Abstract
Endometrial stromal sarcoma (ESS) of the uterus is a rare uterine malignancy that has not been characterized in detail. To characterize the phenotype of ESS of the uterus, we extracted RNA from ESS and the stroma of normal endometrium using a tissue microdissection system and compared the expression profiles in the two tissues. After suppression subtractive hybridization and differential screening, we detected the metastasis‐associated lung adenocarcinoma transcript 1 (MALAT‐1) gene as one of the major genes upregulated in ESS, and a full‐length placental cDNA clone (CS0DI066YJ10) as one of the major genes downregulated. The results were confirmed by in situ hybridization in four resected specimens of ESS and 36 biopsy specimens of normal endometrial tissue. All ESS (4/4) and all cases of endometrial stromal cells in the proliferative phase (13/13) were positive for MALAT‐1, but samples of normal stroma in the secretory phase and menopausal state included some that were negative or weakly positive for MALAT‐1 (5/13 and 3/10, respectively). In contrast, all ESS and 12 of 13 cases of stromal cells in the proliferative phase were negative for the full‐length placental cDNA clone but 10 of 13 cases of endometrial stromal cells in the secretory phase were positive for transcripts of the gene (P < 0.05). These results indicated that endometrial stromal cells have different phenotypic characteristics between proliferative and secretory phases and the tumor cells of ESS have the phenotypic character of endometrial stromal cells in the proliferative phase. (Cancer Sci 2006; 97: 106 – 112)
Endometrial stromal sarcoma (ESS) is a rare uterine malignancy.( 1 , 2 , 3 ) It accounts for approximately 13% of uterine sarcomas and less than 1% of all uterine malignancies. Histologically, ESS is composed of homogeneous spindle‐shaped cells that are similar to normal endometrial stromal cells. ESS has traditionally been classified into two grades: low‐grade and high‐grade malignancy, depending on the mitotic counts. The grade of malignancy and stage are related to the prognosis of ESS patients.( 4 ) The overall survival rate of ESS is 80% in ESS with low‐grade malignancy and 60% in ESS with high‐grade malignancy. Immunohistochemically, the tumor cells are characterized by a positive reaction for CD10, the estrogen receptor and the progesterone receptor.( 5 , 6 , 7 , 8 , 9 )
Because ESS is not a major histological subtype of malignant uterine tumors, few molecular analyses of these tumors have been carried out. In cytogenetic studies, translocations of chromosomes 6, 7, 17 and X have been reported.( 10 , 11 , 12 , 13 ) In particular, Koontz et al. reported that fusion of the JAZF1/JJAZ1 gene caused by t(7;17)(p15;q21) is frequently detected in ESS.( 10 ) Hrzenjak et al. examined the gene expression profile of ESS and reported that expression of frizzled‐related protein 4 and β‐catenin was decreased in high‐grade ESS.( 14 )
Our aim was to elucidate the unique characteristics of ESS by comparative expression analysis. We analyzed and compared the expression profiles of ESS and normal endometrial stroma by a suppression subtractive hybridization (SSH) method, and we found that ESS tumor cells show similar expression patterns to normal stromal cells in the proliferative phase.
Materials and Methods
Tissue samples
One ESS tissue sample and a normal endometrial sample were obtained from surgical specimens. The ESS patient was a 58‐year‐old female. Two nodular lesions had been detected bilaterally in the lobes of the lung by chest X‐ray, and she was admitted to the University Hospital of Tsukuba (Ibaraki, Japan). Nine years before this admission, she had undergone hysterectomy for ESS. Wedge resection of the right middle lobe of the lung and partial resection of the left lung were carried out, and the resected tumors were diagnosed histologically as metastatic ESS. Fresh ESS tissue was obtained from the tumor in the right lung. Fresh normal endometrium was collected from the hysterectomy specimen of a 40‐year‐old female admitted to the Tsukuba Medical Center Hospital (Ibaraki, Japan) for leiomyoma of the uterus. Multiple leiomyomas were detected and hysterectomy was carried out. The two fresh specimens were embedded in Tissue‐Tek OCT Compound (Sakura Finetek Japan, Tokyo, Japan), frozen in dry ice/acetone, and stored at −80°C until analysis. Approval from the institutional review board and written informed consent from the patients were obtained.
