of astatine-211 in thyroid cancer tumors with and without the expression of the sodium iodide symporter (K1-NIS/K1, respectively) was evaluated through in vivo imaging. The K1-NIS tumors had significantly higher astatine-211 activity (sign test, p = 0.031, n = 6) and significantly decreased post-treatment tumor volume (Student’s t-test, p = 0.005, n = 6). The concurrent examination of intratumor drug distribution and treatment outcome could be performed with the same mice.
Keywords: astatine-211, radiotheranostics, dosimetry, in vivo imaging, quantitative imaging
1. Introduction
Radiotheranostics has garnered remarkable attention in nuclear medicine in recent years [1,2,3]. Agents labeled with alpha-ray-emitting radionuclides ( -emitters) have gained prominence [4,5]. As the radiation dose to tissues correlates with therapeutic and side effects, estimating its distribution and absorbed dose before administration is a standard practice. As the radiation sources in radiotheranostics are radiopharmaceuticals, the radiation dose of a given tissue is primarily influenced by their pharmacokinetics and decay. Importantly, alpha particles are markedly more cytotoxic than X-rays and -rays [6]; thus, a precise understanding of radiopharmaceutical pharmacokinetics and biodistribution is required for drug development and clinical application. For pharmacokinetic evaluation, the radiation dose of a radiopharmaceutical in animal tissues is often determined by sacrificing several animals at various time points, collecting tissue samples, and plotting time–activity curves for target tissues. The disadvantages of this method include the requirement to sacrifice numerous mice and the inability to monitor them postsacrifice. Moreover, this method is not feasible for human studies due to tissue sample constraints. Quantitative imaging addresses these limitations to an extent.
X/ -ray camera imaging enables simultaneous analysis of the distribution of radiopharmaceuticals and their quantification in vivo. This method uses X/ -rays emitted by radionuclides for imaging and appears useful as many, if not all, therapeutic radionuclides emit X/ -rays available for quantitative imaging. In -emitter-labeled therapeutic agents, where quantitative imaging is possible to some extent, clinical reports have revealed a correlation between absorbed dose and therapeutic efficacy in certain cancers [7,8,9,10,11]. However, quantitative imaging of -emitter-labeled therapeutics is challenging. The main reason for this challenge is the low dose for imaging. Due to the higher linear energy of -rays, the dosage of -emitter-labeled radiopharmaceuticals is markedly lower than that of diagnostic radiopharmaceuticals. The low intensity of X/ -rays suitable for X/ -ray camera imaging is also another reason. For instance, the -ray intensity of 99mTc is suitable for imaging (i.e., the intensity of 141 keV -ray) is 89% [12]. In contrast, 211At, a promising -emitter, emits X-rays suitable for imaging (approximately 80 keV of lines and approximately 90 keV of lines); however, their combined intensity is only approximately 40% [13]. The current X/ -ray camera and single-photon emission computed tomography (SPECT) are not designed to address these issues, highlighting the need to enhance both sensitivity and the signal-to-noise ratio (SN-ratio) for accurate quantification. In addition, small animal imaging requires a higher spatial resolution than humans due to their smaller size. Higher spatial resolution reduces sensitivity. Therefore, innovations in small animal imaging is essential to maintain adequate sensitivity. In summary, an optimal imager requires a high SN-ratio, suitable spatial resolution for the target, and elevated sensitivity. Augmentation of the SN-ratio requires the utilization of a detector with a higher energy resolution. Furthermore, to enhance sensitivity without an excessive reduction in spatial resolution, it is imperative to establish the minimal spatial resolution requisite and to optimize the thickness and length of the collimator septa, thereby maximizing photon yield.
To address these challenges, we have been developing a planar imager for small animals that have high sensitivity and high SN-ratio. This imager is equipped with a cadmium telluride (CdTe) detector with high energy resolution [14] and a tungsten 3D-printed precision collimator optimized for a specific radionuclide type. 211At, which is a promising therapeutic radionuclide that emits -rays [15], was selected as the target radionuclide for optimizing the collimator. Thus, a collimator tailored for X-rays from 211At (the and emission lines of 211Po) [16] was fabricated. However, the imaging of 211At using cancer cells and animal models has not been validated effectively. This method requires verification in terms of quantification and the practicality of imaging times in biological experiments. Moreover, the utility of imaging At also requires validation, similar to how the imaging of beta-emitting radionuclides has been clinically demonstrated to be effective.
Astatine is a halogen. Moreover, the astatine ion is known to exhibit pharmacokinetics similar to those of the iodide ion (which is also a halogen) [17]. 131I-NaI is known to be effective in the treatment of thyroid cancer [18,19,20]. 211At-NaAt has shown similar efficacy in this regard, as demonstrated in our previous studies [21,22]. Therefore, an experimental system using 211At-NaAt for thyroid cancer is suitable for examining the validity and utility of quantitative imaging.
In this study, we aimed to present the performance of our planar imager, equipped with a further optimized collimator to increase sensitivity. Thereafter, experiments with thyroid-cancer-bearing mice treated with 211At-NaAt were performed to evaluate biodistribution using in vivo imaging and highlight the utility and significance of in vivo imaging for therapeutic radiopharmaceuticals.
2. Results
2.1. Specification of the Imager
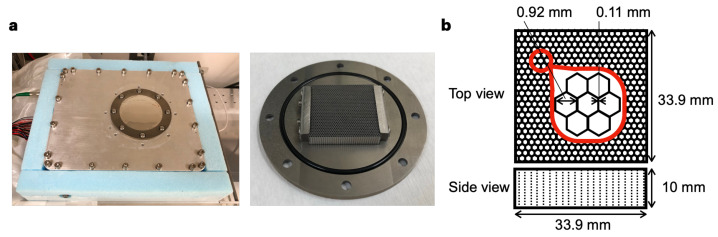
Figure 1a shows the bench-top-sized planar imager (L 25 × W 20 × H 15 cm). This imager is equipped with a 3D-printed tungsten collimator, which has a thickness of 10 mm, a septa thickness of 0.11 mm, and a hole diameter of 0.92 mm (Figure 1b). The efficiency of the collimator was improved by reducing its thickness from 20 mm reported in our previous study to 10 mm, which resulted in an increase in collimator efficiency from 1.4 × 10−4 [16] to 4.5 × 10−4. The hole diameter of the collimator was designed to maximize photon collection while maintaining adequate spatial resolution for identifying small organs, such as the thyroid gland.
