Abstract
Developmental process to gastric cancer by Helicobacter pylori infection consists of three steps: (1) H. pylori infection; (2) gastric atrophy development; and (3) carcinogenesis. In each step, genetic traits may influence the process, interacting with lifestyle. In the step of H. pylori infection, two lines of genetic polymorphisms were assumed: one influencing gastric acid inhibition interacting with smoking, and the other concerning innate immune response attenuation. The former includes functional polymorphisms of IL‐1B (C‐31T or tightly linked T‐511C), and TNF‐A (T‐1031C and C‐857T), and the latter possibly includes NQO1 C609T. In the step to gastric atrophy, polymorphisms pertaining to the signal transduction from cytotoxin‐associated gene A (PTPN11 A/G at intron 3) and to T‐cell responses (IL‐2 T‐330G and IL‐13 C‐1111T) were hypothesized. There are a limited number of epidemiological genotype studies on the final step of literal carcinogenesis, potentially interacting with smoking, a low vegetable and fruit intake, and salty foods, the well‐documented risk factors. In past case‐control studies on the associations between genotype and gastric cancer risk, the cases consisted of H. pylori‐related and unrelated gastric cancer patients and the controls consisted of individuals including the uninfected (H. pylori unexposed and exposed) and the infected with and without gastric atrophy. Accordingly, it was not clear whether the observed risk was for H. pylori‐related or ‐unrelated gastric cancer, nor which step was involved in the observed associations even when nearly all cases were H. pylori‐related. In order to elucidate the genetic traits of H. pylori‐related gastric cancer, stepwise evaluation will be required. (Cancer Sci 2006; 97: 1129–1138)
Helicobacter pylori is a gram‐negative bacterium that colonizes the human gastric mucosa.( 1 ) It is well known that the bacterium increases the risk of gastric diseases, including peptic ulcers and stomach cancer.( 2 , 3 ) In areas where gastric cancer is highly prevalent, such as Japan, Korea and China, the great majority of gastric cancers are H. pylori‐related. In Japan, the cumulative gastric cancer incidence rate of 0–84‐year‐olds was estimated to be 21.2% for infected males and 8.0% for infected females, under the conditions that half of the population are infected and the infected people have a five‐times higher risk of gastric cancer than uninfected people.( 4 ) As H. pylori‐unrelated gastric cancer may develop in a different set of genetic or environmental factors from H. pylori‐related cases, epidemiological and biological studies to elucidate the process should be conducted separately.
In H. pylori‐related gastric cancer, the process has three steps: (1) H. pylori infection; (2) gastric atrophy development; and (3) carcinogenesis. In each step, genetic traits may influence the process, interacting with lifestyle. Fig. 1 shows the genetic polymorphisms with possible biological mechanisms and interacting lifestyle factors for the three steps. This paper briefly reviews the epidemiological findings, the biological background, and polymorphism studies according to these steps. Additionally, previous polymorphism studies of gastric cancer that did not consider these processes are reviewed. Although the pathological process model includes intestinal metaplasia and dysplasia,( 5 ) epidemiological studies using serum pepsinogens (PG) as markers of gastric atrophy cannot distinguish the two pathologically defined stages, so that they are not regarded as different steps in the present paper. In the present paper, human leukocyte antigen (HLA) types were not included in polymorphism genotypes.( 6 )
Figure 1.
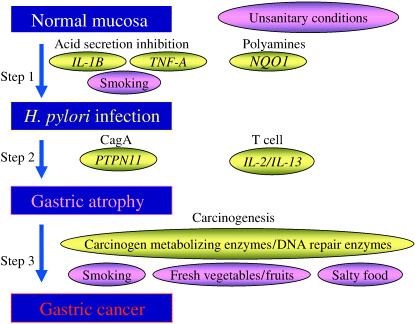
Steps in Helicobacter pylori‐related gastric cancer. IL, interleukin; NQO1, NAD(P)H : quinone oxidoreductase 1; TNF, tumor necrosis factor.
Epidemiology of H. pylori infection
Helicobacter pylori transmits from person to person, largely depending on sanitary conditions, especially in childhood.( 7 , 8 , 9 , 10 ) Lifestyle factors such as salty food intake,( 11 ) fruit intake( 12 ) and smoking( 12 , 13 , 14 , 15 , 16 ) were also reported to influence the persistence of H. pylori infection. Meanwhile, a twin study reported that the concordance of anti‐H. pylori antibody status was higher in monozygotic twin pairs than in dizygotic twin pairs,( 17 ) strongly underscoring the role of genetics in infection.
The bacterium is classified into two main species, in terms of the cytotoxin‐associated gene A (CagA) protein, a toxin injected through a type IV infection system into gastric epithelium: CagA negative and CagA positive. The CagA‐positive species are more virulent than the CagA‐negative species, and have stronger associations with gastric atrophy and gastric cancer.( 18 , 19 , 20 )
Biology of H. pylori infection
Gram‐negative bacteria, including H. pylori, have a cell wall containing lipopolysaccharide (LPS). The innate immune response, a preprogrammed non‐specific first line of defense responsible for eliminating pathogens at the site of entrance into the host, recognizes LPS with a pattern recognition receptor, CD14, on the cell surface. CD14 is a glycosylphospatidylinositol‐anchored receptor lacking an intracellular domain, which binds LPS with high affinity. The LPS–CD14 complex then activates Toll‐like receptor 4 (TLR4) with an intracellular domain for signal transduction. TLR4 is stabilized in the form of a homodimer by MD‐2. The signal from LPS is transduced through myeloid differentiation factor 88 (MyD88), interleukin (IL)‐1 receptor‐associated kinase (IRAK), tumor necrosis factor (TNF) receptor‐associated factor 6 (TRAF6) and inhibitory κB kinase (IKK) to nuclear factor (NF)‐κB (Fig. 2).( 21 )
Figure 2.
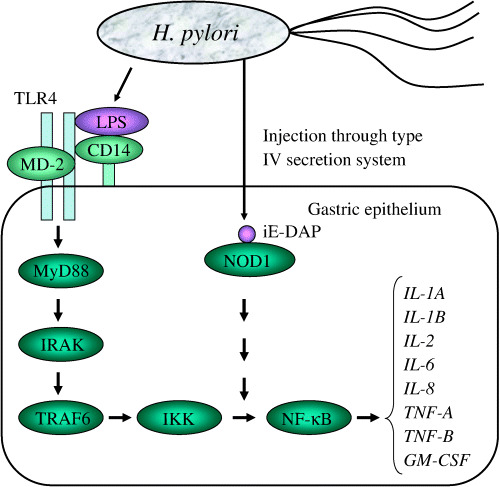
Signal pathway from Helicobacter pylori to cytokine gene expression. iE‐DAP, α‐d‐glutamyl‐meso‐diaminopimelic acid; IKK, inhibitory κB kinase; IL, interleukin; IRAK, interleukin‐1 receptor‐associated kinase; LPS, lipopolysaccharide; MyD88, myeloid differentiation factor 88; NF‐κB, nuclear factor κB; NOD1, nucleotide‐binding oligomerization domain protein; TLR, Toll‐like receptor; TRAF6, TNF receptor‐associated factor 6.
A recent study delineated that the main signal transduction to NF‐κB is through the peptidoglycan‐derived peptide α‐d‐glutamyl‐meso‐diaminopimelic acid (iE‐DAP), which is injected through a type IV secretion system. iE‐DAP is a ligand of nucleotide‐binding oligomerization domain protein 1 (NOD1), which is encoded by the caspase‐recruitment domain 4 gene (CARD4) and expressed in epithelial cells.( 22 ) It is hypothesized that the signal is transduced through several molecules to NF‐κB.( 23 )
NF‐κB is a group of proteins (NF‐κB/REL proteins) that bind a common sequence motif known as the κB site.( 24 ) They transcript inflammation‐related genes such as IL‐1A, IL‐1B, IL‐2, IL‐6, IL‐8, TNF‐A, TNF‐B and GM‐CSF.( 25 ) Other pathways of LPS signaling may also exist for IL‐1B,( 26 ) and for TNF‐A through extracellular signal‐regulated kinase (ERK).( 27 ) LPS‐induced IL‐1β and TNF‐α induce other cytokines and enzymes for inflammation as well as IL‐1β and TNF‐α themselves through the NF‐κB pathway.( 28 ) The IL‐1 receptor antagonist coded by IL‐1RN disturbs IL‐1β binding to IL‐1 receptor I (IL‐1RI), resulting in the inhibition of IL‐1β function.
