Abstract
Homologs of the Epstein-Barr virus (EBV) SM protein exist in several human and nonhuman herpesviruses. Structure and function differ significantly among these proteins. We have cloned and characterized the human herpesvirus 8 (HHV8) gene, KS-SM, which is homologous to the EBV SM and herpes simplex virus ICP27 genes, from an HHV8-infected primary effusion lymphoma. KS-SM is shown to be a posttranscriptional activator of gene expression in cotransfection studies. KS-SM activated gene expression in a gene-specific, promoter-independent manner. In particular, KS-SM enhanced the expression of KDR/flk-1, a receptor for vascular endothelial growth factor (VEGF), in cotransfection studies. Since expression of KDR/flk-1 is increased in Kaposi's sarcoma and HHV8-infected cell cultures and VEGF enhances the proliferation of HHV8-infected cells, KS-SM may play a pathogenic role in Kaposi's sarcoma.
The newly discovered human herpesvirus, Kaposi's sarcoma (KS)-associated herpesvirus or human herpesvirus 8 (HHV8), is causally associated with KS, primary effusion lymphoma, and multicentric Castleman's disease (for a review, see reference 1 and references therein). The role of HHV8 infection in the development of KS has not been fully characterized. However, several aspects of HHV8 gene expression are likely to be central to the pathogenesis of KS. First, many HHV8 gene products expressed during the lytic cycle of replication have angiogenic and antiapoptotic properties (1). Second, many of these proteins are secreted viral homologs of cellular cytokines (53). Expression of lytic cycle proteins has been demonstrated both in KS tumor biopsy specimens and in HHV8-infected human umbilical vein endothelial cells (14, 52). Thus, it is likely that formation of KS tumors does not conform to the paradigm of an abnormally proliferating clone of virus-transformed cells. Rather, a subset of infected cells that are permissive of lytic HHV8 replication may secrete factors that enhance proliferation of neighboring uninfected and latently infected cells by a paracrine mechanism (12, 14). Several lines of evidence also indicate that dysregulation of the vascular endothelial growth factor (VEGF)-VEGF receptor axis plays a critical role in the development of KS. KS cells express high levels of VEGF; VEGF receptors are upregulated in KS cell cultures, HHV8-infected human umbilical vein endothelial cells, and tumor tissues (3, 14, 33); downregulation of VEGF expression inhibits growth of KS cells in vitro and in nude mice (30).
Epstein Barr virus (EBV) encodes a protein (SM, also known as BMLF1, Mta, and EB2) that is a posttranscriptional regulator of gene expression (5, 8, 9, 45, 57). Homologous genes have been described in human alpha-, beta-, and gammaherpesviruses. Examples include herpes simplex virus (HSV) ICP27/IE63, human cytomegalovirus (CMV) UL69, varicella-zoster virus open reading frame (ORF) 4, and herpesvirus saimiri (HVS) IE52/ORF57 (7, 11, 20, 31, 34, 41). Despite similarity among these various proteins, they exhibit considerable functional and structural diversity, which is likely to be related to differences in the biologic behavior and host cell tropism of their parent viruses (32, 37, 46, 47, 55, 56). Because of the potential importance of an HHV8 lytic cycle protein that could activate other HHV8 lytic genes as well as host cell genes, we sought to identify and characterize the function of the HHV8 member of this family of proteins. Sequence analysis of the HHV8 genome had revealed the presence of an ORF (ORF57) beginning with a methionine that is homologous to the carboxy-terminal portions of the EBV SM and HVS IE52 genes (13, 43). However, the size of this ORF is only 218 amino acids, compared to more than 400 amino acids in the saimiri and EBV homologs, suggesting the existence of one or more upstream exons. We have cloned and expressed the complete cDNA for the HHV8 gene, termed KS-SM, and characterized its functional properties in transfection assays. Several aspects of KS-SM activity that are potentially important for activation of HHV8 genes and host cell genes that are critical for angiogenesis and KS tumor growth are described.
Cloning and structure of the KS-SM gene.
