Key Points
Question
Are androgen receptor signaling inhibitors associated with increased risk of cardiovascular events in men with advanced and metastatic prostate cancer?
Findings
In this systematic review and meta-analysis of 24 randomized clinical trials involving 22 166 patients, the addition of androgen receptor signaling inhibitors alongside standard androgen deprivation therapy was associated with significantly increased risk of men experiencing cardiovascular events.
Meaning
These results suggest that patients with prostate cancer should be counselled and monitored for an increased risk of cardiovascular events with initiation of androgen receptor signaling inhibitors alongside conventional hormonal therapy.
This systematic review and meta-analysis investigates the incidence of cardiovascular events with addition of novel androgen receptor signaling inhibitors (ARSI) to standard of care in locally advanced and metastatic prostate cancer.
Abstract
Importance
Cardiovascular (CV) events remain a substantial cause of mortality among men with advanced and metastatic prostate cancer (PCa). The introduction of novel androgen receptor signaling inhibitors (ARSI) has transformed the treatment landscape of PCa in recent years; however, their associated CV toxic effects remains unclear.
Objective
To assess the incidence of CV events with addition of ARSI to standard of care (SOC) in locally advanced (M0) and metastatic (M1) PCa.
Data Sources
Systematic searches of PubMed, Scopus, Web of Science, EMBASE, and ClinicalTrials.gov were performed from inception up to May 2023.
Study Selection
Randomized clinical trials of ARSI agents (abiraterone, apalutamide, darolutamide, enzalutamide) that reported CV events among individuals with M0 and M1, hormone-sensitive prostate cancer (HSPC) and castration-resistant prostate cancer (CRPC).
Data Extraction and Synthesis
A systematic review was performed in accordance with PRISMA guidance. Two authors screened and independently evaluated studies eligible for inclusion. Data extraction and bias assessment was subsequently performed.
Main Outcomes and Measures
A random-effects meta-analysis was performed to estimate risk ratios for the incidence of all grade and grade 3 or higher CV events (primary outcomes), in addition to hypertension, acute coronary syndrome (ACS), cardiac dysrhythmia, CV death, cerebrovascular event, and venous thromboembolism (secondary outcomes). Sources of heterogeneity were explored using meta-regression.
Results
There were 24 studies (n = 22 166 patients; median age range, 63-77 years; median follow-up time range, 3.9-96 months) eligible for inclusion. ARSI therapy was associated with increased risk of all grade CV event (risk ratio [RR], 1.75; 95% CI, 1.50-2.04; P < .001) and grade 3 or higher CV events (RR, 2.10; 95%, 1.72-2.55; P < .001). ARSI therapy also was associated with increased risk for grade 3 or higher events for hypertension (RR, 2.25; 95% CI, 1.74-2.90; P < .001), ACS (RR, 1.93; 95% CI, 1.43-1.60; P < .01), cardiac dysrhythmia (RR, 1.64; 95% CI, 1.23-2.17; P < .001), cerebrovascular events (RR, 1.86; 95% CI, 1.34-2.59; P < .001) and for CV-related death (RR, 2.02; 95% CI, 1.32-3.10; P = .001). Subgroup analysis demonstrated increased risk of all CV events across the disease spectrum (M0 HSPC: RR, 2.26; 95% CI, 1.36-3.75; P = .002; M1 HSPC: RR, 1.85; 95% CI, 1.47-2.31; P < .001; M0 CRPC: RR, 1.79; 95% CI, 1.13-2.81; P = .01; M1 CRPC: RR, 1.46; 95% CI, 1.16-1.83; P = .001).
Conclusions and Relevance
This systematic review and meta-analysis found that the addition of ARSIs to traditional ADT was associated with increased risk of CV events across the prostate cancer disease spectrum. These results suggest that patients with prostate cancer should be advised about and monitored for the potential of increased risk of CV events with initiation of ARSI therapy alongside conventional hormonal therapy.
Introduction
Androgen deprivation therapy (ADT) is a key component in standard of care (SOC) for men with locally advanced or metastatic prostate cancer (PCa). More recently, the addition of novel androgen receptor signaling inhibitors (ARSI) to ADT used in combination with local site radiotherapy (RT), when appropriate, has resulted in substantial benefits in survival in locally advanced (M0) and metastatic (M1) PCa.1,2,3 Since 2012, more than half a million patients have been prescribed ARSI agents.4 It is therefore essential to understand whether improvement in survival attributed to intensified treatment is also associated with emergence of serious adverse effects.
Cardiovascular (CV) events cause substantial morbidity and mortality among men with PCa.5,6 Several factors contribute to this increased risk of CV events, of which, alterations in lipid profile, altered body composition and preexisting CV disease are most well defined.7,8,9,10 ADT monotherapy has known adverse effects, of which CV toxic effects are most critical,11 though current evidence linking ADT use to CV toxic effects is conflicting.12,13 Given that addition of ARSI agents to traditional ADT has resulted in improved survival, patients with PCa using combination therapies are exposed to an increased period of intense androgen deprivation. Therefore, there is a greater need to better understand the risk of CV toxic effects with combination therapy.
Previous meta-analyses of randomized clinical trials in this setting have largely focused on oncological end points, rather than adverse events (AEs) including CV events in sufficient detail.14,15,16,17,18,19 Furthermore, the data do not currently reflect the nature of contemporary treatments in modern clinical trials, where ARSI agents are increasingly used in earlier stages of disease.20,21,22 There is therefore need for a contemporary meta-analysis that synthesizes the most recent clinical trial data on the CV outcomes of combination ARSI therapy across the spectrum of PCa to provide an evidence-based framework to inform clinical decision-making.
We therefore evaluated the risk of CV events with ADT intensification with ARSIs among patients with high-risk nonmetastatic hormone-sensitive prostate cancer (M0 HSPC), metastatic hormone-sensitive prostate cancer (M1 HSPC), nonmetastatic castration-resistant prostate cancer (M0 CRPC), and metastatic castration-resistant prostate cancer (M1 CRPC).
Methods
Protocol and Registration
This systematic review and meta-analysis was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplement 1).23 A protocol for this study was registered and published in advance on the International Prospective Register of Systematic Reviews (PROSPERO Registration ID CRD 408514).
Search Strategy
Systematic searches of PubMed, Scopus, Web of Science, EMBASE, and ClinicalTrials.gov were performed to identify randomized clinical trials (RCTs) assessing ARSI agents in the treatment of PCa, from inception through to May 2023 (eTable 1 in Supplement 1). Two authors individually performed initial screening (O.E., S.T.) and independently evaluated studies eligible for inclusion. Any disagreements were resolved by consensus in discussion with a third author (A.S.).
Inclusion and Exclusion Criteria
Eligible RCTs including patients with M0 HSPC, M1 HSPC, M0 CRPC and M1 CRPC treated with novel (abiraterone acetate) and second-generation (enzalutamide, apalutamide, darolutamide) ARSIs and either a placebo or SOC as per trial protocol. All nonrandomized studies and those including an ARSI agent in the control group were excluded. Where multiple published reports were available for the same trial, the most recent and updated version was included in the final analysis. Prior and concurrent docetaxel use was considered acceptable for inclusion.