RNA extraction
Total RNA was extracted from the ESS tissue with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's instructions. A microdissection system was not used for the ESS specimen because the tumor tissue was histologically homogeneous (Fig. 1a). Normal endometrial stromal cells were obtained selectively by laser capture microdissection with an LM200 system (Arcturus, Mountain View, CA, USA) because the normal endometrial tissue was mixed with stromal and epithelial cells (Fig. 1b). Several 9 µM‐thick sections were made from the frozen endometrial tissue, which were mounted on uncoated glass slides. They were fixed with an ethanol/acetic acid (19 : 1) mixture for 10 min and washed with diethylpyrocarbonate‐treated water. The sections were then dehydrated and desiccated with ethanol, xylene and a vacuum desiccator. Normal endometrial stromal tissue was captured onto the exclusive capsure (a plastic tool used to collect cells; cells stick to the flat adhesion side) using a 7.5 µm‐diameter laser shot and 50 mV laser power (Fig. 2). Total RNA was extracted from the microdissected endometrial stromal tissue using the same method as used for the ESS tissue.
Figure 1.
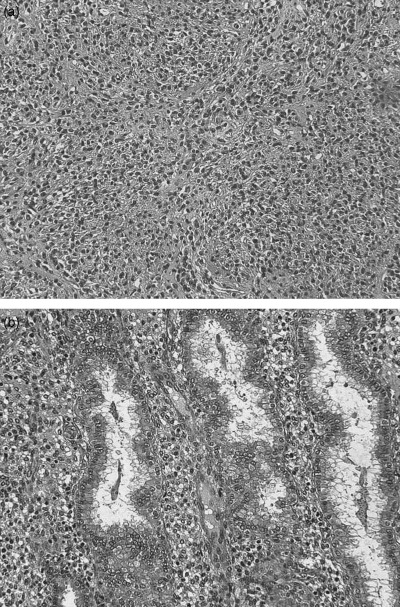
Histology of (a) endometrial stromal sarcoma and (b) normal endometrium in secretory phase (HE, ×200). Frozen specimens of two cases were examined by suppression subtractive hybridization.
Figure 2.
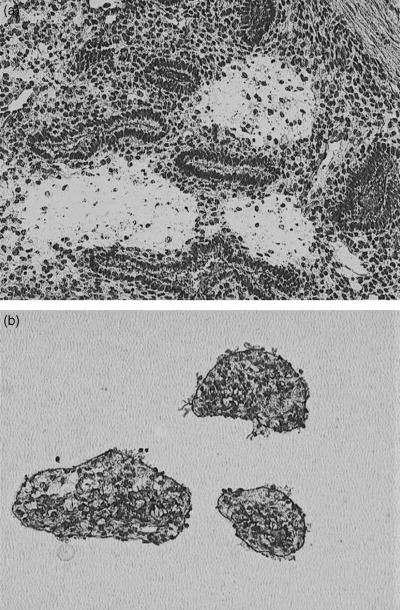
Histology of normal endometrium. (a) Frozen section after laser capture microdissection, and (b) captured cells on a capsure (×100).
The mRNAs extracted from normal endometrial stroma and ESS tissues were amplified by the TALPAT (T7 RNA polymerase‐mediated transcription, adaptor ligation, and polymerase chain reaction [PCR] amplification followed by T7 transcription) method.( 15 , 16 ) The TALPAT method is based on a T7 RNA polymerase‐mediated RNA amplification reaction combined with an adaptor ligation‐mediated PCR, and enables very small amounts of mRNA to be amplified with faithful maintenance of relative levels of mRNA expression.
Suppression subtractive hybridization
We carried out SSH between the TALPAT samples of normal endometrial stroma and ESS, using a PCR‐Select cDNA Subtraction Kit (BD Biosciences Clontech, Palo Alto, CA, USA).( 17 ) Two‐directional subtractive hybridization products were obtained.
Both the forward subtraction product (ESS minus normal stroma) and reverse subtraction product (normal stroma minus ESS) were cloned into the PCR 2.1 vector (Invitrogen). In each case, 500 bacterial colonies were picked up and their inserted cDNAs were amplified by PCR. Subsequent differential screening was carried out using a PCR‐Select Differential Screening Kit (BD Biosciences Clontech).