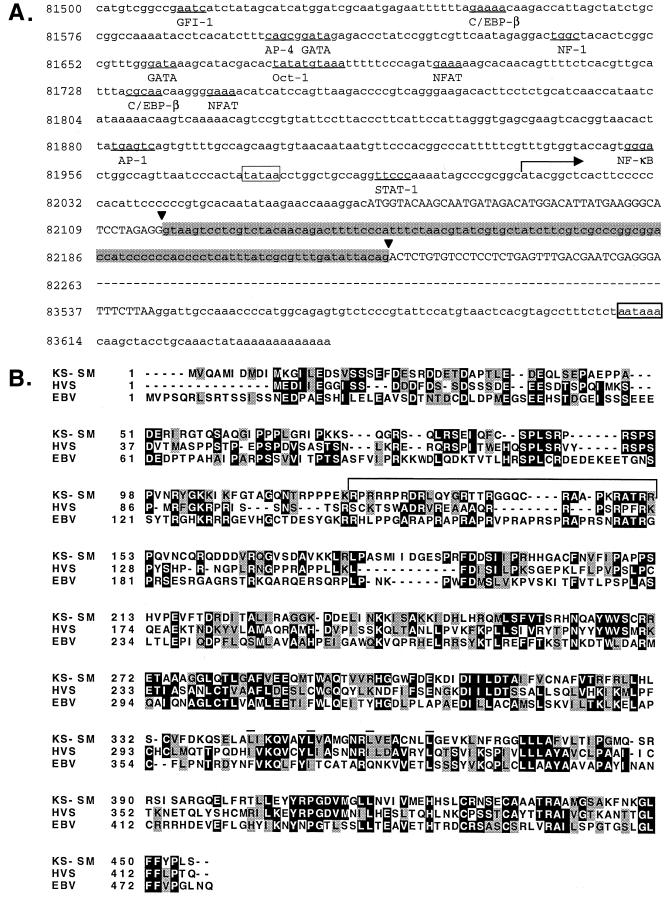
The amino terminus of the KS-SM gene was obtained by 5′ rapid amplification of cDNA ends (RACE) of unfractionated RNA from BCBL1 cells that were treated with 12-tetradecanoyl phorbol acetate (TPA) to induce HHV8 replication as previously described (39, 44). Reverse transcription was performed with a primer in ORF57 consisting of nucleotides (nt) 82883 to 82864 of the HHV8 genome (43), and nested primers complementary to nt 82416 to 82397 and 82428 to 82447 were used to amplify the amino terminus of the KS-SM gene. A full-length cDNA was independently isolated from an oligo(dT) primed BCBL1 library that has been previously described (6). Sequencing of both RACE products and cDNA library clones demonstrated that the KS-SM gene consists of a 49-bp exon spliced to a 1,316-bp exon at canonical splice donor and acceptor sites (Fig. 1A). The gene is predicted to encode a 455-amino-acid protein. Analysis of 700 bp flanking the putative transcriptional start site with the TSSW and MatInspector promoter analysis programs (38, 51) identified a TATA box 88 bp 5′ to the first ATG in the spliced gene and numerous transcription factor binding sites, as shown in Fig. 1A. It should be noted that four potential initiator methionines are present in the first 11 amino acids of the predicted protein (Fig. 1B). Although it is not possible to predict a priori which are used to initiate translation in vivo, all four have a purine in the −3 position and thus may be considered to be in a strong context for initiation (25). The first and third methionines also contain a guanine in the +4 position and may therefore be in a particularly favorable context for initiation.
FIG. 1.
Structure of the KS-SM gene. (A) The KS-SM gene is predicted to encode a 455-amino-acid protein. The first exon (nt 82069 to 82117) and the coding region of the second exon (nt 82226 to 83541) are separated by a 108-bp intron (shaded box). Intron-exon boundaries are shown with vertical arrows. A horizontal arrow shows the approximate transcriptional start site at nt 82014 of the HHV8 genome. A TATA box at −38 bp relative to the transcriptional start site and a canonical hexanucleotide polyadenylation signal 63 bp after the stop codon are enclosed in open boxes. Potential transcription factor-binding sites are underlined, and the corresponding transcription factors are shown below the nucleotide sequence. The nt 82263 to 83534 (represented by a dashed line) are identical to the published BC-1 HHV8 sequence (43) except for the presence of a C instead of a T at position 82959 that does not change the predicted amino acid sequence. (B) Comparison of the amino acid sequence of KS-SM and homologs in HVS and EBV. Amino acids that are identical or similar to KS-SM are shaded black or gray, respectively. A potential leucine zipper in KS-SM is shown with the four component leucines (bars), each separated by six amino acids. The arginine-rich region is bracketed.
The KS-SM gene is similar to its homologs in other human herpesviruses, particularly HVS IE52 (Fig. 1B). Overall, the identity at the amino acid level is approximately 30% among KS-SM, EBV SM, and HVS IE52. As in ICP27 (19), an arginine-rich region and arginine-serine dipeptides characteristic of many RNA-binding proteins (28) are present in KS-SM. Several differences are also present that may be functionally important. First, KS-SM does not contain a leucine-rich nuclear export signal motif potentially capable of binding CRM1 (exportin 1) (15, 16, 35, 54) as found in EBV SM, ICP27, and HVS IE52 (2, 34, 46). Unlike its homologs in other herpesviruses, KS-SM contains a leucine zipper motif between amino acids 343 and 364, suggesting a possible role in dimerization and DNA binding (24, 26) that has not been described for its homologs.
