Abstract
Background:
Sleep disturbances are prevalent in major depressive disorder (MDD). MDD and sleep disturbances are both linked to cognitive impairments. Studies exploring the mechanisms and impact of sleep disturbances on neurocognitive functioning in depressed patients are lacking and proper assessment and therapeutic interventions for sleep disturbances are not part of clinical management of MDD.
Aim:
We investigated the association between subjective sleep quality and neurocognitive dysfunction in patients with MDD.
Materials and Methods:
Patients with moderate MDD episode were matched and assigned to two groups with poor and good sleep quality. We used Pittsburgh Sleep Quality Index (PSQI) to assess sleep quality. To measure frontotemporally mediated cognitive functioning, following tests were administered: Wisconsin Card Sorting Test (WCST) and degraded continuous performance test (CPT-DS). Two-tailed independent samples t tests or Mann–Whitney U tests and Pearson’s correlation coefficient were performed for the statistical analysis of sleep latency, sleep duration, overall sleep quality, CPT d’ value, WCST correct answers, errors, and perseverative errors.
Results:
Participants with MDD and poor sleep quality performed worse on cognitive tests compared to patients with MDD and good sleep quality. Scores of subjective sleep on PSQI positively correlated with WCST errors (r (60) =0.8883 P = .001) and negatively correlated with WCST correct answers (r (60) = -.869 P = .001) and measures of CPT-DS d’ value (r (60) = -.9355 P = .001).
Conclusions:
Poor sleep quality, notably sleep duration and sleep latency, worsens the neurocognitive impairments of MDD patients. As these impairments are found to be associated with treatment outcomes, sleep disturbances should be additionally assessed and treated in MDD episode.
Keywords: Continuous attention, executive functioning, major depression, sleep disturbance
In the recent years, neurocognitive aspects of depression have gained an increasing scientific attention. A substantial number of studies focusing specifically on cognitive functioning and its neurobiological or clinical correlates revealed that depressed patients showed an impairment on a variety of neurocognitive tasks in comparison with healthy controls.[1,2,3] More importantly, these impairments have been found to be associated with poor social function and treatment outcomes.[4,5] According to the 5th edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-V),[6] a decreased ability to concentrate is one of the diagnostical criteria of major depressive disorder (MDD). The underlying mechanisms of these cognitive deficits are still studied, although several suggestions have been made during the recent decades. Based on the review of studies on cognitive processing, Hartlage et al.[7] proposed that depressed patients have a reduced capacity to process information, leading to difficulties accomplish effortful cognitive tasks.[8] Other authors concluded that some specific cognitive deficits, like impairments of working memory and problem solving, cannot be separated from general deficits of motivation and attention in depressed patients.[9,10]
In recent years, some neuroimaging studies succeed to show an impairment of brain functions among depressed patients, specifically in the frontal lobes,[11] which play an important role in cognitive performance. This indicates that cognitive dysfunction in depression may have a particular neurobiological basis.[12] Several studies demonstrated that frontotemporally mediated cognitive functions, like attention and executive functions, are significantly impaired in patients with MDD.[13,14] Attentional disturbance has been proposed to be a hallmark of cognitive impairments in depression.[3,15] The continuous performance test (CPT) is widely used in psychiatric research to measure attentional problems in various disorders including MDD.[16,17,18] Depressed patients were also found to have impairment of executive functioning.[19] Corresponding with the functional brain abnormalities in depression, classical executive tests sensitive to frontal lobe dysfunction[20,21] like Wisconsin Card Sorting Test can be used in depressive patients to measure executive functioning.[22]
The data of several studies showed that cognitive disturbances appear to be impacted by a variety of clinical factors, including severity, comorbidity, duration of illness, and subtypes of depression.[23,24]
Sleep disturbances are common and various among patients with MDD, giving the substantial clinical heterogeneity of depression.[25,26] According to DSM-V, diagnosis of MDD is made when a patient has any out of nine symptoms, among which are sleep disturbances from insomnia to hypersomnia, corresponding to the broad range of sleep disturbances. From the previous research, we know that sleep itself is essential for cognitive performance. People who are exposed to sleep deprivation usually experience a decline in cognitive performance, namely, in attention and working memory, and mood changes.[26,27,28,29] Both attention and working memory are linked to the functioning of frontal lobes. Since the frontal brain areas are vulnerable to sleep deprivation,[29,30] it can be hypothesized that both attention and executive functioning are impaired during poor sleep quality.