The differentially screened clones were reblotted onto nylon membranes and rescreened with probes that were the 32P‐labeled TALPAT products of the normal stroma and the ESS tumor (virtual reverse Northern hybridization). The hybridized membranes were exposed to X‐ray films and evaluated relatively against the intensity of glyceraldehyde‐3‐phosphate dehydrogenase with an imaging densitometer (Bio‐Rad Laboratories, Hercules, CA, USA). The PCR products of the inserted fragments were sequenced with a BigDye terminator v3.1 cycle sequencing ready reaction kit and an ABI PRISM 310 genetic analyzer (both from Applied Biosystems Japan, Tokyo, Japan).
In situ hybridization
For the in situ hybridization and immunohistochemistry, we used formalin‐fixed and paraffin‐embedded materials. They included four ESS and 13, 13 and 10 endometrial tissues in proliferative phase, secretory phase and menopausal state, respectively. The clinicopathological summary of the ESS cases examined is shown in Table 1. Furthermore, we used 10 placental tissues and 10 normal adult tissues, including liver, kidney, pancreas, lung, thyroid gland, adrenal gland, stomach, colon, esophagus and trachea, to examine the expression of the selected gene. Overexpressed and underexpressed cDNAs that had been subcloned into plasmids were amplified by PCR with T7 RNA polymerase promoter‐attached primers. The PCR products were then transcribed to antisense or sense cRNA probes with T7 RNA polymerase. The cRNA probes were hybridized with formalin‐fixed and paraffin‐embedded material in accordance with previous reports.( 16 , 18 ) The sense‐cRNA probe was also hybridized as a negative control. Immunodetection of the in situ hybridization signal was carried out with a 400× alkaline phosphatase‐labeled antidigoxigenin antibody (DakoCytomation, Kyoto, Japan).
Table 1.
Endometrial stromal sarcoma cases examined in this study
| Case | Age (years) | Site | size (cm) | Pathological stage † | Histological grade | Mitotic index |
|---|---|---|---|---|---|---|
| 1 | 48 | Primary | 6 | I | Low | <1 |
| 2 | 62 | Primary | 14 | Ib | High | 35 |
| 3 | 53 | Metastatic ‡ | 10 | IV | High | 30 |
| 4 | 58 | Metastatic | 2 | (I) § | Low | 1 |
Pathological staging was evaluated using the staging classification of the FIGO.( 21 )
Metastatic nodule in the retroperitoneum was used for in situ hybridization (case 3).
§ The fresh material of case 4 (metastatic lung tumor) was used for RNA subtraction analysis. When the primary tumor was treated, it was estimated as pathological stage I.
Immunohistochemistry
Immunohistochemical staining was carried out using 4 µm‐thick sections from formalin‐fixed, paraffin‐embedded tissues mounted on silane‐coated glass slides. The tissues were deparaffinized in xylene and dehydrated through a graded alcohol series. The slides were heated in 10 mM citrate buffer by autoclaving for 10 min at 120°C. Then, monoclonal goat antibody (1:100) against granulysin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied overnight in a humid chamber at 4°C. The signal was detected with a LSAB+ kit (DakoCytomation) in accordance with the manufacturer's instructions.
Statistical analysis
The statistical difference was evaluated using Fischer's exact test. Differences were considered statistically significant if the P‐value was <0.05.
Results
The resected specimen of the lung was diagnosed histologically as low‐grade ESS (Fig. 1a). Tumor cells had proliferated in the tumor and had nuclei with moderate atypia. The cells had invaded the surrounding vessels. The mitotic index was 1 per 10 high‐powered fields. The endometrial tissue of the hysterectomy specimen was diagnosed as normal endometrium in the late secretory phase (Fig. 1b).
Total RNA (100 ng) extracted from ESS was amplified to 7.4 mg cRNA using the TALPAT method. We extracted 90 ng of total RNA from approximately 1900 normal endometrial stromal cells collected by laser capture microdissection (Fig. 2). These samples were amplified using the TALPAT method, and 11 mg cRNA was obtained.