Expression and localization of KS-SM in HHV8-infected and KS-SM-transfected cells.
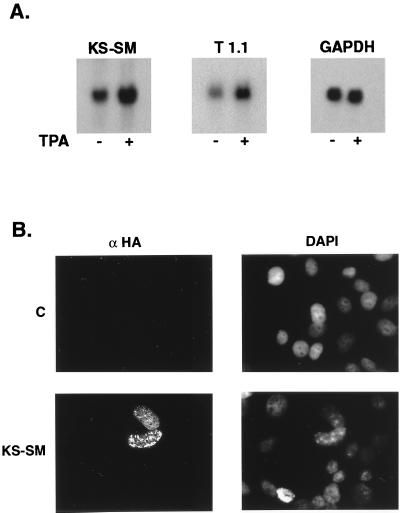
Other herpesvirus genes homologous to KS-SM are expressed during the lytic phase of viral replication. In order to determine whether KS-SM is also expressed in a similar manner, we examined the expression of the KS-SM gene in BCBL1 cells, which are derived from an EBV-negative primary effusion B-cell lymphoma (39). RNA was isolated from untreated BCBL1 cells and from cells treated with TPA to induce lytic replication as previously described (44). Northern blotting demonstrated the presence of a single transcript of 1.8 kb, which correlates well with the predicted size of the cDNA described above (Fig. 2A). Zhu et al. have described a butyrate-inducible, cycloheximide-sensitive 1.5-kb transcript in BC-3 cells that hybridizes to DNA containing a portion of the second exon of KS-SM (59). This transcript is likely to be the mRNA for KS-SM in BC-3 cells. The level of KS-SM induction in BCBL1 cells is similar to that seen with the T1.1 lytic RNA transcript (Fig. 2A). The level of KS-SM expression seen in uninduced cells is likely due to the small percentage of BCBL1 cells that is spontaneously permissive of lytic replication (39).
FIG. 2.
KS-SM expression in HHV8-infected and KS-SM-transfected cells. (A) KS-SM is expressed during lytic replication of HHV8. BCBL1 cells were induced to permit lytic replication of HHV8 with 20 ng of TPA per ml. RNA was harvested after 48 h from induced (+) or uninduced cells (−). Each RNA (10 μg) was electrophoresed, blotted, and probed with 32P-labeled KS-SM cDNA. The blot was stripped and reprobed for T1.1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. (B) Nuclear localization of KS-SM in transfected Cos-7 cells. KS-SM tagged with influenza virus HA was transfected into Cos-7 cells. Cells were stained with anti-HA monoclonal antibody, 16B12 (Babco-Covance). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) stain. Diffuse speckled intranuclear staining was seen in HA–KS-SM-transfected Cos-7 cells (lower left panel) but not in control-transfected cells (upper left panel). Corresponding DAPI-stained nuclei are shown in the upper right (control) and lower right (KS-SM) panels.
In order to study the intracellular location of KS-SM, we performed immunofluorescence microscopy of cells transfected with a plasmid in which epitope-tagged KS-SM expression was driven by the CMV IE promoter (pCDNA3; Invitrogen Corp.). Cos-7 cells were transfected with influenza hemagglutinin epitope (HA)-tagged KS-SM and examined 48 h after transfection as previously described (2). As shown in Fig. 2B, KS-SM localizes to the nucleus in a speckled pattern similar to that seen with EBV SM and ICP27. We have previously shown that EBV SM translocates from nucleus to cytoplasm upon overexpression of the cellular exportin CRM1 (2). EBV SM has also been shown to shuttle from nucleus to cytoplasm in heterokaryon assays (49). Similarly, ICP27-mediated RNA export is nuclear export signal dependent and is blocked by an inhibitor of CRM1 complex formation (46; S. Silverstein, personal communication). In order to determine whether KS-SM interacts with CRM1 in a similar manner, we overexpressed CRM1 in Cos-7 cells by cotransfection with HA–KS-SM. Unlike the results with EBV SM, no cytoplasmic translocation was observed (data not shown). Thus, although we cannot rule out nucleocytoplasmic shuttling by KS-SM, its interaction with cellular export factors is likely to differ from that of homologs in other herpesviruses.
KS-SM activates gene expression by a posttranscriptional mechanism.