Previous studies have used different self-administered questionnaires for measuring subjective sleep quality and cognitive performance in various study groups. The findings of those studies were controversial. Some of them showed the association between working memory,[31] decision-making performance,[32] and executive functions,[33,34] though other studies found no link between subjective sleep quality and cognitive performance.[35] The substantial number of these studies investigated diverse age groups mainly of healthy population, whereas studies conducted in clinical groups are lacking. At the same time, cognitive impairment is gaining increasing attention in predicting the treatment outcomes of major depression.[36]
Here, to fill that gap, we investigate association between subjective sleep quality and neurocognitive dysfunction in patients with MDD. We predicted that poor sleep quality among patients with MDD would be associated with lower scores on objective measures of frontotemporally mediated neurocognitive functions. Additionally, we investigated the association of subjective sleep components, such as sleep latency and sleep duration, with objective measures of sustained attention and executive functions.
MATERIALS AND METHODS
The study was conducted from January 2017 to May 2021 in Tbilisi Mental Health Center, Tbilisi, Georgia. All participants had normal or corrected-to-normal vision, with a visual acuity superior or equal to 0.8 out of 2 determined for both eyes with setup and pre-processing pipeline. Procedure details can be found in da Cruz et al.[37] All participants signed an informed consent after the study protocol was fully explained and were informed that they could quit the experiment at any time. All procedures complied with the Declaration of Helsinki and were approved by the Medical Ethical Committee of the Tbilisi State Medical University.
Study participants
Patients with MDD were recruited in the study either from: 1. Outpatient service of Tbilisi Mental Health Centre, 2. Psychiatric private practices, 3. Through the screening surveys conducted among medical students. All participants had MDD, recurrent episode, according to the DSM-V, by means of an interview based on the Structured Clinical Interview and the study of the medical records. The age of patients was restricted from 18 to 65 due to avoid age-related biases of vision and cognitive functions.
Exclusion criteria were drug or alcohol abuse, neurological or other somatic illnesses influencing the subjects’ mental state, severe or mild depressive episode, treatment with benzodiazepines, or second-generation antipsychotics (SGAs). Psychopathology was assessed by an experienced psychiatrist. The Brief Psychiatric Rating Scale (BPRS)[38] was administered to exclude psychiatric comorbidities. The severity of the depression was measured by means of the 17-item version of the Hamilton Depression Rating Scale (HDRS).[39] Patients having moderate depressive episode (scores 18-24 on HDSR) were recruited in the study.
To measure subjective sleep quality participants completed the Pittsburgh Sleep Quality Index (PSQI),[40] which is a self-administered, effective instrument used to measure the quality and patterns of sleep in adults.[35] It differentiates “poor” from “good” sleep quality by measuring seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction over the last month. On PSQI, total score of 5 or greater corresponds to poor sleep quality, whereas total score lower than 5 corresponds to good sleep quality.
Following variables were analyzed: sleep latency, sleep duration, and overall sleep quality.
All patients took antidepressants (fluoxetine, fluvoxamine, clomipramine, venlafaxine, escitalopram, duloxetine, and trazodone). Three patients were receiving mood stabilizer lamotrigine as an adjunctive treatment.
Overall, 62 patients with moderate recurrent depressive episode were recruited in the study.
Study groups
Participants were divided into two groups: One group consisted of patients having MDD and poor sleep quality (total score of 5 or greater on PSQI). Second group consisted of patients having MDD and good sleep quality (total score lower than 5 on PSQI).
