Abstract
Antiretroviral therapy (ART) is needed across the lifetime to maintain viral suppression for people living with HIV. In South Africa, obstacles to reliable access to ART persist and are magnified in rural areas, where HIV services are also typically costlier to deliver. A recent pilot randomized study (the Deliver Health Study) found that home-delivered ART refills, provided at a low user fee, effectively overcame logistical barriers to access and improved clinical outcomes in rural South Africa. In the present costing study using the payer perspective, we conducted retrospective activity-based micro-costing of home-delivered ART within the Deliver Health Study and when provided at-scale (in a rural setting), and compared to facility-based costs using provincial expenditure data (covering both rural and urban settings). Within the context of the pilot Deliver Health Study which had an average of three deliveries per day for three days a week, home-delivered ART cost (in 2022 USD) $794 in the first year and $714 for subsequent years per client after subtracting client fees, compared with $167 per client in provincial clinic-based care. We estimated that home-delivered ART can reasonably be scaled up to 12 home deliveries per day for five days per week in the rural setting. When delivered at scale, home-delivered ART cost $267 in the first year and $183 for subsequent years per client. Average costs of home delivery further decreased when increasing the duration of refills from three-months to six- and 12-month scripts (from $183 to $177 and $135 per client, respectively). Personnel costs were the largest cost for home-delivered refills while ART drug costs were the largest cost of clinic-based refills. When provided at scale, home-delivered ART in a rural setting not only offers clinical benefits for a hard-to-reach population but is also comparable in cost to the provincial standard of care.
Keywords: HIV/AIDS, sub-Saharan Africa, HIV treatment, ART, costing
Introduction
Until a cure for HIV is available, reliable access to antiretroviral therapy (ART) is needed across the lifetime to maintain viral suppression. South Africa has one of the highest burdens of HIV worldwide with an HIV prevalence of 16% among adults aged 15 and older and a total of 7.8 million people with HIV who require lifetime ART [1]. In the past two decades, the South African government has invested heavily in their HIV programme, and has made great progress towards achieving the UNAIDS 95-95-95 treatment targets: as of 2022, 90% of South African adults with HIV knew their status, 91% of adults who knew their status were on ART, and 94% of people on ART were virally suppressed [1]. However, the status of HIV control varies considerably across the nation, with KwaZulu-Natal province continuing to have the highest HIV prevalence in the country (22%) [1] and rural farming communities reporting higher HIV prevalence than urban areas (18% vs. 13%) [2]. Access to HIV care is further constrained by slowing government funding which has not matched the level of demand [3], particularly in rural areas where the cost of successfully treating HIV is higher due to logistical barriers, supply chain considerations, and staffing challenges [4]. In light of these rural/urban disparities in HIV outcomes and costs, novel and low-cost strategies to address gaps in HIV care in rural South Africa are critically needed.
A number of strategies have been proposed to improve HIV outcomes and reduce the burden of care associated with one to three-month ART refill visits for both clients and health systems [5]. In particular, the World Health Organization now recommends Differentiated Service Delivery (DSD) for HIV care, which involves tailoring programs to accommodate client needs to improve HIV treatment outcomes and reduce health system burden [6]. Various client-centered DSD mechanisms are being proposed to increase viral suppression by overcoming barriers to accessing HIV care including long wait times at clinics, transportation barriers, and social stigma [7–9]. As a result, alternative ART dispensing mechanisms which reduce clinic burden including home delivery of medications are being explored and have had demonstrated feasibility and success in improving client adherence in South Africa [5,10–12].
The recent Deliver Health Study found that home-delivered ART and monitoring for a small user fee increased viral suppression by 21% compared to standard facility-based care among 162 participants in rural KwaZulu-Natal [13]; however, the costs of this program have not previously been quantified. The objective of the present study was to estimate the cost of home-delivered ART for adults living with HIV in rural South Africa (1) within the context of the pilot study and (2) when provided at-scale, compared to standard clinic-based ART resupply. A secondary objective was to identify cost drivers for each mode of ART delivery. In a sensitivity analysis, we also estimated cost savings when home-delivered ART used multi-month refill scripts. This study aims to inform the South African National Department of Health’s (NDoH) strategy to improve HIV treatment outcomes and reduce health system burden in rural South Africa.
Materials and Methods
Deliver Health Study
The Deliver Health Study (NIH R21MH115770; ClinicalTrials.gov Identifier: NCT04027153) was a pilot randomized trial of home ART delivery, monitoring, and ART resupply conducted in three rural and peri-urban communities in uMgungundlovu District, KwaZulu-Natal from October 2019 to December 2020. The detailed study procedures have been previously published [14], and are also summarized in the S1 File flow map. Briefly, 162 ART-eligible [15] adults aged 18 or over with HIV were identified through home and mobile van HIV testing and counselling and at HIV clinics. Following the enrollment and ART initiation visit, clients were randomized to receive either home delivery of HIV care which was provided for a small user fee of 30–90 South African Rand (ZAR) (2–7 United States Dollars [USD]), or standard clinic-based ART follow-up care at a health center of choice within the study catchment area. Home delivery of HIV care was provided leveraging an optimized delivery algorithm to reduce travel times and associated costs, described further in the S2 File. For the home delivery arm of the pilot study, study staff made deliveries three days per week, spent an average of 26 minutes per home delivery of ART (including transportation time), and made an average of three deliveries a day. Home delivery was at first implemented by a team of three (nurse, driver, data collector), but staffing was later reduced to just the nurse due to pandemic social distancing restrictions (S1 File). All Deliver Health Study participants received the TDF/FTC/EFV ART regimen for the treatment of HIV.
Ethics statement and declarations
Ethical approval was obtained from the Human Sciences Research Council Research Ethics Committee in South Africa (REC 1/21/11/18) and the University of Washington Institutional Review Board in Seattle, Washington, United States (STUDY00005739). All study participants provided written informed consent per local ethics committee requirements.
Access to Deliver Health Study data was originally granted on March 8, 2022 to AST. AST had access to information that could potentially identify participants after data collection, specifically participant locator information.
Study population
Deliver Health Study participant demographics
A total of 155 adults living with HIV completed follow-up in the Deliver Health Study, 81 (52%) of whom received home-delivered ART refills and monitoring and 74 (48%) of whom received clinic-based care. The median age of participants was 38 years (interquartile range: 12), and 72 (47%) were women. Seventy-one (88%) people living with HIV in the home-delivery group were virally suppressed at study exit compared to 55 (74%) people living with HIV in the clinic-based care group. The average monthly salary of participants randomized to the home delivery group, self-reported at baseline, was 1376 ZAR (108 USD) per individual with a standard deviation of 2247 ZAR (176 USD).
