Abstract
BACKGROUND
Induction of general anaesthesia has many potential triggers for peri-operative myocardial ischaemia including the acute disturbance of blood gases that frequently follows alterations in breathing and ventilation patterns. Free-breathing oxygenation-sensitive cardiovascular magnetic resonance (OS-CMR) imaging may provide the opportunity to continuously quantify the impact of such triggers on myocardial oxygenation.
OBJECTIVE
To investigate the impact of breathing patterns that simulate induction of general anaesthesia on myocardial oxygenation in awake healthy adults using continuous OS-CMR imaging.
DESIGN
Prospective observational study.
SETTING
Single-centre university hospital. Recruitment from August 2020 to January 2022.
PARTICIPANTS
Thirty-two healthy volunteers younger than 45 years old were recruited. Data were analysed from n = 29 (69% male individuals).
INTERVENTION
Participants performed a simulated induction breathing manoeuvre consisting of 2.5 min paced breathing with a respiration rate of 14 breaths per minute, followed by 5 deep breaths, then apnoea for up to 60s inside a magnetic resonance imaging scanner (MRI). Cardiac images were acquired with the traditional OS-CMR sequence (OSbh-cine), which requires apnoea for acquisition and with two free-breathing OS-CMR sequences: a high-resolution single-shot sequence (OSfb-ss) and a real-time cine sequence (OSfb-rtcine).
MAIN OUTCOME MEASURES
Myocardial oxygenation response at the end of the paced breathing period and at the 30 s timepoint during the subsequent apnoea, reflecting the time of successful intubation in a clinical setting.
RESULTS
The paced breathing followed by five deep breaths significantly reduced myocardial oxygenation, which was observed with all three techniques (OSbh-cine −6.0 ± 2.6%, OSfb-ss −12.0 ± 5.9%, OSfb-rtcine −5.4 ± 7.0%, all P < 0.05). The subsequent vasodilating stimulus of apnoea then significantly increased myocardial oxygenation (OSbh-cine 6.8 ± 3.1%, OSfb-ss 8.4 ± 5.6%, OSfb-rtcine 15.7 ± 10.0%, all P < 0.01). The free-breathing sequences were reproducible and were not inferior to the original sequence for any stage.
CONCLUSION
Breathing manoeuvres simulating induction of general anaesthesia cause dynamic alterations of myocardial oxygenation in young volunteers, which can be quantified continuously with free-breathing OS-CMR. Introducing these new imaging techniques into peri-operative studies may throw new light into the mechanisms of peri-operative perturbations of myocardial tissue oxygenation and ischaemia.
VISUAL ABSTRACT
KEY POINTS
Induction of general anaesthesia has many potential triggers for peri-operative myocardial ischaemia including acute disturbances of blood gases that frequently follow alterations in breathing and ventilation patterns. Pinpointing the underlying cause is difficult because of a lack of measuring techniques.
Myocardial oxygen-supply mismatch can now be spatially mapped, continuously and noninvasively, using free-breathing oxygenation-sensitive cardiovascular magnetic resonance (OS-CMR) imaging.
In a prospective observational study, free-breathing OS-CMR was used to image spontaneously breathing healthy individuals to investigate the effects of voluntary breathing manoeuvres simulating an anaesthesia induction sequence on myocardial oxygenation.
Paced breathing followed by five deep breaths resulted in myocardial de-oxygenation, while subsequent apnoea exerts an opposite effect by increasing myocardial oxygenation.
Free-breathing OS-CMR imaging techniques will now be used peri-operatively to investigate the dynamics of myocardial oxygenation and possibly the evolution of peri-operative ischaemia in patients undergoing induction of general anaesthesia.
Introduction
There is increasing awareness of the association between anaesthesia and peri-operative myocardial injury in cardiac (MICS) and noncardiac surgery (MINS).1,2 Some of these events can be attributed to complications such as type I myocardial infarction, accompanied by a rise in cardiac troponins.1,3 However, the majority of cases are thought to be linked to type II myocardial ischaemia caused by oxygen supply and demand mismatch,3,4 even in the absence of a stenotic coronary artery. Pinpointing type II myocardial ischaemia as the underlying cause for MINS is difficult because of a lack of techniques to both spatially and temporally map its onset, and to quantify the imbalance between oxygen supply and demand.
There are several factors that may contribute to type II ischaemia. Anaemia, hypotension, tachycardia and hypercoagulation are all known contributors, however, their sequence and relative importance may differ from patient to patient.5–7 Recently the role of blood gas alterations has been discussed. Hypocapnia and hyperoxia both have vasoconstrictive effects on the coronary vasculature, whereas hypercapnia is a vasodilator. Altering blood gases by inducing hyperventilation and apnoea induces reversible ischaemia in spontaneously breathing patients with known coronary artery disease, something that may be aggravated by inter-territorial steal.8–10 Although most studies investigate the stable phases of general anaesthesia, blood gas changes are especially pronounced during induction. Spontaneously breathing patients will often be asked to breathe at a higher rate or depth prior to induction to promote preoxygenation.11 During induction, hypoventilation or apnoea may ensue, followed by occasionally overly vigorous or inadequate manual bag ventilation and then apnoea for intubation. Little has been reported on the influence of such respiratory patterns on the oxygenation balance of myocardial tissue owing to a lack of noninvasive techniques for high resolution in-vivo interrogation of tissue oxygenation in thoracic organs.
