Graphical abstract
Keywords: Ceramide-1-phosphate, Hypoxia, Pulmonary edema, Circadian rhythm, Mitochondrial dynamics
Highlights
-
•
CERK inhibition worsened the severity of HAPE, while exogenous C1P treatment had a protective effect on HAPE.
-
•
CERK inhibition disrupted the circadian rhythm by inducing ARNTL autophagic degradation.
-
•
CERK inhibition reduced ARNTL expression, affecting mitochondrial dynamics, causing oxidative stress and mitophagy defects.
-
•
Exogenous C1P has the potential to be an effective therapeutic strategy for preventing or treating HAPE.
Abstract
Introduction
High-altitude pulmonary edema (HAPE) is a severe and potentially fatal condition with limited treatment options. Although ceramide kinase (CERK)-derived ceramide-1-phosphate (C1P) has been demonstrated to offer protection against various pulmonary diseases, its effects on HAPE remain unclear.
Objectives
Our study aimed to investigate the potential role of CERK-derived C1P in the development of HAPE and to reveal the molecular mechanisms underlying its protective effects. We hypothesized that CERK-derived C1P could protect against HAPE by stabilizing circadian rhythms and maintaining mitochondrial dynamics.
Methods
To test our hypothesis, we used CERK-knockout mice and established HAPE mouse models using a FLYDWC50-1C hypobaric hypoxic cabin. We utilized a range of methods, including lipidomics, transcriptomics, immunofluorescence, Western blotting, and transmission electron microscopy, to identify the mechanisms of regulation.
Results
Our findings demonstrated that CERK-derived C1P played a protective role against HAPE. Inhibition of CERK exacerbated HAPE induced by the hypobaric hypoxic environment. Specifically, we identified a novel mechanism in which CERK inhibition induced aryl hydrocarbon receptor nuclear translocator-like (ARNTL) autophagic degradation, inducing the circadian rhythm and triggering mitochondrial damage by controlling the expression of proteins required for mitochondrial fission and fusion. The decreased ARNTL caused by CERK inhibition impaired mitochondrial dynamics, induced oxidative stress damage, and resulted in defects in mitophagy, particularly under hypoxia. Exogenous C1P prevented ARNTL degradation, alleviated mitochondrial damage, neutralized oxidative stress induced by CERK inhibition, and ultimately relieved HAPE.
Conclusions
This study provides evidence for the protective effect of C1P against HAPE, specifically, through stabilizing circadian rhythms and maintaining mitochondrial dynamics. Exogenous C1P therapy may be a promising strategy for treating HAPE. Our findings also highlight the importance of the circadian rhythm and mitochondrial dynamics in the pathogenesis of HAPE, suggesting that targeting these pathways may be a potential therapeutic approach for this condition.
Introduction
High-altitude pulmonary edema (HAPE) as a specific medical condition
High-altitude pulmonary edema (HAPE) is a serious and potentially fatal condition that can occur when a person climbs to an altitude exceeding 2500 m [1]. It is a subtype of acute high altitude illness (AHAI) and is acute and noncardiogenic in nature [2]. HAPE is characterized by various clinical symptoms, including shortness of breath, bluish skin color, dry cough during physical exertion, pink foamy sputum, difficulty breathing while lying flat, and fever [3].
The alveolar epithelium comprises type I and type II cells, with type I alveolar epithelial (AT1) cells being the most abundant cell type, covering over 95% of the alveolar surface and forming the membrane barrier between the air and blood compartments of the lung [4]. Damage to the alveolar structure can lead to damage to AT1 cells, resulting in impaired respiratory function, which is a manifestation of lung injury [5]. The function of AT1 cells is to reabsorb excess lining fluid from the alveolar surface [6], which is associated with the occurrence and progression of pulmonary edema. HAPE is a type of pulmonary edema that occurs at high altitudes. Although the precise mechanism of HAPE is not fully understood, it is believed to be related to the hypoxic vasoconstriction of pulmonary arterioles, which leads to increased pulmonary capillary pressure and fluid leakage into the alveolar space [7], [8]. Therefore, it is our belief that the selection of AT1 cells was appropriate for studying HAPE.
The mortality rate of untreated HAPE is approximately 50% [9]. Current treatment strategies for HAPE, such as acetazolamide, nifedipine, sildenafil, salmeterol, and dexamethasone, are effective but have side effects. Acetazolamide is a diuretic that is commonly used to treat HAPE, but it can cause side effects such as hypotension, dyspnea, tremor, and tachycardia. Other medications, such as nifedipine and sildenafil, can be used to reduce pulmonary artery pressure and improve oxygenation, but they can also cause side effects such as headache, dizziness, and flushing [10]. Despite extensive research conducted over several years, treatment options for HAPE still have several limitations and challenges [11]. Therefore, research on new, safe, and effective therapeutic targets for HAPE is necessary.
Ceramide-1-phosphate (C1P) and its role in pulmonary diseases
Ceramide-1-phosphate (C1P) is a vital signaling molecule that promotes cellular growth and proliferation [12], while regulating inflammation and cancer [13], [14]. It plays a crucial role in maintaining vascular and epithelial integrity [15]. Various studies have demonstrated the significant role of C1P in the pathogenesis of pulmonary diseases such as asthma [15], chronic obstructive pulmonary disease (COPD) [16], and pulmonary fibrosis [17]. C1P is synthesized intracellularly through direct phosphorylation of ceramide by ceramide kinase (CERK) [18], which is the sole pathway for C1P biosynthesis in mammalian cells [18]. Several studies have suggested that CERK has a cytoprotective effect by producing C1P [19]. However, there is no published evidence to date on the potential involvement of CERK/C1P in mitigating the symptoms of HAPE.
Circadian clock and its impact on respiratory diseases
The circadian clock is responsible for regulating physiological functions based on a 24-hour rhythm, enabling organisms to synchronize internal processes in response to external changes [20]. These clocks exist in most cells, including hepatocytes [21], fibroblasts [22], monocytes [23], and cardiac myocytes [24]. In mammals, the circadian clock modulates gene expression and cell function through transcriptional and translational feedback loops. The transcription factor aryl hydrocarbon receptor nuclear translocator-like/brain and muscle ARNT-like 1 (ARNTL/BMAL1) plays a central role in regulating the expression of other clock-controlled genes [25]. Heterodimers of CLOCK:ARNTL activate transcription of the Period (Per) and Cryptochrome (Cry) genes through E-box elements, and this transcriptional activation can be inhibited by Per and Cry proteins [26]. The lung displays significant circadian rhythms, and the severity of several respiratory diseases varies at different times of the day [27]. For instance, asthma and COPD symptoms worsen in the early morning in humans [28], and the survival of mice with influenza depends on the timing of infection [29].
CERK-derived C1P as a promising therapeutic target for alleviating HAPE
In this study, we have discovered a novel molecular mechanism underlying the action of CERK-derived C1P in alleviating HAPE. We observed that C1P deficiency resulting from CERK inhibition exacerbated the severity of HAPE under hypobaric hypoxic conditions, while exogenous C1P supplementation was able to alleviate the condition. Specifically, CERK inhibition induced circadian misalignment by increasing the degradation of ARNTL, leading to dysregulation of the expression of mitochondrial fission and fusion proteins, which resulted in mitochondrial fragmentation and oxidative stress damage. However, treatment with exogenous C1P was able to restore the abnormal circadian rhythms and mitochondrial damage caused by CERK inhibition, ultimately leading to a reduction in HAPE under hypobaric hypoxia. Our findings provide the first mechanistic basis for CERK-derived C1P in alleviating HAPE and identify a promising target for the treatment of this condition.
Materials and methods
Reagents
The reagents were described in table S2.
Generation of CERK-/- C57BL/6 mice by CRISPR/Cas9 technology
CERK-knockout C57BL/6 mice were generated using CRISPR/Cas9 technology. To generate genetically modified mice, Cas9 mRNA and gRNA were injected into the fertilized eggs using microinjection techniques, resulting in the F0 generation. The genetically modified F0 mice were then identified using PCR amplification and sequencing methods before being bred with wild-type mice to produce the F1 generation carrying the desired genetic modifications. Heterozygous mice were then self-crossed to produce gene-knockout homozygous mice (CERK-/-). Transgenic mice were obtained from the Shanghai Model Organisms Center in Shanghai, China. The primers for identifying mouse DNA were designed and used accordingly, and their sequences are provided in Table S3.
Animal care
The animals were maintained in a controlled environment with a constant humidity of 50 ± 5% and temperature of 23 ± 2℃, and 12-hour light/dark cycles, ensuring stable and standardized living conditions throughout the study. To establish the HAPE model mice, we followed the protocol laid out by Qian Ni et al. [30] and our previous study [31] by exposing the mice to hypobaric hypoxic conditions for 3 days. To simulate a 5,500-meter-high atmospheric environment, we utilized a FLYDWC50-1C hypobaric hypoxia cabin from Guizhou Fenglei Air Ordnance LTD in Guizhou, China.
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the Chinese PLA General Hospital (Approval no.SQ2021218).
Cell culture
Type Ⅰ alveolar epithelial (AT1) cells were cultured in DMEM supplemented with 10% FBS and 1% antibiotics (penicillin and streptomycin). To mimic hypoxic conditions, the cells were exposed to an oxygen concentration of 1% for a period of 24 h.
Measurement of ceramide and ceramide-1-phosphate
Total lipids were extracted from cells by the method of Bligh and Dyer, with modification, as described previously [32]. Quantification of ceramide and C1P was performed using LC-ESI-MS/MS following established protocols [32], [33], [34]. For ceramide analysis, MS/MS transitions with m/z values of 538 → 264 for C16-cer, 650 → 264 for C24-cer, and 652 → 264 for C24:1-cer were used. The C1P MS/MS transitions with m/z values of 618 → 264 for C16-C1P, 730 → 264 for C24-C1P, and 732 → 264 for C24:1-C1P were utilized. Data acquisition was performed by Analyst 1.7.1 software.
HE staining
The lung tissue were fixed in formalin overnight and subsequently embedded in paraffin, and then sectioned into slices that were six micrometers thick. The sections were stained with the hematoxylin and eosin (HE) method, wherein they were first immersed in hematoxylin stain for 5 min and then in eosin stain for 3 min. Images were captured using an optical microscope (Nikon, Japan).
