Abstract
Introduction
The German Registry of central serous chorioretinopathy (CSC) collects data on CSC patients in a nationwide multicenter approach to analyze epidemiology, risk factors, clinical presentations, as well as diagnosis and treatment patterns.
Methods
In this multicenter cohort study, patients with CSC were enrolled in nine tertiary referral centers in Germany between January 2022 and June 2023. After consenting to the study, demographic data, risk factors, reported symptoms, best-corrected visual acuity (BCVA), funduscopic findings, disease severity, and diagnostic and treatment decisions were recorded and analyzed.
Results
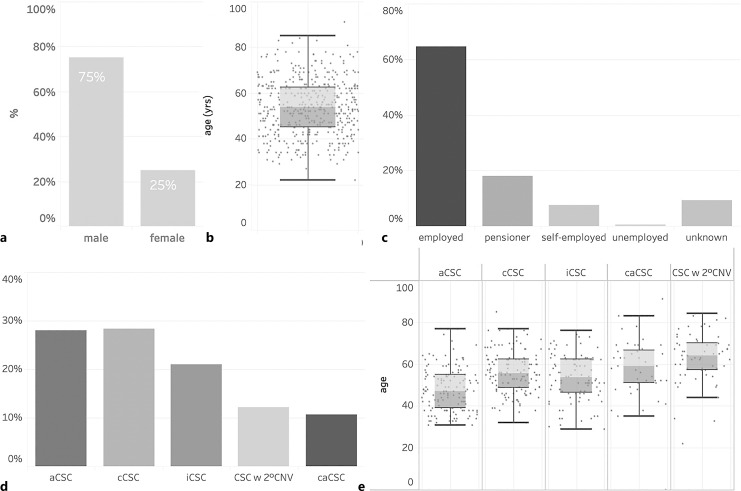
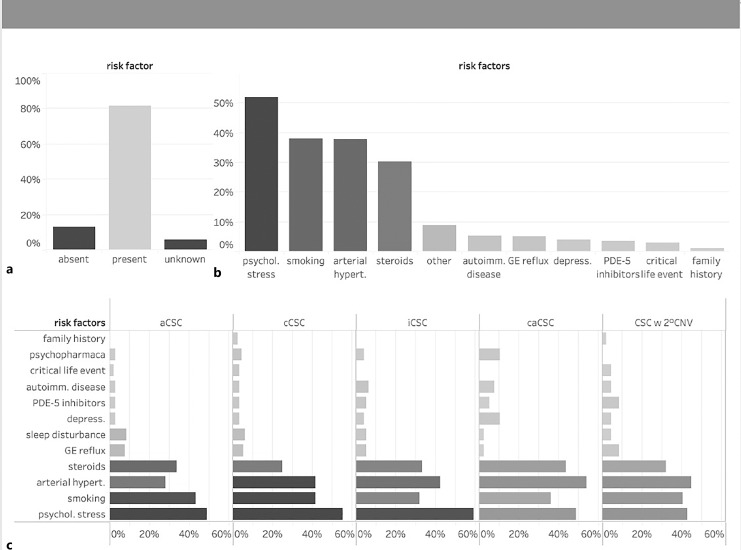
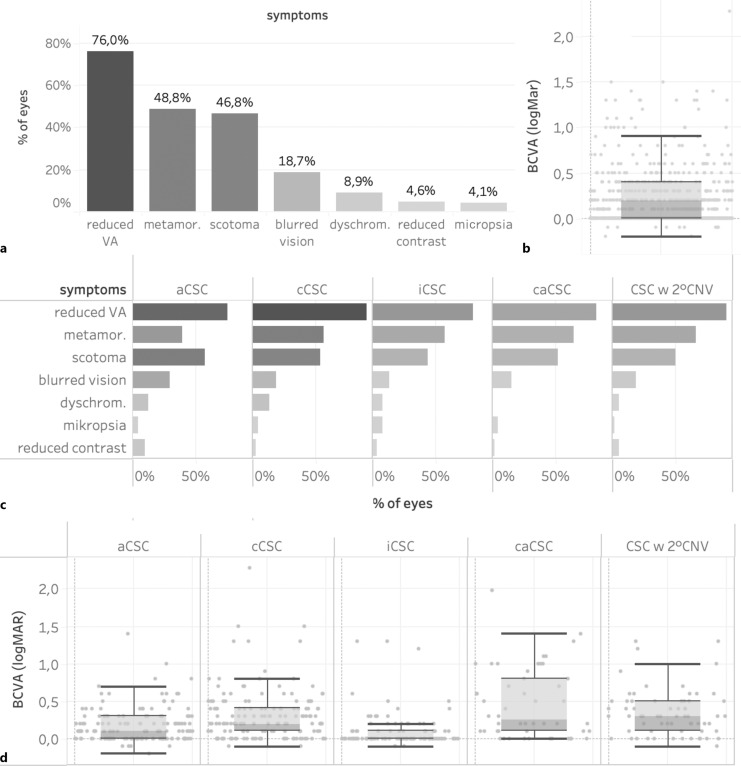
A total of 539 eyes of 411 CSC patients were enrolled in this study including 308 males (75%) and 103 females (25%). Patients were predominantly of Caucasian origin and had a mean age of 55.5 years (IQR 41.0–70.0). 28% of eyes were classified as acute (<4 months duration) CSC, 28% as chronic (>4 months duration) CSC, 21% as inactive CSC, 11% as chronic atrophic CSC, and 12% as CSC with secondary CNV. 128 patients (31%) demonstrated bilateral CSC. The most common risk factors reported were psychological stress (52%), smoking (38%), arterial hypertension (38%), and a history of or current use of steroids (30%). Most frequently encountered symptoms included decreased visual acuity (76%), metamorphopsia (49%), relative scotoma (47%), blurred vision (19%), and dyschromatopsia (9%). The mean logMAR BCVA on initial examination was 0.2 (≈20/30, IQR 0.2–0.4) but showed significant variation with a tendency of lower BCVA in chronic cases. At the baseline visit, 74% of the overall cohort received no treatment, while 19% underwent local treatment and only 2% underwent systemic treatment. Of the local therapies, anti-VEGF injections were the most frequently performed procedure (33%, mainly for secondary CNV), followed by micropulse laser (28%), focal nonpulsed laser (23%), verteporfin photodynamic therapy (14%), and nonsteroidal anti-inflammatory eye drops (2%). Among intravitreal anti-VEGF agents, aflibercept was used most frequently, followed by bevacizumab and ranibizumab.