Noninvasive oxygenation-sensitive cardiac magnetic resonance (OS-CMR) imaging has now proven suitable for investigating such effects, displaying high-resolution changes in myocardial oxygenation on a millimetre scale. In-vitro, translational and patient studies have already employed OS-CMR to investigate responses to typical peri-operative events such as controlled changes of FiO2 and CO2 partial pressure, haematocrit, blood flow and arterial pressure.9,12–15 However, previous CMR techniques were often limited to image acquisition only during apnoea as chest motion from significant breathing reduced image quality or required long acquisition times. This approach could not be employed to investigate blood gas changes in continuously breathing or ventilated patients. Advances with CMR have now led to multiple techniques that can overcome the requirement for apnoeic periods while acquiring data with every heartbeat.16,17 For the purposes of conducting research related to anaesthesia, we have optimized these free-breathing techniques to continuously interrogate myocardial oxygenation. OS-CMR may now open a window for quantifying and linking peri-operative myocardial ischaemia to specific triggers during anaesthesia.18
The goal of this study was to investigate the effects of voluntary breathing manoeuvres simulating an anaesthesia induction sequence, on myocardial oxygenation in healthy adults using free-breathing OS-CMR imaging. We hypothesised that deep paced breathing would reduce myocardial oxygenation in healthy individuals, whereas apnoea would have an opposing effect. Secondary goals were to compare the free-breathing sequences to the gold-standard breath-hold imaging sequence and to investigate the reproducibility of free-breathing OS-CMR imaging during induction-like breathing. These goals were intended to validate the use of the free-breathing technique for a future CMR-based study into the onset of prodromal stages of myocardial ischaemia, and their association with events during general anaesthesia.
Methods
Study design
Thirty-two healthy awake adults were prospectively enrolled in a single centre observational study. Inclusion criteria included the ability to give informed consent, age between 18 and 44 years, general good health and absence of known cardiovascular or respiratory disease or risk factors, and no contraindications to CMR. This study was approved by the Cantonal Research Ethics Board of Bern, Switzerland (#2020-00738) on 02 June 2020.
Experimental procedure
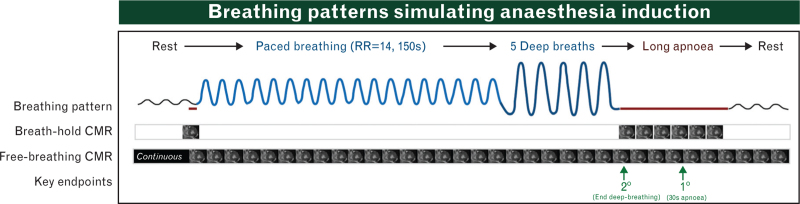
Participants underwent a single CMR visit (3.0 tesla clinical scanner, Siemens MAGNETOM Prisma, Siemens Healthineers, Germany). Baseline images were acquired while participants breathed ambient air at their natural pace and depth, without any specific instruction. Afterwards, participants were instructed to perform a breathing manoeuvre of paced breathing at a respiration rate of 14 for 150 s followed by five deep breaths and then apnoea at end-expiration (Fig. 1). A second rapid pacing breathing protocol was also performed for comparison (Supplemental Figure 1). For consistency, an audio file with voice commands and metronomes guiding the pace of the breathing was played to headphones worn by the participant inside the MRI. The breathing manoeuvre was then repeated and imaged with different OS-CMR imaging techniques (detailed imaging settings are provided in Supplemental Methods).
Fig. 1.
Breathing manoeuvre protocol.
Participants were instructed to follow the breathing patterns depicted in the figure with paced breathing guided by a metronome. Apnoea was performed in end-expiration and held for up to 60 s if possible. The OSbh-cine requires acquisition during a breath-hold period and is thus unable to follow oxygenation changes during breathing. The free-breathing sequences (OSfb-ss and OSfb-rtcine) acquired data continuously during sustained respiration (black bars).
CMR imaging
Technique 1: breath-hold cine (OSbh-cine)
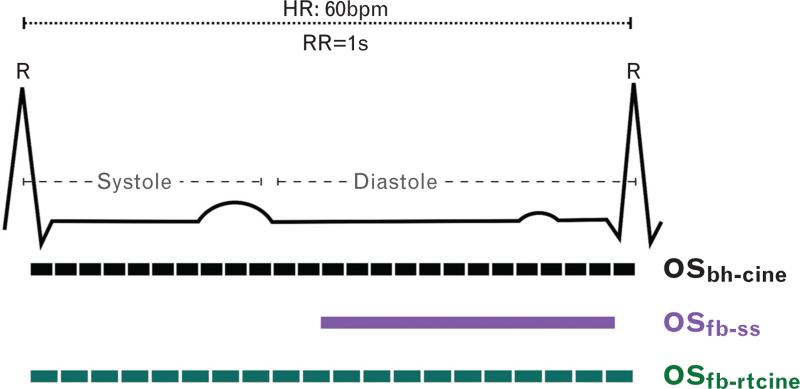
This traditional acquisition requires a breath-hold as it is based on a segmented approach that reconstructs images over four to six heartbeats. The heart must be in a consistent position with a regular heartbeat interval. Voxel size was 2.0 × 2.0 × 10.0 mm3, and a cine of the cardiac cycle was obtained with a temporal resolution of 40.7 ms (corresponding to a framerate of 25 images per second, Fig. 2).19,20
Fig. 2.
Cine vs. single-shot.
The pictogram displays when images are acquired throughout the cardiac cycle with each bar indicating a single frame. With a heart rate of 60 bpm, the duration of the cardiac cycle is 1 s (RR, interval between two R-spikes in the electrocardiogram). Both the breath-hold (OSbh-cine, frame rate = 25 s−1) and real-time (OSft-rtcine, frame rate 21 s−1) cines acquire multiple images throughout the cardiac cycle, whereas the single shot sequence only acquires one single frame in diastole requiring a longer acquisition window (OSfb-ss, one still image).
