Abstract
N‐myc Downstream‐Regulated Gene 1 (NDRG1) is known as a differentiation‐related gene that plays important roles in cell differentiation, organ formation, and embryonic development. NDRG1 has recently been shown to be associated with carcinogenesis and tumor progression in a wide variety of tumors. Phosphatase and tensin homolog deleted from chromosome (PTEN), a phosphatase and tensin homolog located on chromosome 10, is shown to be a tumor suppressor and is often mutated or deleted in various tumor cells, particularly in endometrial carcinoma. Using an immunohistochemical approach, we investigated the expression of NDRG1 and PTEN in normal endometrium, atypical hyperplasia, and endometrial carcinoma. All tumor tissues harvested in this study were derived from endometrioid carcinoma Type I, that were estrogen‐related. Our results demonstrate that the expression of NDRG1 was up‐regulated in 5/40 (12.5%), 18/34 (52.94%), and 86/103 (83.5%) normal endometrium, atypical hyperplasia, and endometrial carcinoma cases, respectively (P < 0.01), while in 6/40 (15%), 20/34 (58.82%), and 89/103 (86.41%) normal endometrium, atypical hyperplasia, and endometrial carcinoma cases, respectively. PTEN expression was significantly decreased (P < 0.01). Statistical analyzes demonstrated a positive correlation between NDRG1 up‐regulation and PTEN down‐regulation (P < 0.01). The expression of NDRG1 had no correlation with the differentiation degree of the tumor cells, lymph‐node metastasis, and/or abdominal cavity implantation (P > 0.05). Our results indicated that development of endometrial carcinoma is associated with an overexpression of NDRG1 and the loss of PTEN expression. Identification of changes in the NDRG1 and PTEN expression may be a significant diagnostic tool for the early detection of endometrial carcinoma. (Cancer Sci 2008; 99: 706–710)
Endometrial carcinoma is the most common gynecologic malignancy in the United States and many other countries of the developed world.( 1 ) Generally, two distinct clinical‐pathological types are recognized. The estrogen‐related Type I is characterized by the endometrioid histology and represents 70–80% of newly diagnosed cases of endometrial carcinoma in the United States. Type II endometrial carcinoma are nonestrogen‐related and have a nonendometrioid histology. They typically have an aggressive clinical course.( 2 )
The inactivation and mutation of tumor suppressor genes and the abnormal activation of proto‐oncogenes may play important roles in the carcinogenesis and progression of endometrial carcinoma. N‐myc Downstream‐Regulated Gene 1 (NDRG1) has been mapped to human chromosome 8q24.2 with a 60 kb sequence and consists of 16 exons and 15 introns. It is present in all cells of the human body and is considered to encode a protein with a general, housekeeping function related to growth arrest, cell differentiation, organ formation, embryonic development, and maintaining the differentiating status. The expression of NDRG1 is regulated by several factors, including androgen, p53, and N‐myc. Hypoxic conditions also induce NDRG1 expression via a mechanism that most likely involves nickel that interacts with the oxygen sensory pathway.( 3 ) Pathologically, the expression of NDRG1 was previously shown to be up‐regulated by the induction of differentiation in a colon carcinoma cell line.( 4 ) More recently, NDRG1 has been associated with the progression of various human cancers, including colorectal carcinogenesis,( 5 ) but it also seems to play an important role in the endometrial transformations leading to the completion of each menstrual cycle in humans.( 6 , 7 , 8 ) It remains, however, controversial whether its overexpression suppresses or promotes tumor metastasis.( 5 , 9 , 10 )
The phosphatase and tensin homolog deleted from chromosome 10 (PTEN) gene was originally identified as a candidate tumor suppressor gene, located on chromosome 10q23.3. The PTEN gene encodes a phosphatase that dephosphorylates phosphatidylinositol‐3,4,5‐triphosphate.( 11 ) It is known to play a key role in controlling cell growth, differentiation, and apoptosis.( 12 ) Recently, the PTEN gene has been shown to be mutated or deleted in various human cancer cells.( 13 ) The latter include prostate, breast, lung, glioblastoma, melanoma, and especially endometrial carcinoma, where deletion of PTEN was observed in more than 90% of the cases.( 14 , 15 ) PTEN has been shown to be inactivated in a wide variety of cancers and its role as a tumor suppressor has been well established.( 16 , 17 ) Interestingly, PTEN expression was correlated with the expression of differentiation related gene 1 in prostate and breast carcinoma which suggests that both genes could be developed as diagnostic tools for the early detection of those carcinoma.( 10 )
To better understand the relationship between PTEN and NDRG1 in the progression of estrogen‐related endometrioid Type I carcinoma, their expression was studied in normal endometrium, atypical hyperplasia, and endometrial carcinoma using an immunohistochemical approach. The relationship between the two markers, as well as the correlation with tumor differentiation and metastasis in lymph node and/or abdominal cavity implantation were evaluated.