Outcome Measures
The primary outcomes were all grade and grade 3 or higher composite CV events. To classify as a composite event, a study was required to report on more than 1 CV condition. Secondary outcomes included clinically relevant conditions subclassified within the CV disease spectrum, including the presence of grade 3 or higher AEs for hypertension, acute coronary syndrome (ACS), cardiac dysrhythmia, cerebrovascular event, and venous thromboembolism and CV-related death. These terms were defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and confirmed by consulting the European guidelines for CV disease, as the onset of any adverse CV event (eTable 2 in Supplement 1).24,25 AEs were cross-referenced with ClinicalTrials.gov when not reported.
Data Extraction and Quality Assessment
Baseline characteristics, interventions, and follow-up duration as well as primary and secondary outcome data were independently extracted by authors (O.E., S.T.). Authors were contacted in the case of missing data. Assessment of study quality and risk of bias was performed independently by the same 2 authors using the Cochrane Handbook for Systematic Reviews of Interventions Risk of Bias tool (RoB version 2.0), and results were visualized using the Robvis package26 in R version 4.1.2 (R Project for Statistical Computing). Assessment of small study effects was visualized using funnel plots and quantified using the Egger test for funnel plot asymmetry.27 Quality of evidence and strength of recommendation was assessed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.28
Statistical Analysis
A pairwise meta-analysis was performed using the meta package in R. Dichotomous data were analyzed using the Mantel-Haenszel–weighted random-effects model with the restricted maximum likelihood estimator of τ2, regardless of calculated heterogeneity, and expressed as a risk ratio (RR) with 95% CI. Statistical heterogeneity was quantified using Cochran Q test and prediction intervals with inconsistency between studies measured using the I2 statistic. Statistical analyses used a 2-sided method: P < .05 was considered statistically significant.
Subgroup and Sensitivity Analysis
A preplanned subgroup analysis based on disease state and type of ARSI treatment was conducted. Sensitivity analyses were performed to investigate the potential effects of uncertainties related to evidence selection. Weighted meta-regression analysis with a random-effects model was performed to quantify the potential moderating influence of participant and trial characteristics. We analyzed the association of age, ECOG performance status, follow-up duration, combination ARSI treatment, and prior or protocol docetaxel with the estimated log RR of CV events. Restricted maximum likelihood with Hartung and Knapp adjustment was used to obtain the F test statistic and P value.
Results
Studies Evaluated
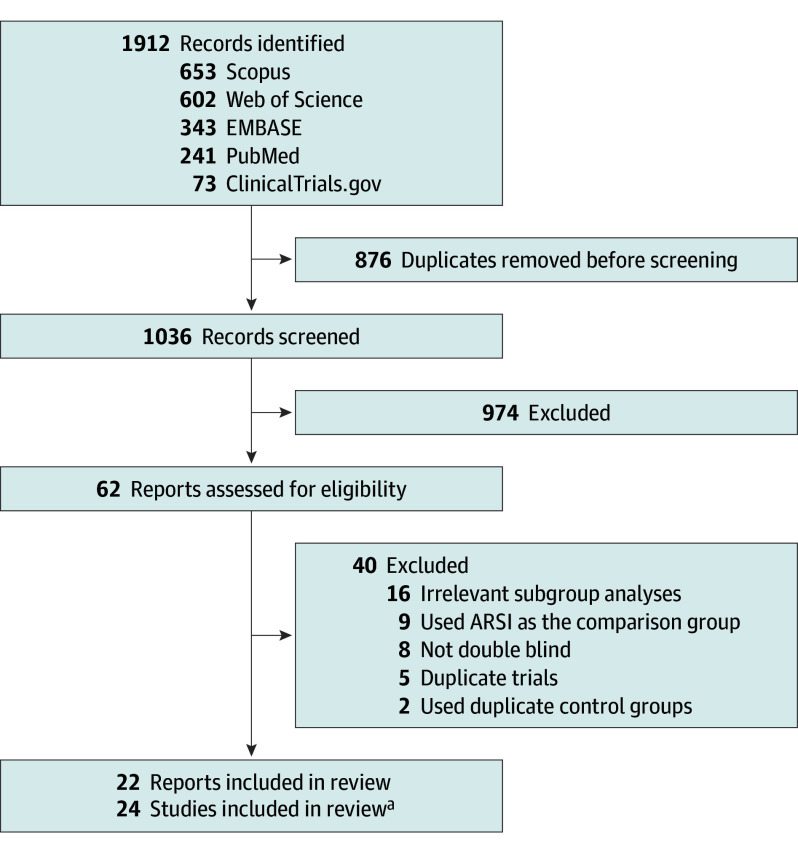
Data from 24 eligible RCTs (n = 22 166) were included (Figure 1); M0 HSPC (3 RCTs, n = 2171),1,29 M1 HSPC (9 RCTs, n = 8994),2,30,31,32,33,34,35,36 M0 CRPC (3 RCTs, n = 4256),37,38,39 M1 CRPC (9 RCTs, n = 6745).40,41,42,43,44,45,46,47,48 One study had patients spanning different disease states, so these populations were split for analysis according to their separate publications.45,49 The multiarm STAMPEDE trial presented data from 4 groups in 2 publications; these studies were considered separately given their different disease populations and control groups.1,2 The median follow-up ranged from 3.9 to 96 months, and median age ranged from 63 to 77 years (Table; eTable 3 in Supplement 1). CV-related AEs were reported using CTCAE criteria for all but 1 study which did not specify the terminology criteria used.42
Figure 1. PRISMA Flow Diagram.
ARSI indicates androgen receptor signaling inhibitors.
aFour trials from the STAMPEDE trial platform were reported across 2 reports.
Table. Characteristics of Included Studies.