Conclusion
This registry represents one of the largest cohorts of European patients with CSC to date. Patient age and the proportion of women were higher than expected and bilateral active disease was lower than anticipated, highlighting that neither age nor gender should be overemphasized when diagnosing CSC. Therapeutic interventions are heterogeneous and include verteporfin photodynamic therapy, micropulse laser, and anti-VEGF injections in case of secondary CNV.
Keywords: Central serous chorioretinopathy, Registry, Retina.net, Incidence, Demographics, Treatment
Introduction
More than 150 years after its first description [1], central serous chorioretinopathy (CSC) remains an enigmatic disease of unknown etiology, with insufficient therapeutic options and poorly standardized treatment patterns [2–4]. It is characterized by unilateral or bilateral accumulation of subretinal fluid (SRF) in the macular area, leading to scotoma, metamorphopsia, and visual loss. The prognosis of CSC is considered favorable in general, with the resorption of SRF and recovery of visual acuity within 6 months in 84% of acute cases [5]. However, in some cases, SRF can persist for a longer period of time and lead to irreversible atrophy of photoreceptors and the underlying retinal pigment epithelium (RPE) and choriocapillaris, resulting in permanent visual impairment [6, 7]. Initial treatment of the disease usually consists of waiting for spontaneous resolution and, if no improvement is seen, often includes laser treatments or drug therapy. However, the ideal timing of treatment and the best treatment method remain controversial [2–4].
CSC is considered the fourth most common retinopathy after age-related macular degeneration, diabetic retinopathy, and retinal vein occlusion. With an incidence of about 9.9 per 100,000 men and 1.7 per 100,000 women, it is nevertheless considered a rare disease [8]. Due to its heterogeneity including a frequently self-limiting disease course, it remains difficult to perform adequately powered studies on CSC [9]. Since preferred treatment patterns and diagnostic criteria also vary between centers, large multicenter studies are needed to systematically collect and evaluate data on patient demographics, clinical symptoms, multimodality imaging features, disease progression, and outcomes of different treatment options [3].
For this reason, the multicentric Retina.net CSC Registry (www.ccs-register.de) was initiated in Germany, to which all German centers that see CSC patients on a regular basis are invited. The overall aim of this registry is to assess the clinical features and risk factors of CSC patients, to explore the course of the disease in different subgroups over time, and to investigate the relationships between different therapies, their complications, and associated outcomes. This first report summarizes the demographic data, risk factors, reported symptoms, best-corrected visual acuity (BCVA), funduscopic findings, disease severity, and diagnosis and treatment decisions on the day of study inclusion of patients enrolled in the first 18 months of the registry.
Methods
Registry Structure
The German CSC Registry was established under the auspices of the German research association Retina.net as a multicenter database to document the disease course of patients suffering from CSC that were seen at the participating centers. The registry was initiated and was being coordinated by the University of Freiburg, Germany, and the Macular Monitor Münster Institute (M3) at the St. Franziskus Hospital in Münster, Germany. The study is conducted in accordance with the World Medical Association Declaration of Helsinki and ethics approval was obtained from the Ethics Committees of all participating centers prior to participation (leading Ethics Committee: Freiburg University Ethics Committee, Application No. 21-1376). The study is registered with the German Clinical Trials Registry (DRKS00027270), a World Health Organization (WHO)-recognized primary clinical trials registry, which meets the requirements of the International Committee of Medical Journal Editors (ICMJE). Written informed consent was obtained from all participants prior to study inclusion. The registry is noninterventional and only collects data obtained in routine clinical practice of the participating centers without affecting clinical decisions. It is divided into a retrospective part, recording the patient's ocular history, and a prospective, pseudonymized part. This first study report summarizes patient characteristics of included patients at the baseline visit to describe epidemiology, risk factors, diagnosis, and treatment patterns. Retina.net CSC Registry study group members who participated in the study are listed at the end of this article. Further information and instructions for participation can be found on the registry website (www.ccs-register.de).
Main Inclusion and Exclusion Criteria
All CSC patients above age of 18 that were seen at any of the participating centers were eligible for study inclusion. Written informed consent was obtained before study inclusion for each patient. The diagnosis of CSC was based on treating clinician’s discretion and based on previous definitions of the disease [10]. The only exclusion criterion was an unwillingness or inability to give informed consent.
Affected eyes of each included patient were classified by the respective investigator into different CSC subgroups based on a modified definition of previous reports [4, 6, 11], which included the following:
Acute CSC (aCSC): SRF with symptoms shorter than 4 months and no evidence of chronicity such as the significant RPE atrophy.