Materials and Methods
Tissue specimens. Formaldehyde‐fixed and paraffin‐embedded tissue specimens from 40 cases of normal endometrium (20 cases of proliferative phase and 20 cases of secretory phase), 34 cases of atypical hyperplasia (i.e. 6 cases of simple atypical hyperplasia and 28 cases of complex atypical hyperplasia), and 103 cases of endometrial carcinoma were collected between January 2003 and December 2005 in our department at The First People's Hospital (Shanghai Jiaotong University, Shanghai, China). Initially, cases with normal endometrium were divided into two subgroups: 20 proliferative phase cases and 20 secretary phase cases. Evaluation of the expression of NDRG1 and PTEN demonstrated that no differences could be observed among the two subgroups (data not shown). Therefore, both groups were combined as the normal group for the continuation of this study. The age range of the subjects involved was 32 years to 81 years with 18 cases of <50 years old and 85 cases of ≥50 years old. The clinical stage distribution was as follows: 65 cases at stage I–II and 38 cases at stage III–IV.
All tumor tissues harvested herein were estrogen‐related endometrioid carcinoma Type I with 35 cases of grade I, 38 cases of grade II, and 30 cases of grade III (i.e. classified according to World Health Organization Tumor Pathology and Genetics, 2003). In total, 103 radical samples of lymph nodes were obtained and 26 of them were found lymph node metastasis. The expression of NDRG1 and PTEN in lymph node metastasis and/or abdominal cavity implantation was characteristic for 26/103 cases (in a mixed tumor population). All procedures described herein were approved by the human research committee of the First People's Hospital.
Immunohistochemical staining. Consecutive paraffin sections of 4‐µm thickness were mounted onto silanized slides and dried overnight at 37°C followed by 2 h at 60°C. The sections were deparaffinized in xylene and rehydrated in a graded series of alcohol solutions.
The Avidin Biotin‐Peroxidase Complex (ABC) method was applied for NDRG1 according to goat ABC Staining System (sc‐2023, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Slides were incubated for 10 min in 0.1–1% hydrogen peroxide diluted in phosphate‐buffered saline (PBS) to quench endogenous peroxidase activity, for 1 h in 1.5% blocking serum in PBS and incubated with purified goat polyclonal primary antibody NDRG1 prepared against the N‐terminus of NDRG1 of a peptide from human origin (IgG; N‐19: sc‐19464, Santa Cruz Biotechnology), 1:80, overnight at 4°C. The specificity of the anti‐NDRG1 antibody N‐19 has been demonstrated by Western blot analyzes conducted by the producer, Santa Cruz Biotechnology, and by its use in a recent study( 18 ) focused on impaired mast cell maturation and degranulation and attenuated allergic responses in NDRG1‐deficient mice in which N‐19 was used in immunohistochemical localization studies and Western blot analyzes. Sections were incubated for 30 min with biotinylated secondary antibody at approximately 1 µg/mL, 30 min with Avidin Biotin enzyme reagent, and 20–30 min with peroxidase substrate. Sections were counterstained with hematoxylin for 5–10 s.
The two‐step method was applied for PTEN. Immunohistochemical staining was performed using the mouse monoclonal primary antibody PTEN (Antibody Diagnostica, Inc., New York, NY, USA), 1:60, for 60 min, followed by a monoclonal secondary antibody (DakoCytomation Envision System Labelled Polymer‐HRP Anti‐Mouse). The sections were visualized with the EnVision Detection Kit (DAKO, Glostrup, Denmark) using diaminobenzidine chromogen as substrate and counterstained with hematoxylin. Controls for the immunostaining were performed using normal endometrial tissue control sections and positive normal cell compartments (i.e. stroma) within test sections. All immunostaining slides and corresponding hematoxylin slides were evaluated independently by two expert pathologists in our department who did not have access to the clinical data.
