Abstract
Although adeno-associated virus type 2 (AAV) has gained attention as a potentially useful alternative to the more commonly used retrovirus- and adenovirus-based vectors for human gene therapy, efficient gene transfer and transgene expression by AAV vectors require that the following two obstacles be overcome. First, the target cell must express the receptor and the coreceptor for AAV infection, and second, the cell must allow for viral second-strand DNA synthesis. We now describe a third obstacle, impaired intracellular trafficking of AAV to the nucleus, which results in the lack of transgene expression in murine fibroblasts which do express the AAV receptor and the coreceptor and which are permissive for viral second-strand DNA synthesis. We document that AAV vectors bind efficiently and gain entry successfully into NIH 3T3 cells, but trafficking into the nucleus is significantly impaired in these cells. In contrast, viral trafficking to the nucleus in cells known to be efficiently transduced by AAV vectors is both rapid and efficient. The demonstration of yet another obstacle in AAV-mediated gene transfer has implications for the optimal use of these vectors in human gene therapy.
Adeno-associated virus type 2 (AAV) is a nonpathogenic human parvovirus which requires coinfection with a helper virus, such as adenovirus, for its optimal replication (3). In the absence of a helper virus, the wild-type AAV genome integrates in a site-specific way into human chromosome 19 and establishes a latent infection (14, 15, 27). AAV possesses a wide host range that transcends the species barrier (18). These properties of AAV have been instrumental in the development of recombinant AAV vectors for their use in human gene therapy (4–6, 18, 33–35, 40). We and others have reported AAV-mediated transduction and transgene expression both in vitro and in vivo (1, 2, 9, 10, 12, 13, 17, 19–26, 28, 30, 41). However, the transduction efficiency of AAV vectors varies greatly in different cell types. This problem has been attributed to the following two obstacles that AAV must overcome. First, AAV must bind to a cellular receptor as well as to a coreceptor for successful entry into the target cell (19, 21, 24, 36, 37), and second, because AAV is a single-stranded DNA-containing virus, the target cell must allow for the conversion of the single-stranded viral genome to a transcriptionally active double-stranded intermediate (7, 8). We have documented the existence of a host cell protein, which we have designated the single-stranded D sequence-binding protein (ssD-BP), which plays a crucial role in viral second-strand DNA synthesis (23, 25). The ssD-BP is phosphorylated at tyrosine residues by epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK) activity, and the phosphorylated form of the ssD-BP prevents viral second-strand DNA synthesis and consequently AAV-mediated transgene expression (16). In this report, we provide evidence for the existence of a third obstacle, impaired intracellular trafficking into the nucleus, which AAV must also overcome prior to high-efficiency transduction.
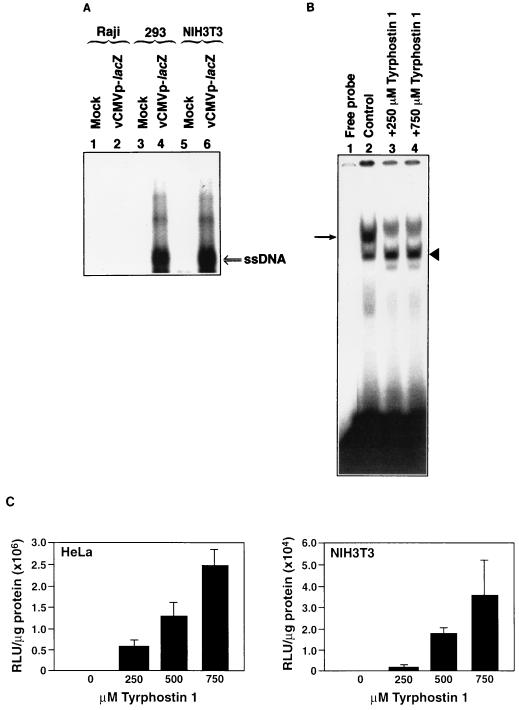
We recently documented that AAV binds to murine NIH 3T3 cells efficiently but that little transgene expression occurs (24). This observation was interpreted as the inability of AAV to enter these cells. However, Southern blot analyses (31) of low-Mr DNA isolated from NIH 3T3 cells soon after infection with a recombinant AAV vector containing the human cytomegalovirus (CMV) immediate-early gene promoter-driven bacterial β-galactosidase (lacZ) reporter gene revealed the presence of AAV single-stranded DNA. These results are shown in Fig. 1A. In these experiments, equivalent numbers of Raji (nonpermissive for AAV binding and entry) (24), 293 (permissive for viral binding and entry) (23), and NIH 3T3 cells were either mock infected or infected with 104 particles/cell of cesium chloride density gradient-purified recombinant vCMVp-lacZ vector for 2 h at 37°C, following which cells were treated with 0.05% trypsin and washed extensively with phosphate-buffered saline (PBS) to remove any virus particles adsorbed to cellular receptors in the plasma membrane, and low-Mr DNA samples were isolated. Equivalent amounts were analyzed on Southern blots with a 32P-labeled DNA probe specific for lacZ gene sequences as previously described (19, 24). These data suggest that AAV does indeed gain entry into NIH 3T3 cells. The apparent lack of transgene expression in NIH 3T3 cells noted previously (24) was not due to the inactivity of the CMV promoter, since abundant expression of the lacZ gene could be detected in plasmid DNA transfection experiments (data not shown).
