Abstract
The aim of this clinical trial was to investigate the feasibility of intra‐arterial infusion of in vitro‐expanded Vα24 natural killer T (NKT) cells combined with submucosal injection of α‐galactosylceramide (KRN7000; αGalCer)‐pulsed antigen‐presenting cells (APC). A phase I clinical study was carried out in patients with head and neck squamous cell carcinoma (HNSCC). Patients with locally recurrent HNSCC refractory to standard therapy were eligible. Eight patients received super‐selective transcatheter intra‐arterial infusion of activated Vα24 NKT cells into tumor‐feeding arteries and nasal submucosal injections of αGalCer‐pulsed APC twice with a 1‐week interval. Vα24 NKT cell‐specific immune responses, safety, and antitumor effects were evaluated. The number of Vα24 NKT cells and interferon‐γ‐producing cells in peripheral blood mononuclear cells increased in seven out of eight patients enrolled. Grade 3 toxicity with a pharyngocutaneous fistula related to local tumor reduction was observed in one patient and mild adverse events with grade 1–2 symptoms occurred in seven patients. Regarding the clinical responses, three cases exhibited a partial but significant response, four were classified as stable disease, and one patient continued to develop progressive disease. The use of the intra‐arterial infusion of activated Vα24 NKT cells and the submucosal injection of αGalCer‐pulsed APC has been shown to induce significant antitumor immunity and had beneficial clinical effects in the management of advanced HNSCC. The use of such therapeutic modalities may be helpful in the management of tumors and therefore needs to be explored in further detail. The clinical trial registration number was UMIN000000722. (Cancer Sci 2009; 100: 1092–1098)
Abbreviations:
- APC
antigen‐presenting cells
- CT
computed tomography
- DC
dendritic cells
- ELISPOT
enzyme‐linked immunospot
- αGalCer
α‐galactosylceramide
- HLA‐DR
human leukocyte antigens‐DR
- HNSCC
head and neck squamous cell carcinoma
- IFN
interferon
- IL
interleukin
- mAb
monoclonal antibody
- NK
natural killer
- NKT cells
natural killer T cells
- PBMC
peripheral blood mononuclear cells
- PD
progressive disease
- PE
phycoerythrin
- PR
partial response
- SD
stable disease
- SFC
spot‐forming cells
- TCR
T‐cell receptor
The management of most patients with head and neck cancer generally involves a combination of chemotherapy, radiation therapy, and surgery. Concurrent chemotherapy and radiotherapy has been suggested to have a survival benefit and allow organ preservation postoperatively for patients at high risk for recurrence.( 1 , 2 , 3 , 4 ) However, the increased toxicity and extensive functional morbidity induced by such combination therapy can severely impair the patients’ quality of life, and the prognosis for these patients remains poor.( 5 ) Therefore, the development of new treatment strategies for head and neck cancer is of critical importance.
Invariant NKT cells are unique lymphocytes characterized by the coexpression of an invariant TCR and a NK receptor.( 6 , 7 , 8 ) Human Vα24 NKT cells express the invariant Vα24JαQ chain paired with the Vβ11 antigen receptor and they are activated by a specific glycolipid antigen, αGalCer (KRN7000), presented by a monomorphic major histocompatibility complex‐like molecule, CD1d.( 9 , 10 , 11 , 12 , 13 ) After activation, human Vα24 NKT cells exert strong antitumor activity against various malignant tumors both in vitro and in vivo.( 14 , 15 , 16 , 17 , 18 , 19 ) Activated Vα24 NKT cells also induce cell death in tumor cells by the expression of a wide variety of cell death‐inducing effector molecules, including perforin, Fas ligand, and tumor necrosis factor‐related apoptosis‐inducing ligand, in a manner similar to other cytotoxic cells such as NK cells and CD8 cytotoxic T cells.( 20 , 21 ) Activated Vα24 NKT cells produce high levels of cytokines, such as IFN‐γ and IL‐4, thereby activating other antitumor effector cells.( 22 , 23 , 24 ) Therefore, the use of Vα24 NKT cells in cancer immunotherapy has attracted attention as a potential therapeutic agent.( 25 , 26 , 27 , 28 )
Antigen‐presenting cells such as DC play a crucial role in the induction of primary T cell‐dependent immune responses, and APC‐based cancer immunotherapy induces significant immunological responses and some clinical improvement in patients with different malignant diseases.( 29 , 30 , 31 , 32 , 33 ) Head and neck cancers arise from the mucosal surface of the upper respiratory or digestive tracts and the lymph nodes of the neck, which are normally defined as regional. In a previous study, isotope‐labeled tumor‐specific peptide‐pulsed APC were administered into the nasal submucosa of patients with head and neck cancer.( 34 ) These cells quickly migrated to the regional neck lymph nodes. Tumor antigen‐specific IFN‐γ‐secreting cells and cytotoxic T cells were detected in the ipsilateral neck lymph nodes, but not in the contralateral lymph nodes. A phase I study of αGalCer‐pulsed APC administration to the nasal submucosa in unresectable or recurrent head and neck cancer was carried out with two administrations of 1 × 108αGalCer‐pulsed APC at a 1‐week interval, and significant NK responses and negligible adverse events were observed.( 35 ) The blood supply of most head and neck cancers is provided by a terminal artery, such as a branch of the external carotid artery. Therefore, selective intra‐arterial infusions of anticancer drugs are used widely as a standard treatment.( 36 ) It would be interesting to elucidate whether the intra‐arterial administration of activated effector cells to the cancer‐feeding vessels can provide efficient targeting of these cells to the cancer tissue and give more effective antitumor responses.
