Abstract
Human T‐cell leukemia virus type‐1 (HTLV‐1)‐specific T‐cell immunity, a potential antitumor surveillance system in vivo, is impaired in adult T‐cell leukemia (ATL). In this study, we aimed to clarify whether the T‐cell insufficiency in ATL is present before the disease onset or occurs as a consequence of the disease. We investigated T‐cell responses against Tax protein in peripheral blood mononuclear cells (PBMCs) from individuals at earlier stages of HTLV‐1‐infection, including 21 asymptomatic HTLV‐1 carriers (ACs) and four patients with smoldering‐type ATL (sATL), whose peripheral lymphocyte count was in normal range. About 30% of samples tested showed clear Tax‐specific interferon (IFN)‐γ producing responses. Proviral loads in this group were significantly lower than those in the other less‐specific response group. The latter group was further divided to two subgroups with or without emergence of Tax‐specific responses following depletion of CC chemokine receptor 4 (CCR4)+ cells that contained HTLV‐1‐infected cells. In the PBMCs with Tax‐specific responses, CD8+ cells efficiently suppressed HTLV‐1 p19 production in culture. The remaining group without the emergence of Tax‐specific response after CCR4+ cell‐depletion included at least two sATL and one AC samples, which spontaneously produced HTLV‐1 p19 in culture, where tetramer‐binding, Tax‐specific cytotoxic T‐lymphocytes were either undetectable or unresponsive. Our results indicated that HTLV‐1‐specific T‐cell responsiveness widely differed among HTLV‐1 carriers, and that impairment of HTLV‐1‐specific T‐cell responses was observed not only in advanced ATL patients but also in a subpopulation at earlier stages, which was associated with insufficient control of HTLV‐1. (Cancer Sci 2009; 100: 481–489)
Human T‐cell leukemia virus type 1 (HTLV‐1) is the etiological agent of adult T‐cell leukemia (ATL).( 1 , 2 ) Although the majority of HTLV‐1‐infected individuals remain asymptomatic throughout their lives, about 5% develop ATL during or after middle age and another small population develops HTLV‐1‐associated myelopathy/tropical spastic paraparesis (HAM/TSP) and a variety of chronic inflammatory diseases.( 3 , 4 , 5 , 6 , 7 ) Several epidemiological risk factors have been suggested to be associated with ATL development, including vertical transmission, gender (greater incidence in males than in females),( 4 , 8 ) and increased numbers of abnormal lymphocytes associated with elevated HTLV‐1 proviral loads.( 9 , 10 ) However, elevation in HTLV‐1 proviral loads is also a feature of HAM/TSP patients.( 7 , 11 )
ATL is known to be an immunosuppressive condition.( 3 ) Recent reports have shown that ATL cells frequently express Foxp3 and the chemokine receptor CCR4, in addition to CD4 and CD25.( 12 , 13 , 14 , 15 , 16 ) These molecules are also expressed in regulatory T‐cells (Tregs).( 17 , 18 , 19 , 20 ) Although isolated ATL cells do not always exhibit suppressive functions in vitro, the common phenotypes shared by ATL cells and Tregs suggest that ATL cells may share a common origin with Tregs, or possess immunoregulatory properties.( 21 ) General immunosuppression may be present not only in ATL patients, but also in asymptomatic HTLV‐1 carriers (ACs) to some extent.( 22 , 23 )
There is a clear difference between ATL and HAM/TSP patients in the host T‐cell responses against HTLV‐1. Outgrowth of CD8+ HTLV‐1‐specific cytotoxic T‐lymphocytes (CTLs) in response to in vitro stimulation is frequently found in peripheral blood mononuclear cell (PBMC) cultures from HAM/TSP patients, but rarely observed in those from ATL patients.( 24 , 25 , 26 ) These CTLs have anti‐HTLV‐1 effects, as elimination of CD8+ cells among PBMCs from HAM/TSP patients induces HTLV‐1 expression during subsequent cell culture.( 27 , 28 ) HTLV‐1 Tax‐specific CTL responses are strongly activated in some ATL patients who obtained complete remission after hematopoietic stem cell transplantation (HSCT), but are not observed in the same patients before transplantation.( 29 ) These findings suggest that Tax‐specific CTLs may play a role in immunosurveillance for HTLV‐1 leukemogenesis.
Studies on a rat model have indicated that the otherwise‐elevated proviral loads in orally HTLV‐1‐infected rats could be reduced by restoration of HTLV‐1‐specific T‐cell responses.( 30 , 31 ) Furthermore, DNA vaccines or peptide vaccines targeting Tax, the major target antigen recognized by HTLV‐1‐specific T‐cells, can induce antitumor immunity and eradicate HTLV‐1‐infected lymphomas.( 32 , 33 ) These observations imply that antitumor therapeutic vaccines targeting Tax might be promising.
It is important to clarify the immunological status of ACs, since insufficiency in host T‐cell responses against HTLV‐1 could be an immunological risk factor for ATL. HTLV‐1‐specific CTL responses are also detectable in ACs.( 34 , 35 ) However, because a wide survey for HTLV‐1‐specific T‐cell immunity has never been carried out, the questions of the proportion of ACs with proper levels of immune responses and the possible existence of a population of ACs with insufficient anti‐HTLV‐1 responses before ATL onset, remain unresolved. One reason for the poor status of such immunological surveys among ACs is the absence of simple methods for measuring HTLV‐1‐specific T‐cell responses, as they are restricted by individual human leukocyte antigens (HLAs).
We recently established a detection system for HTLV‐1‐specific T‐cell responses using recombinant Tax proteins fused to glutathione‐S‐transferase (GST), in which Tax antigens are processed by antigen‐presenting cells and capable of stimulating both CD4+ and CD8+ T‐cells among self PBMCs.( 36 ) In this study, by using this assay, we analyzed HTLV‐1‐specific T‐cell responses in unselected ACs and smoldering‐type ATL (sATL) patients. We examined sATL samples together because their peripheral lymphocyte numbers are in the normal range (< 4000/µL) and the prognoses vary among individuals.( 37 , 38 ) Here, we demonstrated wide diversity in T‐cell response patterns against HTLV‐1 in ACs and sATL patients. Among them, we found some individuals exhibiting impaired Tax‐specific T‐cell responses associated with poor control of HTLV‐1 both in ACs and sATL patients.
Materials and Methods
Subjects. A total of 21 ACs, five HAM/TSP patients, four sATL patients, two chronic‐type ATL (cATL) patients, and two acute ATL patients in long‐term remission (>2 and >5 years) after allogeneic HSCT donated peripheral blood samples after providing written informed consent. PBMCs were isolated by Ficoll‐Paque PLUS (GE Healthcare UK, Buckinghamshire, UK) density gradient centrifugation, and either used immediately or stored frozen in liquid nitrogen in Bambanker stock solution (NIPPON Genetics Co., Tokyo, Japan).