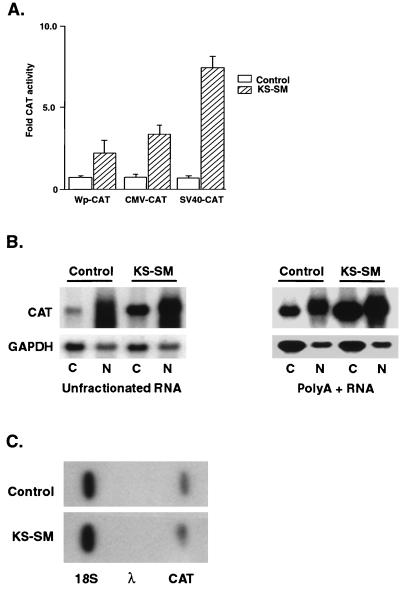
The EBV SM gene, HSV ICP27, and ORF57/IE52 genes all activate expression of other genes via posttranscriptional mechanisms (5, 10, 21, 44, 47, 55). In order to determine whether KS-SM has similar properties, we examined the effect of KS-SM in chloramphenicol acetyltransferase (CAT) reporter assays. BJAB cells, which are derived from an EBV-negative Burkitt lymphoma, were transfected by electroporation with a series of CAT reporter plasmids and either KS-SM expression vector (KS-SM cDNA cloned in pCDNA3) or control vector (Fig. 3A). Cotransfection of KS-SM led to a 4- to 10-fold increase in CAT activity, compared to the control. As has been demonstrated previously for EBV SM, KS-SM was capable of activating CAT transcribed from a variety of promoters (5, 22, 27, 57).
FIG. 3.
Posttranscriptional regulation of gene expression by KS-SM. (A) BJAB cells (from an EBV-negative Burkitt lymphoma) were cotransfected with 10 μg of either KS-SM expression plasmid or control plasmid and intronless CAT reporter constructs driven by either the EBV latent promoter Wp, the CMV IE promoter, or the SV40 late promoter. CAT assays were performed on lysates from cells harvested 18 h after transfection. CAT activity was calculated as percent CAT conversion per microgram of protein, and the results are expressed as fold activation relative to control. Data represent the means ± the standard errors of the means of three independent experiments. (B) KS-SM increases steady-state RNA levels of the target gene. BJAB cells were cotransfected by electroporation with 10 μg of CMV-CAT and either KS-SM or control plasmid. Unfractionated RNA or poly(A) RNA from cytoplasmic and nuclear fractions was harvested 48 h after transfection. Each RNA (10 μg) was electrophoresed, blotted, and probed for CAT expression. (C) KS-SM has no effect on the transcript initiation rate of CMV-CAT. BJAB cells were transfected with either KS-SM or control plasmid and CMV-CAT. Nuclei were harvested 48 h after transfection. In vitro-labeled nuclear transcripts were hybridized to immobilized cDNA corresponding to 18S RNA, λ phage (negative control), or CAT.
To determine if KS-SM-mediated activation occurs at the RNA level, steady-state CAT mRNA levels in KS-SM or control-transfected cells were measured by Northern blotting. RNA was isolated from transfected BJAB cells 48 h after transfection with intronless CMV-CAT and either KS-SM or control plasmids and processed exactly as previously described (44). As shown in Fig. 3B, both nuclear and cytoplasmic levels of the target CAT mRNA were increased in KS-SM-transfected cells. The fact that nuclear levels of target CAT mRNA are also increased suggests that KS-SM-mediated activation of CAT is not simply due to enhanced nucleocytoplasmic transport of CAT mRNA. However, in the presence of KS-SM, the amounts of cytoplasmic CAT poly(A) RNA were increased more than nuclear CAT poly(A) RNA. Direct radiometric quantitation with an Instant Imager (Packard Instruments, Meriden, Conn.) revealed that KS-SM led to a 6-fold increase in cytoplasmic poly(A)+ CAT mRNA versus a 2.6-fold increase in nuclear CAT poly(A)+ mRNA. This finding suggests that KS-SM may facilitate nucleocytoplasmic transport of target mRNAs. Nuclear run-on transcription assays were performed to determine whether KS-SM directly activated transcription (44). As shown in Fig. 3C, no difference in CAT transcript initiation rate was observed in BJAB cells cotransfected with CMV-CAT and KS-SM, compared to control transfected cells. Although it is not possible to rule out direct activation of other promoters by KS-SM, these data indicate that the activating effect seen for CAT expressed from the CMV promoter is posttranscriptional.
Activation by KS-SM is gene-dependent.