Figure 1.
Overview of the imager. (a) Exterior appearance of the imager (left) and appearance of the collimator (right). (b) Layout of the parallel-hole collimator.
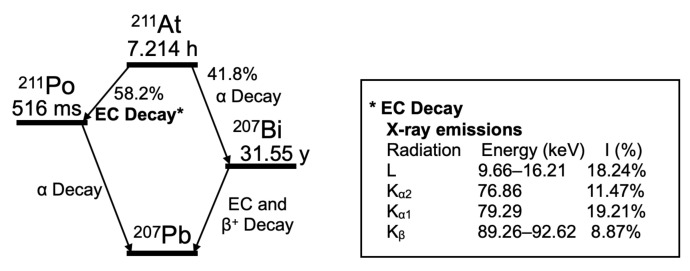
Figure 2 illustrates the decay scheme of 211At, based on the decay data standards from the Lund/LBNL Nuclear Data Search, Version 2.0 [13]. The septa thickness was designed to be thick enough to shield the 211At X-rays (the and lines of 211Po, approximately 80–90 keV). 201Tl emits X-rays at approximately 70 keV. It is used clinically as a diagnostic nuclide. Therefore, X-rays of 80–90 keV (which is close to the energy of X-rays of 201Tl) are suitable for animal imaging.
Figure 2.
Decay scheme of 211At. The and lines of the X-rays derived from electron capture (EC) decay are used for imaging. I (%); intensity per decay (%).
2.2. Performance of the Imager with Respect to 211At X-rays
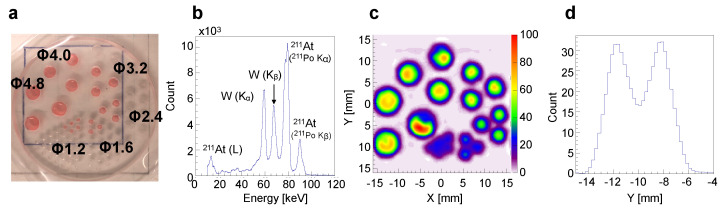
The spatial resolution at approximately 80 keV was evaluated using a Derenzo phantom (Figure 3a) filled with a red-colored solution containing 211At anion in each hole. The acquired spectral data (Figure 3b, count vs. energy) revealed distinct spectral peaks from the 211At X-rays (the and lines of 211Po), which are clearly distinguishable from the fluorescent X-rays of tungsten (W( ) and W( ) in Figure 3b). The phantom image using 75–85 keV (the line) photons (Figure 3c) shows that each hole with a diameter of 1.6 mm or larger can be discernible separately. This observation aligns with the cross-sectional view through the center of the 1.6 mm-diameter hole (Figure 3d, count vs. position), which clearly displays two peaks from 211At in the two holes. Based on our findings, reducing the thickness of the collimator to increase sensitivity has a minimal effect on the spatial resolution.
Figure 3.
Evaluation of the spatial resolution and spectrum using 211At solution. (a) Derenzo phantom loaded with 211At solution (red). indicates the hole diameter (mm). (b) Spectrum obtained from phantom. (c) Image generated by using the 211At X-rays (the emission line of 211Po, 75–85 keV). (d) Cross-sectional view (count vs. position) through the center of the 1.6 mm-diameter holes in (c).
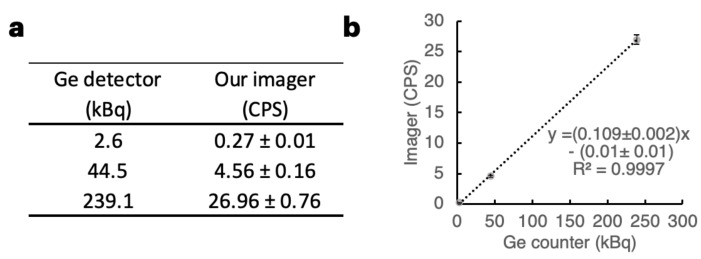
To obtain a reference curve for 211At, three sample solutions at three points of 211At dosage were prepared. The samples were measured with both our imager (75–85 keV) and a Ge detector (Figure 4a). A strong correlation was observed between the photon counts from our imager and the activity using a Ge detector. This is shown in Figure 4b ( = 0.9997).
Figure 4.
Reference curve for 211At. (a) Data of the three 211At samples regarding the photon count from our imager and dose data using a Ge detector. The count data represent the acquired values ± S.D. (S.D. is derived from 211At decay). CPS: count per second. (b) Plot illustrating the strong linear correlation between the activity measured using a dose calibrator and the count per second obtained with our imager.
Table 1 delineates the minimum dose required to achieve the desired accuracy (statistical error) for imaging target tissue within a specified acquisition time. To maintain statistical errors within ±15%, ±10%, and ±5%, at least 44, 100, and 400 photons are necessary, respectively. The minimal count per second (CPS) required was calculated by dividing the required photon counts by the acquisition time. The minimal dose for each acquisition time was then determined by dividing the CPS by the slope of the linear equation in Figure 4b. When an acquisition time is set to 30 min (the acquisition time for this study), an activity over 2.04 kBq is crucial to ensure a statistical error margin of ±5%, which matches the accuracy provided by a dose calibrator. For data requiring a statistical error of 15% or less in 30 min of acquisition time, the estimated minimum accumulation dose is 0.23 kBq.
Table 1.