Separation of PBMC fractions. CD4+ or CD8+ cells were depleted from PBMCs by negative selection using 10‐fold numbers of Dynabeads M‐450 CD4 or CD8 (Dynal Biotec, Oslo, Norway), respectively, according to the manufacturer's instructions. CCR4+ cells were depleted from PBMCs using Dynabeads goat antimouse IgG (Dynal Biotec) following incubation with carboxyfluorescein‐conjugated anti‐CCR4 monoclonal antibody (mAb) for 45 min at 4°C. The resulting contamination by CD4+, CD8+, or CCR4+ cells was between 0.02% and 3.90% of the total lymphocytes, as analyzed by flow cytometry. The PBMC concentrations were adjusted to 1 × 106 cells/mL before depletion, and the resulting CD4+, CD8+, or CCR4+ cell‐depleted fractions were resuspended in medium with the same initial volume, irrespective of the remaining cell numbers.
Recombinant proteins and peptides. GST‐fusion proteins of HTLV‐1 Tax‐A, Tax‐B, and Tax‐C (corresponding to the N‐terminal, central, and C‐terminal regions of HTLV‐1 Tax, respectively) were prepared as described previously.( 36 ) Briefly, partially overlapping DNA fragments designated Tax‐A, Tax‐B, and Tax‐C were inserted into pGEX‐2T (GE Healthcare UK) to express the corresponding proteins fused to GST. DH5α competent cells were transformed with these plasmids, and cultured in 2xYT medium supplemented with ampicillin and isopropyl‐β‐D‐thiogalactopyranoside (IPTG) for protein expression. Individual GST–Tax proteins in inclusion bodies were extracted by sonication and purified using Glutathione Sepharose 4B affinity columns (GE Healthcare UK), followed by size exclusion gel chromatography. The purified proteins were stored at –80°C. The concentrations used were 12.5 µg/mL for GST and 18.75 µg/mL for a mixture of GST–Tax A, B, and C proteins (6.25 µg/mL for each protein). In some experiments, a synthetic peptide corresponding to Tax 301‐309 (SFHSLHLLF) and Tax 88‐96 (KVLTPPITH), representing the major CTL epitopes restricted by HLA‐A24 and A11, respectively, was used as an antigen at 10 µM in PBMC cultures.( 29 , 39 )
Assay for T‐cell responses. Whole PBMCs (2 × 105 cells/well) or various cell‐depleted PBMC fractions starting from the same number of whole PBMCs were incubated with various antigens in 96‐well round‐bottom culture plates in duplicate wells. The culture medium was RPMI‐1640 supplemented with 10% heat‐inactivated fetal bovine serum (FBS), 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 2 mg/mL of sodium bicarbonate. To avoid the potential influence of endotoxin contamination of the recombinant proteins, 10 µg/mL of polymyxin B was added to all assays. After 4 days of culture, the supernatants were harvested and stored at –20°C until analysis. The concentrations of interferon (IFN)‐γ in the supernatants were measured using a Human IFN‐γ ELISA Kit (BioSource, Camarillo, CA, USA) or OptEIA Human IFN‐γ ELISA Set (BD Biosciences). The absorbances at 450 nm were measured using a microplate reader and analyzed with the Microplate Manager III software (Bio‐Rad Laboratories). In some experiments, a Human Th1/Th2 Cytokine Kit for a Cytokine Beads Assay (CBA) (BD Biosciences) was used to measure various cytokines, including IFN‐γ.
Flow cytometry. For cell surface phenotyping, phycoerythrin (PE)‐Cy5‐conjugated anti‐CD4 and PE‐Cy5‐conjugated anti‐CD8 mAbs, carboxyfluorescein‐conjugated anti‐CCR4 mAb, and appropriate isotype controls were used. Uncultured PBMCs were incubated with these mAbs individually or in combination for 30 min at 4°C, before being washed in phosphate‐buffered saline (PBS) containing 1% FBS and fixed with 1% formaldehyde in PBS. For tetramer staining, PBMCs were stained with PE‐Cy5‐conjugated anti‐CD8 mAb for 30 min at 4°C, and then with PE‐conjugated HLA‐A*1101/Tax88‐96, HLA‐A*1101/Tax272–280, or HLA‐A*2402/Tax301‐309 tetramers (National Institute of Allergy and Infectious Diseases Tetramer Facility, Emory University Vaccine Center, Atlanta, GA, USA) for 45 min at 4°C.( 29 , 39 ) The samples were analyzed using a FACSCalibur and the CellQuest software (BD Biosciences).
HTLV‐1 antibody titer. The titers of HTLV‐1‐specific antibodies in the plasma samples were determined by the particle agglutination method by using Serodia HTLV‐1 (FUJIREBIO, Tokyo, Japan) according to the manufacturer's instructions.
HTLV‐1 proviral loads. HTLV‐1 proviral loads in PBMCs were measured by quantitative real‐time polymerase chain reaction (PCR) with HTLV‐1 Tax‐specific primers through the clinical diagnostic services of SRL Inc. (Tokyo, Japan) or the Group of Joint Study on Predisposing Factors of ATL Development (JSPFAD, Japan) as described previously.( 40 , 41 ) Proviral DNA copy numbers in various fractions of PBMC samples were measured by SYBR Green quantitative real‐time PCR methods using Tax‐specific primers (forward: 5′‐cggatacccagtctacgtgtttggagactgt‐3′, reverse: 5′‐gagccgataacgcgtccatcgatggggtcc‐3′) and control beta‐globin primers (forward: 5′‐acacaactgtgttcactagc‐3′, reverse: 5′‐caacttcatccacgttcacc‐3′).
Statistical analysis. The Mann–Whitney U‐test was used to examine the statistical difference in HTLV‐1 proviral loads between two groups by using the Graphpad Prism 4 (Graphpad Software). P‐values <0.05 were considered significant.
Results
Detection of different patterns of Tax‐specific T‐cell responses in various diseases associated with HTLV‐1 infection. In order to obtain typical patterns of T‐cell responses detected by the Tax protein‐based assay, we examined PBMCs from HTLV‐1‐infected patients with various clinical conditions (Fig. 1a). Two ATL patients (#37, #253) who had been in long‐term complete remission after HSCT showed clear Tax‐specific IFN‐γ production, only against GST–Tax protein but not against control GST protein. PBMCs from HAM/TSP patients (#254, #259) produced high levels of IFN‐γ in response to GST–Tax, but also in the presence of medium alone or the control GST. In contrast, PBMCs from two cATL patients (#227, #249) showed very weak responses to any stimulation. Although the PBMCs from uninfected individuals showed low levels of background responses that might involve macrophages or natural killer cells, the levels of IFN‐γ production in cATL samples were even lower than the background responses.
Figure 1.
