Abstract
In patients with breast cancer, taxane as well as anthracycline play central roles in systemic chemotherapy. By evaluating the pathological response, we can gauge sensitivity to primary chemotherapy. However, biomarkers that would predict a response to taxane have not yet been established. We conducted a prospective randomized trial to evaluate whether selecting patients using sensitivity testing based on the gene expression of the tumor might enhance the probability of the pathological response. Five genes were identified as biomarkers derived from a microarray of DNA gene profiles from microdisected breast tumors. In the experimental arm (B1), 12 cycles of weekly paclitaxel, 80 mg/m2, were preoperatively given when the sensitivity test was positive and therefore judged to be sensitive to paclitaxel. When the test was negative, meaning insensitive to paclitaxel, four cycles of FEC100 were given (arm B2). In the control arm (A), paclitaxel was administered weekly without the use of the sensitivity test. A total of 92 patients were enrolled and 86 patients were analyzed. The pathological response rate (pRR) of each arm was 36.4% in B1 (expected sensitive to paclitaxel), 21.1% in A (control) and 12.5% in B2, respectively. Weekly paclitaxel‐treated patients selected by the sensitivity test did not enhance the pRR. The study failed to validate sensitivity testing using five gene expressions for primary chemotherapy with paclitaxel in patients with breast cancer. However, this study suggests that a randomized phase II study is a robust tool for obtaining a rapid conclusion on the usefulness of biomarkers and could be the foundation for further large clinical trials. (Cancer Sci 2011; 102: 130–136)
Trastuzumab, a molecular targeted agent, has greatly improved the survival rate in patients with breast cancer.( 1 ) Trastuzumab binds human epidermal growth factor receptor type 2 (HER2) and downregulates cell proliferation signaling. Trastuzumab enriches its activity by selecting patients with HER2‐overexpressed breast cancer. Biomarkers can both maximize activity and minimize toxicities. Cytotoxic agents such as taxane or anthracycline also play a crucial role in systemic chemotherapy for breast cancer.( 2 ) To date, no specific biomarker of cytotoxic chemotherapeutic agents has been established.
Primary chemotherapy with anthracycline and taxane is standard care for patients with early‐stage breast cancers to obtain breast conservation and survival benefit.( 3 ) Primary chemotherapy informs us of its sensitivity by evaluation of the pathological response. The probability of a pathological complete response (pCR) from a single administration of taxane is no more than 20%.( 4 ) In our experience, primary treatment with paclitaxel weekly produced a 7% pCR with complete disappearance of intraductal lesions and a 30% pathological response with more than two‐thirds reduction in invasive lesions.( 5 ) Taxane induces microtubule bundling, formation of multipolar spindles, mitotic arrest and apoptosis. Resistance to taxane derives from overexpression of ATP‐binding cassette (ABC) transporter, for example, P‐glycoprotein,( 6 ) somatic mutation of β‐tubulin,( 7 )βIII‐tubulin isoform( 8 ) or low expression of tubulin‐binding protein tau.( 9 ) However, the clinical usefulness of these biomarkers has not been determined. The DNA microarray provides a unique molecular portrait or signature regarding clinical behavior and drug responsiveness.( 10 , 11 , 12 , 13 , 14 ) The expression pattern of selected genes, if found to be related to the sensitivity of cytotoxic agents, could yield a biomarker to predict the clinical response and outcome. We have developed a sensitivity test using quantitative RT‐PCR of five selected genes to predict the response to paclitaxel. Commonly, retrospective studies have been used to find predictive biomarkers, but their level of evidence is low. To our knowledge, there have been few randomized trials directly addressing biomarkers in a prospective fashion.
Therefore, we have conducted a prospective randomized trial on whether the selection of patients using a sensitivity test to predict paclitaxel based on the gene expression of the tumor might enhance the probability of the pathological response. The current study aimed to validate the genetic diagnosis to predict sensitivity in primary chemotherapy with paclitaxel in women with breast cancer.