Interleukin‐1β and TNF‐α inhibit gastric acid secretion.( 29 ) The inhibited acid secretion causes H. pylori distribution to the corpus, resulting in gastric atrophy.( 30 ) Accordingly, the level of cytokines could influence the persistence of H. pylori infection.( 31 ) IL‐8 is a CXC chemokine that mediates the activation and migration of neutrophils into tissue from peripheral blood. As is the case with IL‐1β and TNF‐α, IL‐8 induced in gastric epithelial cells,( 1 ) and in neutrophils,( 32 ) by H. pylori serves to trigger the inflammation. It binds CXCR‐1 (previously called IL‐8RA) and CXCR‐2 (IL‐8RB) with similar affinity. IL‐10, a cytokine produced by type 2 T‐helper cells (Th2 cells), inhibits the production of IL‐1β and IL‐8.( 33 , 34 ) In mice, cytokine expression by Helicobacter felis is modified by concurrent infection of the enteric helminth Heligmosomoides polygyrus, which drives the immune response through Th2 cells. Co‐infection increases the mRNA of IL‐10 in comparison with Helicobacter felis infection alone, resulting in reduced Helicobacter‐associated gastric atrophy and high Helicobacter colonization.( 35 ) These findings suggest that a high level of IL‐10 and a lower level of IL‐8 create favorable conditions for prolonging H. pylori infection in human gastric mucosa. Myeloperoxidase (MPO) is a lysosomal enzyme in polymorphonuclear leukocytes and monocytes. Hypochlorous acid produced by MPO shows microbicidal activity against a wide range of organisms,( 36 ) producing tissue inflammation. It was reported that H. pylori water extract activates neutrophils( 37 ) and enhances the secretion of MPO.( 32 )
Another line of innate immune response relating to persistent H. pylori infection is polyamine synthesis. H. pylori induces arginase II generating ornithine, as well as ornithine decarboxylase (ODC) generating polyamines (Fig. 3). Polyamines, especially spermine, restrain the immune response by inhibiting inducible nitric oxide synthase (iNOS) translation and nitric oxide (NO) production,( 38 ) which are upregulated by H. pylori. The ODC level is regulated with antizyme, a polyamine‐induced protein. NAD(P)H : quinone oxidoreductase 1 (NQO1) binds and stabilizes ODC. The regulation of ODC stability by NQO1 is prominent under oxidative stress.( 39 )
Figure 3.
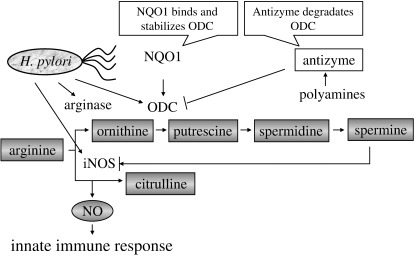
Arginase–ornithine carboxylase (ODC) pathway to attenuate innate immune response by nitric oxide. iNOS, inducible nitric oxide synthase; NQO1, NAD(P)H : quinone oxidoreductase 1.
The mechanisms of H. pylori binding to gastric epithelium may be related to genetic traits of susceptibility to persistent H. pylori infection. H. pylori with the babA2 gene is attached to gastric mucosa with blood group antigen‐binding adhesion (BabA).( 40 , 41 ) BabA binds both Lewis b and H type I blood group carbohydrate structures on the foveolar epithelium of human gastric mucosa. Type I precursor is converted to H type I antigen by fucosyltransferase 2 (FUT2, secretor enzyme), then to Lewis b antigen by fucosyltransferase 3 (FUT3, Lewis enzyme). FUT3 also converts type I precursor to Lewis a antigen.
Genotype and H. pylori seropositivity
Molecules in related pathways seem to have the potential to enhance susceptibility to H. pylori infection at childhood, causing lifetime persistent infection. Table 1 shows the gene polymorphisms of such molecules reported for association with H. pylori infection.( 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 ) In these polymorphisms, relatively consistent associations were found with IL‐1B and TNF‐A. The possible biological mechanism may be related to inhibition of gastric acid secretion. NQO1 C609T also seems to have the potential to be a genetic trait for persistent H. pylori infection through stabilization of ODC.
Table 1.
Polymorphisms reported in association with Helicobacter pylori infection and odds ratio (OR) or seropositive percentage (HP%)
| Polymorphism | Subjects | OR or HP% |
|---|---|---|
| CD14 C‐159T | 1374 Japanese( 42 ) | TT, TC:0.94, CC:1.16 |
| CXCR2 C785T | 241 Japanese( 42 ) | CC(65%), CT(63%), TT(56%) |
| FUT2 Se/se | 241 Japanese( 43 ) | SeSe, Sese:0.79, sese:0.35* |
| 679 Japanese( 44 ) | SeSe, Sese:1.51*, sese:1.50 | |
| 465 Japanese( 44 ) | SeSe, Sese:1.57, sese:1.29 | |
| FUT3 Le/le | 241 Japanese( 43 ) | LeLe, Lele:1.95*, lele:2.80 |
| 679 Japanese( 44 ) | LeLe, Lele:0.98, lele:1.31 | |
| 465 Japanese( 44 ) | LeLe, Lele:1.06, lele:1.40 | |
| IL‐1 A C‐889T | 241 Japanese( 45 ) | CC(62%), CT/TT(68%) |
| IL‐1B C‐31T | 241 Japanese( 45 ) | CC, CT:2.32*, TT:2.46* |
| 55 smokers | CC, CT:6.18*, TT:22.9* | |
| 465 Japanese( 46 ) | CC, CT:0.97, TT:1.73* | |
| 80 ever smokers | CC, CT:1.68, TT:5.29* | |
| 547 Japanese( 47 ) | CC, CT:1.32, TT:1.35 | |
| 127 smokers | CC, CT:1.12, TT:1.01 | |
| 963 Japanese Brazilians( 48 ) | CC, CT:1.30, TT:1.45* | |
| 124 smokers | CC, CT:2.45, TT:3.49* | |
| IL‐1B C‐511T | 499 Japanese( 49 ) | CC(53%), CT(54%), TT(52%) |
| 474 Koreans( 50 ) | CC(88%), CT(86%), TT(86%) | |
| IL‐1RI C‐116T | 241 Japanese( 42 ) | CC(65%), CT(58%), TT(72%) |
| IL‐1RN VNTR | 241 Japanese( 45 ) | 4rpt/4rpt(62%), others(67%) |
| 474 Koreans( 50 ) | non‐2rpt(87%), 2rpt(80%) | |
| IL‐2 T‐330G | 454 Japanese( 51 ) | GG, TG:1.10, TT:1.15 |
| IL‐4 C‐33T | 454 Japanese( 51 ) | CC, CT:1.43, TT:1.25 |
| IL‐8 T‐251A | 454 Japanese( 52 ) | TT, TA:0.86, AA:0.70 |
| IL‐10 T‐819C | 454 Japanese( 52 ) | TT, TC:0.67, CC:0.82 |
| IL‐8 & IL‐10 | 454 Japanese( 52 ) | TT & TT, others:0.62* |
| 65 smokers | TT & TT, others:0.13* | |
| 241 Japanese( 42 ) | TT & TT, others:1.04 | |
| 55 smokers | TT & TT, others:0.45 | |
| 679 Japanese( 42 ) | TT & TT, others:1.49* | |
| 158 smokers | TT & TT, others:0.89 | |
| IL‐13 C‐1111T | 454 Japanese( 51 ) | CC, CT:0.73, TT:1.09 |
| MPO G‐463A | 241 Japanese( 53 ) | GG, GA/AA:0.69 |
| 55 smokers | GG, GA/AA:0.21* | |
| 454 Japanese( 54 ) | GG, GA/AA:0.84 | |
| 64 smokers | GG, GA/AA:0.83 | |
| NF‐KB2–10G I/D | 1374 Japanese( 42 ) | II, ID:1.03, DD:1.15 |
| NQO1 C609T | 241 Japanese( 55 ) | TT, TC:1.13, CC:2.42* |
| 454 Japanese( 55 ) | TT, TC:1.57, CC:1.70 | |
| PPAP Pro12Ala(C/G) | 104 Chinese( 56 ) | CC(62%), CG/GG(33%) |
| PTPN11 G/A at intron 3 | 454 Japanese( 57 ) | GG(58%), GA(49%), AA(47%) |
| TCRBV6S1 A/B | 383 Germans( 58 ) | Significant association* |
| TNF‐A T‐1031C | 1374 Japanese( 59 ) | TT, TC:0.62, CC:0.43* |
| 963 Japanese Brazilians( 60 ) | CC, TC:0.96, TT:1.18 | |
| TNF‐A C‐857T | 1374 Japanese( 59 ) | CC, CT:1.06, TT:1.69 |
| 963 Japanese Brazilians( 60 ) | CC, CT:1.17, TT:1.21 | |
| TNF‐A−1031 & −857 | 1374 Japanese( 59 ) | CC & CC, TT & CC:2.37*, TC & CT:2.84*, TT & TT:3.63* |
| 963 Japanese Brazilians( 60 ) | CC & CC, TT & CC:1.08, TC & CT:1.03, TT & TT:1.27 | |
| 253 ever smokers CC & CC, TT & CC:2.01, TC & CT:1.76, TT & TT:2.30 | ||
| TNF‐A G‐308A | 393 Germans( 61 ) | GG(52%), GA(56%), AA(56%) |
| 792 Italians( 62 ) | GG(54%), GA(61%*), AA(–) | |
| 474 Koreans( 50 ) | GG(86%), GA/AA(89%) | |
| TNF‐B A252G | 1374 Japanese( 59 ) | AA, AG:1.05, GG:1.05 |
Statistically significant (P < 0.05).
