Abstract
Angiogenesis is essential for the development, growth and advancement of solid tumors. Angiogenesis is induced by hypoxia with angiogenic transcription factor hypoxia inducible factors (HIF). This prompted us to study the clinical implications of HIF relative to angiogenesis in uterine cervical cancers. Although there was no significant difference in HIF‐1α histoscores and mRNA levels according to histopathological type or lymph node metastasis, HIF‐1α histoscores and mRNA levels increased significantly with advancing cancer stages. The prognosis of 30 patients with high HIF‐1α in uterine cervical cancers was poor (73% survival), whereas the 24‐month survival rate of the other 30 patients with low HIF‐1α was 93%. HIF‐1α histoscores and mRNA levels were correlated with the levels of the angiogenic factors thymidine phosphorylase and interleukin‐8, and HIF‐1α might be linked with these factors in cervical cancer tissue. HIF‐1α is a candidate for prognostic indicator as an angiogenic mediator in uterine cervical cancer. (Cancer Sci 2006; 97: 861–867)
Angiogenesis is essential for the development, growth and advancement of solid tumors.( 1 ) Angiogenesis is induced by hypoxia with angiogenic transcription factor hypoxia inducible factors (HIF). The transcription factor HIF‐1 belongs to the Per‐Ahr/Arnt‐Sim (PAS) family of basic helix‐loop‐helix proteins. It consists of HIF‐1α and HIF‐1β (aryl hydrocarbon receptor nuclear translocator) or HIF‐2α and HIF‐1β, as heterodimers using helix‐loop‐helix domain.( 2 ) Although HIF‐1α is constitutively reduced by the proteasome after ubiquitinization, hypoxia stabilizes and activates HIF‐1α.( 3 , 4 ) In contrast, HIF‐2α is expressed abundantly in various organs under normoxia, although the transcriptional activation properties of HIF‐2α are similar to those of HIF‐1α.( 5 ) HIF‐1α may be responsible for vascular endothelial growth factor (VEGF) expression mediated by hypoxia, and may be closely related to tumor angiogenesis.( 6 , 7 , 8 )
In uterine cervical cancers, the angiogenic factors VEGF, basic fibroblast growth factor (bFGF), platelet‐derived endothelial cell growth factor (PD‐ECGF), thymidine phosphorylase (TP) and interleukin (IL)‐8 were all identified to have a role in angiogenesis.( 9 , 10 , 11 , 12 , 13 , 14 ) VEGF, particularly its VEGF165 and VEGF121 isomers, is expressed predominantly in cancer cells, especially in adenocarcinomas, but its levels do not correlate with patient prognosis.( 9 ) bFGF is expressed in both cancer and interstitial cells of the uterine cervix, and its levels correlate with clinical stage but not with patient prognosis.( 10 ) TP is expressed predominantly in interstitial cells of uterine cervical cancers, and its levels correlate with patient prognosis in primary tumors of uterine cervical cancers, especially in the metastatic lymph nodes.( 11 , 12 ) Most noteworthy, serum TP can be used as a tumor marker of both squamous cell carcinoma and adenocarcinoma of the uterine cervix.( 13 ) IL‐8 was derived from tumor‐associated macrophages that infiltrated near cancer cells, and its levels correlated with patient prognosis.( 14 )
The HIF‐1 complex might link to specific angiogenic factors to activate angiogenesis for tumor advancement in each tumor. This prompted us to study which isoform of the angiogenic transcription factor HIF‐1 works on tumor advancement leading to poor patient prognosis, and which angiogenic factors link to the main HIF‐1 isoform to promote angiogenesis involved in tumor advancement and poor patient prognosis in uterine cervical cancers.