FIG. 1.
(A) Southern blot analysis of viral DNA entry into cells. Equivalent numbers of Raji, 293, and NIH 3T3 cells were either mock infected or infected with the recombinant vCMVp-lacZ vector (104 particles/cell), trypsinized, and washed extensively, and low-Mr DNA samples were isolated and analyzed with the 32P-labeled lacZ DNA probe as described previously (19, 24). ssDNA, viral single-stranded DNA genomes. (B) EMSA for detection of the phosphorylation status of the ssD-BP in NIH 3T3 cells. Equivalent numbers of cells were either mock treated or treated with the indicated amounts of tyrphostin 1 at 37°C for 2 h. Whole-cell extracts were prepared and used in EMSAs with the 32P-labeled single-stranded AAV DNA probe, as described previously (16, 25). Phosphorylated and dephosphorylated forms of the ssD-BP are denoted by the arrow and the arrowhead, respectively. (C) AAV-mediated transgene expression. Equivalent numbers of HeLa (left) and NIH 3T3 (right) cells were infected with 5 × 103 particles/cell of vCMVp-lacZ and then treated with the indicated amounts of tyrphostin 1 for 2 h under identical conditions. Forty-eight hours postinfection, β-galactosidase activity was determined as described in the text.
Although treatment of NIH 3T3 cells with tyrphostin 1, a specific inhibitor of EGFR-PTK (16), led to dephosphorylation of the ssD-BP as determined by electrophoretic mobility shift assays (EMSAs), transgene expression in tyrphostin 1-treated NIH 3T3 cells could not be detected by a cytochemical staining method (24). EMSAs were performed as previously described (16, 25). Briefly, 10 μg of each whole-cell extract was preincubated with 2 μg of poly(dI-dC), 2 μg of bovine serum albumin (BSA), and 12% glycerol in HEPES buffer (pH 7.9) for 10 min at 25°C. Following preincubation, 10,000 cpm of 32P-labeled d(−) sequence synthetic oligonucleotide (5′-AGGAACCCCTAGTGATGGAG-3′) was added to the reaction mixture and incubated for 30 min at 25°C. The bound complexes were separated from the free probe by electrophoresis on 4% polyacrylamide gels. The ratio of dephosphorylated to phosphorylated forms of ssD-BP was determined by densitometric scanning of autoradiograms with a Digital Imaging System Alphaimager (Alpha Innotech Corp., San Leandro, Calif.). These results are shown in Fig. 1B. Tyrphostin 1 treatment of NIH 3T3 cells resulted in a change of the ratio of dephosphorylated to phosphorylated forms of ssD-BP from 0.8 to 1.2. Accordingly, when NIH 3T3 cells were infected with the recombinant AAV vector (5 × 103 particles/cell) by a more sensitive assay than cytochemical staining, a low level of AAV-mediated transgene expression could be detected 48 h later in these cells treated with higher concentrations of tyrphostin 1. In these experiments, β-galactosidase activity was measured with the Galacto-Light Plus chemiluminescent reporter assay (Tropix, Inc., Bedford, Mass.) according to the manufacturer's instructions; results were within the linear range. These data, expressed as relative light units (RLU) per microgram of total protein, are shown in Fig. 1C. HeLa cells were used as a positive control in these experiments, since tyrphostin 1 treatment has previously been shown to significantly augment AAV-mediated transgene expression in these cells by decreasing the ratio of phosphorylated to dephosphorylated forms of ssD-BP (16). Whereas there was a dose-dependent increase in AAV transduction efficiency in both cell types, the extent of transgene expression was roughly 2 orders of magnitude lower in NIH 3T3 cells. These results corroborate that AAV does indeed enter the NIH 3T3 cells.