Materials and Methods
Patients. Eligible patients were women with histologically confirmed invasive carcinomas of the breast with a tumor size 3 cm or more in stages IIA, IIB, IIIA or IIIB (T1‐4, N0‐1 and M0). All patients were younger than 70 years and had performance status (Eastern Cooperative Oncology Group performance status) 0 or 1; life expectancy 6 months or more; adequate organ function; white blood cell count 4.0 × 109/L or absolute neutrophil count 2.0 × 109/L; hemoglobin 9 g/dL; platelets 100 × 109/L; blood urea nitrogen (BUN) and serum creatinine within normal limits; aspartate transaminase (AST), alanine transaminase (ALT) twice the upper limit of normal; total bilirubin 1.5 mg/dL; and electrocardiography (ECG) within normal limits. Excluded patients were those with non‐invasive or microinvasive breast cancer, stage IIIC or IV; inflammatory breast cancer; male gender; previous chemotherapy, hormone therapy or radiotherapy; active double cancer; serious complication with infection, cardiac disease, pulmonary fibrosis, interstitial pneumonitis, bleeding, hepatitis type B and its carrier; uncontrolled diabetes; heavy history of drug allergy, history of allergic reaction to drugs using the vehicle cremophor; pregnant, nursing or willing to become pregnant; or otherwise judged inadmissible by the investigators. The research ethics committee of Cancer Institute Hospital approved the study, and all patients gave written, informed consent.
Sensitivity testing. How the sensitivity testing was developed has been described in previous papers.( 15 , 16 ) Basically, specimens were obtained by core needle biopsy before primary chemotherapy. To minimize the influence of stromal cells, pure populations of tumor cells were collected by laser captured microdissection. After RNA extraction, we performed gene expression profiling of 21 000 genes by DNA microarray to select the candidate genes. Surgically resected primary breast tumors were examined to determine the pathological response to chemotherapy. All clinical and genomic data were entered into an integrated database and analyzed to identify predictive factors. Differentially expressed genes were selected between the paclitaxel‐resistant group and the paclitaxel‐sensitive group. Then the expression of selected candidate genes was quantified by RT‐PCR to confirm the array data and increase reliability. Furthermore, we narrowed the candidate genes down to establish a prediction system based on real‐time RT‐PCR. Finally, we identified a set of five genes predictive of patient response to paclitaxel in primary chemotherapy (Table 1). Before clinical application, the prediction system was validated retrospectively, revealing that in 51 patients the sensitivity testing using the expression of five genes produced 90% accuracy and a 9.8% error rate.
Table 1.
Five genes identified as biomarkers
| Gene ID | Affy probe | GenBank | UniGene | Gene Symbol | Uni‐title |
|---|---|---|---|---|---|
| 03921 | 223235 | NM022138 | Hs.487200 | SMOC2 | Secreted protein, acidic, cystein‐rich related modular calcium binding 2 |
| 05918 | NA | BG928645 | Hs.494395 | C9orf121 | Chromosome 9 open reading frame 121 |
| 06334 | 205009 | NM003225 | Hs.162807 | TFF1 | Trefoil factor 1 |
| 19403 | 224968 | NM080667 | Hs.264208 | CCDC104 | Coiled‐coil domain containing 104 |
| 20850 | 229580 | BX097190 | Hs.7413 | NA | Transcribed locus |
NA, not applicable.
Study design. To validate the predictiveness of the pathological response by the sensitivity test in primary chemotherapy with paclitaxel, we conducted a prospective randomized trial, as shown in Fig. 1. Patients were stratified according to the status of HER2, estrogen receptor (ER), progesteron receptor (PgR), nuclear grade and tumor size. Participating patients were randomly assigned to receive arm A or B with a ratio of 1:4. For patients in arm A, we did not use the genetic diagnosis for sensitivity to paclitaxel, but they received primary chemotherapy with paclitaxel. For patients in arm B, we did use the genetic diagnosis for sensitivity to paclitaxel. When patients were diagnosed as sensitive to paclitaxel, they received primary chemotherapy with paclitaxel. Patients diagnosed as insensitive to paclitaxel received primary chemotherapy with FEC100.
Figure 1.
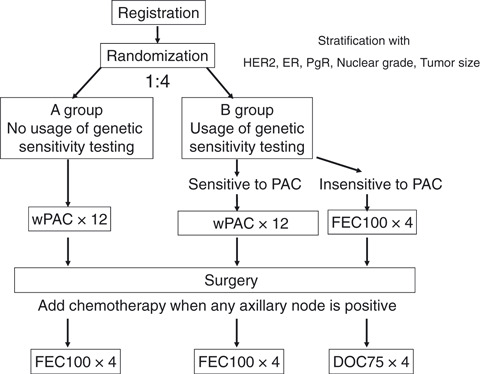
Study design. Patients were stratified according to the status of human epidermal growth factor receptor type 2 (HER2), estrogen receptor (ER), progesteron receptor (PgR), nuclear grade and tumor size. Patients were randomly assigned to receive arm A or B with a ratio of 1:4. In arm A, patients received primary chemotherapy with paclitaxel without selection by genetic sensitivity testing. For patients in arm B, we used the genetic diagnosis for sensitivity to paclitaxel. In arm B1, patients diagnosed as sensitive to paclitaxel received paclitaxel. In arm B2, patients diagnosed as insensitive to paclitaxel received FEC100. When any axillary node was positive for cancer after curative breast surgery, additional chemotherapy was used. Patients pretreated with paclitaxel received FEC100. Patients pretreated with FEC100 received docetaxel. wPAC × 12, 12 cycles of weekly paclitaxel 80 mg/m2; FEC100 × 4, four cycles of combination chemotherapy with fluorouracil 500 mg/m2, epirubicin 100 mg/m2 and cycrophosphamide 500 mg/m2 every 3 weeks; DOC75 × 4, four cycles of docetaxel 75 mg/m2 every 3 weeks.