IL‐1β. IL‐1β is encoded by IL‐1B on chromosome 2q14, whose three polymorphisms (C‐511T, C‐31T and C3954T) have been studied in many diseases. Among Caucasians, the polymorphisms tend to be associated with gastric cancer risk, but not among Orientals.( 49 , 63 , 64 , 65 , 66 , 67 ) In any ethnic group, 511T and −511C are tightly linked with −31C and −31T, respectively.( 45 , 63 ) Although −31T, which makes a TATA box in the promoter region, seems to be a high‐expression allele, there is controversy in its function. An electrophoretic mobility‐shift assay demonstrated that transcription factors combine with −31T, not −31C,( 63 ) and that the IL‐1β level of antrum gastric mucosa was higher in −511CC (that is, −31TT) carriers than in −511TT carriers among H. pylori‐infected Japanese.( 68 ) A similar association was found among gastric cancer patients infected with H. pylori in Korea.( 65 ) However, other studies have reported opposite findings among H. pylori‐infected Japanese,( 69 ) and among H. pylori‐infected people in Thailand.( 70 ) Among those with IL‐1A −889TT in Finland, serum IL‐1β levels were reported to be higher in IL‐1B −511T allele carriers than in non‐carriers.( 71 ) A report on IL‐1β mRNA showed no difference among IL‐1B C‐511T genotypes.( 72 ) Concerning the function of C3954T, few biological studies have been reported, although its association with disease risk has been reported.
The effects of IL‐1β can be modulated by the IL‐1 receptor antagonist encoded by IL‐1RN, IL‐1 receptor I encoded by IL‐1R1, and IL‐1 receptor II encoded by IL‐1R2,( 73 ) so that polymorphisms of these molecules could affect persistent H. pylori infection. However, reports on the associations with H. pylori infection are limited.
TNF‐α. TNF‐A encoding TNF‐α is located between HLA‐B and HLA‐DR on chromosome 6p21.3. In the promoter area, G‐238A, G‐244A, G‐308A, C‐857T, C‐863A and T‐1031C were reported.( 74 , 75 ) The alleles −238A, −244A and –308A were rare (2.0%,( 74 ) 0.0%( 75 ) and 1.7%,( 74 ) respectively, among Japanese people), and C‐863A was tightly linked with T‐1031C.( 76 ) The function of these alleles is still controversial, but −308A is regarded as a high‐expression allele.( 77 ) As shown in Table 1, H. pylori infection tended to be more frequent among those with the −308A allele than among those without the allele. Our study of 1374 participants from three datasets showed that those with TNF‐A −857TT and −1031TT had the highest risk of being H. pylori seropositive, and those with TNF‐A −857CC and −1031CC the lowest,( 59 ) although the association was not clear among Japanese Brazilians.( 60 )
NQO1. NQO1 is an obligate two‐electron reductase whose gene is located in chromosome 16q22.( 78 ) The gene has a functional polymorphism C609T (Pro187Ser); the T allele has null enzyme activity.( 79 ) Our study found that the CC genotype favors persistent H. pylori infection.( 55 ) As the molecules in connection with the innate immune response through the ODC–iNOS pathway were not fully examined in terms of their polymorphisms, further screening may detect the other polymorphisms associated with persistent H. pylori infection in this pathway.
Gene–environment interactions with smoking for H. pylori seropositivity
It is well known that the H. pylori eradication rate is lower among smokers.( 80 ) One possible explanation is that it elevates gastric acid secretion. We have examined the interactions between genotypes and smoking for seropositivity. The published interactions concern IL‐1B, IL‐8 and IL‐10, MPO and TNF‐A (Table 1). Concerning IL‐1B C‐31T,( 45 , 46 , 47 , 48 ) the odds ratios (OR) of the −31TT genotype tended to be higher among smokers, with one exception.( 47 ) The first dataset showed a marked elevation of H. pylori seropositivity for the combination of IL‐8 −251TT and IL‐10 −819TT among current smokers.( 52 ) Subsequent datasets similarly produced an elevated OR, though insignificant.( 42 ) A marginal interaction was observed for MPO (GG vs GA/AA) and smoking (current vs non‐current) (OR = 4.57 and P = 0.08).( 53 ) No difference in OR was observed between current smokers and never smokers in another dataset.( 54 ) In a study on Japanese Brazilians, smokers with TNF‐A −857TT and −1031TT tended to have an insignificantly higher OR (2.30) of infection than the whole subjects including both smokers and non‐smokers (OR = 1.27).( 60 )
Epidemiology of gastric atrophy
There is no doubt that gastric atrophy is a result of inflammation induced by H. pylori infection.( 81 , 82 , 83 , 84 , 85 , 86 , 87 ) In epidemiological studies, PG have been used as a marker of gastric atrophy( 88 ) because of its less invasive method. To date, several risk factors including salty food intake,( 89 ) low vegetable intake( 81 , 87 ) and low vitamin C( 82 ) have been reported for gastric atrophy among those with and without H. pylori infection. A recent study reported that rice, miso soup, cod roe and cuttlefish were high‐risk foods among 1071 infected Japanese, indicating that traditional Japanese foods are a high‐risk diet for gastric atrophy.( 90 ) Another study showed that frequent rice intake significantly increased the risk of atrophic gastritis among 291 infected Japanese Brazilians.( 91 ) A double‐blind randomized controlled intervention study in Japan demonstrated that 5‐year 500 mg of vitamin C supplementation slightly prevented the decrease in average PGI/II ratio relative to 50 mg of supplementation, with no difference in reduction of H. pylori seropositivity percentage between the two groups.( 92 )
Biology of gastric atrophy following H. pylori infection
CagA injected through a type IV secretion system from H. pylori into the gastric epithelial cells seems to play a pivotal role in gastric atrophy development (Fig. 4). The injected CagA is phosphorylated by Src family kinase, which binds SHP‐2 (Src homology 2 domain‐containing protein tyrosine phosphatase) at the phosphorylated site, then transduces its signal to other molecules.( 93 ) Phosporylated CagA activates C‐terminal Src kinase (Csk), which inhibits Src family kinase. This negative feedback regulates the signal from CagA.( 94 ) Among the CagA, the East Asian type and Western type were recognized. The former is more virulent than the latter, and almost all H. pylori in Japan were reported to be East Asian CagA positive.( 95 )
Figure 4.
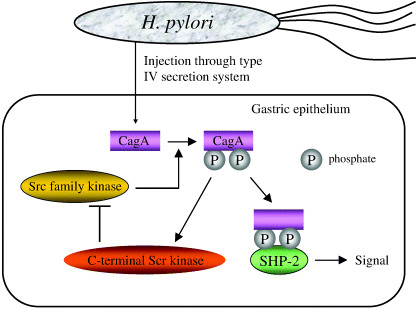
Interaction of cytotoxin‐associated gene A (CagA) with Src homology 2 domain‐containing protein tyrosine phosphatase (SHP‐2).
IL‐1β and TNF‐α induced by H. pylori lead to chronic inflammation, resulting in gastric atrophy. Although the process to gastric atrophy has not been fully elucidated, immunological responses are involved through lifelong H. pylori infection. Extracellular bacterial infections typically induce a Th2 immune response, whereas H. pylori induces proinflammatory cytokines, indicating a Th1 immune response.( 96 , 97 , 98 ) IL‐2 is a multifunction cytokine with an autocrine activity to proliferate helper T cells. IL‐4 causes Th0 cells to differentiate into Th2 cells, and is produced by Th2 cells. IL‐13 is also a Th2 cytokine, which regulates inflammation, mucus production, tissue remodeling and fibrosis.( 99 )
Genotype and gastric atrophy measured with pepsinogens
Studies on the association between genotype and gastric atrophy among the infected are relatively limited (Table 2).( 100 , 101 , 102 ) Significant associations were reported for PNPN11, and for IL‐2 and IL‐13 polymorphisms.
Table 2.
Polymorphisms reported in association with gastric atrophy (GA) among Helicobacter pylori seropositives, as well as odds ratio (OR) and/or gastric atrophy percent (GA%)
| Polymorphism | Subjects | OR and/or GA% |
|---|---|---|
| IL‐1B C‐31T | 253 Japanese( 46 ) 455 Japanese Brazilians( 100 ) | CC(54%), CT(52%), TT(56%) CC, CT:0.61, TT:0.58 CC(36%), CT(31%), TT(21%) |
| IL‐2 T‐330G | 244 Japanese( 101 ) | GG, TG:1.64, TT:2.78* GG(38%), TG(50%), TT(62%) |
| IL‐4 C‐33T | 249 Japanese( 101 ) | CC, CT:2.47, TT:1.80 CC(38%), CT(60%), TT(53%) |
| IL‐13 C‐1111T | 248 Japanese( 101 ) | CC, CT/TT:0.41* CC(59%), CT/TT(45%) |
| PTPN11 G/A at intron 3 | 248 Japanese( 57 ) | GG, GA:0.70, AA:0.09* GG(59%), GA(49%), AA(11%) |
| RANTES C‐471T | 344 Germans( 102 ) | No association |
| TNF‐A T‐1031C | 455 Japanese Brazilians( 60 ) | CC(29%), TC(33%), TT(34%) |
| C‐857T | 456 Japanese Brazilians( 60 ) | CC(32%), CT(36%), TT(39%) |
| −1031 & −857 | 455 Japanese Brazilians( 60 ) | CC & CC(29%), TT & CC(33%), TC & CT(43%), TT & TT(39%) |
Statistically significant (P < 0.05).
