Graphical abstract
Keywords: Mycotoxins, Fumonisins, Control strategies, Food safety, Decontamination, Biotechnology
Highlights
-
•
Fumonisins (FUMs) contamination occurs globally in food and causes significant economic losses.
-
•
Strategies from the points of pre- and post-harvest are developed to manage FUMs contamination.
-
•
Mechanisms of some major strategies based on prevention and decontamination are summarized.
-
•
Application of biotechnological approaches and emerging technologies is discussed.
-
•
Recommendation and prospects are provided for management of fumonisins contamination.
Abstract
Background
Fumonisins (FUMs) are among the most common mycotoxins in plant-derived food products. FUMs contamination has considerably impacted human and animal health, while causing significant economic losses. Hence, management of FUMs contamination in food production and supply chains is needed. The toxicities of FUMs have been widely investigated. FUMs management has been reported and several available strategies have been developed successfully to mitigate FUMs contamination present in foods. However, currently available management of FUMs contamination from different phases of food chains and the mechanisms of some major strategies are not comprehensively summarized.
Aim of review
This review comprehensively characterize the occurrence, impacts, and management of FUMs contamination across food production and supply chains. Pre- and post-harvest strategies to prevent FUMs contamination also are reviewed, with an emphasis on the potential applications and the mechanisms of major mitigation strategies. The presence of modified FUMs products and their potential toxic effects are also considered. Importantly, the potential application of biotechnological approaches and emerging technologies are enunciated.
Key scientific concepts of review
Currently available pre- and post-harvest management of FUMs contamination primarily involves prevention and decontamination. Prevention strategies are mainly based on limiting fungal growth and FUMs biosynthesis. Decontamination strategies are implemented through alkalization, hydrolysis, thermal or chemical transformation, and enzymatic or chemical degradation of FUMs. Concerns have been raised about toxicities of modified FUMs derivatives, which presents challenges for reducing FUMs contamination in foods with conventional methodologies. Integrated prevention and decontamination protocols are recommended to control FUMs contamination across entire value chains in developed countries. In developing countries, several other approaches, including cultivating, introducing Bt maize, simple sorting/cleaning, and dehulling, are suggested. Future studies should focus on biotechnological approaches, emerging technologies, and metagenomic/genomic identification of new degradation enzymes that could allow better opportunities to manage FUMs contamination in the entire food system.
Introduction
Mycotoxins are secondary fungal metabolites that are toxic to plants, animals and humans. The widespread contamination of mycotoxins in food products is becoming an important public health issue and carries substantial social and economic impacts [1]. Consequently, extensive efforts have been made toward practical science-based mycotoxin management strategies [2]. FUMs are a class of the most common mycotoxins and are ubiquitous contaminants of cereal grains globally, especially in maize [3]. FUMs are produced primarily by the Fusarium species, F. verticillioides and F. proliferatum [4]. In addition, some Aspergillus (e.g., A. niger) produce fumonisin B2 (FB2) and FB4 while Tolypocladium species (e.g., T. cylindrosporum) produce fumonisin B1 (FB1) and FB2 [5], [6]. Nevertheless, these non-Fusarium fungi do not produce high enough levels of FUMs to cause health concerns. Moreover, some Alternaria strains produce toxins that show structural and toxicological similarity to FUMs [7]. The identification of FUMs produced by other fungi would improve our understanding of FUMs contamination and biosynthesis.
Approximately 30 fumonisin derivatives have been reported that can be divided into four major groups (A, B, C, and P) based on their chemical structures, with B type fumonisins (FBs) being the most common [8], [9]. Among these, FB1 is the most prevalent and most toxic type accounting for 70–80% of all FB contaminations [10]. FB2 and FB3 are the second and third most common FBs, respectively [3], and are identical structures other than the location of hydroxyl side groups. FB4 contains a single hydroxyl side group, and FB5 has two hydroxyl groups. Overall, most attention is paid to FB1 due to its relatively high frequency of occurrence and its high toxicity.
Modified FBs may be produced by either fungal biotransformation or by thermal or chemical treatment of foods [11]. The European Food Safety Authority (EFSA) defines biotransformation to include hydrolysis of the parent toxin (phase I metabolites: hydrolysed FBs) and conjugation with endogenous molecules (phase II metabolites: O-fatty acyl-FB1, N-fatty acyl-FB1 and N-fatty acyl-hydrolysed FB1 and FB2) [11]. Among process-derived modified FBs, hydrolysed or partially hydrolysed FUMs are produced under thermal conditions after reaction with reducing sugars, including the production of N-(carboxymethyl)-fumonisin B1 (NCM-FB1) or N-(1-deoxy-D-fructos-1-yl)-fumonisin B1 (NDF-FB1). Other modified forms of FUMs also exist, including, for example, N-acetyl-FB1 and N-palmitoyl hydrolysed-FB1, as described by the EFSA discussion of Fumonisin HBGVs [12]. Modified FUMs are typically undetected using conventional analytical and commercial screening methods for the parent mycotoxins [13]. Indeed, analytical standards for modified FBs are not commercially available, with the exception of those for HFB1 [12]. Importantly, some modified FBs, e.g. N-fatty acyl-FBs, are more cytotoxic in vitro than the original FBs [12]. Therefore, modified FBs can substantially alter assessments of overall FUMs occurrence and toxicity. Thus, more attention should be paid to managing modified FBs when considering the effectiveness of FUMs contamination mitigation efforts in the food system, as discussed below.
Occurrence and impacts
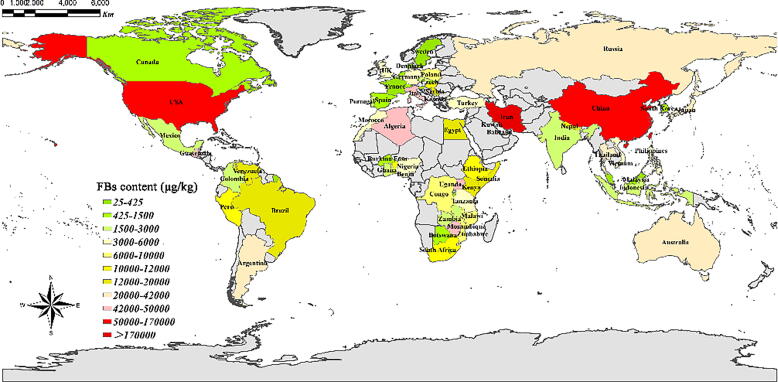
FUMs contamination frequently occurs globally in different plant-derived foods, but primarily in maize. The World Health Organization (WHO) has previously reported that about 50% of global maize and maize-based products were contaminated by FB1 to various extents [14]. In particular, FUMs contamination has become a critical issue in Sub-Saharan Africa, South America, Latin America, and South/Southeast Asia, where an especially high demand for maize and maize-based food products has grown in recent years [14]. In South Africa, approximately 90% of maize contained FB1 at levels up to 118 mg/kg. Samples of home-brewed maize beer from the former Transkei region of South Africa contained between 38 and 1,066 ng/mL of FB1 [15]. A recent review of mycotoxin contamination in China found FUMs contamination of maize country-wide [16]. Further, two surveys demonstrated that total fumonisin levels of some maize samples from Guizhou (FB1 + FB2), Sichuan (FB1 + FB2), Gansu (FB1 + FB2), and Shandong (FB1 + FB2 + FB3) provinces were higher than the maximum limit (2,000 µg/kg) set by the United States Food and Drug Administration (FDA) [17]. A study conducted during 2007–2010 in Brazil indicated that FB1 and FB2 were detected in 82% and 51% of examined corn-based food products, respectively [18]. In a study in the United States, FB1 was identified in all 10 corn grit samples and 15 of 16 corn meal samples that were collected from grocers’ shelves, with mean levels of 0.6 and 1 ppm, respectively, while FB1 was detected in canned and frozen sweet corn in 35 of 97 samples at maximum levels of 0.2 and 0.4 ppm, respectively [19]. A survey of 29 nationally distributed brands of beer in the United States also found 86% were positive for FB1 or FB2 in a range from 0.3 to 12.7 ng/mL for FB1 [19].
The overall global FUMs contamination in corn or corn-based products is shown in Fig. 1. Importantly, developing countries face greater FUMs contamination risk than developed countries (Fig. 1). In developing countries, regulatory measures are either lacking or poorly enforced, which is particularly evident in rural subsistence farming communities with informal market [20]. In addition, when high-quality foods are exported and poor quality foods are left in developing countries, the risk of FUMs exposure further increases. Developed countries have high standards for major food suppliers and the regulatory controls on FUMs contamination that greatly limit the importation and marketing of heavily contaminated products [20]. Hence, management of FUMs in these two major crops, particularly in developing countries, is critically important.
Fig. 1.
Overview of the worldwide occurrence of FUMs in maize or maize-based foods. FBs: FUMs incorporate FB1 or FB2 only, or the sum of different FUMs. The contents of FUMs represent the maximum levels reported by previous research and Rapid Alert System for Food and Feed (RASFF) public database (https://webgate.ec.europa.eu/rasff-window/screen/search). The contents of FUMs and related references used for this Figure were listed in Table S1.
Other FUMs contaminated products include sorghum, white bean, barley, soybean, rice, black tea, and even medicinal herbs [14]. Besides, postharvest fruit may be particularly susceptible to FUMs contamination due to the prevalence of FUMs-producing fungal species in some of these products [21], [22]. Additionally, both FB1 and FB2 have been identified in dried figs [23], [24], suggesting the occurrence of FUMs in some processed fruit products, though contamination is limited to a single type. Nevertheless, the natural occurrence of FUMs in some postharvest fruits broadens the scope of concern for mycotoxin-related food safety issues beyond staple crop.
FUMs contamination can impact human and animal health. The toxic effects of FUMs are attributable to the specific inhibition of ceramide synthase, which disrupts de novo sphingolipid biosynthesis and turnover, thereby interfering with sphingolipid-mediated signal transduction [25], [26]. The impacts of FUMs on animal health (e.g. intestinal toxicity) have been confirmed in laboratory [27]. Among farm animals, FB1 may cause leukoencephalomalacia in horses and porcine pulmonary edema in pigs [28]. In addition, FB1 is hepatotoxic in mice, rats, equids, rabbits, pigs, and primates [29]. Moreover, FUMs are also nephrotoxic in pigs, rats, sheep, mice, and rabbits [29]. Consequently, farmers may experience additional costs for veterinary drugs to treat indirect health problems caused by FUMs contamination.
Based on the above observations, the International Agency for Research on Cancer (IARC) has classified FB1 as a group 2B possible human carcinogen [30]. Further, the WHO has indicated that a primary concern regarding FUMs is their potential to contribute to human cancer (WHO/NHM/FOS/RAM/18.2). An association between FUMs and a risk of carcinogenicity as well as esophageal squamous cell carcinoma (ESCC) has been reported in humans by different studies [31], [32]. Increasing evidence associates fumonisin exposure with child growth impairment and neural tube defects (NTDs) [8], [25]. Consequently, the EFSA has listed FUMs as mycotoxins of highest significance for both human health and agroeconomics based on risk assessments [33]. The EFSA Panel on Contaminants in the Food Chain suggested a tolerable daily intake of 1.0 μg FB1/kg body weight per day [12]. Clearly, increasing levels of FUMs contamination results in considerable global health and food losses. Munkvold et al. [34] reported that losses caused only by FUMs were $11 million/year or even higher in Iowa, USA. The above observations and the broad distribution of FUMs in food products alongside their evident effects on agricultural products and public health have led to the listing of FUMs as one of the most important mycotoxins in food supply chains. Consequently, enhanced management of FUMs contamination is vital to ensure global food safety.