For negative controls, PBS was used to replace the primary antibody. For positive controls, kidney tissues were selected for NDRG1, and the stromal cell nuclei in endometrial carcinoma were selected for PTEN (provided by the commercialized kits).
In case of a positive signal, NDRG1 protein was located both in the cytoplasm and the membrane of the cells. A semiquantitative method( 19 ) was used: total score was A × B; A denoted the number of positive cells: ≤5% as 0, 6–25% as 1, 26–50% as 2, 51–75% as 3, and >75% as 4; B indicated the positive intensity: colorless as 0, light yellow as 1, yellow as 2, brown as 3. Score 0 as negative (–), 1–4 as weak positive (+), 5–8 as positive (++), and 9–12 as strong positive (+++). If the reading difference between the two pathologists was greater than 3, scoring was repeated. PTEN was located in the nuclei. PTEN expression in all the glands was classified as PTEN alterations negative. Any PTEN‐null gland was scored as PTEN alterations positive (included of loss and mutation).( 15 )
Statistical analyzes. Categorical data were presented by frequency. The association of the expression of genes in several tissue types, the association of NDRG1 and PTEN in endometrial carcinoma, the relationship between NDRG1 and the differentiation degree of endometrial carcinoma, and the relationship between NDRG1 and lymph node metastasis and/or abdominal cavity implantation were examined by the Fisher's exact test. All analyzes were performed by using StatXact and the exact probabilities are presented.
Results
The expression of NDRG1 and PTEN in distinct tissues. The expression of NDRG1 and PTEN in normal endometrium, atypical hyperplasia, and endometrial carcinoma cases was evaluated (Table 1).
Table 1.
The association of the expression of genes in distinct tissue types
| Tissue type | NDRG1a | PTEN* | ||
|---|---|---|---|---|
| +(up‐regulation) | –(non) | +(loss) | –(non) | |
| Normal endometrium | 5 | 35 | 6 | 34 |
| Atypical hyperplasia | 18 | 16 | 20 | 14 |
| Endometrial carcinoma | 86 | 17 | 89 | 14 |
P < 0.001. NDRG1, N‐myc Downstream‐Regulated Gene 1; PTEN, Phosphatase and tensin homolog deleted from chromosome 10.
NDRG1 was not expressed in 35/40 (87.5%) normal endometrium cases, except for very few positive cells in the epithelial and stroma (≤ 5%). Only 5/40 (12.5%) of normal endometrium cases contained focal‐positive cells in the endometrium. The number of cases with enhanced NDRG1 expression increased in cases with atypical hyperplasia, with 18/34 cases (52.94%) exhibiting up‐regulated NDRG1 expression. Most of the endometrial carcinoma cases, 86/103 (83.5%) cases, exhibited an increased NDRG1 expression. Representative images illustrating the immunolocalization of NDRG1 in normal endometrium and endometrial carcinoma are shown in Figure 1a and b, respectively. It was observed that NDRG1 positive areas were primarily located in the carcinoma glands while regions where no NDRG1 expression was observed included the normal glands nearby (Fig. 2). The expression levels of NDRG1 among the three groups were significantly different (P < 0.01). In addition, expression of NDRG1 was seen in squamous metaplasia (Fig. 3). In fact, our preliminary data suggest that the expression of NDRG1 was higher in the squamous metaplasia area than in adenocarcinoma (data not shown).
Figure 1.
