Abstract
A major problem in high‐dose chemotherapy with autologous hematopoietic stem cell transplantation is insufficient function of reconstituted bone marrow that limits the efficacy of post‐transplantation chemotherapy. Because transduction of hematopoietic stem cells with the multidrug resistance 1 (MDR1) gene might circumvent this problem, we conducted a pilot study of MDR1 gene therapy against metastatic breast cancer. Peripheral blood stem cells were harvested, and one‐third of the cells were transduced with MDR1 retrovirus. After the reconstitution of bone marrow function, the patients received high‐dose chemotherapy with transplantation of both MDR1‐transduced and unprocessed peripheral blood stem cells. The patients then received docetaxel chemotherapy. Two patients received transplantation of the MDR1‐transduced cells in 2001. Peripheral blood MDR1‐transduced leukocytes were 3–5% of the total cells after transplantation, but decreased gradually. During docetaxel chemotherapy, an increase in the rate of MDR1‐transduced leukocytes (up to 10%) was observed. Comparison of docetaxel‐induced granulocytopenia in the two patients suggested a bone marrow‐protective effect of the MDR1‐transduced cells. No serious side‐effect was observed, and the patients were in complete remission for more than 3 years. The MDR1‐transduced cells gradually decreased and disappeared almost entirely by the end of 2004. Results of linear amplification‐mediated polymerase chain reaction of the MDR1‐transduced leukocytes suggested no sign of abnormal amplification of the transduced cells. A third patient received transplantation of the MDR1‐transduced cells in 2004. These results suggest the feasibility of our MDR1 gene therapy against metastatic breast cancer, and follow‐up is ongoing. (Cancer Sci 2007; 98: 1609–1616)
Although breast cancer is sensitive to chemotherapy and endocrine therapy, the prognosis of advanced or relapsed breast cancer is not satisfactory. The response rates of advanced breast cancers to most combination chemotherapies are between 40 and 70%, with complete response (CR) rates being 10–30%. The duration of response is 9–18 months for CR and 7–10 months for partial response (PR). Rates of long‐term survivors, of more than 10 years, are less than 5%.( 1 , 2 )
High‐dose chemotherapy (HDCT) with autologous hematopoietic stem cell transplantation (AHST) in chemotherapy‐responsive diseases has been tried in various neoplasms, and has resulted in higher cure rates and longer survival than conventional chemotherapy in relapsed non‐Hodgkin lymphoma( 3 ) and myeloma( 4 ) patients. HDCT against advanced breast cancer has also shown high CR rates (up to 50%), and 10–15% of patients have enjoyed enduring remission.( 5 , 6 , 7 ) However, most patients relapse following transplantation. The first reported randomized study comparing HDCT with conventional chemotherapy against advanced breast cancer showed no significant differences between the two groups in progression‐free survival or overall survival.( 8 ) Although more recent studies have shown that HDCT results in longer progression‐free survival than conventional chemotherapy,( 9 , 10 ) the median survival time so far appears to be no better than that achieved with conventional chemotherapy in most studies.
The reasons for the insufficient results of HDCT may be attributable to the difficulty of eradicating minimal residual disease. The patients are treated with HDCT for only 3–5 days, and further effective chemotherapy protocol does not exist because of insufficient bone marrow function after the AHST. One possible approach to overcoming the current limitation in HDCT would be multiple courses of HDCT followed by AHST, which were reported to be effective in myeloma patients.( 11 ) But this strategy failed in a small randomized trial for metastatic breast cancer.( 12 ) Another approach would be the transduction of autologous hematopoietic stem cells with an anticancer drug‐resistance gene and reinfusion, so that normal bone marrow cells would be protected from the toxic effects of anticancer drugs. The human multidrug resistance 1 (MDR1) gene encodes plasma membrane P‐glycoprotein (P‐gp), which consists of two transmembranous domains and two ATP‐binding domains. P‐gp excretes various drugs such as anthracyclines, vinca alkaloids and taxanes from cells in an ATP‐dependent manner.( 13 ) The MDR1 gene seems to be a good candidate for drug resistance gene therapy as chemotherapeutic drugs such as docetaxel and paclitaxel, which have good clinical activity in the treatment of breast cancer, are effluxed efficiently by P‐gp.
Using a retroviral vector, Sorrentino et al.( 14 ) transplanted MDR1‐transduced bone marrow into irradiated mice and then treated them with paclitaxel. This treatment increased MDR1‐transduced leukocytes in peripheral blood (in vivo amplification), and MDR1‐transduced mice showed reduced bone marrow suppression by paclitaxel (bone marrow protection). Then, several groups started clinical studies of MDR1 gene therapy against advanced breast cancer or other neoplasms.( 15 , 16 , 17 ) Abonour et al. showed in vivo amplification of MDR1‐transduced leukocytes by etoposide treatment in their clinical study on germ cell tumors.( 18 ) Here we present the promising results of our MDR1 gene therapy against metastatic breast cancer.
Materials and Methods
Institutional review. The whole protocol for this clinical study was reviewed and approved by our institutional review board and the governmental gene therapy committee, Japan.
Patient selection. The protocol outline is shown in Fig. 1. Histologically confirmed, metastatic breast cancer patients who achieved good PR or CR to a precedent conventional dose chemotherapy regimen (using anthracycline and/or taxane) were selected. Other eligibility criteria were patients: (1) who had signed informed consent; (2) who had no serious active disease other than breast cancer; (3) whose age was 20–60 years; (4) whose Eastern Cooperative Oncology Group performance status was 0 or 1; (5) patients whose life expectancy was 3 months; (6) who had adequate physiological functions (white blood cells [WBC] = 3 500/µL, hemoglobin [Hb] = 9.0 g/dL, platelets = 100 000/µL, creatinine clearance = 60 mL/min [24 h], total serum bilirubin = 1.0 mg/dL, serum glutamic oxaloacetic transaminase [GOT], glutamic pyruvic transaminase [GPT] = 100 U/L, left ventricular ejection fraction = 60%, electrocardiogram [ECG] within normal limits); (7) whose bone marrow aspirate and biopsy showed no cancer cells; and (8) who had neither a past history of serious allergic reaction, bone metastases, brain metastases, irradiation for pelvis, were not pregnant or lactating, or had active infection.