| Study and phase | Year | No. of patients, (ITT) | Treatment | Age, median (range), y | Median follow-up, mo | Grade 1-5 CV events (%) | Grade ≥3 CV events (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |||
| M0 HSPC | ||||||||||||
| STAMPEDE M0 (Comparison AG)1 phase 3 | 2022 | 459 | 455 | Abiraterone acetate + prednisolone + ADT | Placebo + ADT | 68 (44-84) | 67 (48-83) | 72.0 | 155 (34.3) | 90 (19.8) | 31 (6.9) | 11 (2.41) |
| STAMPEDE M0 (Comparison AJ)1 phase 3 | 2022 | 527 | 533 | Abiraterone acetate + prednisolone + Enza + ADT | Placebo + ADT | 68 (46-86) | 69 (64-73) | 60.0 | 303 (59.0) | 108 (20.3) | 82 (15.9) | 19 (3.6) |
| Spetsieris et al29 phase 2 | 2021 | 99 | 98 | Abiraterone acetate + prednisolone + ADT | Placebo + prednisolone + ADT | 65 (44-80) | 65 (42-85) | 64.4 | NR | NR | NR | NR |
| M1 HSPC | ||||||||||||
| STAMPEDE M1 (Comparison AJ)2 phase 3 | 2023 | 462 | 454 | Abiraterone acetate + prednisolone + enzalutamide + ADT | Placebo + prednisolone + ADT | 69 (48-84) | 68 (37-83) | 72.0 | 347 (77.9) | 121 (26.7) | 109 (24.5) | 29 (6.4) |
| STAMPEDE M1 (Comparison AG)2 phase 3 | 2023 | 501 | 502 | Abiraterone acetate + prednisolone + ADT | Placebo + ADT | 67 (42-85) | 67 (39-84) | 96.0 | 290 (58.2) | 123 (24.5) | 60 (12.0) | 18 (3.6) |
| ENZAMET30 phase 3 | 2023 | 563 | 562 | Enzalutamide + ADT | ADT + NSAA | 69 (IQR, 63-74) | 69 (IQR, 64-75) | 68.0 | 301 (53.5) | 175 (31.4) | 116 (20.6) | 70 (12.5) |
| ARCHES31 phase 3 | 2022 | 574 | 576 | Enzalutamide + ADT | ADT + Placebo | 70 (46-92) | 70 (42-92) | 44.6 | 144 (25.2) | 69 (12.0) | 57 (9.9) | 39 (6.8) |
| ARASENS32 phase 3 | 2022 | 651 | 654 | Darolutamide + docetaxel +ADT | Placebo + docetaxel + ADT | 67 (41-89) | 67 (42-86) | 43.7 | 177 (27.0) | 147 (22.6) | NR | NR |
| PEACE-133 phase 3 | 2022 | 583 | 589 | Abiraterone acetate + prednisolone + ADT ± docetaxel ± RTx | Placebo + ADT ± docetaxel ± RTx | 67 (37-94) | 66 (43-87) | 42.0 | NR | NR | NR | NR |
| TITAN34 phase 3 | 2021 | 525 | 527 | Apalutamide + ADT | Placebo + ADT | 68 (45-94) | 69 (43-90) | 44.0 | 152 (29.0) | 107 (20.3) | 72 (13.7) | 55 (10.4) |
| LATITUDE35 phase 3 | 2019 | 597 | 602 | Abiraterone acetate + prednisolone + ADT | Placebo + prednisolone + ADT | 66 (8.5)a | 67 (8.7)a | 51.8 | 339 (56.8) | 201 (33.4) | 160 (26.8) | 80 (13.2) |
| Vaishampayan et al36 phase 2 | 2021 | 36 | 35 | Enzalutamide + ADT | Bicalutamide + ADT | 66 (54-86) | 63 (51-84) | 39.0 | NR | NR | 3 (8.6) | 1 (2.9) |
| M0 CRPC | ||||||||||||
| SPARTAN37 phase 3 | 2021 | 806 | 401 | Apalutamide + ADT | Placebo + ADT | 74 (48-94) | 74 (52-97) | 52.0 | NR | NR | 172 (21.4) | 59 (14.8) |
| PROSPER38 phase 3 | 2020 | 933 | 468 | Enzalutamide + ADT | Placebo + ADT | 74 (50-95) | 73 (53-92) | 48.0 | 227 (24.4) | 50 (10.8) | NR | NR |
| ARAMIS39 phase 3 | 2020 | 955 | 554 | Darolutamide+ ADT | Placebo + ADT | 74 (48-95) | 74 (50-92) | 17.9 | 219 (22.9) | 89 (16.1) | 92 (9.6) | 28 (5.1) |
| STRIVE M049 phase 3 | 2022 | 70 | 69 | Enzalutamide + ADT | Bicalutamide + ADT | 73.5 (50-92) | 77 (58-91) | 17.0 | NR | NR | NR | NR |
| M1 CRPC | ||||||||||||
| PREVAIL Asia40 phase 3 | 2022 | 198 | 190 | Enzalutamide + ADT | Placebo + ADT | 71 (51-89) | 71 (50-88) | 60.0 | 31 (15.7) | 8 (4.2) | NR | NR |
| PREVAIL41 phase 3 | 2020 | 872 | 845 | Enzalutamide + ADT | Placebo + ADT | 71 (43-93) | 71 (42-93) | 69.0 | NR | NR | 155 (17.8) | 54 (6.4) |
| ABI-PRO-300242 phase 3 | 2017 | 157 | 156 | Abiraterone acetate + prednisolone + ADT | Placebo + prednisolone + ADT | 69.7 (8.7)a | 70.8 (8.7)a | 3.9 | 39 (24.8) | 28 (17.9) | 8 (5.1) | 4 (2.6) |
| TERRAIN43 phase 2 | 2016 | 184 | 191 | Enzalutamide + ADT | Placebo + ADT | 71 (50-96) | 71 (48-91) | 20.0b | 52 (28.4) | 23 (12.2) | 31 (16.9) | 14 (7.4) |
| Sun et al44 phase 3 | 2016 | 143 | 71 | Abiraterone acetate + prednisolone + ADT | Placebo + prednisolone + ADT | 68.2 (8.3)a | 67.7 (7.8)a | 12.9 | 38 (26.6) | 18 (25.4) | NR | NR |
| STRIVE M145 phase 3 | 2016 | 127 | 129 | Enzalutamide + ADT | Bicalutamide + ADT | 72 (46-92) | 74 (50-91) | 16.7 | NR | NR | NR | NR |
| COU-AA-30246 phase 3 | 2015 | 546 | 542 | Abiraterone acetate + prednisolone + ADT | Placebo + prednisolone + ADT | 71 (44-95) | 70 (44-90) | 49.2 | 311 (57.4) | 215 (39.8) | 106 (19.6) | 63 (11.7) |
| COU-AA-30147 phase 3 | 2012 | 797 | 398 | Abiraterone acetate + prednisolone + ADT | Placebo + prednisolone + ADT | 69 (42-95) | 69 (39-90) | 20.2 | 235 (29.7) | 91 (23.1) | 72 (9.1) | 23 (5.8) |
| AFFIRM48 phase 3 | 2012 | 800 | 399 | Enzalutamide + ADT | Placebo + ADT | 69 (41-92) | 69 (49-89) | 14.4 | 119 (14.9) | 54 (13.5) | NR | NR |
Abbreviations: ADT, androgen deprivation therapy; AG, groups A and G from the STAMPEDE trial; AJ, groups A and J from the STAMPEDE trial; CV, cardiovascular; CTCAE, Common Terminology Criteria for Adverse Events; ITT, intention to treat; NR, not reported; NSAA, nonsteroidal antiandrogen; M0 HSPC, nonmetastatic hormone-sensitive prostate cancer; M1 HSPC, metastatic hormone-sensitive prostate cancer; M1 CRPC, metastatic castration-resistant prostate cancer; M0 CRPC, nonmetastatic castration-resistant prostate cancer; RR, risk ratio; RT, radiotherapy.
Mean (SD) are reported.
Median follow-up in the control group was 16.7 months.
Studies investigated SOC used with ARSI monotherapy or combined with another ARSI or docetaxel. The following agents were combined with SOC therapy: abiraterone acetate and prednisolone (herein referred to as abiraterone acetate) (n = 9),1,2,29,33,35,42,44,46,47 abiraterone acetate and docetaxel (n = 1),33 enzalutamide alone (n = 9),30,31,36,38,40,41,43,45,48,49 abiraterone acetate and enzalutamide (n = 2),1,2 darolutamide alone (n = 1),39 darolutamide and docetaxel (n = 1),32 and apalutamide alone (n = 2)34,37 were studied. Of the 24 eligible studies, 18 reported CV events (75%): only 16 reported grade 3 or higher CV events (67%).
Quality Assessment
The overall risk of bias was low (eFigure 1 in Supplement 1). However, among 24 eligible studies, 6 (25%) did not report all grade CV events, and 8 (33%) did not report grade 3 or higher CV events. Nonetheless, only 3 studies were deemed at high risk of bias due to missing outcome data.29,33,45 Funnel plots were grossly symmetric across all variables (eFigure 2 in Supplement 1). Based on linear regression, Egger statistic did not demonstrate significant funnel plot asymmetry for either all grade or grade 3 or higher CV events.
