Abstract
Tremendous progress has been made in the development of delivery carriers for small RNA therapeutics. However, most achievements have focused on the treatment of liver-associated diseases because conventional lipid and lipidoid nanoparticles (LNPs) readily accumulate in the liver after intravenous (i.v.) administration. Delivering RNAs to other organs and tumor tissues remains an ongoing challenge. Here, we utilized a 540-member combinatorial functional polyester library to discover nanoparticles (NPs) that enable efficacious siRNA delivery to A549 lung cancer cells in vitro and in vivo. PE4K-A13-0.33C6 and PE4K-A13-0.33C10 NPs were efficiently internalized into A549-Luc cells within 4 hours. The addition of PEG 2000 DMG lipid or Pluronic F-127 onto the surface of the polyplexes reduced the surface charge of NPs, resulting in an increase of serum stability. We then explored aerosol delivery of stabilized PE4K-A13-0.33C6 and PE4K-A13-0.33C10 NPs to implanted orthotopic lung tumors. We found that by altering the administration route from i.v. to aerosol, the NPs could avoid liver accumulation and instead be specifically localized only in the lungs. This resulted in significant gene silencing in the A549 orthotopic lung tumors. Due to the ability to deliver siRNA to non-liver targets, this approach provides a privileged route for gene silencing in the lungs.
Keywords: functional polyesters, siRNA, cancer, drug delivery, nanoparticles
1. Introduction
Small RNAs are being evaluated as therapeutics for diverse classes of diseases because they can directly modulate gene expression in cells [1]. Still, their utility has largely been limited to liver disorders [2]. In cancer, short interfering RNAs (siRNA) can silence oncogenes that drive cancer progression and microRNAs (miRNA) can restore natural tumor suppressors [1, 3]. Small RNAs have been explored for attractive targets which are undruggable by conventional small molecules [3-6]. To realize the potential of small RNA therapeutics, tremendous effort has been spent on the development of safe and effective delivery methods. Among these, lipid and lipidoid nanoparticles (LNPs) are the most efficacious for gene silencing in the liver [7-11]. With the remarkable progress in cationic lipid development, the efficacy of LNPs for silencing Factor VII (FVII) in liver hepatocytes has dramatically increased over 3 orders of magnitude in the past 10 years [8-10], with an EC50 of 0.002 mg/kg now realized [10]. Some of these leading materials are being used in ongoing clinical trials for the treatment of hepatic diseases, such as transthyretin-mediated amyloidosis and liver cancer [1, 12-14]. LNPs readily accumulate in the liver because of the discontinuous features of hepatic vasculature [2], typical 80-100 nm diameters of LNPs [1], adsorption of apolipoproteins [10, 15], and can be metabolized following specified lipid metabolism pathways. Intravenous (i.v.) administration of NPs typically results in >80% injected dose accumulation in the liver [2, 16, 17]. Therefore, most all top-performing nanoparticle-based RNA delivery is largely limited to the treatment of liver diseases [2]. In contrast, small RNA delivery to other organs and tumors remains challenging [3, 6]. The development of alternative and efficacious delivery methods to non-liver tissues are thus urgently needed [18-22].
We recently reported the discovery of functional polyesters that are capable of delivering siRNA drugs selectively to lung cancer cells without entering into normal lung cells [23]. Key to this project was the generation of an siRNA delivery platform with tremendous diversity in chemical properties. The biocompatible polymers were built on a degradable polyester backbone where 840 unique modifications were performed to modulate the tertiary amines (pKa), alkyl groups (hydrophobic packing), and polymer length (binding and stability). Selective polyplex nanoparticles (NPs) were identified by high-throughput library screening on a unique pair of matched cancer/normal cell lines obtained from a single patient. Remarkably, the cancer selective nanoparticles were retained inside of tumors in mice for more than one week, while nonselective control nanoparticles were cleared within only a few hours. This translated to improved siRNA-mediated cancer cell death and significant suppression of tumor growth.
The discovery that nanoparticles can be selective to certain cells based only on their physical and chemical properties could potentially alter patient outcomes in the clinic [23]. Academic cancer centers are already using biomarkers, gene expression profiles, and bioinformatics analysis to estimate patient responses to drugs before making therapy decisions. It may be true that patient responses to NP carriers, such as Doxil or Abraxane, should also be modeled and estimated to personalize treatment and increase drug delivery accuracy. A recent study using a positron emission tomography (PET) liposomal nanoreporter correlated Doxil responses to the heterogeneity of tumors [24]. This kind of analysis may benefit nanoparticle therapeutics that show selectivity to particular organs and cells.