Unless their disease progressed, patients were treated with 12 weeks of paclitaxel or four cycles of FEC100 and then underwent standard surgery. When any axillary node was positive for cancer, additional chemotherapy was used after surgery. Patients pretreated with paclitaxel received FEC100 and those pretreated with FEC100 (diagnosed as insensitive to paclitaxel) received docetaxel after surgery. In a partial resection of the breast, radiation was performed. If cancer was positive in four or more axillary nodes, prophylactic radiation was performed to the chest and regional nodes. Radiation was applied after completion of chemotherapy. In cases with positive estrogen receptor and/or progesterone receptor, appropriate endocrine treatment of tamoxifen or aromatase inhibitors was used after completion of chemotherapy. Patients with HER2‐overexpressed breast cancer received tri‐weekly trastuzumab at a dose of 8 mg/kg followed by 6 mg/kg for 1 year after completion of surgery or postsurgical chemotherapy. Adjuvant trastuzumab was used subsequent to February 2008, which was the approval date in Japan.
Treatment. Paclitaxel was administrated at a dose of 80 mg/m2 as an intravenous infusion over a period of 1 h every week for 12 weeks. Dexamethasone 10 mg, ranitidine 50 mg and granisetoron 3 mg were given intravenously 30 min before paclitaxel. Diphenhydramine 50 mg was given orally just before infusion. FEC100 consisting of fluorouracil (500 mg/m2), epirubicin (100 mg/m2) and cyclophosphamide (500 mg/m2) was administered intravenously every 3 weeks for four cycles. Dexamethasone 20 mg and granisetoron 3 mg were given intravenously before FEC100. Docetaxel was intravenously administrated at a dose of 75 mg/m2 for a 1 h infusion every 3 weeks for four cycles. Dexamethasone 8 mg was given as an intravenous infusion on day 1 followed by oral intake on days 2 and 3.
End‐points. The primary end‐point targeted improvement of the pathological response rate (pRR) as the percentage of patients with grade 2 and 3 as shown by sensitivity testing. The pathological response with grade 2 or 3 was defined as more than a two‐third reduction in invasive lesions or complete disappearance of tumors, including intraductal lesions, respectively.( 17 ) Secondary end‐points examined the pathological complete response rate (probability of pathological response with grade 3), clinical response rate by the Response Evaluation Criteria in Solid Tumors guidelines (RECIST),( 17 ) breast conservation rate, disappearance rate of axillary node metastasis, distant‐metastasis‐free survival, disease‐free survival and overall survival. Adverse events and laboratory parameters were graded according to the National Cancer Institute, Common Toxicity Criteria, version 3.0.
Statistical analysis. Validity was defined as accuracy of the prediction system for sensitivity testing. Improvement of the pathological response was judged as high accuracy of the prediction system for sensitivity testing. The pathological response rates to paclitaxel in patients who were diagnosed as positive by sensitivity testing were compared with those in patients treated with paclitaxel who did not receive sensitivity testing. The difference in response rate in the two groups was assessed by the Fisher exact test for 2 × 2 contingency tables. The pathological response rate in the experimental arm was estimated as 80% compared with 30% in the control arm, which was calculated from 29% (15/51) of the pathological response rate in previous unpublished data. A sample size of 21 assessable patients in each arm (A and B1) was required to achieve 90% power with 5% error (two sided). A sample size of arm B (B1 + B2) required 72 (21 × 100/29) patients. The number of cases that dropped out for any reason including inadequate sampling was estimated as 15%. A total of 109 patients were required in the current study. Patients were randomly assigned to receive arm A or B with a ratio of 1:4. An interim analysis was planned when at least 10 pathological assessable patients were obtained in arm B1. Disease‐free survival and overall survival were calculated by the Kaplan–Meier method.