Neurocognitive tests and measurements
To measure executive functions, we administered a computerized version of the Nelson Test,[41] which is a modified Wisconsin Card Sorting Test (WCST) with 48 cards. We recorded and measured the number of categories that participants went through and numbers of correct responses, errors, and preservative errors.[42]
To measure sustained attention and vigilance, we administered a degraded stimulus continuous performance test CPT-DS[24] to both groups. Computerized CPT-DS with three blocks (720 digits, 10% targets, degradation 40%) with a total duration of 12 minutes was performed in a quite environment. Observers had to detect the pair “1-9” and push the button when the target was appearing on the screen. The digits were presented randomly with a rate of one per second with a presentation time of 50 milliseconds. We measured d’ value, which is an average reaction time and coefficient of variance for both correct reactions and commission errors. Higher scores of d’ value correspond to better performance.[42,43]
Statistical analysis
To examine group differences on demographic, clinical, and neuropsychological scores, two-tailed independent samples t tests or Mann–Whitney U tests were performed, depending on whether data were normally distributed. Associations between PSQI subscale scores and neurocognitive test measures were calculated by Pearson’s correlation coefficient. For all statistical analyses, a P value of <0.05 was used to indicate a significant effect. The statistical analyses were performed by SPSS for Mac.
RESULTS
A total of 34 patients compose one group of participants with mean age/SD = 35.8 ± 7.2: 21 female and 13 male had MDD and poor sleep quality, whereas 28 patients from second group with mean age/SD = 33.7 ± 9.1: 17 female and 11 male had MDD and good sleep quality.
No significant differences between groups were found in regard to education years, gender ratio, visual acuity, handedness, illness duration, and episode severity [Table 1].
Table 1.
Characteristics (mean±SD) for the group 1 (poor sleep quality) and group 2 (good sleep quality)
| Group 1 (n=34) | Group 2 (n=28) | |
|---|---|---|
| Gender (F/M) | 21/13 | 17/11 |
| Age (years) | 35.8±7.2 | 33.7±9.1 |
| Education (years) | 14.6±2.4 | 14.3±2.1 |
| Handedness (L/R) | 4/30 | 2/26 |
| Visual Acuity | 1.6±0.4 | 1.4±0.4 |
| Illness Duration (years) | 6.2±4.3 | 5.8±3.2 |
| BPRS | 30.1±5.0 | 30.2±4.8 |
| HDRS total | 22.6±3.4. | 21.9±2.7 |
| PSQI total | 8.55±2.29 | 2.17±1.05 |
*BPRS, Brief Psychiatric Rating Scale; HDRS, Hamilton Depression Rating Scale; PSQI, Pittsburgh Sleep Quality Index
Two participants dropped out from the first group due to exacerbation of depressive symptoms.
Statistically significant differences were found between the groups on neurocognitive test performance. The outcome measures of d’ value and WCST correct answers were significantly higher in the study group with good sleep quality, whereas WCST errors were significantly lower in the same group. No statistically significant difference was established on WCST perseverative errors [Table 2].
Table 2.
Neurocognitive parameters (mean±SD) and differences in performance of tests between study groups
| P | Group 1 (PSQI ≥5) | Group 2 (PSQI <5) | t/U |
|---|---|---|---|
| d’ value | 1.51±0.42 | 3.86±0.74 | -7.08=0.001b |
| WCST correct (n) | 14.85±3.55 | 33.75±6.38 | -14.7=0.01a |
| WCST errors (n) | 27.94±4.72 | 10.7±5.57 | 13.66=0.01a |
| WCST pers (n) | 5.26±2.13 | 4.25±3.13 | 1.4=0.07a |
*aP-value based on a (two-tailed) Student’s t-test. bP-value based on a (two-tailed) Mann–Whitney U test. PSQI, Pittsburgh Sleep Quality Index; WCST, Wisconsin Card Sorting Test
In regard to associations between PSQI and cognitive test performance measures, here we present only statistically significant correlations. Sleep latency, sleep duration, and overall sleep quality scores on PSQI found to be statistically significantly correlated with specific objective measures of neurocognitive test performance in both groups. Sleep latency scores negatively correlated with number of correct responses on WCST (r (n) (60) = -.7793 P = .05) and with measures of d’ value on CPT-DS (r (n) (60) = -.9275, P = .001). Positive correlation between the number of error responses on WCST and sleep latency scores was established (r (n) (60) = 0.8145 P = .05) [Figure 1, Panel A]. Negative correlation was found between sleep duration scores and WCST correct answers (r (n)(60) = -0.817 P = .01), as well as with CPT-DS d’ value measures (r (n) (60) = -.9195 P = .001). Number of errors on WCST and sleep duration were positively correlated (r (n) (60) =0.849 P = .01) [Figure 1, Panel B].
Figure 1.