The ratio of lung dry and wet weight
To assess the extent of pulmonary edema, we calculated the ratio of dry lung weight to wet lung weight. Firstly, after euthanizing the animals, we immediately performed dissection. We opened the chest cavity, removed the lung tissue, and recorded the total lung weight. To ensure the accuracy of lung weight, we thoroughly cleaned the lung surface before recording the lung weight, in order to remove any contaminants that might affect weight measurement. After cleaning the lung surface, we recorded the lung wet weight. Next, we placed the lung tissue in an oven and dried it for 72 h at 160 °C until the lung tissue was completely dry. After the lung tissue was completely dry, we recorded the lung dry weight. Finally, the dry and wet weight ratio was then calculated to determine the severity of pulmonary edema.
RNA-seq library preparation, sequencing, and processing
Lung tissues were dissected from the mice in each group, immediately frozen. Total RNA was extracted from the samples using the TRIzol method, following the manufacturer's instructions (Total RNA Extraction Reagent, Vazyme, Jiangsu, China). The quality of the extracted RNA was assessed using an RNA Nano 6000 Assay kit on a Bioanalyzer 2100 system, which provides accurate and quantitative measurements of RNA purity and integrity (Agilent Technologies, CA, USA).
After enrichment with polyA, mRNA library preparation was conducted with TruSeq Stranded mRNA (Illumina, CA, USA). High-quality libraries were prepared for sequencing using the HiSeq-2000 platform (Illumina, CA, USA). To ensure the accuracy and reliability of the data, low-quality reads and adaptor sequences were removed from the sequencing data using SOAPnuke (https://github.com/BGI-flexlab/SOAPnuke). The remaining clean reads were aligned to the mouse reference genome (GRCm38) using BOWTIE2 software (version 2.2.5). Gene expression levels were quantified using the RSEM program (version 1.2.12). Differential gene expression was evaluated by DESeq2 utilizing the Wald test. Genes that exhibited a log fold change greater than 0.5 and an adjusted p-value<0.05 were identified as differentially expressed genes (DEGs).
Gene enrichment testing
ClusterProfiler (v4.6.0) was used for the functional enrichment of the differentially expressed genes in Gene Ontology (GO) categories and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. We considered enrichment analysis results with a false discovery rate (FDR) below 0.05 to be statistically significant. The functional analysis of the DEGs was analyzed according to biological process (BP), molecular function (MF), and cellular component (CC) GO categories. Additionally, gene set enrichment analysis of the KEGG pathway was conducted using ClusterProfiler.
E-box motif sequence alignment of gene promoter regions
The [−1000 bp, 100 bp] interval of the transcription start site (TSS) was delineated as the gene promoter region and the promoter coordinate intervals for the genes of interest were obtained using GENCODE gene annotation (gencode.vM25.annotation.gtf). Bedtools (v2.26.0) was utilized to obtain the FASTA sequence of the gene promoter region based on the mouse reference genome. Finally, seqkit (v2.3.1) was employed to align the E-box motif (5′-CANNTG-3′) sequence with the promoter sequence.
qPCR analysis
Total RNA was extracted from lungs using TRIzol reagent (TRIzol™ (15596026), ThermoFisher Scientific, USA). Total mRNA was first converted to cDNA (GoTaq® qPCR Master Mix (A6001), Promega, USA). The resulting cDNA was then subjected to real-time PCR (Bio-Rad, USA). Gene specific primers, whose sequences are listed in Table S4.
Western blotting
Protein lysates were extracted from the samples using RIPA buffer and phenylmethylsulfonyl fluoride. The protein concentration was determined using a BCA assay. Twenty micrograms of protein per condition was loaded onto 4–12% SDS-polyacrylamide gels and subjected to electrophoresis. The separated proteins were then transferred to nitrocellulose membranes for further analysis. After blocking the membranes with 5% milk for 2 h, they were incubated overnight at 4 °C with primary antibodies specific to the protein(s) of interest. Primary antibodies against ceramide kinase (CERK, NB100-2911, 1:500 dilution) was purchased from Novus Biologicals (USA). Aryl hydrocarbon receptor nuclear translocator-like (Arntl or Bmal1, 14020, 1:1000 dilution), mitofusin 2 (MFN2, 9482, 1:1000 dilution), and LC3A/B (4108, 1:1000 dilution) from Cell Signaling Technology (USA). Dynamin 1-like (DRP1, ab56788, 1:1000 dilution) and beta-actin (ACTB, ab6276, 1:10000 dilution) from Abcam (USA). Mitochondrial fission factor (MFF, 66527, 1:5000 dilution) from Proteintech (USA). The membranes were washed three times and then incubated with secondary antibodies. The bands were visualized using an Amersham Imager 600 instrument (GE Healthcare Life Sciences, USA). The relative protein levels of each band were quantified using ImageJ software (Madison, WI, USA) by normalizing to the control ACTIN band.
CO-Immunoprecipitation analysis
Protein lysates were extracted from the samples using RIPA buffer supplemented with phenylmethylsulfonyl fluoride. The protein concentration was determined using a BCA assay. Subsequently, 5 μg of target protein antibody was added to 500 μl of cell lysate containing 500 μg total protein and gently mixed overnight at 4 °C. The following day, 5 μl of Protein A and 5 μl of Protein G dynabeads were added to the mixture and rotated at room temperature for 1 h to allow the antibody to bind to the target protein. The beads-antibody-protein complex was separated using a magnetic rack and washed three times with lysis buffer to remove non-specifically bound proteins. The complex was then gently resuspended in 20–40 μl of 2 × SDS loading buffer and heated at 95–100 °C for 10 min. Finally, Dynabeads and supernatant were separated for Western blot analysis.
Cell activity measurements
To assess cell viability, AT1 cells were seeded in a 96-well plate at a density of 8000 cells per 100 μl per well. We used a Cell Counting Kit/CCK-8 solution (Dojindo Laboratories, Japan) to measure cell viability. The cells were incubated at 37 °C for a specified period of time, and the absorbance was measured at 450 nm using a microplate reader from BioTek (USA). To determine the cytotoxicity of NVP231 (S6501, Selleck, USA) on cells, different concentrations ranging from 0 nM to 1000 nM were cocultured with cells, and the resultant cell activity was calculated.
siRNA and cDNA transfection
To perform siRNA or cDNA transfection, we utilized Lipofectamine 3000 (no. L3000-015, Thermo Fisher Scientific, USA) according to the following protocol: Cells were seeded to be 70%-90% confluent at the time of transfection. Then, 2.5 μg of cDNA or siRNA was mixed with 125 μl of Opti-MEM™ Medium, which could contain or not contain P3000™ Reagent (2 µl/µg DNA). In addition, 3.75 μl of Lipofectamine 3000 reagent was mixed with 125 μl of Opti-MEM™ Medium. The two mixtures were then mixed separately and further combined in a 1:1 ratio to prepare the DNA-lipid complex. The complex was incubated at room temperature for 10 min. In addition, 250 μl of DNA-lipid complex was added to each well of a 6-well plate, and the plate was incubated at 37 °C for 4 h for transfection. The transfection protocol was performed according to the manufacturer's recommended guidelines with modifications as described above.
Immunofluorescence
To prepare the cells for confocal immunofluorescence analysis, we first fixed them in 4% formaldehyde for 10 min to preserve cellular structures and proteins. Next, the cells were permeabilized with 0.2% Triton X-100 for 5 min to allow antibody penetration into the cells. To prevent non-specific binding of antibodies, the cells were then blocked in 3% BSA for 2 h. we used CoraLite®488-conjugated TOM20 monoclonal antibody (CL488-66777, Proteintech, USA) and CoraLite®594-conjugated LAMP1 monoclonal antibody (CL594-67300, Proteintech, USA), both at a dilution of 1:200. The incubation was carried out overnight at a temperature of 4 °C to allow for optimal antibody binding to the target proteins. To visualize and capture the antibody-labeled cells, we added an antifade mounting medium with DAPI to enhance the fluorescence signal and prevent photobleaching. The cells were then imaged using an OLYMPUS FV1000 inverted confocal microscope (Japan).
Mitochondrial respiration
To prepare AT1 cells for further experimentation, they were seeded in a 24-well cell culture plate and incubated at 37 °C with 5% CO2 overnight to allow for optimal cell attachment and growth. Oligomycin (1.0 μM), FCCP (1.0 μM), and a mix of rotenone and antimycin A (0.5 μM) were injected into the cell culture medium. The oxygen consumption rate (OCR) was calculated by Seahorse XFe/XF24 Analyzers (Agilent Technologies, USA) according to the manufacturer's instructions.
Intracellular reactive oxygen species synthesis assay
To measure ROS levels, we used a fluorescent probe called DCFH-DA. The probe was first diluted to a final concentration of 10 μM. Next, logarithmically growing cells were harvested and adjusted to a concentration of 10^6 cells/mL. The cells were incubated in the dark for 30 min. After incubation, To remove DCFH-DA and prepare the cells for ROS analysis, we washed the cells three times with serum-free medium. The cell lines were then harvested and subjected to flow cytometry using a Becton Dickinson instrument (USA) to detect ROS levels. Excitation was set at 488 nm, and emission was recorded at 525 nm.
JC-1 assay for mitochondrial membrane potential (Δψm)
To measure the Δψm, we used JC-1, a fluorescent dye that accumulates in mitochondria in a potential-dependent manner. To load the cells with the JC-1 dye, we prepared a loading solution with a final concentration of 2 μM. The cells were then incubated in this solution 30 min and protected from light. After washing three times with phosphate-buffered saline, the cells with DAPI antifade mounting medium to enhance the fluorescence signal and prevent photobleaching. The cells were then imaged using an OLYMPUS FV1000 inverted confocal microscope (Japan).
Transmission electron microscopy (TEM)
For ultrastructural analyses, freshly harvested AT1 cells were processed as described previously [35]. Briefly, cells were fixed in 1% glutaraldehyde in 0.1 M phosphate-buffered saline overnight. After fixation, the cells were washed with phosphate buffer and distilled water and then stained with 2% osmium tetroxide for 60 min. Next, the cells were dehydrated using a series of acetone concentrations, including 50%, 70%, 90%, and 100% (2 × 10 min each), and embedded (45345, Sigma Aldrich, USA). The sections with a thickness of 70 nm were examined through a transmission electron microscope (Hitachi, HT7800) for ultrastructural analysis.