Technique 2: free-breathing single-shot imaging (OSfb-ss)
This variant was acquired over a single heartbeat and has a higher spatial resolution of 1.4 × 1.4 × 6.0 mm3. However, the entire cardiac cycle is not imaged and only a single frame in diastole is obtained, requiring 450 to 475 ms for this frame.
Technique 3: free breathing real-time cine (OSfb-rtcine)
A full cardiac cycle cine was acquired within a single heartbeat at a temporal resolution of 49.8 ms (framerate of 20 images per second), and voxel size of 2.5 x 2.5 x 8.0 mm3 [Video 1: Oxygenation-Sensitive Sequences. The three sequence variants are shown during apnoea and deep breathing. While the current breath-hold cine (left) cannot acquire good images while breathing, the two new free-breathing variants (centre and right) can acquire data with chest motion, with the real-time cine (right) also acquiring multiple images throughout the cardiac cycle].
All participants (n = 32) were imaged with the gold standard breath-hold cine (OSbh-cine) and single shot sequences [free-breathing single-shot imaging (OSfb-ss)]. Of these, 12 underwent all three variants, including the real-time imaging [free breathing real-time cine (OSfb-rtcine)]. A free-breathing acquisition was then repeated a second time with the OSfb-ss or OSfb-rtcine sequence to investigate reproducibility of the new techniques. This was performed in random order, with at least a 5-min pause between runs. Using heart rate, pulse oximetry and verbal feedback from participants, recovery between experimental periods was confirmed and a new baseline was acquired.
CMR image analysis
Images were analysed throughout the breathing manoeuvres, and the signal was reported as %-change from the corresponding stage. For calculating the change in signal because of paced breathing, the baseline image obtained prior to the breathing manoeuvre was used as the reference. All cardiac cycles during apnoea were analysed and for the change in signal during apnoea, the reference was the image obtained at the start of apnoea. For the free breathing periods, because of movement of the chest from the breathing motion, only images with the correctly angled short-axis anatomy (in-plane with end-expiratory images of the left ventricle, Supplemental Figure 3) were analysed. Analysis was performed with cvi42 (version 5.14, Circle Cardiovascular Imaging, Calgary, Canada) by researchers with two (CDU) and greater than 10 years (KF) experience in reading and interpreting these specific OS-CMR images. Within an individual participant, at least 4 weeks passed between the analysis of each of the runs. For the statistical analysis, the myocardial oxygenation response was assessed at predefined key timepoints, at the end of the paced breathing period and at the 30 s timepoint during the subsequent apnoea. The latter was considered the primary endpoint as this was the point most likely to reflect the time of successful intubation in a clinical setting, which usually marks the end of the induction sequence with commencement of mechanical ventilation. Myocardial de-oxygenation was defined as a drop (<0%) in signal intensity between two OS-CMR images.
Statistical analysis
General linear models were used to assess if an oxygenation response was detected by the sequences for each of the breathing manoeuvre steps. The myocardial response between the newly developed free-breathing sequences (OSfb-ss and OSfb-rtcine) were compared with the standard breath-hold cine (OSbh-cine), using ANOVA accounting for multiple comparisons. Reproducibility of the free-breathing sequences was assessed with intraclass correlations (ICC) for absolute agreement and with Bland–Altman testing. GraphPad Prism version 9.0 (GraphPad Software, USA), and IBM SPSS Statistics 26 (IBM, USA) were used for statistical analysis.
Results
Participant characteristics
Thirty-two participants were recruited. Three were excluded, one because of protocol development, one because of heart-rate gating errors and the third because the breath-hold in end-expiration was not consistently performed. In the remaining 29 patients, all OS images could be analysed at the predefined time-points. The mean [range] age was 29 years [21 to 43], mean ± SD BMI was 23.7 ± 2.8 kg m−2 and n = 20 (69%) were male individuals.
Myocardial oxygenation during paced breathing
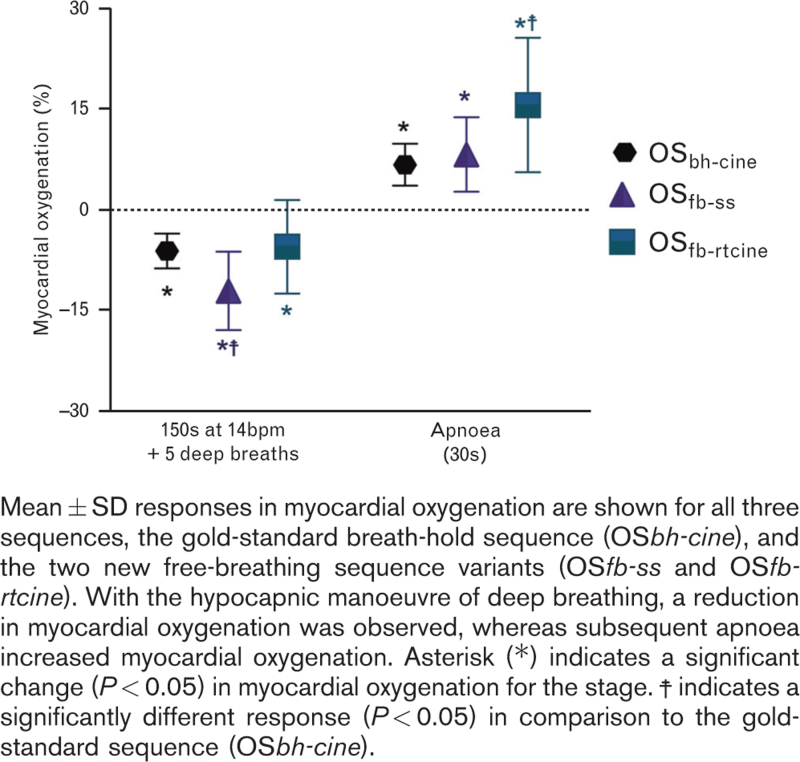
The induction simulation with induction-like paced breathing with five paced deep breaths resulted in a significant decrease in myocardial oxygenation measured by all three imaging methods (OSbh-cine −6.0 ± 2.6%, P < 0.01; OSfb-ss −12.0 ± 5.9%, P < 0.01; OSfb-rtcine −5.4 ± 7.0%, P = 0.01; Fig. 3; Supplemental Figure 2). During this phase, heart rate increased from 57 ± 8 to 71 ± 13 bpm (P < 0.01).