Results
Patient characteristics. Ninety‐two patients were registered and assessed between February 2006 and February 2009 at the Cancer Institute Hospital. Six patients had too few tumor specimens to evaluate sensitivity testing. Eighty‐six patients were randomized. In two patients, we were not able to assess the pathological response in the resected breast tumors, because of progression during primary chemotherapy and a withdrawal of consent to additional post‐surgical chemotherapy. A total of 85 patients were assessed for pathological response at surgery. The median follow‐up time of patients was 40.0 months, and the range was 17.0–49.8 months. All patients were Japanese women. The demographic characteristics of the present study population are presented in Table 2. The median age was 52.5 years (range, 31–68). Median size of tumor estimated as an invasive lesion was 3.75 cm (range, 3.0–9.9). While 81% of patients were T2, 41% of patients had no clinical axillary lymph node metastasis. Histology showed papillotubular carcinoma (8%), solid‐tubular carcinoma (24%) or scirrhous carcinoma (65%). In 24% of patients, we found nuclear grade 3. Estrogen receptor or PgR was positive in 71% or 47% of patients, respectively. Positive HER2 status was defined as immunohistological (Hercep test) score 3+ (>10%) or FISH positive (ratio >2.0). Twenty‐one percent of patients were HER2 positive. Intrinsic subtypes were divided as follows. Luminal A was defined as negative HER2 status with ER positive and/or PgR positive. Luminal B was defined as positive HER2 status with ER positive and/or PgR positive. HER2 subtype was positive HER2 status with both ER and PgR negative. Triple negative was HER2 negative, ER negative and PgR negative. Luminal A, Luminal B, HER2 subtype or triple negative was 64%, 8%, 13% or 15%, respectively. The background of arms A and B (B1 + B2) was mostly balanced except for a slight tendency towards more patients with papillotubular carcinoma, HER2 positive or luminal B, and fewer patients with grade 3 in arm A. The background in arms A and B1 was different because of selection by sensitivity testing.
Table 2.
Patient characteristics
| Sensitivity testing | A | B1 | B2 | Subtotal of patients in B (B1 + B2) | All patients |
|---|---|---|---|---|---|
| Not performed | Performed | ||||
| Sensitive to paclitaxel | Insensitive to paclitaxel | ||||
| Treatment | Paclitaxel | Paclitaxel | FEC100 | ||
| No. randomized patients | 19 | 11 | 56 | 67 | 86 |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Median age | 50 | 57.0 | 52.0 | 53.0 | 52.5 |
| T | |||||
| T2 | 14 (74) | 8 (73) | 48 (86) | 56 (84) | 70 (81) |
| T3 | 4 (21) | 3 (27) | 5 (9) | 8 (12) | 12 (14) |
| T4 | 1 (5) | 0 (0) | 3 (5) | 3 (4) | 4 (5) |
| Median size (cm) | 3.6 | 4.2 | 3.6 | 3.8 | 3.75 |
| Range (cm) | 3.0–5.7 | 3.0–5.8 | 3.0–9.9 | 3.0–9.9 | 3.0–9.9 |
| N | |||||
| 0 | 7 (37) | 4 (36) | 24 (43) | 28 (42) | 35 (41) |
| 1 | 11 (58) | 6 (55) | 31 (55) | 37 (55) | 48 (56) |
| 2 | 1 (5) | 1 (9) | 1 (2) | 2 (3) | 3 (3) |
| Stage | |||||
| IIA | 7 (37) | 4 (36) | 25 (45) | 29 (43) | 36 (42) |
| IIB | 7 (37) | 4 (36) | 23 (41) | 27 (40) | 34 (40) |
| IIIA | 4 (21) | 3 (27) | 6 (11) | 9 (13) | 13 (15) |
| IIIB | 1 (5) | 0 (0) | 2 (3) | 2 (3) | 3 (3) |
| Histology | |||||
| Invasive ductal carcinoma | |||||
| Papillotubular carcinoma | 3 (16) | 0 (0) | 4 (7) | 4 (6) | 7 (8) |
| Solid tubular carcinoma | 4 (21) | 6 (55) | 11 (20) | 17 (25) | 21 (24) |
| Scirrhous carcinoma | 11 (58) | 5 (45) | 40 (71) | 45 (67) | 56 (65) |
| Others | 1 (5) | 0 (0) | 1 (2) | 1 (1) | 2 (2) |
| Nuclear grade | |||||
| 1 | 10 (53) | 1 (9) | 34 (61) | 35 (52) | 45 (52) |
| 2 | 5 (26) | 3 (27) | 11 (20) | 14 (21) | 19 (22) |
| 3 | 4 (21) | 7 (64) | 10 (18) | 17 (25) | 21 (24) |
| Undetermined | 0 (0) | 0 (0) | 1 (2) | 1 (1) | 1 (1) |
| Estrogen receptor | |||||
| Positive | 12 (63) | 2 (18) | 47 (84) | 49 (73) | 61 (71) |