Materials and Methods
Patients
Prior informed consent for the following studies was obtained from all patients and the Research Committee for Human Subjects, Gifu University School of Medicine. Sixty patients ranging from 35 to 79 years of age with uterine cervical cancers (stage I, 20 cases; stage II, 20 cases; and stage III, 20 cases), squamous cell carcinoma (48 cases) and adenocarcinoma (12 cases) underwent curative surgery at the Department of Obstetrics and Gynecology, Gifu University School of Medicine, between September 2000 and August 2002. None of the patients had received any therapy before the uterine cervical cancer tissue was taken. A sample of tissue from each of the uterine cervical cancers was snap‐frozen in liquid nitrogen for the determination of HIF‐1α, HIF‐2α and HIF‐1β mRNA levels and IL‐1α, IL‐1β, tumor necrosis factor (TNF)‐α, IL‐8, bFGF, VEGF and TP levels, and a neighboring part of the tissue was submitted for histopathological study, including immunohistochemical staining for HIF‐1α. The clinical stage of uterine cervical cancers was determined using the International Federation of Obstetrics and Gynecology classification.( 15 )
Real‐time reverse transcription–polymerase chain reaction to amplify HIF‐1α, HIF‐2α and HIF‐1β mRNA
Total RNA was extracted from tissue specimens using the acid‐phenol guanidinium method (ISOGEN; Nippon Gene, Tokyo, Japan). Total RNA (3 µg) was reverse transcribed in a 20 µL volume for 1 h at 37°C with a mixture of 200 IU of Moloney murine leukemia virus reverse transcriptase (MMLV‐RTase, Gibco BRL) and the following reagents: 50 mM Tris‐HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 40 IU of RNAsin (Toyobo, Osaka, Japan), 10 mM dithiothreitol (DTT), 0.5 mM dNTPs and 30 pmol random primer. The reaction was incubated for 5 min at 94°C to inactivate MMLV‐RTase. Real‐time polymerase chain reaction (PCR) was carried out in a final volume of 25 µL containing the reverse transcribed RNA or each cDNA as standards, PCR buffer (2 mM Tris‐HCl [pH 8.0], 10 mM KCl, 10 µM ethylenediamine tetraacetic acid, 0.1 mM DTT, 0.05% Tween‐20, 0.05% Nonidet P‐40, 5% glycerol, 3 mM MgCl2), SYBR Green I (1 : 30 000 dilution; BioWhittaker Molecular Applications, Rockland, ME, USA), 0.3 mM dNTPs, 0.3 µM of each of PCR primer and 1.25 IU of Takara Ex Taq R‐PCR (Takara Suzo, Otsu, Japan). The mixture was amplified in a Smart Cycler (Cepheid, Sunnyvale, CA, USA) for 45 cycles with the following conditions: 94°C for 10 s for denaturing, 55°C for 5 s for annealing and 72°C for 20 s for extension. The PCR primers designed from each cDNA( 16 , 17 , 18 ) were as follows: HIF‐1α‐S, 5′‐GACTCAGCTATTCACCAAAG‐3′; HIF‐1α‐AS, 5′‐TGGGTAATGGAGACATTGCC‐3′; HIF‐2α‐S, 5′‐CCCAATAGCCCTGAAGACTA‐3′; HIF‐2α‐AS, 5′‐CACCAAGTCCAGCTCATTGA‐3′; HIF‐1β‐S, 5′‐TTGGCCCTGTGAAAGAAGGA‐3′; and HIF‐1β‐AS, 5′‐GGGATTCTGGAATGTGAAGC‐3′.
Immunohistochemistry
Sections of formalin‐fixed paraffin‐embedded tissue (4 µm) from the uterine cervical cancers were cut with a microtome and dried overnight at 37°C on a silanized slide (Dako, Carpinteria, CA, USA). Samples were deparaffinized in xylene at room temperature for 80 min and washed with a graded ethanol/water mixture and then with distilled water. Immunohistochemical staining for factor VIII‐related antigen, which is synthesized by vascular endothelial cells, is specific for the endothelial cells of blood vessels( 19 ) and is useful for detecting tumor angiogenesis.( 20 ) The samples for HIF‐1α antigen determination were soaked in a citrate buffer and then microwaved at 100°C for 10 min, and those for factor VIII‐related antigen and CD34 determination were treated with 0.3 µg/mL trypsin in phosphate buffer at room temperature for 20 min. The protocol for the LSAB2 Kit, Peroxidase (Dako) was followed for each sample. Rabbit antihuman HIF‐1α (Chemicon International, Temecula, CA, USA), rabbit antifactor VIII‐related antigen (Zymed, San Francisco, CA, USA) and mouse CD34 (Dako) were used at dilutions of 1 : 20, 1 : 2 and 1 : 40, respectively, as the primary antibodies. For the negative controls of HIF‐1α, factor VIII‐related antigen and CD34, the corresponding preimmune animal serums (rabbit and mouse; both from Dako Cytomation, Glostrup, Denmark) were added instead of the primary antibodies.
The immunohistochemical analyses used three blocks from each patient, and three sections from each tissue block. The results of immunohistochemical staining for HIF‐1α were evaluated semiquantitatively, as described by McCarty et al.( 21 ) Each stained section was given a histochemical score (histoscore, HS) calculated using the formula:
| ∑(i + 1) × Pi, |
where i = nuclear staining intensity (range 1–4, 0 indicates no staining) and Pi = percentage of stained cells. Vessels were counted in the five highest density areas at 200× magnification (using a combination of 20× objective and 10× ocular, 0.785 mm2/field). Microvessel counts were expressed as the mean number of vessels in these areas.( 22 ) Microvessel density was evaluated by the counting of microvessels.