Fig. 3.
Myocardial oxygenation changes during induction like breathing.
Myocardial oxygenation during apnoea
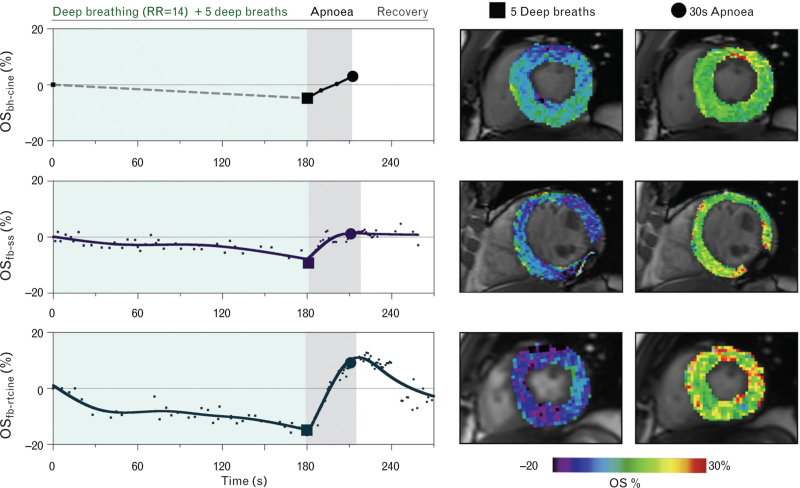
Apnoea following paced breathing resulted in a significant recovery of myocardial oxygenation at the 30 s time-point (OSbh-cine: 6.8 ± 3.1%, OSfb-ss: 8.4 ± 5.6%, OSfb-rtcine: 15.7 ± 10.0 all P < 0.01, Fig. 3). This also coincided with a normalisation of the heart rate to 60 ± 8 bpm. For all breathing manoeuvre components, the free-breathing variants performed as well or better than the gold-standard OSbh-cine method (Fig. 3). An example of an individual participant is shown in Fig. 4.
Fig. 4.
Case example of myocardial oxygenation changes.
The change in myocardial oxygenation is shown for all three sequences in the same individual during three repeated runs of a simulated induction breathing protocol. Dashed line indicates that data were not acquired during the paced breathing for the original breath-hold oxygenation-sensitive cine (top, OS-bhcine), whereas data were acquired throughout the entire manoeuvre for both free-breathing variants, the single-shot (middle, OSfb-ss) and real-time cine (bottom, OSfb-rtcine). Colour maps depict the %-change in myocardial oxygenation between baseline and at the end of five deep breaths, and for the change from the five deep breaths to 30 s of apnoea. Cines are displayed at end-systolic frames, whereas only a diastolic frame was available for the single shot. RR, respiration rate.
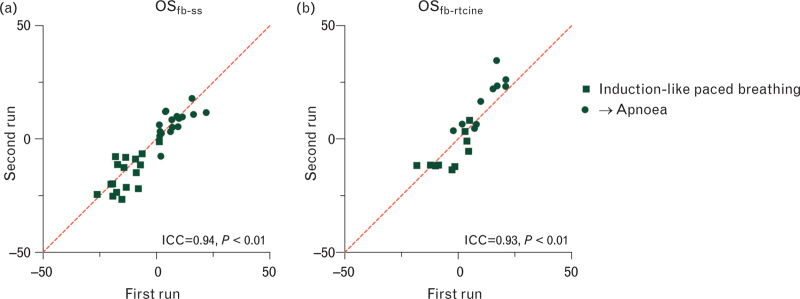
Reproducibility of free-breathing sequences
With this OSfb-ss sequence, an ICC of 0.94, P < 0.01 (Fig. 5) was observed between the two runs with a bias of 1.3 ± 5.7% from the first to the second run. Repetitive acquisitions for the OSfb-rtcine, yielded an ICC of 0.93, P < 0.01 and bias of −1.0 ± 7.0%.
Fig. 5.
Reproducibility of free-breathing oxygenation-sensitive sequences.
Both the free breathing single shot sequence [(a) OSfb-ss] and the free-breathing real-time cine [(b) OSfb-rtcine] demonstrate excellent ICC between repeated runs of the breathing manoeuvres. Line of identity (x = y) depicted by red.
Discussion
In spontaneously breathing healthy individuals, a voluntary breathing protocol simulating typical respiratory manoeuvres during induction of general anaesthesia had a significant and dynamic effect on myocardial oxygenation. A significant reduction in myocardial oxygenation was induced by the 2.5 min period of paced breathing followed by five deep breaths, a manoeuvre similar to the breathing patterns that patients are instructed to follow prior to induction. During the subsequent apnoea, which is representative of conditions occurring prior to and during airway management and intubation, myocardial oxygenation significantly increased. These changes were measured and spatially mapped, continuously and noninvasively, using free-breathing OS-CMR imaging.