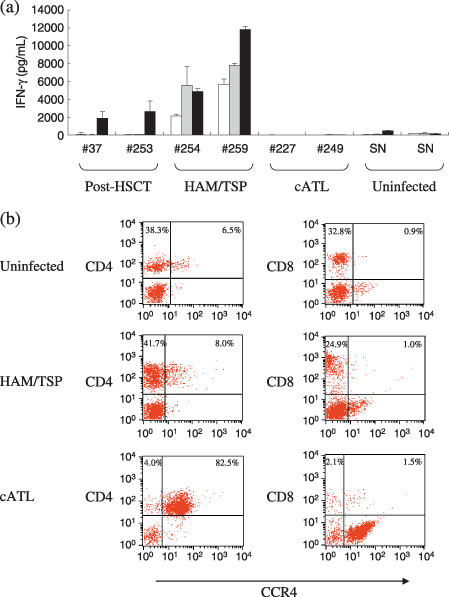
Different patterns of Tax‐specific T‐cell responses in various diseases associated with human T‐cell leukemia virus type‐1 (HTLV‐1) infection. (a) Peripheral blood mononuclear cells (PBMCs) (2.0 × 105 cells/well) from two post‐hematopoietic stem cell transplantation (HSCT) adult T‐cell leukemia (ATL) patients in long‐term remission (#37, #253), two HTLV‐1‐associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients (#254, #259), two chronic‐type ATL (cATL) patients (#227, #249), and two seronegative uninfected subjects (SN) were cultured alone (open bars), with glutathione‐S‐transferase (GST) (gray bars) or with a mixture of GST–Tax proteins (black bars) in a total volume of 200 µL of medium/well for 4 days. Interferon (IFN)‐γ in the supernatants was measured by enzyme‐linked immunoabsorbent assay. The results represent the mean ± SD of duplicate wells. (b) Uncultured PBMCs from an uninfected subject, a HAM/TSP patient (#255), and a cATL patient (#227) were stained with carboxyfluorescein‐conjugated CCR4 monoclonal antibody (mAb) together with phycoerythrin (PE)‐Cy5‐labeled CD4 (left) or CD8 (right) mAbs. Values indicate the percentages of positive cells in each quadrant analyzed by flow cytometry for a total of 10 000 gated lymphocytes.
HTLV‐1‐infected cells have been reported to express the chemokine receptor CCR4 frequently.( 13 ) As shown in Fig. 1(b), PBMCs of an uninfected individual contained 44.8% CD4+ cells and 6.5% CD4+CCR4+ cells. In the cATL patient (#227), the proportion of CD4+CCR4+ cells was markedly elevated, consistent with previous reports.( 13 ) PBMCs from this cATL patient contained 86.5% CD4+ cells and 82.5% CD4+CCR4+ cells. In contrast, the proportion of CD4+CCR4+ cells in the PBMCs from the HAM/TSP patient (#255) was comparable with that in the uninfected individual.
Since the proportion of HTLV‐1‐infected cells in the peripheral lymphocytes was elevated in cATL, the poor T‐cell responses in these samples may be partly explained by the relative scarcity of normal lymphocytes and antigen presenting cells. Such leukemic PBMCs are not suitable for evaluation with a protein‐based assay, and we only used PBMCs with lymphocyte numbers within the normal range hereafter.
Diversity in Tax‐specific T‐cell responses in ACs and sATL patients. Using the GST–Tax protein‐based assay system, we next examined the T‐cell responses of 21 ACs and four sATL patients. One of the sATL patients (#213) had polyclonal HTLV‐1‐infected cells but was categorized as sATL because of the number of abnormal lymphocytes was >5%. The hematologic and virologic profiles of the donors tested are summarized in Table 1. The numbers of peripheral lymphocytes of all ACs and sATL patients were within the normal range.
Table 1.
Blood samples from asymptomatic HTLV‐1 carriers (ACs) and smoldering ATL (sATL) patients tested
| ID | Age | Sex | Clinical status | WBC/µL | Lymphocyte (%) | Abnormal lymphocyte (%) | Provirus DNA copies/1000 PBMCs | Plasma HTLV‐1 antibody titer † |
|---|---|---|---|---|---|---|---|---|
| #211 | 20s | F | AC | 4500 | 34 | 0 | 33 | >8192 |
| #213 | 50s | F | sATL ‡ | 7500 | 36 | 8 | 200 | 4096 |
| #215 | 20s | M | AC | 8000 | 55 | 0 | 13 | 2048 |
| #216 | 70s | F | AC | 4200 | 31 | 3 | 39 | >8192 |
| #217 | 70s | F | AC | 6800 | 51 | 0 | 14 | >8192 |
| #218 | 50s | F | AC | 7500 | 55 | 0 | <1 | 1024 |
| #219 | 60s | F | AC | 7200 | 43 | 0 | <1 | 1024 |
| #220 | 50s | M | sATL | 4800 | 28 | 13 | 277 | >8192 |
| #223 | 60s | M | AC | 4200 | 26 | 0 | <1 | 1024 |
| #226 | 50s | M | AC | 5700 | 38 | 0 | 28 | 2048 |
| #228 | 60s | M | AC | 5900 | 59 | 0 | <1 | 256 |
| #232 | 30s | F | AC | 5800 | 46 | 0 | <1 | >8192 |
| #236 | 30s | F | AC | 6500 | 39 | 0 | 22 | >8192 |
| #238 | 60s | F | AC | 5700 | 51 | 0 | 2 | 1024 |
| #243 | 50s | F | AC | 4100 | 58 | 0 | 3 | 2048 |
| #244 | 50s | F | AC | 4900 | 27 | 3 | 63 | 1024 |
| #245 | 40s | F | AC | 5000 | 46 | 1 | 58 | 1024 |
| #246 | 50s | M | AC | 4600 | 37 | 0 | 2 | >8192 |
| #251 | 60s | M | AC | 4800 | 50 | 0 | 2 | 2048 |
| #252 | 50s | F | sATL | 4100 | 38 | 11 | 207 | >8192 |
| #258 | 60s | M | AC | 6400 | 30 | 3 | 8 | 256 |
| #263 | 60s | M | AC | 3900 | 49 | 2 | 1 | 2048 |
| #264 | 50s | F | AC | 3000 | 37 | 0 | <1 | 256 |
| #265 | 60s | F | sATL § | 4700 | 38 | 1 | 2 | 2048 |
| #277 | 50s | F | AC | 4100 | 29 | 0 | <1 | 256 |
Measured by a particle agglutination method.
Diagnosed as sATL because of increased abnormal lymphocyte number, although the infected cells were polyclonal.
Diagnosed as sATL because of the presence of skin lesions.
HTLV‐1, human T‐cell leukemia virus type‐1; ACs, asymptomatic HTLV‐1 carriers; ATL, adult T‐cell leukemia; sATL, smoldering‐type ATL; PMBC, peripheral blood mononuclear cell; WBC, white blood cell.