Enzyme immunoassay for the determination of bFGF, VEGF, TP and IL‐8 antigens
All steps were carried out at 4°C. Tissues of uterine cervical cancers (wet weight: 10–20 mg) were homogenized in HG buffer (5 mM Tris‐HCl [pH 7.4], 5 mM NaCl, 1 mM CaCl2, 2 mM ethyleneglycol‐bis‐[β‐aminoethyl ether]‐N,N,N′, N′‐tetraacetic acid, 1 mM MgCl2, 2 mM DTT, 25 µg/mL aprotinin, and 25 µg/mL leupeptin) with a Polytron homogenizer (Kinematics, Luzern, Switzerland). This suspension was centrifuged in a microfuge at 10 000 g for 3 min to obtain the supernatant. The protein concentration of samples was measured using the method of Bradford( 23 ) to standardize VEGF, bFGF, TP and IL‐8 antigen levels.
Basic fibroblast growth factor, VEGF and IL‐8 antigen levels in the samples were determined using the sandwich enzyme immunoassay Human bFGF Quantikine kit, Human VEGF Quantikine kit and Human IL‐8 Quantikine kit (all from R&D System, Minneapolis, MN, USA), respectively. TP antigen levels were determined using the method described by Nishida et al.( 24 ) The levels of bFGF, VEGF, TP and IL‐8 were standardized with the corresponding cellular protein concentrations.
Determination of apoptotic index
Apoptosis studies were carried out using Apoptag, an apoptosis detection kit (S7100‐kit; Oncor, Gaithersburg, MD, USA) for the terminal deoxynucleotidyl transferase (TdT)‐mediated deoxyuridine triphosphate (dUTP)‐biotin nick‐end labeling (TUNEL) procedure. Briefly, 4 µm‐thick paraffin‐embedded tissue sections were mounted on silanized‐slides (Dako). Tissue sections were deparaffinized with xylene and rehydrated in a graded ethanol series similar to the tissues stained for immunohistochemistry. Protein digestion was carried out with 20 mg/mL proteinase K (Sigma Chemical, St Louis, MO, USA) for 15 min at room temperature. Tissue sections were rinsed in distilled water for 2 min followed by quenching of endogenous peroxidase with 3% H2O2 in phosphate‐buffered saline (PBS) for 30 min at room temperature. Samples were rinsed in PBS, excess liquid was removed by blotting, and a 1× equilibrium buffer was applied for 10–15 s at room temperature. Residues of digoxigenin‐nucleotide (digoxigenin‐11‐dUTP and dATP) were added to DNA using the TdT enzyme in aeration buffer and by incubating the samples in a humidified chamber at 37°C. Then, samples were incubated with antidigoxigenin conjugated with peroxidase and visualized using diaminobenzidine (DAB), followed by counterstaining with Mayer's hematoxylin and dehydration with a graded ethanol series.
Using light microscopy, Apoptag‐labeled sections of uterine cervical cancers tissues were evaluated for the presence of DAB‐stained (brown) fragmented nuclear material. The hematoxylin–eosin‐stained sections were also evaluated for the presence of apoptotic nuclei using morphological criteria such as intensely basophilic nuclei (compaction of chromatin), cell membrane blebbing, karyorrhexis, cell shrinkage with formation of apoptotic bodies, absence of significant inflammatory response, and phagocytosis of dying cells by adjacent normal cells and macrophages, which are used to diagnose apoptosis.( 25 ) The cells were examined by light microscopy and divided into four to 10 regions. A minimum of 100 cells were counted in each region and the apoptotic index was determined as the mean percentage of positively stained cells in a section.( 26 )
Statistics
Survival curves were calculated using the Kaplan–Meier method and analyzed using the log‐rank test. The levels of HIF‐1α, HIF‐2α and HIF‐1β mRNA, VEGF, bFGF, TP and IL‐8 were measured in three parts taken from each tissue, and the assay for each sample was carried out in triplicate. Differences were considered significant when P was less than 0.05.
Results
Although there was no significant difference in HIF‐1α mRNA levels according to histopathological type and lymph node metastasis, HIF‐1α mRNA levels increased significantly with increasing disease stage in uterine cervical cancers (Fig. 1). There was no significant difference in HIF‐2α or in HIF‐1β mRNA levels according to clinical stage, histopathological type or lymph node metastasis in uterine cervical cancers (2, 3).
Figure 1.
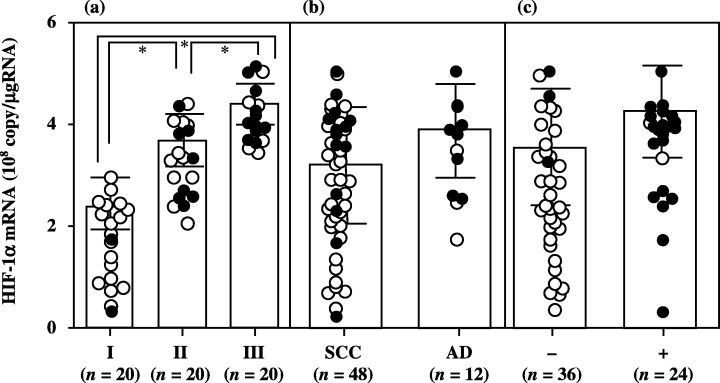
Hypoxia inducible factor (HIF)‐1α mRNA levels in uterine cervical cancers classified according to (a) clinical stage, (b) histopathological type and (c) lymph node metastasis. Clinical stages of uterine cervical cancer are in accordance with the International Federation of Obstetrics and Gynecology classification. Each level is the mean of nine determinations. The bar indicates SD. Alive and deceased cases are shown as ○ and •, respectively. AD, adenocarcinoma; SCC, squamous cell carcinoma.