A phase I study was designed with the intra‐arterial administration of in vitro‐expanded Vα24 NKT cells to patients with recurrent refractory HNSCC, in combination with nasal submucosal injection of αGalCer‐pulsed APC. The development of NKT and NK cell‐specific systemic immune responses was examined, and the tolerability and outcome of any beneficial clinical responses were examined.
Materials and Methods
Cell preparation. APC containing DC and activated Vα24 NKT cells from peripheral blood were prepared as described previously.( 25 , 26 , 35 , 37 ) Briefly, collected PBMC were cultured with granulocyte‐macrophage colony‐stimulating factor (800 U/mL; Primmune, Kobe, Japan) and IL‐2 (100 JRU/mL; Imunace, Shionogi, Osaka, Japan) to generate APC, or with IL‐2 (100 U/mL) and αGalCer (100 ng/mL KRN7000; Kyowa Hakko Kirin, Gunma, Japan) to produce in vitro‐activated Vα24 NKT cells. The cultured APC were pulsed with 100 ng/mL αGalCer on the day before use.
Patient selection. Patients with locally recurrent HNSCC refractory to standard therapy were eligible for the study. The subjects ranged in age from 20 to 80 years. The subjects had an Eastern Cooperative Oncology Group performance status score of 0–2, had received no therapy for at least 4 weeks, and were expected to survive for at least 6 months or more. The routine laboratory profile of these subjects included: white blood cell (WBC) count ≥ 3000/µL; platelet count ≥ 75 000/µL; serum creatinine ≤ 1.5 mg/dL; total bilirubin ≤ 1.5 mg/dL; and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 × upper limit of normal. The Vα24 NKT cells were detected by flow cytometry at a level of at least 10 cells in 1 mL of peripheral blood and expanded more than 100 times in vitro.
The subjects who were excluded from participation in this study included: patients with microbiological or immunological evidence of infection with human immunodeficiency virus, hepatitis B or C virus, or human T‐lymphotropic virus; evidence of any active autoimmune disease; a history of hepatitis, pregnancy, or lactation; concurrent corticosteroid therapy; or the presence of any other malignant neoplasm.
Study design. The study was conducted at the Department of Otorhinolaryngology/Head and Neck Surgery, Chiba University Hospital, Chiba, Japan, according to the standards of Good Clinical Practice for Trials on Medicinal Products in Japan. The protocol was approved by the Institutional Ethics Committee (no. 421) and conformed to the provisions of the Declaration of Helsinki in 1995. In addition, the trial underwent ad hoc reviews by the Chiba University Quality Assurance Committee on Cell Therapy. The study design was a phase I, single‐arm open‐label trial to investigate the immunological response and test the tolerability of intra‐arterial infusion of in vitro‐expanded Vα24 NKT cells and submucosal injection of αGalCer‐pulsed APC in patients with locally recurrent HNSCC. Written informed consent was obtained from all patients before they underwent a screening evaluation to determine eligibility.
After screening and registration, the patients underwent leukapheresis for the collection of PBMC on day 0. Thereafter, on days 7 and 13 all patients received an injection of 1 × 108αGalCer‐pulsed APC into the nasal submucosa, and then received super‐selective trans‐catheter arterial infusion of Vα24 NKT cells (5 × 107) into a tumor‐feeding artery on day 14 (Fig. 1). The activated Vα24 NKT cells could be infused to more than a single artery as multiple tumor feeding vessels were identified. The amounts of infused cells were determined according to the blood flow of each feeding vessel. Clinical and laboratory tests were conducted weekly for 5 weeks. These tests included a complete physical examination and determination of standard laboratory values. PBMC collected at each time point were used for flow cytometric analysis and frozen for a subsequent ELISPOT assay. All patients underwent assessment of their tumor status by CT at baseline and 5 weeks after entry into the study. The responses were assessed using the Response Evaluation Criteria in Solid Tumors guidelines. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Figure 1.
Study timeline of combination therapy of in vitro‐expanded Vα24 natural killer T (NKT) cells and α‐galactosylceramide (αGalCer)‐pulsed antigen‐presenting cells (APC). The patients underwent leukapheresis on day 0, and received αGalCer‐pulsed APC on days 7 and 13 in the nasal submucosa and activated Vα24 NKT cells on day 14 via tumor‐feeding arteries. The timing of leukapheresis and cell administration is shown.