Chronic CSC (cCSC): persistent SRF for more than 4 months with subtle RPE changes but no evidence of the significant RPE atrophy.
Inactive CSC (iCSC): eyes with a history of CSC but no SRF at the time of inclusion in the study.
CSC with secondary choroidal neovascularization (CSC w 2° CNV): CSC complicated by secondary CNV based on multimodal imaging.
Chronic atrophic CSC (caCSC): eyes with SRF showing extensive RPE atrophy and/or posterior cystoid retinal degeneration.
Patients that did not fit into one of the groups were defined as “others,” while incomplete records of individual eyes were considered “not defined.”
Outcomes
The registry used an electronic case report form (eCRF) specifically set up for this registry to collect demographics and risk factors, subjective symptoms, visual acuity, funduscopic findings, which multimodal imaging was attained, and treatment decisions in all enrolled CSC patients at baseline. No additional visits or examinations were required for participation in the registry due to the noninterventional, real-world data collection. In the current analysis, data of the day of study inclusion of patients enrolled between January 2022 and June 2023 are presented.
Statistics
Data were collected in a standardized fashion based on eCRF forms. Due to the multicentric setting, only descriptive statistics on items included in the eCRF were used. Graphical presentation, data visualization, and statistics were performed using Tableau Desktop, Professional Edition (Tableau, CA, USA). For continuous variables, these are number (n), mean and standard deviation (SD) for normally distributed and median and interquartile range (IQR) for non-normally distributed variables. For categorical variables, frequencies of occurrence are reported.
Results
Patient Demographics
In total, 539 eyes of 411 patients were enrolled in the CSC Registry between January 2022 and June 2023 from 9 participating centers in Germany (Table 1). The study cohort included 308 male (75%) and 103 female (25%) patients. The median patient age at enrollment was 55.5 years (IQR 41.0–70.0 years) and did not differ significantly between men (53 years, IQR 43.5–62.0) and women (57.5 years, IQR 51.0–64.0). The vast majority of patients were of Caucasian origin (95%), and only 3% were of Asian and African-American origin (online suppl. Fig. 1; for all online suppl. material, see https://doi.org/10.1159/000535930). Most patients (62%) were employed, while 17% of patients were retired and 7% were self-employed (Fig. 1c).
Table 1.
Patients’ characteristics
| Group | Patients, n | Eyes, n | Age (median) |
|---|---|---|---|
| Total | 411 | 539 | |
| aCSC | 136 | 149 | 47 |
| cCSC | 132 | 152 | 55 |
| iCSC | 97 | 113 | 53 |
| CSC w 2° CNV | 61 | 65 | 64 |
| caCSC | 43 | 57 | 59 |
| Not defined | 9 | 0 | 53 |
| Others | 2 | 3 | 76 |
aCSC, acute CSC; cCSC, chronic CSC; iCSC, inactive CSC; caCSC, chronic atrophic CSC; CSC w 2° CNV, CSC complicated by secondary choroidal neovascularization.
Fig. 1.
Epidemiology of included patients on the day of informed consent. Male/female distribution (a), boxplot of age distribution within the whole cohort (b), and employment status of participants (c). Distribution (d) and age (e) of different CSC subgroups. aCSC, acute CSC; cCSC, chronic CSC; iCSC, inactive CSC; caCSC, chronic atrophic CSC; CSC w 2° CNV, CSC with secondary CNV.
CSC Classification
Of the total cohort, 149 eyes (28%) of 137 patients were classified as acute CSC and 152 eyes (28%) of 133 patients were classified as chronic CSC. Inactive CSC (i.e., no SRF at the baseline visit) was diagnosed in 113 eyes (21%) of 97 patients, while 65 eyes (12%) of 62 patients had a secondary CNV due to CSC, and 57 eyes (11%) of 43 patients had caCSC (Table 1; Fig. 1d). At baseline, 128 patients (31%) were diagnosed with CSC bilaterally. The median age at enrollment differed significantly between subgroups and was 47.0 (IQR 39.0–55.0) for patients with acute CSC, 55.5 years (IQR 48.5–62.0) for chronic CSC, and 53.5 years (IQR 46.5–62.0) for inactive CSC. Patients with caCSC and CSC complicated by secondary CNV tended to be older, with a median age of 59.0 years (IQR 51.0–66.5) and 64.0 years (IQR 57.0–70.0, Kruskal-Wallis p value across all subgroups <0.00001), respectively.
Risk Factors
Eighty-one percent of patients (n = 367) included in this study reported risk factors for the development of CSC that are known from the literature [12], whereas 12 and 7% of patients denied or were unaware of any risk factors, respectively (Fig. 2a). The most common reported risk factors were psychological stress (52%), smoking (38%, mean of 2 packyears IQR 1.0–5.3 packyears), arterial hypertension (38%), history of or current use of steroids (30%). Less common reported risk factors included gastroesophageal reflux, sleep disturbance, and depression among others (Fig. 2b). 13% of patients reported working night shifts (online suppl. Fig. 1). Risk factors did not differ significantly between all subgroups of CSC patients (Fig. 2c, all p values >0.05). Of note, psychological stress was reported to a similar extent in patients with chronic CSC (55%), inactive CSC (59%), and even caCSC (48%) compared to acute CSC (49%, p = 0.3). Among the group of patients with CSC, 10% of patients reported suffering from depression and received psychopharmaceutic medication, compared with only 3% of patients in the acute CSC group. Arterial hypertension was seen more frequently in patients with chronic CSC (42%), secondary CNV (45%), and caCSC (54%) than in acute caCSC (28%), which could, however, also be explained by the different age distribution across cohorts. A history of steroid use was present in 34% of patients with acute CSC and in 25% of patients with chronic CSC compared with 44% of patients with long-standing caCSC.