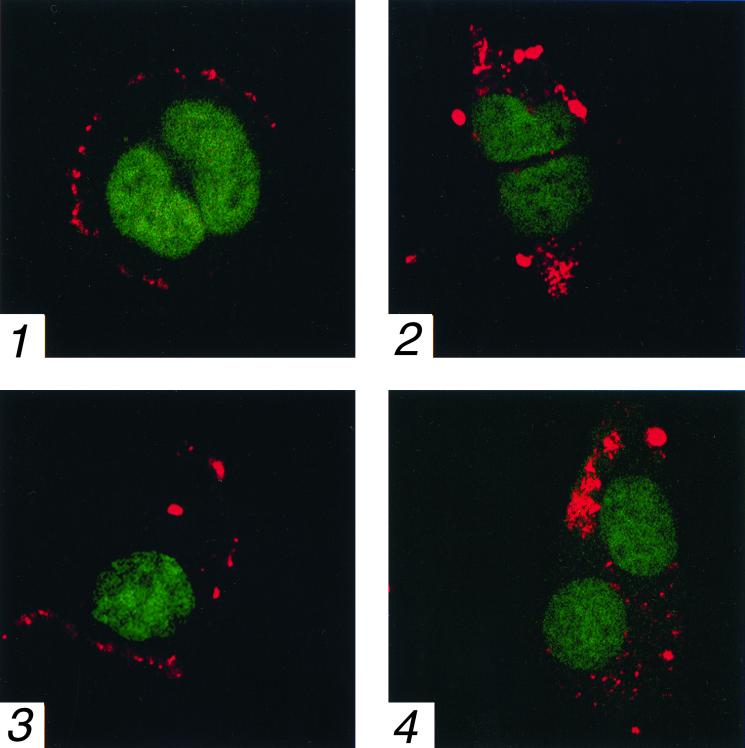
Entry of the virus into NIH 3T3 cells was further confirmed with AAV that was fluorescently labeled with Cy3 (Amersham Life Sciences, Pittsburgh, Pa.) as described previously (2). 293 and NIH 3T3 cells were plated onto polylysine-treated coverslips and 24 h later were infected at 37°C with 104 particles per cell of Cy3-labeled AAV in medium containing 0.1% BSA. At various time points, cells were washed three times with medium containing 0.1% BSA and fixed at 4°C for 20 min with PBS containing 2% formaldehyde and 0.2% glutaraldehyde. Cellular nucleic acid was subsequently counterstained with 1 μM Syto-16 (Molecular Probes, Inc., Eugene, Oreg.) for 30 min at 25°C. Cells were then washed three times with PBS, mounted on glass slides, and visualized with a Zeiss LSM 510 confocal microscope. A series of 0.3-μm optical sections were made through the cells and images representative of the center of the cells were compared to assess the localization of viral particles. These results are shown in Fig. 2. It is evident that within 15 min, AAV could bind efficiently to both 293 (Fig. 2, panel 1) and NIH 3T3 (panel 3) cells. By 2 h, entry of AAV into 293 (panel 2) and NIH 3T3 (panel 4) cells was also clearly seen. As expected, M07e cells, known not to bind AAV due to lack of expression of one of the coreceptors (16, 21, 24), failed to bind the fluorescent-labeled virus (data not shown).
FIG. 2.