All eight patients with HNSCC completed the study protocol and were included in the analysis. The patient characteristics are summarized in Table 1. All patients had previously received radiation therapy and five had undergone surgical resection. Six patients had local recurrent lesions that were not indicated for surgical resection because of direct invasion of the carotid artery (patients 1, 2, 3, 5, and 8) or vertebral bone (patient 4), and two patients (patients 2 and 6) had lung metastasis.
Table 1.
Patients and tumor characteristics
| Case | Age (years) | Sex | PS | Diagnosis | Disease site | Prior treatment |
|---|---|---|---|---|---|---|
| 1 | 61 | F | 0 | Recurrent hypopharyngeal cancer | Pharynx | ST, RT, CT |
| 2 | 67 | M | 1 | Recurrent laryngeal cancer | Cervical esophagus | ST, RT, CT |
| Lung metastasis | Lung | |||||
| 3 | 40 | M | 1 | External auditory canal cancer with invasion to skull bone | Temporal bone | RT, CT |
| 4 | 74 | M | 1 | Recurrent laryngeal cancer | Larynx | RT, CT |
| 5 | 38 | F | 1 | Recurrent tongue cancer | Tongue | ST, RT, CT |
| 6 | 58 | M | 1 | Recurrent laryngeal cancer | Skin flap | ST, RT, CT |
| Lung metastasis | Lung | |||||
| 7 | 65 | F | 1 | Recurrent gingival cancer | Maxillae | ST, RT |
| 8 | 77 | M | 1 | Recurrent cervical esophageal cancer | Cervical esophagus | RT, CT |
CT, chemotherapy; PS, performance status; RT, radiation therapy; ST, surgical treatment.
Flow cytometric analysis. The phenotypes of peripheral blood lymphocytes, in vitro‐activated Vα24 NKT cells, and αGalCer‐pulsed APC were determined as described previously.( 25 , 26 , 35 ) The following mAb were used: fluorescein isothiocyanate‐labeled anti‐TCR Vα24 mAb (Immunotech, Marseilles, France), anti‐HLA‐DR (BD Biosciences, Franklin Lakes, NJ, USA), PE‐labeled anti‐TCR Vβ11 mAb (Immunotech), anti‐CD56 mAb (BD Biosciences), anti‐CD86 (BD Biosciences), and allophycocyanin‐labeled anti‐CD3 mAb (BD Biosciences), and anti‐CD11c (BD Biosciences). Isotype‐matched control mAb were used as negative controls in all flow cytometry analyses. At least 100 Vα24+Vβ11+ NKT cell events were acquired.
Single‐cell ELISPOT assay for detecting IFN‐k‐producing cells in PBMC. Frozen PBMC collected at each time point were thawed and cultured for 6 h. The cultured cells (5 × 105 per well) were washed and transferred to 96‐well filtration plates (Millipore, Danvers, MA, USA) coated with anti‐IFN‐γ capture antibody (Mabtech AB, Stockholm, Sweden) for 16 h. Stimulation was carried out with the addition of 100 ng/mL αGalCer or vehicle as described previously.( 26 , 35 ) Concanavalin A was used as a positive control. Biotinylated antihuman IFN‐γ antibody (Mabtech AB) was added. Two hours later, spots were detected with an avidin–biotin–peroxidase complex (Mabtech AB) and aminoethylcarbazole solution. In this system, the majority of IFN‐γ‐producing cells are NK and NKT cells but not T cells, as described previously.( 26 ) SFC were quantified objectively using an ImmunoScan computer system and ImmunoSpot software (CTL, Cleveland, OH, USA). To calculate the IFN‐γ‐producing SFC from NK and NKT cells, we subtracted the number of SFC generated by vehicle stimulation from those of SFC by αGalCer stimulation.
Results
Characteristics of αGalCer‐pulsed APC. The surface phenotypes of αGalCer‐pulsed APC were analyzed by flow cytometry. From the percentages of HLA‐DR/CD11c/CD86 triple‐positive cells, the actual number of HLA‐DR+CD11c+CD86+ DC was calculated (Table 2, left and middle). The ranges of the DC population were from 25 to 53% (mean ± SD = 37.5% ± 7.81%), and day 7 APC appear to contain relatively increased numbers of HLA‐DR+CD11c+CD86+ DC compared with day 13 APC (day 7; 40.4%± 8.41%, and day 13; 34.6% ± 5.89%). In addition, the ranges of CD83‐expressing cells and CD1d‐expressing cells were 23 to 57% and 8.1 to 20% (data not shown). The APC contained some CD40+ cells (2–10%), but no detectable CD14+ cells (data not shown).
Table 2.