Required minimal dose accumulation (kBq) to a target tissue according to accuracy and acquisition time.
| Accuracy (Statistical Error) | Acquisition Time (min) | |||
|---|---|---|---|---|
| 10 | 20 | 30 | 60 | |
| 5% | 6.12 | 3.06 | 2.04 | 1.02 |
| 10% | 1.53 | 0.76 | 0.51 | 0.25 |
| 15% | 0.68 | 0.34 | 0.23 | 0.11 |
The effective doses of 211At-labeled agents, which are administered without causing serious adverse effects, range from 100 to 1000 kBq. The maximum %IDs to target tumors range from approximately a few percent up to 23% [21,23,24,25,26,27,28]. When referring to these reports, the most challenging scenario for quantitative imaging in terms of administration dose and tumor distribution rate is 100 kBq of administration dose and a tumor distribution rate of 1.0%. Then, if the half-life of 211At (7.2 h) is not considered, the accumulation of the drug in the tumor is 1.0 kBq in the scenario. Therefore, the minimum requirement was defined that a 1.0 kBq target can be quantitatively imaged under the conditions allowed in Table 1. The condition with the lowest accuracy and longest acquisition time in Table 1 is that with 15% statistical error and 60 min of acquisition time. The minimum dose required in this condition is 0.11 kBq, and 1.0 kBq is well above this value; thus, quantitative imaging with the required accuracy is possible even in the challenging scenario described above. Therefore, this imaging system can be applied in many cases regarding the %ID of the drug to targets, dosage, and timing of imaging after administration under the conditions of 15% statistical error and 60 min of acquisition time.
2.3. Cell Imaging Using 211At-NaAt Solution
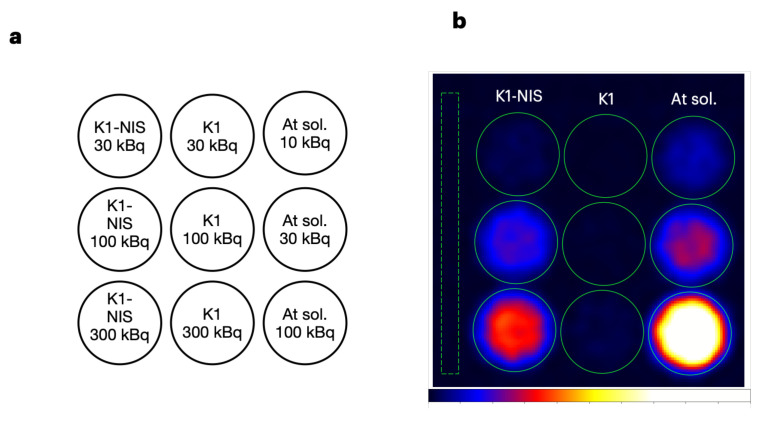
Our previous study revealed that 211At-NaAt was effective against sodium iodide symporter (NIS)–expressing thyroid cancer cells [22] and a xenograft model [21]. The NIS, also known as SLC5A5, is responsible for the uptake of and [29]. K1-NIS cells, which are NIS-transfected K1 cells (human papillary thyroid carcinoma with low expression of NIS), were used in the studies. Kaneda-Nakashima et al. reported that (1) K1 cells display increased 211At uptake according to the expression level of NIS (from approximately 0 to 10 kBq per 1.0 × 105 cells), and (2) the therapeutic effect is also observed according to the amount of 211At uptake [22]. Watabe et al. reported that the activity concentration of 211At in K1-NIS tumors was 22.5 ± 10.4 %ID at 3 h after administration [21]. In the present study, we investigated whether the differences in 211At accumulation in K1/K1-NIS cells could be assessed quantitatively from the images obtained with our imager using cultured cells. Accordingly, 3.0 × 104 K1 cells and K1-NIS cells seeded in a 96-well plate were incubated with the medium containing 211At-NaAt (30, 100, and 300 kBq to each well; n = 1 each; Figure 5a), washed with PBS, and imaged using our imager with an acquisition time of 30 min. The K1-NIS cells produced images with varying intensities correlating with the dose of 211At. Meanwhile, the K1 cells showed a significantly low intensity (Figure 5b). The dose data obtained by setting the region of interest (ROI) on the image of each well demonstrated an 211At uptake rate of approximately 11–24% for K1-NIS cells, whereas K1 cells had an uptake rate of less than 1% (Table 2). An image analysis yielded results consistent with the previous studies. The quantitative data obtained from the imaging of wells without cells were in alignment with the dose calibrator data, considering the accuracy of the dose calibrator (±5%) and errors associated with pipetting.
Figure 5.
Cellular imaging. (a) K1-NIS cells were seeded in the left-column wells, and an equivalent number of K1 cells were seeded in the middle-column wells. No cells were seeded in wells in the right column (the wells for reference). Medium containing the specified activity of 211At-NaAt was added, as shown in the figure. After 5 min of incubation, the medium was removed and the cells were washed once with PBS. The wells in the right column contained the medium comprising 211At-NaAt. (b) Planar image of the well plate. Image was obtained using the 211At (211Po) X-rays (75–85 keV) with an acquisition time of 30 min. The leftmost dashed rectangle indicates the ROI for the background.
Table 2.
Cellular imaging data. Photon counts in cells were obtained from the ROIs assigned to each well. Cell activity was determined by converting the count data. CPS: count per second; n.d.: not detected; n.a.: not available.
| Well | Loaded Activity (kBq) | CPS | Activity (kBq) | Uptake Rate (%) |
|---|---|---|---|---|
| K1-NIS cell | 300 | 4.77 | 44.07 | 14.69 |
| 100 | 2.58 | 23.94 | 23.94 | |
| 30 | 0.35 | 3.34 | 11.13 | |
| K1 cell | 300 | 0.28 | 2.7 | 0.9 |
| 100 | 0.08 | 0.83 | 0.83 | |
| 30 | n.d. | n.a. | n.a. | |
| Reference (no cell) | 100 | 11.58 | 106.84 | - |
| 30 | 3.3 | 30.55 | - | |
| 10 | 1.11 | 10.36 | - |
2.4. In Vivo Imaging in 211At Radionuclide Therapy
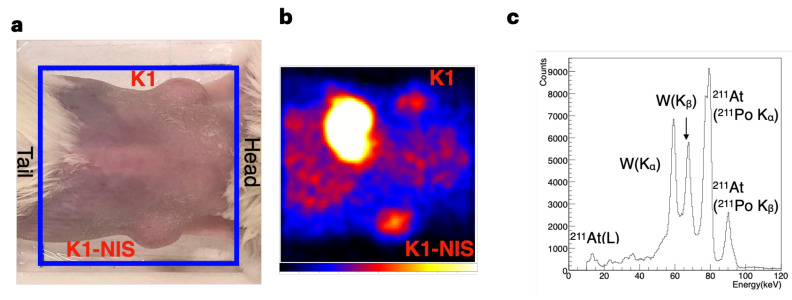
In vivo imaging was performed in the therapeutic experiments using K1 and K1-NIS cells. Cancer-bearing mice with K1 cells transplanted into their left flank and K1-NIS cells transplanted into their right flank (Figure 6a) were administered approximately 1.0 MBq of 211At-NaAt through the tail vein. Image data were acquired 1 h after the administration (n = 6). A representative image is shown in Figure 6b, and the images of all mice are shown in Figure S1: all images of the six mice. The spectrum obtained with our imager is shown in Figure 3c. Here, the scattering component from the mouse is not clearly recognizable with reference to Figure 3b, which does not include it.