| Negative | 7 (37) | 9 (82) | 9 (16) | 18 (27) | 25 (29) |
| Progesterone receptor | |||||
| Positive | 10 (53) | 0 (0) | 30 (54) | 30 (45) | 40 (47) |
| Negative | 9 (47) | 11 (100) | 26 (46) | 37 (55) | 46 (53) |
| HER2 | |||||
| Positive (IHC 3+ or FISH+) | 6 (32) | 4 (36) | 8 (14) | 12 (18) | 18 (21) |
| Negative | 13 (68) | 7 (64) | 48 (86) | 55 (82) | 68 (79) |
| Intrinsic subtype | |||||
| Luminal A | 10 (53) | 2 (18) | 43 (77) | 45 (67) | 55 (64) |
| Luminal B | 3 (16) | 0 (0) | 4 (7) | 4 (6) | 7 (8) |
| HER2 subtype | 3 (16) | 4 (36) | 4 (7) | 8 (12) | 11 (13) |
| Triple negative | 3 (16) | 5 (45) | 5 (9) | 10 (15) | 13 (15) |
FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor type 2; IHC, immunohistochemistry.
Pathological response and clinical outcome. Interim analysis was performed after 11 patients were assessable for pathological response in arm B1. As shown in Table 3, the patients in arm B1, diagnosed as sensitive to paclitaxel, demonstrated 36.4% (4/11) of the pathological response rate, whereas patients who did not use sensitivity testing of paclitaxel showed 21.1% (4/19). The difference between arms A and B1 was not significant (P = 0.627). Since the pathological response rate (36.4%) of the experimental arm (B1) was far below the expected rate of 80% despite achievement with 82% (89/109) of the planned accrual number, the committee decided to terminate the study. In arm B2, the patients who were treated with FEC100 judged as insensitive to paclitaxel showed 12.5% (7/56) of the response rate. A pathological complete response was seen in 3.6% (2/56) of FEC100 (B2), but no complete response in the paclitaxel arms (A1 or B1). Pathological metastasis in resected lymph nodes at surgery was absent in 27% (3/11) of arm B1, in which the mean number of pathological positive nodes was 3.8. In one out of four pathological responders with grade 2 and 3, all axillary nodes disappeared. The clinical response rate of the paclitaxel‐sensitive group (B1) was not improved at 55% (6/11) as compared with 53% (10/19) of the control arm (A) or 54% (30/56) in patients who were treated with FEC100 (B2). The breast conservation rate was not improved at 36% in arm B1, compared with arm A (32%) or arm B2 (52%). Disease‐free survival and overall survival at 3 years in all patients (n = 86) were 81.2% and 94.6%, respectively (Fig. 2). Disease‐free survival at 3 years in arms A, B1 and B2 was 72.3%, 62.3% and 87.6%, respectively. Adverse events are summarized in Table 4. One patient, who dropped out after five cycles of preoperative paclitaxel, was excluded to evaluate toxicity. A total of 85 patients were assessed for toxicity. Grade 3 or 4 of adverse events in the preoperative paclitaxel (A + B1) or FEC100 (B2) was 1.0% and 8.1%, respectively. There was no difference in the profile of adverse events between arms A and B (data not shown). No unexpected adverse events were observed.
Table 3.
Response and clinical outcome
| Sensitivity testing | A | B1 | B2 | Subtotal of patients in B (B1 + B2) (n = 67) | All patients (n = 86) |
|---|---|---|---|---|---|
| Not performed | Performed | ||||
| Sensitive to paclitaxel | Insensitive to paclitaxel | ||||
| Treatment | Paclitaxel (n = 19) | Paclitaxel (n = 11) | FEC100 (n = 56) | ||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Pathological response Grade 2 + Grade 3 | 4 (21.1) | 4 (36.4) | 7 (12.5) | 11 (16.4) | 15 (17.4) |
| Pathologically free metastasis in resected lymph nodes | 7 (36.8) | 3 (27) | 26 (46.4) | 29 (43.2) | 36 (41.8) |
| Mean no. pathological positive nodes | 3.9 | 3.8 | 2.2 | 2.5 | 2.8 |
| Pathological disappearance of axillary nodes in pathological responders (grade 2 + 3) | 3 (75) | 1 (25) | 5 (71) | 6 (55) | 9 (60) |
| Clinical response (RECIST) CR + PR | 10 (53) | 6 (55) | 30 (54) | 36 (54) | 46 (54) |
| Breast conservation | 6 (32) | 4 (36) | 29 (52) | 33 (49) | 39 (45) |
RECIST, response evaluation criteria in solid tumors.