The IFN‐γ production levels by whole PBMCs from the ACs and sATL patients in the absence or presence of GST or GST–Tax proteins are shown in Fig. 2(a). The levels and patterns of IFN‐γ production varied widely among individuals. We divided the samples into two groups, according to Tax‐specificity of the responses. This was assessed by calculating the ratio of IFN‐γ produced in response to GST–Tax proteins divided by IFN‐γ produced in response to GST alone for each sample (Tax/GST ratios). Tax‐specific patterns were observed in samples from seven ACs (#228, #232, #238, #251, #258, #264, #277) and one sATL patient (#265) who had a localized skin lesion but was without apparent abnormal lymphocytes in the peripheral blood. The remaining samples showed less‐specific patterns of IFN‐γ production due to increased non‐specific IFN‐γ production or low IFN‐γ production to any stimulation.
Figure 2.
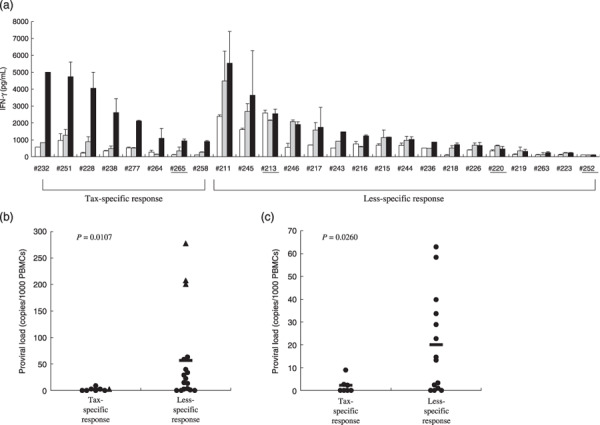
Diversity in Tax‐specific T‐cell responses among asymptomatic human T‐cell leukemia virus type‐1 (HTLV‐1) carriers (ACs) and smoldering‐type adult T‐cell leukemia (sATL) patients. (a) Peripheral blood mononuclear cells (PBMCs) isolated from 21 ACs and 4 sATL patients (underline) were incubated alone (open bars), with glutathione‐S‐transferase (GST) (gray bars) or with a mixture of GST–Tax proteins (black bars) for 4 days, and interferon (IFN)‐γ amounts in the supernatants were measured by enzyme‐linked immunoabsorbent assay and a Cytokine Beads Assay in part. The results were divided into Tax‐specific or less‐specific response groups depending on the ratios of anti‐GST–Tax/anti‐GST IFN‐γ production were more than 2.5 or not, and then aligned in the order of absolute values of IFN‐γ production against GST–Tax proteins in each group. (b, c) HTLV‐1 proviral loads of all the ACs and sATL patients tested (b) or ACs alone (c) were indicated. The mean values of proviral loads (bars) in Tax‐specific and less‐specific response groups were 2.0 and 56.9 copies/1000 PBMCs, respectively (P = 0.0107) in (b), and 1.9 and 20.1 copies/1000 PBMCs, respectively (P = 0.0260) in (c). AC, closed circle; sATL: closed triangle.
We compared the individual proviral loads between the two groups with Tax‐specific and less‐specific T‐cell responses (Fig. 2b,c). The group with clear Tax‐specific T‐cell response possessed significantly lower proviral loads than the less‐specific response group either in the total of ACs and sATL patients (P = 0.0107) or in ACs alone (P = 0.0260). The three sATL patients (#213, #252, #220) with highest proviral loads among those tested (>100 copies/1000 PBMCs), and several ACs with moderate levels of proviral loads (10–100 copies/1000 PBMCs) were in the less‐specific response group. However there were also some ACs who exhibited low T‐cell responses and low proviral loads (<10 copies/1000 PBMCs).
Involvement of CCR4+ cells in high background IFN‐γ production by PBMCs from HAM/TSP patients. We next assessed what cells in the PBMCs were responsible for the high background IFN‐γ production observed in HAM/TSP patients by depleting CD4+, CD8+, or CCR4+ cells from PBMCs prior to the assay. In order to see the effects of cell depletion, the cell concentrations were equalized before cell depletion, and the resulting cell fractions were resuspended in the same volume, irrespective of the final cell numbers. The results are shown in Fig. 3(a). Whole PBMCs from a HAM/TSP patient (#255) showed high levels of non‐specific IFN‐γ production with or without GST–Tax proteins. When the CD4+ cells were depleted, decreased but significant levels of IFN‐γ were only produced in response to GST–Tax proteins. CD8+ cell depletion did not markedly alter the non‐specific responses. CCR4+ cell depletion reduced the non‐specific responses, but retained Tax‐specific IFN‐γ production at a level comparable to that of whole PBMCs. These results indicated that CCR4+ PBMC population from this patient mainly contributed to the non‐specific responses in the assay, and that the effector of the Tax‐specific responses was included in the CCR4− population that contained CD4+ and CD8+ cells.
Figure 3.
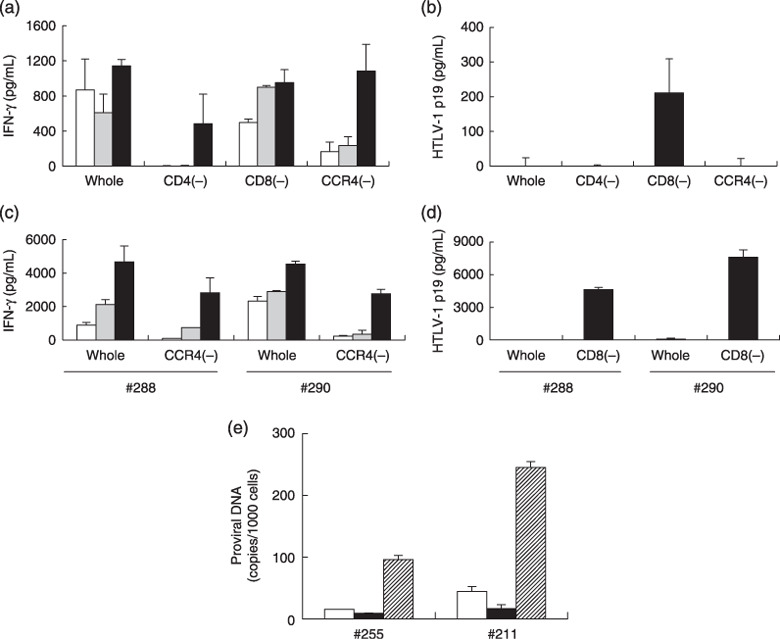
Involvement of CCR4+ cells in high background interferon (IFN)‐γ production by peripheral blood mononuclear cells (PBMCs) from human T‐cell leukemia virus type‐1 (HTLV‐1)–associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. PBMCs from a HAM/TSP patient (#255) were equally divided into four fractions. One fraction was used directly (whole) and the other three fractions were used after depletion of CD4+[CD4(–)], CD8+[CD8(–)], or CCR4+[CCR4(–)] cells. The cell numbers in each fraction were 2.0 × 105, 0.8 × 105, 1.1 × 105, and 1.3 × 105 cells/well, respectively. (a) The fractions were cultured alone (open bars), with glutathione‐S‐transferase (GST) (gray bars), or with a mixture of GST–Tax proteins (black bars) for 4 days. IFN‐γ in the supernatants was measured by enzyme‐linked immunoabsorbent assay (ELISA). (b) The levels of HTLV‐1 p19 antigen in the culture supernatants of the PBMC fractions in the absence of any stimulation were measured by ELISA at 7 days after the initiation of culture. The results represent the mean ± SD of duplicate wells. (c, d) Whole and fractionated PBMCs from two other HAM/TSP patients (#288 and #290) were similarly examined for IFN‐γ production for 4 days against medium (open bars), GST (gray bars), or GST–Tax proteins (black bars) (c), and HTLV‐1 p19 production for 7 days of culture (d). The cell numbers of whole, CCR4(–), and CD8(–) PBMCs in each well were 2.0 × 105, 1.7 × 105, and 1.2 × 105 for patient #288, and 2.1 × 105, 0.9 × 105, and 0.9 × 105 for patient #290, respectively. (e) HTLV‐1 provirus numbers (copies/1000 cells) in whole (open bars), CCR4+ cell‐depleted (closed bars), and CCR4+ (hatched bars) PBMC fractions before culture from subjects #255 (HAM/TSP) and #211 (AC) were measured by real‐time polymerase chain reaction methods. The results represent the mean ± SD of duplicate samples.