Figure 2.
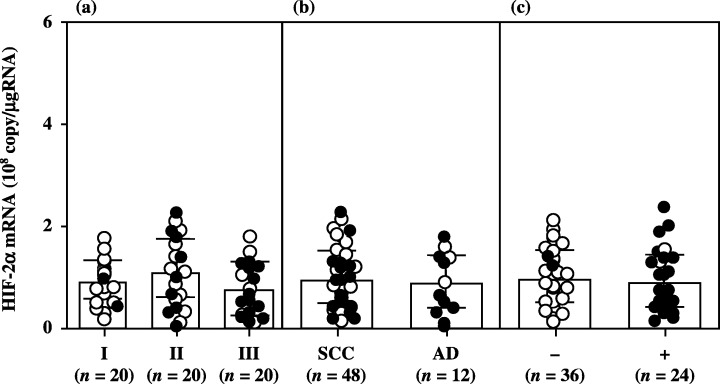
Hypoxia inducible factor (HIF)‐2α mRNA levels in uterine cervical cancers classified according to (a) clinical stage, (b) histopathological type and (c) lymph node metastasis. Clinical stages of uterine cervical cancer are in accordance with the International Federation of Obstetrics and Gynecology classification. Each level is the mean of nine determinations. The bar indicates SD. Alive and deceased cases are shown as ○ and •, respectively. AD, adenocarcinoma; SCC, squamous cell carcinoma.
Figure 3.
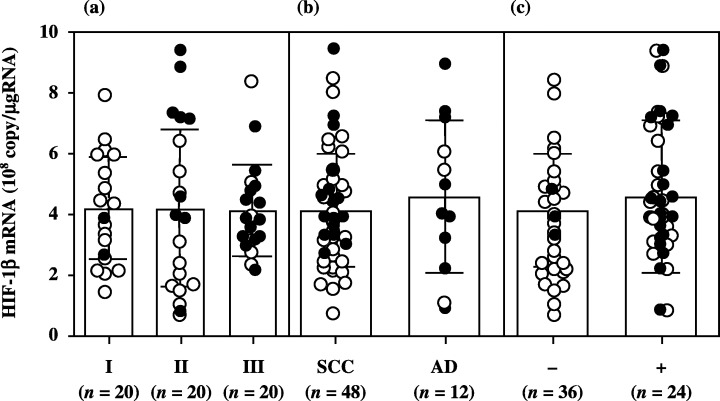
Hypoxia inducible factor (HIF)‐1β mRNA levels in uterine cervical cancers classified according to (a) clinical stage, (b) histopathological type and (c) lymph node metastasis. Clinical stages of uterine cervical cancer are in accordance with the International Federation of Obstetrics and Gynecology classification. Each level is the mean of nine determinations. The bar indicates SD. Alive and deceased cases are shown as ○ and •, respectively. AD, adenocarcinoma; SCC, squamous cell carcinoma.
Immunohistochemical staining revealed that the localization of HIF‐1α was predominantly in the nucleus of cancer cells (Fig. 4). Although there was no significant difference in HIF‐1α histoscores according to histopathological type and lymph node metastasis, HIF‐1α histoscores (Fig. 5) increased significantly with increasing disease stage in uterine cervical cancers, as did HIF‐1α mRNA (Fig. 1).
Figure 4.
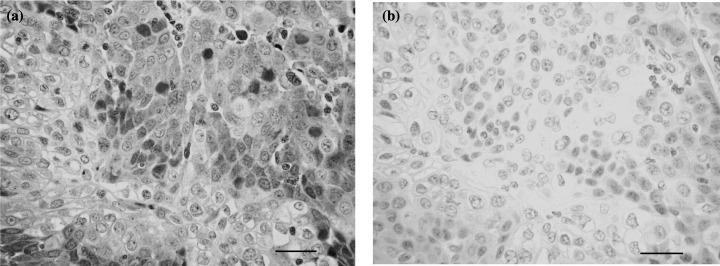
Immunohistochemical staining for hypoxia inducible factor (HIF)‐1α in uterine cervical cancer. (a) A case of squamous cell carcinoma of the uterine cervix and (b) negative control. Rabbit antihuman HIF‐1α was used at a dilution of 1 : 20 as the primary antibody (original magnification ×200). Scale bars = 50 µm.
Figure 5.