CV Event Risk With ARSI Therapy
All Grade CV Events
The incidence of all grade CV events was 22.0% (1717 of 7813) in patients receiving SOC and 36.6% (3479 of 9513) with addition of an ARSI (n = 18 RCTs, n = 17 326 patients; RR, 1.75; 95% CI, 1.50-2.04; P < .001) (Figure 2; eFigure 3 in Supplement 1). An increased risk of all grade CV events was associated with addition of an ARSI across all disease states: M0 HSPC (RR, 2.26; 95% CI, 1.36-3.75; P = .002), M1 HSPC (RR, 1.85; 95% CI, 1.47-2.31; P < .001), M0 CRPC (RR, 1.79; 95% CI, 1.13-2.81; P = .01), and M1 CRPC (RR, 1.46; 95% CI, 1.16-1.83; P = .001). There was no significant difference in the pooled RR across disease states (χ2 = 3.49; P = .32).
Figure 2. Meta-Analysis of Relative Risk for All Grade Cardiovascular Events Associated With Commencement of Androgen Receptor Signaling Inhibitors (ARSI) Therapy.
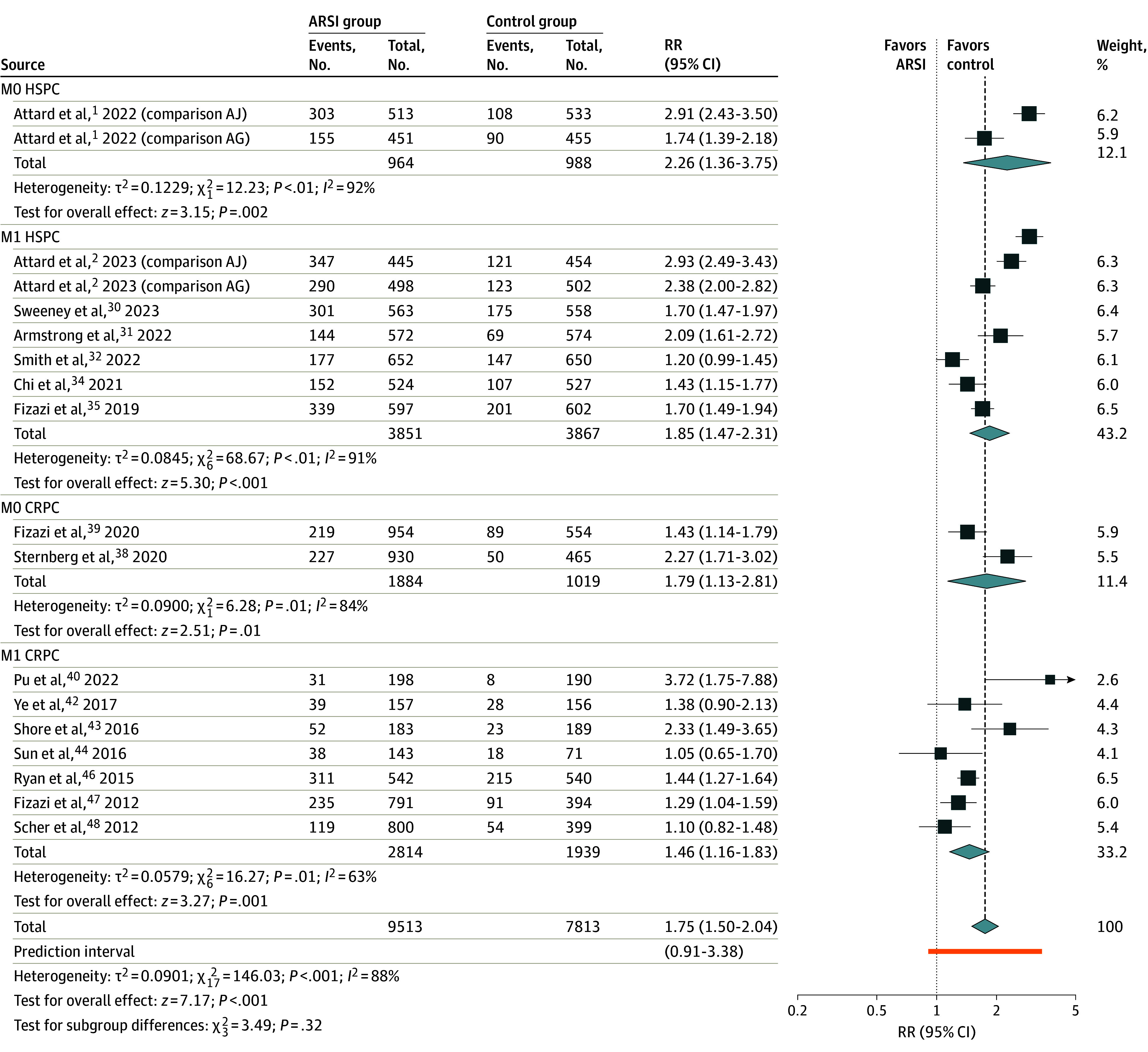
AG indicates groups A and G from the STAMPEDE trial; AJ, groups A and J from the STAMPEDE trial; CRPC, castration-resistant prostate cancer; HSPC, hormone-sensitive prostate cancer; M0, locally advanced; M1, metastatic; RR, risk ratio.
Increased risk of CV events was observed across all ARSI agents, although for apalutamide, there was only a single study available for comparison (eFigure 4 in Supplement 1). Subgroup differences were noted (χ2 = 78.30, P < .001) with highest CV event rate observed with a combination of abiraterone acetate and enzalutamide (RR, 2.92; 95% CI, 2.59-3.30; P < .001), and lowest CV event rate with darolutamide (RR, 1.30; 95% CI, 1.09-1.54; P = .003).
Grade 3 or Higher CV Events
ARSI use was associated with increased risk of grade 3 or higher CV events, from 7.8% to 15.6% (16 RCTs: n = 15 813 patients; RR, 2.10; 95% CI, 1.72-2.55; P < .001) (Figure 3; eFigure 3 in Supplement 1). An association with increased grade 3 or higher CV events was observed across all disease subgroups: M0 HSPC (RR, 3.80; 95% CI, 2.48-5.84; P < .001), M1 HSPC (RR, 2.06; 95% CI, 1.47-2.87; P < .001), M0 CRPC (RR, 1.59; 95% CI, 1.23-2.06; P < .001), M1 CRPC (RR, 2.03; 95% CI, 1.53-2.68; P < .001). There was a significant difference in the pooled RR across disease states (χ2 = 11.72; P = .008).
Figure 3. Meta-Analysis of Relative Risk for Grade 3 or Higher Cardiovascular Events Associated With Commencement of Androgen Receptor Signaling Inhibitors (ARSI) Therapy.