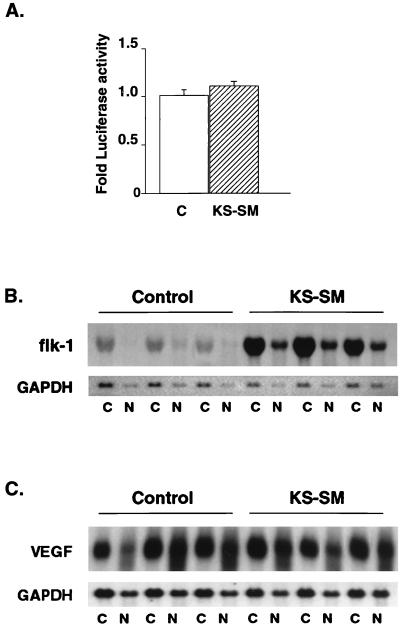
It has previously been shown that the activating effect of EBV SM and ICP27 is gene dependent. For example, EBV SM does not activate β-galactosidase, luciferase, or certain lytic EBV genes in cotransfection assays (21, 29, 49; V. Ruvolo and S. Swaminathan, unpublished observations). Similarly, ICP27 does not activate all HSV genes equally (40, 50). The difference in the sensitivity of various genes to activation by EBV SM and ICP27 remains to be completely explained. However, these differences may be due to multiple factors, including the sequence of the gene itself, as well as the sequence of the 3′ untranslated region (4, 23, 44, 49). In order to determine whether KS-SM function is also gene dependent, we performed cotransfection assays with KS-SM and intronless luciferase expression constructs. KS-SM did not activate luciferase expression (Fig. 4A).
FIG. 4.
KS-SM-mediated activation is gene dependent. (A) BJAB cells were cotransfected by electroporation with either KS-SM or control plasmid and an SV40-luciferase reporter plasmid (pGL3 promoter; Promega). Cells were harvested 48 h after transfection, and the luciferase assay was performed per the manufacturer's protocol. The results are represented as fold luciferase activity relative to control. Data represent the means ± the standard errors of the means of three independent experiments. (B) KS-SM upregulates KDR/flk-1 expression. BJAB cells were cotransfected with KS-SM or control and KDR/flk-1 cDNA expression plasmid. RNA from cytoplasmic or nuclear fractions was isolated 18 h after transfection. Each RNA (10 μg) was electrophoresed, blotted, and probed with 32P-labeled flk-1 cDNA. The blot was stripped and reprobed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (C) KS-SM does not affect VEGF expression. BJAB cells were cotransfected with CMV-VEGF and either KS-SM or control. RNA from cytoplasmic or nuclear fractions was isolated and probed with radiolabeled VEGF cDNA as described for panel B.
Since increased expression of the VEGF receptor KDR/flk-1 may be a necessary step in proliferation and transformation of HHV8-infected cells (14), we examined the effect of KS-SM on the expression of VEGF and KDR/flk-1. Cotransfection assays were performed with CMV-driven expression vectors that contained either VEGF or KDR/flk-1 cDNAs (42, 58) and either KS-SM or control plasmid. Northern blots of RNA from transfected BJAB cells revealed that KS-SM did not activate VEGF expression. Quantitative measurements indicated that the ratio of VEGF mRNAs in the presence or absence of KS-SM was 1.1 (Fig. 4C). However, KS-SM expression led to an approximately sixfold increase in KDR/flk-1 expression (Fig. 4B). These data confirm that KS-SM-mediated activation is gene dependent. The fact that KDR/flk-1 expression is enhanced by KS-SM suggests that KS-SM may play a role in mediating changes in endothelial cell gene expression associated with the HHV8-transformed phenotype. However, it should be noted that these results were obtained in transient transfection experiments with KDR/flk-1 cDNA. The KDR/flk-1 gene is multiply spliced, and its expression is highly restricted to cells of endothelial lineage (36, 58). Therefore, the effect of KS-SM on KDR expression in endothelial cells and its physiological role in endothelial cell growth and VEGF responsiveness remain to be directly determined.
Effect of introns on activation by KS-SM.
Several members of the SM family, including ICP27, HVS IE52, and EBV SM, appear to inhibit the expression of genes containing introns. Such inhibition may be due to interference with the normal processing of intron-containing pre-mRNAs (4, 17, 18). In order to determine whether the presence of introns similarly interferes with KS-SM-mediated activation, we performed CAT activation assays with several intron-containing reporter constructs. Cotransfection experiments were performed with intron-containing CMV-CAT, Wp-CAT, or simian virus 40 (SV40) CAT and KS-SM or control plasmid. In each case, a slight decrease in reporter activity was found in the presence of KS-SM (Fig. 5A). The inhibition was not as marked as seen in similar experiments with EBV SM (44). However, in no instance was there an increase in CAT activity as seen with reporter plasmids that were identical except for the absence of the intron (Fig. 3A).
FIG. 5.