Study setting
KwaZulu-Natal was the province in South Africa with the highest estimated HIV prevalence of 22% among adults aged 15 years and older in 2022 [1], totaling approximately 1.9 million adults [16]. An estimated 52% of the population in KwaZulu-Natal resided in rural areas [17], and rural districts of KwaZulu-Natal had the highest estimated HIV prevalence in South Africa of greater than 28% [18]. At the end of 2022, 31% of 15–64 year olds residing in KwaZulu-Natal were unemployed [19] and the average monthly earnings of employees in the formal non-agricultural sector was 26,032 ZAR across South Africa [20].
Cost estimation
Our primary analysis compared the cost of quarterly home delivery of ART (the pilot Deliver Health Study intervention) with the Standard of Care (SOC), i.e., clinic-based care with three-month refill scripts, in KwaZulu-Natal province. Due to limited capacity for costing during the trial (which was conducted during the COVID-19 pandemic), costs were collected retrospectively, and the home-delivery and SOC costs relied on different data sources representing different regions of KwaZulu-Natal, described further below. All costs were estimated from the payer perspective to inform the South African NDoH’s financial planning and strategy. To quantify resource use [21], we first outlined program activities in a detailed Narrative Summary for the Deliver Health Study (S1 File) which further informed our assumptions regarding programmatic implementation (S2 File). Our primary outcomes of interest were:
Average annual cost per client: the average annual cost per person living with HIV in the Deliver Health Study or in KwaZulu-Natal
Average annual cost per client virally suppressed: the average annual cost per person living with HIV who was virally suppressed in the Deliver Health Study or in KwaZulu-Natal. In our secondary objective, costs for each mode of service delivery were disaggregated by cost categories (personnel, vehicles and fuel, drugs, etc.) to understand the main cost drivers (detailed methods in S2 File).
Costs were reported in 2022 USD. For costs originally reported in ZAR, we used the World Bank GDP implicit deflator for South Africa [22] and the average ZAR-to-USD exchange rate in 2022 [23]. We deflated costs reported in USD using the US annual average Consumer Price Index [24]. Capital items and other costs with a useful life of more than one year were discounted 3%. Full details of these adjustments are provided in S2 File.
Costing of home delivery of ART and monitoring
For home-delivered refills, we conducted activity-based micro-costing for home-delivered ART and monitoring in the Deliver Health Study in rural uMgungundlovu District of KwaZulu-Natal. We excluded costs for research-specific procedures (e.g., data collection, research-related personnel costs). Personnel salaries were obtained from the Deliver Health Study and South Africa Department of Public Service and Administration [25]. To estimate personnel time, vehicle costs, and fuel, we used recorded travel logs for the home delivery trips. We assumed the home-delivery intervention was delivered by a team of a nurse, a driver, and a community outreach worker. Full cost assumptions including staff time and vehicle costs are detailed in S2 File.
Costing of clinic-based ART refills and care
To estimate costs for clinic-based care, we used provincial government expenditure data for the full province of KwaZulu-Natal and scaled it to ART-specific care. We obtained actual HIV/AIDS spending (i.e., the rectified budget) for financial years 2019 and 2020 from the South African National Treasury [26]. We then estimated the proportion of the HIV program budget used for ART using a 2020 government HIV/AIDS spending report [27] and applied this proportion to the total 12-month HIV program budget. Full details for these calculations are in the S2 File. Of note, clinic expenditure data covered both rural as well as urban areas in KwaZulu-Natal (e.g., the major cities of Durban and Newcastle), where ART costs may be lower than in rural regions [4].
Scenarios
For the home-delivered ART intervention, we modelled two sets of scenarios over a 12-month time horizon: programmatic and at-scale. Our baseline estimation assumed three-month (quarterly) refills; in a sensitivity analysis we also estimated the costs of home delivery for six- and 12-month ART refill scripts starting after the first quarter. Costs of home-delivered ART for all scenarios were compared to the SOC, i.e., clinic-based care with three-month ART refill scripts. Full details of the estimation for all scenarios are in S2 File.
Programmatic cost estimation
The primary cost was the programmatic scenario for home-delivered ART, which we estimated to reflect the resource requirements if home-delivered ART were implemented by the South African NDoH. Accordingly, we substituted staff salaries paid under the Deliver Health Study with corresponding salaries reported by the South African government [25]. The programmatic scenario assumed that ART was delivered by a team of two (nurse and driver) who spent approximately 3.5 hours a day for 3 days a week making home deliveries to support 81 clients in the Deliver Health Study, and that the program was also supported by a community outreach worker and other operational staff (S2 File). In a sensitivity analysis, we also estimated a second programmatic “as-observed” scenario using staff salaries from within the Deliver Health Study.
At-scale cost estimation
Given the low client volume within the pilot Deliver Health Study, we estimated a scenario where home delivery of ART was scaled up to accommodate a larger client volume. Granted that within the Deliver Health Study, the maximum number of deliveries made in a given day was 16 visits and the average time per visit was 26 minutes (including driving), we estimated that the same team could reasonably make 12 home deliveries a day for five days per week, spending approximately six hours of their working day making home deliveries to clients and about two hours to load and unload the delivery vehicle at the central office. This estimation assumed a 40-hour work week and 214 workdays a year (excluding weekends, holidays, and other leave). In addition, we assumed an ART initiation visit (which includes HIV counselling) would take three times as long as an ART refill visit, which was qualitatively informed by time-and-motion observations conducted during January–February 2023 for an ongoing follow-up trial to the Deliver Health Study (S2 File).
Results
Programmatic scenarios
For the 81 clients receiving home-delivered ART in the Deliver Health Study, cost of home delivery was substantially higher than provincial estimates for annual per-client facility-based ART costs. Specifically, we estimated that in the context of the pilot study the average annual cost per client receiving home-delivered ART refills was $794 per client and $907 per client virally suppressed in the first year of intervention and $714 per client and $815 per client virally suppressed for subsequent years, after subtracting paid client fees (Tables 1 and 2). In comparison, the SOC was estimated to cost an average of $167 annually per client and $254 per client virally suppressed. “As-observed” costs for home-delivered ART in the Deliver Health Study were somewhat higher in the primary programmatic scenario due to differences in assumptions around staff salaries ($819 per client and $935 per client virally suppressed for quarterly refills in the first year of intervention). Multi-month scripting scenarios (with six- and 12-monthly refills) further reduced the cost of home-delivered ART, as shown in Table 2.