Management of FUMs contamination
The wide occurrence of FUMs and the concomitant lack of adequate prevention technologies have led to food safety problems. Multiple international organizations have significant concerns about FUMs contamination in plant-based food products and have accordingly set maximum advisory levels. For example, the European Commission regulation (EC) specifies a maximum level for total FUMs (FB1 and FB2) in different human foods at between 200 µg/kg (food for infants and young children) to 4,000 µg/kg (raw-unprocessed maize) (Commission Regulation EC No. 1126/2007). The Codex Alimentarius Commission specifies the maximum level for FUMs in raw maize in international trade as 4,000 µg/kg (WHO/NHM/FOS/RAM/18.2). Likewise, the USA FDA specifies a maximum FUMs level of 2,000 µg/kg for maize and maize-based products intended for human consumption [35]. In addition, the FDA specifies a range of FUMs concentrations from 1 to 50 mg kg−1 is allowable for animal feed, depending on the animal species [35]. The maximum recommended levels of total FUMs for animal feed often are higher than those for human foods, and some corn-based products unfit for human food can be used as animal feed [3]. Biofuel production is another use of corn that could allow higher levels of FUMs than allowed for human food, as levels up to 37.4 mg/g have no effect on fermentation rates or ethanol yields [36]. However, FUMs enrichment has been predicted in Distiller Dried Grains (DDGs) that is the co-product of ethanol industry. Hence, the amounts retained in the DDGs must be considered when DDGs is utilized as valuable livestock feed component [36]. Thus, appropriate utilization strategies are important management strategy for FUMs-contaminated agricultural products.
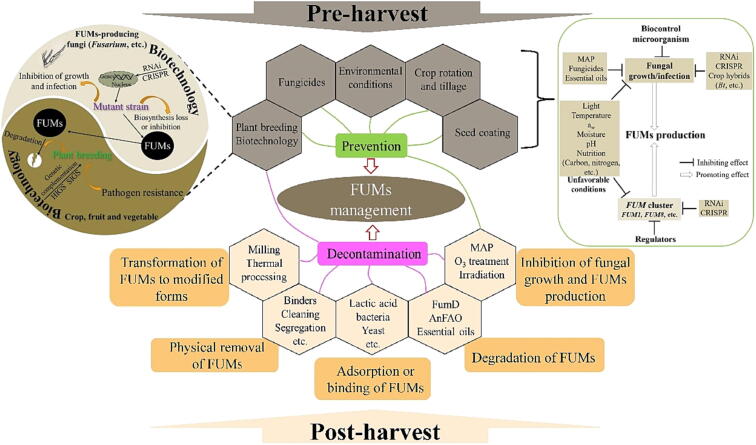
However, in some countries, such as countries in Central America and Subsaharan Africa, corn is a staple food and an important trading commodity. FBs are the main mycotoxin contamination risk in many places and the presence of FBs in some maize exceeds the maximum limits set by the FDA and EC, whereas Maximum Residue Limit (MRLs) or regulations have not been established for FUMs in human food [16], [37]. Due to the lack of high-volume alternative uses for corn, FUMs should be managed at safe levels or completely eliminated through integrated practices. Controlling mycotoxin contamination includes pre-and post-harvest strategies [1] as shown in Fig. 2, Table 1, Table 2. In fact, the control of FUMs contamination almost runs through the entire maize supply chains including farmers, maize traders, feed millers and retailers of maize based products.
Fig. 2.
The major practices and mechanisms for management of FUMs contamination. MAP: Modified atmosphere packaging. HIGS: Host induced gene silence. SIGS: Spray-induced gene silencing. CRISPR: Clustered regularly interspaced short palindromic repeats. RNAi: RNA interference.
Table 1.
The major pre-harvest managements of FUMs contamination.
| Treatments | Application | Mechanisms of action | Application status | Reference | |
|---|---|---|---|---|---|
| Agronomic practices | Crop rotation and tillage | Crops | Inhibiting fungal growth and then FB1 production | Already applied in field and partially successful | [40] |
| Field conditions (Light, Temperature, aw, moisture) | Corn, rice and wheat | Regulating fungal growth and FUM expression | Scientifically conceivable and can be applied in field | [45], [46], [47] | |
| Insecticides | Maize | Inhibiting fungal growth and then FUMs production | Already applied in field but not recommended | [39] | |
| Plant breeding | Bt maize hybrids | Maize | Less Fusarium infection and reduced fumonisin levels via reducing corn-borer damage | Already applied in field and partially successful | [20] |
| Conventional breeding | Maize | Higher genetic resistance to FUMs contamination | Applied in the field and effective | [49] | |
| Molecular breeding | Maize | Increased resistance to fungi or FUMs | Scientifically conceivable with limited application in field | [34] | |
| Genomic selection | Maize | Improving Fusarium ear rot resistance | Scientifically conceivable with limited application in field | [51] | |
| Enriched flavonoids maize hybrid | Maize | Reduced appearance of Fusarium ear rot symptom and lower level of FUMs | Demonstrated experimentally | [52] | |
| iological practices | “Fusaclean” and “Biofox C” (non-pathogenic F. oxysporum) | Vegetables | Inhibiting fungal growth | Already applied as seed coating | [20] |
| “Epic” and “Kodiak” (B. subtilis) | Cotton and legumes | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| “Intercept” (Pseudomonas cepacia) | Maize, vegetables and cotton | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| “Mycostop” (Streptomyces griseoviridis) | Ornamental and vegetables crops | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| T-22G and T-22HB (Trichoderma harziatum) | Grains, soya, cotton and vegetables | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| “Biofungus” (Trichoderma spp) | Citrus and pome fruit | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| “Blue circle (Burkholderia cepacia) | Vegetables | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| “Deny” (B. cepacia) | Grain crops | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| “Cedomon” and “Cerall” (Pseudomonas chlororaphis) | Wheat, rye and triticale | Inhibiting fungal growth | Already applied as seed coating | [20] | |
| Debaryomyces hansenii | Maize grains | Inhibiting growth and FB1 production | Already applied as seed coating | [60] | |
| Afla-Guard® (non-aflatoxigenic A. flavus strain (NRRL21882)) | Maize seed | Reducing the frequency of F. verticillioides through competition for substrate or space, and consequently, fumonisin production | Already applied as seed coating | [58] | |
| Biotechnology | HIGS/SIGS | Potential application in plant-derived food | Biosynthesis inhibition (FUM cluster regulation) | Scientifically conceivable and will be promising | [65], [71] |
Table 2.
The major post-harvest managements of FUMs contamination.
| Treatments | Food commodity | Mechanisms of action | Application status | Reference | |
|---|---|---|---|---|---|
| Physical practices | Thermal processing | Corn flour, kernels, grits, maize-based meal and bread | Reducing FUMs through transformation or modification of | Demonstrated experimentally and partially successful in application | [76] |
| Modified atmospheres (high CO2 and low O2 concentration) | Corn | Inhibiting fungal growth and FB1 production | Demonstrated experimentally and can be applied | [89], [90] | |
| Milling | Corn grits | Redistributing the existing fumonisins into different fractions | Already applied and partially successful | [80] | |
| λ-irradiation | Corn | Hydrolysis and decomposing | Demonstrated experimentally and can be applied | [82] | |
| Dehulling | Maize | Direct remove | Already applied and partially successful | [81] | |
| Cleaning and sorting | Maize | Direct remove | Already applied and partially successful | [81] | |
| Adsorbents | Animal feeds | Bind or reduce bioavailability | Demonstrated experimentally and can be applied | [91] | |
| Chemical practices | Essential oil | Not application in planta | Degrading fumonisin B1 | Demonstrated experimentally and lack information for application | [106] |
| Essential oils | Corn and foodstuff, maize grain | Inhibiting growth and then, FB1 production | Demonstrated experimentally and can be applied | [104] | |
| Nixtamalization | maize meals | Alkaline hydrolysis of FBs | Demonstrated experimentally and can be applied | [103] | |
| Biological practices | Yeast | Animal Feed | Adsorption | Demonstrated experimentally and can be applied | [20] |
| Lactic acid bacteria | Maize meal and maize kernels | Adsorption or binding | Demonstrated experimentally and can be applied | [111] | |
| FUMzyme® | Maize, Feeds for pigs and poultry | FB esterase FumD: hydrolyze the tricarballylic acid groups | Already applied | [120], [121], [126] | |
| FumI | Potential application in food and feed | Aminotransferase: deaminating hydrolyzed fumonisin B1 | Demonstrated experimentally and lack in vivo information | [123] | |
| AnFAO | Potential application in food and feed | Oxidatively deaminate intact fumonisins | Demonstrated experimentally and lack in vivo information | [122] | |
Pre-harvest practice
Pre-harvest practices to prevent FUMs contamination include agronomic practices and use of chemical fungicides, which can inhibit fungal infections and FUMs production, in addition to breeding FUMs-resistant varieties. Biotechnological approaches provide an alternative approach to inhibit FUMs biosynthesis or degradation, although these approaches have not been widely tested under field conditions.
Agronomic practice
Agronomic practices focus on growing healthy plants that are not excessively stressed by environmental factors, thereby reducing mycotoxigenic fungal infections. Infection by FUM-producing fungi usually occurs in the field, and thus good agricultural practices to prevent or control pre-harvest FUMs contamination are particularly important. For example, Fusarium ear rot caused by F. moniliforme through silk and kernel infection is one of the prominent diseases of maize worldwide, thus resulting in frequent FUMs contamination [38]. Meanwhile, agrochemical treatment with insecticide can also decrease FUMs levels in maize grains by controlling insects stress and reducing corn-borer damage [39].
Crop rotation and tillage procedures are the primary agronomic techniques that can prevent the growth of toxigenic fungi and reduce mycotoxin accumulation in infected grains [40]. For example, conventional tillage (soil prepared with a disc plough and heavy-disc harrow followed by a light-disc harrow) reduced FUMs contamination in corn in a study conducted in Brazil over two growing seasons [41]. An analysis of corn samples from 16 fields in north-eastern Spain revealed the lack of a clear effect of tillage system on FUMs levels [42]. The advantages of conventional tillage, including reducing fungal inoculation and alleviating drought stress, suggest that it is beneficial for farmers, and especially farmers that primarily utilize mechanized operations. Higher F. verticillioides incidence in addition to FB1 and FB3 content have been detected in maize-maize crop rotation system [43]. Further, forage maize should be harvested as soon as it reaches the middle dent stage to reduce FUMs contamination risks [44]. Field-grown crops are highly sensitive to environmental conditions, such as light, temperature and moisture, and all of these affect fungal growth and FUMs production [45], [46], [47] (Fig. 2). Thus, good agronomic practices, alongside hazard analysis and critical control point (HACCP) practices can help prevent FUMs contamination by reducing fungal growth and FUMs production.
Breeding practice
Breeding strategies represent another important pre-harvest practice for FUMs control. A previous study of 369 maize samples collected from across China demonstrated that FUMs contamination can be greatly reduced using insect- and pathogen-resistant maize varieties [48]. Conventional breeding programs including backcross breeding, pedigree selection approaches and recurrent selection have been applied in the field to generate higher genetic resistance to FUMs contamination [49]. Importantly, high-yielding hybrids obtained by conventional breeding alone have contributed low rates of fungal infection and mycotoxin contamination. Furthermore, commercial hybrid breeding in many parts of the world has been very effective in controlling fumonisin contamination (Table 1). Likewise, development of new hybrids that are resistant to abiotic stresses through conventional breeding methods can also help mitigate FUMs contamination of maize in the field [50]. Hybrid selection during breeding can help eliminate highly susceptible genotypes to ear-rot pathogens, then contributes to reduce FUMs production [34]. Conventional breeders should leverage abundant breeding materials, large-scale tests, and long-term screening of resistant genotypes to select for resistant genotypes that can reduce FUMs contamination.
Molecular biology techniques also have provided effective approaches to conventional breeding. Various techniques, including transcriptome profiling, genotyping-by-sequencing and genome-wide association (GWAS) methods, have been used to identify molecular markers linked to quantitative trait loci (QTL) and candidate genes associated with FUMs contamination resistance [34]. Significant progress has been made in recent years by using molecular markers to bring in partial resistance. However, applications of marker-assisted breeding for improving resistance to FUMs are still limited to the laboratory scale, with only a few putative minor genes. Major genes are yet to be identified [34], [51]. It is worth to note that genomic selection (GS) is an emerging tool to improve disease resistances in maize. Considering that GS has been used to predict selection response including lethal necrosis resistance, Diplodia ear rot resistance, and Northern corn leaf blight resistance in different maize lines, it seems to be promising for FER resistance breeding [51]. Certainly, there are shortcomings to overcome, though GS can reduce cost, time and labour in breeding programs.