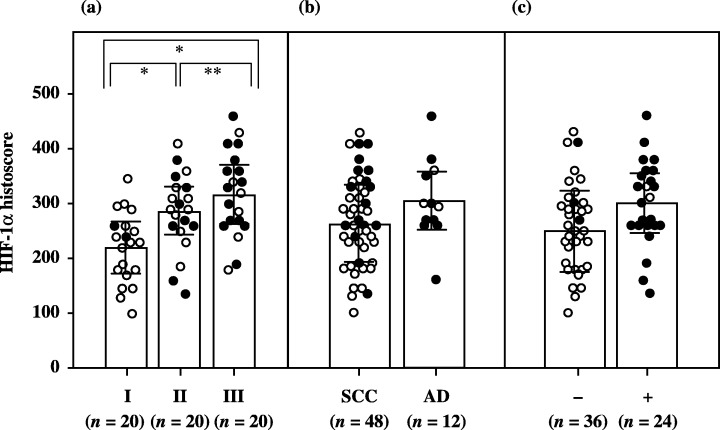
Hypoxia inducible factor (HIF)‐1α histoscores in uterine cervical cancers classified according to (a) clinical stage, (b) histopathological type and (c) lymph node metastasis. Clinical stages of uterine cervical cancer are in accordance with the International Federation of Obstetrics and Gynecology classification. Each level is the mean of nine determinations. The bar indicates SD. Alive and deceased cases are shown as ○ and •, respectively. AD, adenocarcinoma; SCC, squamous cell carcinoma.
Furthermore, the 60 patients who underwent curative resection were divided arbitrarily into two groups with 30 cases in each, based on high/low HIF‐1α histoscores and mRNA levels, with the midpoint being a histoscore of 270 and 3.6 × 108 copies/µg total RNA, respectively. The high group determined by HIF‐1α histoscores consisted of the same patients as the high group broken down by mRNA levels, and the low group by HIF‐1α histoscores comprised the same patients as the low group by mRNA levels. The prognosis of the 30 patients with high HIF‐1α (>270 histoscore and >3.6 × 108 copies/µg total RNA) in uterine cervical cancers was poor (40%), whereas the 24‐month survival rate of the other 30 patients with low HIF‐1α (<270 histoscore and <3.6 × 108 copies/µg total RNA) was 77% (Fig. 6).
Figure 6.
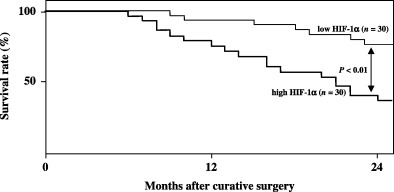
Survival rate after curative resection for uterine cervical cancers. Patient prognosis was analyzed with a 24‐month survival rate. High hypoxia inducible factor (HIF)‐1α: histoscore >270 and >3.6 × 108 copies/µg total RNA. Low HIF‐1α: histoscore <270 and <3.6 × 108 copies/µg total RNA.
The HIF‐1α histoscores and HIF‐1α mRNA levels correlated with the levels of TP (r = 0.430, P < 0.001 and r = 0.500, P < 0.001, respectively) and IL‐8 (r = 0.477, P < 0.001 and r = 0.533, P < 0.001, respectively), but not with the those of bFGF (data not shown) or VEGF (r = –0.003 and r = −0.107, respectively) (Fig. 7).
Figure 7.
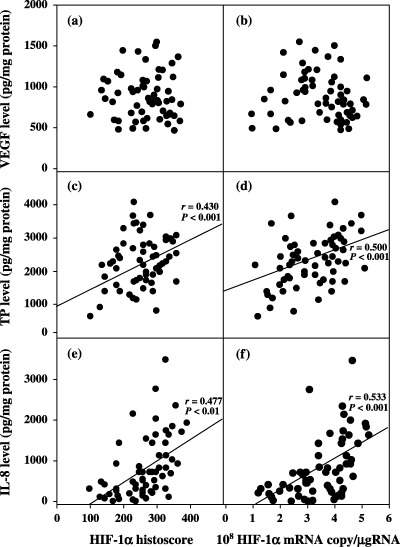
Correlation between hypoxia inducible factor (HIF)‐1α histoscore and levels of (a) vascular endothelial growth factor (VEGF), (c) thymidine phosphorylase (TP) and (e) interleukin (IL)‐8, and between mRNA level and levels of (b) VEGF, (d) TP and (f) IL‐8 in uterine cervical cancers.
The microvessel counts by immunohistochemical staining for factor VIII‐related antigen (MVC‐F8) and by staining for CD34 (MVC‐CD34) to indicate the level of angiogenesis correlated significantly with HIF‐1α histoscores (MVC‐F8: r = 0.338, P < 0.001; and MVC‐CD34: r = 0.392, P < 0.001), TP levels (MVC‐F8: r = 0.523, P < 0.001; and MVC‐CD34: r = 0.445, P < 0.001) and IL‐8 levels (MVC‐F8: r = 0.410, P < 0.001; and MVC‐CD34: r = 0.475, P < 0.001) (Fig. 8). There was no correlation between the rate of apoptosis and HIF‐1α histoscores (Fig. 9).