Table 1.
Average annual cost per client and average annual cost per virally suppressed client by refill method within the context of the Deliver Health Study, under the programmatic scenario with 3-month ART refill scripts.
| Cost category | Average annual cost per client | Average annual cost per client virally suppressed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Home deliverya | Clinicc,d N=1,925,698 |
Home deliverye | Clinicc,d N=1,270,961 |
|||||||||
| First yearb N=81 |
Subsequent years N=81 |
First yearb N=71 |
Subsequent years N=71 |
|||||||||
| 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | |
| Cost (% of total cost) | ||||||||||||
| ART drugs | 1,150 (9) | 70 (9) | 1150 (10) | 70 (10) | 1150 (42) | 70 (42) | 1,312 (9) | 80 (9) | 1,312 (10) | 80 (10) | 1,743 (42) | 106 (42) |
| Buildings and administrative overhead | 2,189 (17) | 134 (17) | 2,091 (18) | 128 (18) | 58 (2) | 4 (2) | 2,497 (17) | 152 (17) | 2,386 (18) | 146 (18) | 88 (2) | 5 (2) |
| Communication | 1,269 (10) | 78 (10) | 1,269 (11) | 78 (11) | 6 (0.22) | 0.36 (0.22) | 1,448 (10) | 88 (10) | 1,448 (11) | 88 (11) | 9 (0.22) | 0.55 (0.22) |
| Equipment | 1,200 (9) | 73 (9) | 150 (1) | 9 (1) | 2 (0.06) | 0.11 (0.06) | 1,369 (9) | 84 (9) | 172 (1) | 10 (1) | 3 (0.06) | 0.16 (0.06) |
| Hiring and training | 657 (5) | 40 (5) | 657 (6) | 40 (6) | 2 (0.09) | 0.15 (0.09) | 749 (5) | 46 (5) | 749 (6) | 46 (6) | 4 (0.09) | 0.22 (0.09) |
| Materials and supplies | 753 (6) | 46 (6) | 590 (5) | 36 (5) | 561 (20) | 34 (20) | 859 (6) | 52 (6) | 674 (5) | 41 (5) | 850 (20) | 52 (20) |
| Personnel wages and benefits | 4,354 (33) | 266 (33) | 4,354 (37) | 266 (37) | 960 (35) | 59 (35) | 4,968 (33) | 303 (33) | 4,968 (37) | 303 (37) | 1454 (35) | 89 (35) |
| Vehicles and fuel | 1,491 (11) | 91 (11) | 1,489 (13) | 91 (13) | 0.26 (0.01) | 0.02 (0.01) | 1,701 (11) | 104 (11) | 1,699 (13) | 104 (13) | 0.40 (0.01) | 0.02 (0.01) |
| Total f | 13,006 | 794 | 11,694 | 714 | 2,740 | 167 | 14,846 | 907 | 13,349 | 815 | 4,151 | 254 |
Abbreviations: ART = antiretroviral therapy, ZAR = South African Rand, USD = United States Dollars, HIV = Human Immunodeficiency Virus, UNAIDS = Joint United Nations
Programme on HIV/AIDS.
There were 81 people living with HIV on ART in the home-delivered ART refills group of the Deliver Health Study.
Includes startup costs.
The standard-of-care in South Africa is 3-month ART refills at clinics.
The total number of adults (aged 15 years and older) living with HIV in KwaZulu-Natal in September 2022 was 1,925,698 people. Source: UNAIDS HIV sub-national estimates viewer.
There were 71 people living with HIV on ART who were virally suppressed at study exit in the home-delivered ART refills group of the Deliver Health Study.
The average fee for home delivery service, about 4 USD, paid by clients in the home delivery intervention of the Deliver Health Study was subtracted from the programmatic costs of implementing home delivery.
Table 2.
Estimated cost implications of multi-month home-delivered and standard clinic-based ART refills and monitoring as-observed by the South African National Department of Health with South African government salaries, under the programmatic scenario with 3-, 6-, and 12-month ART refill scripts
| Scenario | Average annual cost per client | Average annual cost per client virally suppressed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Home delivery | Clinicb N=1,925,698 |
Home delivery | Clinicb N=1,270,961 |
|||||||||
| First yeara N=81 |
Subsequent years N=81 |
First yeara N=71 |
Subsequent years N=71 |
|||||||||
| 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | |
| Programmatic costs with 3-month refills | 13,006 | 794 | 11,694 | 714 | 2,740 | 167 | 14,846 | 907 | 13,349 | 815 | 4,151 | 254 |
| Programmatic costs with 6-month refills | 10,014 | 612 | 9,337 | 570 | 11,424 | 698 | 10,652 | 651 | ||||
| Programmatic costs with 12-month refills | 8,261 | 504 | 7,521 | 459 | 9,424 | 576 | 8,581 | 524 | ||||
Abbreviations: ART = antiretroviral therapy, ZAR = South African Rand, USD = United States Dollars, HIV = Human Immunodeficiency Virus, UNAIDS = Joint United Nations
Programme on HIV/AIDS.
Includes startup costs.
The standard-of-care in South Africa is 3-month ART refills at clinics.