Figure 1.
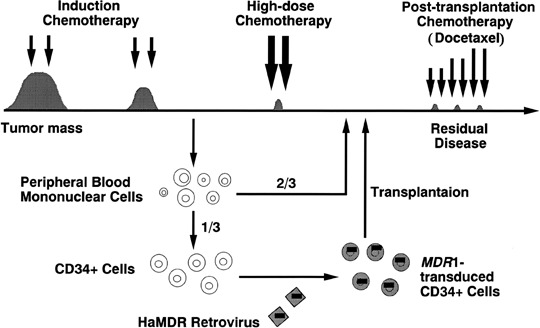
The outline of multidrug resistance 1 (MDR1) gene therapy. After remission induction chemotherapy for metastatic breast cancer, peripheral blood stem cells (PBSC) were harvested from patients in remission, and one‐third were transduced with retroviral MDR1 vector. The patients then received high‐dose chemotherapy with transplantation of both MDR1‐transduced and unprocessed PBSC. After bone marrow reconstitution, the patients received docetaxel chemotherapy.
Hematopoietic stem cell harvest, CD34‐positive cell selection and gene transfer. The HaMDR retrovirus used in the present study carries wild‐type (Gly‐185) MDR1 cDNA isolated from a human placenta cDNA library( 19 ) in the Harvey sarcoma virus backbone. The HaMDR producer clone 3P26, with a retrovirus titer of 107 c.f.u./mL, was established in our laboratory. The clinical‐grade HaMDR retrovirus was prepared by MAGENTA (BioReliance, Rockville, MD, USA) under a contract with our foundation. GMP‐grade human cytokines, stem cell factor (SCF), Flk2/Flt3‐ligand (FL‐ligand), interleukin (IL)‐6 soluble receptor (sIL‐6R) and thrombopoietin (TPO), were obtained from R&D Systems (Minneapolis, MN, USA). GMP‐grade human IL‐6 was a generous gift of Serono Japan (Tokyo, Japan).
Patients were treated with 2 g/m2 cyclophosphamide, and then 300 µg/day granulocyte colony‐stimulating factor [G‐CSF] at days 10–15. Peripheral blood stem cells (PBSC) were harvested at days 13–15 using the mononuclear cell collection procedure of the COBE Spectra Cell Separator (Gambro BCT, Lakewood, CO, USA). In this protocol, one‐third of the total cells were transduced with the MDR1 gene, and two‐thirds were returned to the patients without genetic modification. To do this, cells harvested at day 14 were used for CD34 selection and gene transfer, and cells harvested at days 13 and 15 were frozen directly in liquid nitrogen. Harvest goals were a minimum of 2 × 106 CD34‐positive cells/kg for non‐transduced cells. CD34‐positive cells were isolated from freshly harvested PBSC of the patient at day 14 by magnetic microbead selection using Isolex 50 Stem Cell Reagent kit (Nexell Therapeutics, Irvine, CA, USA) in the cell‐processing center of our institution. The CD34‐positive cells were subsequently cultured in a medium containing 50 ng/mL SCF, 100 ng/mL FL‐ligand, 300 ng/mL sIL‐6R, 20 ng/mL TPO and 100 ng/mL IL‐6. At 36 h after the initiation of the ex vivo cultures, cells were cultured with HaMDR retrovirus on plates coated with CH‐296 (Takara Bio, Otsu, Japan) for 4 h, then without the virus for 8 h. The culture was repeated three times over 36 h. The cells were then washed and cryopreserved. The expression of human P‐gp protein in the transduced cells was examined by fluorescence‐activated cell sorter [FACS] analysis. Contamination of breast cancer cells was also checked by cytocentrifugation of 106 PBSC and immunohistochemical examination using an EPIMET epithelial cell detection kit (Baxter, Unterschleissheim, Germany) after CD34 selection (day 14) and after MDR1 gene transfer (day 17).
Transplantation and post‐transplantation chemotherapy. One to two months after the final PBSC harvest, the patients received HDCT with 2 g/m2 cyclophosphamide, 67 mg/m2 thiotepa and 533 mg/m2 carboplatin per day for 3 days. Three additional days after the completion of HDCT, the MDR1‐transduced CD34‐positive cells (approximately one‐third of the transplanted CD34‐positive cells) and unmodified cells (approximately two‐thirds of the total transplanted CD34‐positive cells) were reinfused to the patient simultaneously.
After bone marrow was reconstituted, patients were treated with 30 mg/m2 docetacel (50% of the standard dose in Japan) per 3 weeks, and then the docetaxel dose was planned to be increased to 45 mg/m2, then to 60 mg/m2, if grade 4 neutropenia was not observed.
P‐glycoprotein expression in the peripheral mononuclear cells of the patient. After transplantation, peripheral blood of patients was obtained at each time point. Mononuclear cells (MNC) were separated by Ficoll‐Hypaque density gradient centrifugation. A portion of the MNC were used for P‐gp FACS and MDR1 polymerase chain reaction (PCR) analyses. The rest were cryopreserved for other experiments. To examine the expression levels of human P‐gp on the surface of the MDR1‐transduced hematopoietic cells, FACS analysis was carried out. Cells were incubated with the F(ab′)2 fragment of MRK16 (100 µg/mL),( 20 ) then washed and incubated with R‐phycoerythrin‐conjugated streptavidin (Becton Dickinson Biosciences, San Jose, CA, USA). The fluorescence staining level was analyzed using FACScan (Becton‐Dickinson Biosciences).
Polymerase chain reaction from the peripheral MNC of the patient. Genomic DNA was extracted using a DNA Blood Mini kit (QIAGEN, Valencia, CA, USA) according to the manufacturer's instructions. HaMDR provirus DNA was amplified with the following PCR primers: forward 5′‐TGGGGGTTGGGGATTTAG‐3′ and reverse 5′‐GCACCAAAATGAAACCTG‐3′. The PCR conditions were as follows: 95°C for 9 min; then 40 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 1 min; and final extension of 15 min at 72°C. Then the second PCR was carried out with the same procedure, using 10% of the first PCR product as template DNA. PCR products were analyzed on agarose gels.