Fig. 2.
Risk factor analysis on the day of informed consent. a 82% of patients reported known CSC-related risk factors. b Distribution of risk factors in % for all patients (b) and patients in different subgroups (c). aCSC, acute CSC; cCSC, chronic CSC; iCSC, inactive CSC; caCSC, chronic atrophic CSC; CSC w 2° CNV, CSC complicated by 2° choroidal neovascularization.
Symptoms and Visual Acuity in CSC
The most common symptoms reported were decreased visual acuity (76%), metamorphopsia (49%), relative scotoma (47%), blurred vision (19%), and dyschromatopsia (9%, Fig. 3a). Even patients who were classified as having inactive CSC complained of persisting symptoms including reduced BCVA (80%), scotoma (44%), and metamorphopsia (57%). Reported symptoms seemed to be relatively equally distributed across all subgroups of CSC patients (Fig. 3d). However, patients with chronic CSC (91%) and caCSC (83%) tended to report reduced visual acuity more frequently than patients with acute CSC (75%) and inactive CSC (80%). Patients with 2° CNV, on the other hand, reported symptoms such as decreased visual acuity (91%) and metamorphopsia (64%) more frequently than patients with acute CSC (74 and 39%, respectively, Fig. 3d). Median logMAR BCVA as assessed on the day of initial examination was 0.2 (≈20/32 Snellen equivalent, IQR 0.2–0.4) across all patients but showed significant variation, ranging from −0.1 (≈20/15) to 2.3 (≈ hand motion, Fig. 3b). There was a trend toward poorer median BCVA in chronic CSC compared to acute CSC cases (Fig. 3c). Mean visual acuity was 0.1 logMar (≈20/25, IQR 0.0–0.3) in eyes with acute CSC, 0.0 logMar (≈20/20, IQR 0.0–0.1) in eyes with inactive CSC, 0.2 logMar (≈20/32, IQR 0.1–0.4) in eyes with chronic CSC, 0.25 logMar (≈20/32–20/40, IQR 0.1–0.8) in eyes with caCSC, and 0.3 logMar (≈20/40, IQR 0.1–0.5) in eyes with chronic CSC complicated by a CNV (Kruskal-Wallis p value <0.00001, online suppl. Fig. 2).
Fig. 3.
Presenting symptoms at the time of inclusion and best-corrected visual acuity (BCVA) in all patients (a, b) and in different subgroups (c, d). aCSC, acute CSC; cCSC, chronic CSC; iCSC, inactive CSC; caCSC, chronic atrophic CSC; CSC w 2° CNV, CSC complicated by a secondary choroidal neovascularization.
Clinical Findings and Imaging Modalities
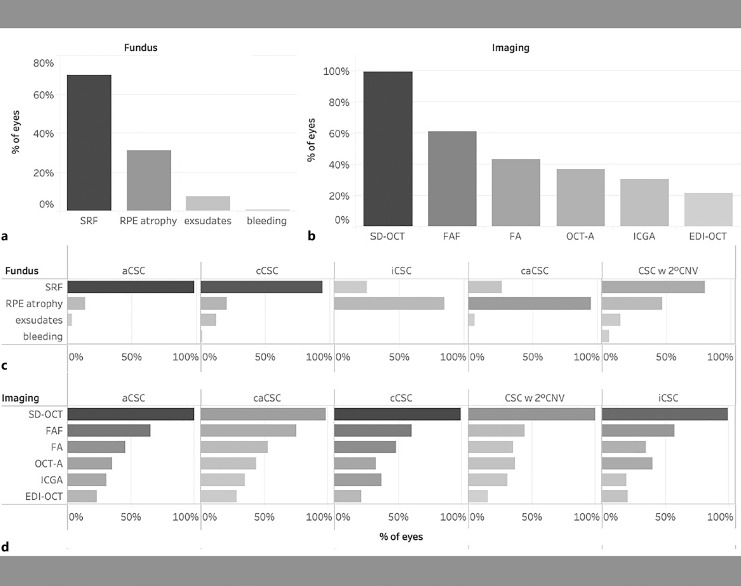
On fundoscopy, SRF and signs of RPE changes and atrophy were recorded by the clinician in 70 and 31% of patients, respectively. Exudates and retinal hemorrhages were observed in only 8 and 1% of patients, respectively (Fig. 4a). When focusing on the different subgroups, while SRF was funduscopically visible on almost all acute CSC patients (98%), it was seen less frequently in caCSC (26%) and inactive CSC (25%). Exudates were equally distributed across all subgroups except inactive CSC, while a retinal hemorrhage was only reported in 1 cCSC patient and 2 patients with secondary CNV. RPE atrophy was rarely reported in acute CSC (14%) and often reported in caCSC (95%), which corresponds to the current definition of these disease entities. Interestingly, a high proportion of iCSC patients (84%) showed signs of RPE atrophy, which may indicate persisting RPE damage even after SRF resolution.
Fig. 4.
Fundus findings and imaging modalities performed for all patients (a, b) and in different subgroups (c, d). SRF, subretinal fluid; RPE, retinal pigment epithelium; SD-OCT, spectral-domain optical coherence tomography; FAF, fundus autofluorescence; FA, fluorescein angiography; OCT-A, optical coherence tomography angiography; ICGA, indocyanine green angiography; EDI-ODT, enhanced-depth imaging optical coherence tomography; aCSC, acute CSC; cCSC, chronic CSC; iCSC, inactive CSC; caCSC, chronic atrophic CSC; CSC w 2° CNV, CSC complicated by secondary choroidal neovascularization.