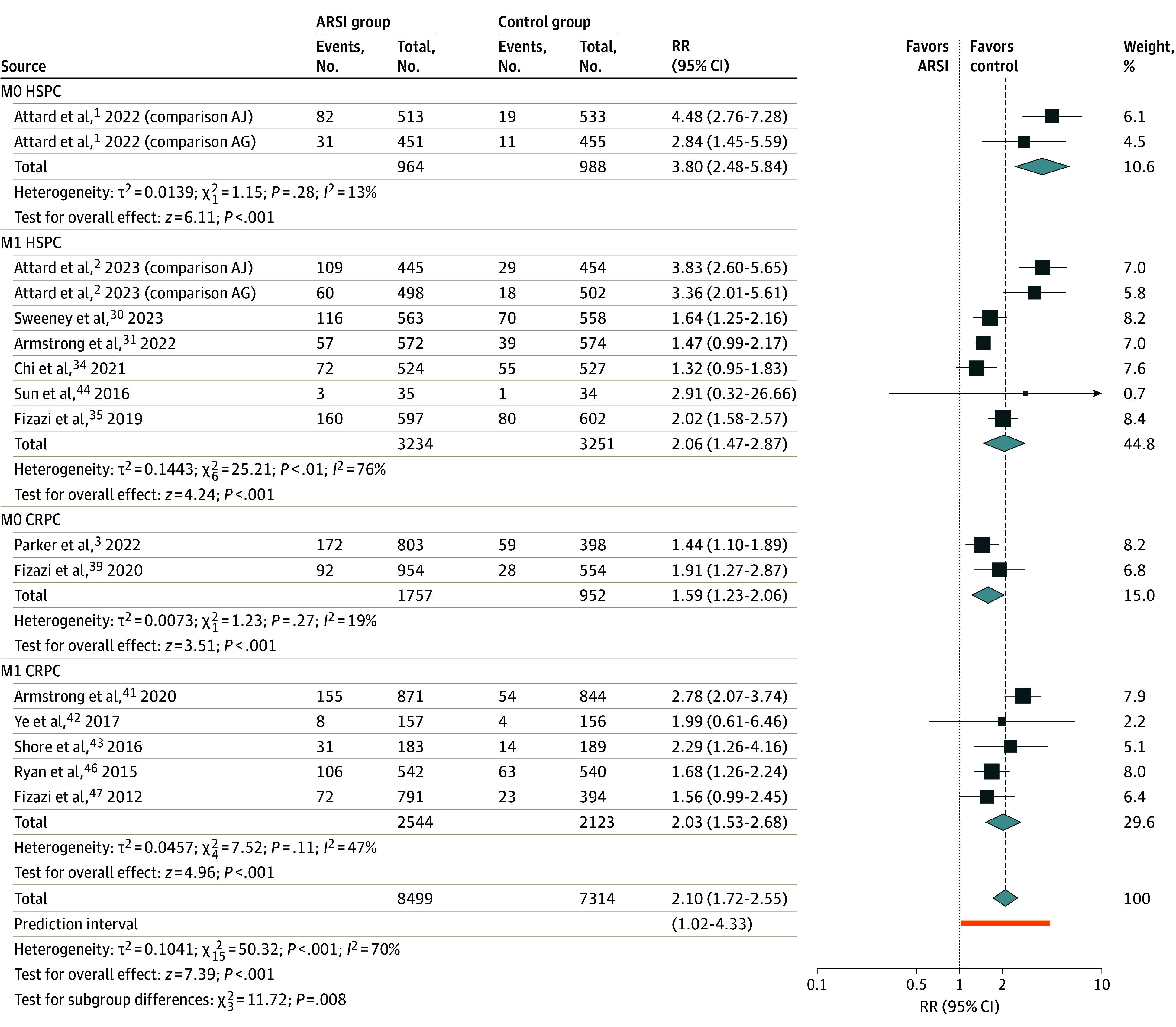
AG indicates groups A and G from the STAMPEDE trial; AJ, groups A and J from the STAMPEDE trial; CRPC, castration-resistant prostate cancer; HSPC, hormone-sensitive prostate cancer; M0, locally advanced; M1, metastatic; RR, risk ratio.
All ARSI agents were associated with an increased risk of grade 3 or higher CV events, although in patients taking darolutamide, there was only a single study available for comparison (eFigure 5 in Supplement 1). Subgroup differences were observed based on ARSI agent (χ2 = 32.87; P < .001). The greatest risk of grade 3 or higher CV events was observed among patients treated with a combination of abiraterone acetate and enzalutamide (RR, 4.08; 95% CI, 3.01-5.52; P < .001), and lowest risk with apalutamide (RR, 1.39; 95% CI, 1.13-1.72; P < .001).
Secondary Outcomes
Secondary outcomes of grade 3 or higher events within CV subcategories were available for most included studies: hypertension (n = 22 studies, 92%), ACS (n = 19 studies, 79%), cardiac dysrhythmia (n = 17 studies, 71%), cerebrovascular (n = 17 studies, 71%), CV-related death (n = 17 studies, 71%), and venous thromboembolism (n = 17 studies, 71%). ARSI use was associated with increased risk of grade 3 or higher hypertension (RR, 2.25; 95% CI, 1.74-2.90; P < .001), ACS (RR, 1.93; 95% CI, 1.43-1.60; P < .001), cardiac dysrhythmia (RR, 1.64; 95% CI, 1.23-2.17; P < .001), cerebrovascular events (RR, 1.86; 95% CI, 1.34-2.59; P < .001), and CV-related death (RR, 2.02; 95% CI, 1.32-3.10; P = .001). There was, however, no difference in the RR of grade 3 or higher venous thromboembolism with ARSI use (Figure 4; eFigure 3 in Supplement 1).
Figure 4. Meta-Analysis of Relative Risk for Secondary Outcomes Associated With Commencement of Androgen Receptor Signaling Inhibitors (ARSI) Therapy.
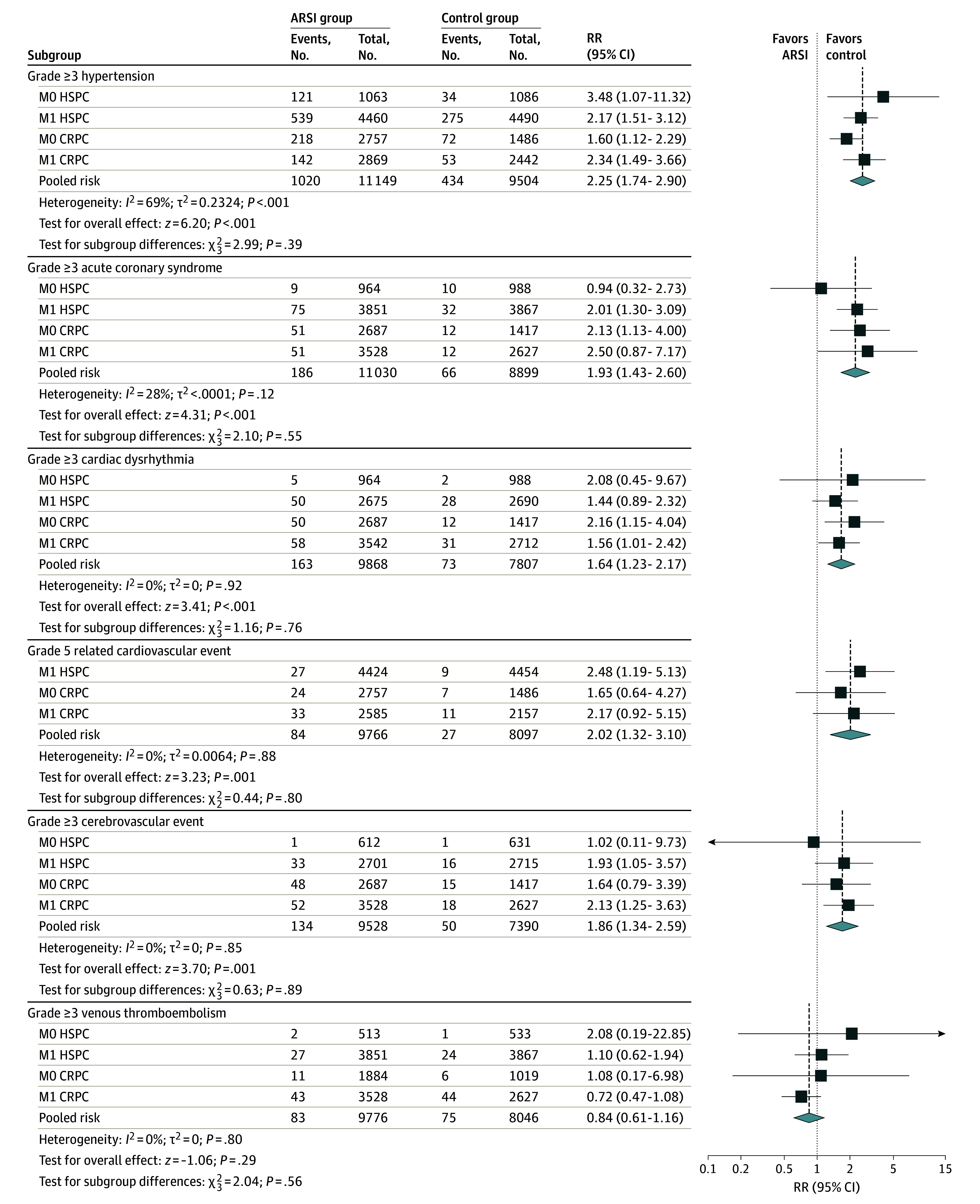
CRPC indicates castration-resistant prostate cancer; HSPC, hormone-sensitive prostate cancer; M0, locally advanced; M1, metastatic; RR, risk ratio.