Figure 8.
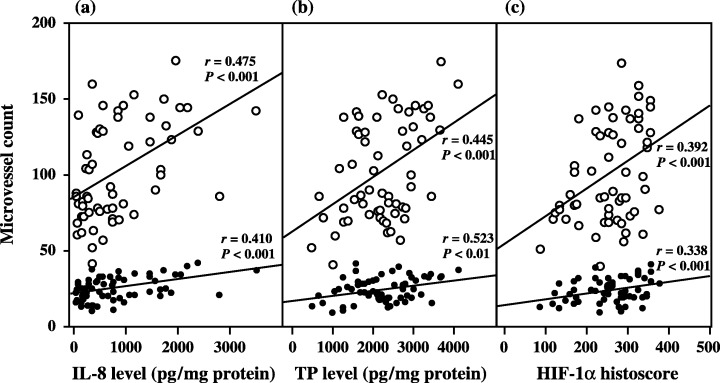
Correlation between microvessel count (MVC) and (a) interleukin (IL)‐8 levels, (b) thymidine phosphorylase (TP) levels and (c) hypoxia inducible factor (HIF)‐1α histoscores in uterine cervical cancers. MVC, indicative of the level of angiogenesis, were evaluated by immunohistochemistry for factor VIII‐related antigen (•) and for CD34 (○) in the same specimens as for immunohistochemistry of HIF‐1α.
Figure 9.
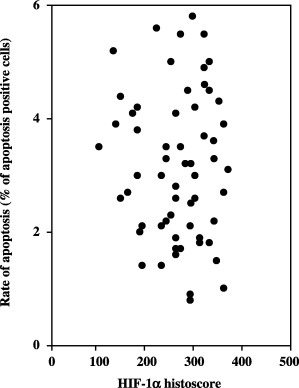
Correlation between rate of apoptosis and hypoxia inducible factor (HIF)‐1α histoscores in uterine cervical cancers.
Discussion
Based on the immunohistochemical analysis, patients with strong expression of HIF‐1α had shorter overall and disease‐free survival times compared with those with moderate to absent HIF‐1α expression. HIF‐1α expression is a strong independent prognostic marker in early stage cervical cancer.( 27 ) Strong and moderate expression of HIF‐1α as measured by immunohistochemistry is associated with partial response to radiotherapy and is deemed to be an independent prognostic factor for shorter progression‐free survival.( 28 ) A multivariate analysis revealed HIF‐1α expression by immunohistochemistry to be an independent factor for overall survival. HIF‐1α was expressed in the vast majority of patients with advanced cervical cancer and had a prognostic significance.( 29 ) In the present study, HIF‐1α protein and mRNA levels correlated with increasing disease stage of uterine cervical cancers and poor patient prognosis. HIF‐1α expression by immunohistochemistry was correlated with disease recurrence, recurrence‐free survival rate, and with distant metastasis after radiation therapy for patients with stage IIIB squamous cell carcinoma of the cervix. Therefore, HIF‐1α is an important prognostic factor, especially for predicting future metastasis.( 30 ) Integrally, HIF‐1α might be a reliable indicator for prognosis from early to advanced stage with distant metastasis of uterine cervical cancer. In contrast, HIF‐2α and HIF‐1β mRNAs are both expressed constitutively, regardless of disease stage and histopathological type, and neither transcription factor could be used as a prognostic indicator.
Although HIF‐2α is expressed abundantly regardless of hypoxia,( 5 ) HIF‐1α is induced by stabilization and activation with hypoxia, but the level of mRNA is not raised under these circumstances, nor is the translation activity from the mRNA.( 3 , 4 ) There was no correlation between HIF‐1α expression and median oxygen tension or hypoxic fractions, indicating that HIF‐1α should not be used as an endogenous marker of tumor hypoxia in locally advanced squamous cell carcinoma of the uterine cervix.( 31 ) In the present study, HIF‐1α levels increased with tumor advancement. Therefore, factors other than hypoxia might induce HIF‐1α levels with advancement of uterine cervical cancers. Furthermore, hypoxia induces VEGF expression in various tissues, especially in tumors.( 6 , 7 , 8 ) In uterine cervical cancers, some authors have reported that VEGF expression correlates with hypoxic status of the tumors.( 32 , 33 ) However, others have reported no correlation between VEGF expression and hypoxic status.( 34 , 35 ) In the present study, HIF‐1α expression did not correlate with VEGF expression. The linkage of hypoxia to VEGF expression might not be stable and may involve various conditions. Therefore, HIF‐1α expression might be mainly induced not only by hypoxia, but also by various other factors.