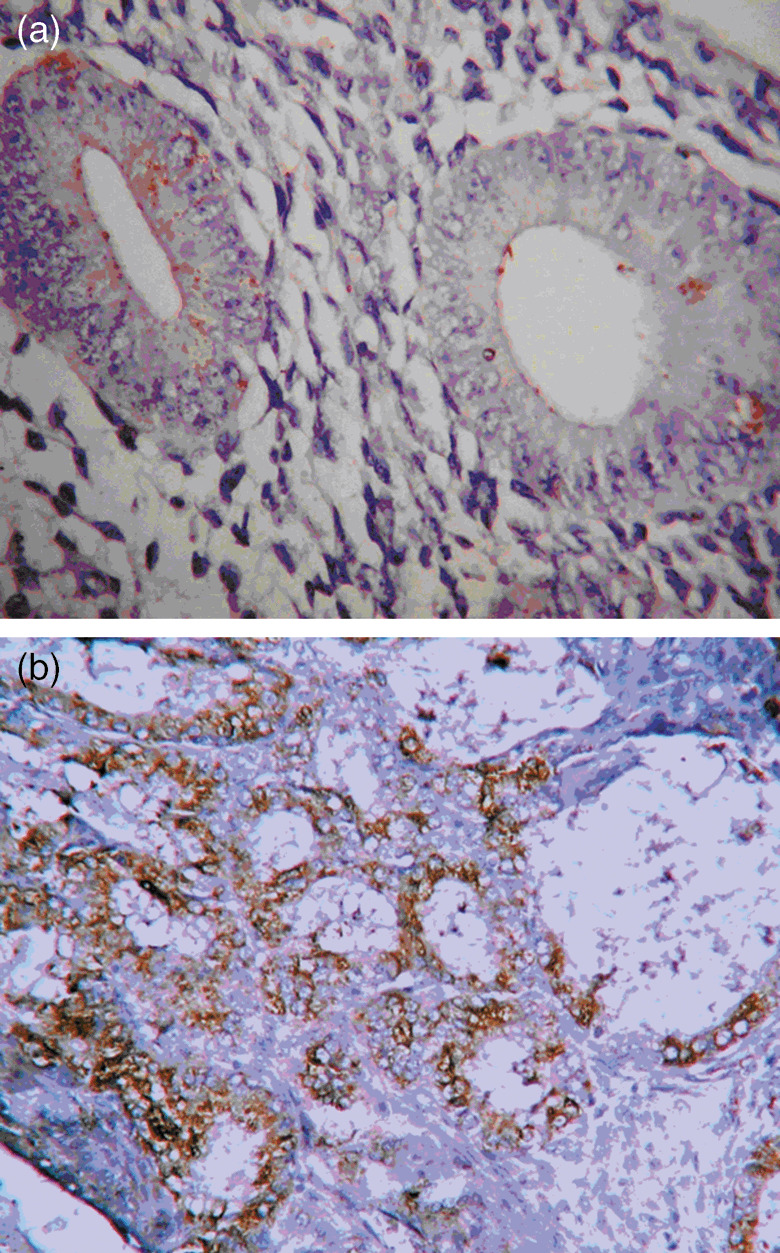
Immunolocalization of N‐myc Downstream‐Regulated Gene 1 in normal endometrium, 400× magnification (a) and endometrial carcinoma, 200× magnification (b).
Figure 2.
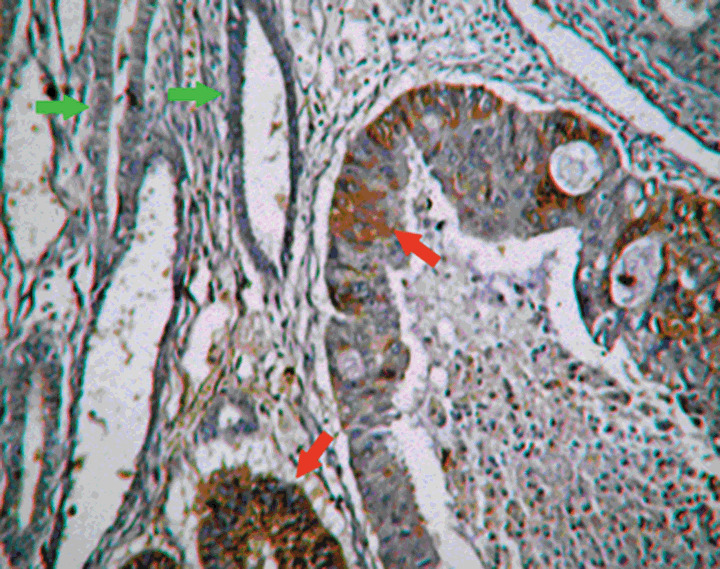
Immunolocalization of N‐myc Downstream‐Regulated Gene 1 was positive in the carcinoma glands (red arrows) while negative in proximal normal glands (green arrows), 200× magnification.
Figure 3.