We also measured the HTLV‐1 p19 levels in the supernatants of these PBMC cultures after 7 days, and found that HTLV‐1 p19 became detectable only in the CD8+ cell‐depleted fraction but not in the whole or CD4+ cell‐depleted or CCR4+ cell‐depleted fractions (Fig. 3b). Since the starting PBMC number before cell‐depletion in each fraction was equivalent, the increase in p19 production in the CD8+ cell‐depleted fraction indicated that CD8+ cells served as suppressors of viral expression in culture.
Reduction in the non‐specific IFN‐γ production by CCR4+ cell‐depletion and enhancement in p19 production by CD8+ cell‐depletion were also observed in the samples from two other HAM/TSP patients (#288 and #290) (Fig. 3c,d).
The prevalence of HTLV‐1‐infected cells in the CCR4+ cell fraction was further confirmed by a real‐time PCR method in uncultured PBMCs from subjects #255 (HAM/TSP) and #211 (AC), although there were still detectable levels of proviruses left in the CCR4+ cell‐depleted fractions (Fig. 3e).
Poor effects of CCR4+ cell depletion on Tax‐specific T‐cell responses in PBMC cultures from some ACs and sATL patients. We then assessed whether ACs and sATL samples with apparently less‐specific responses actually possessed Tax‐specific responses, similarly to the samples from HAM/TSP patients. We examined the effects of CCR4+ cell‐depletion on Tax‐specific T‐cell responses and the effects of CD8+ cell‐depletion on HTLV‐1 p19 production in the PBMC samples from several available ACs and sATL patients with less‐specific responses and elevated proviral loads (Fig. 4).
Figure 4.
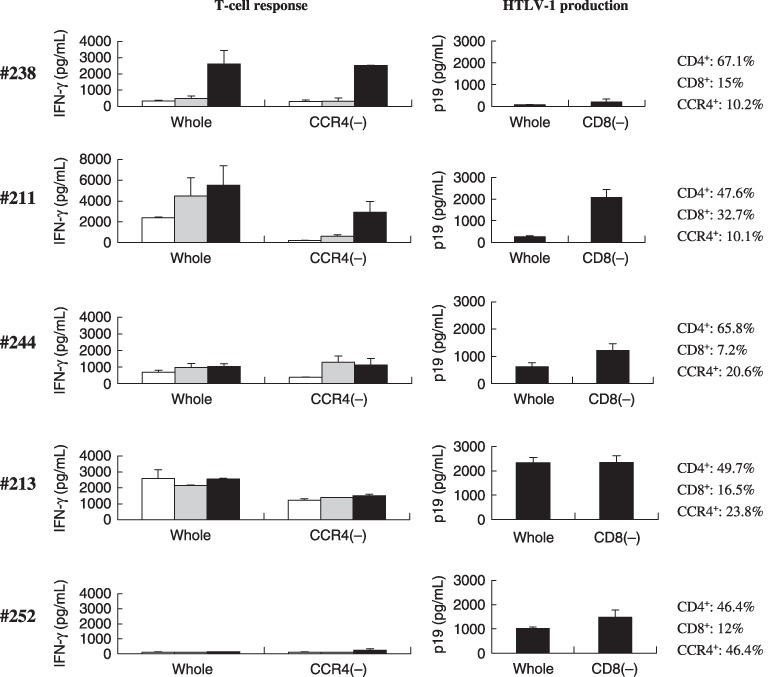
Tax‐specific T‐cell response associated with CD8+ cell‐mediated control of human T‐cell leukemia virus type‐1 (HTLV‐1) production in peripheral blood mononuclear cell (PBMC) cultures from asymptomatic HTLV‐1 carriers (ACs) and smoldering‐type adult T‐cell leukemia (sATL) patients. Whole and CCR4+ cell‐depleted [CCR4(–)] PBMC fractions prepared from subjects #238 (AC), #211 (AC), #244 (AC), #213 (sATL), and #252 (sATL) were cultured alone (open bars), with glutathione‐S‐transferase (GST) (gray bars), or with a mixture of GST–Tax proteins (black bars) for 4 days. Interferon (IFN)‐γ produced in the supernatants was measured by enzyme‐linked immunoabsorbent assay (left panels). The levels of HTLV‐1 p19 production in whole and CD8+ cell‐depleted [CD8(–)] PBMC cultures from the same donors were measured on day 7 (right panels). The ratios of CD4+, CD8+, and CCR4+ cells (%) in uncultured PBMCs from each subject were indicated at right, as determined by flow cytometry.
PBMCs from AC #238 were examined as a control that exhibited clear Tax‐specific T‐cell responses. As shown in Fig. 4, the Tax‐specific pattern in this sample was not altered by CCR4+ cell depletion. HTLV‐1 p19 production in the PBMC culture of #238 was very low, consistent with the low proviral load in this subject.
In the sample from AC #211, which had a moderately elevated level of proviral load (10–100 copies/1000 PBMCs), whole PBMCs showed a less‐specific response pattern with high background IFN‐γ production. This was improved to a Tax‐specific pattern by depletion of CCR4+ cells. HTLV‐1 p19 production in CD8+ cell‐depleted fraction from this subject was 10 times higher than that in whole PBMCs, indicating that CD8+ cells efficiently suppressed HTLV‐1 p19 production in culture.