Among all participating centers, the most common imaging at baseline was SD-OCT, which was performed in almost all patients (99%), while the second most common imaging was Fundus autofluorescence, which was performed in 61% of patients. Fluorescein angiography (43%) and OCT-angiography (37%) were performed in a minority of patients, while indocyanine green angiography was performed less frequently (30%). Although CSC is considered a part of the pachychoroid disease spectrum, enhanced-depth imaging-OCT, which provides better visualization of the choroid, was used relatively infrequent (22%, Fig. 4d).
Treatment Decisions
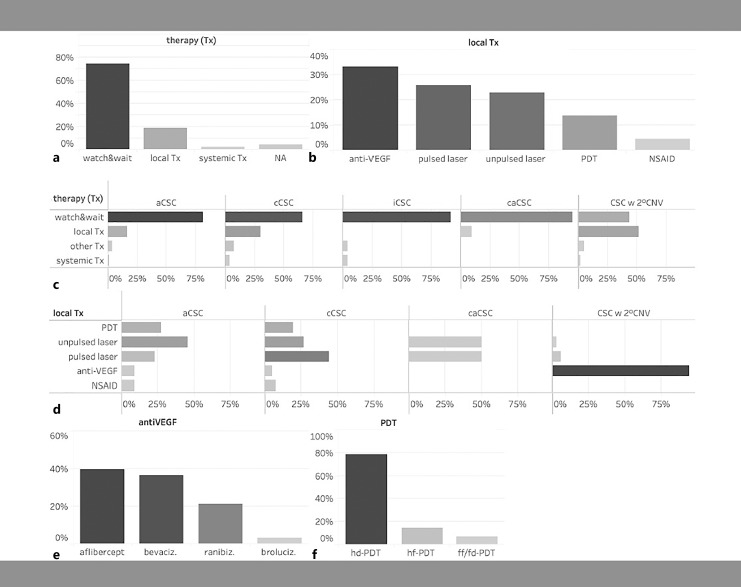
On the day of enrollment, 74% of the total cohort received no treatment and a watch-and-wait strategy was recommended, while 19% received local treatment and only 2% received systemic treatment. A watch-and-wait strategy was particularly advised in patients with acute CSC (81%), inactive CSC (92%), and caCSC (95%), whereas patients with chronic CSC and CSC complicated by 2° CNV were more likely to receive local treatments in 30 and 52% of cases, respectively. The most common local therapies included anti-VEGF injections (33% of all local therapies), micropulse lasers (26%), focal nonpulsed lasers (23%), verteporfin photodynamic therapy (14%), and nonsteroidal anti-inflammatory eye drops (5%). As expected, anti-VEGF injections were by far the most common treatment for secondary CNV, whereas they were rarely (<10%) used in the other subgroups. Surprisingly, caCSC patients did not receive treatment more often than other groups, possibly indicating the more complex treatment decisions in this group (Fig. 5d). Among anti-VEGF agents, aflibercept was used most frequently, followed by bevacizumab and ranibizumab injections, whereas brolucizumab was used less frequently (Fig. 5e). Verteporfin photodynamic therapy was most commonly used as half-dose PDT, followed by half-fluence, and only in a single case as full-dose and -fluence PDT (Fig. 5f).
Fig. 5.
Therapeutic decisions on the day of study inclusion for all patients (a) and different CSC subgroups (c). Local treatment decision on the day of study inclusion for all patients (b) and different CSC subgroups (d). Anti-VEGF drugs (e) and type of verteporfin photodynamic therapy (f) applied. Tx, treatment; VEGF, vascular endothelial growth factors; NSAID, nonsteroidal anti-inflammatory drug; aCSC, acute CSC; cCSC, chronic CSC; iCSC, inactive CSC; caCSC, chronic atrophic CSC; CSC w 2° CNV, CSC complicated by a secondary choroidal neovascularization; hd, half-dose; hf, half-fluence; ff/fd, full-fluence, full-dose.
Discussion
Even though CSC was first described in 1886 and has been studied extensively for over 100 years, controversy remains regarding its pathogenesis, classification, and therapy. Due to the low incidence of about 1:10,000 and the often favorable natural course of the disease, multicenter registries are needed to collect sufficient patient numbers to study CSC. To the best of our knowledge, there is only one other CSC registry study from Hong Kong that is currently recruiting patients (NCT05278169). Our cohort thus adds to the current evidence base 1 of the largest patient cohorts to date with predominantly European ancestry. Since CSC incidence and possibly pathogenesis can differ between the Asian and European populations, these differences can be important when interpreting our results.
The Retina.net CSC Registry was established as a collaboration of large German tertiary referral centers that specialize in CSC to capture data on patient demographics, medical history, diagnostic work-up, and therapeutic decisions in a standardized way. Both the initial visit as well as all follow-up visits can be recorded. The first results published in this report focus on the data documented at the patient’s initial visit. Due to the referral structure in Germany, this may lead to fewer patients with acute, self-limiting disease being included in this report because these patients do not always reach a tertiary treatment center. This may explain some of the differences seen between our data and previously published reports on CSC cohorts.