Statistical analysis
To analyze the data and perform statistical tests, we used GraphPad Prism 8.0 Software (GraphPad Software Inc., USA). The measurement data were presented as the means ± standard deviation (SD) of at least three biological replicates. Before statistical analysis, we assessed the normality of the data distribution using the Shapiro-Wilk test. For normally distributed continuous variables, we used an independent-sample T-test to evaluate the statistical significance between the two experimental groups. To test for statistical significance between multiple experiments, we used a one-way analysis of variance (ANOVA) followed by Dunnett's test to determine which groups were significantly different from each other. For non-normally distributed continuous variables, we employed a Mann-Whitney U test instead. P value (P) < 0.05 was considered to indicate statistical significance. All statistical analyses were performed with a two-tailed test.
Results
CERK-knockout impaired lung structure and aggravated HAPE in hypobaric hypoxia
As an initial step to investigate the potential role of CERK in HAPE, we examined the expression levels of CERK mRNA and protein in the lungs of HAPE model mice. Our findings, based on mRNA and Western blot analyses, revealed a significant reduction in both CERK mRNA and protein expression levels in HAPE models compared to control groups (Fig. 1A-B).
Fig. 1.
CERK-knockout impaired lung structure and aggravated HAPE in hypobaric hypoxia. A: CERK mRNA expression in lung tissues of normoxic control and hypobaric hypoxia intervention mice. B: Upper panel: Western blot analysis of CERK protein expression in lung tissue of normoxic control and hypobaric hypoxia intervention mice. ACTB was used as a loading control. Lower panel: Quantification of Western blot analysis from the upper panel. C: Upper panel: Western blot analysis of CERK protein expression in lung tissue. ACTB was used as a loading control. Lower panel: Quantification of Western blot analysis from the upper panel. D: The ceramide contents of the lungs and serum were analyzed by LC–MS. E: The C1P contents of the lungs and serum were analyzed by LC–MS. F: Microscopic images of lung tissues stained with hematoxylin and eosin (H&E). Scale bar: 100 μm. G: Lung dry and wet weight ratio. These was normalized to that in the Ctrl group. The results were obtained from three independent experiments and are presented as the mean ± SD. n = 3/4. P < 0.05, **P < 0.01.
Based on our observation of decreased CERK expression in HAPE models, we formulated a hypothesis that CERK deficiency might contribute to the pathogenesis of HAPE. To test this hypothesis, we utilized CRISPR/Cas9 technology to generate CERK gene knockout mice, as depicted in Fig. S1 A. The genotype and protein expression of CERK-knockout mice was confirmed via PCR analysis (Fig. S1B) and Western blotting (Fig. 1C), respectively. To evaluate the impact of CERK-knockout on ceramide and C1P production in vivo, we measured their concentrations in the lungs and serum. Our findings indicated a significant increase in the levels of ceramide species, such as C16:0 Cer (C16Cer), C24:0 Cer (C24 Cer), and C24:1 Cer (C24:1 Cer) by approximately 1.5–3-fold in CERK-knockout mice (Fig. 1D). Furthermore, the levels of C16:0 C1P (C16C1P), C24:0 C1P (C24 C1P), and C24:1 C1P (C24:1 C1P) in the lungs and serum of CERK-knockout mice were reduced by approximately 70% compared to those in the wild-type controls (Fig. 1E).
Histological analysis using hematoxylin-eosin staining (HE staining) revealed that the pulmonary tissue of CERK-knockout mice exhibited destroyed alveolar structure and thickened alveolar walls compared to those of wild-type lungs (Fig. 1F). Notably, hypobaric hypoxia intervention resulted in more significant pathological alterations in CERK-knockout lungs, including pulmonary capillary congestion, massive inflammatory cytokine infiltration in the alveolar cavity, and interstitial edema, than in wild-type lungs. We further evaluated the severity of HAPE by measuring the dry and wet weight ratio (D/W ratio). In normoxia, there was no significant difference in the D/W ratio between wild-type and CERK-knockout mice. However, under hypobaric hypoxia intervention, HAPE was aggravated in CERK-knockout mice, as reflected by a more significant decrease in the D/W ratio compared to that in wild-type mice (Fig. 1G). These findings indicated that C1P deficiency caused by CERK-knockout induced pulmonary injury and aggravated HAPE in hypobaric hypoxia.
CERK-knockout caused lung injury by disrupting the circadian rhythm
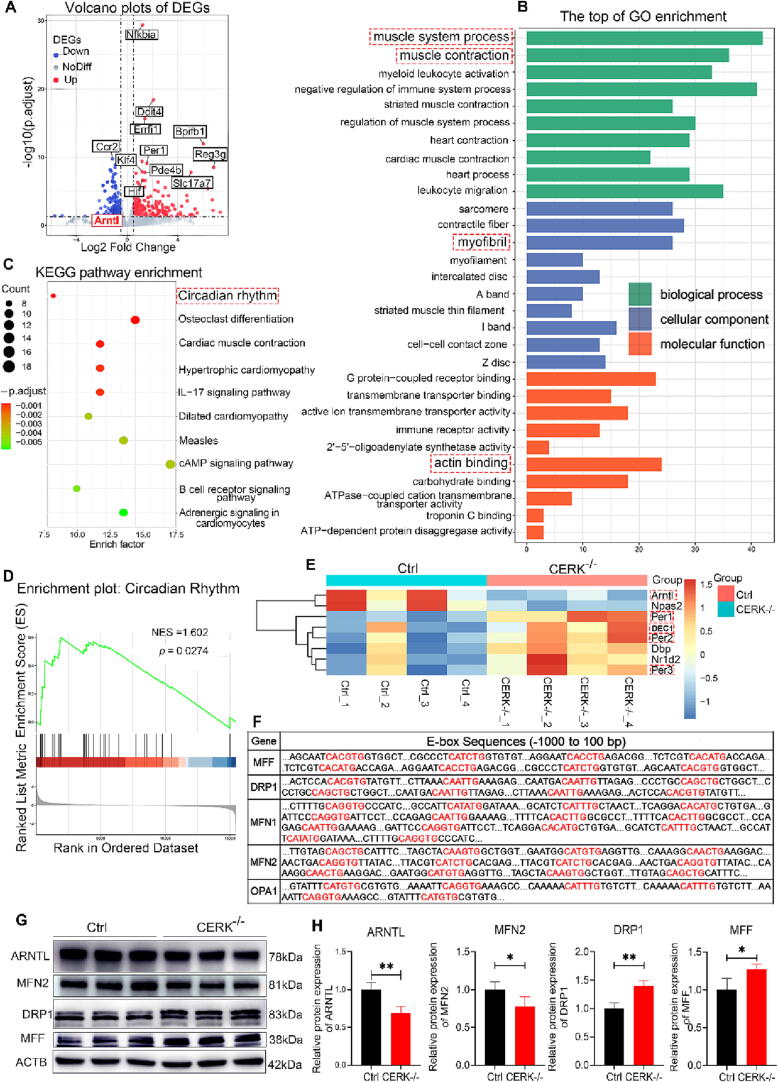
To investigate the mechanism underlying CERK-knockout-induced lung injury, we conducted high-throughput RNA sequencing on lung tissue from wild-type and CERK-knockout mice. We identified a total of 528 DEGs (Fig. 2A and Table S1). GO analysis of the DEGs revealed enrichment of terms related to muscle contraction, including muscle system process, myofibril, and actin-binding (Fig. 2B). KEGG pathway analysis showed an enrichment of pathways related to the circadian rhythm (Fig. 2C). Using gene set enrichment analysis (GSEA) with the KEGG database, we identified an enriched pathway related to the circadian rhythm (normalized enrichment score; NES = 1.602, p = 0.0274) (Fig. 2D). The heatmap of DEGs in the circadian rhythm pathway was shown in Fig. 2E. ARNTL is a central component of the mammalian circadian clock [25]. Our study revealed that CERK-knockout led to a decrease in the expression of ARNTL, while upregulating its negative regulator proteins, PER and DEC, which inhibited ARNTL expression in the circadian rhythm pathway (Fig. 2E). Disruptions in circadian rhythms have been linked to the pathogenesis of various diseases [23], [36], [37]. Additionally, GSEA showed an enrichment of the HIF-1 signaling pathway and chemical carcinogenesis-reactive oxygen species (Fig. S1), suggesting that CERK-knockout might affect mitochondrial activity. Our results also demonstrated that many of the DEGs involved in mitochondrial dynamics contained canonical or noncanonical E-box elements (CANNTG) for CLOCK: ARNTL (Fig. 2F) (24, 38). To further investigate this, we analyzed the abundance of ARNTL and mitochondrial dynamic proteins (MFN2, DRP1, and MFF) by Western blotting. As shown in Fig. 2G-H, the expression of ARNTL and MFN2 was decreased, while the expression of DRP1 and MFF was upregulated in CERK-knockout lungs compared to wild-type controls. In conclusion, our findings suggested that CERK-knockout-induced lung injury was due to disrupted expression of core proteins involved in circadian rhythms and impaired mitochondrial dynamics.
Fig. 2.