Figure 2.
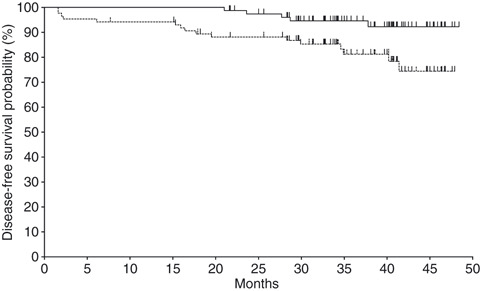
Kaplan–Meier plot for disease‐free survival (dashed line) and overall survival (solid line) in all randomized patients (n = 86).
Table 4.
Adverse events following preoperative chemotherapy
| Paclitaxel (n = 29) | FEC100 (n = 56) | |||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Anorexia | 6 (21) | 0 | 0 | 25 (45) | 1 (2) | 0 |
| Fatigue | 22 (76) | 1 (3) | 1 (3) | 44 (79) | 2 (4) | 0 |
| Nausea | 7 (24) | 0 | 0 | 36 (64) | 0 | 0 |
| Vomiting | 2 (7) | 0 | 0 | 23 (41) | 4 (7) | 0 |
| Diarrhea | 11 (38) | 0 | 0 | 13 (23) | 3 (5) | 0 |
| Constipation | 15 (52) | 0 | 0 | 31 (55) | 1 (2) | 0 |
| Mucositis | 0 (0) | 0 | 0 | 13 (23) | 1 (2) | 0 |
| Dysgeusia | 2 (7) | 0 | 0 | 4 (7) | 0 | 0 |
| Peripheral neuropathy | 26 (90) | 1 (3) | 0 | 16 (29) | 0 | 0 |
| Alopecia | 29 (100) | NA | NA | 56 (100) | NA | NA |
| Hand‐foot syndrome | 0 (0) | 0 | 0 | 2 (4) | 0 | 0 |
| Rash | 3 (10) | 0 | 0 | 1 (2) | 0 | 0 |
| Allergic reaction | 1 (3) | 0 | 0 | 0 | 0 | 0 |
| Itching | 1 (3) | 0 | 0 | 0 | 0 | 0 |
| Phlebitis | 0 (0) | 0 | 0 | 1 (2) | 0 | 0 |
| Myalgia | 2 (7) | 0 | 0 | 0 | 0 | 0 |
| Infection | 2 (7) | 0 | 0 | 13 (23) | 5 (9) | 2 (4) |
| Febrile neutropenia | 0 (0) | 0 | 0 | 7 (13) | 2 (4) | 0 |
| Leukopenia | 19 (65) | 1 (3) | 0 | 53 (95) | 28 (50) | 15 (27) |
| Neutropenia | 14 (48) | 3 (10) | 0 | 53 (95) | 2 (4) | 49 (88) |
| Anemia | 15 (52) | 0 | 0 | 37 (66) | 2 (4) | 0 |
| Thrombocytopenia | 0 (0) | 0 | 0 | 3 (5) | 1 (2) | 0 |
| AST elevation | 15 (52) | 0 | 0 | 23 (41) | 0 | 0 |
| ALT elevation | 13 (45) | 1 (3) | 0 | 22 (39) | 0 | 0 |
| Total bilirubin elevation | 3 (10) | 0 | 0 | 3 (5) | 0 | 0 |
| Creatinine elevation | 1 (3) | 0 | 0 | 1 (2) | 0 | 0 |
| Hyperglycemia | 3 (10) | 0 | 0 | 14 (25) | 0 | 0 |
| All events | 212 (27.0) | 7 (0.9) | 1 (0.1) | 494 (32) | 52 (3.6) | 66 (4.5) |
ALT, alanine transaminase; AST, aspartate transaminase; NA, not applicable.