Confocal microscopy for localization of AAV particles in 293 and NIH 3T3 cells. At 15 min (panels 1 and 3) and 2 h (panels 2 and 4) postinfection with 104 particles/cell of Cy3-labeled AAV, 293 (panels 1 and 2) and NIH 3T3 (panels 3 and 4) cells were visualized as described in the text. Cy3-labeled AAV (red) and cellular nucleic acids (green) are shown in images representative of the center of the cells. Magnification, ×630.
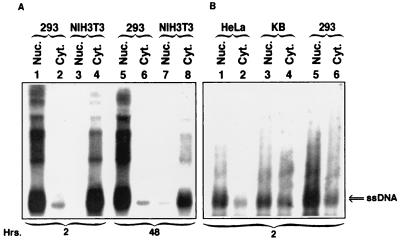
Since these data are more qualitative than quantitative and do not take into account the kinetics of intracellular trafficking and uncoating of AAV in the two cell types being compared, we hypothesized that despite successful entry, a significant fraction of AAV vectors fails to enter the nucleus in NIH 3T3 cells. This hypothesis was experimentally tested as follows. Southern blot analyses of low-Mr DNA isolated at various times postinfection were carried out with cytoplasmic as well as nuclear fractions isolated from 293 and NIH 3T3 cells infected under identical conditions with the CMVp-lacZ vector (104 particles/cell) as described above. Nuclear and cytoplasmic fractions were prepared as described previously (32) from equivalent numbers of cells, followed by isolation of low-Mr DNA. These results are shown in Fig. 3A. It is evident that within 2 h, a substantial amount (∼76%) of the input single-stranded AAV DNA is present in the nucleus in 293 cells, whereas essentially all (∼99%) of the signal is detected in the cytoplasm in NIH 3T3 cells, as determined by densitometric scanning of autoradiographs. By 48 h, a small portion (∼18%) of the input AAV DNA does enter the nucleus in NIH 3T3 cells, but the majority (∼82%) of the signal is still in the cytoplasm. In contrast, ∼78% of the input viral DNA is in the nucleus, and ∼22% of the signal is in the cytoplasm of 293 cells 48 h postinfection. The purity of each fraction was determined to be >95%, as measured by the absence of acid phosphatase activity (in the nuclear fraction) and the absence of histone H3 (in the cytoplasmic fraction), by Western blot analysis with αH3 antibody (Upstate Biotechnology, Lake Placid, N.Y.) (data not shown).
FIG. 3.
(A) Southern blot analysis of subcellular distribution of the viral DNA in 293 and NIH 3T3 cells. Approximately 2 × 106 cells of each type were either mock infected or infected with the recombinant vCMVp-lacZ vector (104 particles/cell), trypsinized, and washed extensively. Low-Mr DNA samples were isolated at various indicated time points from purified nuclear (Nuc.) and cytoplasmic (Cyt.) fractions and analyzed with the 32P-labeled lacZ DNA probe as described in the legend to Fig. 1. (B) Southern blot analyses of AAV entry and trafficking into the nucleus of permissive human cell lines. Equivalent numbers of HeLa, KB, and 293 cells were infected with the recombinant vCMVp-lacZ vector, trypsinized, and washed extensively, and low-Mr DNA samples were isolated 2 h postinfection from purified nuclear and cytoplasmic fractions. The rest of the analysis on Southern blots was performed as described above.
We have previously reported that while AAV binds to HeLa and KB cells more efficiently than to 293 cells, AAV-mediated transduction of HeLa and KB cells is significantly lower than that of 293 cells (23). Therefore, we compared the efficiency of AAV trafficking into the nucleus of these three cell types. These results are shown in Fig. 3B. It is clear that despite less-efficient binding, the extent of AAV entry as well as trafficking into the nucleus in 293 cells is significantly higher than that in HeLa and KB cells. Thus, in addition to the phosphorylation status of the cellular ssD-BP (23), AAV transduction efficiency among permissive human cells also correlates well with the extent of viral trafficking into the nucleus. Taken together, these studies establish that efficient translocation to the nucleus is essential for successful transduction of cells by AAV vectors.