CERK knockout caused lung injury by disrupting the circadian rhythm. A: Volcano plots of DEGs between wild-type lungs and CERK-knockout lungs. B: Gene Ontology assignment of the top 10 biological processes (BPs), molecular functions (MFs), and cellular components (CCs) of DEGs between wild-type lungs and CERK-knockout lungs. C: Kyoto Encyclopedia of Genes and Genomes pathway enrichment of DEGs between wild-type lungs and CERK-knockout lungs. D: Enrichment gene set showed the circadian rhythm pathway. E: Heatmap showing the DEGs in circadian rhythm between wild-type lungs and CERK-knockout lungs. F: ARNTL target genes with E-box elements. Both canonical and noncanonical E-box sequences were identified and highlighted in red for easy visualization; G: Western blot analysis of ARNTL, MFN2, DRP1, and MFF protein expression compared to that of the ACTB loading control in wild-type and CERK-knockout lungs. H: Quantification of the Western blot results. The relative expression of each protein was normalized to that of ACTB. The results were obtained from three independent experiments and are presented as the mean ± SD. n = 3/4. *P < 0.05, **P < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
CERK inhibition induced mitochondrial oxidative stress and fragmentation in AT1 cells
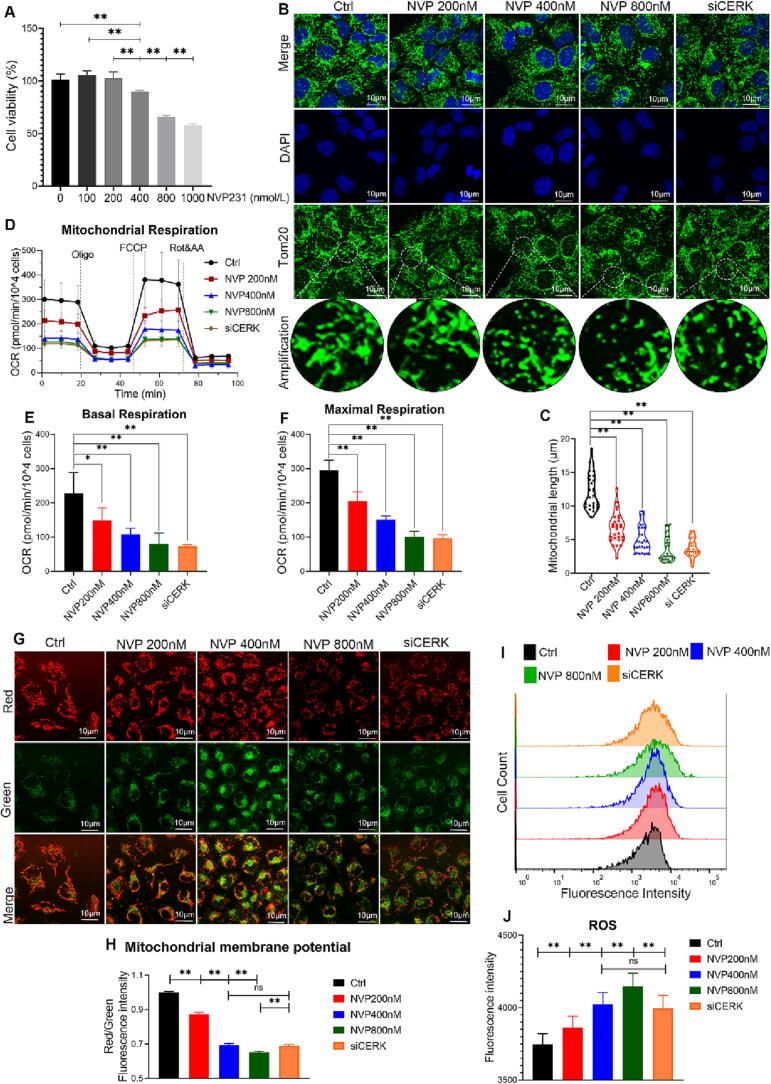
To investigate the effect of CERK inhibition on mitochondrial health and function, we examined the dysregulation of several genes essential for mitochondrial dynamics in CERK-knockout mice. We established two CERK inhibition models using CERK inhibitor (NVP-231) treatment or CERK downregulation by siRNA in vitro. Our results showed that the selective CERK inhibitor NVP-231 significantly decreased cell activity in a dose-dependent manner (Fig. 3A), indicating that CERK-derived C1P was necessary for AT1 cells to maintain activity. We also observed the mitochondrial morphology by labeling the Tom20 protein, which was specifically localized in the mitochondrial outer membrane. Both NVP-231 treatment and CERK siRNA impaired mitochondrial morphology, with the mitochondrial network showing extensive fragmentation and the average length of mitochondria being reduced (Fig. 3B-C). These abnormal structures might impair mitochondrial function. To measure the mitochondrial respiratory capacity, we used the Seahorse Extracellular Flux Analyzer and observed impaired mitochondrial respiration, with the oxygen consumption rate (OCR) significantly decreasing after CERK inhibition (Fig. 3D-F). Reactive oxygen species (ROS) are toxic byproducts of oxidative phosphorylation that accumulate in damaged mitochondria, and their accumulation is often accompanied by a loss of membrane potential (Δψm) [39]. Additionally, we found that CERK inhibition induced Δψm loss (Fig. 3G-H) and increased ROS production, suggesting increased oxidative stress (Fig. 3I-J). Taken together, these findings indicated that CERK inhibition induced mitochondrial damage by dysregulating mitochondrial dynamics, driving mitochondrial fragmentation, and increasing oxidative stress.
Fig. 3.
CERK inhibition induced mitochondrial oxidative stress and fragmentation in AT1 cells. A: AT1 cells were treated with NVP231 (0–1000 nmol/L) for 24 h and then used in a Cell Counting Kit-8 assay to detect cell activity. B: The cells were coincubated with the Tom20 antibody followed by image acquisition on a confocal microscope; scale bar: 10 μm. C: The mitochondrial length was calculated by ImageJ software. D: Mitochondrial respiratory function. At the end of the experiment, OCR values were normalized to the number of cells cultured per well. E: Basal respiration-associated OCR was calculated. F: The maximal respiration-associated OCR was calculated. G: Δψm was incubated with JC-1 followed by image acquisition on a confocal microscope; scale bar: 10 μm. H: Δψm was calculated by the ratio of the red/green fluorescence intensity. I: The intracellular ROS level was evaluated using a DCFH-DA probe by flow cytometry. J: The ROS analysis was calculated as the mean fluorescence intensity. The results were obtained from three independent experiments and are presented as the mean ± SD. *P < 0.05, **P < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
CERK inhibition aggravated the dysregulation of mitochondrial dynamics under hypoxic conditions
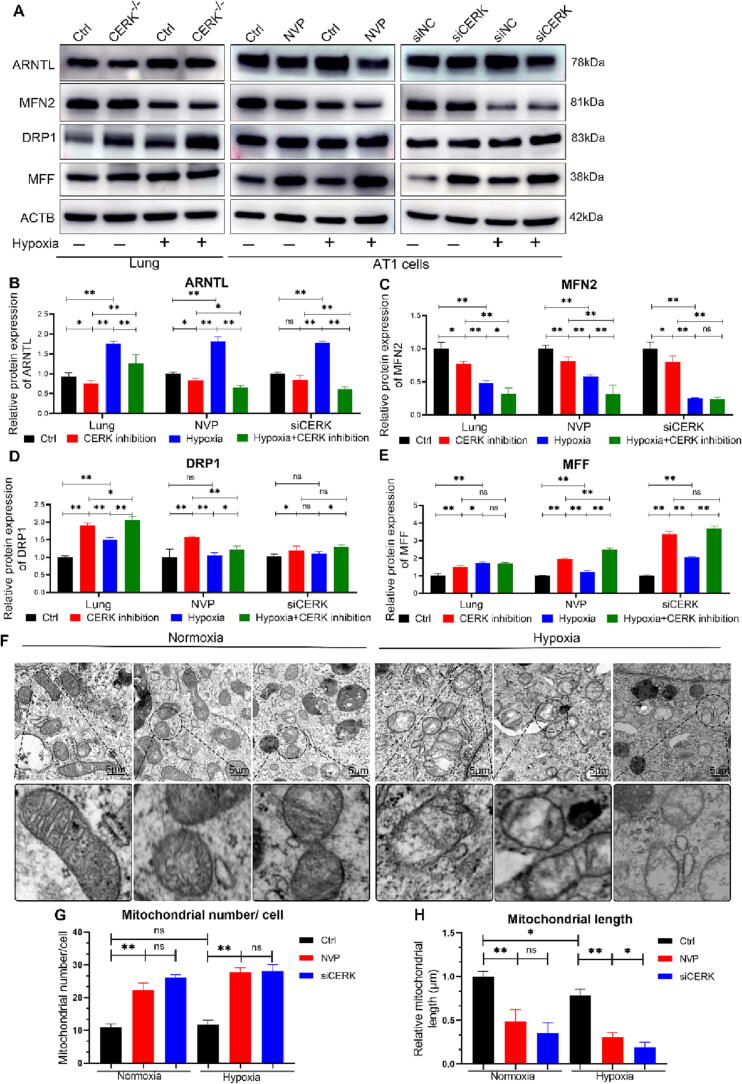
To evaluate the significance of CERK in pathological conditions, we investigated the impact of CERK inhibition under hypoxic conditions. We conducted high-throughput RNA sequencing of lung tissues and found that the dysregulation of DEGs in the circadian pathway caused by CERK inhibition worsened under hypoxia compared with normoxia (Fig. S2). Consistent with these results, we observed that CERK inhibition consistently reduced ARNTL expression both in normoxia and hypoxia (Fig. 4A-B). Furthermore, CERK inhibition induced upregulation of mitochondrial fission marker proteins (DRP1 and MFF) and downregulation of fusion protein (MFN2) expression. The observed changes in mitochondrial morphology indicated increased fission and decreased fusion events, suggesting that impaired mitochondrial dynamics caused by CERK inhibition were exacerbated under hypoxic conditions (Fig. 4A, C-E). Additionally, transmission electron microscopy (TEM) analysis revealed that CERK inhibition resulted in smaller mitochondria with a significantly increased mitochondrial number, with a disintegrated mitochondrial membrane and disappearance of inner cristae during hypoxia (Fig. 4F-H). These findings suggested that CERK played a crucial role in regulating mitochondrial homeostasis in AT1 cells during hypoxia and that CERK inhibition exacerbated the dysregulation of mitochondrial dynamics under hypoxic conditions.
Fig. 4.
CERK inhibition aggravated mitochondrial fragmentation under hypoxic conditions. A: Western blot analysis of ARNTL, MFN2, DRP1, and MFF protein expression compared to that of the ACTB loading control in control and CERK-inhibited lungs or AT1 cells. B-E: Quantification analysis of Western blots. The relative expression of each protein was normalized to that of ACTB. F: Transmission electron microscopy (TEM) images of mitochondria were obtained at a magnification of 12000X. Scale bars: 5 µm. G: The number of mitochondria per cell from TEM images was calculated. H: The relative length of mitochondria was normalized to Ctrl. The results were obtained from three independent experiments and are presented as the mean ± SD. *P < 0.05, **P < 0.01.