Sensitivity Analysis
Substantial heterogeneity was observed in the RR of all grade (I2 = 88%, P < .001) and grade 3 or higher (I2 = 70%, P < .001) CV events. Previous studies on docetaxel use reported a possible increase in CV toxic effects.50 Three trials30,32,33 administered docetaxel as part of SOC, while 7 permitted the use of early or prior docetaxel.2,31,34,44,47,48 We found no statistically significant subgroup difference attributed to docetaxel exposure for all grade (χ2 = 1.02, P = .31) and grade 3 or higher (χ2 = 0.24, P = .62) CV events (eFigure 6 in Supplement 1).
Combination therapy with doublet ARSI was investigated in the STAMPEDE trial using a combination of abiraterone acetate and enzalutamide in both M0 HSPC and M1 HSPC settings.1,2 We found a statistically significant subgroup difference attributed to combination ARSI use for both all grade (χ2 = 42.78, P < .001) and grade 3 or higher (χ2 = 20.29, P < .001) (eFigure 6 in Supplement 1) CV events.
Furthermore, metaregression analysis suggested that length of follow-up and combination ARSI use were significantly associated with a higher RR of CV events among ARSI-treated patients (eTable 4 in Supplement 1).
Quality of Evidence
The quality of evidence was summarized using the GRADE approach (eTable 5 in Supplement 1). We judged the certainty of evidence as moderate for all grade CV events and high for grade 3 or higher CV events based on high-quality RCTs, low risk of bias, sample size, and the magnitude of treatment effect.
Discussion
To our knowledge, this is the largest meta-analysis highlighting the increased risk of CV toxic effects associated with ARSI therapy across the PCa disease spectrum. The inclusion of contemporary HSPC trials made it possible, for the first time, to demonstrate a 2-fold increase in CV morbidity associated with the addition of an ARSI, and up to a 4-fold risk associated with the combined use of 2 ARSIs. We observed an increased risk of experiencing grade 3 or higher hypertension, ACS, cardiac dysrhythmia, and cerebrovascular events. Notably, a 2-fold increase in risk of a CV-related death was also observed with addition of an ARSI, though absolute risk of CV-related death was low. These findings are consistent with previous retrospective and observational studies49,50 and should be discussed while counselling patients when commencing ARSI therapies.
Our results provide robust data compared with previous smaller meta-analyses based predominantly on CRPC trials.18,19,20,21,22 We observed a higher RR of CV events with ARSI therapy in comparison with previous studies, which may be due to longer trial follow-up, inclusion of additional HSPC studies, and variations in definitions of CV toxic effects used across studies. Uniquely, we report contemporary data from 12 HSPC trials, highlighting increased CV risk with earlier initiation of ARSI therapy compared with CRPC. One potential explanation for this finding may be a greater lifetime exposure to intensive androgen suppression with use of ARSI therapy in the HSPC setting, and the inclusion of recent studies using dual ARSI therapy.
Our study highlights the potential variation in CV events among different therapeutic antiandrogenic drug combinations. We found that treatment with enzalutamide and abiraterone acetate together was the most cardiotoxic, followed by use of either enzalutamide or abiraterone alone. In patients receiving dual ARSI treatment, we found that there was up to a 4-fold increase in grade 3 or higher CV events, which was also observed in the PLATO study.51 Both darolutamide and apalutamide were the least cardiotoxic agents based on currently available trial data. However, these results should be interpreted with caution as they may reflect patient selection and shorter trial follow-up, rather than a true difference in CV event risk with individual ARSI agents.
Physiological consequences of complete blockade of androgen production or androgenic action include insulin resistance, loss of muscle and bone mass, and an increase in fat distribution/composition, which are thought to accelerate the development of atherosclerosis and CV events.52 When applying these results to everyday clinical practice, it is important to understand that these drugs may affect patients with preexisting CV conditions more adversely than the clinical trial patient population, with health systems data illustrating up to 67% of patients receiving an ARSI have at least 1 CV comorbidity. Patients with 3 or more CV morbidities have been shown to experience a higher all-cause mortality than otherwise similar ARSI-treated patients without CV disease (RR 1.56; 95% CI, 1.29-1.88).53 This highlights the importance of conducting outcome evaluation among patients not meeting strict trial eligibility criteria in the clinical setting. Notably, 17.8% of ADT-treated patients with preexisting CV disease in the HERO trial experienced a major adverse CV event within 1 year of treatment initiation,54 and 71% of patients who experienced a fatal CV event in the PROSPER trial had a history of CV disease.38 Risk scores, such as the Framingham Risk Score or QRISK2 score, have previously been used to estimate 10-year CV event risk in patients on ADT.55,56 There is, however, a clear need for further data to stratify patients planned to commence ARSIs by preexisting comorbidities. By estimating baseline CV risk, clinicians can assess additional risks posed by ARSI therapy and consider appropriate management strategies depending on individual patient factors, including the potential future requirement for CV medications and their possible drug-drug interactions.57,58,59 It is clear from our results that this ethos of risk management is even more important when considering combination ADT/ARSI use. Thus, due to the current lack of supporting evidence, comprehensive baseline CV risk assessment and implementation of evidence-based preventative strategies are crucial prior to initiating ARSI treatment in patients with preexisting CV risk factors. This highlights the importance of a multidisciplinary approach involving cancer physicians, primary care physicians and cardio-oncologists to ensure longitudinal CV care, especially for patients at increased CV risk.