In the present study, the microvessel density to evaluate angiogenic status correlated with the expression levels of HIF‐1α, TP and IL‐8 in uterine cervical cancers. Furthermore, we found an important correlation between HIF‐1α expression and that of IL‐8 and TP. HIF‐1α expression, along with IL‐8 and TP levels, increased with increasing disease stage of uterine cervical cancers. Although TP was expressed in stromal cells( 11 ) and IL‐8 was expressed in infiltrated macrophages( 12 ) in stroma that involves the capillary network of uterine cancer tissue, HIF‐1α was expressed predominantly in the nucleus of cancer cells. Carbonic anhydrase‐9 induced by hypoxia with upregulation of HIF‐1α significantly coexpressed TP, and relates to angiogenic pathways and to poor outcome in non‐small cell lung cancer.( 36 ) Acidic pH activates nuclear factor‐κB and activator protein‐1 in human ovarian cancer cells and, in doing so, increases IL‐8 gene expression.( 37 ) HIF‐1α activation induces substantial heme oxygenase‐1 expression, which is associated with attenuated proinflammatory chemokine IL‐8 production by the microvascular endothelium.( 38 ) Although no direct evidence exists linking HIF‐1α to IL‐8 and TP induction, an HIF‐1α‐induced factor in cancer cells might translocate to the stroma and induce IL‐8 and TP expression near microvessels in cancer tissue to activate angiogenesis. In addition, as the ratio of apoptotic cells did not correlate with the expression levels of HIF‐1α, HIF‐1α might not be involved in apoptosis in uterine cervical cancers.
Because chemotherapy and radiation are often not very specific to cancer cells, they produce severe effects on normal cells as well, especially bone marrow cells. In contrast, anti‐angiogenic therapy is specific to the rapidly growing vascular endothelial cells in tumors, without affecting slow‐growing vascular endothelial cells in normal tissues and other normal cells. If TP and IL‐8 can be suppressed as an anti‐angiogenic therapy, patient prognosis should be improved remarkably without the severe side‐effects seen with chemotherapy and radiotherapy. However, if the main angiogenic factors IL‐8 and TP are suppressed by anti‐angiogenic therapy for a long period, other angiogenic factors, for example VEGF, might be induced by an alternately linked angiogenic pathway, which is recognized as tolerance. Therefore, suppression of the major angiogenic factors along with suppression of HIF‐1α induction might be more effective as an anti‐angiogenic therapy than mere suppression of major angiogenic factors.
In conclusion, HIF‐1α might be a reliable indicator for prognosis from early to advanced stages of uterine cervical cancers, and may become an important target for anti‐angiogenic therapy to avoid tolerance in response to suppression of the main angiogenic factors for a long period of time.
Acknowledgments
The authors wish to thank Mr John Cole for proofreading the English of this manuscript.
References
- 1. Folkman J. Tumor angiogenesis. Adv Cancer Res 1985; 43: 175–203. [DOI] [PubMed] [Google Scholar]
- 2. Wang GL, Semenza GL. Purification and characterization of hypoxia‐inducible factor 1. J Biol Chem 1995; 270: 1230–7. [DOI] [PubMed] [Google Scholar]
- 3. Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia‐inducible transcription factor depends primarily upon redox‐sensitive stabilization of its alpha subunit. J Biol Chem 1996; 271: 32253–9. [DOI] [PubMed] [Google Scholar]
- 4. Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia‐inducible factor 1α: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA 1997; 94: 5667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii‐Kuriyama Y. A novel bHLH‐PAS factor with close sequence similarity to hypoxia‐inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 1997; 94: 4273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maxwell PH, Dachs GU, Gleadle JM et al. Hypoxia‐inducible factor‐1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA 1997; 94: 8104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carmeliet P, Dor Y, Herbert JM et al. Role of HIF‐1α in hypoxia‐mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394: 485–90. [DOI] [PubMed] [Google Scholar]
- 8. Ryan HE, Lo J, Johnson RS. HIF‐1α is required for solid tumor formation and embryonic vascularization. EMBO J 1998; 17: 3005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujimoto J, Sakaguchi H, Hirose R, Ichigo S, Tamaya T. Expression of vascular endothelial growth factor (VEGF) and its mRNA in uterine cervical cancers. Br J Cancer 1999; 80: 827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujimoto J, Ichigo S, Hori M, Hirose R, Sakaguchi H, Tamaya T. Expression of basic fibroblast growth factor and its mRNA in advanced uterine cervical cancers. Cancer Lett 1997; 111: 21–6. [DOI] [PubMed] [Google Scholar]