Characteristics of administered cells
| Case | αGalCer‐pulsed APC (day 7) | αGalCer‐pulsed APC (day 13) | Activated Vα24 NKT cells (day14) | |||||
|---|---|---|---|---|---|---|---|---|
| Total | † DC | Total | † DC | Total | † NKT cells | † NK cells | CD3+ cells | |
| 1 | 1.0 × 108 | 3.6 × 107 | 1.0 × 108 | 3.0 × 107 | 5.0 × 108 | 5.0 × 107 | 1.4 × 108 | 3.5 × 108 |
| 2 | 1.0 × 108 | 5.3 × 107 | 1.0 × 108 | 4.1 × 107 | 8.5 × 109 | 5.0 × 107 | 2.8 × 109 | 5.5 × 109 |
| 3 | 1.0 × 108 | 3.2 × 107 | 1.0 × 108 | 3.2 × 107 | 4.2 × 108 | 5.0 × 107 | 1.5 × 107 | 4.0 × 108 |
| 4 | 1.0 × 108 | 3.8 × 107 | 1.0 × 108 | 3.4 × 107 | 7.0 × 108 | 5.0 × 107 | 3.7 × 107 | 6.2 × 108 |
| 5 | 1.0 × 108 | 4.0 × 107 | 1.0 × 108 | 3.3 × 107 | 1.1 × 108 | 5.0 × 107 | 1.2 × 107 | 9.5 × 107 |
| 6 | 1.0 × 108 | 2.7 × 107 | 1.0 × 108 | 2.5 × 107 | 7.4 × 108 | 5.0 × 107 | 1.3 × 108 | 5.6 × 108 |
| 7 | 1.0 × 108 | 4.9 × 107 | 1.0 × 108 | 3.7 × 107 | 6.2 × 108 | 5.0 × 107 | 1.4 × 108 | 4.3 × 108 |
| 8 | 1.0 × 108 | 4.8 × 107 | 1.0 × 108 | 4.5 × 107 | 5.0 × 109 | 5.0 × 107 | 2.8 × 109 | 1.7 × 109 |
HLA‐DR+CD11c+CD86+ cells, CD3+Vα24+Vβ11+ cells and CD3−CD56+ cells were defined as dendritic cells (DC), natural killer T cells (NKT cells), and natural killer (NK) cells. APC, antigen‐presenting cells.
Characteristics of in vitro‐activated Vα24 NKT cells. The prepared activated Vα24 NKT cell population was analyzed by flow cytometry. NK cells were defined as CD3−CD56+ cells, and T cells, containing Vα24 NKT cells, were defined as CD3+ cells. Vα24 NKT cells were defined by the coexpression of CD3, Vα24, and Vβ11 because this combination has been demonstrated to be specific for αGalCer‐reactive invariant Vα24 NKT cells.( 26 , 38 ) Freshly isolated PBMC contained very small percentages of CD3+Vα24+Vβ11+ NKT cells relative to lymphocytes (e.g. 0.085% in case 1). After a 2‐week cultivation in the presence of αGalCer and IL‐2, this population expanded to approximately 10% (11.2%± 12.4%) of the cultured cells. The total numbers of administered cells and the actual numbers of CD3+Vα24+Vβ11+ NKT cells from all patients are summarized in Table 2. Each patient received an injection of 5 × 107 CD3+Vα24+Vβ11+ NKT cells.
Immunological monitoring. Immunological assays were conducted using samples from eight patients. The absolute numbers of peripheral blood Vα24 NKT cells were calculated using a flow cytometric analysis and automated full blood counts. Because the blood sample was collected before the injection of αGalCer‐pulsed APC on day 7, the values on day 7 should be the baseline level in each patient. As shown in 2, 3 patients showed moderate increases (≥2 × 2) in the number of circulating Vα24 NKT cells (case 1, ×2.4 on day 14; case 3, ×3.0 on day 21; case 5, ×2.3 on day 21), and four patients showed greater increases (≥ ×4) (case 2, ×4.4 on day 28; case 4, ×5.2 on day 21; case 6, ×9.5 on day 28; case 8, ×4.5 on day 21). In these four patients, the increase was observed 1 or 2 weeks after the activated Vα24 NKT cell injection. Case 7 did not show an obvious increase in the number of Vα24 NKT cells.
Figure 2.