PTPN11 G/A at intron 3 (IMS‐JST057927, rs2301756). IMS‐JST057927 is a G‐to‐A single nucleotide polymorphism 223 bp upstream of exon 4 of PTPN11 gene encoding SHP‐2 on chromosome 12q24.1. The function of this polymorphism has not been reported. The first dataset showed that one (11.1%) out of nine infected individuals with the AA genotype had gastric atrophy, while 134 (56.1%) out of 239 infected individuals with the G allele had gastric atrophy.( 57 ) If the polymorphism is functional or linked to a functional one, the association can be biologically explained by the strength of signal transduction through the CagA–SHP2 complex. According to rs2301756 of the National Center for Biotechnology Information (NCBI) dbSNP, the frequency of the G allele (high‐risk allele for gastric atrophy) is 0.802 among 1484 Japanese, 0.917 among 48 Chinese, 0.348 among 46 African American, and 0.064 among 46 Caucasians. This indicates that Japanese and Chinese become high‐risk ethnic groups through CagA‐positive H. pylori infection.
IL‐2 T‐330G and IL‐13 C‐1111T. T‐330G of the IL‐2 gene on chromosome 4q26‐27 was reported to be a functional polymorphism,( 103 ) and IL‐2 production is higher in the GG genotype than in the TT genotype.( 104 ) The TT genotype was at a higher risk of gastric atrophy,( 101 ) and was less frequent among Asians (38% out of 29 individuals) than among Caucasians (51% out of 199 individuals).( 105 )
The IL‐13 gene on chromosome 5q31 has several polymorphisms; at least three at the promoter region, two at intron 1 (Arg130Gln) and four at the 3′ untranslated region of exon 4.( 106 ) The −1111TT genotype was reported to have increased binding of nuclear proteins, and to be associated with asthma.( 106 , 107 ) Concerning gastric atrophy, −1111TT was a low risk genotype.( 101 ) The biological mechanism has not yet been elucidated.
Genotype and advanced precancerous lesions
Some H. pylori‐infected individuals go on to develop advanced precancerous lesions. A study in China showed no significant differences in genotype frequencies of CYP2E1, GSTM1, GSTP1, GSTT1, ALDH2 and ODC between those with mild chronic atrophic gastritis (including 29.7% of H. pylori‐negative patients) and those with deep intestinal metaplasia or dysplasia (including 20.2% of H. pylori‐negative patients), but did show a significant interaction between CYP2E1 DraI and smoking.( 108 ) In Germany, harboring both IL‐1B −511T and IL‐1RN 2rpt alleles relative to lacking IL‐1B −511T or/and IL‐1RN 2rpt alleles was significantly associated with atrophic gastritis, intestinal metaplasia and severe inflammation.( 109 )
Epidemiology of gastric cancer
There are many epidemiological studies on risk factors for gastric cancer,( 110 , 111 ) but studies on the gastric cancer factors among those with gastric atrophy are limited. The plausible risk factors among those with gastric atrophy are smoking, salty food and a lower intake of fresh fruit and vegetables, as well as family history of gastric cancer.( 112 , 113 )
Smoking elevates the risk of gastric cancer,( 114 , 115 , 116 ) as well as of precancerous lesions, intestinal metaplasia and dysplasia.( 117 , 118 , 119 ) Smoking was reported to promote the grade of atrophic gastritis in infected Japanese.( 120 ) In addition to these epidemiological findings, biological studies on tobacco smoke carcinogenesis indicate that smoking plays a role also in the final step of carcinogenesis of H. pylori‐related gastric cancer.( 121 )
Genotype and gastric cancer risk
To date, many genetic polymorphisms have been examined for associations with gastric cancer in case‐control studies with mixed cases (H. pylori‐related and H. pylori‐unrelated) and controls at different stages (unexposed to H. pylori, exposed but uninfected, infected but without gastric atrophy, and with gastric atrophy), as shown in Fig. 5. As those case‐control studies compared genotype frequencies between the mixed cases and heterogeneous controls, the estimated OR did not reflect any distinct step to gastric cancer. Controls unexposed to H. pylori have the same genotype frequency as the average among the exposed, which reduces the difference in the genotype frequency between the uninfected and infected. In order to measure the associations between genotypes and H. pylori infection, studies should be conducted at a region where exposure to the bacterium is highly prevalent. Usual case‐control studies could provide estimates for the final step (i.e. literal carcinogenesis), when genotype frequency is different between gastric atrophy and gastric cancer, and the same among the uninfected, infected and those with gastric atrophy.
Figure 5.
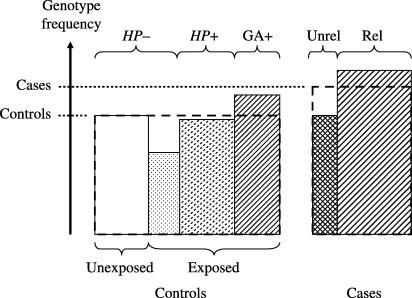
Heterogeneity of subjects in case‐control studies. Exposed, exposed to Helicobacter pylori; GA+, gastric atrophy positive; HP–, H. pylori negative; HP+, H. pylori positive; Rel, H. pylori‐related gastric cancer; Unexposed, unexposed to H. pylori; Unrel, H. pylori‐unrelated gastric cancer. In this case‐control study, the average percentages of the targeted genotype for mixed cases and heterogeneous controls were measured.
Table 3 lists the polymorphisms reported for gastric cancer risk, adopted from Gonzalez et al.( 122 ) and recent studies.( 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 ) The OR were listed if they were significant. Accordingly, it should be noted that there were many insignificant studies behind Table 3.
Table 3.
Polymorphisms reported in association with gastric cancer risk: only significant associations are with odds ratio (OR) and 95% confidence interval (95%CI)
| Polymorphism | Country | OR (95%CI) |
|---|---|---|
| ACE I/D | Germany( 123 ) | DD, DI:0.55 (0.31–0.96), II:0.20 (0.08–0.54) |
| Cyclin D1 G870A | Germany( 124 ) | Significant association (P = 0.003) |
| CYP1A1 Ile/Val | China( 125 ) | IleIle, ValVal:4.84 (1.24–22.07) |
| CYP2C6 *1/*4 | Japan( 126 ) | *1*1/*1*4, *4*4 : 3.14 (1.05–9.41) |
| CYP2C19 *1/*2/*3 | Japan( 127 ) | *1*1, no*1:1.98 (1.07–3.65) |
| CYP2E1 RsaI | Japan † | |
| Brazil † | ||
| China( 128 ) | ||
| E‐cadherin C‐160a | Taiwan( 129 ) | CC, AA:0.20 (0.06–0.56) |
| EGF A61G | Japan( 130 ) | |
| GSTM1 present/null | UK † | Present, null:2.9 (1.25–6.73) |
| Japan † | Present, null:1.70 (1.05–2.8) | |
| Iran † | Present, null:2.3 (1.15–4.95) | |
| Poland † | ||
| China( 125 ) | Present, null:2.81 (1.39–5.71) | |
| Taiwan( 131 ) | Present, null:1.75 (1.04–2.96) | |
| GSTM3 IVS6del3 | Poland † | |
| GSTP1 I105V | Japan † | |
| Poland † | ||
| GSTT1 present/null | China † | Present, null:2.5 (1.01–6.2) |
| Poland † | Present, null:3.1 (1.5–6.5) among current smokers | |
| Japan † | ||
| IL‐1B C‐1473G | Korea( 131 ) | |
| C‐511T | Poland( 63 ) | CC, CT:1.8 (1.3–2.4), TT:2.6 (1.7–3.9) |
| Portugal † | CC, CT/TT:1.7 (1.1–2.7) | |
| Taiwan( 116 ) | ||
| C3954T | Poland( 63 ) | |
| IL‐1RN 86‐bp VNTR | Poland( 63 ) | 4rpt4rpt, 2rp2rp:3.7 (2.4–5.7) |
| Taiwan( 116 ) | ||
| IL‐1B C‐511T +IL‐1RN 86‐bp VNTR | Portugal † | CC + LL/L2rpt, CT/TT+2rpt2rp:9.0 (3.5–23.0) |
| IL‐2 G‐384T, G114T | China( 133 ) | |
| IL‐4 C‐590T | Taiwan( 116 , 134 ) | |
| RP1/RP2 | Taiwan( 134 ) | |
| IL‐4R Ile50Val | Taiwan( 116 ) | |
| Gln576Arg | Taiwan( 116 ) | |
| IL‐10 G‐1082A | Taiwan( 116 ) | AA, AG:2.14 (1.07–4.30) |
| China( 133 ) | ||
| T‐819C | Taiwan( 116 ) | TT, TC:1.83 (1.23–2.71), CC:1.95 (1.03–3.69) |
| China( 133 ) | ||
| MK G‐2669A | Taiwan( 134 ) | |
| MTHFR C677T | China † | CC, TT:1.87 (1.00–3.48) |
| Mexico( 135 ) | CC, TT:1.62 (1.00–2.59) | |
| C677T, A1298C | Korea( 136 ) | |
| MUC1 VNTR | Portugal † | Large, small: 4.3 (1.8–10.5) |
| MUC6 VNTR | Portugal † | Large, small: P < 0.05 |
| MYCL1(L‐myc) EcoRI | Japan † | LL, LS:1.55 (1.03–2.34) |
| Japan † | LL, LS/SS:3.09 (1.33–7.21) | |
| NAT1 | UK † | Slow, rapid:2.6 (1.3–5.3) |
| Japan † | ||
| NAT2 | UK † | |
| Japan † | ||
| NQO1 C609T | Japan( 137 ) | |
| OGG1 Ser327Cys | Japan † | |
| Brazil † | ||
| p16 INK4A C540G | Germany( 124 ) | |
| C570G | Taiwan( 134 ) | |
| p21 codon31 | Taiwan( 134 ) | |
| p53 codon72 | Taiwan( 134 ) | Significant association (P = 0.02) |
| PPAR Pro12Ala(C/G) | China( 56 ) | CC, CG/GG:2.5 (1.1–5.8) |
| TFF2 VNTR | Portugal † | |
| TNF‐A G‐308A | Korea † | |
| Taiwan( 116 ) | ||
| G‐238A | Korea † | |
| Taiwan( 116 ) | ||
| XRCC1 Arg194Trp | Brazil( 138 ) | |
| Arg399Gln | Brazil( 138 ) | |
| Arg194Trp + Arg399Gln | China † | TrpTrp + ArgArg, ArgArg + ArgGln/GlnGln:1.73 (1.12–2.69) |
| XRCC3 Thr241Met | Brazil( 138 ) |
Studies cited in the review by Gonzalez et al.( 122 ); L, alleles longer than 2rpt.