CERK inhibition aggravated mitophagy defects under hypoxic conditions
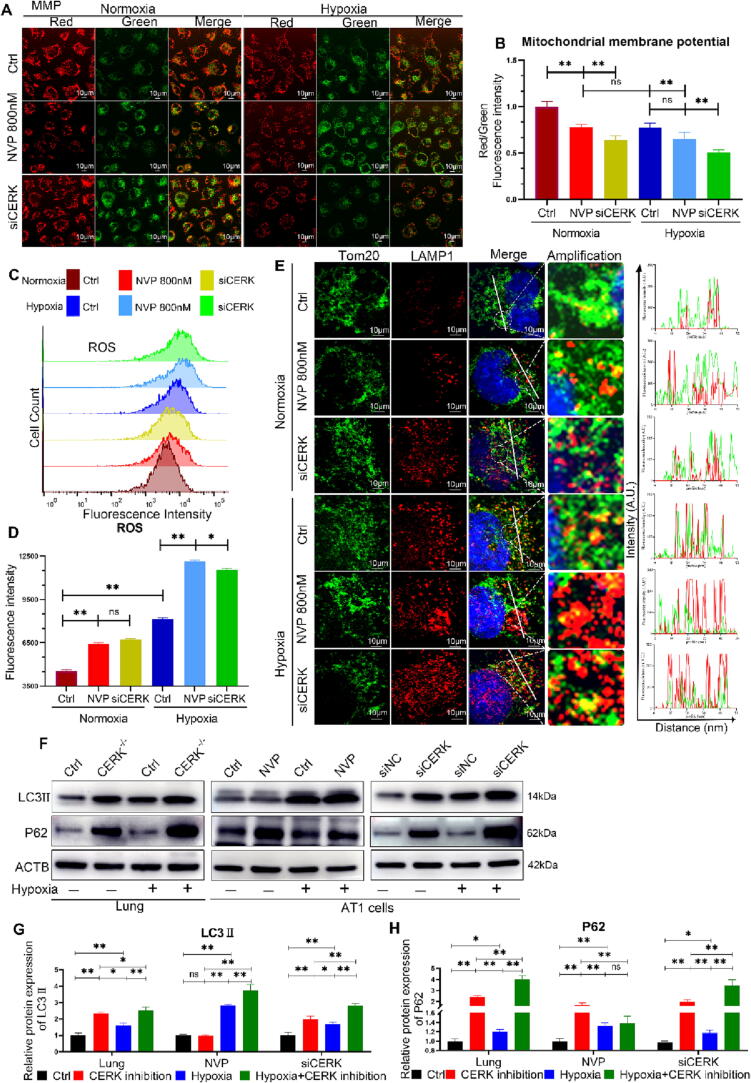
Disruption of the balance between mitochondrial fission and fusion can affect the function and health of mitochondria [40]. Our results showed that CERK inhibition induced Δψm depolarization and ROS accumulation, which were worsened under hypoxia (Fig. 5A-D), indicating severe mitochondrial damage. To maintain homeostasis, damaged mitochondria need to be cleared by a specific form of autophagy - mitophagy [41]. Co-immunofluorescence microscopy detection showed that in control cells, mitochondria were spindle-shaped with punctate lysosomes (red) occasionally overlapping with mitochondria (green). However, in CERK-inhibited cells, the mitochondrial network structure was absent, with numerous mitochondrial fragments appearing that barely overlapped with lysosomes, and this was worsened in hypoxia (Fig. 5E). LC3II is a mammalian autophagosome protein that can be used to evaluate autophagosome formation [42]. The expression level of LC3-II was significantly increased in CERK-inhibited cells (Fig. 5F-G), indicating the increased formation of autophagosomes or their defective fusion with lysosomes [43]. P62 is another common marker used to study the formation of autolysosomes [42]. However, accumulation of P62 indicated defective mitophagy flux (Fig. 5F, 5H), consistent with confocal microscopy results. These findings suggested that inhibition of CERK induced mitophagy defects and inhibited the clearance of damaged mitochondria.
Fig. 5.
CERK inhibition disrupts mitophagy under hypoxic conditions. A: Δψm was determined by incubation with JC-1 followed by image acquisition on a confocal microscope; scale bar: 10 μm. B: Δψm was calculated by the ratio of the red/green fluorescence intensity. C: The intracellular ROS level was evaluated using a DCFH-DA probe by flow cytometry. D: The ROS analysis was calculated as the mean fluorescence intensity. E: The cells were coincubated with Tom20 and LAMP1 antibodies followed by image acquisition on a confocal microscope; scale bar: 10 μm. F: Western blot analysis of LC3II and P62 protein expression in control and CERK-inhibited lungs or AT1 cells compared to the ACTB loading control. G-H: Quantification analysis of Western blots. The relative expression of each protein was normalized to that of ACTB. The results were obtained from three independent experiments and are presented as the mean ± SD. *P < 0.05, **P < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
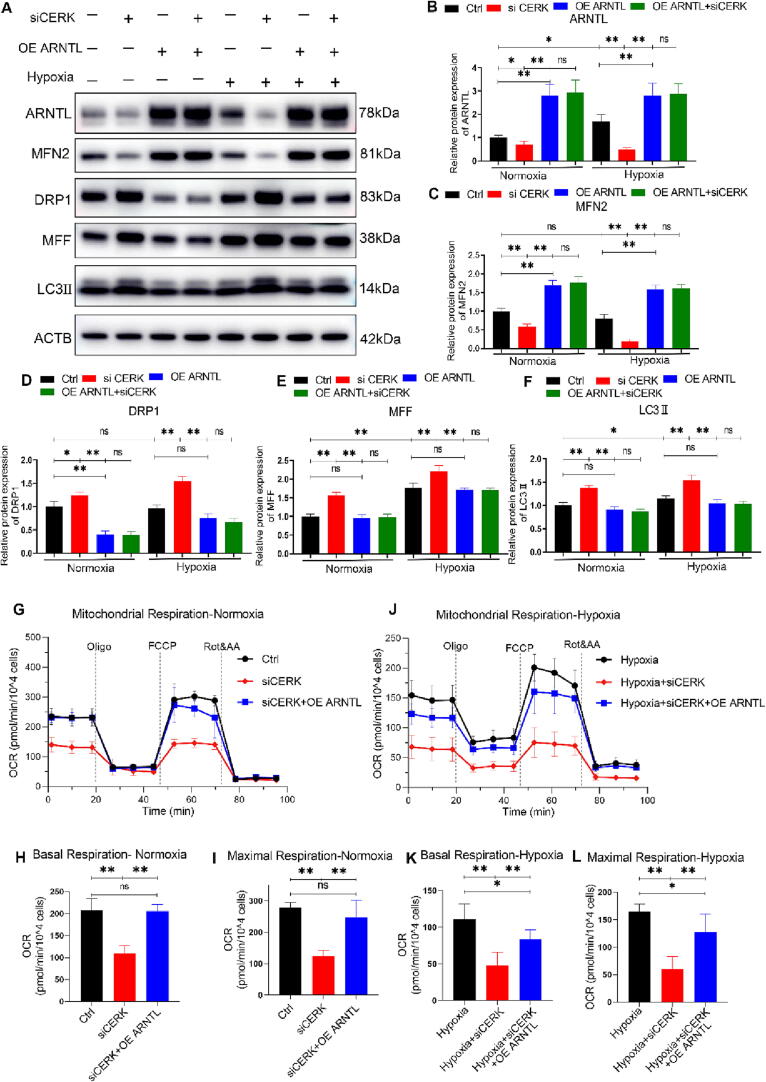
Overexpression of ARNTL alleviated the mitochondrial damage caused by CERK inhibition
To investigate whether ARNTL downregulation was necessary for CERK inhibition to induce mitochondrial damage, we conducted a series of experiments. Firstly, we measured the expression of mitochondrial dynamic proteins when ARNTL was overexpressed by transfecting specific plasmids. Western blot analyses confirmed increased ARNTL expression after plasmid transfection (Fig. 6A-B). Subsequently, we found that ARNTL overexpression significantly reversed the abnormal expression of mitochondrial dynamics proteins caused by CERK inhibition under normoxic and hypoxic conditions. Specifically, the reduced expression of MFN2 and upregulation of DRP1/MFF caused by CERK inhibition were reversed by ARNTL overexpression compared to the control group (Fig. 6A-E). Furthermore, we observed that ARNTL overexpression alleviated the mitophagy defects induced by CERK inhibition, as shown by the protein expression of LC3b-II (Fig. 6A, 6F). Additionally, ARNTL overexpression ameliorated the decreased basal and maximal OCR caused by CERK inhibition (Fig. 6G-L). These results suggested that ARNTL overexpression restored the impaired mitochondrial respiratory capacity caused by CERK inhibition under normoxic and hypoxic conditions. In summary, our findings indicated that ARNTL downregulation was downstream of CERK inhibition and dysregulated mitochondrial dynamics, leading to impaired mitochondrial respiratory function.
Fig. 6.
Overexpression of ARNTL blocked mitochondrial damage caused by CERK inhibition. A: Western blot analysis of ARNTL, MFN2, DRP1, MFF, and LC3II protein expression compared to that of the ACTB loading control in AT1 cells. B-F: Quantification analysis of Western blots. The relative expression of each protein was normalized to that of ACTB. G-I: Mitochondrial respiratory function was measured by using the Seahorse Extracellular Flux Analyzer in normoxia. Basal respiration-associated OCR (H) and maximal respiration-associated OCR (I) were calculated. J-L: Mitochondrial respiratory function was measured in hypoxia. Basal respiration-associated OCR (K) and maximal respiration-associated OCR (L) were calculated. The results were obtained from three independent experiments and are presented as the mean ± SD. *P < 0.05, **P < 0.01.
Exogenous C1P blocked ARNTL downregulation and mitochondrial damage caused by CERK inhibition in vitro
In our previous study, we demonstrated that CERK knockout led to C1P deficiency, while CERK inhibition exacerbated HAPE by downregulating ARNTL and impairing mitochondrial function. We hypothesized that C1P might play a role in preventing ARNTL reduction and maintaining mitochondrial dynamics, and could be a promising target for HAPE treatment. To test this hypothesis, we conducted in vitro experiments using AT1 cells preincubated with C16-C1P (10 μM), which is the major intracellular C1P species in mammalian cells [44], for 10 min[45]. After 24 h of normoxia/hypoxia treatment, we collected cells for protein extraction and found that exogenous C1P prevented the downregulation of ARNTL caused by CERK inhibition (Fig. 7A-B). Furthermore, C1P supplementation rectified the abnormal expression of proteins specific to mitochondrial dynamics and mitophagy, such as MFN2, DRP1, MFF, and LC3II, which were disrupted by CERK inhibition (Fig. 7A, C-F). We also observed that C1P alleviated the mitochondrial fragmentation and length reduction induced by CERK inhibition through fluorescence microscopy (Fig. 7G-H). These results indicated that exogenous C1P supplementation neutralized ARNTL reduction and prevented mitochondrial dysfunction caused by CERK inhibition.
Fig. 7.
Exogenous C1P blocked ARNTL downregulation and mitochondrial damage caused by CERK inhibition. A: Western blot analysis of ARNTL, MFN2, DRP1, MFF, and LC3II protein expression compared to that of the ACTB loading control in AT1 cells. B-F: Quantification analysis of Western blots. The relative expression of each protein was normalized to that of ACTB. G: The cells were coincubated with the Tom20 antibody and then subjected to image acquisition on a confocal microscope; scale bar: 10 μm. H: The mitochondrial length was calculated by ImageJ software. The results were obtained from three independent experiments and are presented as the mean ± SD. *P < 0.05, **P < 0.01.