Linear amplification‐mediated PCR. Possible clonal expansion of the MDR1‐tranduced cells was examined by linear amplification‐mediated (LAM)‐PCR.( 21 ) The genomic–proviral junction sequence was preamplified by 50 cycles of primer extension using 0.25 pmol of vector‐specific, 5′‐biotinylated primer LTR1 (5′‐AGCTGTTCTATCTGTTCTTGGCCCT‐3′) with AmpliTaq Gold DNA Polymerase (2.5 U; Applied Biosystems, Foster, CA, USA) from 100 ng of each sample DNA. Selection of biotinylated extension products was carried out with a µMACS Streptavidin Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The samples were incubated with Klenow polymerase (4.4 U; Invitrogen, Carlsbad, CA, USA), dNTPs (300 µM; Applied Biosystems), and random hexanucleotide mixture (Roche Diagnostics, Mannheim, Germany) in a volume of 50 µL for 1 h at 37°C. Samples were washed on the magnetic particle concentrator and incubated with MaeII endonuclease (16 U in 100 µL; Roche Diagnostics) for 1 h at 50°C. After an additional wash step, 100 pmol of a double‐stranded asymmetric linker cassette and T4 DNA Ligase (5 U; Invitrogen) were incubated with the beads in a volume of 40 µL at 4°C overnight. Then the ligase was purified with the magnetic beads. Each ligation product was amplified with AmpliTaq Gold DNA Polymerase, 25 pmol of vector‐specific primer LTR2 (5′‐GACCTTGATCTGAACTTCTC‐3′), and linker cassette primer LC1 (5′‐GACCCGGGAGATCTGAATTC‐3′), using the same conditions as the PCR of the MDR1 gene. Each PCR product served as a template for a second, nested PCR with internal primers LTR3 (5′‐TCCATGCCTTGTAAAATGGC‐3′; QIAGEN) and LC2 (5′‐GATCTGAATTCAGTGGCACAG‐3′; QIAGEN) under the same conditions. PCR products were analyzed on agarose gels.
Results
Clinical courses. Three patients have were treated under the protocol. The first and second patients received transplantation in 2001, and the third in 2004. The characteristics of the patients who received MDR1‐transduced CD34‐positive cells are shown in Table 1.
Table 1.
Patient characteristics
| Patient no. | Date of recurrence | Site of disease | Protocol | Response | Date of transplantaion | Response | Comments |
|---|---|---|---|---|---|---|---|
| 1 | March 2000 | Lung | FAC | PR | 4/01 | CR | PD April 2007 |
| 2 | March 1999 | Lymph node | FAC | PR | 10/01 | CR | PD July 2005 |
| 3 | October 2001 | Lymph node, liver | DOX, DTX | Near CR | 8/04 | Near CR | PD March 2005 |
CR, complete response; DOX, doxorubicin; DTX, docetaxel; FAC, fluorouracil, doxorubicin, cyclophosphamide; NED, no evidence of disease; PD, progressive disease; PR, partial response.
Patient 1. This patient was diagnosed with breast cancer and underwent surgery on 14 May 1990. Pathological diagnosis was scirrhous adenocarcinoma. In March 2000, multiple lung nodules were detected by computed tomography (CT) scan, and transbronchial biopsy revealed lung metastases of breast cancer. From April 2000 until September 2000, the patient received fluorouracil, doxorubicin, cyclophosphamide (FAC) chemotherapy and entered into PR. After informed consent, the first and the second PBSC harvest and MDR1 gene transfer were done in November 2000 and February 2001, respectively. In April 2001, the patient received HDCT and the transplantation of MDR1‐transduced CD34‐positive cells. From June 2001 until February 2002, the patient received 10 cycles of docetaxel chemotherapy. The patient entered into CR after five cycles of docetaxel in October 2001. In April 2007, pleural nodules appeared at CT scan, and positron emission tomography (PET) scan confirmed breast cancer relapse. The patient showed no sign of secondary malignancies.
Patient 2. This patient was diagnosed with breast cancer and underwent surgery on 29 August 1990. Pathological diagnosis was solid tubular adenocarcinoma. In May 1999, left supraclavicular and parasternal lymph nodes were detected by ultrasonography and CT scan, and aspiration biopsy revealed metastases of breast cancer. From July 1999 to November 1999, the patient received six cycles of FAC chemotherapy and entered into PR. The patient then received six cycles of docetaxel and irradiation to supraclavicular lymph nodes. After informed consent, the first, second and third PBSC harvests and MDR1 gene transfer were done in August 2000, January 2001 and April 2001, respectively. In October 2001, the patient received HDCT with transplantation of MDR1‐transduced hematopoietic stem cells. The patient entered into CR after HDCT in November 2001. From May 2002 until August 2002 the patient received five cycles of docetaxel chemotherapy. In July 2005, the patient showed parasternal lymph node swelling at ultrasound and fine needle aspiration biopsy showed breast cancer relapse.
The third patient received transplantation of the MDR1‐transduced cells in 2004, 18 months after the harvest, due to the adverse event of the X‐linked severe combined immune deficiency (X‐SCID) gene therapy in France. The patient then began receiving post‐transplantation chemotherapy of vinorelbine. During chemotherapy, the patient developed liver metastases in March 2005. The patient was treated with some chemotherapeutic and endocrine therapies since then.
CD34 selection and MDR1 gene transduction of PBSC. CD34‐positive cells from the three patients were transduced with clinical‐grade HaMDR retrovirus. After HDCT, the MDR1‐transduced cells were reinfused to the patients. Results of CD34 selection and transduction efficiency in the three patients are summarized in Table 2. In this protocol, the total number of untransduced CD34‐positive cells should be more than 2 × 106/kg. The number of untransduced cells in all of the patients was large enough to satisfy the requirement. Transduction efficiency of in vitro MDR1 gene transfer to PBSC was 8–17% by flow cytometry (Fig. 2). Transplanted P‐gp‐expressing cells were 1.1–3.7 × 105/kg, which was approximately 3–7% of the transplanted CD34‐positive cells. Safety examinations of the transduced cells (including the sarcoma‐positive/leukemia‐negative [S+L−] test) were carried out at SRL (Tokyo, Japan). The S+L− tests of the MDR1‐transduced cells of each patient were all negative. Contamination of breast cancer cells into the transduced cells was also ruled out by cytokeratin immunohistochemistry (data not shown).