Basics of the OS-CMR signal
Although the application of OS-CMR is relatively new to anaesthesia research, these techniques were introduced into neuroimaging studies and then cardiac imaging studies in the 1990s, and have since been validated with numerous invasive and noninvasive comparators.9,21–26 OS-CMR relies on the paramagnetic properties of the local de-oxyhaemoglobin (dHb) fraction, which acts as an endogenous contrast agent.12,22 When a physiological stimulus is applied, the corresponding change in signal intensity in comparison to a baseline image at a predefined steady state depicts the change in tissue oxygenation. If the dHb fraction in the microcirculation of the myocardium increases, the signal intensity of the myocardium will decrease. This will occur if blood flow or oxygen content of the tissue declines, adversely tipping the delicate oxygen balance. This decline in signal intensity is what we observed with the paced breathing followed by five deep breaths.
Conversely, factors leading to excess or luxury perfusion, such as blood flow exceeding tissue demand created by uncoupling of blood flow from metabolism through specific stimuli like apnoea-induced hypercapnia, will diminish the relative dHb fraction and a subsequent rise in signal intensity can be quantified in the images. This has been validated in anaesthetised swine, where OS-CMR was compared with myocardial oxygen delivery (DO2) and demand (MVO2), which were invasively derived from coronary blood flow and simultaneous arterial and coronary sinus blood gas measurements. When DO2 outweighed MVO2, the signal intensity in OS-CMR images increased. However, when this relationship inversed, signal intensity dropped depicting a tissue oxygen deficit.27 In patients, OS-CMR has been compared with other modalities quantifying oxygen supply or demand including invasive haemodynamic measurements of fractional flow reserve,28,29 and to noninvasive measurements of myocardial perfusion, and myocardial energetics.30,31 OS-CMR has also been used to investigate how other physiological stimuli may lead to temporary myocardial de-oxygenation, including reduced haematocrit in which there is a lower oxygen carrying potential, how hypotension and hypertension influence perfusion and workload and how underlying myocardial injury with oedema and fibrosis influence the response.12,14,31–34 The majority of studies apply pharmacological coronary vasodilators as a stimulus, and in patients with coronary artery disease, OS-CMR discriminates healthy myocardium from myocardium subtended to a stenosed vessel, which responds with an attenuated response or even a fall in signal intensity because of the pharmacological stimulus.23,24,28,29 Myocardium has a particularly high baseline oxygen extraction. Therefore, even a small decrease in tissue oxygenation may indicate a critical O2 deficit in patients with an already compromised myocardial oxygen supply.35 Already small deflections in PaCO2 significantly affect myocardial perfusion and oxygenation to the same extent or more than pharmacological vasodilators.13,19,20,36 Vasoactive breathing manoeuvres exploiting the coronary vasomotor effect of systemic PaCO2 changes even detected myocardial de-oxygenation in patients with coronary artery disease, heart failure, hypertrophic cardiomyopathy and cardiac allograft vasculopathy.17,20,33,37,38 These de-oxygenation events have been localised to the onset of transient regional diastolic and systolic dysfunction, signifying that such breathing manoeuvres can induce ischaemia detectable with OS-CMR.39 These studies investigated shorter breathing protocols, and the data presented in this study are the first to show that breathing manoeuvres similar to those used in general anaesthesia induction protocols also have a significant impact on myocardial oxygenation fluctuations.
Impact of simulated induction breathing manoeuvres on oxygenation of healthy myocardium
The goal of deep breathing during preoxygenation is to use the functional residual capacity of the lungs as an oxygen reserve during subsequent apnoea. This is a safety measure to avoid hypoxaemia during intubation or other intervention for airway management. Although some centres advocate 3 min of preoxygenation with normal or deep breathing, there are studies suggesting that a few deep breaths over 60 s or less may be equivalent to preoxygenation with 3 min of normal breathing in generating an appropriate safety margin.40 In patients with a difficult airway, both approaches might be appropriate. Despite maintaining blood oxygenation, the accompanying hypocapnic stimulus decreases blood flow to critical levels in CO2-sensitive vascular beds with tissues prone to ischaemia.15,34,41 Thus, blood oxygenation as monitored by pulse oximetry may not be indicative of normal tissue oxygenation.15,34 OS-CMR offers a unique opportunity to interrogate myocardial tissue regarding oxygenation. In this study, we could quantify that the paced breathing followed by five deep breaths simulating preoxygenation led to a short period of myocardial de-oxygenation, even in healthy myocardium. Therefore, changes in breathing patterns overseen by anaesthetists every day may be predisposing patients to inducible ischaemia. Our results demonstrate that with these two free-breathing OS-CMR sequence versions, it is now possible to take this method one step further and investigate how induction of anaesthesia affects myocardial oxygen balance.