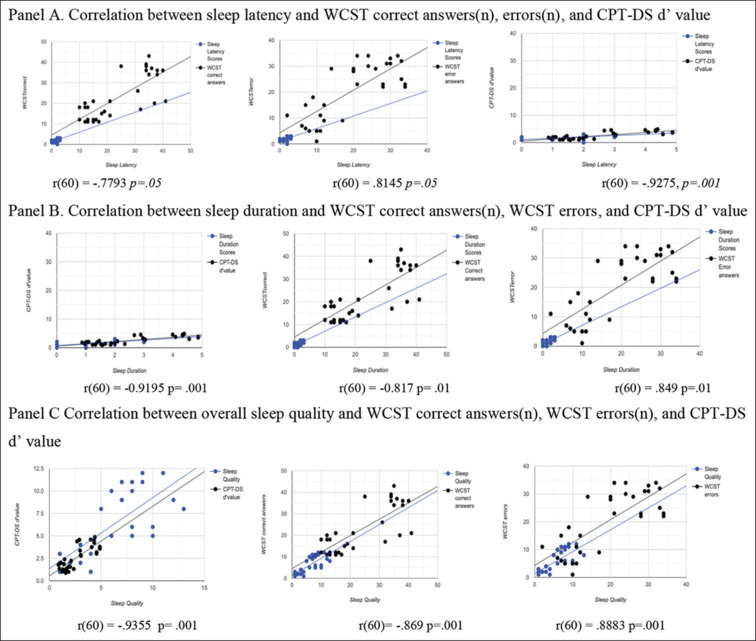
Correlation between sleep latency, duration, and quality with WCST correct answers (n), errors (n), and CPT-DS d’ value. Abbreviations: WCST: Wisconsin card sorting test; CPT-DS: Degraded stimulus continuous performance test
Overall scores of subjective sleep on PSQI positively correlated with number of WCST errors (r(n) (60) = 0.8883 P = .001), whereas the same PSQI scores found to be in negative correlation with WCST correct answers (r (n) (60) = -.869 P = .001) and measures of CPT-DS d’ value (r (n) (60) = -.9355 P = .001) [Figure 1, Panel C].
DISCUSSION
Results of this study suggest that sleep disturbances reported by patients have a substantial impact on neurocognitive performance. Some specific components of sleep quality, such as sleep latency and sleep duration, appeared to be more related to objective parameters of frontotemporally mediated neurocognitive performance. Sustained attention and executive functions tend to be significantly worse in patients with MDD experiencing prolonged sleep latency and short sleep duration.
Our findings are in line with several previous research results investigating sleep quality and sleep duration. Ma et al.[44] based on their large cohort longitudinal study found that insufficient or excessive sleep duration is associated with cognitive decline. Study on individuals with diabetes revealed that short sleep duration was associated with impairment of executive functioning.[45] Nebes et al.[33] showed that self-reported good and poor sleeper older adults differed on tests of working memory, attentional set shifting, and abstract problem solving.
Some empirical studies showed no statistically significant association between subjective sleep quality and cognitive performance in healthy young population.[35] That might indicate that age and comorbidities play an important role and among healthy younger adults sleep disturbances are not causing significant measurable cognitive impairments. However, older adults and persons with other disorders like diabetes or depression are more vulnerable to the sleep disturbances. Overlapping neurobiological mechanisms of depression and sleep disturbances in MDD may be an explanation. Involvement of frontal lobe areas in both pathologies is established by neuroimaging studies. Thus, decreased sleep duration and depression may have a negative cumulative effect on executive cognitive functions, which are also mediated by frontal lobe areas.
Prolonged sleep latency is related to decrease slow-wave sleep (SWS)[44] that is known to be already diminished during depressive episodes. Lack of SWS leads to fatigue, which can be considered an intermediate variable[46] affecting sustained attention and vigilance causing impaired performance on CPT-DS.
The study has several limitations. We have not administered objective measurements of sleep disturbances, like actigraphy or polysomnography. Additionally, we did not investigate the impact of medications on cognitive performance among participants, though it is known that most of the antidepressants have no impact on slow-wave sleep[47] and do not alter significantly cognitive performance.
Strength of the study should be also mentioned. The samples were homogenous and comparable in regard to possible extraneous variables like age, illness duration, and episode severity.