Table 2.
Reinfused cells
| Patient no. | Total no. CD34+ cells reinfused (×106/kg) | Untreated CD34+ cells reinfused (×106/kg) | HaMDR‐ transduced CD34+ cells (× 106/kg) | Transduction efficiency (% P‐gp+) | Estimated no. of HaMDR‐transduced cells reinfused (×105/kg) | Ratio of P‐gp+ cells in total reinfused cells (% P‐gp+) |
|---|---|---|---|---|---|---|
| 1 | 5.5 | 3.5 | 2.0 | 17 | 3.7 | 6.7 |
| 2 | 3.0 | 2.2 | 0.8 | 14 | 1.1 | 3.7 |
| 3 | 9.4 | 6.0 | 3.4 | 8 | 2.6 | 2.8 |
P‐gp, P‐glycoprotein.
Figure 2.
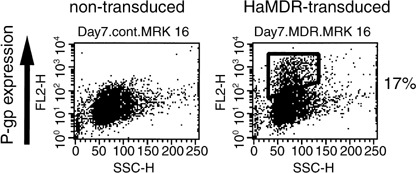
Ex vivo multidrug resistance 1 (MDR1) transduction of peripheral blood stem cells (PBSC) in patient 1. CD34‐positive cells were isolated from freshly harvested PBSC of the patients using an Isolex 50 Stem Cell Reagent kit. The CD34‐enriched cells were then stimulated with granulocyte colony‐stimulating factor (G‐CSF), thrombopoietin (TPO), interleukin (IL)‐6, sIL‐6R and Flk2/Flt3‐ligand (FL‐ligand), and transduced with HaMDR viral supernatant. Non‐transduced or HaMDR‐transduced cells were incubated with the F(ab′)2 fragment of MRK16, and were washed and incubated with R‐phycoerythrin‐conjugated streptavidin. The fluorescence staining level was analyzed using FACScan. The analysis was carried out after 2 days of stem cell amplification, 2 days of viral transfer, and then 2 days of further culture. P‐gp, P‐glycoprotein.
Increase in MDR1‐transduced leukocytes during docetaxel chemotherapy. Patients received HDCT with transplantation of the MDR1‐transduced cells. After the recovery of bone marrow function, the patients were treated with docetaxel. Expression of P‐gp in the peripheral leukocytes was investigated by FACS analysis. In patient 1 (upper panel of Fig. 3B), P‐gp‐positive cells were not detected until day 5, but started to increase at day 7, culminated near 5% at day 12, then decreased. The ratio of P‐gp‐positive cells decreased to 1% in 2 months. Docetaxel treatment was started at a dose of 30 mg/m2 and increased to 45 mg/m2, but further dose increases were not done because of grade 4 neutropenia during the second dose. In the first or second cycles of docetaxel treatment at 45 mg/m2, the ratios of P‐gp‐positive cells in the peripheral blood of the patients at day 4 were approximately 1%. They increased transiently to 2–3% at day 8, and then decreased again within 2 weeks (upper panel of Fig. 3B). In the fourth to tenth cycles of docetaxel, the ratios of P‐gp‐positive cells prior to the chemotherapy were 3–4%, increasing to 6–10% after docetaxel treatment, and then fell to 3–4% within 2 weeks. This basal level remained unchanged over the next half year following completion of the treatment. In patient 2 (middle panel of Fig. 3B), P‐gp‐positive cells in the peripheral blood appeared at day 10 and increased to 4%, then decreased. In accordance with the patient's wishes, docetaxel chemotherapy was delayed until 200 days after transplantation. In the meantime, P‐gp‐positive cells continued to decrease and became undetectable. However, during five cycles of docetaxel chemotherapy (30–45 mg/m2), P‐gp‐positive cells in the peripheral blood became detectable. They then became undetectable again after treatment with five cycles of docetaxel. These data clearly show that MDR1‐transduced leukocytes were enriched by docetaxel chemotherapy in vivo. In patient 3, we observed 3% of P‐gp‐positive cells in the peripheral blood 7–12 days after the transplantation. They then decreased to less than 1% at day 19 and became very low in 2 months, and did not seem to increase with vinorelbine therapy (lower panel of Fig. 3B).
Figure 3.
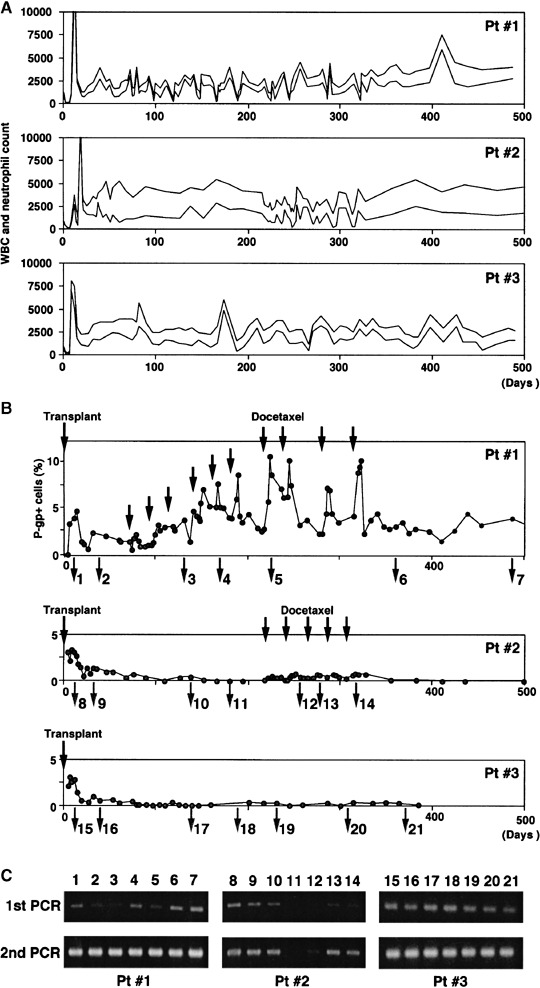
Changes in (A) white blood cells (WBC) and neutrophils, and (B) P‐glycoprotein (P‐gp)‐positive cells after peripheral blood stem cell transplantation (PBSCT) and (C) semiquantitative polymerase chain reaction (PCR) of HaMDR DNA in peripheral blood cells of the three patients. Docetaxel chemotherapy was started 60 days after PBSCT in patient 1 and 208 days after PBSCT in patient 2. The patients were then treated with 30 mg/m2 (first course) or 45 mg/m2 (second course and later) docetaxel (black arrow). (A) Plots of total white blood cells (upper line) and neutrophils (lower line). (B) Mononuclear cells (MNC) were separated from peripheral blood by Ficoll‐Hypaque density gradient centrifugation, incubated with the F(ab′)2 fragment of MRK16, and washed and incubated with R‐phycoerythrin‐conjugated streptavidin. The fluorescence staining level was analyzed using FACScan. (C) Genomic DNA was extracted from peripheral blood of the three patients at various time points. Multidrug resistance 1 (MDR1) genes were amplified with the PCR primers for 40 cycles. Then the second PCR was carried out with the same primers. PCR products were analyzed on agarose gels. Patient 1: 1, day 12 after PBSCT; 2, day 40; 3, day 131 (after third docetaxel chemotherapy); 4, day 170 (after fifth docetaxel); 5, day 226 (after seventh docetaxel); 6, day 362 (after 10th docetaxel); 7, day 488. Patient 2: 8, day 12 after PBSCT; 9, day 33; 10, day 138; 11, day 170; 12, day 247 (after second docetaxel chemotherapy); 13, day 268 (after third docetaxel); 14, day 310 (after fifth docetaxel). Patient 3: 15, day 12 after PBSCT; 16, day 40; 17, day 139; 18, day 188; 19, day 231; 20, day 308; 21, day 371.