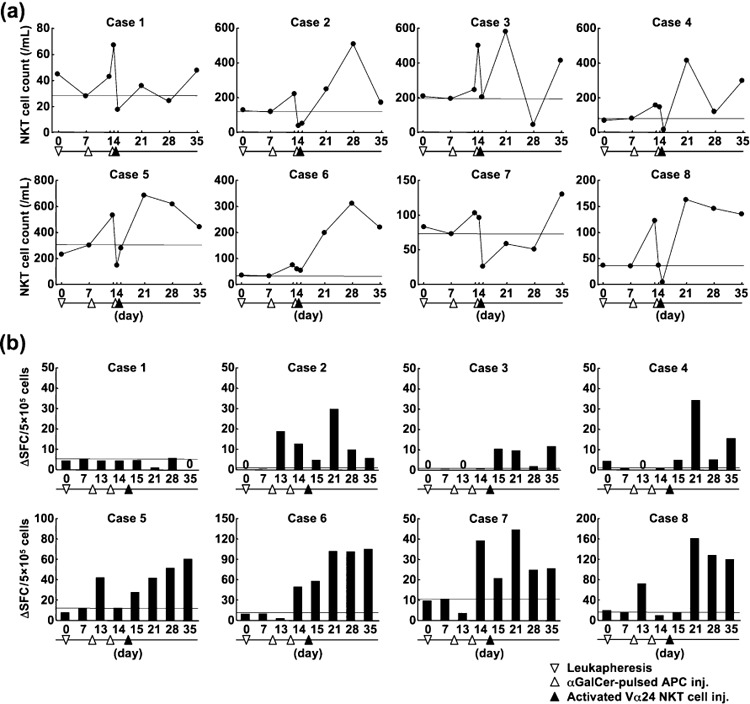
Immunological assays of peripheral blood samples from eight patients. (a) The kinetics of circulating Vα24 natural killer T (NKT) cells during the course of treatment. The thin black horizontal line represents the level of Vα24 NKT cell numbers on day 7 (before the first antigen‐presenting cell [APC] injection). (b) The number of interferon (IFN)‐γ spot‐forming cells (SFC) induced by in vitro stimulation with α‐galactosylceramide (αGalCer) during the course of treatment. Frozen peripheral blood mononuclear cells (PBMC) were thawed and stimulated for 16 h with αGalCer or vehicle. The mean numbers of IFN‐γ spots were determined from triplicate cultures. Subtracted values of SFC (those induced by αGalCer‐stimulation minus those by vehicle stimulation) are shown.
Figure 3.
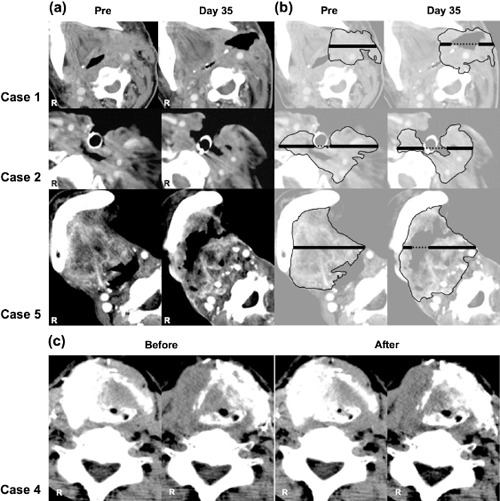
Computed tomography (CT) images. (a) The real CT images of three partial response cases are shown. (b) The cancer region is surrounded by a thin line and the longest diameter is indicated by a bold line. (c) Perfusion CT images before and after the intra‐arterial infusion of the activated Vα24 natural killer T cells are shown (case 4).
The ability of PBMC samples to produce IFN‐γ was assessed using an ELISPOT assay (Fig. 2b). Four patients showed moderate increases (≥ ×4) in IFN‐γ SFC (case 5, ×5.0 on day 35; case 6, ×9.8 on day 35; case 7, ×4.1 on day 21; case 8, ×9.7 on day 21) and three patients showed greater (≥ ×50) elevations in SFC (case 2, ×180 on day 21; case 3, ×72 on day 35; case 4, ×69 on day 21). One patient (case 1) showed no increase in the number of IFN‐γ‐producing cells. Collectively, seven of eight patients (87.5%) appeared to have systemic NKT or NK cell‐specific immunological responses initiated by the treatment.
Adverse events. The adverse events during the study are documented in Table 3. One subject developed a grade 3 fistula (pharynx‐skin), initially detected on day 28, 2 weeks after intra‐arterial infusion of Vα24 NKT cells. In this patient, tumor growth gradually decreased after the administration of Vα24 NKT cells, and the lesion with central low density was induced from the center of the tumor. Finally, rapid collapse of the tumor resulted in a fistula.
Table 3.
Observed adverse events and clinical responses
| Case | Adverse event | Clinical course | Tumor size (%) | ||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |||
| 1 | Lymphopenia | Fistula (Pharynx) | PR | 48 | |
| 2 | Lymphopenia | Fever | PR | 65 | |
| 3 | Pain (external ear) | SD | 104 | ||
| 4 | Fever | SD | 109 | ||
| Headache | |||||
| 5 | Fever | Dehydration, pain (oral cavity) | PR | 65 | |
| 6 | Fatigue | PD | 125 | ||
| Dizziness | |||||
| 7 | Pain (oral cavity) | Pain (back) | SD | 104 | |
| 8 | Fever Fatigue | SD | 110 | ||
PD, progressive disease; PR, partial response; SD, stable disease.
No major toxicity (grade > 2) or severe side effects were observed in the other patients. Four other patients experienced low‐grade fever for less than 24 h after the administration of cells, one subject complained of a headache, and two other subjects of fatigue. However, no additional treatment was required and the symptoms resolved spontaneously. Three patients had an aggravation of cancer pain, which required an increased dose of morphine. One patient had back pain after angiography because she was lying in the same position for 3 h, but the pain disappeared by the next day.