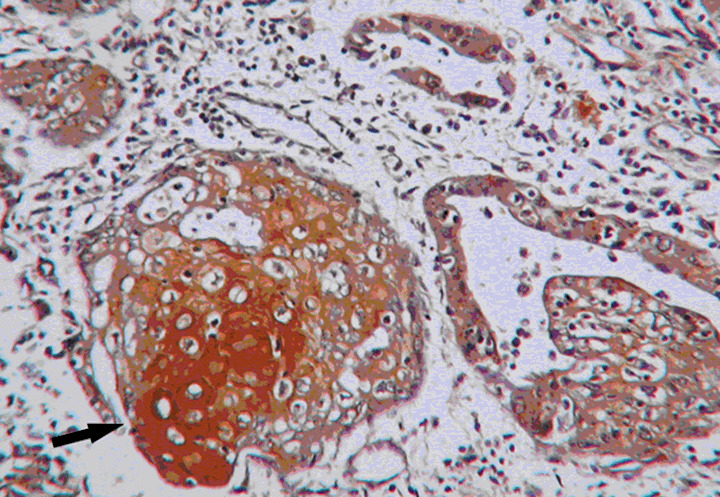
Expression of N‐myc Downstream‐Regulated Gene 1 in squamous metaplasia (black arrow), 200× magnification.
Significant PTEN expression was observed in most of the normal endometrium cases (34/40) which is obvious based on the immunolocalization studies (Fig. 4a). PTEN down‐regulation was apparent in 20/34 (58.82%), and 89/103 (86.41%) cases with atypical hyperplasia, and endometrial carcinoma, respectively (Table 1 and Fig. 4b) (P < 0.001).
Figure 4.
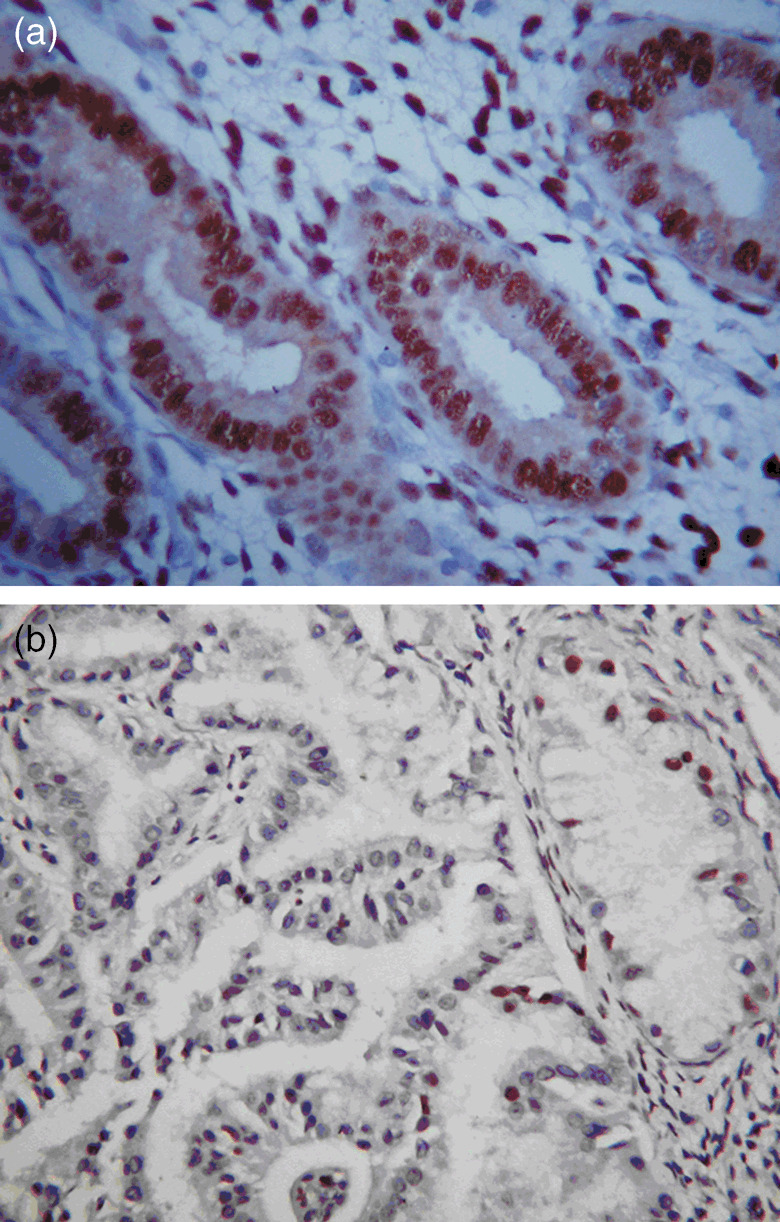
Immunostaining of phosphatase and tensin homolog deleted from chromosome was positive in the normal endometrium (a) and loss in the carcinoma gland (b), 400× magnification.