To summarize, poor sleep quality, notably sleep duration and sleep latency, significantly worsens the neurocognitive impairments of MDD patients. As these impairments are found to be associated with treatment outcomes,[4,5] sleep disturbances should be assessed and treated in MDD episode, which is not the case in daily clinical practice. Self-addressed questionnaires like PSQI appear to be useful and could be easily implemented. Emphasizing the importance of subjective sleep quality in depressed patients, we suggest that pharmacological and nonpharmacological interventions managing the associated sleep disturbances should be the part of first-line treatment for MDD. Consequently, further studies are needed to find the exact underlying neurobiological mechanisms and novel treatment strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Roca M, Monzón S, Vives M, López-Navarro E, Garcia-Toro M, Vicens C, et al. Cognitive function after clinical remission in patients with melancholic and non-melancholic depression: A 6 month follow-up study. J Affect Disord. 2015;171:85–92. doi: 10.1016/j.jad.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Thomas P, Goudemand M, Rousseaux M. Divided attention in major depression. Psychiatry Res. 1998;81:309–22. doi: 10.1016/s0165-1781(98)00123-1. [DOI] [PubMed] [Google Scholar]
- 3.Williams RA, Hagerty BM, Cimprich B, Therrien B, Bay E, Oe H. Changes in directed attention and short-term memory in depression. J Psychiatr Res. 2000;34:227–38. doi: 10.1016/s0022-3956(00)00012-1. [DOI] [PubMed] [Google Scholar]
- 4.Cohen RM, Greenberg JM, IsHak WW. Incorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression to measure treatment impact and recovery in MDD. JAMA Psychiatry. 2013;70:343–50. doi: 10.1001/jamapsychiatry.2013.286. [DOI] [PubMed] [Google Scholar]
- 5.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med. 2014;44:2029–40. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association . 5th. Washington: APA; 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 7.Hartlage S, Alloy LB, Vázquez C, Dykman B. Automatic and effortful processing in depression. Psychol Bull. 1993;113:247–78. doi: 10.1037/0033-2909.113.2.247. [DOI] [PubMed] [Google Scholar]
- 8.Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen RM, Weingartner H, Smallberg SA, Pickar D, Murphy DL. Effort and cognition in depression. Arch Gen Psychiatry. 1982;39:593–7. doi: 10.1001/archpsyc.1982.04290050061012. [DOI] [PubMed] [Google Scholar]
- 10.Hart RP, Wade JB, Calabrese VP, Colenda CC. Vigilance performance in Parkinson’s disease and depression. J Clin Exp Neuropsychol. 1998;20:111–7. doi: 10.1076/1380-3395(199802)20:1;1-P;FT111. [DOI] [PubMed] [Google Scholar]
- 11.Veiel HO. A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol. 1997;19:587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- 12.Lam RW, Kennedy SH, Mclntyre RS, Khullar A. Cognitive dysfunction in major depressive disorder: Effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59:649–54. doi: 10.1177/070674371405901206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo YS, Rosenblat JD, Kakar R, Bahk WM, McIntyre RS. Cognitive deficits as a mediator of poor occupational function in remitted major depressive disorder patients. Clin Psychopharmacol Neurosci. 2016;14:1–16. doi: 10.9758/cpn.2016.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. 2012;140:113–24. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am. 2004;27:19–36. doi: 10.1016/S0193-953X(03)00106-0. , vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasmer OB, Mjeldheim K, Førland W, Hansen AL, Syrstad VE, Oedegaard KJ, et al. Linear and non-linear analyses of Conner’s Continuous Performance Test-II discriminate adult patients with attention deficit hyperactivity disorder from patients with mood and anxiety disorders. BMC Psychiatry. 2016;16:284. doi: 10.1186/s12888-016-0993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29:65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- 18.Nelson EB, Sax KW, Strakowski SM. Attentional performance in patients with psychotic and nonpsychotic major depression and schizophrenia. Am J Psychiatry. 1998;155:137–9. doi: 10.1176/ajp.155.1.137. [DOI] [PubMed] [Google Scholar]
- 19.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci Ther. 2018;24:994–1003. doi: 10.1111/cns.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 22.Fossati P, Ergis AM, Allilaire JF. Neuropsychologie des troubles des fonctions exécutives dans la dépression: Une revue de la littérature [Executive functioning in unipolar depression: A review] L’Encephale. 2002;28:97–107. [PubMed] [Google Scholar]