To determine whether ARNTL was necessary for C1P-mediated mitochondrial protection, we inhibited ARNTL expression by shRNA transfection. We found that shARNTL blocked all protein expression changes induced by C1P (Fig. 7), indicating that ARNTL was a necessary downstream protein for C1P to maintain mitochondrial dynamics and function. Overall, our findings suggest that C1P may be a promising therapeutic target for HAPE treatment by preventing ARNTL reduction and maintaining mitochondrial function.
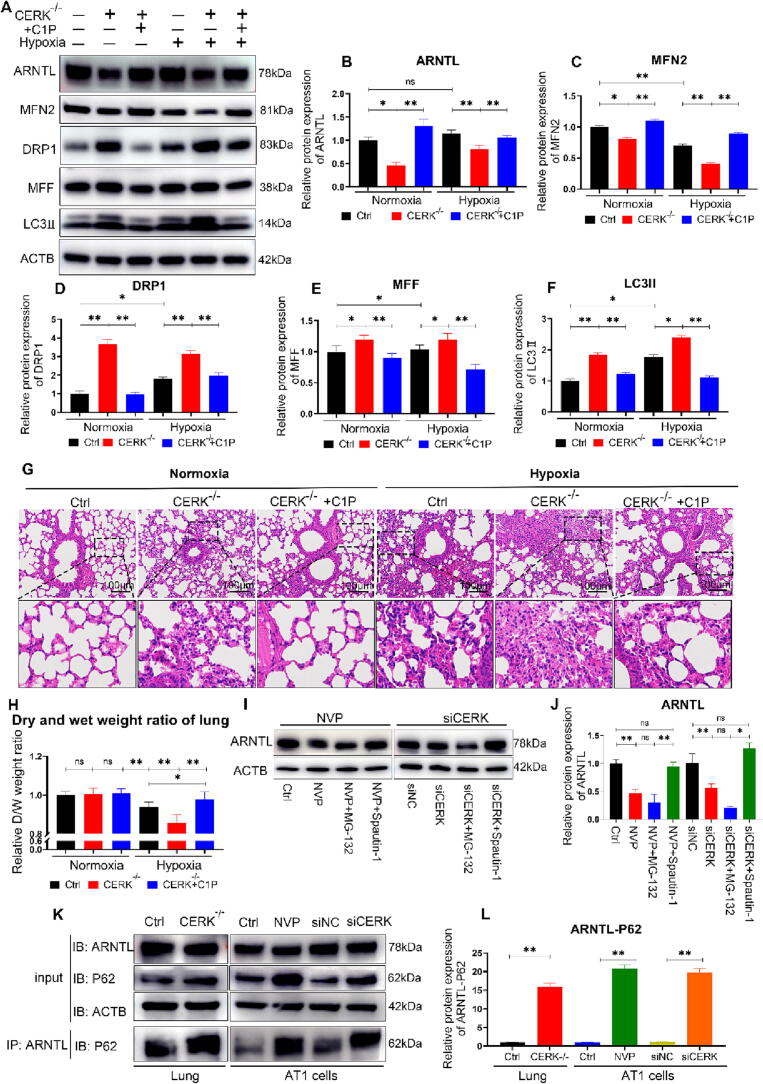
Exogenous C1P attenuated mitochondrial damage and alleviated HAPE in hypobaric hypoxia in vivo
To further investigate the potential therapeutic effects of exogenous C1P on HAPE in vivo, we conducted a subcutaneous injection of C16-C1P (200 µl, 1 µM [45], [46]), which is the major intracellular C1P species in mammalian cells, and found that C1P blocked the downregulation of ARNTL caused by CERK-knockout (Fig. 8A-B). Moreover, C1P reversed the MFN2 downregulation DRP1/MFF/LC3II upregulation caused by CERK-knockout, indicating that C1P supplementation neutralized the mitochondrial damage caused by CERK-knockout in mice (Fig. 8A-F). Additionally, C16-C1P relieved pulmonary capillary congestion, inflammatory cytokine infiltration, and interstitial edema caused by CERK knockout under hypobaric hypoxic conditions (Fig. 8G). The decrease in the D/W weight ratio in CERK-knockout lungs was reversed by C16-C1P injection under hypobaric hypoxia, indicating relief of pulmonary edema (Fig. 8H). Overall, our results suggested that exogenous C1P alleviated HAPE by stabilizing ARNTL expression and mitigating mitochondrial damage in vivo.
Fig. 8.
Exogenous C1P restored mitochondrial damage and alleviated HAPE in hypobaric hypoxia in vivo. A: Western blot analysis of ARNTL, MFN2, DRP1, MFF and LC3II protein expression compared to that of the ACTB loading control in lungs. B-F: Quantification analysis of Western blots. The relative expression of each protein was normalized to that of ACTB. G: Microscopic images of lung tissue sections stained with hematoxylin and eosin (H&E). Scale bar: 100 μm. H: The dry and wet weight ratio of lungs. The relative D/W ratio was normalized to the ratio of the Ctrl group. I: Western blot analysis of ARNTL protein expression compared to that of the ACTB loading control in AT1 cells. J: Quantification analysis of Western blots. K: CO-Immunoprecipitation analysis of ARNTL-P62. L: Quantification analysis of CO-Immunoprecipitation. The results were obtained from three independent experiments and are presented as the mean ± SD. n = 3/4. *P < 0.05, **P < 0.01.
Finally, we investigated the mechanism by which CERK inhibition leads to ARNTL downregulation. The ubiquitin–proteasome system and autophagy are two pathways that mediate protein degradation in mammals [47]. Our results demonstrated that the degradation of ARNTL caused by CERK inhibition was blocked by Spautin-1 (an autophagy inhibitor) but not by MG-132 (a proteasome inhibitor) (Fig. 8I-J indicating that CERK inhibition induced ARNTL degradation via autophagy rather than proteasomes. P62 is a multifunctional cargo receptor implicated in the autophagic degradation of proteins and organelles [48]. The Co-Immunoprecipitation analysis revealed that CERK inhibition increased the protein–protein interaction between ARNTL and P62, leading to ARNTL autophagic degradation (Fig. 8K-L).
Discussion
CERK-derived C1P alleviated HAPE and its deficiency aggravated the pathophysiological processes
HAPE is a growing concern, and there is an urgent need to develop new safe, effective, and cost-effective drug candidates for its treatment or prevention [10]. Previous studies have demonstrated that CERK-derived C1P is involved in regulating several pulmonary diseases and has protective properties [15], [16], [17]. Our study revealed that CERK-derived C1P could alleviate HAPE and that its deficiency caused by CERK inhibition worsened the disease severity under hypobaric hypoxic conditions. We discovered a novel molecular mechanism in which CERK and C1P alleviated HAPE by regulating the circadian rhythm. CERK inhibition led to ARNTL degradation, which disrupted mitochondrial dynamics, blocked mitophagy flux, and triggered oxidative stress. Exogenous C1P injection neutralized the damage caused by CERK inhibition and alleviated HAPE. Therefore, targeting C1P may present a potential therapeutic avenue for the treatment of HAPE.
C1P has been shown to have both proinflammatory and anti-inflammatory properties, depending on the cell type [18], [49]. Notably, C1P has a potent anti-inflammatory impact in animal models of emphysema and may be a potential treatment for COPD, asthma, or lung fibrosis [50], [51], [52]. In our study, we observed decreased expression of CERK mRNA and protein in HAPE model mice. CERK-knockout mice exhibited disrupted alveolar architecture and lower C1P concentrations in their lungs compared to wild-type mice. Furthermore, CERK-knockout mice also had aggravated HAPE after hypobaric hypoxia intervention. Baudiß et al. (46) reported that C1P reduced cigarette smoke (CS) induced acute and chronic lung inflammation and the development of emphysema in mice. Additionally, C1P protected mice from lung edema development and lethal Staphylococcus aureus sepsis by inhibiting A-SMase activity [53]. Overall, our results confirmed that C1P had a protective effect against HAPE, and its deficiency could exacerbate the severity of HAPE.
Inhibition of CERK decreased circadian ARNTL protein and induced mitochondrial damage
In this study, we discovered a novel mechanism in which CERK inhibition impaired the circadian rhythm by downregulating the expression of the circadian core component protein ARNTL. Circadian misalignment is present in many diseases, including cardiac injury [24], pancreatic fibrosis progression [54], and obesity [55]. Loss of ARNTL leads to abnormal physiological rhythms due to low-amplitude rhythms in the expression of multiple genes [37]. Our findings demonstrated that CERK inhibition increased ARNTL degradation through the protein autophagy pathway, which was specifically blocked by Spautin-1. ARNTL primarily regulates mitochondrial dynamics and oxidative metabolism [24], [38]. Hepatic ARNTL gene deletion results in dysfunctional mitochondria and may cause fatty liver and insulin resistance due to increased oxidative stress, decreased mitochondrial responsiveness to metabolic input, and increased ROS levels [21]. Our findings indicated that CERK inhibition dysregulated mitochondrial dynamics, including upregulating the mitochondrial fission proteins DRP1 and MFF and downregulating the fusion protein MFN2. Mitochondrial dynamics involve a delicate balance between fission and fusion. The balance of division and fusion maintains mitochondrial morphology and structure [56]. These mitochondrial dysfunctions contributed to extensive mitochondrial fragmentation, as identified through transmission electron microscopy and confocal microscopy. Furthermore, CERK inhibition promoted mitochondrial oxidative stress by decreasing the Δψm and increasing ROS production. These findings indicated that ARNTL downregulation leading to impaired mitochondrial quality control could be the mechanism by which CERK inhibition induces lung injury.