- 11. Fujimoto J, Ichigo S, Sakaguchi H et al. The expression of platelet‐derived endothelial cell growth factor in uterine cervical cancers. Br J Cancer 1999; 79: 1249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujimoto J, Sakaguchi H, Hirose R, Ichigo S, Tamaya T. Clinical implication of expression of platelet‐derived endothelial cell growth factor (PD‐ECGF) in metastatic lesions of uterine cervical cancers. Cancer Res 1999; 59: 3041–4. [PubMed] [Google Scholar]
- 13. Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. The value of platelet‐derived endothelial cell growth factor as a novel predictor of advancement of uterine cervical cancers. Cancer Res 2000; 60: 3662–5. [PubMed] [Google Scholar]
- 14. Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res 2000; 60: 2632–5. [PubMed] [Google Scholar]
- 15. FIGO News. Int J Gynecol Obstet 1989; 28: 189–93. [Google Scholar]
- 16. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia‐inducible factor 1 is a basic‐helix‐loop‐helix‐PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997; 11: 72–82. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman EC, Reyes H, Chu FF et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 1991; 252: 954–8. [DOI] [PubMed] [Google Scholar]
- 19. Bell DA, Flotte TJ. Factor VIII related antigen in adenomatoid tumors. Cancer 1982; 50: 932–8. [DOI] [PubMed] [Google Scholar]
- 20. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis − correlation in invasive breast carcinoma. New Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 21. McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Laboratory Med 1985; 109: 716–21. [PubMed] [Google Scholar]
- 22. Maeda K, Chung Y, Ogawa Y et al. Thymidine phosphorylase/platelet‐derived endothelial cell growth factor expression associated with hepatic metastasis in gastric carcinoma. Br J Cancer 1996; 73: 884–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradford MA. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 1976; 72: 315–23. [DOI] [PubMed] [Google Scholar]
- 24. Nishida M, Hino A, Mori K et al. Preparation of anti‐human thymidine phosphorylase monoclonal antibodies useful for detecting the enzyme levels in tumor tissues. Biol Pharm Bull 1996; 19: 1407–11. [DOI] [PubMed] [Google Scholar]
- 25. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995; 146: 3–15. [PMC free article] [PubMed] [Google Scholar]
- 26. Del Vecchio S, Zannetti A, Ciarmiello A et al. Dynamic coupling of 99mTc‐MIBI efflux and apoptotic pathway activation in untreated breast cancer patients. Eur J Nucl Med 2002; 29: 809–14. [DOI] [PubMed] [Google Scholar]
- 27. Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia‐inducible factor 1α is a marker for an unfavorable prognosis in early‐stage invasive cervical cancer. Cancer Res 2000; 60: 4693–6. [PubMed] [Google Scholar]
- 28. Bachtiary B, Schindl M, Potter R et al. Overexpression of hypoxia‐inducible factor 1α indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res 2003; 9: 2234–40. [PubMed] [Google Scholar]
- 29. Burri P, Djonov V, Aebersold DM et al. Significant correlation of hypoxia‐inducible factor‐1α with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 494–501. [DOI] [PubMed] [Google Scholar]
- 30. Ishikawa H, Sakurai H, Hasegawa M et al. Expression of hypoxic‐inducible factor 1α predicts metastasis‐free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2004; 60: 513–21. [DOI] [PubMed] [Google Scholar]
- 31. Mayer A, Wree A, Hockel M, Leo C, Pilch H, Vaupel P. Lack of correlation between expression of HIF‐1α protein and oxygenation status in identical tissue areas of squamous cell carcinomas of the uterine cervix. Cancer Res 2004; 64: 5876–81. [DOI] [PubMed] [Google Scholar]
- 32. Chiarotto JA, Hill RP. A quantitative analysis of the reduction in oxygen levels required to induce up‐regulation of vascular endothelial growth factor (VEGF) mRNA in cervical cancer cell lines. Br J Cancer 1999; 80: 1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ziemer LS, Koch CJ, Maity A, Magarelli DP, Horan AM, Evans SM. Hypoxia and VEGF mRNA expression in human tumors. Neoplasia 2001; 3: 500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raleigh JA, Calkins‐Adams DP, Rinker LH et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res 1998; 58: 3765–8. [PubMed] [Google Scholar]
- 35. West CM, Cooper RA, Loncaster JA, Wilks DP, Bromley M. Tumor vascularity: a histological measure of angiogenesis and hypoxia. Cancer Res 2001; 61: 2907–10. [PubMed] [Google Scholar]
- 36. Giatromanolaki A, Koukourakis MI, Sivridis E et al. Expression of hypoxia‐inducible carbonic anhydrase‐9 relates to angiogenic pathways and independently to poor outcome in non‐small cell lung cancer. Cancer Res 2001; 61: 7992–8. [PubMed] [Google Scholar]
- 37. Xu L, Fidler IJ. Acidic pH‐induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res 2000; 60: 4610–16. [PubMed] [Google Scholar]
- 38. Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA 3rd, Kukreja RC. HIF‐1 activation attenuates postischemic myocardial injury: role for heme oxygenase‐1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol 2005; 289: 542–8. [DOI] [PubMed] [Google Scholar]