Clinical response. A CT scan for evaluating tumor size was carried out a few days before enrollment. The longest diameter of the measurable lesion was measured as the baseline value. The objective response on day 35 was assessed on the same slice by a review panel including some experienced radiologists using the Response Evaluation Criteria in Solid Tumors guidelines (Table 3). Three patients (1, 2, and 5) showed a PR, four had SD, and one patient had a PD. The PR cases are shown in Figure 3(a,b). In case 1, who had a recurrent hypopharyngeal cancer, the longest tumor diameter of 56.1 mm at baseline decreased to 27.2 mm (48% of the original diameter), so that fistula was generated. Case 2 had a recurrent laryngeal cancer of the cervical esophagus, surrounding a tracheostoma, thus making it difficult to change the tracheal cannula. The longest tumor diameter was 66.6 mm before the treatment, and this was reduced to 43.6 mm (65% of the baseline value) after the infusion of activated Vα24 NKT cells; this reduction in tumor size made it much easier to change the tracheal cannula. In case 5, the enhanced area in CT decreased, and a lesion with low density was apparent in the center of the tumor on day 21. On day 35, the tumor diameters were reduced from 58.3 to 37.7 mm (65%). In addition to the three cases with PR who exhibited clear lesions with central low density, a small low‐density lesion was found in case 3 who showed SD. No obvious necrotic change was observed in any other cases including three SD and one PD.
To confirm that the clinical response was not simply a result of the embolization of the tumor‐feeding artery, a perfusion CT scan was taken just before and after the intra‐arterial infusion of the activated Vα24 NKT cells. Perfusion CT is a relatively new technique that allows rapid qualitative and quantitative evaluation of blood flow. The tumor site was equally enhanced before and after the infusion, and the embolization of tumor‐feeding arteries was not observed. Regardless of the levels of clinical responses (i.e. PR, SD and PD) there was no difference in the picture before and after the infusion. Typical images are shown in Figure 3(c).
Two patients (2 and 6) had metastatic lesions at entry, which were investigated for clinical responses in addition to the local responses. The lesions had not enlarged after treatment in either of the cases and were judged to be SD responses.
Discussion
In our previous study, we injected αGalCer‐pulsed APC (1 × 108) into the nasal submucosa twice with a 1‐week interval. The treatment was well tolerated, and both the increased immune responses and some clinical responses were observed.( 35 ) In addition, we injected activated Vα24 NKT cells intravenously to lung cancer patients. The treatment was well‐tolerated and the increased Vα24 NKT cell immune responses were observed.( 26 ) Based on these findings, we designed this study with the intra‐arterial infusion of activated Vα24 NKT cells (5 × 107) combined with the submucosal injection of αGalCer‐pulsed APC (1 × 108).
Invariant NKT cells are commonly activated by αGalCer in mammals and this treatment could be applied to any patients as an innate immunity. The culture protocol does not need complicated procedures and enough cells can be prepared easily in the short term. Selective intra‐arterial infusion of anticancer drugs has been used widely as a popular treatment in head and neck cancer and the method of selective intra‐arterial infusion has already been established. The mean percentage of CD3+Vα24+Vβ11+NKT cells in intra‐arterially administered cells was approximately 15%, and each patient identically received 50 million of activated NKT cells. In the culture medium, Vα24 NKT cells in PBMC were activated by αGalCer and the activated Vα24 NKT cells produced a large amount of IFN‐γ, which would stimulate the other effector cells (e.g. cytotoxic T lymphocytes (CTL) or NK cells). These non‐NKT cells might act as antitumor factors and therefore these effects could be contained in the NKT cell‐mediated antitumor effects.
Combination therapy with intra‐arterial infusion of activated Vα24 NKT cells and submucosal injection of αGalCer‐pulsed APC was found to be well tolerated, although a pharyngocutaneous fistula was observed in one patient. Several adverse events that were observed in this study (Table 3) were also reported in previous studies using αGalCer‐pulsed APC or in vitro‐activated Vα24 NKT cells.( 25 , 26 , 27 , 28 , 35 ) These adverse events were transient and easily managed by standard supportive treatment. Exacerbation of liver function, which has been observed in a murine model of Vα14 NKT cell activation,( 39 , 40 ) was not observed in this study.