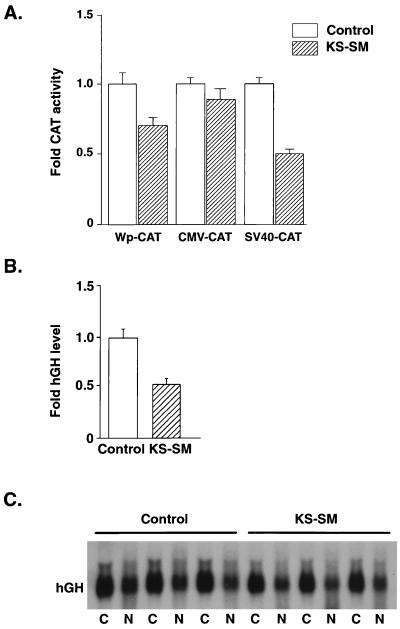
KS-SM does not activate intron-containing reporter genes. (A) BJAB cells were cotransfected with 10 μg of KS-SM or control plasmid and a CAT reporter plasmid driven by either the EBV latent promoter Wp, the CMV IE promoter, or the SV40 late promoter. CAT assays were performed as described in the legend for Fig. 3. Data represent the means ± the standard errors of the means of three independent experiments. (B) Effect of KS-SM on a gene with multiple introns. BJAB cells were transfected with a plasmid (pXGH5) encoding genomic hGH, which contains four introns (48) and either KS-SM or control plasmid. Radioimmunoassays for hGH were performed by using the medium from cells 48 h after transfection. (C) Effect of KS-SM on hGH RNA levels. BJAB cells were cotransfected with pXGH5 and either KS-SM or control plasmid as above. RNA isolated from cytoplasmic or nuclear fractions was electrophoresed, blotted, and probed with 32P-labeled hGH cDNA.
We also examined the effect of KS-SM on an expression construct that encodes a genomic copy of the human growth hormone (hGH) gene containing four introns (48). Expression of the hGH gene has previously been shown to be markedly inhibited by EBV SM at the posttranscriptional level (44). Reporter assays were performed with BJAB cells transfected with intron-containing hGH reporter plasmid and KS-SM or control plasmid. hGH secreted by the transfected cells was then measured by radioimmunoassay of the growth medium (Nichols Institute, San Juan Capistrano, Calif.). In addition, hGH mRNA levels were measured by Northern blotting. In both cases, there was a moderate inhibition of hGH expression by KS-SM. Secreted hGH levels in the presence of KS-SM were 51% of those of the control (Fig. 5B), and hGH RNA levels in KS-SM-transfected cells were 82% of those in control transfected cells (Fig. 5C). These results indicate that although the presence of introns may interfere with activation by KS-SM, KS-SM may not possess a strong intrinsic inhibitory function compared with EBV SM or ICP27.
In summary, we have cloned the KSHV/HHV8 member of a family of herpesvirus gene regulatory proteins that includes HSV ICP27 and EBV SM, among others. This HHV8 gene (KS-SM) is shown to be a nuclear protein expressed during lytic replication that posttranscriptionally activates the expression of reporter genes in B lymphocytes. KS-SM leads to increased accumulation of target mRNAs, particularly in the cytoplasm, although we have no direct evidence of a role for KS-SM in nuclear mRNA export. Activation by KS-SM is also gene dependent. In addition, the presence of introns in the target gene appears to interfere with KS-SM-mediated activation. Such an effect may be important in selectively enhancing expression of HHV8 genes, which are predominantly intronless. However, the net effect of KS-SM on the expression of a specific gene is likely to depend on multiple gene-specific factors. These include the presence or absence of introns as well as the 3′ untranslated region and the coding sequence of the gene. These data suggest that KS-SM, like its counterparts in HSV, EBV, and HVS, may be important for the activation of other viral lytic genes, particularly those that are encoded as unspliced ORFs, and for facilitation of the lytic cascade. Our data also suggest that KS-SM could play a role in enhancing proliferation of HHV8-infected endothelial cells by upregulating host cell genes such as KDR/flk-1. Although the KDR/flk-1 gene is spliced from an extremely long transcript in vivo (36), the strongly stimulatory effect on expression of KDR/flk-1 cDNA leaves open the possibility that KS-SM enhances KDR/flk-1 expression in vivo and thereby increases VEGF-mediated angiogenesis. Physiological expression of cellular KDR/flk-1 expression is highly restricted to cells of endothelial origin (36). Therefore, any potential role for KS-SM in enhancing cellular expression of KDR/flk-1 needs to be validated in endothelial cells. Nevertheless, KS-SM activation of HHV8 and critical host cell genes may play an important role in the pathogenesis of KS and provide potential targets for specific antiviral and antiangiogenic therapy.
Acknowledgments
This work was supported by Public Health Service grants CA 81133-01 and CA 82985-01 from the National Cancer Institute (S.S.), grants HL 03658-01 and HL 61656-01 from the National Heart, Lung and Blood Institute (C.P.), and a grant from the John Sealy Memorial Endowment Fund (S.S.).