Semiquantitative PCR of HaMDR DNA in the peripheral blood cells also showed in vivo enrichment of MDR1‐transduced leukocytes following docetaxel chemotherapy (Fig. 3C). In patient 1, first PCR showed a relative decrease of HaMDR DNA from days 12 to 40, but the PCR band widened during docetaxel chemotherapy. In patient 2, the PCR band decreased after transplantation, and became undetectable even with the second PCR at day 170. However, during docetaxel chemotherapy, HaMDR DNA became detectable at the first PCR. In patient 3, vinorelbine chemotherapy did not seem to increase HaMDR DNA.
Changes in peripheral neutrophil counts after docetaxel chemotherapy. When we compared neutrophil recovery after docetaxel chemotherapy in patient 1, who had a higher ratio of MDR1‐transduced peripheral blood leukocytes, and patient 2, who had a lower ratio, neutrophil recovery in patient 2 was delayed even with G‐CSF administration (Fig. 4).
Figure 4.
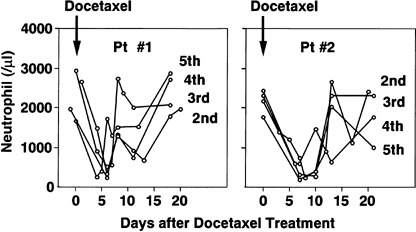
Comparison of neutrophil count after docetaxel chemotherapy in patients 1 and 2. The changes in neutrophil count after the second to fifth docetaxel chemotherapy (45 mg/m2) in patients 1 and 2 are shown.
Follow‐up of the three patients. After cessation of docetaxel chemotherapy, two patients were in CR and were followed without chemotherapy. They received annual systemic follow‐up (CT, bone scintigraphy, ultrasonography and tumor markers) and showed no sign of relapse 3 years after transplantation. Peripheral blood counts and blood chemistry showed no serious adverse events. The first patient showed relapse at pleura in April 2007, and started aromatase inhibitor. In July 2005, the second patient showed relapse in the parasternal lymph node, and started capecitabine monotherapy. She has had stable disease since then. The third patient was treated with capecitabine, mitomycin C and methotrexate, then anastrozole after vinorelbine, but liver metastases has progressed gradually. She complained of dyspnea at July 2006, and showed massive pleural metastases. She died from respiratory failure due to pleural metastases in December 2006. All three patients showed no sign of secondary malignancy.
Clonality analysis of HaMDR‐transduced leukocytes. The MDR1‐transduced cells of the first and second patients decreased gradually and almost disappeared by the end of 2004. Using LAM‐PCR, we identified several MDR1‐tranduced peripheral blood leukocyte clones at various time points after transplantation (Fig. 5). However, there was no indication of the amplification of specific clones. Analysis of insertion sites of MDR1 retrovirus are ongoing in our laboratory (Mitsuhashi et al., unpublished data).
Figure 5.
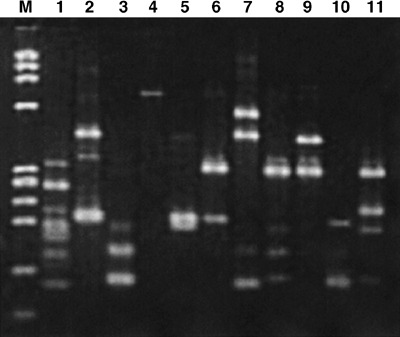
Linear amplification‐mediated (LAM)‐polymerase chain reaction (PCR) analysis of HaMDR integration sites in the peripheral blood leukocytes of patient 1. Genomic DNA was extracted from peripheral blood of the first patient at various time points. The genomic–proviral junction sequence was preamplified by repeated primer. Biotinylated extension products were selected, treated with Klenow fragment and incubated with MaeII. The product was then treated with T4 DNA Ligase and amplified with AmpliTaq Gold DNA polymerase with vector‐specific primer and linker cassette primer, then further amplified with the same primer set. PCR products were analyzed on agarose gels. 1, day 12 after PBSCT; 2, day 40; 3, day 112 (after second docetaxel chemotherapy); 4, day 152 (after fourth docetaxel); 5, day 196 (after sixth docetaxel); 6, day 257 (after eighth docetaxel); 7, day 327 (after 10th docetaxel); 8, day 586; 9, day 727; 10, day 762; 11, day 790.