Figure 6.
In vivo imaging. (a,b) Representative mouse image from the back (a) and the corresponding planar image of 211At (75–85 keV) (b). K1 cells and NIS-transfected K1(K1-NIS) cells were transplanted into the left (top) and right (bottom) flanks, respectively. Blue squares indicate the field of observation. Strong accumulation of 211At was observed not only in the NIS-expressing tumor but also in the stomach, which expresses NIS. (c) Representative spectrum obtained by mouse imaging.
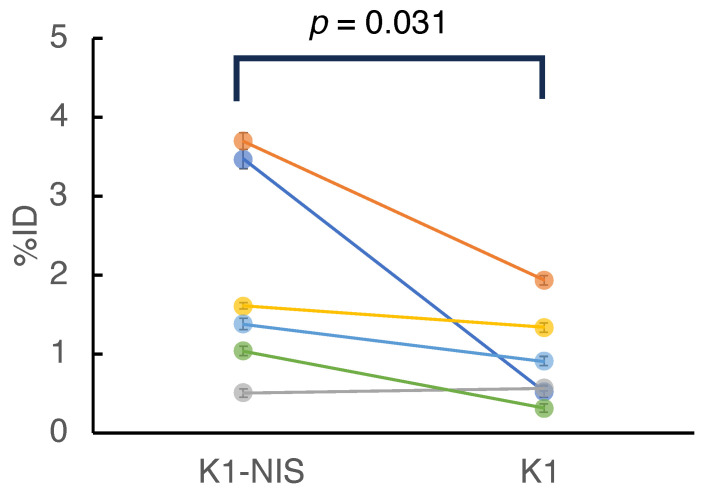
For further data analysis, a region of interest (ROI) was designated, which corresponded to the tumor site on each image. Detailed data, including doses, counts, and activity for all mice, are available in Table 3. The activity (%ID) of 211At in the tumors is shown in Figure 7. In five of the six mice, K1-NIS cell tumors had a higher activity than the K1 cell tumors, with no activity difference found in one case. The activity (%ID) of the two groups (K1 vs. K1-NIS) was significantly different in the sign test (n = 6, K1-NIS > K1, p = 0.031).
Table 3.
Activity of 211At in tumors. Data represent the acquired values ± S.D. (S.D. is derived from 211At decay).
| Mouse No. | Dose (MBq) | K1-NIS | K1 | ||
|---|---|---|---|---|---|
| Activity (kBq) | %ID (%) | Activity (kBq) | %ID (%) | ||
| 1 | 1.16 | 40.34 ± 1.31 | 3.47 ± 0.11 | 6.13 ± 0.69 | 0.53 ± 0.06 |
| 2 | 1.07 | 39.68 ± 1.29 | 3.7 ± 0.12 | 20.81 ± 0.9 | 1.94 ± 0.08 |
| 3 | 1.07 | 5.49 ± 0.44 | 0.51 ± 0.04 | 6.14 ± 0.59 | 0.57 ± 0.06 |
| 4 | 1.05 | 16.91 ± 0.7 | 1.61 ± 0.07 | 14.07 ± 0.65 | 1.34 ± 0.06 |
| 5 | 1.04 | 14.32 ± 0.62 | 1.38 ± 0.06 | 9.48 ± 0.52 | 0.91 ± 0.05 |
| 6 | 1.07 | 11.08 ± 0.56 | 1.04 ± 0.05 | 3.46 ± 0.36 | 0.32 ± 0.03 |
Figure 7.
Activity of 211At in the tumors (n = 6). Each color indicates each pair of tumors in the mouse. Error bars represent the S.D. arising from the atomic decay of 211At. The 211At activity (%ID) of K1-NIS tumors was significantly lower than that of K1 tumors (sign test, p = 0.031, n = 6).
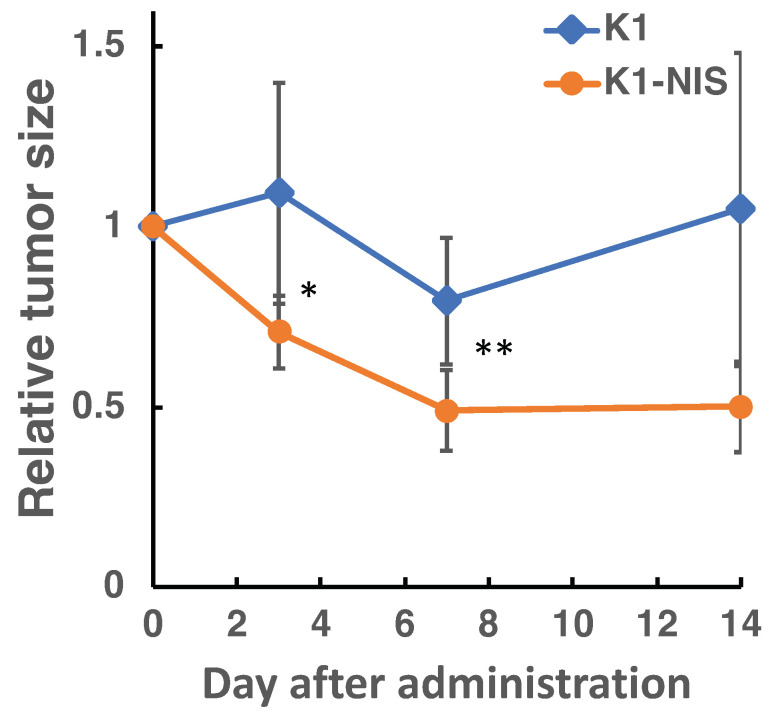
The time course of tumor size following treatment is shown in Figure 8. The K1-NIS tumors were significantly smaller than those of the K1 tumors at both day 3 and 7 post-treatment (n = 6, the Student’s t-test, p = 0.015, p = 0.005, respectively). This finding aligned with the observed 211At activity in the tumors.