Reverse genetics can also be used to evaluate the effects of pathway disturbance on crop resistance to FUMs contamination. For example, a maize hybrid with enriched flavonoids in the kernel pericarps reduced Fusarium ear rot (FER) symptoms and exhibited lower levels of FUMs than the isogenic hybrid without flavonoids [52]. Certainly, other factors should be considered in breeding programs of flavonoids-enriched maize such as palatability. Similarly, Diaz-Gomez et al. [53] observed that increased carotenoid content in maize grains reduces levels of FUMs, suggesting that carotenoids are resistant to FER and FUMs production. Differences in starch content between carotenoid-enriched maize lines and isogenic lines suggest that the application of this genetically engineered corn hybrid requires further investigation, since starch content is associated with FUMs contamination resistance. Whether the amount of starch present or the chemical form plays a role in FUMs reduction need to study in detail.
Genetic modification of maize crops (e.g. Bt maize) also provides a safe and highly effective method for insect control and reduced Fusarium infection and/or FUMs production [20]. Bt crop-derived foods have been approved by numerous global regulatory agencies. Importantly, transgenic Bt maize exhibits less FUMs contamination than corresponding non-Bt isolines [54]. It is worth to note that Bt products are not directly targeted to FUMs reduction. Due to less insect damage, Bt corn has fewer holes that prevents fungal access to the interior of the plant, thus is more resistant to FUMs contamination.
Although considerable benefits exist for low FUMs contamination, production of Bt maize has faced increasing challenges in recent years, due to increasing insect resistance and unstable effectiveness. For example, low concentrations of FUMs were observed in Bt maize during the season when the European corn borer (O. nubilalis Hübner) was prevalent, but not when the corn earworm (H. zea Boddie) was prevalent [20]. Furthermore, the high cost of Bt hybrid seed limits its use by farmers in rural areas compared to other varieties, such as herbicide tolerant seed.
Plant breeding has generally led to significant progress over the past decade in increasing resistance to Fusarium ear rot in commercial hybrids. Despite that plant breeding is the most recommended, cost-effective and ecologically safe method for managing FUMs-producing fungal infections, it has still been challenging for multiple reasons. Firstly, resistant selection involves the consideration of multiple factors, including breeding material, climatic conditions, labour requirements, and time costs. Secondly, the commercial or public deployment of improved hybrids is limited and some corn hybrids via molecular breeding have been identified only at the lab-scale in a few countries. Thus, significant efforts are still needed to produce hybrids that effectively prevent FUMs contamination in the field. Concomitantly, plant breeding should be used to achieve a balance between FUMs-resistance and high quality or yields of crops.
Biological practice
The early development of seed coatings using bio-agents is considered an environmentally friendly strategy to prevent mycotoxin contamination. Biocontrol has been reported as an effective method for aflatoxin control while some commercial aflatoxin biocontrol products have been registered at different times and in different countries [55], [56]. Most of aflatoxin biocontrol practices do not effectively reduce FUM contents [57]. Interestingly, a non-aflatoxigenic A. flavus strain (NRRL 21882) is the active ingredient of Afla-Guard® and has been demonstrated as a promising biocontrol agent to prevent F. verticillioides growth and FUMs production in maize [58]. Likewise, the use of Serratia marcescens SerEW01 was efficient in suppressing F. proliferatum growth and FUMs accumulation in rice medium [59]. S. marcescens SerEW01 application is particularly promising for the development of seed coatings to control FUMs contamination in some crops. In addition, Debaryomyces hansenii is effective against Aspergillus sp., F. proliferatum and F. subglutinans infection, as well as reducing FUMs production in maize grains [60]. A significant concern remains in how to improve the efficacy of these biocontrol agents under field conditions or during storage. Important commercially used biological practices for preventing effectively FUMs contamination are shown in Table 1.
Similar to Bt products, some bio-agents also exhibit side effects rather than directly target to FUMs reduction. For example, Afla-Guard® application results in a competition for substrate or space between a non-aflatoxin-producing A. flavus and F. verticillioides strains, thus reducing the frequency of F. verticillioides and FUMs production in maize [58].
Application of biotechnology
Biotechnological approaches are widely accepted as effective controls of fungal disease and mycotoxin production in food systems [61]. Significant advances have been made in the identification of FUMs biosynthesis pathways [62]. Hence, management could be achieved by engineering crops to regulate FUMs biosynthesis. Degradation of FUMs can also be achieved through biotechnological protocols. Current biotechnology applications primarily involve in heterologous expression of foreign genes in crops and gene-editing within crops. An additional strategy, host-induced gene silencing (HIGS), involves host expression of foreign genes that target pathogens, which has emerged as a novel transgenic approach for breeding mycotoxin resistant cultivars [63]. Further, RNA interference (RNAi) via double-stranded RNA (dsRNA) synthesis is an effective strategy for inhibiting fungal secondary metabolism [64]. For example, Johnson et al. [65] used dsRNA constructs to efficiently inhibit FB1 production by downregulating the expression of FUM1 and FUM8 in F. verticillioides. Thus, transgenic crops produced through RNAi-mediated HIGS can lead to overexpression of dsRNA complementary to a fungal gene critical for biosynthesis, thereby reducing FUMs production.
Several studies have found that FUMs contribute to fungal virulence in crops while FUMs-producing defective strains are less virulent for crops, although results are inconsistent in different hosts [66], [67]. Hence, transgenic approaches may simultaneously inhibit FUMs synthesis and generate fungal resistant cultivars. The effects on target genes caused by dsRNA are particularly prominent.
Another potential application of dsRNA is RNA fungicides. Spray-induced gene silencing (SIGS) based on RNAi technologies is environmentally-friendly and sustainable, but does not induce resistant or tolerant mutated pathogenic strains [68]. SIGS is a promising implementation of dsRNA technology considering public concern for production of genetically modified crops (GMOs) and provides an alternative strategy for commercial application in corn production. However, current regulatory practices don’t provide a standardized legal framework worldwide for SIGS [69]. For example, the legal framework for SIGS-based products in Australia is more inspiring than that in the European Union [69]. Thus, developing ‘RNA fungicides’ by targeting genes vital to biosynthesis of FUMs can open avenues for environmentally-friendly control of FUMs contamination.
There is no doubt that SIGS could accelerate elimination of FUMs in crops and its commercial application will be imminent in future. It is consequently important to understand biosynthesis of FUMs to promote the development of biotechnology approaches for preventing FUMs contamination, including inhibiting the growth and infection of FUMs-producing fungi in the field (Fig. 2). The rapid development of biotechnological approaches, such as clustered regularly interspaced short palindromic repeat (CRISPR)/ CRISPR-associated (Cas) systems that delete FUMs-biosynthetic gene clusters instead of single gene modifications, can provide a powerful solution for making non-toxigenic fungal strains to compete with fumonisin-producing Fusarium strains for the eventual elimination of FUMs production [70]. Importantly, the dual-plasmid CRISPR/Cas system can efficiently and stably provide knockouts without later restoration. It is worth to note that other genomic effects of knockout strains caused by CRISPR need to be tested because CRISPR can induce DNA changes outside the target area as well as within it. Meanwhile, a huge amount of work need to be done, making this approach extremely challenging.
In addition to what had previously described, rapid advances in gene-editing technology have also made targeted and precise genetic manipulation of crops a reality [71]. Even though acceptance of genetically modified foods can be difficult for consumers and eventual approval must be gained at different regulatory levels [72], gene engineering of crops still exhibits great potential for better preventing FUMs contamination in the future. 9-LOX-derived oxylipins in plants are required by some fungi to accelerate conidiation and mycotoxin production, suggesting that host oxylipin pathways could be useful targets for genetic modification [73]. Gao et al. [73] reported that ZmLOX3 (lipoxygenase) inactivation resulted in drastically reduced levels of conidia and FUMs production by F. verticillioides on kernels at laboratory scale, providing proof of concept for successful gene engineering approaches in preventing FUMs contamination. FBs-degrading enzymes have also been identified and genetically engineered maize varieties that detoxify FUMs via these enzymes have been developed in the laboratory [74].
Overall, effective control of FUMs contamination in crops can potentially be achieved by genetic engineering of pathogen or host crop (Fig. 2). Although biotechnological approaches based on FUMs-degrading enzymes hold great promises for future management of FUMs contamination, few examples of their implementation are currently available. Additionally, the possible negative impacts on nutritional quality, texture, or flavor of foods remain to be critically evaluated. Successful application of these approaches will largely depend on detoxification ability, localization, stability, and activity of FUMs-degrading enzymes, as well as the toxicity of the FUMs breakdown products generated pre- and post-harvest [20].
Post-harvest practice
Physical practice
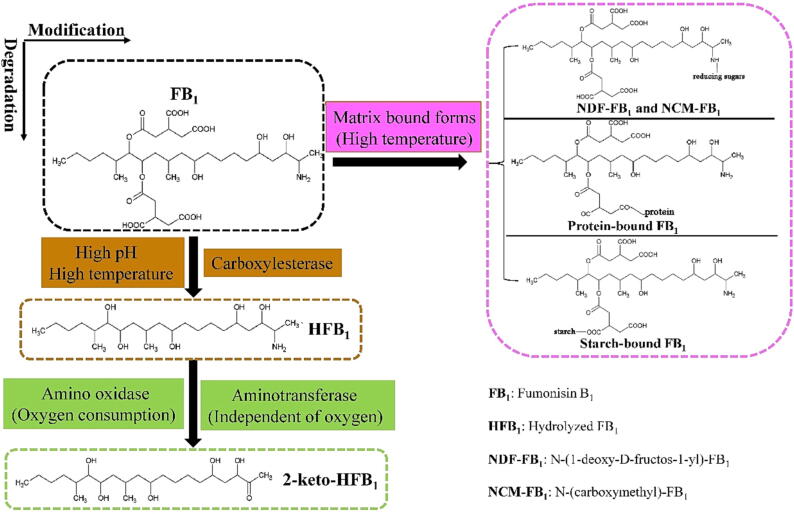
Physical practices receive considerable attention due to their simplicity and the lack of need for chemical use. Physical methods are primarily used to control FUMs contamination through detoxification (thermal processing, drying, sorting, milling, and binders) and prevention via irradiation and modified atmosphere packaging. Thermal processing is a traditional method that can be used to reduce contaminants in food production. Notably, the production of most maize-based foods involving one or multiple heat treatments and hydrothermal treatment promotes the binding of FB1 with some non-macro-digestible components of foods [75]. In addition, most of the bound FB1 resisted the in vitro digestion [75]. A recent review summarized the effects of thermal processing on FUMs contamination in food that primarily include baking, roasting, frying, high-pressure cooking and extrusion cooking [76]. The authors suggested that extrusion cooking of maize flour and high-pressure cooking of maize grits during cornflake production could lower FB1 and FB2 levels to comply with the EU ML of 800 µg/kg when the raw materials also were compliant (i.e., not exceeding the maximum recommended levels for raw-unprocessed maize: 4,000 µg/kg). Thermal treatments result in reduced FUMs or promote mycotoxin-matrix interactions to mitigate FUMs contamination in food production (Table 2). The reduction of FUMs contamination by thermal processing is primarily caused by transformation of FUMs to modified forms such as NDF-FB1 and NCM-FB1 (Fig. 3) that are undetectable [37]. NDF-FB1 and NCM-FB1 conjugates are rather stable in the in vitro model system while NDF -FB1 is less toxic than FB1 [77]. Variable reduction of FUMs is caused by different processing procedures. For example, obvious changes in the FB1 levels of maize flour were not observed during domestic pressure cooking while significant reductions of FB1 and FB2 levels occurred during laboratory and industrial pressure cooking of spiked flaking grits [76]. Similarly, reduced FB1 levels in maize also occur during baking, in conjunction with NCM-FB1 formation. Concomitantly, FB1 contamination decreases over time and with higher temperature [76]. Notably, the formation of modified FUMs by thermal processing cannot be determined by standard analytical procedures in some cases [78], suggesting that disappearance of the parent FUMs does not necessarily indicate decontamination. Thus, improved detection and monitoring, in addition to evaluating the bioavailability and toxicological impacts of modified FUMs in foods and raw materials, will be a significant focus for future food industry research.