The whole PBMCs from AC #244, possessing a proviral load similar to that of subject #211, also showed a less‐specific response pattern. CCR4+ cell depletion reduced spontaneous IFN‐γ production but did not improve the difference between the responses to control GST and GST–Tax by much. Whole PBMCs from subject #244 spontaneously produced a substantial amount of HTLV‐1 p19, which was slightly enhanced by depletion of CD8+ cells.
PBMCs from sATL patient #213, who had a high proviral load (>100 copies/1000 PBMCs), spontaneously produced significant levels of IFN‐γ irrespective of the stimulation. Depletion of CCR4+ cells slightly reduced the general levels of IFN‐γ production, but did not improve the non‐specific pattern. Unfractionated PBMCs from this subject produced high levels of HTLV‐1 p19, and CD8+ cell depletion did not alter the levels of HTLV‐1 p19 at all.
PBMCs from sATL patient #252, who also had a high proviral load, showed very low levels of IFN‐γ production in response to GST or GST–Tax proteins. CCR4+ cell depletion from these PBMCs did not improve the scale or specificity of the IFN‐γ production by these PBMCs. Whole PBMCs from subject #252 spontaneously produced a significant amount of HTLV‐1 p19, which was slightly increased by CD8+ cell depletion.
Thus, CCR4+ cell depletion revealed Tax‐specific T‐cell responses in some, but not all, samples with less‐specific responses. Samples that did not show a Tax‐specific pattern after CCR4+ cell depletion were associated with insufficient control of HTLV‐1 production in culture.
Unresponsiveness of Tax‐specific CTLs in PBMCs from an AC. Finally, we investigated whether the five subjects shown in Fig. 4 had Tax‐specific CTLs, using a flow cytometric analysis with tetramers. As shown in Fig. 5(a), the PBMCs from AC #238 contained HLA‐A*1101/Tax88‐96 tetramer‐binding CTLs, and ACs #211 and #244 were positive for HLA‐A*2402/Tax301‐309 tetramer‐binding CTLs. In the PBMCs from subjects #213 and #252 (sATL), tetramer‐binding cells were not detectable, although these subjects were positive for HLA‐A24 and A11, and HLA‐A11, respectively (data not shown).
Figure 5.
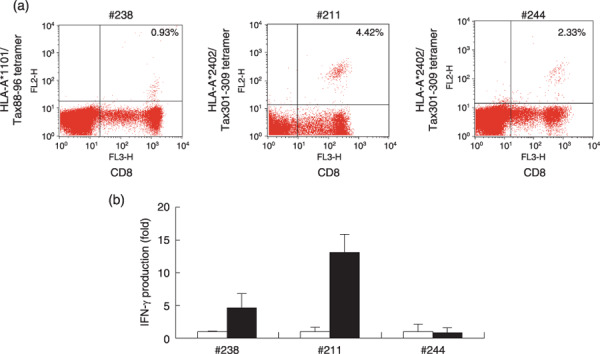
Discrepancy between the presence of Tax‐specific cytotoxic T‐lymphocytes (CTLs) and interferon (IFN)‐γ production in peripheral blood mononuclear cells (PBMCs) from asymptomatic human T‐cell leukemia virus type‐1 (HTLV‐1) carriers (ACs). (a) Uncultured PBMCs from indicated subjects were stained with phycoerythrin (PE)‐Cy5‐conjugated CD8 monoclonal antibody (mAb) together with PE‐conjugated HLA‐A*1101/Tax88‐96 tetramers for subject #238, and HLA‐A*2402/Tax301‐309 tetramers for subjects #211 and #244. The percentages of tetramer+ cells among CD8+ cells are indicated, as analyzed by flow cytometry. (b) CCR4+ cell‐depleted PBMCs from the same subjects were incubated with control dimethylsulfoxide (DMSO) (open bars) or 10 µM of Tax peptides (closed bars) for 4 days, and the amounts of IFN‐γ in the supernatants were measured by enzyme‐linked immunoabsorbent assay. Tax peptides used were Tax 88‐96 for subject #238 and Tax 301‐309 for subjects #211 and #244. The values represent folds of IFN‐γ production against control DMSO, and indicated as the mean ± SD of duplicate samples.
We examined the activity of Tax‐specific CTLs by stimulating CCR4+ cell‐depleted PBMCs from three ACs, which contained tetramer‐binding CTLs, with oligopeptides corresponding to the tetramers in culture. As shown in Fig. 5(b), CCR4+ cell‐depleted PBMCs from subjects #238 and #211 produced IFN‐γ in response to Tax88‐96 and Tax301‐309 peptides, respectively, whereas those from subject #244 did not respond to Tax 301‐309 peptides. These results are consistent with the results of the assay using GST–Tax proteins and suggest that Tax‐specific CTLs in subject #244 might be in an anergic state.
Discussion
In the present study, we investigated HTLV‐1‐specific T‐cell responses in unselected HTLV‐1‐infected individuals without any bias of HLAs by using a GST–Tax protein‐based assay system. This assay detected clear Tax‐specific responses in ATL patients in complete remission, less‐specific responses in HAM/TSP patients, and very weak responses in cATL patients. Interestingly, all of these patterns were observed in PBMCs from ACs and sATL patients, indicating wide diversity in Tax‐specific T‐cell responsiveness at these stages. Most importantly, some individuals at these stages exhibited impaired Tax‐specific T‐cell responses and elevated proviral loads, indicating that such conditions are present before an overt leukemia stage.
High background responses have been an obstacle to evaluate T‐cell responses in HTLV‐1‐infected individuals. This may be similar to the phenomenon known as ‘spontaneous proliferation’ of PBMCs from HAM/TSP patients and ACs.( 42 , 43 , 44 ) In our study, depletion of CCR4+ cells containing HTLV‐1‐infected cells from the PBMCs reduced this spontaneous response and revealed a Tax‐specific T‐cell response pattern in the samples from several HAM/TSP patients and ACs with strong non‐specific responses (3, 4). The sources of spontaneous IFN‐γ production may be either HTLV‐1‐specific T‐cells reacting with coexisting infected cells in culture, or HTLV‐1‐infected cells themselves, or both.( 45 , 46 ) As CCR4+ cell depletion from the PBMCs reduced the number of HTLV‐1‐infected cells without losing CD8+ cells, T‐cell response against exogenously added Tax proteins became clearly detectable. CD8+ cells in these PBMCs effectively suppressed HTLV‐1 p19 production in culture, presumably by killing infected cells.
Although the number of samples available for detailed analysis was limited, PBMCs from at least two sATL patients (#213 and #252) with high proviral loads (>100 copies/1000 cells) exhibited impaired T‐cell responses even after CCR4+ cell depletion. CD8+ cells of these subjects had poor effects on HTLV‐1 p19 production (Fig. 4). This was not attributable to the scarcity of non‐ATL cells, as these PBMCs contained 16.5% and 12.0% CD8+ cells, respectively. HTLV‐1‐infected cells were the most likely source of IFN‐γ production in whole and CCR4− PBMC cultures from subject #213, as HTLV‐1 p19 was detected even in the CCR4+ cell‐depleted fraction during further culture (data not shown), presumably because of expansion of the remaining infected cells in the initially CCR4− cell culture, in the absence of any anti‐Tax response. HTLV‐1‐infected cells from subject #252 expressed viral antigens but not IFN‐γ.