Figure 8.
Changes in relative tumor size after treatment. Data are presented as the mean ± S.D., (n = 6, the Student’s t-test, *p < 0.05, ** p < 0.01).
3. Discussion
By performing in vitro experiments in this study, the performance of our imager in terms of sensitivity, spatial resolution, and energy resolution, was confirmed to meet the requirements for in vivo imaging of 211At. Although the experiment was straightforward, we investigated the accumulation of 211At in cells using imaging. An image that reflects the activity of 211At and that of accumulated 211At according to the expression level of NIS (the target molecule of the therapeutic agent in this study) could be obtained. The capability to obtain both imaging data on drug accumulation and quantitative data on the drug in cell-based experiments is of high utility in drug discovery. The accumulation of a tracer in cells can be measured by a -counter using cell suspensions. However, this method is somewhat complicated because it requires the washing and centrifugation of samples and the removal of buffer multiple times, depending on the number of specimens. In quantitative cell imaging, a series of operations can be easily and quickly performed because the cells are attached to the culture plate, and both the activity and the image can be obtained. Furthermore, if the cells are fixed in formalin, quantitative imaging can be followed by fluorescent immunostaining analysis. For instance, Kaneda-Nakashima et al. reported a positive correlation between an accumulated dose of 211At and the number of H2AX foci in immunofluorescence images [22], which is a marker molecule of DNA double-strand break. In this study, the cell accumulation dose per applied dose and the number of H2AX foci per applied dose were obtained by individual experiments. Our imaging system allows these experiments to be performed as a sequence of experiments using the same cells. Simplicity and low cost are important in drug screening. This imaging system satisfies these criteria.
A summary of the requirements for the system and the achieved results is shown in Table 4. In the animal experiment, the activity of 211At in NIS-positive and NIS-negative xenografts was evaluated, and the correlation with the treatment response could be evaluated. The difference in 211At activity in the K1-NIS and K1 tumors was less than anticipated, considering the findings of the previous study, where the 211At activity concentration in K1-NIS tumor was 22.5 ± 10.4 %ID of 211At, despite being measured 3 h after injection [21]. However, even under such circumstances, a correlation could be found between the 211At activity in the tumors and treatment outcome. Administering the same dose does not always guarantee identical drug distribution within tumors. Hence, as demonstrated in this study, the activity of therapeutic agents within tumors should be evaluated using quantitative imaging, ensuring a more effective assessment of drug efficacy.
Table 4.
Summary of requirements and achieved results.
| Requirements | Achieved Results | |
|---|---|---|
| Sensitivity | Is quantitative imaging of many therapeutics possible concerning dose and tumor accumulation rate? | The system is capable of handling many cases as detailed in Table 1 and the results. |
| Spatial resolution | Is spatial resolution sufficient to distinguish mouse organs? | The spatial resolution is better than 1.6 mm, which is sufficient to identify small organs such as the thyroid gland. |
| Energy resolution | Is the energy resolution sufficient to distinguish it from noise signals? | Its high energy resolution makes it possible to distinguish between X-rays from 211At and tungsten fluorescent X-rays. |
There is growing interest in the use of 211At as an -emitter for targeted radionuclide therapy [15]. For instance, 211At-NaAt [21], 211At-labeled antihuman epidermal growth factor receptor 2 antibody [23], 211At-astato-benzylguanidine [24], 211At-phenylalanine [25], 211At-prostate-specific membrane antigen [26], 211At-octreotide [27], and 211At- -methyl-l-tyrosine [28] have been reported as promising agents. In these studies, the minimum effective doses of 211At-labeled agents are 100 kBq, and the minimum %ID to target tumors is approximately a few percent. If high accuracy is not required, many cases can be accommodated by our system. On the other hand, for agents that accumulate in tumors to a lesser extent, imaging conducted 24 h after administration, or situations requiring high-accuracy imaging of low-activity targets in a short acquisition time, may not be suitable. To obtain the absorbed dose, it is necessary to obtain the dose in tumors after 24 h of administration. However, 24 h is more than three half-lives of that of 211At (7.2 h), reducing it to less than one-eighth of the original activity. Therefore, to obtain absorbed doses with high accuracy, the sensitivity of the device needed to be improved.
Sensitivity is not the only important aspect of quantitative imaging. This study underscores the necessity of accurately quantifying radiopharmaceuticals for drug development. A method to achieve this is to use sensors with a high energy resolution. The energy resolution of a CdTe sensor is higher than those of CdZnTe sensors and the NaI(Tl) scintillator (1.6%, 5.3%, and 8.7% at 140 keV, respectively) [14], which are currently used in SPECT imaging. This study demonstrates that the high energy resolution of the CdTe sensor enables differentiation between X-rays from 211At and fluorescent X-rays from tungsten, thereby achieving superior quantitative accuracy. Another approach is to mitigate noise signals, such as scattering components, which increase with an increase in body sizes. Therefore, noise reduction methods are crucial. We have developed a method to remove noise signals by applying a spectroscopic analysis method to spectral data obtained from CdTe sensors [30].