The correlation between NDRG1 and PTEN expression. The increased expression of NDRG1 appeared to be associated with the decrease in PTEN expression (Table 2). In the consecutive slides that were analyzed during the immunolocalization studies, a negative correlation between the expression of NDRG1 and PTEN was observed. Their expression in normal endometrium was with NDRG1 negative and PTEN positive, associated in the endometrial carcinoma with NDRG1 positive and PTEN negative. The difference between the NDRG1 and PTEN expression levels was significant (P < 0.001).
Table 2.
The association of NDRG1 and PTEN in endometrial carcinoma
| NDRG1 | PTEN Loss* | |
|---|---|---|
| +(loss) | –(non) | |
| +(upregulation) | 83 | 3 |
| –(non) | 6 | 11 |
P < 0.0001. NDRG1, N‐myc Downstream‐Regulated Gene 1; PTEN, Phosphatase and tensin homolog deleted from chromosome 10.
The relationship between NDRG1 and PTEN expression and endometrial carcinoma differentiation. One hundred and three cases of endometrial carcinoma were classified into three groups, according to the degree of differentiation. The scores were evaluated by the semiquantitative method described in Materials and Methods. Increased expression of NDRG1 in endometrial carcinoma cases was classified as follows: 29/35 (82.86%) cases in Grade I, 31/38 (81.58%) cases in Grade II, and 26/30 (81.58%) cases in Grade III (Table 3). No significant differences were observed among the three groups (P = 0.8618). Similar to NDRG1, no correlation between PTEN expression and endometrial carcinoma differentiation could be observed (Table 4).
Table 3.
NDRG1 and the differentiation degrees of endometrial carcinoma
| Carcinoma degrees † | NDRG1* expression score | |||
|---|---|---|---|---|
| – | + | ++ | +++ | |
| Grade I | 6 | 12 | 10 | 7 |
| Grade II | 7 | 15 | 8 | 8 |
| Grade III | 4 | 8 | 11 | 7 |
P = 0.8618.
See Materials and Methods for an explanation of the carcinoma degrees.
NDRG1, N‐myc Downstream‐Regulated Gene 1.
Table 4.
Correlation between PTEN and NDRG1 expression, and differentiation degree, lymph node metastasis, and tumor stage †
| Clinical factors | Case No. | PTEN (Loss) | NDRG1 (upregulation) | ||||
|---|---|---|---|---|---|---|---|
| 89 (+) (loss) | 14 (–) | P | 89 (+) (upregulation) | 17 (–) | P | ||
| Age | |||||||
| <50 | 18 | 15 (16.85) | 3 (21.43) | 0.708 | 14 (16.28) | 4 (23.53) | 0.492 |
| ≥50 | 85 | 74 (83.15) | 11 (78.57) | 72 (83.72) | 13 (76.47) | ||
| Differentiation degree | |||||||
| I | 35 | 31 (34.83) | 4 (28.57) | 0.935 | 29 (33.72) | 6 (35.29) | 0.894 |
| II | 38 | 32 (35.96) | 6 (42.86) | 31 (36.05) | 7 (41.18) | ||
| III | 30 | 26 (29.21) | 4 (28.57) | 26 (30.23) | 4 (23.53) | ||
| Lymph node metastasis | |||||||
| No | 77 | 67 (75.28) | 10 (71.43) | 0.748 | 62 (72.09) | 15 (88.24) | 0.227 |
| Yes | 26 | 22 (24.72) | 4 (28.57) | 24 (27.91) | 2 (11.76) | ||
| Tumor stage | |||||||
| I–II | 65 | 56 (62.92) | 9 (64.29) | 1.000 | 57 (66.28) | 8 (47.06) | 0.171 |
| III–IV | 38 | 33 (37.08) | 5 (35.71) | 29 (33.72) | 9 (52.94) | ||
All values refer to Fisher's exact test. NDRG1, N‐myc Downstream‐Regulated Gene 1; PTEN, Phosphatase and tensin homolog deleted from chromosome 10.