ACs with less‐specific T‐cell responses and moderate levels of proviral loads (10–100 copies/1000 PBMCs) seemed to be a mixed population in regards to Tax‐specific response. CCR4+ cell‐depleted PBMCs showed Tax‐specific T‐cell response in subject #211, but did not markedly do so in subject #244. Similarly, CD8+ cell‐mediated control on HTLV‐1 p19 production in #211 was more efficient than in #244. The results of peptide stimulation also indicated impaired status of Tax‐specific response in #244 (4, 5). However, the level of p19 production in the CD8+ cell‐depleted cell fraction was even higher in #211. Nevertheless, #211 and #244 had comparable levels of proviral loads (33 and 63 copies/1000 PBMCs, respectively). These results suggested that proviral load might represent equilibrium between growth capabilities of infected cells and the T‐cell response against them.
It is of note that some ACs (#219, #223, #263) exhibited very low anti‐Tax T‐cell responses that were not improved by CCR4+ cell depletion, and also had low proviral load. HTLV‐1‐infected cells in these individuals might have low proliferative abilities. Since these subjects maintained considerable levels of anti‐HTLV‐1 antibodies in the plasma (1:1024–2048, Table 1), poor T‐cell responsiveness cannot be explained merely by the scarcity of viral antigens. The mechanisms of general and/or HTLV‐1‐specific immune suppression in HTLV‐1 infection remain to be clarified.
In conclusion, ACs and sATL patients consist of diverse subpopulations with different patterns of T‐cell responses against HTLV‐1, containing a subpopulation exhibiting impaired HTLV‐1‐specific T‐cell responses and insufficient control of HTLV‐1‐infected cells. This strongly suggests that impairment of HTLV‐1‐specific T‐cell response is not a consequence of advanced ATL but present at early stages or even before the disease onset. Although there must be multiple steps towards ATL development, impaired HTLV‐1‐specific T‐cell response could be one of the underlying conditions that allows expansion of HTLV‐1 infected cells in vivo. Reactivation of HTLV‐1‐specific T‐cell response by immunotherapeutic strategies such as vaccines in the subpopulation with insufficient T‐cell response might contribute to the recovery of host control on HTLV‐1‐infected cells in vivo.
Acknowledgments
We thank Ms. Minako Nakashima and Ms. Yasuko Tsuji (Imamura Bun‐in Hospital, Kagoshima, Japan) for coordination of clinical samples. This study was supported by Grant 17013028 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a grant for an anticancer project from the Ministry of Health, Welfare, and Labor of Japan; and the Public Trust Haraguchi Memorial Cancer Research Fund. The authors have no financial conflict of interest.
References
- 1. Hinuma Y, Nagata K, Hanaoka M et al . Adult T‐cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA 1981; 78: 6476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T‐cell lymphoma. Proc Natl Acad Sci USA 1980; 77: 7415–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia. clinical and hematologic features of 16 cases. Blood 1977; 50: 481–92. [PubMed] [Google Scholar]
- 4. Tajima K. The 4th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. The T‐ and B‐cell Malignancy Study Group. Int J Cancer 1990; 45: 237–43. [DOI] [PubMed] [Google Scholar]
- 5. Arisawa K, Soda M, Endo S et al . Evaluation of adult T‐cell leukemia/lymphoma incidence and its impact on non‐Hodgkin lymphoma incidence in southwestern Japan. Int J Cancer 2000; 85: 319–24. [DOI] [PubMed] [Google Scholar]
- 6. Gessain A, Barin F, Vernant JC et al . Antibodies to human T‐lymphotropic virus type‐I in patients with tropical spastic paraparesis. Lancet 1985; 2: 407–10. [DOI] [PubMed] [Google Scholar]
- 7. Osame M, Usuku K, Izumo S et al . HTLV‐1 associated myelopathy, a new clinical entity. Lancet 1986; 1: 1031–2. [DOI] [PubMed] [Google Scholar]
- 8. Hisada M, Okayama A, Spiegelman D, Mueller NE, Stuver SO. Sex‐specific mortality from adult T‐cell leukemia among carriers of human T‐lymphotropic virus type I. Int J Cancer 2001; 91: 497–9. [DOI] [PubMed] [Google Scholar]
- 9. Hisada M, Okayama A, Tachibana N et al . Predictors of level of circulating abnormal lymphocytes among human T‐lymphotropic virus type I carriers in Japan. Int J Cancer 1998; 77: 188–92. [DOI] [PubMed] [Google Scholar]
- 10. Okayama A, Stuver S, Matsuoka M et al . Role of HTLV‐1 proviral DNA load and clonality in the development of adult T‐cell leukemia/lymphoma in asymptomatic carriers. Int J Cancer 2004; 110: 621–5. [DOI] [PubMed] [Google Scholar]
- 11. Nagai M, Usuku K, Matsumoto W et al . Analysis of HTLV‐1 proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV‐1 carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol 1998; 4: 586–93. [DOI] [PubMed] [Google Scholar]
- 12. Ishida T, Utsunomiya A, Iida S et al . Clinical significance of CCR4 expression in adult T‐cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 2003; 9: 3625–34. [PubMed] [Google Scholar]
- 13. Yoshie O, Fujisawa R, Nakayama T et al . Frequent expression of CCR4 in adult T‐cell leukemia and human T‐cell leukemia virus type 1‐transformed T cells. Blood 2002; 99: 1505–11. [DOI] [PubMed] [Google Scholar]
- 14. Karube K, Ohshima K, Tsuchiya T et al . Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T‐cell leukaemia/lymphoma cells. Br J Haematol 2004; 126: 81–4. [DOI] [PubMed] [Google Scholar]
- 15. Matsubara Y, Hori T, Morita R, Sakaguchi S, Uchiyama T. Phenotypic and functional relationship between adult T‐cell leukemia cells and regulatory T cells. Leukemia 2005; 19: 482–3. [DOI] [PubMed] [Google Scholar]
- 16. Kohno T, Yamada Y, Akamatsu N et al . Possible origin of adult T‐cell leukemia/lymphoma cells from human T lymphotropic virus type‐1‐infected regulatory T cells. Cancer Sci 2005; 96: 527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakaguchi S, Sakaguchi N, Shimizu J et al . Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 2001; 182: 18–32. [DOI] [PubMed] [Google Scholar]
- 18. Sebastiani S, Allavena P, Albanesi C et al . Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol 2001; 166: 996–1002. [DOI] [PubMed] [Google Scholar]