Following SSH analysis, 475 clones from the forward‐subtracted library and 570 clones from the reverse‐subtracted library were chosen randomly. After differential screening for selected clones, 78 forward‐subtracted clones and 255 reverse‐subtracted clones were picked up. Subsequently, virtual reverse Northern hybridization was carried out. Twelve forward‐subtracted clones and 19 reverse‐subtracted clones were finally chosen. The results of sequence analysis of the total 31 clones are shown Table 2. In the 12 cDNA clones overexpressed in ESS, seven were contaminated DNA clones. The remaining five clones were composed of metastasis‐associated lung adenocarcinoma transcript 1 (MALAT‐1), heat shock 70 kDa protein and PNAS‐108. In particular, the clone of the MALAT‐1 was selected three times.( 19 ) Of the 19 cDNA clones underexpressed in ESS, two were contaminated DNA clones. Of the remaining 17 clones that were underexpressed in ESS, eight of the clones selected consisted of granulysin and six were full‐length placental cDNA CS0DI066YJ10 clones.( 20 ) The remaining clones were in the interleukin 1β, aggrecanase‐2 and human chondroitin sulfate proteoglycan genes. We decided to examine the expressions of the major clones that were MALAT‐1, granulysin and CS0DI066YJ10.
Table 2.
cDNA clones overexpressed and underexpressed in endometrial stromal sarcoma
| Description | No. clones selected | Expression ratio of ESS/stromal cells |
|---|---|---|
| 12 overexpressed cDNA clones | ||
| MALAT‐1 | 3 | 2.2–2.4 |
| Heat shock 70 kDa protein | 1 | 2.58 |
| PNAS‐108 | 1 | 2.69 |
| DNA fragments | 7 | (–) |
| 19 underexpressed cDNA clones | ||
| Granulysin | 8 | 2.6–3.7 |
| Placental CS0DI066YJ10 | 6 | 2.8–3.5 |
| Interleukin 1‐β | 1 | 2.5 |
| Aggrecancase‐2 | 1 | 2.4 |
| Human chondroitin sulfate proteoglycan | 1 | 2.6 |
| DNA fragments | 2 | (–) |
MALAT‐1, metastasis associated lung adenocarcinoma transcript 1.
Figure 3 shows the results of in situ hybridization against MALAT‐1 gene expression in representative cases. Interestingly, the positive signals were detected in the nuclei of each specimen. The results indicated that mRNA of MALAT‐1 is non‐coding RNA that is located in the nucleus.( 19 ) All ESS samples (4/4) and all samples of normal stroma in the proliferative phase (13/13) were positive for MALAT‐1, but samples of normal stroma in the secretory phase and menopausal state included some that were negative or weakly positive for MALAT‐1 (5/13 and 3/10 respectively) (Table 3).
Figure 3.
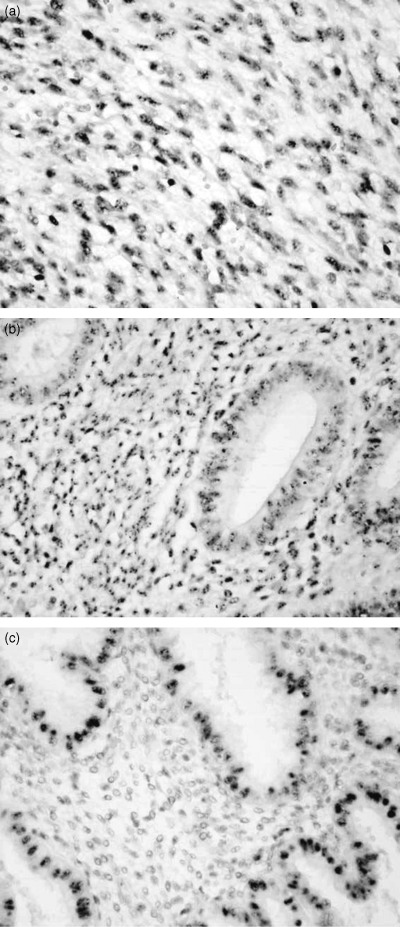
In situ hybridization of metastasis associated lung adenocarcinoma transcript 1 (MALAT‐1) mRNA. (a) The nuclei of the endometrial sarcoma, (b) both epithelial and stromal cells in proliferative phase and (c) epithelial cells in secretory phase were positive for MALAT‐1. However, stromal cells in secretory phase were negative for the gene (×200).
Table 3.
In situ hybridization analysis of the expression of the metastasis associated lung adenocarcinoma transcript 1 (MALAT‐1) gene
| Description | ++ | + | – | Total |
|---|---|---|---|---|
| Endometrial stromal sarcoma | 4 | 0 | 0 | 4 |
| Endometrial stroma (proliferative phase) | 13 | 0 | 0 | 13 |
| Endometrial stroma (secretory phase) | 8 | 4 | 1 | 13 |
| Endometrial stroma (menopausal) | 7 | 0 | 3 | 10 |
++, positive cells >10%; +, positive cells <10%; –, negative.