There are several studies to demonstrate the risks of both gastric atrophy and gastric cancer in comparison with the same controls without gastric atrophy. Individuals with the IL‐8 −251A allele had OR = 1.50 with 95% confidence interval (95% CI) = 0.98–2.23 for gastric atrophy and OR = 1.50 with 95% CI = 1.00–2.25 for gastric cancer, indicating that the risk elevation was due to the risk for gastric atrophy, not for the step from gastric atrophy to gastric cancer.( 139 ) The direct comparisons between controls with gastric atrophy and cases with gastric cancer have been reported; there were no associations with p53 Arg72Pro,( 140 , 141 ) nor with PTPN11 G/A at intron 3.( 57 )
Lifestyle factors may interact with genotype in the final step. Biologically, the interactions of smoking, fresh vegetable and fruit intake, and salty food intake with polymorphisms of carcinogen‐metabolic enzyme and DNA repair enzymes are very plausible.
Conclusions
It is clear that H. pylori‐related gastric cancer develops through several steps, including infection, gastric atrophy (histologically intestinal metaplasia, dysplasia) and cancer. Lifestyle factors such as smoking and diet could influence one or more steps. However, genotypes may be step specific because the biological process is distinct in the different steps. Accumulated findings on the associations between gastric cancer risk and polymorphism genotypes demonstrate that the strength of association varies among the studies. As most case‐control studies examined the mixed effects on these steps, the inconsistent findings may be natural. In addition, the diversity of lifestyle factors interacting with the genotypes among the different study subjects may enlarge the inconsistency. In order to elucidate the genetic traits of H. pylori‐related gastric cancer, studies on each step taking into account lifestyle factors should be conducted. Such studies will produce useful information for gastric cancer prevention.
References
- 1. Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nature Rev Mol Cell Biol 2001; 2: 457–66. [DOI] [PubMed] [Google Scholar]
- 2. Labenz J, Borsch G. Evidence for the essential role of Helicobacter pylori in gastric ulcer disease. Gut 1994; 35: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Infection with Helicobacter pylori . IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans, Vol. 61. Schistosomes, liver flukes, and Helicobacter pylori. Lyon, France: International Agency for Research on Cancer, 1994: 177–241. [Google Scholar]
- 4. Hamajima N, Goto Y, Nishio K et al. Helicobacter pylori eradication as a preventive tool against gastric cancer. Asian Pac J Cancer Prev 2004; 5: 246–52. [PubMed] [Google Scholar]
- 5. Correa P. New strategies for the prevention of gastric cancer: Helicobacter pylori and genetic susceptibility. J Surg Oncol 2005; 90: 134–8. [DOI] [PubMed] [Google Scholar]
- 6. Go MF. What are the host factors that place an individual at risk for Helicobacter pylori‐associated disease? Gastroenterology 1997; 113: S15–20. [DOI] [PubMed] [Google Scholar]
- 7. Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori . J Infect Dis 1993; 168: 219–21. [DOI] [PubMed] [Google Scholar]
- 8. Goodman KJ, Correa P. The transmission of Helicobacter pylori. A critical review of the evidence. Int J Epidemiol 1995; 24: 875–87. [DOI] [PubMed] [Google Scholar]
- 9. Cave DR. How is Helicobacter pylori transmitted? Gastroenterology 1997; 113: S9–14. [DOI] [PubMed] [Google Scholar]
- 10. Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 2000; 22: 283–97. [DOI] [PubMed] [Google Scholar]
- 11. Tsugane S, Tei Y, Takahashi T, Watanabe T, Sugano K. Salty food intake and risk of Helicobacter pylori infection. Jpn J Cancer Res 1994; 85: 474–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fontham ETH, Ruiz B, Perez A, Hunter F, Correa P. Determinations of Helicobacter pylori infection and chronic gastritis. Am J Gastroenterol 1995; 90: 1094–101. [PubMed] [Google Scholar]
- 13. Murray LJ, McCrum EE, Evans AE, Bamford KB. Epidemiology of Helicobacter pylori infection among 4742 randomly selected subjects from Northern Ireland. Int J Epidemiol 1997; 26: 880–7. [DOI] [PubMed] [Google Scholar]
- 14. Woodward M, Morrison C, McColl K. An investigation into factors associated with Helicobacter pylori infection. J Clin Epidemiol 2000; 53: 175–82. [DOI] [PubMed] [Google Scholar]
- 15. Hamajima N, Inoue M, Tajima K et al. Lifestyle and anti‐Helicobacter pylori immunoglobulin G antibody among outpatients. Jpn J Cancer Res 1997; 88: 1038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Namekata T, Miki K, Kimmey M et al. Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. Am J Epidemiol 2000; 151: 820–30. [DOI] [PubMed] [Google Scholar]
- 17. Malaty HM, Engstrand L, Pedersen NL, Graham DY. Helicobacter pylori infection: genetic and environmental influence. A study of twins. Ann Intern Med 1994; 129: 982–6. [DOI] [PubMed] [Google Scholar]
- 18. Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree JE. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol 1998; 51: 225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kikuchi S, Crabtree JE, Forman D, Kurosawa M. Association between infections with CagA‐positive or ‐negative strains of Helicobacter pylori and risk of gastric cancer in young adults. Am J Gastroenterol 1999; 94: 3455–9. [DOI] [PubMed] [Google Scholar]
- 20. Hatakeyama M, Higashi H. Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci 2005; 96: 835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knuefermann P, Nemoto S, Baumgarten G et al. Cardiac inflammation and innate immunity in septic shock. Is there a role for Toll‐like receptors? Chest 2002; 121: 1329–36. [DOI] [PubMed] [Google Scholar]
- 22. Viala J, Chaput C, Boneca IG et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nature Immun 2004; 5: 1166–74. [DOI] [PubMed] [Google Scholar]
- 23. Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature Rev 2006; 6: 9–20. [DOI] [PubMed] [Google Scholar]
- 24. Karin M, Cao Y, Greten FR, Li Z‐W. NF‐κB in cancer: from innocent bystander to major culprit. Nat Rev 2002; 2: 301–10. [DOI] [PubMed] [Google Scholar]
- 25. May MJ, Ghosh S. Rel/NF‐κB and IRB proteins: an overview. Semin Cancer Biol 1997; 8: 63–73. [DOI] [PubMed] [Google Scholar]
- 26. Haddad JJ. Nuclear factor (NF)‐κB blockade attenuates but does not abrogate LPS‐mediated interleukin (IL)‐1β biosynthesis in alveolar epithelial cells. Biochem Biophysic Res Commun 2002; 293: 252–7. [DOI] [PubMed] [Google Scholar]
- 27. Rutault K, Hazzalin CA, Mahadevan LC. Combinations of ERK and p38 MAPK inhibitors ablate tumor necrosis factors‐α (TNF‐α) mRNA induction. J Biol Chem 2001; 276: 6666–74. [DOI] [PubMed] [Google Scholar]
- 28. Maeda S, Yoshida H, Ogura K et al. H. pylori activates NFκB‐inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology 2000; 119: 97–108. [DOI] [PubMed] [Google Scholar]
- 29. Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 1998; 42: 227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuipers EJ, Lundell L, Klinkenberg‐Knol EC et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334: 1018–22. [DOI] [PubMed] [Google Scholar]
- 31. El‐Omar EM. The importance of interleukin 1β in Helicobacter pylori associated disease. Gut 2001; 48: 743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JS, Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori water‐soluble surface proteins activate human neutrophils and up‐regulate expression of CXC chemokines. Dig Dis Sci 2000; 45: 83–92. [DOI] [PubMed] [Google Scholar]