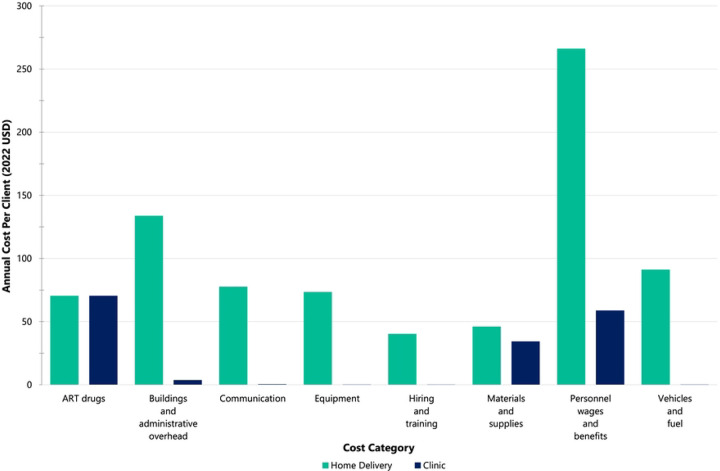
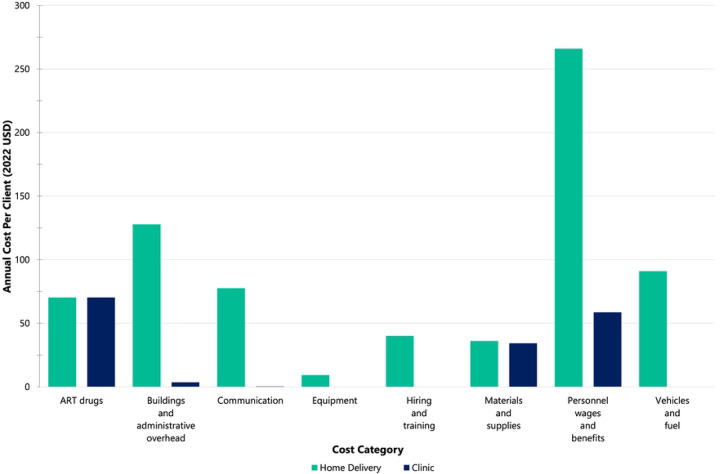
For home-delivered ART, the largest cost categories were personnel costs (33%), buildings and overhead (17%), and vehicles and fuel (11%) for the first year of implementation (Fig 1A). These proportions remained relatively stable for subsequent years (Fig 1B). For the SOC, the largest costs were ART drugs (42%), personnel costs (35%), and materials and supplies (20%) for each year of implementation (Fig 1A). Total annual costs by cost category are reported in the S3 File.
Figure 1. Average annual cost per client (2022 USD) for home-delivered 3-month ART refills vs. clinic-based 3-month ART refills (standard-of-care) by cost category in the programmatic NDoH-implemented scenario.
The NDoH scenario assumes fixed costs as implemented in the Deliver Health Study and public sector clinical staff salaries instead of study salaries.
(A) Home-delivered 3-month ART refills (first year costs) vs. standard-of-care. (B) Home-delivered 3-month ART refills (subsequent year costs) vs. standard-of-care.
At-scale scenarios
We estimated that if the home delivery team was working full-time (using the parameters described in the methods), the team could feasibly make 12 home delivery stops per working day and accommodate a client volume of 367 newly diagnosed people with HIV in the first year and up to 642 clients in subsequent years. We estimated that this at-scale programmatic cost of home delivery would be $267 annually per client for home-delivered ART refills and monitoring in the first year and $183 in subsequent years (Table 3), in comparison to $167 for clinic-based refills. The average annual cost per client virally suppressed receiving home-delivered ART refills would be $303 in the first year and $208 in subsequent years (Table 3) vs. $254 for clients receiving clinic-based care.
Table 3.
Estimated cost implications of multi-month home-delivered and standard clinic-based ART refills and monitoring provided at-scale by the South African National Department of Health with South African government salaries, with 3-, 6-, and 12-month ART refill scripts.
| Scenario | Average annual cost per client | Average annual cost per client virally suppressed | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Home delivery | Clinic N=1,925,698 |
Home delivery | Clinic N=1,270,961 |
|||||||||
| First yearb N=367 |
Subsequent years N=642 |
First yearb N=323 |
Subsequent years N=565 |
|||||||||
| 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | 2022 ZAR | 2022 USD | |
| At-scale programmatic costs with 3-month refillsa | 4,367 | 267 | 2,996 | 183 | 2,740 | 167 | 4,963 | 303 | 3,405 | 208 | 4,151 | 254 |
| At-scale programmatic costs with 6-month refills | 3,681 | 225 | 2,900 | 177 | 4,183 | 255 | 3,296 | 201 | ||||
| At-scale programmatic costs with 12-month refills | 3,921 | 239 | 2,214 | 135 | 4,455 | 272 | 2,516 | 154 | ||||
Abbreviations: ART = antiretroviral therapy, ZAR = South African Rand, USD = United States Dollars, HIV = Human Immunodeficiency Virus, UNAIDS = Joint United Nations Programme on HIV/AIDS.
The standard-of-care in South Africa is 3-month ART refills.
Includes startup costs.
Multi-month scripting further reduced the cost of home-delivered ART so that it was comparable or even lower in cost than SOC. With six-month ART refill scripts, the annual cost per client would be $225 for home-delivered refills in the first year and $177 for subsequent years, (compared to $167 for the SOC), and the average cost per client virally suppressed would be $255 in the first year and $201 in subsequent years (vs. $254 for the SOC). At-scale 12-month ART refills would be cost-saving after the first year: the annual cost per client would be $135 in subsequent years (compared to $167 for the SOC) (Table 3).
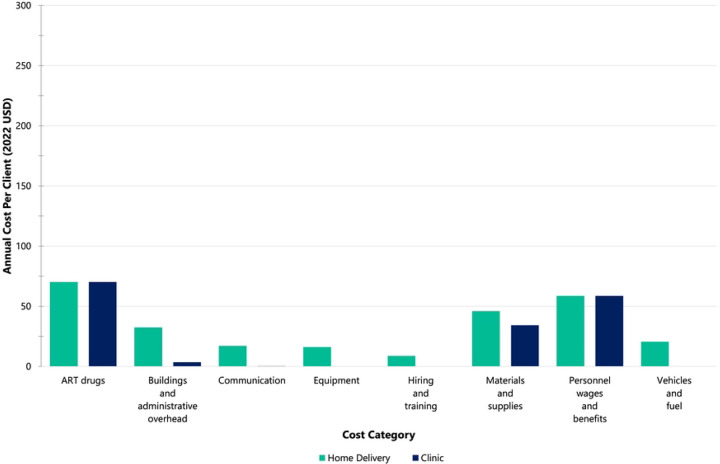
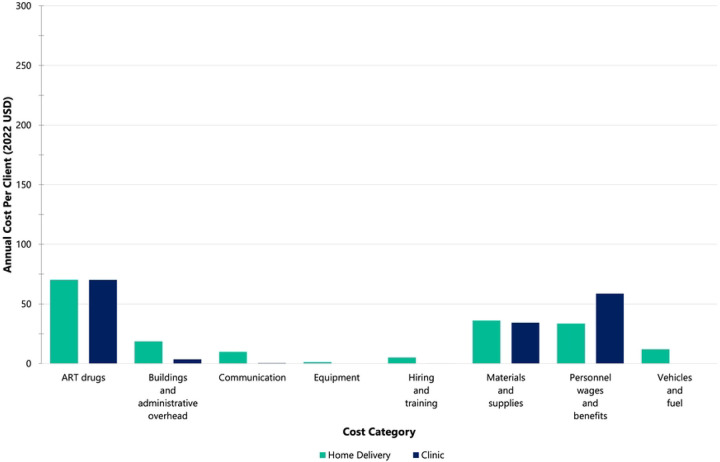
For three-month at-scale home delivered ART, the largest first-year costs were ART drugs (26%), personnel costs (22%), materials and supplies (17%), and buildings and overheads (12%) (Fig 2A). The rankings of cost categories remained the same for subsequent years (Fig 2B). Average client costs per cost category for the six-month refill programmatic and at-scale scenarios are reported in the S3 File, along with additional cost driver figures.