It should be noted that the breathing manoeuvres in this study were performed without supplementary oxygen, although preoxygenation with an FIO2 of 1.0 is widely used in combination with incentive deep breathing prior to induction.42 Oxygen itself is a coronary vasoconstrictor and has been shown to decrease myocardial oxygenation, as measured by OS-CMR in anaesthetised animal studies, in awake healthy controls and in chronic coronary syndrome patients.15,34 Future investigations need to assess whether the combination of hyperoxia and hypocapnia during such periods of preoxygenation will have incremental effects and whether supplementary oxygen blunts vasodilation during apnoea.43 The peri-operative environment is quite complex for the heart because blood gas changes are not the only factors at play. Heart rate, for example, can rise not only during the deep breathing of preoxygenation,18,44 but also during and after induction especially if anaesthesia is too shallow. This can lead to an increase in sympathetic tone with a subsequent increase in heart rate and blood pressure leading to an increase in myocardial oxygen consumption. An increase in heart rate not only limits myocardial perfusion by shortening diastole but is also associated with increased cardiac work exposing the myocardium to a potential oxygenation mismatch, and even ischaemia in patients with a susceptible coronary status.45 Bradycardia can also result from vagal stimulation during intubation, potentially reducing oxygen supply as well. With appropriate dosing of anaesthetics and opioids, these autonomic reflexes should be sufficiently blunted. Additionally, peri-operative disturbances in myocardial perfusion pressure following periods of hypertension or hypotension will contribute to fluctuations in myocardial oxygenation, especially once anaesthetics are administered and normovolaemia becomes compromised.32,46
Advantages of the free-breathing OS-CMR sequences
One of the primary limitations of published OS-CMR approaches was that they required either a breath-hold20 or quite long acquisition times when choosing a free-breathing alternative. This implies that myocardial oxygenation could not be continuously monitored during rapidly changing respiratory patterns, as seen during induction of general anaesthesia. The advantage of the fast single-shot and real-time imaging versions used in this study is that these sequences acquire data from a single heartbeat. They do not rely on reconstruction from multiple heartbeats and are, therefore, less sensitive to misregistration because of breathing motion and arrhythmia. The free-breathing sequences used in this study were initially designed for diagnostic imaging of patients unable to tolerate breath-holds, and not to specifically assess myocardial oxygenation. We optimised the settings of these manufacturer-provided free-breathing sequences, to enhance their oxygenation-sensitivity, creating a sequence variant for monitoring myocardial oxygenation. Our analysis demonstrates that these two new oxygenation-sensitive free-breathing sequences perform just as well as the conventional sequence requiring a breath-hold. Additionally, our reproducibility analysis demonstrated that both new sequence variants yielded similar results on repetition of the respiratory manoeuvres of the induction-like respiratory manoeuvres indicating that first, the latter exert reproducible cardiac effects, and second, that the free-breathing OS-CMR sequences can provide reliable quantification.
The two free-breathing variants employed in this study differ in several aspects. The single-shot sequence (OSfb-ss) acquisition window requires approximately 450 to 475 ms (Fig. 2), and is unable to account for myocardial movement. Images can only be acquired during diastole when heart motion is minimal. If the heart rate increases and diastole is shortened, the image quality worsens. This can create problems as heart rate increases with deep breathing, and will usually also change throughout the course of induction.44,47 Nevertheless, the key advantage of OSfb-ss is that it can acquire smaller voxels and thus provide better spatial resolution, allowing a more detailed regional assessment of the myocardium. In comparison, the real-time sequence (OSfb-rtcine) is a cine. This means that it acquires one image every 49.8 ms throughout the entire cardiac cycle, capturing both motion and the OS signal with good temporal resolution. Therefore, it is much less susceptible to changes in heart rate and cardiac movement. Because of the cine characteristic, both oxygenation and functional data can be acquired simultaneously, potentially allowing a more detailed assessment of the onset and severity of the ischaemia.32,39
Study limitations
A key limitation with OS-CMR is that the signal does not provide an absolute value. Only changes of signal intensity are obtained to follow the myocardial oxygenation response to a given stimulus. Therefore, a baseline or reference image is required. Also, further research is needed to investigate the clinical relevance of the measured de-oxygenation events. Although the images appear on the scanner screen within seconds and thus allow cursory visual assessment of functional changes, quantification of (relative) myocardial oxygenation change still requires off-line analysis and user input, which can introduce an operator bias. Thus, for instance, interventions to improve oxygenation based on emerging OS-CMR findings cannot yet be made in a real-time automated fashion. Rather, this technique currently provides insight into whether and when de-oxygenation does occur, and in the future, will shed light on the potential of preoxygenation routines or haemodynamic changes32 to modulate myocardial oxygen balance during induction of general anaesthesia or sedation. Finally, this research was conducted in awake healthy participants performing a predefined breathing protocol in room air.
Potential for anaesthesia research
CMR is not feasible for the everyday peri-operative environment, but advanced imaging techniques are increasingly present in the operating room. Our findings demonstrate their utility as a comprehensive research tool to assess the cardiac effects of induction of general anaesthesia and other preoperative interventions and procedures. We have recently published the first findings from employing free-breathing OS-CMR sequences to continuously investigate myocardial oxygenation during the induction of general anaesthesia in two patients.18 With this technique, we were able to demonstrate that complex oscillations of myocardial oxygenation occurred in a patient without cardiovascular disease and in one with coronary artery disease. These were triggered by specific interventions during the induction sequence despite maintaining adequate blood-oxygen saturation and they corresponded with changes in end-tidal CO2. Specifically, the patient with a significant coronary stenosis exhibited inducible regional ischaemia, indicated by regional de-oxygenation localised with wall motion abnormalities in the territory at risk.18 These preliminary findings are evidence that OS-CMR can be used in a peri-operative setting as a research tool. This could be important for understanding the onset of ischaemia, as clinical assessment of incipient peri-operative myocardial ischaemia comes with limitations, in part because patients under general anaesthesia cannot inform the medical team about symptoms. ECG abnormalities may not arise or may not appear until late in the ischaemic cascade.48 Similarly, high-sensitivity troponin assays are used to diagnose MINS and MICS but will take at least 90 min after cellular damage has occurred to be detectable.2,4 Consequently, troponin assays cannot offer better time resolution in pinpointing the onset and trigger of myocardial ischaemia than imaging techniques that can provide data every few seconds. Cardiovascular anaesthetists have been able to routinely perform peri-operative echocardiography, and have used this more accessible modality to detect early sequelae of myocardial ischaemia such as new-onset regional diastolic and systolic dysfunction. But echocardiography is not able to explore an oxygenation deficit that precedes new-onset mechanical dysfunction. The OS-CMR is a promising technique for assessing myocardial oxygenation during anaesthesia induction directly without relying on surrogate markers. As a technology, MRI is rapidly moving towards interventional and peri-operative applications,49 and the field of anaesthesia will certainly benefit by incorporating advanced imaging into research studies.