Discussion
The current study failed to validate the sensitivity of testing using the expression of five genes. However, we became aware of the importance of deciding how to incorporate a new biomarker into clinical practice. Evidence levels of a biomarker are commonly derived from retrospective studies,( 18 , 19 ) which harbor strong bias due to differing backgrounds. A large cohort or meta‐analysis is mandatory to establish usefulness. Prospective trials to evaluate biomarkers have rarely been reported. Simon and simon et al. have proposed a refined guideline system for biomarker studies.( 20 , 21 ) The guideline indicates that level 1 evidence may permit reproducible positive results from high‐quality retrospective studies using archived specimens in the prospective trials addressing therapeutic questions, but not biomarkers. However, a prospective trial that would directly address biomarkers is still the gold standard to achieve level 1 evidence. Designing randomized trials for biomarkers presents several challenges.( 21 ) One involves the therapeutic question of accommodation of biomarkers, such as the Tailor X trial of the 21‐gene classifiers. The other involves the biomarker question, such as microarray testing of the 70‐gene classifier. However, these trials require a large number of patients to arrive at a definitive conclusion. It is difficult to conduct such a large trial for all possible biomarkers. A relatively smaller number of patients, approximately 100 like in this study, could be reasonable for evaluation in biomarker study design.
The current study failed to enrich responsive patients to treatment with paclitaxel. We expected that prediction of a pathological response would be more than 80% of sensitivity in the new testing. Unexpectedly, the pathological response of the experimental arm was as low as 36.4%. Since the pathological response rate, 21.1%, of the control arm was also lower than 30% as expected, performing the interim analysis in this study took a long time. We decided to terminate the study because we considered that the enrichment of response by sensitivity testing should be minimally more than 50% for clinically meaningful usage or further evaluation by a randomized large phase III study. We did not plan to address the specificity of gene testing, because the specificity could not be yielded from the data of arm B (B1 + B2). The reason was that patients in arm B2, who were judged as insensitive to paclitaxel, did not receive paclitaxel from an ethical point of view. However, we were able to examine the specificity of gene testing in arm A. In arm A (n = 19), 18 patients could be evaluated by gene testing, because of one sampling that contained no cancer cells. Twelve out of 15 patients who failed to obtain a pathological response exhibited as insensitive to paclitaxel by the gene testing. Therefore, the specificity resulted in 80% (12/15). One out of three patients who achieved a pathological response were revealed as sensitive to paclitaxel by the testing. The sensitivity of arm A resulted in 33.3% (1/3), which was similar to that of arm B1 (36.4%, 4/11). The present study aimed to examine whether the gene testing improved sensitivity, but not specificity. The number was too small to obtain a definitive result of specificity. The current study failed to show an enhanced response rate. However, if we conducted a phase II study with a single arm, we would not have been able to obtain such a clear conclusion as early as we did. Therefore, a small randomized study appears to be a robust tool in obtaining a rapid conclusion to evaluate the usefulness of biomarkers.
The methodology of this randomized trial might need further discussion. We wanted to determine whether the selection of patients by new testing could be useful. Thus, we considered that the selection by itself should be randomized. Namely, we compared the outcome for patients who were selected by testing with that of patients who were not selected. This is different from randomized trials that compare a new treatment with a standard therapy. Unbalanced randomization at a 1:4 ratio would minimize the number of patients in control arm A who were not selected by testing. Patients who wished to receive extensive, maximal primary chemotherapy did not enter this trial. One patient withdrew from this trial during her primary chemotherapy because she wanted to receive additional primary chemotherapy. Patients who wished to receive minimal chemotherapy were likely to participate in this trial. Patients with incomplete clearance of axillary tumors could receive additional chemotherapy after surgery. This ethical issue was discussed and approved by institutional review board.
In the current study, the clinical response rate and the conservation rate of the breast were 55% and 36%, respectively, with 27% of patients free from pathological metastasis in resected lymph nodes. These results were not satisfactory. However, the study did not aim to improve breast conservation and clearance of axillary metastasis, but rather aimed to minimize exposure to cytotoxic chemotherapy for those sensitive to chemotherapy. Patients with axillary nodes involved were treated by adding adjuvant alternative chemotherapy with FEC100 or docetaxel. The results of disease‐free survival and overall survival (81.2% and 94.6% at 3 years) were not assessable for further analysis. The safety profile of paclitaxel or FEC100 was similar to previous reports.( 3 , 4 , 5 ) Both treatments were manageable.