Figure 2. At-scale average annual cost per client (2022 USD) for home-delivered 3-month ART refills vs. clinic-based 3-month ART refills (standard-of-care) by cost category in the programmatic NDoH-implemented scenario.
The NDoH scenario assumes fixed costs as implemented in the Deliver Health Study and public sector clinical staff salaries instead of study salaries.
(A) Home-delivered ART intervention (first year costs) vs. clinic-based ART refills. (B) Home-delivered ART intervention (subsequent year costs) vs. clinic-based ART refills.
Discussion
In this costing study of home-delivered ART refills in a high-prevalence rural setting in South Africa, we estimate that when delivered at scale, home delivery costs in a rural setting are comparable to the SOC of clinic-based refills administered across both rural and urban settings. Specifically, assuming a reasonable client volume of 12 home deliveries per day, we estimate that the annual per-client cost of home-delivered ART is $267 in the first year and $183 in subsequent years, as compared to the province-level average of $167 for clinic-based refills; this corresponds to a per-client incremental cost of $100 in the first year and $16 in subsequent years (Table 3). These incremental costs were predominantly driven by differences in personnel requirements (Fig 1). Importantly, this comparison covers a rural setting for home-delivered ART and a combined rural/urban setting for the SOC – the difference in setting may contribute to an increase in observed incremental costs given prior literature from South Africa which suggests that the cost of delivering ART is on average 7% more costly in rural areas than in urban areas [4]. Multi-month scripting (six- and 12-months) even further reduced costs of home delivery compared with standard clinic-based refills, and were either comparable (six-months) or cost-saving (12-months) when provided at-scale after the first year (Table 3). The published Deliver Health Study results demonstrated clinical effectiveness of home-delivered ART [14], and the present costing study suggests that home-delivered ART can be feasibly scaled up in a high prevalence rural setting in South Africa at modest incremental cost.
Our estimates somewhat differ from other costing studies for DSD and ART delivery in South Africa. Importantly, our estimates for the SOC using KwaZulu-Natal’s provincial budget data ($167 per client annually) are substantially lower than the estimates from the South African HIV investment case, which estimated that the average cost (in 2022 USD) for providing ART per client annually across South Africa was $290 [28]. Using a lower comparator means that our estimates of the incremental cost of home-delivered care are higher than would be if we compared to the South African HIV investment case. Our estimated at-scale annual per-client costs of ART with quarterly refills were higher than those observed in the DO ART Study, a study of community-based ART conducted in the same region of rural South Africa ($183 in this study, compared with $287 in the DO ART Study for subsequent years after adjusting for inflation as shown in S2 File) [29]. While it is expected that home delivery costs might be higher than community-based delivery of ART due to increased personnel and fuel costs, an approach like home delivery would remove the physical access barrier for the client, which could result in better retention in care and ART adherence. Finally, in line with other studies on multi-month scripting from Southern Africa which have also found comparable clinical outcomes [30–33], we found that that increasing the duration of ART scripts to six- and 12-months further reduced costs due to the fewer follow-up visits.
Interestingly, the largest cost category for home-delivered ART was personnel wages and benefits (33% in the baseline scenario) and not vehicle and fuel costs (11%). Personnel was the largest cost for a number of reasons. First, within the Deliver Health Study, staff coordinated and made individual home deliveries based on client delivery preferences. In addition, the protocol required that clients were physically present at the time of delivery (vs. dropping off refills at their front door). Thus, planning of delivery trips and routes driven were not always optimized. With a greater volume of clients, optimized delivery algorithms adapted from routing science-guided delivery in the private sector [34–36] could be useful in managing client delivery preferences and delivery personnel time, and may ultimately lower programmatic costs. This point is evidenced by our findings in the at-scale scenarios, in which ART drugs contributed the largest costs to the home-delivery intervention in both three- and six-month refill scenarios. Second, we assumed that programmatic implementation of home-delivered ART included a full-time professional nurse at grade 2 and a driver. These programmatic nurse salaries were in fact higher than the nurse staff salaries from the Deliver Health Study, which is generally not consistent with the literature [29,37]. Alternative team configurations with task shifting to lower cadre health workers (e.g., lower grade nurses or community health workers) could be tested in future work to determine effectiveness and efficiency. Finally, vehicles did not contribute a substantial portion of total estimated costs due to low diesel and vehicle costs relative to personnel costs.
In the Deliver Health Study, clients paid a one-time fee, tiered based on individuals’ income (US $2, $4, or $6), for a six-month ART home delivery service (which was extended due to COVID-19). User fees in general were well-accepted: in the Deliver Health Study, 98% of clients paid the full user fee for the home-delivery ART service and 100% reported willingness to continue to pay a fee for the service due to its perceived convenience and flexibility [9]. Notably, these client fees for the home delivery service did not substantially offset delivery costs (personnel, vehicle, and fuel) in either the as-observed or at-scale scenarios. Moreover, the fee in the Deliver Health Study covered home ART delivery service for six months, thus an annual service fee per client would likely be higher (e.g., double that of the six-month service) in implementation. However, the user fees still contributed to a modest reduction in programmatic costs and may additionally have contributed to program effectiveness [38,39].