Regarding patient demographics, three factors differ significantly between our report and previously published cohorts. First, while almost all reports show a male preponderance in CSC with a male to female ratio of between 5:1 and 6:1 in predominantly Caucasian cohorts [8, 13], our study finds a gender ratio of 3:1, which is more similar to previous reports on a South Korean cohort (3.4:1) [14]. Second, CSC is often regarded as a disease affecting patients between 40 to 50 years of age [4, 15]. The mean age in our study, however, was 54 (SD 12.3) years and included patients between the ages of 22 and up to 91 years of age. While this does not necessarily mean that patients had their initial CSC diagnosis at such a relatively old age, it emphasizes that CSC can remain an issue throughout life and it not only affects males in mid-life. Third, the previously reported higher mean age of women with CSC compared to men affected by the disease [16] was not evident in our data (online suppl. Fig. 1), which may be due to the higher age of male participants compared to previously reported cohorts. Overall, our data highlight that neither age nor gender should be overemphasized when diagnosing CSC.
The phenotypic variability of CSC is reflected in the distribution of CSC subgroups in this study. Remarkably, acute CSC was diagnosed in only a minority of patients (28%) and was similar to the percentage of patients diagnosed with chronic CSC (28%). As most of the participating centers were tertiary referral centers, the proportion of acute CSC cases was most likely underestimated, as many acute CSC patients may be managed by their primary ophthalmologist. Moreover, a sizeable number of patients showed CSC complicated by a secondary CNV (12%) or caCSC (11%). Regardless of the impact of the referral structure, this highlights the fact that CSC patients need to be followed-up and the disease cannot just be regarded as self-limiting in all cases. This notion is supported by the fact that BCVA in our cohort was worse in patients with more severe phenotypes such as caCSC, of which only 30% received treatment. While the median BCVA in these cases was between 20/32 and 20/40, more than a quarter of caCSC patients showed a BCVA of 20/125 or worse. A major treatment goal should therefore be able to treat SRF before patients develop widespread RPE atrophy, at which stage treatment may still be beneficial, but BCVA may not fully recover [6]. Furthermore, while CSC is often described as a self-limiting and self-healing disease [17], our data show that the majority of CSC patients (80% with decreased visual acuity) have visual symptoms even in the inactive state of the disease. This finding highlights the need for further treatment options that target not only SRF resolution but also photoreceptor integrity and phototransduction.
With regards to risk factors, our study mirrors previous reports that mention psychological stress (52% of patients), arterial hypertension (37%), and especially previous use of corticosteroids (30%). It should, however, be noted that psychological stress was self-reported and was not assessed objectively. Whether stress may therefore represent a cause or a consequence of CSC remains a matter of debate and needs to be elucidated [18]. A prospective substudy of this registry examining the psychological stress levels of CSC patients using standardized and validated questionnaires is currently ongoing. Of note, patients with caCSC had the highest frequency of depression and psychopharmacological medication use in our study. Interestingly, a sizeable number of patients in our cohort were smokers (38%), which has so far received little attention as a possible risk factor for CSC. In total, more than 80% of patients reported at least one presumed risk factor for the disease, highlighting the possible influence of lifestyle and systemic diseases on CSC.
Treatment of CSC remains a clinical challenge and a variety of therapies are currently in clinical use [3]. One of the aims of this registry was therefore to capture the current treatment patterns in participating centers. We found that the most common treatments applied at the baseline visit were micropulse laser, nonpulsed focal laser, and indocyanine green angiography-guided verteporfin photodynamic therapy. This is largely in keeping with the most recent national and international recommendations for the treatment of CSC in Germany, which mention these three treatment options after watch-and-wait for about 4–6 months for acute CSC cases [2]. Since some patients may have had SRF for some time before study inclusion (reported by the referring ophthalmologist), these may be the patients for whom treatment was initiated at the initial visit. Interestingly, MPL and focal laser were performed more frequently than PDT even though recent large randomized controlled trials, such as the PLACE and SPECTRA trial, found PDT to be superior to other treatment options [19, 20]. PDT also shows favorable long-term safety data [21] and can safely be performed bilaterally within a single treatment session [22]. While it is thus often considered the treatment of choice [4, 23], its use in our German study population was surprisingly low. This may be explained by the high administrative burden since reimbursement for PDT treatment must be negotiated with the patient’s German health insurance in an often time-consuming process [24]. Moreover, the recent supply shortage of the photosensitizer verteporfin, which is needed for PDT, resulted in a global treatment delay, which may have influenced treatment decision in our cohort [25]. Of note, anti-VEGF injections were also frequently administered, although primarily in cases of suspected secondary CNV, which in CSC usually presents as a type 1, sub-RPE macular neovascularization [26]. Even though ranibizumab is currently the only agent that received European Medical Agency approval for use in secondary CNV, aflibercept was the most frequently used agent in this cohort. This may be based on reports that show a similar efficacy of both agents in secondary CNV due to CSC [27] and other studies suggesting that switching treatment to aflibercept in patients that do not respond to ranibizumab induces a favorable short-term response [28]. Combined PDT and anti-VEGF treatment, which has also been proposed as a treatment option for secondary CNV, was not administered in any of the recorded cases so far [29], which can be explained by the aforementioned restrictions on the use of PDT in Germany.
As for every registry, the presented study has several limitations. Based on a calculated incidence of 4,640 CSC cases in Germany [8], this report covers only a fraction of all CSC patients in Germany and is therefore representative only of the participating centers. Moreover, COVID-19-related restrictions were still in place in the beginning of 2022, which could have impacted the choice of treatment at the beginning of recruitment. A selection bias is also possible due to the participation being voluntary and the referral system resulting in over-representation of more advanced stages at tertiary referral centers. A broader participation of both primary and secondary treatment centers in the registry and an expansion to more centers across Europe would be desirable.