The immunohistochemical analysis of granulysin expression showed that lymphocytes in the normal endometrium were positive and all stromal cells were negative for the antibody (Fig. 4). These results indicated that the stromal cells did not express granulysin but lymphocytes in the endometrial stroma did express it.
Figure 4.
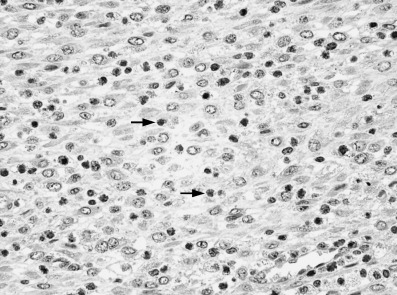
Immunohistochemistry of granulysin protein. Infiltrating lymphocytes (arrows) were positive for the antigranulysin antibody, but stromal cells were negative (×400).
All ESS samples (4/4) were negative for CS0DI066YJ10 (Table 4). The samples of normal stroma in the proliferative phase and menopausal‐state cells were also negative (12/13 and 10/10, respectively), but many samples of normal stroma in the secretory phase were positive (10/13). The positivity of normal stroma in the secretory phase was significantly higher than that of ESS and menopausal‐state cells (P < 0.05). We also examined 10 samples of placental tissue to ascertain which cells in the placenta expressed CS0DI066YJ10 mRNA. Spindle and polygonal decidual cells (maternal components of the placenta) were selectively positive for CS0DI066YJ10, and the other tissue components were negative (Fig. 5). To examine the expression of CS0DI066YJ10 in various normal adult tissues, we used a set of normal adult tissues, including liver, kidney, pancreas, lung, thyroid gland, adrenal gland, stomach, colon, esophagus and trachea. All of the tissues examined were negative for the expression of CS0DI066YJ10.
Table 4.
In situ hybridization analysis of the expression of cDNA of the clone CS0DI066YJ10
| Description | ++ | + | – | Total |
|---|---|---|---|---|
| Endometrial stromal sarcoma | 0 | 0 | 4 | 4 |
| Endometrial stroma (proliferative phase) | 0 | 1 | 12 | 13 |
| Endometrial stroma (secretory phase) | 7 | 3 | 3 | 13 † |
| Endometrial stroma (menopausal) | 0 | 0 | 10 | 10 |
| Decidua, placenta | 8 | 0 | 2 | 10 |
++, positive cells >10%; +, positive cells <10%; –, negative. †The positivity of endometrial stroma (secretory phase) (10/13) is significantly higher than that of endometrial stromal sarcoma (0/4) or endometrial stroma (proliferative phase) (1/13) (P < 0.05).
Figure 5.
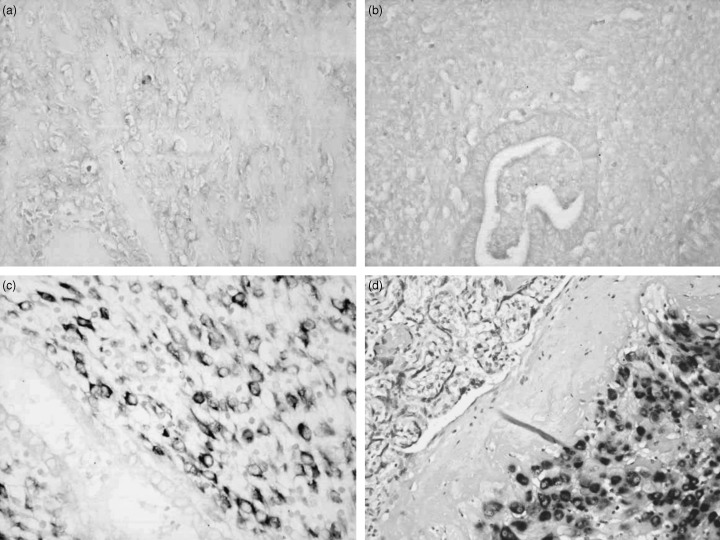
In situ hybridization of CS0DI066YJ10 mRNA. (a) Endometrial stromal sarcoma, (b) endometrial tissue in proliferative phase and (c) epithelial cells in secretory phase were negative for CS0DI066YJ10. The stromal cells in secretory phase (c) and decidual cells in the placenta (d) were selectively positive for the gene (a–c, ×200; d, ×100).