Discussion
A group at the M. D. Anderson Cancer Center (Houston, TX, USA) first reported results of a clinical trial of retroviral MDR1 gene therapy.( 15 ) They carried out retroviral gene transfer without the use of cytokines, either with PBSC alone or PBSC with autologus stromal cells. The in vitro transduction efficiency was 2.8% with the solution method and 5.6% with the stromal method, detected by in situ PCR. However, 3–4 weeks after transplantation, direct PCR assay of peripheral blood leukocytes in the patients showed positive results in 0/10 with the solution method, and 5/8 with the stromal method. These data show insufficient transduction efficiency without using cytokines. The National Cancer Institute (NCI) also reported the results of a clinical trial of retroviral MDR1 gene therapy.( 16 ) They transferred the MDR1 gene into bone marrow mononuclear cells or PBSC stimulated by IL‐3, IL‐6 and SCF. The ex vivo transduction efficiency was 0.2–0.5%. They treated transplanted patients with 175 mg/m2 paclitaxel, but they could not show any enrichment of MDR1‐positive white blood cells by PCR. A group at Columbia University also transferred the MDR1 gene into bone marrow mononuclear cells or peripheral blood stem cells stimulated by IL‐3, IL‐6 and SCF.( 17 ) They showed that 20–70% of burst‐forming unit‐erythroid (BFU‐E) and colony‐forming unit–granulocyte macrophage (CFU‐GM) colonies from MDR1‐transferred CD34‐positive cells were positive for MDR1 by PCR. Bone marrow (BM) from patients 3–12 weeks after transplantation was shown to be MDR1‐positive by PCR in 2/5 patients. They also analyzed P‐gp expression in bone marrow cells using flow cytometry, but they could not show any expression.
A group at Indiana University (Indianapolis, IN, USA) tried to increase transduction efficiency by using recombinant fibronectin fragment (CH‐296).( 18 ) CH‐296 contains specific adhesion domains for hematopoietic progenitor cells and retroviral vectors, and can increase the attachment of retrovirus vector to the target CD34 cells. They transferred the MDR1 gene into PBSC of germ cell tumor patients with SCF/IL‐6 or G‐CSF/TPO/SCF in the presence of CH‐296. The in vitro transduction efficiency by PCR was 4–52% of colonies from transduced PBSC. After transplantation, patients received three cycles of oral etoposide therapy. MDR1 PCR of BM cells after three cycles was positive in 3–10% of colonies in eight patients. They did not investigate P‐gp expression.
We tried to increase transduction efficiency by stimulating peripheral blood CD34‐positive cells with five cytokines (SCF, TPO, IL‐6, FL‐ligand and sIL‐6R) using CH‐296. We also used MRK‐16 flow cytometry to investigate P‐gp expression in tranduced PBSC and peripheral blood leukocytes in addition to PCR assay. Transduction efficiency of in vitro MDR1 gene transfer to PBSC was 8–17% by P‐gp expression. Soon after the transplantation of transduced PBSC (approximately one‐third of total PBSC) and untreated PBSC (approximately two‐thirds), the ratio of P‐gp‐positive cells in peripheral white blood cells increased to 3–5%, which corresponds to the ratio of infused MDR1‐transduced CD34‐positive cells. The ratio of P‐gp‐expressing cells in the peripheral blood decreased to an undetectable level within 6 months. This corresponds to the PCR data, which shows a decrease in PCR product 30 days after transplantation. This might be due to a survival disadvantage of MDR1‐transduced hematopoietic stem cells in bone marrow, or worse transduction efficiency of MDR1 into true hematopoietic stem cells than into more differentiated stem cells.
Docetaxel chemotherapy increased the ratio of P‐gp‐positive white blood cells in patients 1 and 2. This shows the following two things. The first is that MDR1‐transduced leukocytes can be maintained for a long time. In patient 1, MDR1 gene‐transduced white blood cells can be detected even 3 years after transplantation or 2.5 years after cessation of docetaxel chemotherapy (data not shown). MDR1 gene transfer into ‘true’ long‐term repopulating hematopoietic stem cells may be possible, but multilineage engraftment of MDR1‐transduced blood cells has not be proven, because PCR of HaMDR DNA of each cell lineage from peripheral blood is difficult. The second is that enrichment of MDR1 gene‐transduced blood cells and hematopoietic stem cells by P‐gp‐correlated chemotherapeutic drugs may be possible. The transient increase of P‐gp‐positive cells in every cycle of docetaxel might be due to selection of resistant differentiated precursor cells (e.g. CFU‐GM), but the basal increase of P‐gp‐positive cells during several cycles of chemotherapy may be due to selection of P‐gp‐positive hematopoietic stem cells. These results not only demonstrate the possible utility of MDR1 gene therapy for chemotherapy of various cancers, but also suggest the utility of the MDR1 gene for selection of transduced cells for various gene therapies, as has been shown in several animal models.( 22 )
Data from only two patients cannot show whether MDR1 transduction of PBSC protected hematopoietic cells from docetaxel toxicity. However, comparison of patient 1, whose white blood cells had a higher ratio of P‐gp‐positive cells, and patient 2, whose white blood cells had a lower ratio, showed that the higher ratio of P‐gp‐positive cells might correlate with less bone marrow toxicity. It is possible that even MDR1 gene transfer into one‐third of PBSC protects hematopoietic stem cells from chemotherapeutic toxicity. We could not increase the dose of docetaxel to the standard dose because of grade 4 neutropenia at 75% dose. MDR1 gene transfer into total PBSC might protect hematopoietic stem cells more efficiently than transfer into one‐third, and might make standard‐dose chemotherapy after autologous stem cell transplantation easy to carry out.
Those two patients had been in CR for more than 3 years after transplantation of MDR1‐transduced PBSC and docetaxel chemotherapy. The first patient relapsed 6 years after transplantation, and the second patient relapsed 3 years and 9 months after transplantation, but both are presenting in good physical condition. So far there has been no severe side‐effects with MDR1‐transduced PBSC other than those typically accompanying HDCT and peripheral blood stem cell transplantation (PBSCT).