Inhibition of CERK exacerbated mitochondrial damage and impaired mitophagy, particularly under hypoxic conditions
Cells respond to hypoxic or ischemic stress by altering their metabolic demands [57]. Circadian rhythms maintain mitochondrial turnover and biogenesis to ensure healthy mitochondrial pools to meet the metabolic needs of cells. Disrupting circadian rhythms prevents the activation of adaptive mechanisms for maintaining mitochondrial health, leaving cells susceptible to ischemic/hypoxic stress damage [24]. Our research revealed that the reduction in ARNTL and dysregulation of mitochondrial dynamics were responsible for the exacerbation of HAPE by CERK inhibition under hypobaric hypoxia. CERK inhibition induced severe mitochondrial damage, particularly under hypoxic conditions, leading to Δψm depolarization and ROS accumulation. The excessive generation of ROS and the resulting heightened oxidative stress can ultimately compromise the proper function of the mitochondrial quality control machinery [58]. Damaged mitochondria are cleared by mitophagy, which plays an important role in cell survival [41]. Free amino acids and fatty acids produced by autophagic degradation can be used for protein synthesis and energy production to adapt to environmental stress [59]. The process of mitophagy is initiated by the formation of autophagosomes, which are double-membrane structures that engulf and sequester damaged or dysfunctional mitochondria. These autophagosomes then fuse with lysosomes to form autolysosomes, where the contents are degraded and recycled. Lysosomal hydrolases degrade the autophagosome-delivered contents and their inner membranes [60]. Our data showed increased LC3-II and P62 protein levels in CERK-inhibited cells, reflecting defective mitophagy flux. Impairment of mitophagy appears to be closely associated with the progressive accumulation of defective organelles and damaged mitochondria, which is the basis of the pathogeneses of many diseases [56]. CERK inhibition dramatically worsened mitophagy deficiency in hypoxia. Consistent with Nosal et al. [61], who reported that deleting ARNTL aggravates COPD and excessive fibrosis, our results implicated that CERK inhibition disturbed circadian rhythms, impaired mitochondrial dynamics, and induced defective mitophagy as well as oxidative stress, which might be the main mechanism of HAPE aggravation under hypobaric hypoxic conditions.
Exogenous C1P injection stabilized circadian rhythms and alleviated HAPE
The current study demonstrates that exogenous C1P injection could recover circadian rhythms by blocking ARNTL downregulation caused by CERK inhibition and alleviate pulmonary edema under hypobaric hypoxia. Disruption of circadian rhythms causes transient discomfort or exacerbates chronic diseases [62]. Maintaining healthy circadian rhythms can prevent or treat various diseases, including cancer, inflammatory diseases, and metabolic diseases [63], [64], [65]. Herein, we found that exogenous C1P injection stabilized ARNTL expression both in vivo and in vitro. The mitochondrial dynamics changes, which were downstream of ARNTL, were also reversed by exogenous C1P injection. Additionally, exogenous C1P administration reversed pulmonary capillary congestion, interstitial edema, and inflammatory cytokine infiltration and increased the D/W weight ratio. These results suggested that exogenous C1P injection stabilizes circadian rhythms by maintaining ARNTL expression, alleviating mitochondrial damage, and relieving HAPE under hypobaric hypoxia. Pharmacological modulation to stabilize circadian rhythms has proven promising in improving therapeutic benefits in fibrosis, according to Jiang et al. (54). Therefore, exogenous C1P could be a potential therapeutic strategy for preventing or treating HAPE by stabilizing circadian rhythms.
Despite our findings suggesting a protective role of CERK-derived C1P and exogenous C1P in alleviating HAPE under hypobaric hypoxic conditions, there are some limitations to our research. Firstly, multiple pathways for mitophagy have been identified, including PINK1-Parkin-mediate mitophagy, ubiquitin-mediated mitophagy, stress-induced mitophagy, and basal mitophagy. The mechanism by which CERK inhibition impairs mitophagy needs further clarification, as multiple signaling pathways have been identified. Secondly, further study is necessary to understand how disrupted circadian rhythms influence mitochondrial homeostasis through ARNTL degradation-induced mitochondrial dynamics disorder.
CERK-derived C1P maintained circadian rhythms and mitochondrial health as a promising therapeutic approach for HAPE
In conclusion, our results demonstrated that CERK-derived C1P played a protective role in HAPE under hypobaric hypoxic conditions. CERK inhibition increased the protein–protein interaction between circadian protein ARNTL and P62, thereby leading to ARNTL autophagic degradation. Furthermore, ARNTL dysregulated the transcription of many mitochondrial dynamics-regulating genes containing E-box elements, such as MFN2, DRP1, and MFF, resulting in the disorder of mitochondrial dynamics and defective mitophagy. Ultimately, it led to mitochondrial fragmentation and oxidative stress damage. However, exogenous C1P treatment stabilized circadian rhythms and reverses these effects. Therefore, exogenous C1P had the potential to be an effective therapeutic strategy for preventing or treating HAPE by stabilizing circadian rhythms and maintaining mitochondrial dynamics. Mitochondrial health is crucial for a myriad of physiological processes, including energy production, cellular signaling, and the prevention of various diseases [66]. By focusing on the predictive and personalized approach in the context of mitochondrial health, we could develop innovative strategies for disease prevention and treatment. Although there are limitations to our research, these findings shed light on the importance of CERK-C1P signaling and the circadian rhythm in mitigating the development and progression of HAPE.
Compliance with ethics requirements.
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the Chinese PLA General Hospital (Approval no.SQ2021218).
CRediT authorship contribution statement
Liuyang Tian: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Chenghui Zhao: Formal analysis, Software. Yan Yan: Data curation, Project administration. Qian Jia: Data curation. Saijia Cui: Investigation. Huining Chen: Investigation. Xiaolu Li: Investigation. Hongfeng Jiang: Data curation. Yongming Yao: Formal analysis, Funding acquisition, Writing – review & editing. Kunlun He: Conceptualization, Supervision, Funding acquisition. Xiaojing Zhao: Conceptualization, Visualization, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Natural Sciences Foundation of China (grant number 82001994 and 82241062). Support was also provided by the Science and Technology Innovation Special Zone of China (grant number 19-163-12-ZD-037-003-02).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.07.008.
Contributor Information
Yongming Yao, Email: c_ff@sina.com.
Kunlun He, Email: kunlunhe@plagh.org.
Xiaojing Zhao, Email: xjingzhao@126.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Khodaee M., Grothe H.L., Seyfert J.H., VanBaak K. Athletes at high altitude, sports. Health. 2016:126–132. doi: 10.1177/1941738116630948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarada S, Himadri P, Mishra C, Geetali P, Ram MS, Ilavazhagan G. Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema, Exp Biol Med (Maywood). (2008) 1088-1098.doi:10.3181/0712-RM-337. [DOI] [PubMed]
- 3.Bartsch P., Swenson E.R. Clinical practice: acute high-altitude illnesses. N Engl J Med. 2013:2294–2302. doi: 10.1056/NEJMcp1214870. [DOI] [PubMed] [Google Scholar]
- 4.McElroy M.C., Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J. 2004:664–673. doi: 10.1183/09031936.04.00096003. [DOI] [PubMed] [Google Scholar]
- 5.Ware L.B., Matthay M.A. Clinical practice. acute pulmonary edema. N Engl J Med. 2005:2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 6.Baloglu E, Nonnenmacher G, Seleninova A, Berg L, Velineni K, Ermis-Kaya E, et al. The role of hypoxia-induced modulation of alveolar epithelial Na(+)- transport in hypoxemia at high altitude, Pulm Circ. (2020) 50-58.doi:10.1177/2045894020936662. [DOI] [PMC free article] [PubMed]
- 7.Sharma Kandel R, Mishra R, Gautam J, Alaref A, Hassan A, Jahan N. Patchy vasoconstriction versus inflammation: A debate in the pathogenesis of high altitude pulmonary edema, Cureus. 2020 e10371.doi:10.7759/cureus.10371. [DOI] [PMC free article] [PubMed]
- 8.Woods P, Alcock J. High-altitude pulmonary edema, Evol Med Public Health. 2021 118-119.doi:10.1093/emph/eoaa052. [DOI] [PMC free article] [PubMed]
- 9.Yanamandra U., Sharma M., Katoch D., Yanamandra S., Bhattachar S.A., Gupta A., et al. High-altitude pulmonary oedema: newer treatment modalities for an age-old problem. Indian J Med Res. 2019:778–782. doi: 10.4103/ijmr.IJMR_1981_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luks A.M., Auerbach P.S., Freer L., Grissom C.K., Keyes L.E., McIntosh S.E., et al. update. Wilderness Environ Med. 2019:S3–S18. doi: 10.1016/j.wem.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Luks A.M., Hackett P.H. Medical conditions and high-altitude travel. N Engl J Med. 2022:364–373. doi: 10.1056/NEJMra2104829. [DOI] [PubMed] [Google Scholar]
- 12.Leon Y, Magarinos M, Varela-Nieto I. Ceramide kinase inhibition blocks IGF-1-bediated survival of otic neurosensory progenitors by impairing AKT phosphorylation, Front Cell Dev Biol. (2021) 678760.doi:10.3389/fcell.2021.678760. [DOI] [PMC free article] [PubMed]
- 13.Presa N, Gomez-Larrauri A, Dominguez-Herrera A, Trueba M, Gomez-Munoz A. Novel signaling aspects of ceramide 1-phosphate, Biochim Biophys Acta Mol Cell Biol Lipids. (2020) 158630.doi:10.1016/j.bbalip.2020.158630. [DOI] [PubMed]
- 14.Abdelbaset-Ismail A., Cymer M., Borkowska-Rzeszotek S., Brzezniakiewicz-Janus K., Rameshwar P., Kakar S.S., et al. Bioactive phospholipids enhance migration and adhesion of human leukemic cells by inhibiting heme oxygenase 1 (HO-1) and inducible nitric oxygenase synthase (iNOS) in a p38 MAPK-sependent manner. Stem Cell Rev Rep. 2019:139–154. doi: 10.1007/s12015-018-9853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease, Nature. (2014) 58-67.doi:10.1038/nature13475. [DOI] [PMC free article] [PubMed]
- 16.Zulueta A., Caretti A., Campisi G.M., Brizzolari A., Abad J.L., Paroni R., et al. Inhibitors of ceramide de novo biosynthesis rescue damages induced by cigarette smoke in airways epithelia. Naunyn Schmiedebergs Arch Pharmacol. 2017:753–759. doi: 10.1007/s00210-017-1375-2. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T., Koyama K., Takahashi N., Morito K., Ali H., Azuma M., et al. Lysophosphatidic acid, ceramide 1-phosphate and sphingosine 1-phosphate in peripheral blood of patients with idiopathic pulmonary fibrosis. J Med Invest. 2022:196–203. doi: 10.2152/jmi.69.196. [DOI] [PubMed] [Google Scholar]