Correlation between NDRG1 and PTEN expression and lymph node metastasis and/or abdominal cavity implantation. A majority of the cases (24/26, 92.3%) with lymph node metastasis and/or abdominal cavity implantation expressed NDRG1 (Table 4). However, 62/77 (80.5%) cases expressed NDRG1 without metastasis and/or implantation. There was no statistical difference between the two groups (P > 0.05). Similar to NDRG1, no correlation was observed between PTEN expression and the lymph node metastasis or cavity implantation (Table 4).
Discussion
Experimentally, NDRG1 was reported to be associated with carcinogenesis and progression in a wide variety of cancers, but its expression in tumors was different depending on the system. Some reports demonstrate that the expression of NDRG1 is enhanced( 3 ) in tumors while others report a decrease.( 20 ) Consequently, its significance has been reported differently. Here, we observed that NDRG1 was virtually not expressed in normal endometrium, except for very few epithelial and stroma cells. The latter NDRG1‐positive cells were small and resembled undifferentiated mesenchymal cells, thought to be important in the growth and differentiation of endometrial cells. NDRG1 expression was increased in atypical endometrium and markedly increased in endometrial carcinoma. These data suggest that early identification of an enhanced NDRG1 expression in endometrial tissues could be an indication of the initiation of carcinoma development.
Since NDRG1 is a differentiation related gene, we also observed its expression in well‐, moderate‐, and poorly differentiated endometrial carcinoma. No significant difference was found among the three groups which strongly suggest that the regulation of NDRG1 is not significantly affected by the degree of differentiation of the endometrial carcinoma. Further studies are required to demonstrate whether NDRG1 can be recognized as a proto‐oncogene.
It has been controversial whether overexpression of NDRG1 inhibits or promotes cancer metastasis. Guan et al.( 9 ) reported that NDRG1 could inhibit metastasis of colon cancer while Wang et al.( 5 ) reported that it could promote metastasis of colon cancer. Here, we demonstrate that 26/103 cases of endometrial carcinoma were accompanied with lymph node metastasis and/or abdominal implantation in which 24/26 cases expressed NDRG1. Nevertheless, 62/77 non‐metastasis cases also expressed NDRG1. There was no statistical difference between the two groups. Even though the expression of NDRG1 was higher in the squamous metaplasia area than in adenocarcinoma (data not shown), whether or not NDRG1 was associated with the development of metaplasia needs further demonstration.
Endometrial carcinoma can be divided into two broad categories based on clinico‐pathological and molecular characteristics: Type I (endometrioid) showing defects in PTEN mutations and Type II (nonendometrioid such as papillary serous and clear cell) showing p53 mutations.( 2 , 20 ) It was reported that over 90% cases of PTEN deletion were detected in endometrioid adonocarcinoma.( 14 ) Coincidentally, in our study, the rate of PTEN loss was 15% in the normal endometrium, 58.82% in the atypical hyperplasia, and 86.41% in the endometrial carcinoma. The malignant phenotype was increased with the PTEN loss, which suggested that PTEN deletion might be an important cause of carcinogenesis of endometrial carcinoma. Bandyopadhyay et al.( 10 ) reported that PTEN expression correlated with NDRG1 in both prostate and breast cancer and suggested that PTEN up‐regulates NDRG1 by an AKT‐dependent pathway. However, we demonstrated that most NDRG1 positive cases exhibited a down‐regulation of PTEN and most PTEN positive cases exhibited a decrease in NDRG1 expression in endometrial carcinoma. Only a minority of the cases examined herein expressed both markers. The negative correlation between the PTEN and NDRG1 expression patterns indicated an intrinsic association between PTEN loss and NDRG1 overexpression. Whether the deletion of PTEN leads to the decrease in tumor suppressing action and activates NDRG,1 or whether the overexpression of NDRG1 inhibits PTEN, needs to be further scrutinized. Even though, similar to NDRG1, no correlation between PTEN expression and endometrial carcinoma differentiation or metastasis could be observed.
Based on the results presented herein, both the overexpression of NDRG1 and the decrease of PTEN expression were found as early as the stage of atypical hyperplasia implying that abnormal expression of both genes may be associated with the early stages of endometrial carcinoma development; however, no correlation with the lymph node metastasis or abdominal implantation could be demonstrated in endometrial carcinoma. Future studies in animal models will be carried out to further document the direct association between NDRG1 and PTEN, and their role in the initiation of endometrial carcinoma development and eventually in the early diagnosis of endometrial carcinoma.( 3 )
Acknowledgments
The study was supported by Shanghai Science & Technologic Committee, China (04JC14064). The authors thank Dr Ya‐Ping Zhu for providing the clinical information.