It is interesting to note that although AAV transduction efficiency of murine cells in general has been reported to be low (18), the exceptions include the muscle and the brain tissues (10, 12, 17, 41). We have previously suggested that this might be due to overabundant expression of fibroblast growth factor receptor 1, a coreceptor for AAV infection (24), and the lack of expression of the EGFR, PTK activity of which catalyzes the phosphorylation of the ssD-BP, resulting in failure to synthesize the viral second-strand DNA (16). It would be of interest to now examine the kinetics of AAV trafficking into the nucleus in primary murine cells as well as human cells that are transduced differentially by AAV vectors, to substantiate the observations reported here. Indeed, our recent studies suggest that impaired intracellular trafficking of AAV into the nucleus in primary murine hematopoietic progenitor cells limits high-efficiency transduction of these cells, both in vitro and in vivo (39).
Virtually nothing is known about the intracellular trafficking of AAV particles following infection. However, a wealth of information is available on the underlying mechanisms of cytoplasmic transport and nuclear import of other viruses (11). For instance, herpesvirus binds to the cellular protein, dynein, a minus-end-directed motor protein, which transports the viral particle along microtubules toward the nucleus where the viral DNA is released through the nuclear pore complex into the nucleus (29). Adenovirus, on the other hand, first enters the endosomal pathway and, after acidification of the endosome, is released into the cytoplasm where it appears to bind microtubules prior to nuclear entry (38). Since both herpesviruses and adenoviruses provide the helper function for a productive infection by AAV (3, 18) and since both adenovirus and AAV use αVβ5 integrin as a coreceptor (36), it is reasonable to suggest that trafficking of AAV into the nucleus might be accomplished by similar mechanisms as those employed by its helper viruses. Thus, further studies on the mechanisms of postreceptor entry, transport into the nucleus, and uncoating of AAV should allow a clearer understanding of molecular events involved in AAV-mediated high-efficiency transduction which, in turn, should lead to improvements in the optimal use of AAV vectors in human gene therapy.
Acknowledgments
We thank Hal E. Broxmeyer for a critical review of the manuscript as well as for his support. We also thank Johnny He for his helpful suggestions.
This research was supported in part by Public Health Service grants (HL-53586, HL-58881, and DK-49218; Centers of Excellence in Molecular Hematology) from the National Institutes of Health and a grant from the Phi Beta Psi Sorority.
REFERENCES
- 1.Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by recombinant adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. doi: 10.1089/hum.1996.7.7-841. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett J S, Samulski R J, McCown T J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 3.Berns K I, Bohenzky R A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–307. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- 4.Berns K I, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- 5.Carter B J, Flotte T R. Development of adeno-associated virus vectors for gene therapy of cystic fibrosis. Curr Top Microbiol Immunol. 1996;218:119–144. doi: 10.1007/978-3-642-80207-2_8. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Lu D, Podsakoff G, Wong K K., Jr Strategies for efficient gene transfer into hematopoietic cells: the use of adeno-associated virus vectors in gene therapy. Ann N Y Acad Sci. 1995;770:79–90. doi: 10.1111/j.1749-6632.1995.tb31045.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzog R W, Yang E Y, Couto L B, Hagstrom J N, Elwell D, Fields P A, Burton M, Bellinger D A, Read M S, Brinkhous K M, Podsakoff G M, Nichols T C, Kurtzman G J, High K A. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 10.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O'Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–153. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 11.Kasamatsu H, Nakanishi A. How do animal viruses get to the nucleus? Annu Rev Microbiol. 1998;52:627–686. doi: 10.1146/annurev.micro.52.1.627. [DOI] [PubMed] [Google Scholar]