Strengths of this registry include the collection of real-world data from a large cohort of patients with a rare disease, the possibility to track the natural history of the disease over time, and to explore the long-term effectiveness of treatment modalities, which may allow generating new hypotheses for further investigation. The long-term observations in the Retina.net CSC Registry will also enable an investigation into whether CSC characteristics or preferred treatment patterns change over time and whether treatment decisions differ between centers.
Conclusion
This study reports the first data from the German Retina.net CSC Registry on baseline characteristics and treatment patterns of 539 eyes from 411 patients with CSC. It emphasizes that while there is a clear male preponderance in the disease, neither age nor gender should be overemphasized when diagnosing CSC. Therapeutic interventions are heterogeneous and include verteporfin photodynamic therapy, micropulse laser, and anti-VEGF injections in case of secondary CNV. Noninterventional multicenter studies such as the present study fill important gaps in our knowledge regarding the clinical features of CSC, the course of the disease over time, and the preferred treatment patterns. The participation of more centers from across Europe in the Retina.net CSC Registry would be desirable.
Acknowledgments
Actively recruiting members of the Retina.net CSC Registry Study Group:
A. Kieskämper, C. von Schwartzkopf, C. Janning, S. Wirtz-Kirchberg, C. Lange, Department of Ophthalmology, St. Franziskus Hospital, 48145 Münster, Germany.
L. Pauleikhoff, H. Hufnagel, M. Gruber, D. Goos, H. Agostini, Eye Center, Freiburg Medical Center, University of Freiburg, 79106 Freiburg, Germany.
F. Reinking, B. Schworm, S. Priglinger, Department of Ophthalmology, Ludwig-Maximilians-University Munich, Germany.
U. Brocks, M. Spitzer, Department of Ophthalmology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
A. Gamulescu, L. Schreiner, H. Helbig, Department of Ophthalmology, University Hospital of Regensburg, Franz-Josef-Strauss-Allee 11, DE-93042, Regensburg, Germany.
Y. Miura, J. Kempska, G. Bozzini, S. Grisanti, Department of Ophthalmology, University of Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany.
C. Clemens, C. Zurmuehlen, N. Eter, Department of Ophthalmology, University of Münster Medical Center, Münster, Germany.
B. Grundel, A. Stahl, Department of Ophthalmology Greifswald, University Medicine Greifswald, Mecklenburg-Vorpommern, Germany.
T. Lohmann, P. Walter, Department of Ophthalmology, RWTH University Hospital of Aachen, 52074 Aachen, Germany.
Statement of Ethics
The study is conducted in accordance with the World Medical Association Declaration of Helsinki and ethics approval was obtained from the Ethics Committees of all participating centers prior to participation (leading Ethics Committee: Freiburg University Ethics Committee, Application No. 21-1376). The study is registered with the German Clinical Trials Registry (DRKS00027270), a World Health Organization (WHO)-recognized primary clinical trials registry that meets the requirements of the International Committee of Medical Journal Editors (ICMJE). Written informed consent was obtained from all participants prior to study inclusion.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study is supported by Retina.net, a collaborative network of academic institutions in Germany and received financial support from the Dr. Werner Jackstädt Foundation, Germany. LJBP is supported by a personal grant from the German Research Foundation (DFG PA Grant PA 4282/1-1). The funding organizations had no role in the design or conduct of this research.
Author Contributions
Lange and Pauleikhoff were all involved in the design of the study, management of the registry, and initial preparation of the manuscript. Lange and Ohlmeier performed the data analysis. Kiskämper, von Schwarzkopf, Hufnagel, Gruber, Schworm, Brocks, Reinking, Schreiner, Miura, Grundel, Lohmann, Clemens, Gamulescu, Eter, Grisanti, Priglinger, Spitzer, Walter, Agostini, and Stahl secured local permissions for the registry, entered data into the registry, had the opportunity to review and interpret the data, and amended drafts of the manuscript.
Funding Statement
This study is supported by Retina.net, a collaborative network of academic institutions in Germany and received financial support from the Dr. Werner Jackstädt Foundation, Germany. LJBP is supported by a personal grant from the German Research Foundation (DFG PA Grant PA 4282/1-1). The funding organizations had no role in the design or conduct of this research.
Data Availability Statement
Due to data protection and per the study protocol, pseudonymized data for the registry are not publicly available. Further inquiries can be directed to the corresponding authors.