- 33. Moore KW, De Waal Malefyt R, Coffman RL, O’Garra A. Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol 2001; 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 34. Ameixa C, Friedland JS. Down‐regulation of interleukin‐8 secretion from Mycobacterium tuberculosis‐infected monocytes by interleukin‐4 and ‐10 but not by interleukin‐13. Infect Immun 2001; 69: 2470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fox JG, Beck P, Dangler CA et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter‐induced gastric atrophy. Nat Med 2000; 6: 536–42. [DOI] [PubMed] [Google Scholar]
- 36. Foster CB, Lehrnbecher T, Mol F et al. Host defense molecule polymorphisms influence the risk for immune‐mediated complications in chronic granulomatous disease. J Clin Invest 1998; 102: 2146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takemura T, Granger DN, Evans DJ et al. Extract of Helicobacter pylori induces neutrophils to injure endothelial cells and contains antielastase activity. Gastroenterology 1996; 110: 21–9. [DOI] [PubMed] [Google Scholar]
- 38. Bussiere F, Chaturvedi R, Cheng Y et al. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric‐oxide synthase translation. J Biol Chem 2005; 280: 2409–12. [DOI] [PubMed] [Google Scholar]
- 39. Asher G, Bercovich Z, Tsvetkov P, Shaul Y, Kahana C. 20S proteasomal degradation of ornithine decarboxylase is regulated by NQO1. Mol Cell 2005; 17: 645–55. [DOI] [PubMed] [Google Scholar]
- 40. Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993; 262: 1892–5. [DOI] [PubMed] [Google Scholar]
- 41. Ilver D, Arnqvist A, Ögren J et al. Helicobacter pylori adhesin binding fucosylated histo‐blood group antigens revealed by retagging. Science 1998; 279: 373–7. [DOI] [PubMed] [Google Scholar]
- 42. Hamajima N. Persistent Helicobacter pylori infection and genetic polymorphisms of the host. Nagoya J Med Sci 2003; 66: 103–17. [PubMed] [Google Scholar]
- 43. Ikehara Y, Nishihara S, Yasutomi H et al. Polymorphisms of two fucosyltransferase genes (Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti‐Helicobacter pylori IgG antibody. Cancer Epidemiol Biomarkers Prev 2001; 10: 971–7. [PubMed] [Google Scholar]
- 44. Hamajima N, Shibata A, Ikehara Y et al. Lack of consistency in the association of Helicobacter pylori seropositivity with Se and Le polymorphisms among Japanese. Gastric Cancer 2002; 5: 194–200. [DOI] [PubMed] [Google Scholar]
- 45. Hamajima N, Matsuo K, Saito T et al. Interleukin 1 polymorphisms, lifestyle factors, and Helicobacter pylori infection. Jpn J Cancer Res 2001; 92: 383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Katsuda N, Hamajima N, Matsuo K et al. Association between the interleukin 1B (C‐31T) polymorphism and Helicobacter pylori infection in health checkup examinees. Jpn J Public Health 2001; 49: 604–12. (In Japanese.) [PubMed] [Google Scholar]
- 47. Hamajima N, Ito H, Matsuo K, Tajima K, Tominaga S. Helicobacter pylori seropositivity, the interleukin 1B polymorphism, and smoking among first‐visit outpatients. Asian Pac J Cancer Prev, 2002; 3: 23–8. [PubMed] [Google Scholar]
- 48. Uno M, Hamajima N, Ito LS et al. Helicobacter pylori seropositivity and IL‐1B C‐31T polymorphism among Japanese Brazilians. Int J Mol Med 2002; 10: 321–6. [PubMed] [Google Scholar]
- 49. Kato S, Onda M, Yamada S, Matsuda N, Tokunaga A, Matsukura N. Association of the interleukin‐1β genetic polymorphism and gastric cancer risk in Japanese. J Gastroenterol 2001; 36: 696–9. [DOI] [PubMed] [Google Scholar]
- 50. Kim N, Cho S‐I, Yim J‐Y et al. The effects of genetic polymorphisms of IL‐1 and TNF‐A on Helicobacter pylori‐induced gastroduodenal diseases in Korea. Helicobacter 2006; 11: 105–12. [DOI] [PubMed] [Google Scholar]
- 51. Togawa S, Joh T, Itoh M et al. Interleukin‐2 gene polymorphisms associated with increased risk of gastric atrophy from Helicobacter pylori infection. Helicobacter 2005; 10: 172–8. [DOI] [PubMed] [Google Scholar]
- 52. Hamajima N, Katsuda N, Matsuo K et al. High anti‐Helicobacter pylori antibody seropositivity associated with the combination of IL‐8–251TT and IL‐10–819TT: genotype. Helicobacter 2003; 8: 105–10. [DOI] [PubMed] [Google Scholar]
- 53. Hamajima N, Matsuo K, Suzuki T et al. Low expression myeloperoxidase negatively associated with Helicobacter pylori infection. Jpn J Cancer Res 2001; 92: 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katsuda N, Hamajima N, Tamakoshi A et al. Helicobacter pylori seropositivity and the Myeloperoxidase G‐463A polymorphism in combination with interleukin‐1B in Japanese Health Checkup Examinees. Jpn J Clin Oncol 2003; 33: 192–7. [DOI] [PubMed] [Google Scholar]
- 55. Goto Y, Hamajima N, Honda H et al. Association between Helicobacter pylori seropositivity and NAD(P)H quinone oxidoreductase 1 (NQO1) C609T polymorphism observed for outpatients and health checkup examinees. Gastric Cancer 2005; 8: 12–17. [DOI] [PubMed] [Google Scholar]
- 56. Liao S‐Y, Zeng Z‐R, Leung WK et al. Peroxisome proliferator‐activated receptor‐gamma Pro12Ala polymorphism, Helicobacter pylori infection and non‐cardia gatric carcinoma in Chinese. Aliment Pharmacol Ther 2006; 23: 289–94. [DOI] [PubMed] [Google Scholar]
- 57. Goto Y, Ando T, Yamamoto K et al. Association between serum pepsinogens and polymorphism of PTPN11 encoding SHP‐2 among Helicobacter pylori seropositive Japanese. Int J Cancer 2006; 118: 203–8. [DOI] [PubMed] [Google Scholar]
- 58. Kunstmann E, Hardt C, Elitok E et al. The nonfunctional allele TCRBV6S1B is strongly associated with Helicobacter pylori infection. Infect Immun 2000; 68: 6493–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hamajima N, Shibata A, Katsuda N et al. The highest Helicobacter pylori seropositive rate among those with TNF‐A‐857TT and ‐1031TT: genotypes. Gastric Cancer 2003; 6: 230–6. [DOI] [PubMed] [Google Scholar]
- 60. Atsuta Y, Ito LS, Oba‐Shinjo SM et al. Associations of TNF‐A‐1031TT and ‐857TT: genotypes with Helicobacter pylori seropositivity and gastric atrophy among Japanese Brazilians. Int J Clin Oncol 2006; 11: 140–5. [DOI] [PubMed] [Google Scholar]
- 61. Kunstmann E, Epplen C, Elitok E et al. Helicobacter pylori infection and polymorphisms in the tumor necrosis factor region. Electrophoresis, 1999; 20: 1756–61. [DOI] [PubMed] [Google Scholar]
- 62. Zambon C‐F, Basso D, Navaglia F et al. Pro‐ and anti‐inflammatory cytokine gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine 2005; 29: 141–52. [DOI] [PubMed] [Google Scholar]
- 63. El‐Omar EM, Carrington M, Chow W‐H et al. Interleukin‐1 polymorphisms associated with increased risk of gastric cancer. Nature 2000; 404: 398–402. Erratum in: Nature 2001; 412: 99. [DOI] [PubMed] [Google Scholar]
- 64. Machado JC, Pharoah P, Sousa S et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology 2001; 121: 823–9. [DOI] [PubMed] [Google Scholar]
- 65. Chang Y‐W, Jang J‐Y, Kim N‐H et al. Interleukin‐1β (IL‐1β) polymorphisms and gastric mucosal levels of IL‐1β cytokine in Korean patients with gastric cancer. Int J Cancer 2005; 114: 465–71. [DOI] [PubMed] [Google Scholar]
- 66. Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)‐1β, IL‐1RN, IL‐8, IL‐10 and tumor necrosis factor α and risk of gastric cancer in a Chinese population. Carcinogenesis 2005; 26: 631–6. [DOI] [PubMed] [Google Scholar]
- 67. Palli D, Saieva C, Luzzi I et al. Interleukin‐1 gene polymorphisms and gastric cancer risk in a high‐risk Italian population. Am J Gastroenterol 2005; 100: 1941–8. [DOI] [PubMed] [Google Scholar]