Our planar imager is bench-top-sized and is markedly smaller than small animal SPECT. The cost for its development and maintenance is less than that of SPECT. Moreover, the operational simplicity of this imager—requiring users to merely place the mouse on the imager for image acquisition—ensures minimal interference during treatment observation experiments. However, unlike SPECT, our imager lacks 3D information. Depending on the position of the target tissue, overlaps with other tissues can occur in the image, potentially complicating accurate activity acquisition. In scenarios where the target tissue activity is overshadowed by surrounding tissue activity, precise estimates become challenging. Nonetheless, given specific requirements, our planar imaging system remains a viable choice. The key areas for enhancement in the planar imaging system include enlarging the field of view (FOV) and enhancing the sensitivity. Currently, the imager’s FOV accommodates only half of a mouse’s body. This necessitates repositioning to capture the full-body image. An expanded FOV will enable whole-body imaging in a single session. Moreover, heightened sensitivity could shorten the imaging time, thereby improving the efficiency of cellular imaging workflows.
4. Conclusions
We developed a system to analyze the intratumoral drug distribution of the therapeutic candidate 211At-NaAt in radiotheranostics via in vivo imaging and confirmed its utility. This imager enabled us to assess drug distribution and treatment outcome in the same mice. Moreover, our data indicate the utility of quantitative imaging in drug development via cell imaging using 211At-labeled agents. However, it is necessary to accumulate data on the utility of quantitative imaging by performing quantitative imaging of many therapeutic agents. The limitations of this system are that the sensitivity is not sufficient for imaging low-activity samples with high accuracy and that the FOV does not cover the entire mouse body. Theranostics is a coined term for “therapy” and “diagnostics”. We hope that our imaging system will contribute to the advancement of this field from a “diagnostic” perspective.
5. Materials and Methods
5.1. Imager Setup and Its Performance
The basic imaging system configuration was reported previously [16]. The imager consists of a CdTe semiconductor detector imaging sensor, a parallel-hole collimator made of tungsten with a 3D metal printer (Toray Precision Co., Ltd., Shiga, Japan), a Peltier cooling system, a readout system, and a housing. The density of the collimator is measured to be 19.13 g cm−3, which is consistent with that of tungsten metal. The CdTe sensor is a double-sided strip detector (CdTe-DSD), where imaging is based on coincidence detection on signals from strips on both sides of the detector. The CdTe-DSD is a square-shape detector with a thickness of 32 mm on each side. The thickness of the CdTe crystal is 0.75 mm. The detection efficiency is 85% for 59.5 keV. The detector has an energy resolution of 1–2 keV for 10–100 keV and a position resolution of 250 μm. In this study, the collimator thickness was changed from 20 mm in the previous study to 10 mm. Collimator efficiency (g) was calculated by substituting the septa thickness (t), collimator thickness (l), and hole diameter (d) into the following formula [16,31]:
| (1) |
The energy window of 211At was set to 75–85 keV. Spatial resolution was evaluated using a Derenzo phantom with holes of diameters of 1.2, 1.6, 2.4, 3.2, 40, and 4.8 mm. Each hole was filled with an 211At solution. Three sample solutions were prepared at three points of dosage to obtain a reference curve for 211At. The samples were measured with both our imager and a Ge detector (BE2020, Mirion Technologies (Canberra), Zellik Belgium), CT, USA). The reference curve was obtained from photon count data and dose data using linear regression.
5.2. Preparation of 211At Solutions
The 211At solutions were prepared as described previously [21]. Briefly, 211At was procured from the RIKEN Nishina Center for Accelerator-Based Science. 211At was produced by the 209Bi( , 2n)211At reaction, and then separated and purified using a dry distillation method. 211At was dissolved in distilled water containing 1.2% (w/v) ascorbic acid (IWAKI & CO., Ltd., Tokyo, Japan) as a reducing agent.
5.3. Cell Culture
K1 cells (human papillary thyroid carcinoma) were provided by the European Collection of Authenticated Cell Cultures. K1-NIS cells were obtained via transfection using the human SLC5A5 (NIS) gene clone (OriGene, Rockville, MD), as described previously [21]. K1 and K1-NIS cells were maintained in culture medium, D-MEM (Nacalai Tesque, Inc. Kyoto, Japan):Ham F12 (Nacalai Tesque):MCDB 105 (Cell Applications, Inc., San Diego, CA, USA) (2:1:1) supplemented with 2 mM glutamine (Nacalai Tesque) and 10% heat-inactivated fetal bovine serum (Corning, NY, USA).
5.4. Preparation of Animals
NOD-SCID mice (male, 4 weeks old) were purchased from Charles River Japan, Inc. (Atsugi, Japan). Animals were housed under a 12 h light/12 h dark cycle and granted free access to food and water. Tumor xenograft models were established in NOD-SCID mice by subcutaneously injecting K1-NIS cells into the upper left-flank and K1 cells into the upper-right flank. Each injection comprised 1.0 × 107 cells suspended in 0.2 mL of a 1:1 mixture of culture medium and Matrigel (BD Biosciences, San Jose, CA, USA). All animal experiments complied with the guidelines of the Institute of Experimental Animal Sciences. The protocol was approved by the Animal Care and Use Committee of the Osaka University Graduate School of Medicine (Approval No. 30-103-008, approval date 20 February 2019). Tumor size was monitored, and mice were euthanized after 2 weeks via deep anesthesia with isoflurane inhalation.
5.5. Imaging Conditions for Cellular and Animal Imaging
For cellular imaging, 3.0 × 104 of K1 cells and K1-NIS cells were seeded in each well of 96-well plates 1 day prior to the experiment. The cells were incubated with medium containing 30, 100, and 300 kBq of 211At-NaAt, which was measured with a dose calibrator (Curie meter IGC-8B, Hitachi, Tokyo, Japan) with an accuracy of ±5%, for 5 min; rinsed once with PBS; and imaged with our imager (acquisition time of 30 min). The accumulated 211At activity in the cells were determined by subtracting the background activity from the activity in the well ROI. For animal imaging, mice were anesthetized with isoflurane (Viatris Pharmaceuticals Japan, Tokyo, Japan; induction 3%, maintenance 1.5%) during the administration of the 211At solutions and imaging. Each cancer-bearing mouse was administered approximately 1.0 MBq of 211At solution (measured with a dose calibrator) through the tail vein. One hour postadministration, mice were imaged for 30 min. The accumulated 211At activity in the tumors was determined by subtracting the background activity from the activity in the tumor ROI. The background per area was estimated according to the activity of the area surrounding the tumors. In the comparison of 211At activity in K1-NIS/K1 tumors using the sign test, if the activity difference in each mouse exceeded the statistical error due to At decay, + or − was assigned, and those within the statistical error were treated as having no difference. Statistic analysis was carried out using the latest version of ROOT 6 (Cern, Meyrin, Switzerland).