At the end of 2002, occurrence of T‐cell leukemia in two patients after gene therapy of X‐SCID was reported. A genetic defect in the γC gene, which is a common domain of multiple IL receptors (e.g. IL‐2R, IL‐4R and IL‐7R), causes severe defects of T cells and natural killer cells and severe immune deficiency in X‐SCID patients. Retroviral γC gene transfer using autologous CD34‐positive hematopoietic cells in X‐SCID patients restored the immune system in 9 of 11 patients.( 23 ) But T‐cell leukemia occurred in two of those nine patients. In the leukemic cells, retroviral vector was inserted into the LMO2 gene, which causes T‐cell leukemogenesis.( 24 ) The third leukemic French X‐SCID patient was reported at the beginning of 2005. Those adverse events have warned of the risk of carcinogenicity in retrovirus gene therapy. After thorough investigation of retroviral gene therapy trials for hematopoietic stem cells all over the world, no leukemic event has been found in clinical gene therapy trials other than the French X‐SCID trial.( 25 ) Screening of the Mouse Retroviral Cancer Gene database showed that retroviral insertion into the γC and LMO2 genes was found in two leukemic mice, and insertion into both genes was found in one mouse. This fact suggests that both genes are oncogenes, and that the two genes can collaborate.( 26 ) In X‐SCID gene therapy, a double hit with retroviral activation of LMO2 and exogenous activated γC might be necessary for leukemogenesis. Retroviral gene therapy with non‐oncogenic genes may have a low risk of cancer.( 27 )
In a previous study, Bunting et al. showed that transduction of murine bone marrow cells with an MDR1 vector may cause a myeloproliferative syndrome in transplanted mice.( 28 ) They suggested that P‐gp overexpression in bone marrow cells might cause leukemogenesis, because transduction of the dihydrofolate reductase gene into bone marrow cells did not cause myeloproliferative syndrome. However, this suggestion was lately denied by the same group. They showed that leukemias following retroviral transfer of MDR1 were driven by combinatorial insertional mutagenesis due to usage of high titer of retrovirus, not by P‐gp overexpression.( 29 )
We have also started to analyze clonal amplification of each transduced clone. So far, we have not seen any abnormal proliferation. Integration site analyses are ongoing in our laboratory.
Chemotherapy for cancer increases the risk of secondary leukemia. In breast cancer, the use of anthracyclines or cyclophosphamide might cause secondary leukemia or myelodysplastic syndrome, although the risk is not so high (0.2–1.7% in 10 years).( 30 , 31 ) HDCT with autologous hematopoietic stem cell transplantation is reported to increase the risk of leukemia, especially in lymphoma patients (2.5–18% in 5–6 years).( 32 ) However, HDCT for breast cancer is not accompanied by a high risk of secondary leukemia (0.3–0.9%).( 33 ) It is possible that a combination of conventional chemotherapy, HDCT and retroviral integration into special genes such as LMO‐2 causes leukemogenesis, but the probability of that event is thought to be low. So far, an increase of specific clones, MDR1 gene‐positive cells or P‐gp‐positive cells in peripheral blood has not been detected using PCR or flow cytometry in our three patients.
In conclusion, we carried out MDR1 gene therapy for three recurrent breast cancer patients. After HDCT with transplantation of peripheral blood hematopoietic cells transduced with the MDR1 gene, the MDR1‐transduced leukocytes were increased by docetaxel chemotherapy at the level of both DNA PCR and P‐gp protein expression. Two patients reached CR after HDCT or docetaxel treatment. No side‐effects associated with the transplantation of the MDR1‐transduced cells were observed. No evidence of abnormal proliferation of specific clones was obtained. The two patients have been disease‐free for more than 3 years, and have been performance status 0. These results suggest the safety and feasibility of our MDR1 gene therapy.
Acknowledgments
The authors are thankful to Drs Michael M. Gottesman and Ira Pastan, NCI, NIH, for their continuous support. This work was supported by grants from the Ministry of Health, Labor and Welfare, and the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Gregory WM, Smith P, Richards MA, Twelves CJ, Knight RK, Rubens RD. Chemotherapy of advanced breast cancer: outcome and prognostic factors. Br J Cancer 1993; 68: 988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inoue K, Ogawa M, Horikoshi N et al . Evaluation of prognostic factors for 233 patients with recurrent advanced breast cancer. Jpn J Clin Oncol 1991; 21: 334–9. [PubMed] [Google Scholar]
- 3. Philip T, Guglielmi C, Hagenbeek A et al . Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–5. [DOI] [PubMed] [Google Scholar]
- 4. Attal M, Harousseau JL, Stoppa AM et al . A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–7. [DOI] [PubMed] [Google Scholar]
- 5. Ito Y, Mukaiyama T, Ogawa M et al . Epirubicin‐containing high‐dose chemotherapy followed by autologous hematopoietic progenitor cell transfusion for patients with chemotherapy‐sensitive metastatic breast cancer: results of 5‐year follow‐up. Cancer Chemother Pharmacol 1999; 43: 8–12. [DOI] [PubMed] [Google Scholar]
- 6. Peters WP, Dansey RD, Klein JL, Baynes RD. High‐dose chemotherapy and peripheral blood progenitor cell transplantation in the treatment of breast cancer. Oncologist 2000; 5: 1–13. [DOI] [PubMed] [Google Scholar]
- 7. Dunphy FR, Spitzer G, Fornoff JE et al . Factors predicting long‐term survival for metastatic breast cancer patients treated with high‐dose chemotherapy and bone marrow support. Cancer 1994; 73: 2157–67. [DOI] [PubMed] [Google Scholar]
- 8. Berry DA, Broadwater G, Klein JP et al . High‐dose versus standard chemotherapy in metastatic breast cancer: comparison of Cancer and Leukemia Group B trials with data from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol 2002; 20: 743–50. [DOI] [PubMed] [Google Scholar]