Discussion
We examined the metastatic lung tissue of a patient with ESS, as well as the normal endometrium of another patient as a normal control. As we could not use primary ESS tissue and the normal endometrium of the same patient, there were several limitations to our comparison of the expression profiles of the two tissues. However, as 1, 2 reveal, the tumor tissue showed typical low‐grade ESS histology and we were able to collect 100% stromal tissue from normal endometrium by the tissue microdissection method. Therefore, we considered that we could meet the aim of our study.
We found that the MALAT‐1 gene was overexpressed in ESS. MALAT‐1 is a non‐coding RNA reported by Ji et al. in 2003.( 19 ) It is expressed at high levels in non‐small‐cell carcinoma (especially in adenocarcinoma) that metastasizes in the early stages, and patients with tumors that express MALAT‐1 have a poor prognoses. In normal organs, MALAT‐1 is expressed in such tissues as the pancreas, lungs and prostate and is barely expressed in the uterus. Its expression in other malignant tumors has not yet been examined. We observed that all four ESS samples (including both low‐grade and high‐grade) expressed MALAT‐1. Over‐expression of MALAT‐1 is thought to be one of the characteristics of ESS and may be correlated with relapse or metastasis.
We found that full‐length cDNA of the placental clone CS0DI066YJ10 was underexpressed in ESS.( 20 ) The function of this clone has not yet been reported. As the cDNA clone was included in one the cDNA libraries of human placental tissue, we examined the expression of the clone in the normal placenta and a set of various kinds of normal adult tissues by in situ hybridization. Only the decidual cells of the maternal placental tissue were selectively positive for this clone and all of the adult tissues were negative. Therefore, we considered that the gene was selectively expressed in endometrial stromal cells in the secretory phase. Expression of CS0DI066YJ10 was limited to stromal cells in the secretory phase, and all ESS were negative for this gene. CS0DI066YJ10 expression may be under the influence of the hormonal environment and its expression may be useful to maintain good communication between uterus and placenta.
Hrzenjak et al. found that secreted frizzled‐related protein 4 and β‐catenin were underexpressed in high‐grade ESS, but we could not confirm their results.( 14 ) They also refer to several other genes that were highly expressed, but none matched those in our study. One of the reasons for this discrepancy may be differences in the samples examined. We used a low‐grade ESS tissue, and the endometrial stromal cells were extracted from another patient; moreover, this patient's endometrium was in the secretory phase.
Our study showed a high level of expression of MALAT‐1 and no expression of full‐length cDNA of the placental clone CS0DI066YJ10 in ESS. It is also speculated that the endometrial stromal cells have different phenotypic characteristics between the proliferative and secretory phases. According to these results, the tumor cells of ESS have the phenotypic characteristics of endometrial stromal cells in the proliferative phase. This result indicates the possibility that ESS may differentiate to endometrial stromal cells in the proliferative phase. They are speculated to preserve the morphological characteristics of endometrial stromal cells in the proliferative phase and become independent of cyclic estrogen/progesterone stimulation morphologically.