Regarding the systemic immunological responses, remarkably increased Vα24 NKT cell numbers and increased NKT and NK cell activities in peripheral blood occurred in four and five patients, respectively. Seven out of eight patients showed moderate responses in both Vα24 NKT cell numbers and NKT and NK cell activities (Fig. 2). Although the increased numbers of Vα24 NKT cells were not significantly different from those observed in a previous study with only administration of αGalCer‐pulsed APC,( 35 ) IFN‐γ‐producing cells in this combination therapy increased much higher than previous monotherapy. These antitumor immunological responses did not always correlate with the clinical responses. Case 1 had received bilateral neck lymphatic dissection in the previous treatment, which might have prevented migration of the nasal submucosally injected APC and induction of immunological responses in the peripheral blood. Therefore, the application site might need to be reconsidered in patients who had received neck dissection extensively. However, case 1 showed PR in tumor size, which suggested the antitumor effects of the intra‐arterially infused Vα24 NKT cells. The decreased numbers of Vα24 NKT cells in peripheral blood, which were observed in all cases on the day after intra‐arterial infusion of activated Vα24 NKT cells, suggests accumulation in the target cancer tissue, although some might be captured in lung or liver. To clarify these important issues, the pathological study of cancer tissue is indispensable. As the safety and some antitumor activity were shown in the present study, we will apply this combination therapy to patients with resectable recurrent head and neck cancer before radical operation in the next phase II study.
A clinical outcome of PR was achieved in three patients; however, tumor regrowth was observed by CT scan or physical examination 4–5 weeks after the clinical trial. These cases had an extensive unresectable recurrent tumor suspected of direct invasion of the carotid artery. Immunotherapy was applied only once in all cases. Repeated administration of Vα24 NKT cells or αGalCer‐pulsed APC will be planned in the next study. The SD responses for metastatic lesions observed in two cases may be related to increased systemic antitumor activities.
Although the number of patients was limited, the results of the present study of intra‐arterial administration of activated Vα24 NKT cells combined with submucosal injection of αGalCer‐pulsed APC to patients with HNSCC have demonstrated the induction of NKT and NK cell‐specific antitumor activity and some beneficial clinical effects.
Acknowledgments
We would like to acknowledge Kyowa Hakko Kirin Co. for providing clinical‐grade αGalCer (KRN7000), and the Translational Research Informatics Center (Kobe, Japan) for advice about the study protocol design. We would also like to thank Drs Naomi Shimizu and Hideki Hanaoka (Division of Blood Transfusion and Clinical Research Center, Chiba University Hospital, Chiba, Japan) who assisted our clinical trial. This trial was supported by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Scientific Research on Priority Area #17016010 and Cancer Translational Research Project).
References
- 1. Cooper JS, Pajak TF, Forastiere AA et al . Postoperative concurrent radiotherapy and chemotherapy for high‐risk squamous‐cell carcinoma of the head and neck. N Engl J Med 2004; 350: 1937–44. [DOI] [PubMed] [Google Scholar]
- 2. Bernier J, Domenge C, Ozsahin M et al . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350: 1945–52. [DOI] [PubMed] [Google Scholar]
- 3. Tsao AS, Garden AS, Kies MS et al . Phase I/II study of docetaxel, cisplatin, and concomitant boost radiation for locally advanced squamous cell cancer of the head and neck. J Clin Oncol 2006; 24: 4163–9. [DOI] [PubMed] [Google Scholar]
- 4. Forastiere AA, Goepfert H, Maor M et al . Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349: 2091–8. [DOI] [PubMed] [Google Scholar]
- 5. Ledeboer QC, Velden LA, Boer MF, Feenstra L, Pruyn JF. Physical and psychosocial correlates of head and neck cancer: an update of the literature and challenges for the future (1996–2003). Clin Otolaryngol 2005; 30: 303–19. [DOI] [PubMed] [Google Scholar]
- 6. Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol 2003; 21: 483–513. [DOI] [PubMed] [Google Scholar]
- 7. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol 2004; 4: 231–7. [DOI] [PubMed] [Google Scholar]
- 8. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007; 25: 297–336. [DOI] [PubMed] [Google Scholar]
- 9. Kawano T, Cui J, Koezuka Y et al . CD1d‐restricted and TCR‐mediated activation of Vα14 NKT cells by glycosylceramides. Science 1997; 278: 1626–9. [DOI] [PubMed] [Google Scholar]
- 10. Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science 1995; 268: 863–5. [DOI] [PubMed] [Google Scholar]
- 11. Brossay L, Chioda M, Burdin N et al . CD1d‐mediated recognition of an α‐galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med 1998; 188: 1521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spada FM, Koezuka Y, Porcelli SA. CD1d‐restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med 1998; 188: 1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawano T, Tanaka Y, Shimizu E et al . A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int Immunol 1999; 11: 881–7. [DOI] [PubMed] [Google Scholar]