Acknowledgments
211At was procured from RIKEN Nishina Center for Accelerator-Based Science and via Supply Platform of Short-lived Radioisotopes supported by JSPS Grant-in-Aid for Scientific Research on Innovative Areas (Grant Number 16H06278). We would like to thank Takanori Kobayashi and Kazuko Kaneda-Nakashima for their excellent technical assistance.
Abbreviations
The following abbreviations are used in this manuscript:
| SPECT | single-photon emission computed tomography |
| NIS | sodium iodide symporter |
| SN-ratio | signal-to-noise ratio |
| CdTe | cadmium telluride |
| CPS | count per second |
| ROI | region of interest |
| %ID | percentage of injected dose |
| FOV | field of view |
Supplementary Materials
The following supporting information can be downloaded at www.mdpi.com/xxx/s1, Figure S1: All images of the six mice.
Author Contributions
Conceptualization, A.Y. and T.W.; formal analysis, M.K. and S.T.; investigation, M.K., S.T., Y.S., K.O., A.T. and T.W.; resources, T.W.; writing—original draft preparation, A.Y.; writing—review and editing, T.W. and T.T.; supervision, T.W., T.T. and A.Y.; project administration, T.W. and A.Y.; funding acquisition, A.Y., T.T., S.T. and T.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of the Osaka University Graduate School of Medicine (Approval No. 30-103-008, approval date 20 February 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed in this study are included in this published article and the Supplementary Information file.
Conflicts of Interest
A.Y., S.T. and T.T. hold more than 5% stocks of Imagine-X Inc. The other authors declare that they have no conflicts of interest.
Funding Statement
This work was supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan, a matching fund program of Centers for Inter-University Collaboration from ISAS/JAXA, and JSPS KAKENHI (Grant Numbers 23K18233, 20K16692, 18H05463, and 16H02170), and the Qiss program of the OPERA (Grant Number: JPMJOP1721) from the Japan Science and Technology Agency (JST).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bodei L., Herrmann K., Schöder H., Scott A.M., Lewis J.S. Radiotheranostics in oncology: Current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 2022;19:534–550. doi: 10.1038/s41571-022-00652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann K., Schwaiger M., Lewis J.S., Solomon S.B., McNeil B.J., Baumann M., Gambhir S.S., Hricak H., Weissleder R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020;21:e146–e156. doi: 10.1016/S1470-2045(19)30821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pomykala K.L., Hadaschik B.A., Sartor O., Gillessen S., Sweeney C.J., Maughan T., Hofman M.S., Herrmann K. Next generation radiotheranostics promoting precision medicine. Ann. Oncol. 2023;34:507–519. doi: 10.1016/j.annonc.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Jang A., Kendi A.T., Johnson G.B., Halfdanarson T.R., Sartor O. Targeted Alpha-Particle Therapy: A Review of Current Trials. Int. J. Mol. Sci. 2023;24:11626. doi: 10.3390/ijms241411626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eychenne R., Chérel M., Haddad F., Guérard F., Gestin J.F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics. 2021;13:906. doi: 10.3390/pharmaceutics13060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassis A.I., Adelstein S.J. Radiobiologic Principles in Radionuclide Therapy. J. Nucl. Med. 2005;46((Suppl. 1)):4S–12S. [PubMed] [Google Scholar]
- 7.Flux G.D., Haq M., Chittenden S.J., Buckley S., Hindorf C., Newbold K., Harmer C.L. A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:270–275. doi: 10.1007/s00259-009-1261-3. [DOI] [PubMed] [Google Scholar]
- 8.Dewaraja Y.K., Schipper M.J., Shen J., Smith L.B., Murgic J., Savas H., Youssef E., Regan D., Wilderman S.J., Roberson P.L., et al. Tumor-Absorbed Dose Predicts Progression-Free Survival Following 131I-Tositumomab Radioimmunotherapy. J. Nucl. Med. 2014;55:1047–1053. doi: 10.2967/jnumed.113.136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garin E., Tselikas L., Guiu B., Chalaye J., Edeline J., de Baere T., Assenat E., Tacher V., Robert C., Terroir-Cassou-Mounat M., et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021;6:17–29. doi: 10.1016/S2468-1253(20)30290-9. [DOI] [PubMed] [Google Scholar]
- 10.Cremonesi M., Ferrari M.E., Bodei L., Chiesa C., Sarnelli A., Garibaldi C., Pacilio M., Strigari L., Summers P.E., Orecchia R., et al. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:2426–2441. doi: 10.1007/s00259-018-4044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strigari L., Konijnenberg M., Chiesa C., Bardies M., Du Y., Gleisner K.S., Lassmann M., Flux G. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:1976–1988. doi: 10.1007/s00259-014-2824-5. [DOI] [PubMed] [Google Scholar]
- 12.Cherry S.R., Sorenson J.A., Phelps M.E. Physics in Nuclear Medicine. 4th ed. Elsevier; Philadelphia, PA, USA: 2012. Decay Characteristics of Some Medically Important Radionuclides; pp. 449–475. [Google Scholar]
- 13.Chu S.Y.F., Ekström L.P., Firestone R.B. The Lund/LBNL Nuclear Data Search, Version 2.0. 1999. [(accessed on 1 May 2023)]. Available online: http://nucleardata.nuclear.lu.se/toi/
- 14.Takeda S., Orita T., Yagishita A., Katsuragawa M., Yabu G., Tomaru R., Moriyama F., Sugawara H., Watanabe S., Mizuma H., et al. CdTe-DSD SPECT-I: An Ultra-High-Resolution Multi-Isotope Tomographic Imager for Mice. IEEE Trans. Radiat. Plasma Med. Sci. 2023;7:860–870. doi: 10.1109/TRPMS.2023.3291756. [DOI] [Google Scholar]