- 9. Crump M, Gluck S, Stewart D et al . A randomized trial of high‐dose chemotherapy (HDC) with autologous peripheral blood stem cell support (ASCT) compared to standard therapy in women with metastatic breast cancer: a national cancer institute of Canada (NCIC) clinical trials group study. Proc Am Soc Clin Oncol 2001; 20: 21a, #82. [Google Scholar]
- 10. Lotz JP, Cure H, Janvier M et al . High‐dose chemotherapy with haematopoietic stem cell transplantation for metastatic breast cancer patients: final results of the French multicentric randomised CMA/PEGASE 04 protocol. Eur J Cancer 2005; 41: 71–80. [DOI] [PubMed] [Google Scholar]
- 11. Attal M, Harousseau JL, Facon T et al . Single versus double autologous stem‐cell transplantation for multiple myeloma. N Engl J Med 2003; 349: 2495–502. [DOI] [PubMed] [Google Scholar]
- 12. Schmid P, Schippinger W, Nitsch T et al . Up‐front tandem high‐dose chemotherapy compared with standard chemotherapy with doxorubicin and paclitaxel in metastatic breast cancer: results of a randomized trial. J Clin Oncol 2005; 23: 432–40. [DOI] [PubMed] [Google Scholar]
- 13. Ambudkar SV, Kimchi‐Sarfaty C, Sauna ZE, Gottesman MM. P‐glycoprotein: from genomics to mechanism. Oncogene 2003; 22: 7468–85. [DOI] [PubMed] [Google Scholar]
- 14. Sorrentino BP, Brandt SJ, Bodine D et al . Selection of drug‐resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science 1992; 257: 99–103. [DOI] [PubMed] [Google Scholar]
- 15. Hanania EG, Giles RE, Kavanagh J et al . Results of MDR‐1 vector modification trial indicate that granulocyte/macrophage colony‐forming unit cells do not contribute to posttransplant hematopoietic recovery following intensive systemic therapy. Proc Natl Acad Sci USA 1996; 93: 15 346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cowan KH, Moscow JA, Huang H et al . Paclitaxel chemotherapy after autologous stem‐cell transplantation and engraftment of hematopoietic cells transduced with a retrovirus containing the multidrug resistance complementary DNA (MDR1) in metastatic breast cancer patients. Clin Cancer Res 1999; 5: 1619–28. [PubMed] [Google Scholar]
- 17. Hesdorffer C, Ayello J, Ward M et al . Phase I trial of retroviral‐mediated transfer of the human MDR1 gene as marrow chemoprotection in patients undergoing high‐dose chemotherapy and autologous stem‐cell transplantation. J Clin Oncol 1998; 16: 165–72. [DOI] [PubMed] [Google Scholar]
- 18. Abonour R, Williams DA, Einhorn L et al . Efficient retrovirus‐mediated transfer of the multidrug resistance 1 gene into autologous human long‐term repopulating hematopoietic stem cells. Nat Med 2000; 6: 652–8. [DOI] [PubMed] [Google Scholar]
- 19. Kioka N, Tsubota J, Kakehi Y et al . P‐glycoprotein gene (MDR1) cDNA from human adrenal: normal P‐glycoprotein carries Gly185 with an altered pattern of multidrug resistance. Biochem Biophys Res Commun 1989; 162: 224–31. [DOI] [PubMed] [Google Scholar]
- 20. Hamada H, Tsuruo T. Functional role for the 170‐ to 180‐kDa glycoprotein specific to drug‐resistant tumor cells as revealed by monoclonal antibodies. Proc Natl Acad Sci USA 1986; 83: 7785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt M, Carbonaro DA, Speckmann C et al . Clonality analysis after retroviral‐mediated gene transfer to CD34+ cells from the cord blood of ADA‐deficient SCID neonates. Nat Med 2003; 9: 463–8. [DOI] [PubMed] [Google Scholar]
- 22. Sugimoto Y, Tsukahara S, Sato S et al . Drug‐selected co‐expression of P‐glycoprotein and gp91 in vivo from an MDR1‐bicistronic retrovirus vector Ha‐MDR‐IRES‐gp91. J Gene Med 2003; 5: 366–76. [DOI] [PubMed] [Google Scholar]
- 23. Hacein‐Bey‐Abina S, Le Deist F, Carlier F et al . Sustained correction of X‐linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002; 346: 1185–93. [DOI] [PubMed] [Google Scholar]
- 24. McCormack MP, Rabbitts TH. Activation of the T‐cell oncogene LMO2 after gene therapy for X‐linked severe combined immunodeficiency. N Engl J Med 2004; 350: 913–22. [DOI] [PubMed] [Google Scholar]
- 25. Kohn DB, Sadelain M, Dunbar C et al . American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral‐mediated gene transfer to hematopoietic stem cells. Mol Ther 2003; 8: 180–7. [DOI] [PubMed] [Google Scholar]
- 26. Dave UP, Jenkins NA, Copeland NG. Gene therapy insertional mutagenesis insights. Science 2004; 303: 333. [DOI] [PubMed] [Google Scholar]
- 27. Berns A. Good news for gene therapy. N Engl J Med 2004; 350: 1679–80. [DOI] [PubMed] [Google Scholar]
- 28. Bunting KD, Galipeau J, Topham D, Benaim E, Sorrentino BP. Transduction of murine bone marrow cells with an MDR1 vector enables ex vivo stem cell expansion, but these expanded grafts cause a myeloproliferative syndrome in transplanted mice. Blood 1998; 92: 2269–79. [PubMed] [Google Scholar]
- 29. Modlich U, Kustikova OS, Schmidt M et al . Leukemias following retroviral transfer of multidrug resistance 1 (MDR1) are driven by combinatorial insertional mutagenesis. Blood 2005; 105: 4235–46. [DOI] [PubMed] [Google Scholar]
- 30. Fisher B, Rockette H, Fisher ER, Wickerham DL, Redmond C, Brown A. Leukemia in breast cancer patients following adjuvant chemotherapy or postoperative radiation: the NSABP experience. J Clin Oncol 1985; 3: 1640–58. [DOI] [PubMed] [Google Scholar]
- 31. Curtis RE, Boice JD Jr, Moloney WC, Ries LG, Flannery JT. Leukemia following chemotherapy for breast cancer. Cancer Res 1990; 50: 2741–6. [PubMed] [Google Scholar]
- 32. Milligan DW, Ruiz De Elvira MC, Kolb HJ et al . Secondary leukaemia and myelodysplasia after autografting for lymphoma: results from the EBMT. EBMT lymphoma and late effects working parties. European group for blood and marrow transplantation. Br J Haematol 1999; 106: 1020–6. [DOI] [PubMed] [Google Scholar]
- 33. Kroger N, Zander AR, Martinelli G et al . Low incidence of secondary myelodysplasia and acute myeloid leukemia after high‐dose chemotherapy as adjuvant therapy for breast cancer patients: a study by the Solid Tumors Working Party of the European Group for Blood and Marrow Transplantation. Ann Oncol 2003; 14: 554–8. [DOI] [PubMed] [Google Scholar]