Fig. 3.
Proposed mechanisms of FUMs detoxification, taking FB1 for example.
Physical removal during food processing is also well known to reduce mycotoxin levels. The physical removal of mycotoxins is efficient when manual sorting grains, nuts, and dried fruits is conducted by farmers or via automatic sorting [79]. During the processing stage, FUMs contamination in raw maize kernels can be reduced by about 75% after milling [80]. Furthermore, simple sorting of maize grains is also efficient in reducing dietary FB1 for consumers and leads to little or no mycotoxin exposure [81].
Irradiation to inactivate microorganisms is also an effective physical practice for controlling fungal infection and mycotoxin production before storage. Aziz et al. [82] applied λ-irradiation at 5.0 and 7.0 kGy (kGy) levels to reduce FB1 levels in corn by 87% and 100%, respectively. Ozone treatment is also a promising technology to reduce mycotoxin contamination for food preservation and safety. Indeed, some mycotoxins, such as aflatoxins, ochratoxin A, and patulin, are degraded by ozone [79]. For FUMs, results from Ribeiro et al. confirmed the degradation of FUMs by ozone in naturally contaminated maize, though chemical structure and toxic effects of degradation products need to be further evaluated [83]. Gaseous O3 can also be used for controlling F. verticillioides growth and subsequent FBs contamination in maize under intermediate moisture conditions [84]. Hence, ozone is potentially a critical physical tool for controlling FBs contamination. Interestingly, ozone treatment effectively inhibits fungal infection, delays decay, and prolongs shelf life, all without impacting citrus quality [85]. Thus, ozone application is a promising approach for postharvest fruit storage to control mycotoxin contamination. Chlorine gas treatment is also commonly used to detoxify mycotoxins. Degradation of FB1 by chlorine dioxide gas has been reported in lab scale [86]. λ-irradiation and O3 treatment are considered as environmentally friendly alternatives compared to conventional decontamination approaches. However, their widespread utilization by food industries could be restricted due to safety issues, strict design requirements, special equipment that is needed, and handling costs. For example, the grain treated with ozone is not considered to be acceptable as human food, which results in a reduced price for the resulting products. Resolving these limitations will greatly improve management and prevention of FUMs contamination in the food industry.
Modified atmosphere packaging (MAP) is widely used by immediately exchanging gas compositions (mainly CO2 and O2) within food packaging. MAP is effective in reducing quality deterioration and postharvest loss during fruit storage, while also being consistent with appropriate consumer practices [87]. Importantly, MAP is effective in inhibiting fungal growth and controlling mycotoxin contamination [88]. Samapundo et al. showed that modified atmospheres (high CO2 and low O2 concentrations) effectively inhibited fungal growth and FB1 production by F. verticillioides and F. proliferatum on corn during early storage periods [89], [90]. Considering the efficiency, convenience, safety, and side-effects from alcohol or lactic acid fermentation of these technologies, more investigations are needed to determine whether MAP controls FUMs-producing fungi or affects FUMs production. For example, MAP seems unfriendly for long-term storage, which usually requires aeration to maintain grain quality. Moreover, the refinement of optimal conditions for MAP is needed during postharvest fruit storage.
The addition of mycotoxin binders is another physical strategy to manage mycotoxin contamination of food products. In general, these binders can be divided into two major groups, namely, mineral or organic adsorbents and biological adsorbents. The binders primarily operate by adsorbing mycotoxins to their surface. Mineral and organic adsorbents tested include aluminosilicate, diatomite, talc, activated carbon and polymeric resins [91]. Bentonite has been authorized as an adsorbent for aflatoxins adsorption in animal feed [91]. Although combined orange peels (OP) extract and modified organic–inorganic hybrid bentonite (OP-bentonite) materials exhibit a capacity for FB1 adsorption, the adsorption capacity of FB1 is much lower than for aflatoxins [92]. Furthermore, some organically modified clays exhibit a high adsorptive capacity for both FB1 and FB2 due to organic cation activities [93]. Cholestyramine has been demonstrated as an effective binder of FUMs in vitro, which was also confirmed for FUMs in vivo experiments [94]. However, organically modified clays have not been received regulatory approval for commercialization due to a lack of in vivo evidence of their efficacy. Confirmation of the safety and efficacy of mycotoxin binders, followed by regulatory approval will provide customers with quality guarantee. Clay-based technologies may provide novel means for the management of FUMs contamination. Nevertheless, the costs of these binders and their impacts on important nutrients in feed commodities should be considered in future studies [95].
Emerging technologies, including high pressure, non-thermal plasma, ultraviolet light, and pulsed electric fields treatments, have been used in recent years as physical methods for microbial decontamination of food products during processing [61], [96], [97], [98]. Among these nonconventional technologies, plasma, ultrasound, and pulsed electric field methods offer good potential for mycotoxin decontamination at the laboratory scale [98]. Furthermore, plasma-activated water (PAW) also exhibits antifungal activities against fungal spores both in vitro and in actual food products, especially in plant foods, such as fruit [97]. One minute of atmospheric plasma treatment resulted in complete degradation of FUMs [99]. In addition, cold plasma is an effective tool for the FUMs degradation or inactivation of dangerous fungi that are capable of producing FUMs in many food commodities, such as cereals and dairy products [100], [101]. When applied in practice, the plasma faces the biggest problem that it is often quite ineffective against internal contaminants of food commodities. The feasibility and potential benefits of emerging techniques for FUMs decontamination should be further explored. Combining conventional and emerging technologies also should be considered. Considerable research efforts in terms of cost effectiveness, food safety evaluation, and process efficiency are prerequisites for the practical application of these innovative approaches in the food industry.
Chemical practice
Consumers have expressed great concern for food safety in recent years. Thus, new and safe chemical agents for fungal control and mycotoxin management are a priority for future research. For example, butylated hydroxyanisole (BHA) is a commonly used antioxidant in food processing that can effectively reduce FUMs contamination when F. proliferatum was cultured in Czapek’s broth (CB) medium [102]. Nixtamalization is another commonly used chemical process, wherein corn is boiled in a lime solution to produce masa for tortillas and total FUMs content is reduced by 50%, with hydrolyzed FUMs containing aminopolyol backbones as the major product [103]. HFB1 is much less toxic than FB1 based on feeding trials in pigs and poultry [11]. Thus, corn products after nixtamalization treatment can be used for animal feed.
Some plant essential oils (EOs) have been approved as food additives by the USFDA and the roles of plant EOs in preventing fungal growth and reducing mycotoxin contamination have been noted [104]. Alizadeh et al. summarized the prospects of various EOs and pointed that aflatoxin B1, G1, B2, G2, and ochratoxin A are the most investigated toxins during the application of the most used EOs [105]. Till now, the utility of EOs for control of FUMs is at best at the laboratory level.
Ocimum EOs from Kenya effectively inhibited F. verticillioides growth and FB1 accumulation leading to their suggestion as an alternative means to control fungal infestation and mycotoxin contamination in stored corn and foodstuffs [104]. Based on the experimental evidence in laboratory, the direct degradation of FB1 by EOs was also reported by Xing et al. [106]. Recent advances in the control of FUMs contamination also demonstrated that several EOs showed FB1 inhibitory effects [3]. However, most of these promising antifungal agents have only been applied in vitro to control FUMs contamination due to their instability and organoleptic side effects [105]. Thus, plant EOs might be best utilized with antimicrobial packaging surrounding foods after harvest. For example, Tarazona et al. [107] reported an innovative packaging system containing ethylene–vinyl alcohol copolymer films incorporating plant EOs as a promising strategy to control F. proliferatum growth and FB1 and FB2 production.
Due to the possible health risks caused by toxic by-products or the compounds themselves, chemical treatments (such as with EOs) against FUMs production are not authorized and in some cases are banned by the European Union (EU) for food processing. Hence, further research into the safety and practical use of chemical treatments to control FUMs contamination is still needed for the food industry.
Biological practice
Biological control via microorganisms has become an increasingly promising means for mycotoxin decontamination in food production [108]. The bacterial consortium SAAS79 was recently identified with high levels of FB1-degrading activity, providing a new approach for FUMs management [109]. Lactic acid bacteria (LAB) strains have also been observed to successfully control FUMs production. The primary mechanism of LAB activity results from the adsorption or binding of FUMs by cell wall constituents and peptidoglycans [110]. Sadiq et al. [111] summarized possible interactions between FB1 and bacterial cell wall components of different LAB strains, including Lactobacillus paraplantarum, Streptococcus thermophilus, L. plantarum B7, and Lactobacillus Pentosus X8. Interestingly, heat or acid treatment of LAB bacterial strains can enhance their binding capacities for FB1 and FB2 [110]. Thermal treatments are particularly common during processing of agricultural products into foods. Thus, the use of combined LAB and heat treatment might be more effective and practical for controlling FUMs decontamination. More importantly, some LAB strains, including L. plantarum MYS6, L. delbrueckii subsp., lactis DSM 20076 and Pediococcus acidilactici NNRL B-5627, also exhibit protective effects against cellular toxicity and hepatorenal damage in broilers caused by FB1 [110], [112], [113]. Thus, LAB strains can potentially be used to decontaminate FUMs in the food industry.
In addition to bacteria, some yeast strains have been used to control mycotoxin contamination. For example, yeast cell wall extracts combined with clay minerals were effective in adsorption of FUMs within animal feeds [20]. Kolawole et al. [114] also reported that modified yeast cell walls can simultaneously adsorb over 50% of FB1 in animal feeds. Similarly, Armando et al. observed that S. cerevisiae yeast (strain RC016) exhibits high FB1 binding properties and their ability of binding the mycotoxin increases with concentration [115]. Furthermore, Chlebicz et al. [116] reported that 6 S. cerevisiae strains and 12 Lactobacillus strains exhibit detoxification of FUMs in in vitro assays. These strains are all probiotic microorganisms that confer a health benefit on the host when administered in adequate amounts, as defined by the FAO/WHO (2002) [117]. Hence, these yeast strains harbour great potential for use as food additives to detoxify contaminating FUMs. These microbial-based approaches clearly have many advantages. Nevertheless, commercial application requires evaluation of their safety, efficiency, and economic practicality.
In addition to adsorption or binding, some microorganisms can directly degrade FUMs. The direct use of microbial FUMs-degrading enzymes is a practical detoxification technique due to better safety and specificity compared to the application of microorganisms [118]. Function-based screening of microbial communities to identify potential enzymes involved in mycotoxin degradation was recently conducted [119].
Enzymatic transformation of mycotoxins into less-toxic metabolites through targeted modification is an important detoxification approach. Several catabolism-related enzymes can be used for enzymatic detoxification of FUMs in food [120], [121], [122], [123] (Fig. 3). The primary mechanisms of FBs detoxification include enzymatic modification of the free amino group at position C-2 and de-esterification of ester bonds at the C-14 and C-15 positions. Many critical enzymes, including carboxylesterases, amino oxidases and aminotransferases, are suitable for degrading FB1 in food production applications [123], [124], [125]. FUMzyme® (Biomin, Austria) contains a FUMs esterase, FumD (EC 3.1.1.87), that was authorized by the EC in 2014 and deemed safe for human, animal, and environmental exposure by the EFSA [120], [123]. The enzyme can hydrolyse the tricarballylic acid groups of FB1, producing the less-toxic HFB1 compound. The commercial FumD esterase has been subject to wide application in both commercial maize-based practices and home-grown maize products [120], [121], [126]. FUMzyme application can be expanded to enzymes from different microorganisms including FumDSB from Sphingomonadales bacterium [127]. Garnham et al. identified another enzyme, termed AnFAO (A. niger fumonisin amine oxidase), that exhibits robust deamination activity of FUMs and has promising potential for remediating FUMs-contaminated foods [122]. The toxic effects of enzyme products and feeding trials with AnFAO as a feed additive require further study. Nevertheless, enzymatic degradation of FUMs in food production could provide commercially viable opportunities to prevent FUMs contamination.