- 19. Iellem A, Mariani M, Lang R et al . Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4 (+) CD25 (+) regulatory T cells. J Exp Med 2001; 194: 847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2002; 2: 389–400. [DOI] [PubMed] [Google Scholar]
- 21. Chen S, Ishii N, Ine S et al . Regulatory T cell‐like activity of Foxp3+ adult T cell leukemia cells. Int Immunol 2006; 18: 269–77. [DOI] [PubMed] [Google Scholar]
- 22. Katsuki T, Katsuki K, Imai J, Hinuma Y. Immune suppression in healthy carriers of adult T‐cell leukemia retrovirus (HTLV‐1). impairment of T‐cell control of Epstein‐Barr virus‐infected B‐cells. Jpn J Cancer Res 1987; 78: 639–42. [PubMed] [Google Scholar]
- 23. Nakada K, Yamaguchi K, Furugen S et al . Monoclonal integration of HTLV‐1 proviral DNA in patients with strongyloidiasis. Int J Cancer 1987; 40: 145–8. [DOI] [PubMed] [Google Scholar]
- 24. Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV‐1 pX in patients with HTLV‐1 associated neurological disease. Nature 1990; 348: 245–8. [DOI] [PubMed] [Google Scholar]
- 25. Kannagi M, Sugamura K, Kinoshita K, Uchino H, Hinuma Y. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J Immunol 1984; 133: 1037–41. [PubMed] [Google Scholar]
- 26. Arnulf B, Thorel M, Poirot Y et al . Loss of the ex vivo but not the reinducible CD8+ T‐cell response to Tax in human T‐cell leukemia virus type 1‐infected patients with adult T‐cell leukemia/lymphoma. Leukemia 2004; 18: 126–32. [DOI] [PubMed] [Google Scholar]
- 27. Bangham CR, Osame M. Cellular immune response to HTLV‐1. Oncogene 2005; 24: 6035–46. [DOI] [PubMed] [Google Scholar]
- 28. Asquith B, Mosley AJ, Barfield A et al . A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T‐lymphotropic virus type 1 proviral load. J General Virol 2005; 86: 1515–23. [DOI] [PubMed] [Google Scholar]
- 29. Harashima N, Kurihara K, Utsunomiya A et al . Graft‐versus‐Tax response in adult T‐cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res 2004; 64: 391–9. [DOI] [PubMed] [Google Scholar]
- 30. Hasegawa A, Ohashi T, Hanabuchi S et al . Expansion of human T‐cell leukemia virus type 1 (HTLV‐1) reservoir in orally infected rats: inverse correlation with HTLV‐1‐specific cellular immune response. J Virol 2003; 77: 2956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Komori K, Hasegawa A, Kurihara K et al . Reduction of human T‐cell leukemia virus type 1 (HTLV‐1) proviral loads in rats orally infected with HTLV‐1 by reimmunization with HTLV‐1‐infected cells. J Virol 2006; 80: 7375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohashi T, Hanabuchi S, Kato H et al . Prevention of adult T‐cell leukemia‐like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T‐cell leukemia virus type 1 Tax‐coding DNA vaccine. J Virol 2000; 74: 9610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanabuchi S, Ohashi T, Koya Y et al . Regression of human T‐cell leukemia virus type I (HTLV‐1)‐associated lymphomas in a rat model: peptide‐induced T‐cell immunity. J Natl Cancer Inst 2001; 93: 1775–83. [DOI] [PubMed] [Google Scholar]
- 34. Kannagi M, Sugamura K, Sato H, Okochi K, Uchino H, Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus‐bearing cells. J Immunol 1983; 130: 2942–6. [PubMed] [Google Scholar]
- 35. Parker CE, Daenke S, Nightingale S, Bangham CR. Activated, HTLV‐1‐specific cytotoxic T‐lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology 1992; 188: 628–36. [DOI] [PubMed] [Google Scholar]
- 36. Kurihara K, Shimizu Y, Takamori A et al . Human T‐cell leukemia virus type‐I (HTLV‐1)‐specific T‐cell responses detected using three‐divided glutathione‐S‐transferase (GST)‐Tax fusion proteins. J Immunol Meth 2006; 313: 61–73. [DOI] [PubMed] [Google Scholar]
- 37. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol 1991; 79: 428–37. [DOI] [PubMed] [Google Scholar]
- 38. Yamaguchi K, Kiyokawa T, Nakada K et al . Polyclonal integration of HTLV‐1 proviral DNA in lymphocytes from HTLV‐1 seropositive individuals: an intermediate state between the healthy carrier state and smouldering ATL. Br J Haematol 1988; 68: 169–74. [DOI] [PubMed] [Google Scholar]
- 39. Harashima N, Tanosaki R, Shimizu Y et al . Identification of two new HLA‐A*1101‐restricted tax epitopes recognized by cytotoxic T lymphocytes in an adult T‐cell leukemia patient after hematopoietic stem cell transplantation. J Virol 2005; 79: 10 088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sonoda J, Koriyama C, Yamamoto S et al . HTLV‐1 provirus load in peripheral blood lymphocytes of HTLV‐1 carriers is diminished by green tea drinking. Cancer Sci 2004; 95: 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanaka G, Okayama A, Watanabe T et al . The clonal expansion of human T lymphotropic virus type 1‐infected T cells: a comparison between seroconverters and long‐term carriers. J Infect Dis 2005; 191: 1140–7. [DOI] [PubMed] [Google Scholar]
- 42. Itoyama Y, Minato S, Kira J et al . Spontaneous proliferation of peripheral blood lymphocytes increased in patients with HTLV‐1‐associated myelopathy. Neurology 1988; 38: 1302–7. [DOI] [PubMed] [Google Scholar]
- 43. Jacobson S, Zaninovic V, Mora C et al . Immunological findings in neurological diseases associated with antibodies to HTLV‐1: activated lymphocytes in tropical spastic paraparesis. Ann Neurol 1988; 23 (Suppl): S196–200. [DOI] [PubMed] [Google Scholar]
- 44. Kramer A, Jacobson S, Reuben JF et al . Spontaneous lymphocyte proliferation in symptom‐free HTLV‐1 positive Jamaicans. Lancet 1989; 2: 923–4. [DOI] [PubMed] [Google Scholar]
- 45. Sakai JA, Nagai M, Brennan MB, Mora CA, Jacobson S. In vitro spontaneous lymphoproliferation in patients with human T‐cell lymphotropic virus type I‐associated neurologic disease. predominant expansion of CD8+ T cells. Blood 2001; 98: 1506–11. [DOI] [PubMed] [Google Scholar]
- 46. Iwatsuki K, Harada H, Motoki Y, Kaneko F, Jin F, Takigawa M. Diversity of immunobiological functions of T‐cell lines established from patients with adult T‐cell leukaemia. Br J Dermatol 1995; 133: 861–7. [DOI] [PubMed] [Google Scholar]