- 18.Simanshu DK, Kamlekar RK, Wijesinghe DS, Zou X, Zhai X, Mishra SK, et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids, Nature. (2013) 463-467.doi:10.1038/nature12332. [DOI] [PMC free article] [PubMed]
- 19.Payne AW, Pant DK, Pan TC, Chodosh LA. Ceramide kinase promotes tumor cell survival and mammary tumor recurrence, Cancer Res. (2014) 6352-6363.doi:10.1158/0008-5472.CAN-14-1292. [DOI] [PMC free article] [PubMed]
- 20.Dunlap JC. Molecular bases for circadian clocks, Cell. (1999) 271-290.doi:10.1016/s0092-8674(00)80566-8. [DOI] [PubMed]
- 21.Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, et al. Hepatic bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness, Cell Metab. (2015) 709-720.doi:10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed]
- 22.Cox S.L., O’Siorain J.R., He Y., Lordan R., Naik A., Tang S.Y., et al. Circadian disruption in lung fibroblasts enhances NF-kappaB activity to exacerbate neutrophil recruitment. FASEB J. 2023 doi: 10.1096/fj.202201456R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida Y, Matsunaga N, Nakao T, Hamamura K, Kondo H, Ide T, et al. Alteration of circadian machinery in monocytes underlies chronic kidney disease-associated cardiac inflammation and fibrosis, Nat Commun. 2021 2783.doi:10.1038/s41467-021-23050-x. [DOI] [PMC free article] [PubMed]
- 24.Rabinovich-Nikitin I, Rasouli M, Reitz CJ, Posen I, Margulets V, Dhingra R, et al. Mitochondrial autophagy and cell survival is regulated by the circadian Clock gene in cardiac myocytes during ischemic stress, Autophagy. 2021 3794-3812. doi:10.1080/15548627.2021.1938913. [DOI] [PMC free article] [PubMed]
- 25.Yang M., Chen P., Liu J., Zhu S., Kroemer G., Klionsky D.J., et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019:eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowrey P.L., Takahashi J.S. Genetics of circadian rhythms in mammalian model organisms. Adv Genet. 2011:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundar I.K., Yao H., Sellix M.T., Rahman I. Circadian molecular clock in lung pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2015:L1056–L1075. doi: 10.1152/ajplung.00152.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krakowiak K, Durrington HJ. The role of the body clock in asthma and COPD: Implication for treatment, Pulm Ther. (2018) 29-43.doi:10.1007/s41030-018-0058-6. [DOI] [PMC free article] [PubMed]
- 29.Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, et al. Circadian control of lung inflammation in influenza infection, Nat Commun. 2019 4107.doi:10.1038/s41467-019-11400-9. [DOI] [PMC free article] [PubMed]
- 30.Ni Q, Shao Y, Wang YZ, Jing YH, Zhang YC. Impact of high altitude on the hepatic fatty acid oxidation and synthesis in rats, Biochem Biophys Res Commun. 2014 574-579.doi:10.1016/j.bbrc.2014.03.001. [DOI] [PubMed]
- 31.Tian L., Jia Z., Xu Z., Shi J., Zhao X., He K. Transcriptional landscape in rat intestines under hypobaric hypoxia. PeerJ 2021;e11823.doi:10.7717/peerj.11823. [DOI] [PMC free article] [PubMed]
- 32.Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex, Proc Natl Acad Sci U S A. (2016) E1334-1342. doi:10.1073/pnas.1504555113. [DOI] [PMC free article] [PubMed]
- 33.Park K., Elias P.M., Shin K.O., Lee Y.M., Hupe M., Borkowski A.W., et al. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol Cell Biol. 2013:752–762. doi: 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijesinghe D.S., Allegood J.C., Gentile L.B., Fox T.E., Kester M., Chalfant C.E. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res. 2010:641–651. doi: 10.1194/jlr.D000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labuschagne C.F., Cheung E.C., Blagih J., Domart M.C., Vousden K.H. Cell clustering promotes a metabolic switch that supports metastatic colonization. Cell Metab. 2019:720–734 e725. doi: 10.1016/j.cmet.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu M., Zhang G., Qu H., Vu A., Wu R., Tsukamoto H., et al. Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc Natl Acad Sci U S A. 2023 doi: 10.1073/pnas.2214829120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu P., Hamlish N.X., Thakkar A.V., Steffeck A.W.T., Rendleman E.J., Khan N.H., et al. BMAL1 drives muscle repair through control of hypoxic NAD(+) regeneration in satellite cells. Genes Dev. 2022:149–166. doi: 10.1101/gad.349066.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bass J., Takahashi J.S. Circadian integration of metabolism and energetics. Science. 2010:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleele T., Rey T., Winter J., Zaganelli S., Mahecic D., Perreten Lambert H., et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021:435–439. doi: 10.1038/s41586-021-03510-6. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues T., Ferraz L.S. Therapeutic potential of targeting mitochondrial dynamics in cancer. Biochem Pharmacol. 2020 doi: 10.1016/j.bcp.2020.114282. [DOI] [PubMed] [Google Scholar]
- 41.Li Y.F., Ouyang S.H., Tu L.F., Wang X., Yuan W.L., Wang G.E., et al. Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics. 2018:5713–5730. doi: 10.7150/thno.28778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjorkoy G., Lamark T., Pankiv S., Overvatn A., Brech A., Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 43.Fan P., Yu X.Y., Xie X.H., Chen C.H., Zhang P., Yang C., et al. Mitophagy is a protective response against oxidative damage in bone marrow mesenchymal stem cells. Life Sci. 2019:36–45. doi: 10.1016/j.lfs.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Lamour N.F., Stahelin R.V., Wijesinghe D.S., Maceyka M., Wang E., Allegood J.C., et al. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007:1293–1304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Mena H.A., Zubiry P.R., Dizier B., Mignon V., Parborell F., Schattner M., et al. Ceramide 1-phosphate protects endothelial colony-forming cells from apoptosis and increases vasculogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2019:e219–e232. doi: 10.1161/ATVBAHA.119.312766. [DOI] [PubMed] [Google Scholar]
- 46.Baudiss K., Ayata C.K., Lazar Z., Cicko S., Beckert J., Meyer A., et al. Ceramide-1-phosphate inhibits cigarette smoke-induced airway inflammation. Eur Respir J. 2015:1669–1680. doi: 10.1183/09031936.00080014. [DOI] [PubMed] [Google Scholar]
- 47.Shin W.H., Park J.H., Chung K.C. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson's disease. BMB Rep. 2020:56–63. doi: 10.5483/BMBRep.2020.53.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gatica D., Lahiri V., Klionsky D.J. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez-Munoz A, Presa N, Gomez-Larrauri A, Rivera IG, Trueba M, Ordonez M. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate, Prog Lipid Res. (2016) 51-62.doi:10.1016/j.plipres.2015.09.002. [DOI] [PubMed]
- 50.Al-Rashed F., Ahmad Z., Snider A.J., Thomas R., Kochumon S., Melhem M., et al. Ceramide kinase regulates TNF-α-induced immune responses in human monocytic cells. Sci Rep. 2021 doi: 10.1038/s41598-021-87795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim J., Kim Y., Heo J., Kim K.H., Lee S., Lee S.W., et al. Priming with ceramide-1 phosphate promotes the therapeutic effect of mesenchymal stem/stromal cells on pulmonary artery hypertension. Biochem Biophys Res Commun. 2016:35–41. doi: 10.1016/j.bbrc.2016.03.046. [DOI] [PubMed] [Google Scholar]
- 52.Pastukhov O., Schwalm S., Romer I., Zangemeister-Wittke U., Pfeilschifter J., Huwiler A. Ceramide kinase contributes to proliferation but not to prostaglandin E2 formation in renal mesangial cells and fibroblasts. Cell Physiol Biochem. 2014:119–133. doi: 10.1159/000362989. [DOI] [PubMed] [Google Scholar]
- 53.Simonis A., Hebling S., Gulbins E., Schneider-Schaulies S., Schubert-Unkmeir A. Differential activation of acid sphingomyelinase and ceramide release determines invasiveness of Neisseria meningitidis into brain endothelial cells. PLoS Pathog. 2014 doi: 10.1371/journal.ppat.1004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang W., Jin L., Ju D., Lu Z., Wang C., Guo X., et al. The pancreatic clock is a key determinant of pancreatic fibrosis progression and exocrine dysfunction. Sci Transl Med. 2022:eabn3586. doi: 10.1126/scitranslmed.abn3586. [DOI] [PubMed] [Google Scholar]
- 55.Hepler C., Weidemann B.J., Waldeck N.J., Marcheva B., Cedernaes J., Thorne A.K., et al. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science. 2022:276–284. doi: 10.1126/science.abl8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pradeepkiran J.A., Reddy P.H. Defective mitophagy in alzheimer's disease. Ageing Res Rev. 2020 doi: 10.1016/j.arr.2020.101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pascual F., Coleman R.A. Fuel availability and fate in cardiac metabolism: a tale of two substrates. Biochim Biophys Acta. 2016:1425–1433. doi: 10.1016/j.bbalip.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koklesova L., Samec M., Liskova A., Zhai K., Busselberg D., Giordano F.A., et al. Mitochondrial impairments in aetiopathology of multifactorial diseases: common origin but individual outcomes in context of 3P medicine. EPMA J. 2021:27–40. doi: 10.1007/s13167-021-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu X., Long Y.C. Autophagy modulates amino acid signaling network in myotubes: differential effects on mTORC1 pathway and the integrated stress response. FASEB J. 2015:394–407. doi: 10.1096/fj.14-252841. [DOI] [PubMed] [Google Scholar]
- 60.Xie Z., Klionsky D.J. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 61.Nosal C., Ehlers A., Haspel J.A. Why lungs keep time: circadian rhythms and lung immunity. Annu Rev Physiol. 2020:391–412. doi: 10.1146/annurev-physiol-021119-034602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sulli G., Manoogian E.N.C., Taub P.R., Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 2018:812–827. doi: 10.1016/j.tips.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones K.A., Hatori M., Mure L.S., Bramley J.R., Artymyshyn R., Hong S.P., et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol. 2013:630–635. doi: 10.1038/nchembio.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He B., Nohara K., Park N., Park Y.S., Guillory B., Zhao Z., et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016:610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patouret R., Doebelin C., Garcia-Ordonez R.D., Chang M.R., Ruiz C., Cameron M.D., et al. Identification of an aminothiazole series of RORbeta modulators. Bioorg Med Chem Lett. 2018:1178–1181. doi: 10.1016/j.bmcl.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koklesova L., Mazurakova A., Samec M., Kudela E., Biringer K., Kubatka P., et al. Mitochondrial health quality control: measurements and interpretation in the framework of predictive, preventive, and personalized medicine. EPMA J. 2022:177–193. doi: 10.1007/s13167-022-00281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.