Conclusion
Fluctuations in myocardial oxygenation can be monitored continuously and noninvasively using novel free-breathing OS-CMR imaging techniques in awake healthy volunteers. Voluntary breathing manoeuvres simulating the induction sequence of general anaesthesia have a dynamic effect on myocardial oxygenation in spontaneously breathing healthy participants. Paced breathing followed by five deep breaths results in myocardial de-oxygenation, whereas subsequent apnoea exerts an opposite effect by increasing myocardial oxygenation. To assess if these findings translate to a clinical setting, these techniques will now be applied peri-operatively to investigate the dynamics of myocardial oxygenation and possibly the evolution of peri-operative ischaemia in patients undergoing induction of general anaesthesia.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: this study was made possible by the superb work of study nurse Sarah Overney.
Financial support and sponsorship: funding was provided by the Bern University Hospital Department of Anaesthesiology and Pain Medicine Scientific Funds, the European Society of Anaesthesiology and Intensive Care (ESAIC) and the Swiss National Science Foundation (SNSF).
Conflict of interest: none.
Presentation: preliminary data has been presented at Swiss Anaesthesia Congress 2021, Geneva, Switzerland, European Society of Cardiothoracic Anaesthesiology Congress 2022, Napoli, Italy, and Euroanaesthesia 2023, Glasgow, UK.
This manuscript was handled by Dan Longrois.
Footnotes
Supplemental digital content is available for this article.
References
- 1.Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev 2019; 27:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botto F, Alonso-Coello P, Chan MTV, et al. Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group, on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators, Appendix 1. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578. [DOI] [PubMed] [Google Scholar]
- 3.Puelacher C, Gualandro DM, Glarner N, et al. Long-term outcomes of perioperative myocardial infarction/injury after non-cardiac surgery. Eur Heart J 2023; 44:1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafiudeen R, Barlis P, White HD, et al. Type 2 MI and myocardial injury in the era of high-sensitivity troponin. Eur Cardiol Rev 2022; 17:e03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priebe H-J. Triggers of perioperative myocardial ischaemia and infarction. BJA Br J Anaesth 2004; 93:9–20. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Chen L, He J, et al. Perioperative myocardial injury/infarction after noncardiac surgery in elderly patients. Front Cardiovasc Med 2022; 9:910879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit M, Coetzee AR, Lochner A. The pathophysiology of myocardial ischemia and perioperative myocardial infarction. J Cardiothorac Vasc Anesth 2020; 34:2501–2512. [DOI] [PubMed] [Google Scholar]
- 8.Bache RJ, Cobb FR. Effect of maximal coronary vasodilation on transmural myocardial perfusion during tachycardia in the awake dog. Circ Res 1977; 41:648–653. [DOI] [PubMed] [Google Scholar]
- 9.Fischer K, Guensch DP, Shie N, et al. Breathing maneuvers as a vasoactive stimulus for detecting inducible myocardial ischemia - an experimental cardiovascular magnetic resonance study. PLoS One 2016; 11:e0164524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson NP, Kirkeeide RL, Gould KL. Coronary steal: mechanisms of a misnomer. J Am Heart Assoc 2021; 10:e021000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouroche G, Bourgain JL. Preoxygenation and general anesthesia: a review. Minerva Anestesiol 2015; 81:910–920. [PubMed] [Google Scholar]
- 12.Guensch DP, Michel MC, Huettenmoser SP, et al. The blood oxygen level dependent (BOLD) effect of in-vitro myoglobin and hemoglobin. Sci Rep 2021; 11:11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HJ, Yumul R, Tang R, et al. Assessment of myocardial reactivity to controlled hypercapnia with free-breathing T2-prepared cardiac blood oxygen level-dependent MR imaging. Radiology 2014; 272:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guensch DP, Nadeshalingam G, Fischer K, et al. The impact of hematocrit on oxygenation-sensitive cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2016; 18:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guensch DP, Fischer K, Shie N, et al. Hyperoxia exacerbates myocardial ischemia in the presence of acute coronary artery stenosis in swine. Circ Cardiovasc Interv 2015; 8:e002928. [DOI] [PubMed] [Google Scholar]
- 16.Kido T, Kido T, Nakamura M, et al. Compressed sensing real-time cine cardiovascular magnetic resonance: accurate assessment of left ventricular function in a single-breath-hold. J Cardiovasc Magn Reson 2016; 18:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Boomen M, Manhard MK, Snel GJH, et al. Blood oxygen level-dependent MRI of the Myocardium with Multiecho Gradient-Echo Spin-Echo Imaging. Radiology 2020; 294:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guensch DP, Federer J, Schweizer T, et al. First findings from perioperative magnetic resonance imaging of inducible myocardial ischaemia during induction of general anaesthesia. Br J Anaesth 2023; 131:e75–e79. [DOI] [PubMed] [Google Scholar]
- 19.Fischer K, Guensch DP, Friedrich MG. Response of myocardial oxygenation to breathing manoeuvres and adenosine infusion. Eur Heart J Cardiovasc Imaging 2015; 16:395–401. [DOI] [PubMed] [Google Scholar]
- 20.Fischer K, Yamaji K, Luescher S, et al. Feasibility of cardiovascular magnetic resonance to detect oxygenation deficits in patients with multivessel coronary artery disease triggered by breathing maneuvers. J Cardiovasc Magn Reson 2018; 20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci 1990; 87:9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer WR, Nadler W, Bock M, et al. The relationship between the BOLD-induced T (2) and T(2)(∗): a theoretical approach for the vasculature of myocardium. Magn Reson Med 1999; 42:1004–1010. [DOI] [PubMed] [Google Scholar]