Metagenomic analysis as alternative approaches to conventional screening of microbial communities allows more efficient comprehensive screening of microbial genomes to identify new FUMs-degrading enzymes. Further, newly identified FUMs-reducing/degrading enzymes can be introduced into microorganisms for subsequent large-scale fermentation using food-grade recombinant enzymes to degrade FUMs. Additionally, recently developed metabolic engineering technologies have enabled the improvement of more efficient and useful microbial strains. Thus, the identification and implementation of different genes encoding FUMs-degrading enzymes into expression vectors and transforming them into microbe strains through metabolic engineering could help the utilization of combinatory enzymatic degradation of FUMs in the food industry. In addition, P. pentosaceus strain L006 has been shown to produce antifungal and “antimycotoxin” metabolites that reduce FUMs contamination in foods [128]. Metabolic engineering exhibits unique advantages for the yield of non-native desired products in plants [129], while providing new potential to produce “antimycotoxin” metabolites (e.g., inhibitors of FUMs biosynthesis) to counteract FUMs contamination in food system. Although the impacts of metabolic engineering on FUMs decontamination have not been reported, this emerging technology alongside genetic engineering will enhance the potential for the biocontrol of FUMs contamination in the food industry.
Conclusions and future prospects
Control of FUMs contamination in food supply chains is a significant area of focus due to the potential threat to human and animal health. Advances in reducing FUMs effects by prevention and decontamination measures have grown rapidly recently. Prevention strategies are theoretically the first line of defence toward FUMs contamination, while decontamination approaches are the last lines of defence to protect human and animals from the FUMs contamination of food supply chains. Culturally acceptable methods for FUMs management are recommended herein under different conditions based on practicality. In addition, biologically based practices and appropriate chemical and physical treatments can be applied to further reduce FUMs contamination. Integrated strategies, including pre-harvest, post-harvest and marketing are encouraged in developed countries to prevent FUMs contamination and guarantee food safety in supply chains. In contrast, the lack of sophisticated technologies in developing countries leads to the recommendation of decontamination approaches because they are simple and low cost management measures. For example, hand sorting/cleaning and dehulling are culturally acceptable and practical methods to implement in farm communities, and especially where consumer staple diets contaminated with high levels of FUMs.
The growth of Bt maize is another recommendation to control FUMs contamination in both developed and developing countries. Legislation is needed to implement FUMs mitigation protocols in developing countries. Increasing food safety concerns in both developed and developing countries requires communication about FUMs contamination networks along food supply chains [130]. Expanding production of horticultural crops may increase FUMs contamination in fresh fruits and their processed products. MAP methods are recommended to control FUMs contamination for fruit or fruit-based products because the packaging is widely used during storage or transport.
Application of gene-editing technologies to crop varieties to reduce fungal infestation and mycotoxin contamination is a prominent area to begin. New enzymes for FUMs degradation should be identified through metagenomics analysis of microbial communities. The enzymatic degradation products of FUMs require careful evaluation to ensure safety in long-term consumption. Since some modified/degraded FUMs cannot be identified by analytical procedures, collecting toxicological information for these modified products remains a challenge, which must be determined before safety evaluations can be made. If modified FUMs exhibiting similar toxicity, or even greater toxicity than the original FUMs [11], then applications of enzymatic degradations should be limited. For example, ammonization, thermal treatments and chemical treatment methods for FUMs decontamination all face considerable obstacles due to incomplete understanding of the toxicity of the modified FUMs. Hence, risk assessments and the establishment of tolerable limits for these modified FUMs are urgently needed for the eventual purpose of food safety and reduction of health risks. Clearly, the development of efficient management methods to mitigate FUMs still requires extensive research.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Taotao Li: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization. Jiajia Li: Formal analysis, Methodology, Software, Visualization, Writing – original draft. Jiasheng Wang: Conceptualization, Writing – original draft. Kathy S. Xue: Visualization, Writing – original draft. Xinguo Su: Writing – review & editing. Hongxia Qu: Writing – review & editing. Xuewu Duan: Writing – review & editing. Yueming Jiang: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Research and Development Program of China (No. 2021YFD00500), Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2020343), and the Natural Science Foundation of Guangdong Province (No. 2019A1515011481).
Biographies
Taotao Li Dr. Taotao Li is an Associate Professor from South China Botanical Garden, Chinese Academy of Sciences. Dr. Li got his PhD Degree from University of Chinese Academy of Sciences, South China Botanical Garden in 2016. The research interests of Dr. Li mainly focus on: Ripening and senescence mechanism of tropical/sub-tropical fruit. Infection mechanism of postharvest pathogenetic fungi from fruit. Biosynthesis and regulation mechanism of mycotoxin
Jiajia Li. Dr. Jiajia Li is a Lecturer from Pingdingshan University. Dr. Li got his PhD Degree from Nanjing Agricultural University. The research interests of Dr. Li mainly focus on: Natural geography and resource environment. Soil environment and health.
Jiasheng Wang. Dr. Jiasheng Wang is a Full Professor from The University of Georgia. Dr. Wang got his PhD Degree from School of Medicine, Boston University. Dr. Wang is Department Head of Department of Environmental Health Science, College of Public Health, The University of Georgia from 2008. The research interests of Prof. Jiang mainly focus on: Toxicology of natural toxins. Molecular epidemiology and prevention of human esophageal cancer and hepatocellular carcinoma. Human health effects of environmental toxicants, especially for food-borne toxicants. Intervention with natural products on human acute and chronic diseases with environmental linkage
Kathy S. Xue. Dr. Kathy S. Xue is a Post-doctor from Department of Environmental Health Science, College of Public Health, The University of Georgia. The research interests of Dr. Xue mainly focus on: Toxicology of natural toxins Molecular epidemiology and prevention of human esophageal cancer and hepatocellular carcinoma. Human health effects of environmental toxicants, especially for food-borne toxicants. Intervention with natural products on human acute and chronic diseases with environmental linkage.
Xinguo Su. Dr. Xinguo Su is a Full Professor from Guangdong AIB Polytechnic. Dr. Su got his PhD Degree from South China Botanical Garden (former South China Institute of Botany), Chinese Academy of Sciences in 2004. The research interests of Dr. Su mainly focus on Food quality and safety.
Hongxia Qu Dr. Hongxia Qu is a Full Professor from South China Botanical Garden, Chinese Academy of Sciences. Dr. Qu got her PhD Degree from South China Botanical Garden (former South China Institute of Botany), Chinese Academy of Sciences in 2001. The research interests of Dr. Qu mainly focus on: Energy metabolism and regulation in senescent fruit. Postharvest biology and technology of tropical/sub-tropical fruit. Infection mechanism of postharvest pathogenetic fungi from fruit.
Xuewu Duan. Dr. Xuewu Duan is a Full Professor from South China Botanical Garden, Chinese Academy of Sciences. Dr. Duan got his PhD Degree from South China Botanical Garden (former South China Institute of Botany), Chinese Academy of Sciences in 2004. Dr. Duan is the Secretary General of Guangdong Plant Physiology Society. The research interests of Dr. Duan mainly focus on: Biochemistry and Molecular Biology. Postharvest Biology of Horticultural Crop. Development of Storage Technology.
Yueming Jiang. Dr. Yueming Jiang is a Full Professor from South China Botanical Garden, Chinese Academy of Sciences. Dr. Jiang got his PhD Degree from Sun Yat-sen University in 1999. Prof. Jiang was Deputy Director of South China Botanical Garden from 2014 to 2019. The research interests of Prof. Jiang mainly focus on: Postharvest biology of horticulture crop. Comprehensive utilization of plant-derived foods. Evaluation, structure and function of secondary metabolites from fungi. Public Services Vice President of the Chinese Society for Plant Biology (2009-2014), Honorary President of Guangdong Plant Physiology Society, Vice President of Guangzhou International Scientific Technology Communication Society, Deputy editors of Plant Physiology Journal and Jounal of Tropical and Subtropical Botany, and Editorial board members of Subtropical Plant Science, Food Research International, Postharvest Biology and Technology, Journal of Food, Agriculture and Environment, Pakistan Journal of Botany, Journal of Applied Horticulture and The Open Agriculture Journal.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.08.001.
Contributor Information
Jiasheng Wang, Email: jswang@uga.edu.
Yueming Jiang, Email: ymjiang@scbg.ac.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jafarzadeh S., Hadidi M., Forough M., Nafchi A.M., A. Mousavi Khaneghah, The control of fungi and mycotoxins by food active packaging: a review. Crit Rev Food Sci Nutr. 2022:1–19. doi: 10.1080/10408398.2022.2031099. [DOI] [PubMed] [Google Scholar]
- 2.Ndemera M., De Boevre M., De Saeger S. Mycotoxin management in a developing country context: A critical review of strategies aimed at decreasing dietary exposure to mycotoxins in Zimbabwe. Crit Rev Food Sci Nutr. 2020;60:529–540. doi: 10.1080/10408398.2018.1543252. [DOI] [PubMed] [Google Scholar]
- 3.Ponce-Garcia N, Serna-Saldivar SO, Garcia-Lara S. Fumonisins and their analogues in contaminated corn and its processed foods - a review. Food Addit. Contam. Part a-Chem. Anal. Control Exposure & Risk Assess. 35(2018)2183-2203. doi:10.1080/19440049.2018.1502476. [DOI] [PubMed]
- 4.Aoki T., O'Donnell K., Geiser D.M. Systematics of key phytopathogenic Fusarium species: current status and future challenges. J Gen Plant Pathol. 2014;80:189–201. doi: 10.1007/s10327-014-0509-3. [DOI] [Google Scholar]
- 5.Mogensen J.M., Frisvad J.C., Thrane U., Nielsen K.F. Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. J Agric Food Chem. 2010;58:954–958. doi: 10.1021/jf903116q. [DOI] [PubMed] [Google Scholar]
- 6.Zabalgogeazcoa I., Alvarez A., Herrero N., Vazquez-De-Aldana B.R. Production of fumonisins by endophytic strains of Tolypocladium cylindrosporum and its relation to fungal virus infection. Mycotoxin Res. 2018;34:49–57. doi: 10.1007/s12550-017-0298-6. [DOI] [PubMed] [Google Scholar]
- 7.Fernández Pinto V. In: Mycotoxins in fruits and vegetables. Barkai-Golan R., Paster N., editors. Academic Press; San Diego: 2008. Chapter 13 - detection and determination of alternaria mycotoxins in fruits and vegetables; pp. 271–278. [DOI] [Google Scholar]
- 8.Braun M.S., Wink M. Exposure, occurrence, and chemistry of fumonisins and their cryptic derivatives. Compre Rev Food Sci Food Saf. 2018;17:769–791. doi: 10.1111/1541-4337.12334. [DOI] [PubMed] [Google Scholar]
- 9.Rheeder J.P., Marasas W.F.O., Vismer H.F. Production of fumonisin analogs by Fusarium species. Appl Environ Microbiol. 2002;68(5):2101–2105. doi: 10.1128/Aem.68.5.2101-2105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picot A., Barreau C., Pinson-Gadais L., Caron D., Lannou C., Richard-Forget F. Factors of the Fusarium verticillioides-maize environment modulating fumonisin production. Crit Rev Microbiol. 2010;36:221–231. doi: 10.3109/10408411003720209. [DOI] [PubMed] [Google Scholar]
- 11.Helle-Katrine K., Jan A., Lars B., Margherita B., Beat B., Sandra C., et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018;16:5242. doi: 10.2903/j.efsa.2018.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutsen H., Barregrd L., Bignami M., Brüschweiler B., Alexander J. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018;16:5172. doi: 10.2903/j.efsa.2018.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freire L., Sant'Ana A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem Toxicol. 2018;111:189–205. doi: 10.1016/j.fct.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Bryla M, Roszko M, Szymczyk K, Jedrzejczak R, Obiedzinski MW, Sekul J. Fumonisins in plant-origin food and fodder - a review. Food Addit Contam Part a-Chem Anal Control Exposure Risk Assess. 30(2013)1626-1640. doi:10.1080/19440049.2013.809624. [DOI] [PubMed]
- 15.Shephard GS, van der Westhuizen L, Gatyeni PM, Somdyala NIM, Burger HM, Marasas WFO. Fumonisin mycotoxins in traditional xhosa maize beer in South Africa, J Agric Food Chem. 53(24) (2005) 9634-9637.https://doi.org/10.1021/jf0516080. [DOI] [PubMed]
- 16.Sun X.D., Su P., Shan H. Mycotoxin contamination of maize in China. Compre Rev Food Sci Food Saf. 2017;16:835–849. doi: 10.1111/1541-4337.12286. [DOI] [PubMed] [Google Scholar]
- 17.Li R., Tao B., Pang M., Liu Y., Dong J. Natural occurrence of fumonisins B1 and B2 in maize from three main maize-producing provinces in China. Food Control. 2015;50:838–842. doi: 10.1016/j.foodcont.2014.09.034. [DOI] [Google Scholar]
- 18.Martins F.A., Ferreira F.M.D., Ferreira F.D., Bando E., Nerilo S.B., Hirooka E.Y., et al. Daily intake estimates of fumonisins in corn-based food products in the population of Parana, Brazil. Food Control. 2012;26:614–618. doi: 10.1016/j.foodcont.2012.02.019. [DOI] [Google Scholar]
- 19.Williams PL, James RC, Roberts SM. Principles of Toxicology || Properties and effects of natural toxins and venoms, (2000)409-433. doi: 10.1002/0471231800.