We thank B. Chandran (University of Kansas Medical Center, Kansas City, Kans.) for the HHV8 cDNA library.
REFERENCES
- 1.Anonymous. Kaposi's sarcoma herpesvirus/human herpesvirus 8. IARC Monogr Eval Carcinog Risks Hum. 1997;70:375–492. [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle S M, Ruvolo V, Gupta A K, Swaminathan S. Association with the cellular export receptor CRM1 mediates function and intracellular localization of Epstein-Barr virus SM protein, a regulator of gene expression. J Virol. 1999;73:6872–6881. doi: 10.1128/jvi.73.8.6872-6881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown L F, Tognazzi K, Dvorak H F, Harrist T J. Strong expression of kinase insert domain-containing receptor, a vascular permeability factor/vascular endothelial growth factor receptor in AIDS-associated Kaposi's sarcoma and cutaneous angiosarcoma. Am J Pathol. 1996;148:1065–1074. [PMC free article] [PubMed] [Google Scholar]
- 4.Buisson M, Hans F, Kusters I, Duran N, Sergeant A. The C-terminal region but not the Arg-X-Pro repeat of Epstein-Barr virus protein EB2 is required for its effect on RNA splicing and transport. J Virol. 1999;73:4090–4100. doi: 10.1128/jvi.73.5.4090-4100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buisson M, Manet E, Trescol-Biemont M C, Gruffat H, Durand B, Sergeant A. The Epstein-Barr virus (EBV) early protein EB2 is a posttranscriptional activator expressed under the control of EBV transcription factors EB1 and R. J Virol. 1989;63:5276–5284. doi: 10.1128/jvi.63.12.5276-5284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandran B, Smith M S, Koelle D M, Corey L, Horvat R, Goldstein E. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology. 1998;243:208–217. doi: 10.1006/viro.1998.9055. [DOI] [PubMed] [Google Scholar]
- 7.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison III C A, Kouzarides T, Martignetti J A, et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho M S, Jeang K T, Hayward S D. Localization of the coding region for an Epstein-Barr virus early antigen and inducible expression of this 60-kilodalton nuclear protein in transfected fibroblast cell lines. J Virol. 1985;56:852–859. doi: 10.1128/jvi.56.3.852-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook I D, Shanahan F, Farrell P J. Epstein-Barr virus SM protein. Virology. 1994;205:217–227. doi: 10.1006/viro.1994.1637. [DOI] [PubMed] [Google Scholar]
- 11.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 12.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande J P, Weiss R A, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc Natl Acad Sci USA. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleckenstein B, Derosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesvirus. New York, N.Y: Plenum Publishing Corp.; 1992. pp. 253–332. [Google Scholar]
- 14.Flore O, Rafii S, Ely S, O'Leary J J, Hyjek E M, Cesarman E. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 15.Fornerod M, Ohno M, Yoshida M. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 17.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hibbard M K, Sandri-Goldin R M. Arginine-rich regions succeeding the nuclear localization region of the herpes simplex virus type 1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J Virol. 1995;69:4656–4667. doi: 10.1128/jvi.69.8.4656-4667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inchauspe G, Nagpal S, Ostrove J M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989;173:700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 21.Kenney S, Kamine J, Holley-Guthrie E, Mar E C, Lin J C, Markovitz D, Pagano J. The Epstein-Barr virus immediate-early gene product, BMLF1, acts in trans by a posttranscriptional mechanism which is reporter gene dependent. J Virol. 1989;63:3870–3877. doi: 10.1128/jvi.63.9.3870-3877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenney S, Kamine J, Markovitz D, Fenrick R, Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci USA. 1988;85:1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Key S C, Yoshizaki T, Pagano J S. The Epstein-Barr virus (EBV) SM protein enhances pre-mRNA processing of the EBV DNA polymerase transcript. J Virol. 1998;72:8485–8492. doi: 10.1128/jvi.72.11.8485-8492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouzarides T, Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988;336:646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- 25.Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 26.Landschulz W H, Johnson P F, McKnight S L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman P M, O'Hare P, Hayward G S, Hayward S D. Promiscuous trans activation of gene expression by an Epstein-Barr virus-encoded early nuclear protein. J Virol. 1986;60:140–148. doi: 10.1128/jvi.60.1.140-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manley J L, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 29.Markovitz D M, Kenney S, Kamine J, Smith M S, Davis M, Huang E-S. Disparate effects of two herpesviruses immediate-early gene trans-activators on the HIV LTR. Virology. 1989;173:750–754. doi: 10.1016/0042-6822(89)90591-6. [DOI] [PubMed] [Google Scholar]