- 23.Wacker CM, Bock M, Hartlep AW, et al. Changes in myocardial oxygenation and perfusion under pharmacological stress with dipyridamole: assessment using T∗2 and T1 measurements. Magn Reson Med 1999; 41:686–695. [DOI] [PubMed] [Google Scholar]
- 24.Friedrich MG, Karamitsos TD. Oxygenation-sensitive cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013; 15:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shea SM, Fieno DS, Schirf BE, et al. T2-prepared steady-state free precession blood oxygen level-dependent MR imaging of myocardial perfusion in a dog stenosis model1. Radiology 2005; 236:503–509. [DOI] [PubMed] [Google Scholar]
- 26.Guensch DP, Fischer K, Flewitt JA, et al. Impact of intermittent apnea on myocardial tissue oxygenation—a study using oxygenation-sensitive cardiovascular magnetic resonance. PLoS One 2013; 8:e53282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guensch DP, Fischer K, Jung C, et al. Relationship between myocardial oxygenation and blood pressure: Experimental validation using oxygenation-sensitive cardiovascular magnetic resonance. PloS One 2019; 14:e0210098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahnke C, Gebker R, Manka R, et al. Navigator-gated 3D blood oxygen level-dependent CMR at 3.0-T for detection of stress-induced myocardial ischemic reactions. JACC Cardiovasc Imaging 2010; 3:375–384. [DOI] [PubMed] [Google Scholar]
- 29.Luu JM, Friedrich MG, Harker J, et al. Relationship of vasodilator-induced changes in myocardial oxygenation with the severity of coronary artery stenosis: a study using oxygenation-sensitive cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 2014; 15:1358–1367. [DOI] [PubMed] [Google Scholar]
- 30.Karamitsos TD, Leccisotti L, Arnold JR, et al. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging 2010; 3:32–40. [DOI] [PubMed] [Google Scholar]
- 31.Mahmod M, Francis JM, Pal N, et al. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J Cardiovasc Magn Reson 2014; 16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer K, Neuenschwander MD, Jung C, et al. Assessment of myocardial function during blood pressure manipulations using feature tracking cardiovascular magnetic resonance. Front Cardiovasc Med 2021; 8:743849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer K, Guensch DP, Jung B, et al. Insights into myocardial oxygenation and cardiovascular magnetic resonance tissue biomarkers in heart failure with preserved ejection fraction. Circ Heart Fail 2022; 15:e008903. [DOI] [PubMed] [Google Scholar]
- 34.Guensch DP, Fischer K, Yamaji K, et al. Effect of hyperoxia on myocardial oxygenation and function in patients with stable multivessel coronary artery disease. J Am Heart Assoc 2020; 9:e014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feigl EO. Coronary physiology. Physiol Rev 1983; 63:1–205. [DOI] [PubMed] [Google Scholar]
- 36.Yang HJ, Dey D, Sykes J, et al. Arterial CO2 as a potent coronary vasodilator: a preclinical PET/MR validation study with implications for cardiac stress testing. J Nucl Med 2017; 58:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iannino N, Fischer K, Friedrich M, et al. Myocardial vascular function assessed by dynamic oxygenation-sensitive cardiac magnetic resonance imaging long-term following cardiac transplantation. Transplantation 2021; 105:1347–1355. [DOI] [PubMed] [Google Scholar]
- 38.Hillier E, Covone J, Fischer K, et al. Microvascular dysfunction as a possible link between heart failure and cognitive dysfunction. Circ Heart Fail 2023; e010117. [DOI] [PubMed] [Google Scholar]
- 39.Spicher B, Fischer K, Zimmerli ZA, et al. Combined analysis of myocardial deformation and oxygenation detects inducible ischemia unmasked by breathing maneuvers in chronic coronary syndrome. Front Cardiovasc Med 2022; 9:800720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baraka AS, Taha SK, Aouad MT, et al. Preoxygenation: comparison of maximal breathing and tidal volume breathing techniques. Anesthesiology 1999; 91:612–616. [DOI] [PubMed] [Google Scholar]
- 41.Mutch WAC, Ellis MJ, Ryner LN, et al. Canada North Concussion Network, and, University Health Network Cerebrovascular Reactivity Research Group. Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J Neurosurg 2015; 125:648–660. [DOI] [PubMed] [Google Scholar]
- 42.Bignami E, Saglietti F, Girombelli A, et al. Preoxygenation during induction of anesthesia in noncritically ill patients: a systematic review. J Clin Anesth 2019; 52:85–90. [DOI] [PubMed] [Google Scholar]
- 43.Fischer K, Guensch DP, Shie N, et al. Altered blood gas tensions of oxygen and carbon dioxide confound coronary reactivity to apnea. Front Anesthesiol 2022; 1:997836. [Google Scholar]
- 44.Hawkins SM, Guensch DP, Friedrich MG, et al. Hyperventilation-induced heart rate response as a potential marker for cardiovascular disease. Sci Rep 2019; 9:17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu T, Song SY. Perioperative management of left ventricular diastolic dysfunction and heart failure: an anesthesiologist's perspective. Korean J Anesthesiol 2017; 70:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehandale SG, Rajasekhar P. Perfusion index as a predictor of hypotension following propofol induction - a prospective observational study. Indian J Anaesth 2017; 61:990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh R, Choudhury M, Kapoor PM, et al. A randomized trial of anesthetic induction agents in patients with coronary artery disease and left ventricular dysfunction. Ann Card Anaesth 2010; 13:217–223. [DOI] [PubMed] [Google Scholar]
- 48.DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation 2019; 140:1661–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rier SC, Vreemann S, Nijhof WH, et al. Interventional cardiac magnetic resonance imaging: current applications, technology readiness level, and future perspectives. Ther Adv Cardiovasc Dis 2022; 16:17539447221119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.