- 20.Alberts J.F., Zyl W.H., Gelderblom W.C.A. Biologically based methods for control of fumonisin-producing Fusarium species and reduction of the fumonisins. Front Microbiol. 2016;7:548. doi: 10.3389/Fmicb.2016.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail N.A., Mohd M.H., Nor N.M.I.M., Zakaria L. Fumonisin B1-producing Fusarium species from agricultural crops in Malaysia. Crop Prot. 2017;98:70–75. doi: 10.1016/j.cropro.2017.03.014. [DOI] [Google Scholar]
- 22.Li T., Gong L., Jiang G., Wang Y., Gupta V.K., Qu H., et al. Carbon sources influence fumonisin production in Fusarium proliferatum. Proteomics. 2017;17:1700070. doi: 10.1002/pmic.201700070. [DOI] [PubMed] [Google Scholar]
- 23.Karbanc?Oglu-Güler F, Heperkan D. Natural occurrence of fumonisin B1 in dried figs as an unexpected hazard. Food Chem Toxicol. 47(2009)289-292. doi:10.1016/j.fct.2008.11.003. [DOI] [PubMed]
- 24.Senyuva H.Z., Gilbert J. Identification of fumonisin B2, HT-2 toxin, patulin, and zearalenone in dried figs by liquid chromatography-time-of-flight mass spectrometry and liquid chromatography-mass spectrometry. J Food Prot. 2008;71:1500–1504. doi: 10.1111/j.1550-7408.2008.00354.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen C., Riley R.T., Wu F. Dietary fumonisin and growth impairment in children and animals: A Review. Compre Rev Food Sci Food Saf. 2018;17:1448–1464. doi: 10.1111/1541-4337.12392. [DOI] [PubMed] [Google Scholar]
- 26.Riley R.T., Merrill A.H. Ceramide synthase inhibition by fumonisins: a perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J Lipid Res. 2019;60:1183–1189. doi: 10.1194/jlr.S093815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X.R., Cao C.Y., Zhu X.Y., Li X.W., Wang K. Fumonisins B1 exposure triggers intestinal tract injury via activating nuclear xenobiotic receptors and attracting inflammation response. Environ Pollut. 2020;267 doi: 10.1016/J.Envpol.2020.115461. 115461-115461. [DOI] [PubMed] [Google Scholar]
- 28.Fandohan P., Hell K., Marasas W.F.O., Wingfield M.J. Infection of maize by Fusarium species and contamination with fumonisin in Africa. Afr J Biotechnol. 2003;2:570–579. doi: 10.5897/ajb2003.000-1110. [DOI] [Google Scholar]
- 29.Marasas WFO, Miller JD, Riley RT. Environmental Health Criteria: Fumonisin B1, International Programme on Chemical Safty. Geneva: EHC, 219(2000)1-150.
- 30.Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans, Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans, Household Use of Solid Fuels and High-Temperature Frying 95(2010)9-38. doi:10.1080/00207233.2010.544388. [PMC free article] [PubMed]
- 31.Rheeder J.P., Marasas W.F.O., Thiel P.G., Sydenham E.W., Shephard G.S., Vanschalkwyk D.J. Fusarium-moniliforme and fumonisins in corn in relation to human esophageal cancer in transkei. Phytopathology. 1992;82(3):353–357. doi: 10.1094/Phyto-82-353. [DOI] [Google Scholar]
- 32.Xue K.S., Tang L.L., Sun G.J., Wang S.K., Hu X., Wang J.S. Mycotoxin exposure is associated with increased risk of esophageal squamous cell carcinoma in Huaian area, China. BMC Cancer. 2019;19:1218. doi: 10.1186/S12885-019-6439-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.E.F.S.A. (EFSA). Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to fumonisins as undesirable substances in animal feed: Request No EFSA-Q-2003-040, EFSA J 235(2005)1-32. doi:10.1029/RG001i004p00507.
- 34.Munkvold G.P., Arias S., Taschl I., Gruber-Dorninger C. Mycotoxins in corn: occurrence, impacts, and management. Corn (Third Edition) 2019:235–287. doi: 10.1016/B978-0-12-811971-6.00009-7. [DOI] [Google Scholar]
- 35.Food and Drug Administration USA . Center for Food Safety and Applied Nutrition; Center for Veterinary Medicine: 2001. Fumonisin levels in human foods and animal feeds. https://www.fda.gov/regulatory-information/search-fda-guidance-ocuments/guidance-industry-fumonisin-levels-human-foods-and-animal-feeds. [Google Scholar]
- 36.Bowers E., Munkvold G. Fumonisins in conventional and transgenic, insect-resistant maize intended for fuel ethanol production: implications for fermentation efficiency and DDGS co-product quality. Toxins. 2014;6:2804–2825. doi: 10.3390/toxins6092804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misihairabgwi J.M., Ezekiel C.N., Sulyok M., Shephard G.S., Krska R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016) Crit Rev Food Sci Nutr. 2019;59:43–58. doi: 10.1080/10408398.2017.1357003. [DOI] [PubMed] [Google Scholar]
- 38.Munkvold G.P., Desjardins A.E. Fumonisins in maize: Can we reduce their occurrence? Plant Dis. 1997;81(6):556–565. doi: 10.1094/pdis.1997.81.6.556. [DOI] [PubMed] [Google Scholar]
- 39.Folcher L., Jarry M., Weissenberger A., Gerault F., Eychenne N., Delos M., et al. Comparative activity of agrochemical treatments on mycotoxin levels with regard to corn borers and Fusarium mycoflora in maize (Zea mays L.) fields. Crop Prot. 2009;28:302–308. doi: 10.1016/j.cropro.2008.11.007. [DOI] [Google Scholar]
- 40.Chen Y., Kistler H.C., Ma Z.H. Fusarium graminearum trichothecene mycotoxins: biosynthesis, regulation, and management. Annu Rev Phytopathol. 2019;57:15–39. doi: 10.1146/annurev-phyto-082718-100318. [DOI] [PubMed] [Google Scholar]
- 41.Ono E.Y.S., Moreno E.C., Ono M.A., Rossi C.N., Saito G.H., Vizoni E., et al. Effect of cropping systems and crop successions on fumonisin levels in corn from Northern Parana State, Brazil. Eur J Plant Pathol. 2011;131:653–660. doi: 10.1007/s10658-011-9839-6. [DOI] [Google Scholar]
- 42.Arino A., Herrera M., Juan T., Estopanan G., Carraminana J.J., Rota C., et al. Influence of agricultural practices on the contamination of maize by fumonisin mycotoxins. J Food Prot. 2009;72:898–902. doi: 10.4315/0362-028x-72.4.898. [DOI] [PubMed] [Google Scholar]
- 43.Tran T.M., Ameye M., Phan T.K., Devlieghere F., Audenaert K. Impact of ethnic pre-harvest practices on the occurrence of Fusarium verticillioides and fumonisin B1 in maize fields from Vietnam. Food Control. 2020;120:107567. doi: 10.1016/j.foodcont.2020.107567. [DOI] [Google Scholar]
- 44.Okabe I., Hiraoka H., Miki K. Influence of harvest time on fumonisin contamination of forage maize for whole-crop silage. Mycoscience. 2015;56:470–475. doi: 10.1016/j.myc.2015.02.004. [DOI] [Google Scholar]
- 45.Castano S.M., Medina A., Magan N. Impact of storage environment on respiration, dry matter losses and fumonisin B-1 contamination of stored paddy and brown rice. World Mycotoxin J. 2017;10:319–326. doi: 10.3920/Wmj2017.2237. [DOI] [Google Scholar]
- 46.Cendoya E., Farnochi M., Chulze S., Ramirez M.L. Two-dimensional environmental profiles of growth and fumonisin production by Fusarium proliferatum on a wheat-based substrate. Int J Food Microbiol. 2014;182:9–17. doi: 10.1016/j.ijfoodmicro.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 47.Fanelli F., Iversen A., Logrieco A.F., Mule G. Relationship between fumonisin production and FUM gene expression in Fusarium verticillioides under different environmental conditions, Food Addit. Contam Part a-Chem Anal Control Exposure & Risk Assess. 2013;30:365–371. doi: 10.1080/19440049.2012.743039. [DOI] [PubMed] [Google Scholar]
- 48.Wei T., Zhu W., Pang M., Liu Y., Dong J. Natural occurrence of fumonisins B1 and B2 in corn in four provinces of China. Food Addit Contam Part B: Surveill. 2013;6:270–274. doi: 10.1080/19393210.2013.819816. [DOI] [PubMed] [Google Scholar]
- 49.Santiago R., Cao A., Malvar R.A., Butron A. Genomics of maize resistance to Fusarium ear rot and fumonisin contamination. Toxins. 2020;12:431. doi: 10.3390/Toxins12070431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo B.Z., Ji X.Y., Ni X.Z., Fountain J.C., Li H., Abbas H.K., et al. Evaluation of maize inbred lines for resistance to pre-harvest aflatoxin and fumonisin contamination in the field. Crop J. 2017;5:259–264. doi: 10.1016/j.cj.2016.10.005. [DOI] [Google Scholar]
- 51.Gaikpa D.S., Miedaner T. Genomics-assisted breeding for ear rot resistances and reduced mycotoxin contamination in maize: methods, advances and prospects. Theor Appl Genet. 2019;132:2721–2739. doi: 10.1007/s00122-019-03412-2. [DOI] [PubMed] [Google Scholar]
- 52.Venturini G, Babazadeh L, Casati P, Pilu R, Salomoni D, Toffolatti SL. Assessing pigmented pericarp of maize kernels as possible source of resistance to fusarium ear rot, Fusarium spp. infection and fumonisin accumulation, Int J Food Microbiol. 227 (2016)56-62. doi:10.1016/j.ijfoodmicro.2016.03.022. [DOI] [PubMed]
- 53.Diaz-Gomez J., Marin S., Nogareda C., Sanchis V., Ramos A.J. The effect of enhanced carotenoid content of transgenic maize grain on fungal colonization and mycotoxin content. Mycotoxin Res. 2016;32:221–228. doi: 10.1007/s12550-016-0254-x. [DOI] [PubMed] [Google Scholar]
- 54.Rheeder J., Vismer H., van der Westhuizen L., Imrie G., Gatyeni P., Thomas D., et al. Effect of Bt corn hybrids on insect damage, incidence of fumonisin-producing Fusarium species and fumonisin levels in South Africa. Phytopathology. 2005;95(6):S88–S. [Google Scholar]
- 55.Moore G.G. Practical considerations will ensure the continued success of pre-harvest biocontrol using non-aflatoxigenic Aspergillus flavus strains. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1873731. [DOI] [PubMed] [Google Scholar]
- 56.Ortega-Beltran A., Agbetiameh D., Atehnkeng J., Falade T.D.O., Bandyopadhyay R. Does use of atoxigenic biocontrol products to mitigate aflatoxin in maize increase fumonisin content in grains? Plant Dis. 2021;105:2196–2201. doi: 10.1094/Pdis-07-20-1447-Re. [DOI] [PubMed] [Google Scholar]
- 57.Pitt J.I. The pros and cons of using biocontrol by competitive exclusion as a means for reducing aflatoxin in maize in Africa. World Mycotoxin J. 2019;12:103–112. doi: 10.3920/Wmj2018.2410. [DOI] [Google Scholar]
- 58.Reis T.A., Oliveira T.D., Zorzete P., Faria P., Correa B. A non-toxigenic Aspergillus flavus strain prevents the spreading of Fusarium verticillioides and fumonisins in maize. Toxicon. 2020;181:6–8. doi: 10.1016/j.toxicon.2020.04.0191. [DOI] [PubMed] [Google Scholar]
- 59.Guo Z.Q., Zhang X., Wu J.X., Yu J., Xu M.L., Chen D.X., et al. In vitro inhibitory effect of the bacterium Serratia marcescens on Fusarium proliferatum growth and fumonisins production. Biol Control. 2020;143:104188. doi: 10.1016/j.biocontrol.2020.104188. [DOI] [Google Scholar]
- 60.Medina-Cordova N, Lopez-Aguilar R, Ascencio F, Castellanos T, Campa-Cordova AI, Angulo C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.), Biol. Control 97 (2016)70-79. doi:10.1016/j.biocontrol.2016.03.006.