In conclusion, the current study failed to validate sensitivity testing using five‐gene expression for primary chemotherapy with paclitaxel in patients with breast cancer. However, a small prospective randomized study is useful for reaching a rapid conclusion on the usefulness of biomarkers. We consider that the present trial design is a prospective randomized phase II trial directly addressing the predictive biomarker question. The current compact trial could be a hallmark to proceed to further large clinical phase III trials.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by funding from the Ministry of Economy, Trade and Industry. The authors thank the participating medical doctors in our institute: Kokoro Kobayashi, Shinichiro Taira, Chizuko Tsutsumi, Tsutomu Sugihara, Masafumi Oto, Mayuko Ito, Takayo Fukuda, Mari Hosonaga, Masaya Hattori, Hiroyoshi Miura, Taijiro Kosaka, Akiko Kuwayama, Rikako Hashimoto, Kenichi Inoue, Yoshie Nakayama, Yoko Chihara, Ippei Fukada, Natsue Uehiro, Aya Hirano, Yumi Miyagi, Seiichiro Nishimura, Kotaro Iijima, Kiyomi Kimura, Hidetomo Morizono, Takehiko Sakai, Reiko Iwasaki, Yoshimi Ide, Tomo Osako, Naoya Gomi, Keiko Nemoto, Akashi Toshiyasu and Masahiko Oguchi. The authors also thank the data managers: Chie Watanabe, Masumi Yamazaki, Chizuru Suizu, Michiko Yago, Kumiko Yamabe and Kaori Kobayashi.
Name of the trial register: Validation of genetic diagnosis to predict sensitivity in primary systemic chemotherapy with paclitaxel in women with breast cancer.
Registration number: C000000413, UMIN Clinical Trials Registry.
References
- 1. Dahabreh IJ, Linardou H, Siannis F et al. Trastuzumab in the adjuvant treatment of early‐stage breast cancer: a systematic review and meta‐analysis of randomized controlled trials. Oncologist 2008; 13: 620–30. [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–717. [DOI] [PubMed] [Google Scholar]
- 3. Untch M, von Minckwitz G. Recent advances in systemic therapy: advances in neoadjuvant (primary) systemic therapy with cytotoxic agents. Breast Cancer Res 2009; 11: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estévez LG, Gradishar WJ. Evidence‐based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res 2004; 10: 3249–61. [DOI] [PubMed] [Google Scholar]
- 5. Horii R, Akiyama F, Ito Y, Matsuura M, Miki Y, Iwase T. Histological features of breast cancer, highly sensitive to chemotherapy. Breast Cancer 2007; 14: 393–400. [DOI] [PubMed] [Google Scholar]
- 6. Szakács G, Paterson JK, Ludwig JA et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006; 5: 219–34. [DOI] [PubMed] [Google Scholar]
- 7. Hasegawa S, Miyoshi Y, Egawa C et al. Mutational analysis of the class I beta‐tubulin gene in human breast cancer. Int J Cancer 2002; 101: 46–51. [DOI] [PubMed] [Google Scholar]
- 8. Tommasi S, Mangia A, Lacalamita R et al. Cytoskeleton and paclitaxel sensitivity in breast cancer: the role of beta‐tubulins. Int J Cancer 2007; 120: 2078–85. [DOI] [PubMed] [Google Scholar]
- 9. Pusztai L, Jeong JH, Gong Y et al. Evaluation of microtubule‐associated protein‐Tau expression as a prognostic and predictive marker in the NSABP‐B 28 randomized clinical trial. J Clin Oncol 2009; 27: 4287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perou CM, Jeffrey SS, van de Rijn M et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA 1999; 96: 9212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perou CM, Sorlie T, Eisen MB et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–52. [DOI] [PubMed] [Google Scholar]
- 12. Hedenfalk I, Duggan D, Chen Y et al. Gene‐expression profiles in hereditary breast cancer. N Engl J Med 2001; 344: 539–48. [DOI] [PubMed] [Google Scholar]
- 13. Sorlie T, Perou CM, Tibshirani R et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van ‘t Veer LJ, Dai H, van deVijver MJ et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415: 530–6. [DOI] [PubMed] [Google Scholar]
- 15. Nagasaki K, Miki Y. Gene expression profiling of breast cancer. Breast Cancer 2006; 13: 2–7. [DOI] [PubMed] [Google Scholar]
- 16. Nagasaki K, Miki Y. Molecular prediction of the therapeutic response to neoadjuvant chemotherapy in breast cancer. Breast Cancer 2008; 15: 117–20. [DOI] [PubMed] [Google Scholar]
- 17. Sakamoto G, Inaji H, Akiyama F et al. General rules for clinical and pathological recording of breast cancer 2005. Breast Cancer 2005; 12 (Suppl): S1–27. [PubMed] [Google Scholar]
- 18. McShane LM, Altman DG, Sauerbrei W et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 2005; 23: 9067–72. [DOI] [PubMed] [Google Scholar]
- 19. Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J Clin Oncol 2009; 27: 4027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simon R. The use of genomics in clinical trial design. Clin Cancer Res 2008; 14: 5984–93. [DOI] [PubMed] [Google Scholar]
- 21. Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009; 101: 1446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