Limitations
This study’s results should be interpreted in the context of the following limitations. First, we observed substantial heterogeneity despite inclusion of only RCT data and stratification by disease subtype. Potential explanations may include variations in collection of AE data, follow-up frequency, and follow-up duration between trials. We observed potential risk of bias due to method of AE reporting or missing outcome data among some studies. Where feasible, we attempted to account for such heterogeneity statistically and highlighted a greater frequency of CV events with longer trial follow-up. Second, CV outcomes were not consistently prespecified across all studies, including 2 studies where CTCAE v3.0 was used,46,47 which may potentially bias measurement of these outcomes. However, we believe that these factors would be equally balanced between treatment and control groups and therefore unlikely to substantially affect our findings. Third, improved survival with ARSIs could manifest as a higher captured incidence of CV events. It is impossible to account for this given lack of time to event data and may be addressed in future studies using competing risks analysis as part of individual patient data meta-analyses. Additionally, we were also unable to account for established baseline CV risk factors from a majority of the trials and expect a higher frequency of preexisting CV morbidity resulting in an increased risk of CV events in the clinical setting.
Conclusions
This systemic review and meta-analysis provides up-to-date evidence of increased CV events in patients with PCa receiving ARSI with conventional ADT. As use of combination therapy becomes more widespread, particularly in earlier disease settings, there is greater need to evaluate and optimize baseline CV risk prior to commencing ARSI therapy.
eText. Search Strategy
eTable 1. CTCAE v4.0 Definitions for Classifying CV Adverse Events
eTable 2. Characteristics of Included Studies
eTable 3. Meta-Regression Analysis
eTable 4. GRADE Assessment for Certainty of Evidence
eFigure 1. RoB2 Tool Assessing Risk of Bias for CV Events
eFigure 2. Funnel Plots for the Examination of Small Study Effects for (A) All Grade CV Events and (B) Grade ≥ 3 CV Events
eFigure 3. Incidence Rates for (A) Primary Outcomes and (B) Secondary Outcomes
eFigure 4. Sub-Group Analysis of All Grade CV Events Based on ARSI
eFigure 5. Sub-Group Analysis of Grade ≥3 CV Events Based on ARSI
eFigure 6. Sensitivity Analysis of (A) All Grade CV Events and (B) Grade ≥3 CV Events
Data Sharing Statement
References
- 1.Attard G, Murphy L, Clarke NW, et al. ; Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators . Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447-460. doi: 10.1016/S0140-6736(21)02437-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attard G, Murphy L, Clarke NW, et al. ; STAMPEDE investigators . Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023;24(5):443-456. doi: 10.1016/S1470-2045(23)00148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker CC, James ND, Brawley CD, et al. ; STAMPEDE Trial Collaborative Group . Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: long-term results from the STAMPEDE randomised controlled trial. PLoS Med. 2022;19(6):e1003998. doi: 10.1371/journal.pmed.1003998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IMS Institute for Healthcare Informatics . Global oncology trend report: a review of 2015 and outlook to 2020. Published June 2016. Accessed May 7, 2024. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trend-report-2016.pdf
- 5.Veccia A, Maines F, Kinspergher S, Galligioni E, Caffo O. Cardiovascular toxicities of systemic treatments of prostate cancer. Nat Rev Urol. 2017;14(4):230-243. doi: 10.1038/nrurol.2016.273 [DOI] [PubMed] [Google Scholar]
- 6.Epstein MM, Edgren G, Rider JR, Mucci LA, Adami HO. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104(17):1335-1342. doi: 10.1093/jnci/djs299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berardi R, Caramanti M, Savini A, et al. State of the art for cardiotoxicity due to chemotherapy and to targeted therapies: a literature review. Crit Rev Oncol Hematol. 2013;88(1):75-86. doi: 10.1016/j.critrevonc.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 8.Edelman S, Butler J, Hershatter BW, Khan MK. The effects of androgen deprivation therapy on cardiac function and heart failure: implications for management of prostate cancer. Clin Genitourin Cancer. 2014;12(6):399-407. doi: 10.1016/j.clgc.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 9.Su JJ, Park SK, Hsieh TM. The effect of testosterone on cardiovascular disease: a critical review of the literature. Am J Mens Health. 2014;8(6):470-491. doi: 10.1177/1557988314522642 [DOI] [PubMed] [Google Scholar]
- 10.O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243-1251. doi: 10.1200/JCO.2014.59.1792 [DOI] [PubMed] [Google Scholar]
- 11.Gandaglia G, Sun M, Popa I, et al. Cardiovascular mortality in patients with metastatic prostate cancer exposed to androgen deprivation therapy: a population-based study. Clin Genitourin Cancer. 2015;13(3):e123-e130. doi: 10.1016/j.clgc.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 12.Muniyan S, Xi L, Datta K, et al. Cardiovascular risks and toxicity - the Achilles heel of androgen deprivation therapy in prostate cancer patients. Biochim Biophys Acta Rev Cancer. 2020;1874(1):188383. doi: 10.1016/j.bbcan.2020.188383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen PL, Je Y, Schutz FAB, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306(21):2359-2366. doi: 10.1001/jama.2011.1745 [DOI] [PubMed] [Google Scholar]
- 14.Rydzewska LHM, Burdett S, Vale CL, et al. ; STOPCaP Abiraterone Collaborators . Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur J Cancer. 2017;84:88-101. doi: 10.1016/j.ejca.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menges D, Yebyo HG, Sivec-Muniz S, et al. Treatments for metastatic hormone-sensitive prostate cancer: systematic review, network meta-analysis, and benefit-harm assessment. Eur Urol Oncol. 2022;5(6):605-616. doi: 10.1016/j.euo.2022.04.007 [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Mostafaei H, Sari Motlagh R, et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int. 2022;129(4):423-433. doi: 10.1111/bju.15507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao B, Kim M, Reizine NM, Moreira DM. Adverse events and androgen receptor signaling inhibitors in the treatment of prostate cancer: a systematic review and multivariate network meta-analysis. Eur Urol Oncol. 2023;6(3):237-250. doi: 10.1016/j.euo.2023.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Moreira RB, Debiasi M, Francini E, et al. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8(48):84572-84578. doi: 10.18632/oncotarget.20028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roviello G, Sigala S, Danesi R, et al. Incidence and relative risk of adverse events of special interest in patients with castration resistant prostate cancer treated with CYP-17 inhibitors: a meta-analysis of published trials. Crit Rev Oncol Hematol. 2016;101(101):12-20. doi: 10.1016/j.critrevonc.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 20.Iacovelli R, Ciccarese C, Bria E, et al. The Cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645-e653. doi: 10.1016/j.clgc.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 21.Lee HY, Chen HL, Teoh JYC, et al. Abiraterone and enzalutamide had different adverse effects on the cardiovascular system: a systematic review with pairwise and network meta-analyses. Prostate Cancer Prostatic Dis. 2021;24(1):244-252. doi: 10.1038/s41391-020-00275-3 [DOI] [PubMed] [Google Scholar]