- 68. Xuan J, Deguchi R, Watanabe S et al. Relationship between IL‐1β gene polymorphism and gastric mucosal IL‐1β levels in patients with Helicobacter pylori infection. J Gastroenterol 2005; 40: 796–801. [DOI] [PubMed] [Google Scholar]
- 69. Hwang I‐R, Kodama T, Kikuchi S et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1β production in Helicobacter pylori infection. Gastroenterology 2002; 123: 1793–803. [DOI] [PubMed] [Google Scholar]
- 70. Vilaichone R‐K, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Gastric mucosal cytokine levels in relation to host interleukin‐1 polymorphisms and Helicobacter pylori cagA genotype. Scand J Gastroenterol 2005; 40: 530–9. [DOI] [PubMed] [Google Scholar]
- 71. Hullkonen J, Laippata P, Hurme M. A rare allele combination of the interleukin‐1 gene complex is associated with high interleukin‐1β plasma levels in healthy individuals. Eur Cytokine Netw 2000; 11: 251–5. [PubMed] [Google Scholar]
- 72. Rad R, Dossumbekova Neu B et al. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonization during Helicobacter pylori infection. Gut 2004; 53: 1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dinarello CA. Biological basis for interleukin‐1 in disease. Blood 1996; 87: 2095–147. [PubMed] [Google Scholar]
- 74. Kamizono S, Hiromatsu Y, Seki N et al. A polymorphism of the 5′ flanking region of tumour necrosis factor α gene is associated with thyroid‐associated ophthalmopathy in Japanese. Clin Endocrinol 2000; 52: 759–64. [PubMed] [Google Scholar]
- 75. Yamaguchi E, Itoh A, Hizawa N, Kawakami Y. The gene polymorphism of tumor necrosis factor‐β, but not that of tumor necrosis factor‐α, is associated with the prognosis of sarcoidosis. Chest 2001; 119: 753–61. [DOI] [PubMed] [Google Scholar]
- 76. Higuchi T, Seki N, Kamizono S et al. Polymorphism of the 5′‐flanking region of the human tumor necrosis factor (TNF)‐α gene in Japanese. Tissue Antigens 1998; 51: 605–12. [DOI] [PubMed] [Google Scholar]
- 77. D’Alfonso S, Richiardi PM. A polymorphic variation in a putative regulation box of the TNFα promoter region. Immunogenet 1994; 39: 150–5. [DOI] [PubMed] [Google Scholar]
- 78. Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 2000; 129: 77–97. [DOI] [PubMed] [Google Scholar]
- 79. Siegel D, McGuinness SM, Winski SL, Ross D. Genotype–phenotype relationships in studies of a polymorphism in NAD(P)H quinone oxidoreductase 1. Pharmacogenetics 1999; 9: 113–21. [DOI] [PubMed] [Google Scholar]
- 80. Suzuki T, Matsuo K, Ito H et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med 2006; 119: 217–24. [DOI] [PubMed] [Google Scholar]
- 81. Tsugane S, Kabuto M, Imai H et al. Helicobacter pylori, dietary factors, and atrophic gastritis in five Japanese populations with different gastric cancer mortality. Cancer Causes Control 1993; 4: 297–305. [DOI] [PubMed] [Google Scholar]
- 82. Fontham ETH, Ruiz B, Perez A, Hunter F, Correa P. Determinants of Helicobacter pylori infection and chronic gastritis. Am J Gastroenterol 1995; 90: 1094–101. [PubMed] [Google Scholar]
- 83. Kuippers EJ, Uyterlinde AM, Pena AS et al. Long‐term sequelae of Helicobacter pylori gastritis. Lancet 1995; 345: 1525–8. [DOI] [PubMed] [Google Scholar]
- 84. Ozasa K, Kurata JH, Higashi A et al. Helicobacter pylori infection and atrophic gastritis. A nested case‐control study in a rural town in Japan. Dig Dis Sci 1999; 44: 253–6. [DOI] [PubMed] [Google Scholar]
- 85. Kuwahara Y, Kono S, Eguchi H, Hamada H, Shinchi K, Imanishi K. Relationship between serologically diagnosed chronic atrophic gastritis, Helicobacter pylori, and environmental factors in Japanese men. Scand J Gastroenterol 2000; 35: 476–81. [DOI] [PubMed] [Google Scholar]
- 86. Namekata T, Miki K, Kimmey M et al. Chronic atrophic gastritis and Helicobacter pylori infection among Japanese Americans in Seattle. Am J Epidemiol 2000; 151: 820–30. [DOI] [PubMed] [Google Scholar]
- 87. Shibata K, Moriyama M, Fukushima T, Une H, Miyazaki M, Yamaguchi N. Relation of Helicobacter pylori infection and lifestyle to the risk of chronic atrophic gastritis: a cross‐sectional study in Japan. J Epidemiol 2002; 12: 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology 1982; 83: 204–9. [PubMed] [Google Scholar]
- 89. Chen VW, Abu‐Elyazeed RR, Zavala DE et al. Risk factors of gastric precancerous lesions in a high‐risk Colombian population. I. Salt. Nutr Cancer 1990; 13: 59–65. [DOI] [PubMed] [Google Scholar]
- 90. Montani A, Sasazuki S, Inoue M, Higuchi K, Arakawa T, Tsugane S. Food/nutrient intake and risk of atrophic gastritis among the Helicobacter pylori‐infected population of northeastern Japan. Cancer Sci 2003; 94: 372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ito LS, Oba‐Shinjo SM, Marie SKN et al. Lifestyle factors associated with atrophic gastritis among Helicobacter pylori‐seropositive Japanese‐Brazilians in Sao Paulo. Int J Clin Oncol 2003; 8: 362–8. [DOI] [PubMed] [Google Scholar]
- 92. Sasazuki S, Sasaki S, Tsubono Y et al. The effect of 5‐year vitamin C supplementation on serum pepsinogen level and Helicobacter pylori infection. Cancer Sci 2003; 94: 378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hatakeyama M, Higashi H. Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis. Cancer Sci 2005; 96: 835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA. SHP−2 signaling by interaction between CagA and C‐terminal Src kinase. J Biol Chem 2003; 278: 3664–70. [DOI] [PubMed] [Google Scholar]
- 95. Azuma T. Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. J Gastroenterol 2004; 39: 97–103. [DOI] [PubMed] [Google Scholar]
- 96. Bamford KB, Fan X, Crowe SE et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 1998; 114: 482–92. [DOI] [PubMed] [Google Scholar]
- 97. Lundgren A, Trollmo C, Edebo A, Svennerholm AM, Lundin BS. Helicobacter pylori‐specific CD4+ T cells home to and accumulate in the human Helicobacter pylori‐infected gastric mucosa. Infect Immun 2005; 73: 5612–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Taylor JM, Ziman ME, Huff JL, Moroski NM, Vajdy M, Solnick JV. Helicobacter pylori lipopolysaccharide promotes a Th1 type immune response in immunized mice. Vaccine 2006; 24: 4987–94. [DOI] [PubMed] [Google Scholar]
- 99. Mentink‐Kane MM, Wynn TA. Opposing roles for IL‐13 and IL‐13 receptor α2 in health and disease. Immunol Rev 2004; 202: 191–202. [DOI] [PubMed] [Google Scholar]
- 100. Uno M, Ito LS, Oba‐Shinjo SM et al. Possible association of interleukin 1B C‐31T polymorphism among Helicobacter pylori seropositive Japanese Brazilians with susceptibility to atrophic gastritis. Int J Mol Med 2004; 14: 421–6. [PubMed] [Google Scholar]
- 101. Togawa S, Joh T, Itoh M et al. Interleukin‐2 gene polymorphisms associated with increased risk of gastric atrophy form Helicobacter pylori infection. Helicobacter 2005; 10: 172–8. [DOI] [PubMed] [Google Scholar]
- 102. Hellmig S, Mascheretti S, Folsch U, Schreiber S. Functional promoter polymorphism in RANTES gene does not influence the clinical course of Helicobacter pylori infection. Gastroenterology 2005; 20: 405–8. [DOI] [PubMed] [Google Scholar]
- 103. Williams TM, Eisenberg L, Burlein JE et al. Two regions within the human IL‐2 gene promoter are important for inducible IL‐2 expression. J Immunol 1988; 141: 662–6. [PubMed] [Google Scholar]
- 104. Hoffmann SC, Stanley EM, Darrin Cox E et al. Association of cytokine polymorphic inheritance and in vitro cytokine production in anti‐CD3/CD28‐stimulated peripheral blood lymphocytes. Transplantation 2001; 72: 1444–50. [DOI] [PubMed] [Google Scholar]