Acknowledgments
We thank Dr Nakako Sato of the Tsukuba Medical Center Hospital for permitting us to analyze a fresh sample of normal endometrial tissue. This study was supported in part by a Grant‐in‐Aid for Cancer Research (16‐1) from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. De Fusco PA, Gaffy TA, Cha SS et al. Endometrial stromal sarcoma: review of Mayo Clinic experience, 1945–1980. Gynecol Oncol 1989; 35: 8–14. [DOI] [PubMed] [Google Scholar]
- 2. Layfield LJ, Liu K, Dodge R, Barsky SH. Uterine smooth muscle tumors: utility of classification by proliferation, ploidy, and prognostic markers versus traditional histopathology. Arch Pathol Lab Med 2000; 124: 221–7. [DOI] [PubMed] [Google Scholar]
- 3. Chang KL, Crabtree GS, Hendrickson MR et al. Primary uterine endometrial stromal neoplasms: a clinicopathologic study of 117 cases. Am J Surg Pathol 1990; 14: 415–38. [DOI] [PubMed] [Google Scholar]
- 4. Bodner K, Bodner‐Adler B, Obermair A et al. Prognostic parameters in endometrial stromal sarcoma: a clinicopathological study in 31 patients. Gynecol Oncol 2001; 81: 160–5. [DOI] [PubMed] [Google Scholar]
- 5. Chu MC, Mor G, Shwartz PE et al. Low‐grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol 2003; 90: 170–6. [DOI] [PubMed] [Google Scholar]
- 6. Toki T, Shimizu M, Konishi I et al. CD10 is a marker for normal and neoplastic endometrial stromal cells. Int J Gynecol Pathol 2002; 21: 41–7. [DOI] [PubMed] [Google Scholar]
- 7. Zhu XQ, Shi YF, Wu YZ et al. Immunohistochemical markers in differential diagnosis of endometrial stromal sarcoma and cellular leiomyoma. Gynecol Oncol 2004; 92: 71–9. [DOI] [PubMed] [Google Scholar]
- 8. Popiolek D, Yee H, Demopulos RI et al. MIB1 as a possible predictor of recurrence in low‐grade endometrial stromal sarcoma of the uterus. Gynecol Oncol 2003; 90: 353–7. [DOI] [PubMed] [Google Scholar]
- 9. Morifar F, Regitnig P, Tavassolo FA et al. Expression of androgen receptors in benign and malignant endometrial stromal neoplasms. Virchows Arch 2004; 444: 410–14. [DOI] [PubMed] [Google Scholar]
- 10. Koontz JI, Soreng AL, Sklar J et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci USA 2001; 98: 6348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang HY, Ladanyi M, Soslow RA. Molecular detection of JAZF–JJAZ1 gene fusion in endometrial stromal neoplasms with classic and variant histology: evidence for genetic heterogeneity. Am J Surg Pathol 2004; 28: 224–32. [DOI] [PubMed] [Google Scholar]
- 12. Micci F, Walter CU, Heim S et al. Cytogenetic and molecular genetic analysis of endometrial stromal sarcoma: nonrandom involvement of chromosome arms 6p and 7p and confirmation of JAZF1/JJAZ1 gene fusion in t(7;17). Cancer Genet Cytogen 2003; 144: 119–24. [DOI] [PubMed] [Google Scholar]
- 13. Gunawan B, Schulten HJ, Fuzesi L. Identification of a BAC clone overlapping the t(6p12.3) breakpoint in the cell line ESS‐1 derived from an endometrial stromal sarcoma. Cancer Genet Cytogen 2003; 147: 84–6. [DOI] [PubMed] [Google Scholar]
- 14. Hrzenjak A, Tippl M, Denk H et al. Inverse correlation of secreted frizzled‐related protein 4 and β‐catenin expression in endometrial stromal sarcomas. J Pathol 2004; 204: 19–27. [DOI] [PubMed] [Google Scholar]
- 15. Aoyagi K, Terada M, Sasaki H et al. A faithful method for PCR‐mediated global mRNA amplification and its integration into microarray analysis on laser‐captured cells. Biochem Biophys Res Commun 2003; 300: 915–20. [DOI] [PubMed] [Google Scholar]
- 16. Okubo C, Morishita Y, Minami Y et al. Phenotypic characteristics of mouse lung adenoma induced by 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone. Mol Carcinogenesis 2005; 42: 121–6. [DOI] [PubMed] [Google Scholar]
- 17. Baumgart E, Schad A, Fahimi HD et al. Detection of mRNAs encoding peroxisomal proteins by non‐radioactive in situ hybridization with digoxigenin‐labelled cRNAs. Histchem Cell Biol 1997; 108: 371–9. [DOI] [PubMed] [Google Scholar]
- 18. Diachenco L, Lau YC, Siebert PD et al. Suppression subtractive hybridization: a method for generating differentially regulated or tissue‐specific cDNA probes and libraries. Proc Natl Acad Sci USA 1996; 93: 6025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ji P, Diederichs S, Wang W et al. MALT‐1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early‐stage non‐small cell lung cancer. Oncogene 2003; 22: 8031–41. [DOI] [PubMed] [Google Scholar]
- 20. Ota T, Suzuki Y, Nishikawa T et al. Complete sequencing and characterization of 21 243 full‐length human cDNAs. Nature Genet 2004; 36: 40–5. [DOI] [PubMed] [Google Scholar]
- 21. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classification and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynecol Obstet 2000; 70: 208–62 . [PubMed] [Google Scholar]