- 22.Morgans AK, Shore N, Cope D, et al. Androgen receptor inhibitor treatments: cardiovascular adverse events and comorbidity considerations in patients with non-metastatic prostate cancer. Urol Oncol. 2021;39(1):52-62. doi: 10.1016/j.urolonc.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Online). 2021;372:n71. [DOI] [PMC free article] [PubMed]
- 24.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute . Common terminology criteria for adverse events (CTCAE). Published November 27, 2017. Accessed May 8, 2024. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- 25.Visseren FLJ, Mach F, Smulders YM, et al. ; ESC National Cardiac Societies; ESC Scientific Document Group . 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 26.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Springer; 2015. doi: 10.1007/978-3-319-21416-0 [DOI] [Google Scholar]
- 27.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spetsieris N, Boukovala M, Alafis I, et al. Abiraterone acetate plus prednisone in non-metastatic biochemically recurrent castration-naïve prostate cancer. Eur J Cancer. 2021;157(157):259-267. doi: 10.1016/j.ejca.2021.06.017 [DOI] [PubMed] [Google Scholar]
- 30.Sweeney CJ, Martin AJ, Stockler MR, et al. ; ENZAMET trial investigators and Australian and New Zealand Urogenital and Prostate Cancer Trials Group . Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323-334. doi: 10.1016/S1470-2045(23)00063-3 [DOI] [PubMed] [Google Scholar]
- 31.Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616-1622. doi: 10.1200/JCO.22.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith MR, Hussain M, Saad F, et al. ; ARASENS Trial Investigators . Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132-1142. doi: 10.1056/NEJMoa2119115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fizazi K, Foulon S, Carles J, et al. ; PEACE-1 investigators . Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695-1707. doi: 10.1016/S0140-6736(22)00367-1 [DOI] [PubMed] [Google Scholar]
- 34.Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294-2303. doi: 10.1200/JCO.20.03488 [DOI] [PubMed] [Google Scholar]
- 35.Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700. doi: 10.1016/S1470-2045(19)30082-8 [DOI] [PubMed] [Google Scholar]
- 36.Vaishampayan UN, Heilbrun LK, Monk P III, et al. Clinical efficacy of enzalutamide vs bicalutamide combined with androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a randomized clinical trial. JAMA Netw Open. 2021;4(1):e2034633. doi: 10.1001/jamanetworkopen.2020.34633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150-158. doi: 10.1016/j.eururo.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 38.Sternberg CN, Fizazi K, Saad F, et al. ; PROSPER Investigators . Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi: 10.1056/NEJMoa2003892 [DOI] [PubMed] [Google Scholar]
- 39.Fizazi K, Shore N, Tammela TL, et al. ; ARAMIS Investigators . Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. doi: 10.1056/NEJMoa2001342 [DOI] [PubMed] [Google Scholar]
- 40.Pu YS, Ahn H, Han W, et al. Enzalutamide in chemotherapy-naïve metastatic castration-resistant prostate cancer: an Asian multiregional, randomized study. Adv Ther. 2022;39(6):2641-2656. doi: 10.1007/s12325-022-02140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong AJ, Lin P, Tombal B, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol. 2020;78(3):347-357. doi: 10.1016/j.eururo.2020.04.061 [DOI] [PubMed] [Google Scholar]
- 42.Ye D, Huang Y, Zhou F, et al. A phase 3, double-blind, randomized placebo-controlled efficacy and safety study of abiraterone acetate in chemotherapy-naïve patients with mCRPC in China, Malaysia, Thailand and Russia. Asian J Urol. 2017;4(2):75-85. doi: 10.1016/j.ajur.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shore NDD, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153-163. doi: 10.1016/S1470-2045(15)00518-5 [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Zou Q, Sun Z, et al. Abiraterone acetate for metastatic castration-resistant prostate cancer after docetaxel failure: a randomized, double-blind, placebo-controlled phase 3 bridging study. Int J Urol. 2016;23(5):404-411. doi: 10.1111/iju.13051 [DOI] [PubMed] [Google Scholar]
- 45.Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098-2106. doi: 10.1200/JCO.2015.64.9285 [DOI] [PubMed] [Google Scholar]
- 46.Ryan CJP, Smith MRP, Fizazi K, et al. ; COU-AA-302 Investigators . Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152-160. doi: 10.1016/S1470-2045(14)71205-7 [DOI] [PubMed] [Google Scholar]
- 47.Fizazi K, Scher HIP, Molina A, et al. ; COU-AA-301 Investigators . Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983-992. doi: 10.1016/S1470-2045(12)70379-0 [DOI] [PubMed] [Google Scholar]
- 48.Scher HI, Fizazi K, Saad F, et al. ; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 49.Penson DF, Armstrong AJ, Concepcion RS, et al. Enzalutamide versus bicalutamide in patients with nonmetastatic castration-resistant prostate cancer: a prespecified subgroup analysis of the STRIVE trial. Prostate Cancer Prostatic Dis. 2022;25(2):363-365. doi: 10.1038/s41391-021-00465-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilk M, Waśko-Grabowska A, Szmit S. Cardiovascular complications of prostate cancer treatment. Front Pharmacol. 2020;11:555475. doi: 10.3389/fphar.2020.555475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attard G, Borre M, Gurney H, et al. ; PLATO collaborators . Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol. 2018;36(25):2639-2646. doi: 10.1200/JCO.2018.77.9827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2013;189(1)(suppl):S34-S42. doi: 10.1016/j.juro.2012.11.017 [DOI] [PubMed] [Google Scholar]
- 53.Lu-Yao G, Nikita N, Keith SW, et al. Mortality and hospitalization risk following oral androgen signaling inhibitors among men with advanced prostate cancer by pre-existing cardiovascular comorbidities. Eur Urol. 2020;77(2):158-166. doi: 10.1016/j.eururo.2019.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shore ND, Saad F, Cookson MS, et al. ; HERO Study Investigators . Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187-2196. doi: 10.1056/NEJMoa2004325 [DOI] [PubMed] [Google Scholar]
- 55.Leong DP, Fradet V, Shayegan B, et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC Study. J Urol. 2020;203(6):1109-1116. doi: 10.1097/JU.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 56.Ndjavera W, Orange ST, O’Doherty AF, et al. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2020;125(1):28-37. doi: 10.1111/bju.14922 [DOI] [PubMed] [Google Scholar]
- 57.Davey P, Alexandrou K. Assessment and mitigation of cardiovascular risk for prostate cancer patients: a review of the evidence. International Journal of Clinical Practice (Esher). 2022;2022:2976811-2976813. [DOI] [PMC free article] [PubMed]
- 58.Chan JSK, Satti DI, Lee YHA, et al. Temporal trends in cardiovascular burden among patients with prostate cancer receiving androgen deprivation therapy: a population-based cohort study. Br J Cancer. 2023;128(12):2253-2260. doi: 10.1038/s41416-023-02271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyon AR, López-Fernández T, Couch LS, et al. ; ESC Scientific Document Group . 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229-4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eText. Search Strategy
eTable 1. CTCAE v4.0 Definitions for Classifying CV Adverse Events
eTable 2. Characteristics of Included Studies
eTable 3. Meta-Regression Analysis
eTable 4. GRADE Assessment for Certainty of Evidence
eFigure 1. RoB2 Tool Assessing Risk of Bias for CV Events
eFigure 2. Funnel Plots for the Examination of Small Study Effects for (A) All Grade CV Events and (B) Grade ≥ 3 CV Events
eFigure 3. Incidence Rates for (A) Primary Outcomes and (B) Secondary Outcomes
eFigure 4. Sub-Group Analysis of All Grade CV Events Based on ARSI
eFigure 5. Sub-Group Analysis of Grade ≥3 CV Events Based on ARSI
eFigure 6. Sensitivity Analysis of (A) All Grade CV Events and (B) Grade ≥3 CV Events
Data Sharing Statement