- 12.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotin R M, Menninger J C, Ward D C, Berns K I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 15.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L A, Laughlin C A, McLaughlin S K, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mah C, Qing K Y, Khuntirat B, Ponnazhagan S, Wang X-S, Kube D M, Yoder M C, Srivastava A. Adeno-associated virus 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J Virol. 1998;72:9835–9843. doi: 10.1128/jvi.72.12.9835-9843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCown T J, Xiao X, Li J, Breese G R, Samulski R J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 18.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 19.Ponnazhagan S, Mukherjee P, Wang X-S, Qing K Y, Kube D M, Mah C, Kurpad C, Yoder M C, Srour E F, Srivastava A. Adeno-associated virus type 2-mediated transduction of primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J Virol. 1997;71:8262–8267. doi: 10.1128/jvi.71.11.8262-8267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponnazhagan S, Mukherjee P, Yoder M C, Wang X-S, Zhou S Z, Kaplan J, Wadsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1997;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- 21.Ponnazhagan S, Wang X-S, Woody M J, Luo F, Kang L Y, Nallari M L, Munshi N C, Zhou S Z, Srivastava A. Differential expression in human cells from the p6 promoter of human parvovirus B19 following plasmid transfection and recombinant adeno-associated virus 2 (AAV) infection: human megakaryocytic leukaemia cells are non-permissive for AAV infection. J Gen Virol. 1996;77:1111–1122. doi: 10.1099/0022-1317-77-6-1111. [DOI] [PubMed] [Google Scholar]
- 22.Ponnazhagan S, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J Virol. 1997;71:3098–3104. doi: 10.1128/jvi.71.4.3098-3104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qing K Y, Khuntirat B, Mah C, Kube D M, Wang X-S, Ponnazhagan S, Zhou S Z, Dwarki V J, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qing K Y, Mah C, Hansen J, Zhou S Z, Dwarki V J, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 25.Qing K Y, Wang X-S, Kube D M, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci USA. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera V M, Ye X, Courage N L, Sachar J, Cerasoli F, Wilson J M, Gilman M. Long-term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc Natl Acad Sci USA. 1999;96:8657–8662. doi: 10.1073/pnas.96.15.8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samulski R J, Zhu X, Xiao X, Brook J D, Houseman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder R O, Miao C, Meuse L, Tubb J, Donahue B A, Lin H-F, Stafford D W, Patel S, Thompson A R, Nichols T C, Read M S, Bellinger D A, Brinkhous K M, Kay M A. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 29.Sodeik B, Ebersold M, Helenius A. Dynein mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantley M, Muzyczka N, Byrne B J, Atkinson M, Flotte T R. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 32.Sperinde V, Nugent M A. Heparan sulfate proteoglycans control intracellular processing of bFGF in vascular smooth muscle cells. Biochemistry. 1998;37:13153–13164. doi: 10.1021/bi980600z. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava A. Delivery systems for gene therapy: adeno-associated virus 2. In: Quesenberry P J, Stein G S, Forget B G, Weissman S M, editors. Stem cell biology and gene therapy. New York, N.Y: Wiley-Liss, Inc.; 1998. pp. 257–288. [Google Scholar]
- 34.Srivastava A. Parvoviral vectors for human hematopoietic gene therapy. In: Fairbairn L J, Testa N, editors. Blood cell biochemistry. 8. Hematopoiesis and gene therapy. New York, N.Y: Kluwer Academic/Plenum Publishers; 1999. pp. 89–122. [Google Scholar]
- 35.Srivastava A, Wang X-S, Ponnazhagan S, Zhou S Z, Yoder M C. Adeno-associated virus 2-mediated transduction and erythroid lineage-specific expression in human hematopoietic progenitor cells. Curr Top Microbiol Immunol. 1996;218:93–117. doi: 10.1007/978-3-642-80207-2_7. [DOI] [PubMed] [Google Scholar]
- 36.Summerford C, Bartlett J S, Samulski R J. αVβ5 integrin: a co-receptor for adeno-associated virus 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 37.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suomalainen M, Nakano M Y, Keller S, Boucke K, Stidwell R P, Greber U F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan, M. Q., K. Y. Qing, M. C. Yoder, and A. Srivastava. Adeno-associated virus 2-mediated transduction of primary murine hematopoietic progenitor cell in vitro and in vivo. Blood, in press.
- 40.Walsh C E, Liu J M, Miller J L, Nienhuis A W, Samulski R J. Gene therapy for human hemoglobinopathies. Proc Soc Exp Biol Med. 1993;204:289–300. doi: 10.3181/00379727-204-43665. [DOI] [PubMed] [Google Scholar]
- 41.Xiao X, Li L, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]