Our study had a number of limitations. First, as discussed above, our costing of home-delivered ART covered a rural setting while the SOC covered a rural and urban setting, likely leading to an overestimate of incremental costs [40]. In a related point, we did not obtain all costing inputs from a single source; however, we relied on either peer-reviewed studies or published data from the South African government. Third, our study included a low client volume from pilot randomized controlled trial leading to higher costs per person. To account for the small sample size, we modelled at-scale scenarios to accommodate a higher client volume; these findings were more in line with estimates from the literature. Fourth, we estimated costs for TDF/FTC/EFV (the regimen prescribed for Deliver Health Study participants); however, as of 2023, South Africa recommends ART regimens containing DTG for greater adherence and viral suppression [15,41] [42,43] – future costing studies should note this change in clinical guidelines. Fifth, while our study was conducted from the payer perspective to reflect the stakeholder of interest, a societal perspective could better capture benefits to clients (e.g., transportation costs, lost wages due to time spent on clinic visits, childcare [9]). Sixth, this study uses the SOC as the comparison, but does not compare to costs of other DSD models for HIV, such as the use of community package lockers (smart lockers) being tested in an ongoing trial throughout rural KwaZulu-Natal [44]. Finally, while the present study does not incorporate health outcomes, the estimates generated in this study can be leveraged for future cost-effectiveness analysis.
National scale-up of DSD models for HIV treatment, tailored to specific patient populations and geographies, are needed in South Africa. A number of alternative ART dispensing mechanisms have demonstrated improved medication adherence and retention compared to clinic care, including home delivery service, community package lockers, and automated pharmacy dispensing units [45]. These methods, particularly when clients can flexibly choose the most convenient option, can strengthen individuals’ commitment to care [46,47]. Additionally, community-based ART resupply can increase coverage in rural areas [48] and reduce clinic congestion [49]. At scale, home-delivered ART could be optimized using a centralized warehouse to fulfill and dispatch delivery trucks that synchronize deliveries to neighboring locations, similar to what is used in the private sector by Amazon [50]. These warehouses could even be used for other chronic medications as part of the Centralised Chronic Medicine Dispensing and Distribution [51], freeing up clinics and pharmacies to focus on clients with health care needs other than refills. Drone delivery, which has successfully been tested in other African countries including Uganda (for ART) and Malawi (for transportation of lab samples), offers a promising cost-effective solution for rural areas [52,53]. In fact, Amazon has recently begun implementing home delivery of medications via drones in the US [54]. These innovative methods hold promise for addressing the hard-to-reach and high prevalence populations in rural South Africa, where interventions increasing early adoption of and adherence to ART can be highly cost-effective (increases cost-effectiveness relative to the base-case by 23% in a rural areas of South Africa, compared to 1.1% in urban areas) [4]. Future research should explore the costs and outcomes of various innovative approaches compared to traditional clinic-based care.
Conclusion
Our costing study is a novel evaluation to quantify the costs associated with implementing home-delivered ART and monitoring in rural South Africa. Implementing home-delivered ART and monitoring costs more on average annually per client and per client virally suppressed compared to standard three-month clinic-based ART refills in KwaZulu-Natal, but incremental costs were reduced when home delivery was scaled to a larger client volume and potentially even cost-saving when using six- and 12-month refill scripts. The findings of this study could help inform the South African NDoH’s strategy to meet the 95-95-95 targets by focusing on the scale-up of novel DSD methods, such as home-delivered ART, to promote long-term retention in HIV care while reducing patient load at public health clinics.
Acknowledgements
We would like to thank the Deliver Health Study Team and the individuals who participated in the study.
Footnotes
Supporting information captions
S1 File. Narrative summary of the Deliver Health Study.
S2 File. Additional methods.
S3 File. Additional results.
References
- 1.Human Sciences Research Council. SABSSM VI The Sixth South African National HIV Prevalence, Incidence, Behaviour and Communication Survey: Summary Sheet. Cape Town; 2023. [Google Scholar]
- 2.Simbayi L, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017. Cape Town; 2019. Available: https://hsrc.ac.za/uploads/pageContent/10779/SABSSMV.pdf [Google Scholar]
- 3.Malakoane B, Heunis JC, Chikobvu P, Kigozi NG, Kruger WH. Public health system challenges in the Free State, South Africa: a situation appraisal to inform health system strengthening. BMC Health Serv Res. 2020;20: 58. doi: 10.1186/s12913-019-4862-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbonigaba J, Oumar SB. The cost-effectiveness of major HIV/AIDS interventions in rural and urban areas in South Africa. Int J Econ Bus Res. 2017;13: 413. doi: 10.1504/IJEBR.2017.084387 [DOI] [Google Scholar]
- 5.Grimsrud A, Wilkinson L. Acceleration of differentiated service delivery for HIV treatment in sub-Saharan Africa during COVID-19. J Int AIDS Soc. 2021;24. doi: 10.1002/jia2.25704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva; 2021. Available: https://www.who.int/publications/i/item/9789240031593 [PubMed] [Google Scholar]