Supplementary Material
Supplementary Material
References
- 1. Von Graefe A. Über zentrale rezidivierende Retinitis. Graefes Arch Clin Exp Ophthalmol. 1866;12:221. [Google Scholar]
- 2. Lange C, Treumer F, Bertram B, Feltgen N, Hoerauf H, Pauleikhoff D, et al. Chorioretinopathia centralis serosa (CCS). Stellungnahme der Deutschen Ophthalmologischen Gesellschaft, der Retinologischen Gesellschaft und des Berufsverbandes der Augenärzte Deutschlands. Der Ophthalmologe: Zeitschrift Der Deutschen Ophthalmologischen Gesellschaft; 2022. print; last updated.10.2021. [Google Scholar]
- 3. Pauleikhoff L, Agostini H, Lange C. [Central serous chorioretinopathy]. Ophthalmologe. 2021;118(9):967–80. [DOI] [PubMed] [Google Scholar]
- 4. van Rijssen TJ, van Dijk EHC, Yzer S, Ohno-Matsui K, Keunen JEE, Schlingemann RO, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019;73:100770. [DOI] [PubMed] [Google Scholar]
- 5. Daruich A, Matet A, Marchionno L, De Azevedo JD, Ambresin A, Mantel I, et al. Acute central serous chorioretinopathy: factors influencing episode duration. Retina. 2017;37(10):1905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohabati D, van Dijk EH, van Rijssen TJ, de Jong EK, Breukink MB, Martinez-Ciriano JP, et al. Clinical spectrum of severe chronic central serous chorioretinopathy and outcome of photodynamic therapy. Clin Ophthalmol. 2018;12:2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohabati D, van Rijssen TJ, van Dijk EH, Luyten GP, Missotten TO, Hoyng CB, et al. Clinical characteristics and long-term visual outcome of severe phenotypes of chronic central serous chorioretinopathy. Clin Ophthalmol. 2018;12:1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115(1):169–73. [DOI] [PubMed] [Google Scholar]
- 9. Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;2015(12):CD011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Dijk EHC, Boon CJF. Serous business: delineating the broad spectrum of diseases with subretinal fluid in the macula. Prog Retin Eye Res. 2021;84:100955. [DOI] [PubMed] [Google Scholar]
- 11. Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. [DOI] [PubMed] [Google Scholar]
- 12. Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016;36(1):9–19. [DOI] [PubMed] [Google Scholar]
- 13. Gäckle HC, Lang GE, Freißler KA, Lang GK. Clinical, fluorescein angiographic and demographic aspects in central serous chorioretinopathy: klinische, fluoreszeinangiographische und demographische Aspekte*. Ophthalmologe. 1998;95(8):529–33. [DOI] [PubMed] [Google Scholar]
- 14. Rim TH, Kim HS, Kwak J, Lee JS, Kim DW, Kim SS. Association of corticosteroid use with incidence of central serous chorioretinopathy in South Korea. JAMA Ophthalmol. 2018;136(10):1164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992;81(4):379–86. [DOI] [PubMed] [Google Scholar]
- 16. Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103(12):2070–80; discussion 2079–2080. [DOI] [PubMed] [Google Scholar]
- 17. Iacono P, Battaglia Parodi M, Falcomatà B, Bandello F. Central serous chorioretinopathy treatments: a mini review. Ophthalmic Res. 2015;55(2):76–83. [DOI] [PubMed] [Google Scholar]
- 18. Sabel BA, Wang J, Cárdenas-Morales L, Faiq M, Heim C. Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J. 2018;9(2):133–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Dijk EHC, Fauser S, Breukink MB, Blanco-Garavito R, Groenewoud JMM, Keunen JEE, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmology. 2018;125(10):1547–55. [DOI] [PubMed] [Google Scholar]
- 20. van Rijssen TJ, van Dijk EHC, Tsonaka R, Feenstra HMA, Dijkman G, Peters PJH, et al. Half-dose photodynamic therapy versus eplerenone in chronic central serous chorioretinopathy (SPECTRA): a randomized controlled trial. Am J Ophthalmol. 2022;233:101–10. [DOI] [PubMed] [Google Scholar]
- 21. Feenstra HMA, Diederen RMH, Lamme MJCM, Tsonaka R, Fauser S, Yzer S, et al. Increasing evidence for the safety of fovea-involving half-dose photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2023;43(3):379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pauleikhoff LJB, Diederen RMH, Feenstra H, Schlingemann RO, van Dijk EHC, Boon CJF. Single-session bilateral reduced-settings photodynamic therapy for bilateral chronic central serous chorioretinopathy. Retina. 2023;43(8):1356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Dijk EHC, Feenstra HMA, Bjerager J, Grauslund J, Boon CJF, Subhi Y. Comparative efficacy of treatments for chronic central serous chorioretinopathy: a systematic review with network meta-analyses. Acta Ophthalmol. 2023;101(2):140–59. [DOI] [PubMed] [Google Scholar]
- 24. Pauleikhoff L, Rothaus K, Groß-Bölting F, Böhringer D, Lübke J, Agostini H, et al. [Photodynamic therapy in Germany-Quo vadis?] Ophthalmologie. 2023;120(8):818–24. [DOI] [PubMed] [Google Scholar]
- 25. Sirks MJ, van Dijk EHC, Rosenberg N, Hollak CEM, Aslanis S, Cheung CMG, et al. Clinical impact of the worldwide shortage of verteporfin (Visudyne®) on ophthalmic care. Acta Ophthalmol. 2022;100(7):e1522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature Study Group. Ophthalmology. 2020;127(5):616–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lejoyeux R, Behar-Cohen F, Mantel I, Ruiz-Medrano J, Mrejen S, Tadayoni R, et al. Type one macular neovascularization in central serous chorioretinopathy: short-term response to anti-vascular endothelial growth factor therapy. Eye. 2022;36(10):1945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schworm B, Luft N, Keidel LF, Herold TR, Wolf A, Priglinger SG, et al. Ranibizumab non-response in pachychoroid neovasculopathy: effects of switching to aflibercept. Sci Rep. 2020;10(1):8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smretschnig E, Hagen S, Glittenberg C, Ristl R, Krebs I, Binder S, et al. Intravitreal anti-vascular endothelial growth factor combined with half-fluence photodynamic therapy for choroidal neovascularization in chronic central serous chorioretinopathy. Eye. 2016;30(6):805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to data protection and per the study protocol, pseudonymized data for the registry are not publicly available. Further inquiries can be directed to the corresponding authors.