- 105. Hoffmann SC, Stanley EM, Darrin Cox E et al. Ethnicity greatly influences cytokine gene polymorphism distribution. Am J Transplant 2002; 2: 560–7. [DOI] [PubMed] [Google Scholar]
- 106. Howard TD, Whittaker PA, Zaiman AL et al. Identification and association of polymorphisms in the interleukin‐13 gene with asthma and atopy in a Dutch population. Am J Respir Cell Mol Biol 2001; 25: 377–84. [DOI] [PubMed] [Google Scholar]
- 107. Van der Pouw Kraan TC, Van Veen A, Boeije LC et al. An IL‐13 promoter polymorphism associated with increased risk of allergic asthma. Genes Immun 1999; 1: 61–5. [DOI] [PubMed] [Google Scholar]
- 108. You W‐C, Hong J‐Y, Zhang L et al. Genetic polymorphisms of CYP2E1, GSTT1, GSTP1, GSTM1, ALDH2, and ODC and the risk of advanced precancerous gastric lesions in a Chinese population. Cancer Epidemiol Biomarkers Prev 2005; 14: 451–8. [DOI] [PubMed] [Google Scholar]
- 109. Rad R, Prinz C, Neu B et al. Synergistic effect of Helicobacter pylori virulence factors and interleukin‐1 polymorphisms for the development of severe histological changes in the gastric mucosa. J Infect Dis 2003; 188: 272–81. [DOI] [PubMed] [Google Scholar]
- 110. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12: 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J 2005; 81: 419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Inoue M, Tajima K, Kobayashi S et al. Protective factor against progression from atrophic gastritis to gastric cancer − data from a cohort study in Japan. Int J Cancer 1996; 66: 309–14. [DOI] [PubMed] [Google Scholar]
- 113. Shikata K, Kiyohara Y, Kubo M et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: The Hisayama study. Int J Cancer 2006; 119: 196–201. [DOI] [PubMed] [Google Scholar]
- 114. Fujino Y, Mizoue T, Tokui N et al. Cigarette smoking and mortality due to stomach cancer: findings from the JACC study. J Epidemiol 2005; 15: S113–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gonzalez CA, Pera G, Agudo A et al. Smoking and the risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 2003; 107: 629–34. [DOI] [PubMed] [Google Scholar]
- 116. Wu M‐S, Wu C‐Y, Chen C‐J et al. Interleukin‐10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer 2003; 104: 617–23. [DOI] [PubMed] [Google Scholar]
- 117. Kneller RW, You WC, Chang YS et al. Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J Natl Cancer Inst 1992; 84: 1261–6. [DOI] [PubMed] [Google Scholar]
- 118. You WC, Zhang L, Gail MH et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst 2000; 92: 1607–12. [DOI] [PubMed] [Google Scholar]
- 119. Kato I, Vivas J, Plummer M et al. Environmental factors in Heliocobacter pylori‐related gastric precancerous lesions in Venezuela. Cancer Epidemiol Biomarkers Prev 2004; 13: 468–76. [PubMed] [Google Scholar]
- 120. Nakamura M, Haruma K, Kamada T et al. Cigarette smoking promotes atrophic gastritis in Helicobacter pylori‐positive subjects. Dig Dis Sci 2002; 47: 675–81. [DOI] [PubMed] [Google Scholar]
- 121. Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking‐associated cancer. Oncogene 2002; 21: 7435–51. [DOI] [PubMed] [Google Scholar]
- 122. Gonzalez CA, Sala N, Capella G. Genetic susceptibility and gastric cancer risk. Int J Cancer 2002; 100: 249–60. [DOI] [PubMed] [Google Scholar]
- 123. Ebert MP, Lendeckel U, Westphal S et al. The angiotensin I‐converting enzyme gene insertion/deletion polymorphism in linked to early gastric cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 2987–9. [DOI] [PubMed] [Google Scholar]
- 124. Geddert H, Kiel S, Zotz RB et al. Polymorphism of p16 INK4A and cyclic D1 in adenocarcinomas of the upper gastrointestinal tract. J Cancer Res Clin Oncol 2005; 131: 803–8. [DOI] [PubMed] [Google Scholar]
- 125. Li H, Chen X‐L, Li H‐Q. Polymorphism of CYPIA1 and GSTM1 genes associated with susceptibility of gastric cancer in Shandong province of China. World J Gastroenterol 2005; 11: 5757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tsukino H, Kuroda Y, Qio D, Nakao H, Imai H, Katoh T. Effects of cytochrome p450 (CYP) 2A6 gene deletion and CYP2E1 genotypes on gastric adenocarcinoma. Int J Cancer 2002; 100: 425–8. [DOI] [PubMed] [Google Scholar]
- 127. Sugimoto M, Furuta T, Shirai N et al. Poor metabolizer genotype status of CYP2C19 is a risk factor for developing gastric cancer in Japanese patients with Helicobacter pylori infection. Aliment Pharmacol Ther 2005; 22: 1033–40. [DOI] [PubMed] [Google Scholar]
- 128. Gao C, Takezaki T, Wu J et al. Interaction between cytochrome‐450 2E1 polymorphisms and environmental factors with risk of esophageal and stomach cancers in Chinese. Cancer Epidemiol Biomarkers Prev 2002; 11: 29–34. [PubMed] [Google Scholar]
- 129. Wu M‐S, Huang S‐P, Chang Y‐T et al. Associaition of the −160 C > A promoter polymorphism of E‐cadherin gene with gastric carcinoma risk. Cancer 2002; 94: 1443–8. [DOI] [PubMed] [Google Scholar]
- 130. Goto Y, Ando T, Goto H, Hamajima N. No association between EGF gene polymorphism and gastric cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 2454–6. [DOI] [PubMed] [Google Scholar]
- 131. Lai KC, Chen WC, Tsai FJ, Chou MC, Jeng LB. Glutathione S‐transferase M1 gene null genotype and gastric cancer risk in Taiwan. Hepatogastroenterology 2005; 52: 1916–19. [PubMed] [Google Scholar]
- 132. Lee Y‐A, Ki C‐S, Kim H‐J et al. Novel interleukin 1β polymorphism increases the risk of gastric cancer in Korean population. J Gastroenterol 2004; 39: 429–33. [DOI] [PubMed] [Google Scholar]
- 133. Savage SA, Abnet CC, Haque K et al. Polymorphisms in interleukin‐2, ‐6, and ‐10 are not associated with gastric cardia or esophageal cancer in a high‐risk Chinese population. Cancer Epidemiol Biomarkers Prev 2004; 13: 1547–9. [PubMed] [Google Scholar]
- 134. Lai K‐C, Chen W‐C, Jeng L‐B, Li S‐Y, Chou M‐C, Tsai F‐J. Association of genetic polymorphisms of MK, IL‐4, p16, p21, p53 genes and human gastric cancer in Taiwan. Eur J Surg Oncol 2005; 31: 1135–40. [DOI] [PubMed] [Google Scholar]
- 135. Lacasana‐Navarro M, Galvan‐Portillo M, Chen J, Lopez‐Cervantes M, Lopez‐Carrillo L. Methylenetetrahydrofolate reductase 677C > T polymorphism and gastric cancer susceptibility in Mexico. Eur J Cancer 2006; 42: 528–33. [DOI] [PubMed] [Google Scholar]
- 136. Kim JK, Kim S, Han HJ et al. Polymorphisms of 5,10‐methylenetetrahydrofolate reductase and risk of stomach cancer in a Korean population. Anticancer Res 2005; 25: 2249–52. [PubMed] [Google Scholar]
- 137. Hamajima N, Matsuo K, Iwata H et al. NAD(P)H quinone oxidoreductase 1 (NQO1) C609T polymorphism and the risk of eight cancers in Japan. Int J Clin Oncol 2002; 7: 103–8. [DOI] [PubMed] [Google Scholar]
- 138. Duarte MC, Colombo J, Rossit ARB et al. Polymorphisms of DNA repair genes XRCC1 and XRCC2, interaction with environmental exposure and risk of chronic gastritis and gastric cancer. World J Gastroenterol 2005; 11: 6593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Taguchi A, Ohmiya N, Shirai K et al. Interleukin‐8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev 2005; 14: 2487–93. [DOI] [PubMed] [Google Scholar]
- 140. Hiyama T, Tanaka S, Kitadai Y et al. p53 codon 73 polymorphism in gastric cancer susceptibility in patients with Helicobacter pylori‐associated chronic gastritis. Int J Cancer 2002; 100: 304–8. [DOI] [PubMed] [Google Scholar]
- 141. Chung WC, Lee KM, Lee BI et al. p53 genetic polymorphism of gastric cancer in Korea. Korean J Intern Med 2006; 21: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