- 7.Nicol E, Jama NA, Mehlomakulu V, Hlongwa M, Pass D, Basera W, et al. Enhancing linkage to HIV care in the “Universal Test and Treat” era: Barriers and enablers to HIV care among adults in a high HIV burdened district in KwaZulu-Natal, South Africa. BMC Public Health. 2023;23: 1756. doi: 10.1186/s12889-023-16576-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwuji C, Chimukuche RS, Zuma T, Plazy M, Larmarange J, Orne-Gliemann J, et al. Test but not treat: Community members’ experiences with barriers and facilitators to universal antiretroviral therapy uptake in rural KwaZulu-Natal, South Africa. Yotebieng M, editor. PLoS One. 2020;15: e0239513. doi: 10.1371/journal.pone.0239513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ntinga X, Musiello F, Pita T, Mabaso N, Celum C, Szpiro A, et al. People living with HIV’s perspectives of acceptability of fee for home delivery of ART: a qualitative study. BMC Health Serv Res. 2024;24: 88. doi: 10.1186/s12913-023-10533-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brey Z, Mash R, Goliath C, Roman D. Home delivery of medication during Coronavirus disease 2019, Cape Town, South Africa: Short report. African J Prim Heal care Fam Med. 2020;12: e1–e4. doi: 10.4102/phcfm.v12i1.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mash RJ, Schouw D, Daviaud E, Besada D, Roman D. Evaluating the implementation of home delivery of medication by community health workers during the COVID-19 pandemic in Cape Town, South Africa: a convergent mixed methods study. BMC Health Serv Res. 2022;22: 98. doi: 10.1186/s12913-022-07464-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber A, Pascoe S, Nichols B, Long L, Kuchukhidze S, Phiri B, et al. Differentiated Service Delivery Models for HIV Treatment in Malawi, South Africa, and Zambia: A Landscape Analysis. Glob Heal Sci Pract. 2021;9: 296–307. doi: 10.9745/GHSP-D-20-00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnabas R V, Szpiro A, Ntinga X, Mugambi ML, Krows ML, Schaafsma TT, et al. Fee for home delivery and monitoring of ART raises viral suppression in South Africa. Conference on Retroviruses and Opportunistic Infections (CROI). 2021. Available: https://www.croiconference.org/abstract/fee-for-home-delivery-and-monitoring-of-art-raises-viral-suppression-in-south-africa/ [Google Scholar]
- 14.Barnabas R V, Szpiro AA, Ntinga X, Mugambi ML, van Rooyen H, Bruce A, et al. Fee for home delivery and monitoring of antiretroviral therapy for HIV infection compared with standard clinic-based services in South Africa: a randomised controlled trial. Lancet HIV. 2022;9: e848–e856. doi: 10.1016/S2352-3018(22)00254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Republic of South Africa National Department of Health. 2023 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy and Breastfeeding, Adolescents, Children, Infants and Neonates. Pretoria; 2023. Available: https://www.differentiatedservicedelivery.org/wp-content/uploads/National-ART-Clinical-Guideline-2023_06_06-version-3-Web.pdf [Google Scholar]
- 16.UNAIDS. HIV sub-national estimates viewer. In: UNAIDS [Internet]. 2024. [cited 15 Feb 2024]. Available: https://naomi-spectrum.unaids.org/ [Google Scholar]
- 17.South African Department of Water and Sanitation. WSA: PROVINCIAL [NAT] DEMOGRAPHY. 2024. [cited 9 Apr 2024]. Available: https://ws.dws.gov.za/wsks/DefaultList.aspx?SubjectAreaID=1&DataTopicDetailID=92&DisplayTypeId=1&PerspectiveID=0&LvlID=10&DataTopicID=1
- 18.South Africa Estimates and Modelling Technical Working Group. South Africa District HIV Estimates. 2023. [cited 9 Mar 2024]. Available: https://www.hivdata.org.za/
- 19.Statistics South Africa. Statistical Release P0211 Quarterly Labour Force Survey Quarter 4: 2022. Pretoria; 2023. Available: https://www.statssa.gov.za/publications/P0211/P02114thQuarter2022.pdf [Google Scholar]
- 20.Statistics South Africa. Statistical Release P0277 Quarterly employment statistics (QES) December 2022. Pretoria; 2023. Available: https://www.statssa.gov.za/publications/P0277/P0277December2022.pdf [Google Scholar]
- 21.Vassall A, Sweeney S, Kahn J, Gomez GB, Bollinger L, Marseille E, et al. Reference Case for Estimating the Costs of Global Health Services and Interventions. 2017. Available: https://researchonline.lshtm.ac.uk/id/eprint/4653001/1/vassall_etal_2018_reference_case_for_estimating_costs_global_health_services.pdf
- 22.The World Bank. GDP deflator (base year varies by country) - South Africa. In: The World Bank [Internet]. 2024. [cited 10 Dec 2023]. Available: https://data.worldbank.org/indicator/NY.GDP.DEFL.ZS?locations=ZA [Google Scholar]
- 23.Exchange Rates UK. US Dollar (USD) to South African Rand (ZAR) exchange rate history. In: Exchange Rates UK [Internet]. 2024. [cited 10 Dec 2023]. Available: https://www.exchangerates.org.uk/USD-ZAR-exchange-rate-history.html [Google Scholar]
- 24.U.S. Bureau of Labor Statistics. Databases, Tables & Calculators by Subject: Consumer Price Index for All Urban Consumers (CPI-U). In: U.S. Bureau of Labor Statistics [Internet]. 2024. [cited 19 Mar 2024]. Available: https://data.bls.gov/timeseries/CUUR0000SA0 [Google Scholar]
- 25.South Africa Department of Public Service and Administration. Appendices A to H4 to COLA 2021: 2019 and 2021 salary scales, with translation keys, for employees on salary levels 1 to 12 and those employees covered by Occupation Specific Dispensations (OSDs). In: South Africa Department of Public Service and Administration [Internet]. 2021. [cited 8 Feb 2024]. Available: https://www.dpsa.gov.za/dpsa2g/documents/rp/2021/Appendices A to H4 to COLA 2021.xlsx [Google Scholar]
- 26.South African National Treasury. Budgeted and Actual Provincial Expenditure. In: Vulekamali [Internet]. 2024. [cited 29 Jan 2024]. Available: https://vulekamali.gov.za/datasets/budgeted-and-actual-provincial-expenditure/budgeted-and-actual-provincial-expenditure [Google Scholar]
- 27.Davén J, Khoele A, Manamela L. 2020 HIV/AIDS Component of the HIV, TB, Malaria and Community Outreach Grant - an analysis of expenditure per output and options for future reform. Pretoria; 2021. Available: https://www.gtac.gov.za/pepa/wp-content/uploads/2021/11/HIV-TB-Spending-Review-Report.pdf [Google Scholar]