References
- 1. Ryan AJ, Susil B, Jobling TW. Endometrial cancer. Cell Tissue Res 2005; 322: 53–61. [DOI] [PubMed] [Google Scholar]
- 2. Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol 2006; 24: 4783–91. [DOI] [PubMed] [Google Scholar]
- 3. Cangul H. Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC Genet 2004; 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Belzen N, Dinjens WN, Diesveld MP et al . A novel gene which is up‐regulated during colon epithelial cell differentiation and down‐regulated in colorectal neoplasms. Lab Invest 1997; 77: 85–92. [PubMed] [Google Scholar]
- 5. Wang Z, Wang F, Wang WQ et al . Correlation of N‐myc downstream‐regulated gene 1 overexpression with progressive growth of colorectal neoplasm. World J Gastroenterol 2004; 10: 550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lachat P, Shaw P, Gebhard S, Van Belzen Chaubert P, Bosman FT. Expression of NDRG1, a differentiation‐related gene in human tissues. Histochem Cell Biol 2002; 118: 399–408. [DOI] [PubMed] [Google Scholar]
- 7. Wang Z, Liu Q, Chen Q et al . Overexpression of NDRG1: relationship with proliferation activity and invasiveness of breast cancer cell line and breast cancer metastasis. Chin J Pathol 2006; 35: 333–8. [PubMed] [Google Scholar]
- 8. Malette B, Cherry E, Lagace M et al . Large scale validation of human N‐myc downstream gene (NDRG)‐1 expression in endometrium during the menstrual cycle. Mol Hum Reprod 2003; 9: 671–9. [DOI] [PubMed] [Google Scholar]
- 9. Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM, Pardee ABI. Drg‐1 as a differentiation‐related, putative metastatic suppressor gene in human colon cancer 1. Cancer Res 2000; 60: 749–55. [PubMed] [Google Scholar]
- 10. Bandyopadhyay S, Pai SK, Hirota S et al . PTEN up‐regulates the tumor metastasis suppressor gene Drg‐1 in prostate and breast cancer. Cancer Res 2004; 64: 7655–60. [DOI] [PubMed] [Google Scholar]
- 11. Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res 2001; 264: 29–41. [DOI] [PubMed] [Google Scholar]
- 12. Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci 2001; 114: 2375–82. [DOI] [PubMed] [Google Scholar]
- 13. Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3‐kinase/AKT pathway. Proc Natl Acad Sci USA 1999; 96: 4240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taranger‐Charpin C, Carpentier S, Dales JP et al . Immunohistochemical expression of PTEN antigen: a new tool for diagnosis of early endometrial neoplasia. Bull Acad Natl Med 2004; 188: 415–27. [PubMed] [Google Scholar]
- 15. Baak JP, Van Diermen B, Steinbakk A et al . Lack of PTEN expression in endometrial intraepithelial neoplasia is correlated with cancer progression. Hum Pathol 2005; 36: 555–61. [DOI] [PubMed] [Google Scholar]
- 16. Salvesen HB, Stefansson I, Kretzschmar EI et al . Significance of PTEN alterations in endometrial carcinoma: a population‐based study of mutations, promoter methylation and PTEN protein expression. Int J Oncol 2004; 25: 1615–23. [PubMed] [Google Scholar]
- 17. Uegaki K, Kanamori Y, Kigawa J et al . PTEN is involved in the signal transduction pathway of contact inhibition in endometrial cells. Cell Tissue Res 2006; 323: 523–8. [DOI] [PubMed] [Google Scholar]
- 18. Taketomi Y, Sunaga K, Tanaka S et al . Impaired mast cell maturation and degranulation and attenuated allergic responses in Ndrg1‐deficient mice. J Immunol 2007; 178: 7042–53. [DOI] [PubMed] [Google Scholar]
- 19. Rhode A, Jasani B, Barnes DM, Bobrow LG, Miller KD. Reliability of immunohistochemical demonstration of estrogen receptor in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol 2000; 53: 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ando T, Ishiguro H, Kimuraet M et al . Decreased expression of NDRG1 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Dis Esophaqus 2006; 19: 454–8. [DOI] [PubMed] [Google Scholar]