- 30.Masood R, Cai J, Zheng T, Smith D L, Naidu Y, Gill P S. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci USA. 1997;94:979–984. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy A M, McMahan L, Schaffer P A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGregor F, Phelan A, Dunlop J, Clements J B. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J Virol. 1996;70:1931–1940. doi: 10.1128/jvi.70.3.1931-1940.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura S, Murakami-Mori K, Rao N, Weich H A, Rajeev B. Vascular endothelial growth factor is a potent angiogenic factor in AIDS-associated Kaposi's sarcoma-derived spindle cells. J Immunol. 1997;158:4992–5001. [PubMed] [Google Scholar]
- 34.Nicholas J, Gompels U A, Craxton M A, Honess R W. Conservation of sequence and function between the product of the 52-kilodalton immediate-early gene of herpesvirus saimiri and the BMLF1-encoded transcriptional effector (EB2) of Epstein-Barr virus. J Virol. 1988;62:3250–3257. doi: 10.1128/jvi.62.9.3250-3257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 36.Patterson C, Perrella M A, Hsieh C-M, Yoshizumi M, Lee M-E, Haber E. Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J Biol Chem. 1995;270:23111–23118. doi: 10.1074/jbc.270.39.23111. [DOI] [PubMed] [Google Scholar]
- 37.Perera L P, Kaushal S, Kinchington P R, Mosca J D, Hayward G S, Straus S E. Varicella-zoster virus open reading frame 4 encodes a transcriptional activator that is functionally distinct from that of herpes simplex virus homolog ICP27. J Virol. 1994;68:2468–2477. doi: 10.1128/jvi.68.4.2468-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 40.Rice S A, Knipe D M. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988;62:3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice S A, Knipe D M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruef J, Hu Z Y, Yin L Y, Wu Y, Hanson S R, Kelly A B, Harker L A, Rao G N, Runge M S, Patterson C. Induction of vascular endothelial growth factor in balloon-injured baboon arteries. A novel role for reactive oxygen species in atherosclerosis. Circ Res. 1997;81:24–33. doi: 10.1161/01.res.81.1.24. [DOI] [PubMed] [Google Scholar]
- 43.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruvolo V, Wang E, Boyle S, Swaminathan S. The Epstein-Barr virus nuclear protein SM is both a post-transcriptional inhibitor and activator of gene expression. Proc Natl Acad Sci USA. 1998;95:8852–8857. doi: 10.1073/pnas.95.15.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sample J, Lancz G, Nonoyama M. Mapping of genes in BamHI fragment M of Epstein-Barr virus DNA that may determine the fate of viral infection. J Virol. 1986;57:145–154. doi: 10.1128/jvi.57.1.145-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandri-Goldin R M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;63:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 48.Selden R F, Howie K B, Rowe M E, Goodman H G, Moore D D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986;6:3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semmes O J, Chen L, Sarisky R T, Gao Z, Zhong L, Hayward S D. Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene mRNA. J Virol. 1998;72:9526–9534. doi: 10.1128/jvi.72.12.9526-9534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman T M, Sandri-Goldin R M, Silverstein S J. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solovyev V V, Salamov A A. Proceedings of the Fifth International Conference on Intelligent Systems for Molecular Biology. Halkidiki, Greece: AAAI Press; 1997. The Gene-Finder computer tools for analysis of human and model organisms genome sequences; pp. 294–302. [PubMed] [Google Scholar]
- 52.Sun R, Lin S, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss R A, Whitby D, Talbot S, Kellam P, Boshoff C. Human herpesvirus type 8 and Kaposi's sarcoma. J Natl Cancer Inst Monogr. 1998;23:51–54. doi: 10.1093/oxfordjournals.jncimonographs.a024173. [DOI] [PubMed] [Google Scholar]
- 54.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 55.Whitehouse A, Cooper M, Meredith D M. The immediate-early gene product encoded by open reading frame 57 of herpesvirus saimiri modulates gene expression at a posttranscriptional level. J Virol. 1998;72:857–861. doi: 10.1128/jvi.72.1.857-861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winkler M, Rice S A, Stamminger T. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J Virol. 1994;68:3943–3954. doi: 10.1128/jvi.68.6.3943-3954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong K-M, Levine A J. Identification and mapping of Epstein-Barr virus early antigens and demonstration of a viral gene activator that functions in trans. J Virol. 1986;60:149–156. doi: 10.1128/jvi.60.1.149-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin L Y, Wu Y, Ballinger C A, Patterson C. Genomic structure of the human KDR/flk-1 gene. Mamm Genome. 1998;9:408–410. doi: 10.1007/s003359900783. [DOI] [PubMed] [Google Scholar]
- 59.Zhu F X, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]