- 28.Meyer-Rath G, van Rensburg C, Chiu C, Leuner R, Jamieson L, Cohen S. The per-patient costs of HIV services in South Africa: Systematic review and application in the South African HIV Investment Case. McCreesh N, editor. PLoS One. 2019;14: e0210497. doi: 10.1371/journal.pone.0210497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnabas R V, Szpiro AA, van Rooyen H, Asiimwe S, Pillay D, Ware NC, et al. Community-based antiretroviral therapy versus standard clinic-based services for HIV in South Africa and Uganda (DO ART): a randomised trial. Lancet Glob Heal. 2020;8: e1305–e1315. doi: 10.1016/S2214-109X(20)30313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benade M, Nichols BE, Fatti G, Kuchukhidze S, Takarinda K, Mabhena-Ngorima N, et al. Economic evaluation of a cluster randomized, non-inferiority trial of differentiated service delivery models of HIV treatment in Zimbabwe. Pai M, editor. PLOS Glob Public Heal. 2023;3: e0000493. doi: 10.1371/journal.pgph.0000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols BE, Cele R, Lekodeba N, Tukei B, Ngorima-Mabhena N, Tiam A, et al. Economic evaluation of differentiated service delivery models for HIV treatment in Lesotho: costs to providers and patients. J Int AIDS Soc. 2021;24. doi: 10.1002/jia2.25692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Tourneau N, Germann A, Thompson RR, Ford N, Schwartz S, Beres L, et al. Evaluation of HIV treatment outcomes with reduced frequency of clinical encounters and antiretroviral treatment refills: A systematic review and meta-analysis. Newell M-L, editor. PLOS Med. 2022;19: e1003959. doi: 10.1371/journal.pmed.1003959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis L, Sookrajh Y, van der Molen J, Khubone T, Sosibo P, Maraj M, et al. Clinical outcomes after extended 12-month antiretroviral therapy prescriptions in a community-based differentiated HIV service delivery programme in South Africa: a retrospective cohort study. J Int AIDS Soc. 2023;26. doi: 10.1002/jia2.26164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Lam WHK, Chen BY. On-time delivery probabilistic models for the vehicle routing problem with stochastic demands and time windows. Eur J Oper Res. 2016;249: 144–154. doi: 10.1016/j.ejor.2015.08.050 [DOI] [Google Scholar]
- 35.Wang J, Zhou Y, Wang Y, Zhang J, Chen CLP, Zheng Z. Multiobjective Vehicle Routing Problems With Simultaneous Delivery and Pickup and Time Windows: Formulation, Instances, and Algorithms. IEEE Trans Cybern. 2016;46: 582–594. doi: 10.1109/TCYB.2015.2409837 [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Zhang Z, Fries R. Development and implementation of algorithms for vehicle routing during a no-notice evacuation. Loxton R, editor. Cogent Math. 2016;3: 1163767. doi: 10.1080/23311835.2016.1163767 [DOI] [Google Scholar]
- 37.Roberts DA, Barnabas R V, Abuna F, Lagat H, Kinuthia J, Pintye J, et al. The role of costing in the introduction and scale-up of HIV pre-exposure prophylaxis: evidence from integrating PrEP into routine maternal and child health and family planning clinics in western Kenya. J Int AIDS Soc. 2019;22. doi: 10.1002/jia2.25296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashraf N, Berry J, Shapiro JM. Can Higher Prices Stimulate Product Use? Evidence from a Field Experiment in Zambia. Am Econ Rev. 2010;100: 2383–2413. Available: http://www.aeaweb.org/articles.php?doi=10.1257/aer.100.5.2383 [Google Scholar]
- 39.El-Shal A, Cubi-Molla P, Jofre-Bonet M. Are user fees in health care always evil? Evidence from family planning, maternal, and child health services. Econ Anal Policy. 2021;72: 506–529. doi: 10.1016/j.eap.2021.08.009 [DOI] [Google Scholar]
- 40.Statistics South Africa. Statistics South Africa Provincial Profile 2004: KwaZulu-Natal. Pretoria; 2006. Available: https://www.statssa.gov.za/publications/Report-00-91-05/Report-00-91-052004.pdf [Google Scholar]
- 41.World Health Organization. WHO recommends dolutegravir as preferred HIV treatment option in all populations. In: World Health Organization [Internet]. 2019. [cited 7 Mar 2024]. Available: https://www.who.int/news/item/22-07-2019-who-recommendsdolutegravir-as-preferred-hiv-treatment-option-in-all-populations [Google Scholar]
- 42.The NAMSAL ANRS 12313 Study Group. Dolutegravir-Based or Low-Dose Efavirenz–Based Regimen for the Treatment of HIV-1. N Engl J Med. 2019;381: 816–826. doi: 10.1056/NEJMoa1904340 [DOI] [PubMed] [Google Scholar]
- 43.Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019;381: 803–815. doi: 10.1056/NEJMoa1902824 [DOI] [PubMed] [Google Scholar]
- 44.van Heerden A, Szpiro A, Ntinga X, Celum C, van Rooyen H, Essack Z, et al. A Sequential Multiple Assignment Randomized Trial of scalable interventions for ART delivery in South Africa: the SMART ART study. Trials. 2023;24: 32. doi: 10.1186/s13063-022-07025-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mash R, Christian C, Chigwanda R V. Alternative mechanisms for delivery of medication in South Africa: A scoping review. South African Fam Pract. 2021;63: e1–e8. doi: 10.4102/safp.v63i1.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wieber F, Thürmer JL, Gollwitzer PM. Promoting the translation of intentions into action by implementation intentions: behavioral effects and physiological correlates. Front Hum Neurosci. 2015;9. doi: 10.3389/fnhum.2015.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagger MS, Luszczynska A, de Wit J, Benyamini Y, Burkert S, Chamberland P-E, et al. Implementation intention and planning interventions in Health Psychology: Recommendations from the Synergy Expert Group for research and practice. Psychol Health. 2016;31: 814–839. doi: 10.1080/08870446.2016.1146719 [DOI] [PubMed] [Google Scholar]
- 48.Luque-Fernandez MA, Van Cutsem G, Goemaere E, Hilderbrand K, Schomaker M, Mantangana N, et al. Effectiveness of Patient Adherence Groups as a Model of Care for Stable Patients on Antiretroviral Therapy in Khayelitsha, Cape Town, South Africa. Dowdy DW, editor. PLoS One. 2013;8: e56088. doi: 10.1371/journal.pone.0056088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Heerden A, Ntinga X, Lippman SA, Leslie HH, Steward WT. Understanding the factors that impact effective uptake and maintenance of HIV care programs in South African primary health care clinics. Arch Public Heal. 2022;80: 221. doi: 10.1186/s13690-022-00975-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenharo M. The science behind grouping package deliveries. In: Amazon Science [Internet]. 2022. [cited 14 Mar 2024]. Available: https://www.amazon.science/latest-news/the-science-behind-grouping-amazon-package-deliveries [Google Scholar]
- 51.South Africa National Department of Health. dablapmeds. In: South Africa National Department of Health [Internet]. 2024. [cited 7 Mar 2024]. Available: https://www.health.gov.za/ccmdd/ [Google Scholar]
- 52.UNICEF Malawi. The Drones Factsheet. 2018. Available: https://www.unicef.org/malawi/reports/drones-factsheet
- 53.Goad K. How drones are being used to deliver lifesaving HIV drugs to remote areas of the world. Johnson & Johnson. 21 Jul 2021. Available: https://www.jnj.com/innovation/medical-drones-deliver-hiv-medicine [Google Scholar]
- 54.Amazon Staff. Get medications faster with drone delivery from Amazon Pharmacy. Amazon News. Oct 2023. Available: https://www.aboutamazon.com/news/retail/amazonpharmacy-amazon-air-prescription-drone-delivery [Google Scholar]