- 23.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: A systematic review. J Affect Disord. 2011;134:20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 24.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Bühler J, Seemüller F, Läge D. The predictive power of subgroups: An empirical approach to identify depressive symptom patterns that predict response to treatment. J Affect Disord. 2014;163:81–7. doi: 10.1016/j.jad.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 26.Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. Journal of cellular and molecular medicine. 2019;23:2324–32. doi: 10.1111/jcmm.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16:375. doi: 10.1186/s12888-016-1075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maquet P. The role of sleep in learning and memory. Science (New York, N.Y. ) 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 29.Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–67. [PMC free article] [PubMed] [Google Scholar]
- 30.Patrick Y, Lee A, Raha O, Pillai K, Gupta S, Sethi S, et al. Effects of sleep deprivation on cognitive and physical performance in university students. Sleep Biol Rhythms. 2017;15:217–25. doi: 10.1007/s41105-017-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Noort M, Struys E, Perriard B, Staudte H, Yeo S, Lim S, et al. Schizophrenia and depression: The relation between sleep quality and working memory. Asian J Psychiatr. 2016;24:73–8. doi: 10.1016/j.ajp.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Telzer EH, Fuligni AJ, Lieberman MD, Galván A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–83. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyle SD, Sexton CE, Feige B, Luik AI, Lane J, Saxena R, et al. Sleep and cognitive performance: Cross-sectional associations in the UK Biobank. Sleep Med. 2017;38:85–91. doi: 10.1016/j.sleep.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zavecz Z, Nagy T, Galkó A, Nemeth D, Janacsek K. The relationship between subjective sleep quality and cognitive performance in healthy young adults: Evidence from three empirical studies. Sci Rep. 2020;10:4855. doi: 10.1038/s41598-020-61627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groves SJ, Douglas KM, Porter RJ. A systematic review of cognitive predictors of treatment outcome in major depression. Front Psychiatry. 2018;9:382. doi: 10.3389/fpsyt.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Cruz JR, Chicherov V, Herzog MH, Figueiredo P. An automatic pre-processing pipeline for EEG analysis (APP) based on robust statistics. Clin Neurophysiol. 2018;129:1427–37. doi: 10.1016/j.clinph.2018.04.600. [DOI] [PubMed] [Google Scholar]
- 38.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. Br J Psychiatry. 2005;187:366–71. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–24. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 42.Favrod O, da Cruz JR, Roinishvili M, Berdzenishvili E, Brand A, Figueiredo P, et al. Electrophysiological correlates of visual backward masking in patients with major depressive disorder. Psychiatry Res Neuroimaging. 2019;294:111004. doi: 10.1016/j.pscychresns.2019.111004. [DOI] [PubMed] [Google Scholar]
- 43.Chkonia E, Roinishvili M, Reichard L, Wurch W, Puhlmann H, Grimsen C, et al. Patients with functional psychoses show similar visual backward masking deficits. Psychiatry Res. 2012;198:235–40. doi: 10.1016/j.psychres.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y, Liang L, Zheng F, Shi L, Zhong B, Xie W. Association between sleep duration and cognitive decline. JAMA Netw Open. 2020;3:e2013573. doi: 10.1001/jamanetworkopen.2020.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Titova OE, Lindberg E, Tan X, Elmståhl S, Lind L, Schiöth HB, et al. Association between sleep duration and executive function differs between diabetic and non-diabetic middle-aged and older adults. Psychoneuroendocrinology. 2020;111:104472. doi: 10.1016/j.psyneuen.2019.104472. [DOI] [PubMed] [Google Scholar]
- 46.Shrivastava D, Jung S, Saadat M, Sirohi R, Crewson K. How to interpret the results of a sleep study. J Community Hosp Intern Med Perspect. 2014;4:24983. doi: 10.3402/jchimp.v4.24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thase ME. Depression and sleep: Pathophysiology and treatment. Dialogues Clin Neurosci. 2006;8:217–26. doi: 10.31887/DCNS.2006.8.2/mthase. [DOI] [PMC free article] [PubMed] [Google Scholar]