- 14. Kawano T, Nakayama T, Kamada N et al . Antitumor cytotoxicity mediated by ligand‐activated human Vα24 NKT cells. Cancer Res 1999; 59: 5102–5. [PubMed] [Google Scholar]
- 15. Seino K, Fujii S, Harada M et al . Vα14 NKT cell‐mediated anti‐tumor responses and their clinical application. Springer Semin Immunopathol 2005; 27: 65–74. [DOI] [PubMed] [Google Scholar]
- 16. Shin T, Nakayama T, Akutsu Y et al . Inhibition of tumor metastasis by adoptive transfer of IL‐12‐activated Vα14 NKT cells. Int J Cancer 2001; 91: 523–8. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi T, Nieda M, Koezuka Y et al . Analysis of human Vα24+ CD4+ NKT cells activated by α‐glycosylceramide‐pulsed monocyte‐derived dendritic cells. J Immunol 2000; 164: 4458–64. [DOI] [PubMed] [Google Scholar]
- 18. Naiki Y, Nishimura H, Kawano T, Itohara S, Taniguchi M, Yoshikai Y. Regulatory role of peritoneal NK1.1+αβT cells in IL‐12 production during Salmonella infection. J Immunol 1999; 163: 2057–63. [PubMed] [Google Scholar]
- 19. Nakagawa R, Motoki K, Ueno H et al . Treatment of hepatic metastasis of the colon26 adenocarcinoma with an α‐galactosylceramide, KRN7000. Cancer Res 1998; 58: 1202–7. [PubMed] [Google Scholar]
- 20. Kawano T, Cui J, Koezuka Y et al . Natural killer‐like nonspecific tumor cell lysis mediated by specific ligand‐activated Vα14 NKT cells. Proc Natl Acad Sci USA 1998; 95: 5690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nieda M, Nicol A, Koezuka Y et al . TRAIL expression by activated human CD4+Vα24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood 2001; 97: 2067–74. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa R, Nagafune I, Tazunoki Y et al . Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by α‐galactosylceramide in mice. J Immunol 2001; 166: 6578–84. [DOI] [PubMed] [Google Scholar]
- 23. Smyth MJ, Crowe NY, Pellicci DG et al . Sequential production of interferon‐γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α‐galactosylceramide. Blood 2002; 99: 1259–66. [DOI] [PubMed] [Google Scholar]
- 24. Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells – conductors of tumor immunity? Curr Opin Immunol 2002; 14: 165–71. [DOI] [PubMed] [Google Scholar]
- 25. Ishikawa A, Motohashi S, Ishikawa E et al . A phase I study of α‐galactosylceramide (KRN7000)‐pulsed dendritic cells in patients with advanced and recurrent non‐small cell lung cancer. Clin Cancer Res 2005; 11: 1910–17. [DOI] [PubMed] [Google Scholar]
- 26. Motohashi S, Ishikawa A, Ishikawa E et al . A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non‐small cell lung cancer. Clin Cancer Res 2006; 12: 6079–86. [DOI] [PubMed] [Google Scholar]
- 27. Nieda M, Okai M, Tazbirkova A et al . Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood 2004; 103: 383–9. [DOI] [PubMed] [Google Scholar]
- 28. Chang DH, Osman K, Connolly J et al . Sustained expansion of NKT cells and antigen‐specific T cells after injection of α‐galactosyl‐ceramide loaded mature dendritic cells in cancer patients. J Exp Med 2005; 201: 1503–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest 2007; 117: 1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gilboa E, Vieweg J. Cancer immunotherapy with mRNA‐transfected dendritic cells. Immunol Rev 2004; 199: 251–63. [DOI] [PubMed] [Google Scholar]
- 31. Schuler G, Schuler‐Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol 2003; 15: 138–47. [DOI] [PubMed] [Google Scholar]
- 32. Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non‐small‐cell lung cancer. J Clin Oncol 2004; 22: 2808–15. [DOI] [PubMed] [Google Scholar]
- 33. Stift A, Friedl J, Dubsky P et al . Dendritic cell‐based vaccination in solid cancer. J Clin Oncol 2007; 21: 135–42. [DOI] [PubMed] [Google Scholar]
- 34. Horiguchi S, Matsuoka T, Okamoto Y et al . Migration of tumor antigen‐pulsed dendritic cells after mucosal administration in the human upper respiratory tract. J Clin Immunol 2007; 27: 598–604. [DOI] [PubMed] [Google Scholar]
- 35. Uchida T, Horiguchi S, Tanaka Y et al . Phase I study of α‐galactosylceramide‐pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother 2008; 57: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuwa N, Ito Y, Matsumoto A et al . A combination therapy of continuous superselective intraarterial carboplatin infusion and radiation therapy for locally advanced head and neck carcinoma. Phase I Study Cancer 2000; 89: 2099–105. [PubMed] [Google Scholar]
- 37. Ishikawa E, Motohashi S, Ishikawa A et al . Dendritic cell maturation by CD11c− T cells and Vα24+ natural killer T‐cell activation by α‐galactosylceramide. Int J Cancer 2005; 117: 265–73. [DOI] [PubMed] [Google Scholar]
- 38. Karadimitris A, Gadola S, Altamirano M et al . Human CD1d‐glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci USA 2001; 98: 3294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaneko Y, Harada M, Kawano T et al . Augmentation of Vα14 NKT cell‐mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A‐induced hepatitis. J Exp Med 2000; 191: 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Osman Y, Kawamura T, Naito T et al . Activation of hepatic NKT cells and subsequent liver injury following administration of α‐galactosylceramide. Eur J Immunol 2000; 30: 1919–28. [DOI] [PubMed] [Google Scholar]