- 15.Albertsson P., Bäck T., Bergmark K., Hallqvist A., Johansson M., Aneheim E., Lindegren S., Timperanza C., Smerud K., Palm S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front. Med. 2022;9:1076210. doi: 10.3389/fmed.2022.1076210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii H., Ohnuki K., Takeda S., Katsuragawa M., Yagishita A., Yabu G., Watanabe S., Takahashi T. A Pure Tungsten Collimator Manufactured Using 3D Printing Technology for the Evaluation of 211At Radionuclide Therapy. Radioisotopes. 2022;71:141–151. doi: 10.3769/radioisotopes.71.141. [DOI] [Google Scholar]
- 17.Spetz J., Rudqvist N., Forssell-Aronsson E. Biodistribution and dosimetry of free 211At, 125I− and 131I− in rats. Cancer Biother. Radio. 2013;28:657–664. doi: 10.1089/cbr.2013.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maheshwari Y.K., Hill C.S., Jr., Haynie T.P., III, Hickey R.C., Samaan N.A. 131I therapy in differentiated thyroid carcinoma: MD Anderson Hospital experience. Cancer. 1981;47:664–671. doi: 10.1002/1097-0142(19810215)47:4<664::AID-CNCR2820470408>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Maxon H., 3rd, Englaro E.E., Thomas S.R., Hertzberg V.S., Hinnefeld J.D., Chen L.S., Smith H., Cummings D., Aden M.D. Radioiodine-131 therapy for well-differentiated thyroid cancer—A quantitative radiation dosimetric approach: Outcome and validation in 85 patients. J. Nucl. Med. 1992;33:1132–1136. [PubMed] [Google Scholar]
- 20.Ciarallo A., Rivera J. Radioactive iodine therapy in differentiated thyroid cancer: 2020 update. Am. J. Roentgenol. 2020;215:285–1291. doi: 10.2214/AJR.19.22626. [DOI] [PubMed] [Google Scholar]
- 21.Watabe T., Kaneda-Nakashima K., Liu Y., Shirakami Y., Ooe K., Toyoshima A., Shimosegawa E., Fukuda M., Shinohara A., Hatazawa J. Enhancement of 211At Uptake via the Sodium Iodide Symporter by the Addition of Ascorbic Acid in Targeted α-Therapy of Thyroid Cancer. J. Nucl. Med. 2019;60:1301–1307. doi: 10.2967/jnumed.118.222638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneda-Nakashima K., Shirakami Y., Watabe T., Ooe K., Yoshimura T., Toyoshima A., Wang Y., Haba H., Fukase K. Effect to Therapy of Sodium-Iodine Symporter Expression by Alpha-Ray Therapeutic Agent via Sodium/Iodine Symporter. Int. J. Mol. Sci. 2022;23:15509. doi: 10.3390/ijms232415509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takashima H., Ohnuki K., Manabe S., Koga Y., Tsumura R., Anzai T., Wang Y., Yin X., Sato N., Shigekawa Y., et al. Tumor Targeting of 211At-Labeled Antibody under Sodium Ascorbate Protection against Radiolysis. Mol. Pharmaceut. 2023;20:1156–1167. doi: 10.1021/acs.molpharmaceut.2c00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohshima Y., Sudo H., Watanabe S., Nagatsu K., Tsuji A.B., Sakashita T., Ito Y.M., Yoshinaga K., Higashi T., Ishioka N.S. Antitumor effects of radionuclide treatment using α-emitting meta-211At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:999–1010. doi: 10.1007/s00259-017-3919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watabe T., Kaneda-Nakashima K., Shirakami Y., Liu Y., Ooe K., Teramoto T., Toyoshima A., Shimosegawa E., Nakano T., Kanai Y., et al. Targeted alpha therapy using astatine (211At)-labeled phenylalanine: A preclinical study in glioma bearing mice. Oncotarget. 2020;11:1138–1398. doi: 10.18632/oncotarget.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watabe T., Kaneda-Nakashima K., Shirakami Y., Kadonaga Y., Ooe K., Wang Y., Haba H., Toyoshima A., Cardinale J., Giesel F.L., et al. Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: A preclinical evaluation as a novel compound. Eur. J. Nucl. Med. Mol. Imaging. 2023;50:849–858. doi: 10.1007/s00259-022-06016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin S., Yang Y., Zhang J., Yin Y., Liu W., Zhang H., Fan X., Yang M., Yu F. Effective Treatment of SSTR2-Positive Small Cell Lung Cancer Using 211At-Containing Targeted α-Particle Therapy Agent Which Promotes Endogenous Antitumor Immune Response. Mol. Pharmaceut. 2023;20:5543–5553. doi: 10.1021/acs.molpharmaceut.3c00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneda-Nakashima K., Zhang Z., Manabe Y., Shimoyama A., Kabayama K., Watabe T., Kanai Y., Ooe K., Toyoshima A., Shirakami Y., et al. α-Emitting cancer therapy using 211At-AAMT targeting LAT1. Cancer Sci. 2021;112:1132–1140. doi: 10.1111/cas.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindencrona U., Nilsson M., Forssell-Aronsson E. Similarities and differences between free 211At and 125I transport in porcine thyroid epithelial cells cultured in bicameral chambers. Nucl. Med. Biol. 2001;28:41–50. doi: 10.1016/S0969-8051(00)00179-7. [DOI] [PubMed] [Google Scholar]
- 30.Yagishita A., Takeda S.i., Katsuragawa M., Kawamura T., Matsumura H., Orita T., Umeda I.O., Yabu G., Caradonna P., Takahashi T., et al. Simultaneous visualization of multiple radionuclides in vivo. Nat. Biomed. Eng. 2022;6:640–647. doi: 10.1038/s41551-022-00866-6. [DOI] [PubMed] [Google Scholar]
- 31.Cherry S.R., Sorenson J.A., Phelps M.E. Physics in Nuclear Medicine. 4th ed. Elsevier; Philadelphia, PA, USA: 2012. The Gamma camera, performance characteristics; pp. 209–231. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in this published article and the Supplementary Information file.