- 61.Zhang H., Godana E.A., Sui Y., Yang Q., Zhang X., Zhao L. Biological control as an alternative to synthetic fungicides for the management of grey and blue mould diseases of table grapes: a review. Crit Rev Microbiol. 2020;46:450–462. doi: 10.1080/1040841x.2020.1794793. [DOI] [PubMed] [Google Scholar]
- 62.Li T.T., Su X.G., Qu H.X., Duan X.W., Jiang Y.M. Biosynthesis, regulation, and biological significance of fumonisins in fungi: current status and prospects. Crit Rev Microbiol. 2021 doi: 10.1080/1040841X.2021.1979465. [DOI] [PubMed] [Google Scholar]
- 63.Koch A., Kumar N., Weber L., Keller H., Kogel K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14 -demethylase-encoding genes confers strong resistance to Fusarium species. Proc Natl Acad Sci U S A. 2013;110:19324–19329. doi: 10.1073/pnas.1306373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilbert M.K., Majumdar R., Rajasekaran K., Chen Z.Y., Wei Q.J., Sickler C.M., et al. RNA interference-based silencing of the alpha-amylase (amy1) gene in Aspergillus flavus decreases fungal growth and aflatoxin production in maize kernels. Planta. 2018;247:1465–1473. doi: 10.1007/s00425-018-2875-0. [DOI] [PubMed] [Google Scholar]
- 65.Johnson E.T., Proctor R.H., Dunlap C.A., Busman M. Reducing production of fumonisin mycotoxins in Fusarium verticillioides by RNA interference. Mycotoxin Res. 2018;34:29–37. doi: 10.1007/s12550-017-0296-8. [DOI] [PubMed] [Google Scholar]
- 66.Glenn A.E., Zitomer N.C., Zimeri A.M., Williams L.D., Riley R.T., Proctor R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol Plant-Microbe Interact. 2008;21:87–97. doi: 10.1094/Mpmi-21-1-0087. [DOI] [PubMed] [Google Scholar]
- 67.Xie L.H., Wu Y.F., Wang Y., Jiang Y.M., Yang B., Duan X.W., et al. Fumonisin B1 induced aggressiveness and infection mechanism of Fusarium proliferatum on banana fruit. Environ Pollut. 2021;288:117793. doi: 10.1016/J.Envpol.2021.117793. [DOI] [PubMed] [Google Scholar]
- 68.Wang M., Jin H.L. Spray-induced gene silencing: a powerful innovative strategy for crop protection. Trends Microbiol. 2017;25:4–6. doi: 10.1016/j.tim.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Schutter K., Taning C.N.T., Van Daele L., Van Damme E.J.M., Dubruel P., Smagghe G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front Insect Sci. 2022;1 doi: 10.3389/finsc.2021.818037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu W.W., An C.Y., Shu X., Meng X.X., Yao Y.P., Zhang J., et al. A dual-plasmid CRISPR/Cas system for mycotoxin elimination in polykaryotic industrial fungi. Acs Synth Biol. 2020;9:2087–2095. doi: 10.1021/acssynbio.0c00178. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., Pribil M., Palmgren M., Gao C. A CRISPR way for accelerating improvement of food crops. Nat Food. 2020;1:200–205. doi: 10.1038/s43016-020-0051-8. [DOI] [Google Scholar]
- 72.Siegrist M., Hartmann C. Consumer acceptance of novel food technologies. Nat Food. 2020;1:343–350. doi: 10.1038/s43016-020-0094-x. [DOI] [PubMed] [Google Scholar]
- 73.Gao X.Q., Shim W.B., Gobel C., Kunze S., Feussner I., Meeley R., et al. Disruption of a maize 9-lipoxygenase results in increased resistance to fungal pathogens and reduced levels of contamination with mycotoxin fumonisin. Mol Plant-Microbe Interact. 2007;20:922–933. doi: 10.1094/Mpmi-20-8-0922. [DOI] [PubMed] [Google Scholar]
- 74.Duvick J. Prospects for reducing fumonisin contamination of maize through genetic modification. Environ Health Perspect. 2001;109:337–342. doi: 10.2307/3435028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Massarolo K.C., Ferreira C.F.J., Collazzo C.C., Bianchini A., Kupski L., Badiale-Furlong E. Resistant starch and hydrothermal treatment of cornmeal: Factors in aflatoxins and fumonisin B1 reduction and bioaccessibility. Food Control. 2020;114:107274. doi: 10.1016/j.foodcont.2020.107274. [DOI] [Google Scholar]
- 76.Schaarschmidt S., Fauhl-Hassek C. The fate of mycotoxins during secondary food processing of maize for human consumption. Compre Rev Food Sci Food Saf. 2021;20:91–148. doi: 10.1111/1541-4337.12657. [DOI] [PubMed] [Google Scholar]
- 77.Hahn I., Nagl V., Schwartz-Zimmermann H.E., Varga E., Schwarz C., Slavik V., et al. Effects of orally administered fumonisin B-1 (FB1), partially hydrolysed FB1, hydrolysed FB1 and n-(1-deoxy-d-fructos-1-yl) FB1 on the sphingolipid metabolism in rats. Food Chem Toxicol. 2015;76:11–18. doi: 10.1016/j.fct.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z.Q., Nie D.X., Fan K., Yang J.H., Guo W.B., Meng J.J., et al. A systematic review of plant-conjugated masked mycotoxins: Occurrence, toxicology, and metabolism. Crit Rev Food Sci Nutr. 2020;60:1523–1537. doi: 10.1080/10408398.2019.1578944. [DOI] [PubMed] [Google Scholar]
- 79.Karlovsky P., Suman M., Berthiller F., De Meester J., Eisenbrand G., Perrin I., et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016;32:179–205. doi: 10.1007/s12550-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scudamore K.A. Fate of fusarium mycotoxins in the cereal industry: recent UK studies. World Mycotoxin J. 2008;1:315–323. doi: 10.3920/Wmj2008.X034. [DOI] [Google Scholar]
- 81.Matumba L., Van Poucke C., Ediage E.N., Jacobs B., De Saeger S. Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin-contaminated white maize, Food Addit. Contam Part a-Chem Anal Control Exposure & Risk Assess. 2015;32:960–969. doi: 10.1080/19440049.2015.1029535. [DOI] [PubMed] [Google Scholar]
- 82.Aziz N.H., El-Far F.M., Shahin A.A.M., Roushy S.M. Control of Fusarium moulds and fumonisin B1 in seeds by gamma-irradiation. Food Control. 2007;18:1337–1342. doi: 10.1016/j.foodcont.2005.12.013. [DOI] [Google Scholar]
- 83.Ribeiro D.F., Faroni L.R.D., Pimentel M.A., Prates L.H.F., Heleno F.F., De Alencar E.R. Ozone as a fungicidal and detoxifying agent to maize contaminated with fumonisins. Ozone-Sci Eng. 2022;44(1):38–49. doi: 10.1080/01919512.2021.1924616. [DOI] [Google Scholar]
- 84.Mylona K., Kogkaki E., Sulyok M., Magan N. Efficacy of gaseous ozone treatment on spore germination, growth and fumonisin production by Fusarium verticillioides in vitro and in situ in maize. J Stored Prod Res. 2014;59:178–184. doi: 10.1016/j.jspr.2014.08.001. [DOI] [Google Scholar]
- 85.Wu Q.X., Zhu X.R., Gao H.J., Zhang Z.K., Zhu H., Duan X.W., et al. Comparative profiling of primary metabolites and volatile compounds in Satsuma mandarin peel after ozone treatment, Postharvest. Biol Technol. 2019;153:1–12. doi: 10.1016/j.postharvbio.2019.03.008. [DOI] [Google Scholar]
- 86.Chang X., Wang J., Sun C., Liu H., Wu S., Sun J., et al. Research on degradation of chlorine dioxide in primary mycotoxins. J Chin Cereals Oils Assoc. 2016;31(9):113–118. [Google Scholar]
- 87.Celine M., Valerie G., Karine G., Sandrine C., Nathalie G., Stephane G., et al. Consumer behaviour in the prediction of postharvest losses reduction for fresh strawberries packed in modified atmosphere packaging, Postharvest. Biol Technol. 2020;163:111119. doi: 10.1016/j.postharvbio.2020.111119. [DOI] [Google Scholar]
- 88.Liu Y., Yamdeu J.H.G., Gong Y.Y., Orfila C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compre Rev Food Sci Food Saf. 2020;19:1521–1560. doi: 10.1111/1541-4337.12562. [DOI] [PubMed] [Google Scholar]
- 89.Samapundo S, De Meulenaer B, Atukwase A, Debevere J, Devlieghere F. The influence of modified atmospheres and their interaction with water activity on the radial growth and fumonisin B-1 production of Fusarium verticillioides and F-proliferatum on corn. Part I: The effect of initial headspace carbon dioxide concentration, Int. J. Food Microbiol. 114 (2007) 160-167. doi:10.1016/j.ijfoodmicro.2006.09.005. [DOI] [PubMed]
- 90.Samapundo S, De Meulenaer B, Atukwase A, Debevere J, Devlieghere F. The influence of modified atmospheres and their interaction with water activity on the radial growth and fumonisin B-1 production of Fusarium verticillioides and F. proliferatum on corn. Part II: The effect of initial headspace oxygen concentration, Int. J. Food Microbiol. 113 (2007) 339-345. doi:10.1016/j.ijfoodmicro.2006.09.006. [DOI] [PubMed]
- 91.Wielogorska E., MacDonald S., Elliott C.T. A review of the efficacy of mycotoxin detoxifying agents used in feed in light of changing global environment and legislation. World Mycotoxin J. 2016;9:419–433. doi: 10.3920/Wmj2015.1919. [DOI] [Google Scholar]
- 92.Rasheed U, Ul Ain Q, Yaseen M, Fan XS, Yao XH, Tong ZF. et al., Modification of bentonite with orange peels extract and its application as mycotoxins ? binder in buffered solutions and simulated gastrointestinal fluids, J Clean Prod. 267(2020) 122105-122105. doi:10.1016/j.jclepro.2020.122105.
- 93.Baglieri A, Reyneri A, Gennari M, Negre M. Organically modified clays as binders of fumonisins in feedstocks, J Environ Sci Health Part B-Pestic Food Contam Agric. Wastes 48(2013)776-783. doi:10.1080/03601234.2013.780941. [DOI] [PubMed]
- 94.Avantaggiato G., Solfrizzo M., Visconti A. Recent advances on the use of adsorbent materials for detoxification of Fusarium mycotoxins. Food Addit Contam. 2005;22:379–388. doi: 10.1080/02652030500058312. [DOI] [PubMed] [Google Scholar]
- 95.Kolosova A., Stroka J. Substances for reduction of the contamination of feed by mycotoxins: a review. World Mycotoxin J. 2011;4:225–256. doi: 10.3920/Wmj2011.1288. [DOI] [Google Scholar]
- 96.Barros J.H.T., Oliveira L.D., Cristianini M., Steel C.J. Non-thermal emerging technologies as alternatives to chemical additives to improve the quality of wheat flour for breadmaking: a review. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1966380. [DOI] [PubMed] [Google Scholar]
- 97.Xiang Q.S., Fan L.M., Li Y.F., Dong S.S., Li K., Bai Y.H. A review on recent advances in plasma-activated water for food safety: current applications and future trends. Crit Rev Food Sci Nutr. 2022;62:2250–2268. doi: 10.1080/10408398.2020.1852173. [DOI] [PubMed] [Google Scholar]
- 98.Gavahian M., Sheu S.C., Magnani M., Khaneghah A.M. Emerging technologies for mycotoxins removal from foods: Recent advances, roles in sustainable food consumption, and strategies for industrial applications. J Food Process Preserv. 2021;00:e15922. [Google Scholar]
- 99.ten Bosch L., Pfohl K., Avramidis G., Wieneke S., Viol W., Karlovsky P. Plasma-based degradation of mycotoxins produced by Fusarium, Aspergillus and Alternaria species. Toxins. 2017;9:97. doi: 10.3390/Toxins9030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gavahian M., Cullen P.J. Cold plasma as an emerging technique for mycotoxin-free food: efficacy, mechanisms, and trends. Food Rev Int. 2020;36:193–214. doi: 10.1080/87559129.2019.1630638. [DOI] [Google Scholar]
- 101.Gavahian M, Pallares N, Al Khawli F, Ferrer E, Barba FJ. Recent advances in the application of innovative food processing technologies for mycotoxins and pesticide reduction in foods, Trends Food Sci. Technol. 106(2020)209-218. doi:10.1016/j.tifs.2020.09.018.
- 102.Li T.T., Jian Q.J., Chen F., Wang Y., Gong L., Duan X.W., et al. Influence of butylated hydroxyanisole on the growth, hyphal morphology, and the biosynthesis of fumonisins in Fusarium proliferaturn. Front Microbiol. 2016;7:1038. doi: 10.3389/Fmicb.2016.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De la Campa R., Miller J.D., Hendricks K. Fumonisin in tortillas produced in small-scale facilities and effect of traditional masa production methods on this mycotoxin. J Agric Food Chem. 2004;52:4432–4437. doi: 10.1021/jf035160j. [DOI] [PubMed] [Google Scholar]
- 104.Dambolena JS, Zunino MP, Lopez AG, Rubinstein HR, Zygadlo JA, Mwangi JW. et al., Essential oils composition of Ocimum basilicum L. and Ocimum gratissimum L. from Kenya and their inhibitory effects on growth and fumonisin production by Fusarium verticillioides, Innov. Food Sci. Emerg. Technol. 11 (2010) 410–414. doi:10.1016/j.ifset.2009.08.005.
- 105.Alizadeh A.M., Golzan S.A., Mahdavi A., Dakhili S., Torki Z., Hosseini H. Recent advances on the efficacy of essential oils on mycotoxin secretion and their mode of action. Crit Rev Food Sci Nutr. 2021 doi: 10.1080/10408398.2021.1878102. [DOI] [PubMed] [Google Scholar]
- 106.Xing F.G., Hua H.J., Selvaraj J.N., Yuan Y., Zhao Y.J., Zhou L., et al. Degradation of fumonisin B-1 by cinnamon essential oil. Food Control. 2014;38:37–40. doi: 10.1016/j.foodcont.2013.09.045. [DOI] [Google Scholar]
- 107.Tarazona A, Mateo EM, Gomez JV, Gavara R, Jimenez M, Mateo F. Machine learning approach for predicting Fusarium culmorum and F. proliferatum growth and mycotoxin production in treatments with ethylene-vinyl alcohol copolymer films containing pure components of essential oils, Int J Food Microbiol. 338(2021)109012-109012. doi:10.1016/j.ijfoodmicro.2020.109012. [DOI] [PubMed]
- 108.Yang J.Y., Li J., Jiang Y.M., Duan X.W., Qu H.X., Yang B., et al. Natural occurrence, analysis, and prevention of mycotoxins in fruits and their processed products. Crit Rev Food Sci Nutr. 2014;54:64–83. doi: 10.1080/10408398.2011.569860. [DOI] [PubMed] [Google Scholar]
- 109.Zhao Z.Y., Zhang Y.M., Gong A.D., Liu N., Chen S.S., Zhao X.Y., et al. Biodegradation of mycotoxin fumonisin B1 by a novel bacterial consortium SAAS79. Appl Microbiol Biotechnol. 2019;103:7129–7140. doi: 10.1007/s00253-019-09979-6. [DOI] [PubMed] [Google Scholar]
- 110.Niderkorn V, Morgavi DP, Aboab B, Lemaire M, Boudra H. Cell wall component and mycotoxin moieties involved in the binding of fumonisin B-1 and B-2 by lactic acid bacteria, J Appl Microbiol. 106(2009)977-985. doi:10.1111/j.1365-2672.2008.04065.x. [DOI] [PubMed]
- 111.Sadiq F.A., Yan B.W., Tian F.W., Zhao J.X., Zhang H., Chen W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compre Rev Food Sci Food Saf. 2019;18:1403–1436. doi: 10.1111/1541-4337.12481. [DOI] [PubMed] [Google Scholar]
- 112.Deepthi B.V., Somashekaraiah R., Rao K.P., Deepa N., Dharanesha N.K., Girish K.S., et al. Lactobacillus plantarum MYS6 ameliorates fumonisin B1-induced hepatorenal damage in broilers. Front Microbiol. 2017;8:2317. doi: 10.3389/Fmicb.2017.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khalil A.A., Abou-Gabal A.E., Abdellatef A.A., Khalid A.E. Protective role of probiotic lactic acid bacteria against dietary fumonisin B1-induced toxicity and DNA-fragmentation in sprague-dawley rats. Prep Biochem. 2015;45:530–550. doi: 10.1080/10826068.2014.940969. [DOI] [PubMed] [Google Scholar]
- 114.Kolawole O., Meneely J., Greer B., Chevallier O., Jones D.S., Connolly L., et al. Comparative in vitro assessment of a range of commercial feed additives with multiple mycotoxin binding claims. Toxins. 2019;11:659. doi: 10.3390/Toxins11110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Armando M.R., Galvagno M.A., Dogi C.A., Cerrutti P., Dalcero A.M., Cavaglieri L.R. Statistical optimization of culture conditions for biomass production of probiotic gut-borne Saccharomyces cerevisiae strain able to reduce fumonisin B1. J Appl Microbiol. 2013;114:1338–1346. doi: 10.1111/jam.12144. [DOI] [PubMed] [Google Scholar]
- 116.Chlebicz A., Śliżewska K. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 Toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast, Probiotics and Antimicrob. Proteins. 2019;12:289–301. doi: 10.1007/s12602-018-9512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.FAO/WHO, Guidelines for the Evaluation of Probiotics in Food, Food and Agriculture Organization of the United Nations, London Ontario, Canada (2002).
- 118.Patriarca A., Pinto V.F. Prevalence of mycotoxins in foods and decontamination. Current Opin Food Sci. 2017;14:50–60. doi: 10.1016/j.cofs.2017.01.011. [DOI] [Google Scholar]
- 119.Ferrara M., Haidukowski M., D'Imperio M., Parente A., De Angelis E., Monaci L., et al. New insight into microbial degradation of mycotoxins during anaerobic digestion. Waste Manage. 2021;119:215–225. doi: 10.1016/j.wasman.2020.09.048. [DOI] [PubMed] [Google Scholar]
- 120.Alberts J., Schatzmayr G., Moll W.D., Davids I., Rheeder J., Burger H.M., et al. Detoxification of the fumonisin mycotoxins in maize: An enzymatic approach. Toxins. 2019;11:523. doi: 10.3390/Toxins11090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alberts J.F., Davids I., Moll W.D., Schatzmayr G., Burger H.M., Shephard G.S., et al. Enzymatic detoxification of the fumonisin mycotoxins during dry milling of maize. Food Control. 2021;123:107726. doi: 10.1016/j.foodcont.2020.107726. [DOI] [Google Scholar]
- 122.Garnham C.P., Butler S.G., Telmer P.G., Black F.E., Renaud J.B., Sumarah M.W. Identification and characterization of an Aspergillus niger amine oxidase that detoxifies intact fumonisins. J Agric Food Chem. 2020;68:13779–13790. doi: 10.1021/acs.jafc.0c04504. [DOI] [PubMed] [Google Scholar]
- 123.Heinl S., Hartinger D., Thamhesl M., Vekiru E., Krska R., Schatzmayr G., et al. Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J Biotechnol. 2010;145:120–129. doi: 10.1016/j.jbiotec.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 124.Heinl S., Hartinger D., Thamhesl M., Schatzmayr G., Moll W.D., Grabherr R. An aminotransferase from bacterium ATCC 55552 deaminates hydrolyzed fumonisin B. Biodegradation. 2011;22:25–30. doi: 10.1007/s10532-010-9371-y. [DOI] [PubMed] [Google Scholar]
- 125.Hartinger D., Moll W.D. Fumonisin elimination and prospects for detoxification by enzymatic transformation. World Mycotoxin J. 2011;4:271–283. doi: 10.3920/Wmj2011.1285. [DOI] [Google Scholar]
- 126.Grenier B., Schwartz-Zimmermann H.E., Gruber-Dorninger C., Dohnal I., Aleschko M., Schatzmayr G., et al. Enzymatic hydrolysis of fumonisins in the gastrointestinal tract of broiler chickens. Poult Sci. 2017;96:4342–4351. doi: 10.3382/ps/pex280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z.Y., Wang Y., Liu Z.Q., Jin S.Z., Pan K.G., Liu H.H., et al. Biological detoxification of fumonisin by a novel carboxylesterase from Sphingomonadales bacterium and its biochemical characterization. Int J Biol Macromol. 2021;169:18–27. doi: 10.1016/j.ijbiomac.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 128.Dalie D., Pinson-Gadais L., Atanasova-Penichon V., Marchegay G., Barreau C., Deschamps A., et al. Impact of Pediococcus pentosaceus strain L006 and its metabolites on fumonisin biosynthesis by Fusarium verticillioides. Food Control. 2012;23:405–411. doi: 10.1016/j.foodcont.2011.08.008. [DOI] [Google Scholar]
- 129.Shanab S.M.M., Hafez R.M., Fouad A.S. A review on algae and plants as potential source of arachidonic acid. J Adv Res. 2018;11:3–13. doi: 10.1016/j.jare.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leslie J.F., Morris J.B. Perspective: Talking about mycotoxins. Front Sustain Food Syst. 2019;3:109. doi: 10.3389/Fsufs.2019.00109. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.