Given these issues, there remains a need to further develop NPs that could be delivered to targeted organs (such as the lungs) and provide wide distribution without cellular specificity. Our prior work focused on achieving selectivity at the cellular level to specific patient cells in culture and in xenografts [23]. In this paper, we focus on organ level selectivity and have employed surgically implanted orthotopic tumors. We aimed to develop a more general delivery approach that may not need patient customization, and therefore utilized the more common and readily available A549 lung cancer cell line. We developed and optimized aerosol delivery [25-27] of functional polyester NPs to deliver siRNA locally to the lung. Among our candidate polyesters [23], we identified polymer compositions that were able to deliver to lung, cervical, and breast cancer cells without showing selectivity. These two features combined to allow siRNA NPs to effectively accumulate in the lungs after aerosol inhalation and enable efficacious gene silencing in orthotopic lung tumors. This work demonstrates that changing the administration method from i.v. injection to aerosol inhalation can overcome the small RNA biodistribution challenge by re-directing liver accumulating NPs to the lungs. Moreover, this work opens new engineering opportunities to reduce cell specialization and promote broad lung distribution of a variety of cancer therapeutics.
2. Materials and methods
2.1. Materials
Organic solvents were purchased from Fisher Scientific or Sigma-Aldrich and used as received. Amines, thiols, trimethylolpropane allyl ether (TPAE), suberoyl chloride (diACl-C8), 2,2-dimethoxy-2-phenylacetophonone (DMPA), γ-thiobutyrolactone (TBL) Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and phosphate buffered saline (PBS) were purchased from Sigma-Aldrich. 4’,6-Diamidino-2-phenylindole (DAPI), LysoTracker Green, and Quant-iT RiboGreen RNA assay kits were purchased from Life Technologies. The ONE-Glo + Tox luciferase assay kit was purchased from Promega. Matrigel was purchased from BD Biosciences. Custom-synthesized siRNA against Luciferase (siLuc) (sense: 5’-GAUUAUGUCCGGUUAUGU A[dT][dT]-3’; antisense: 5’-UACAUAACCGGACAUAAUC [dT][dT]-3’), and Cy5.5-labeled siRNA (Cy5.5-siRNA) (sense: 5’-GAUUAUGUCCGGUUAUGUA[dT][dT]-Cy5.5-3’; antisense: 5’-UACAUAACCGGACAUAAUC[dT][dT]-3’) were purchased from Sigma-Aldrich. PEO80-PPO27-PEO80 (Pluronic F-68, Mw = 8,400, PDI = 1.06) and PEO101-PPO56-PEO101 (Pluronic F-127, Mw = 12,600, PDI = 1.05) were obtained from Sigma-Aldrich. PEG 2000 DMG lipid (Sunbright GM-020, 1,2-dimyristoyl-sn-glycerol, methoxypolyethylene glycol) was purchased from NOF America.
2.2. Preparation and characterization of siRNA polyplex NPs
Amino thiols, -ene functional polyesters with Mw = 4,200 g/mol (PE4K) and Mw = 8,300 g/mol (PE8K), and the consequent functional polyester library were synthesized according to our previous reports (Scheme S1) [23, 28]. For in vitro siRNA delivery, polyplex NPs of functional polyesters and siRNA were prepared by adding polymer DMSO solution (3 g/L) into citric acid/trisodium citrate buffer (pH 4.2, 10 mM) at a polymer/siRNA ratio of 30:1 (wt/wt) and final siRNA concentration of 2.5 ng/uL. For in vivo siRNA delivery, the NPs were prepared following the same procedure, except that 5 wt. % F-127 was added to the polymer stock solution prior to mixing with siRNA. The NPs were dialyzed against PBS (4 h) for i.v. injection or aerosol inhalation in mice. The size and surface charge of the NPs were measured using a Zetasizer Nano ZS (Malvern, He-Ne laser, λ = 632 nm). siRNA binding was quantified using the Quant-iT RiboGreen RNA assay [6, 23, 29-31]. The measurements were performed in quadruplicate.
2.3. In vitro siRNA delivery to A549-Luc cells
A549 human non-small cell lung cancer (NSCLC) cells stably expressing luciferase (A549-Luc) were generated by lentiviral injection, selected for high expressing clones, and cultured in RPMI 1640 medium with 5% FBS (5% CO2, 37 °C). A549-Luc cells were seeded into opaque white 96-well plates (10,000 cells/well) and incubated for 24 h. Medium was replaced with the fresh RPMI with 5% FBS (200 μL/well) prior to the addition of 20 μL NPs to cells. After 24-hour incubation with NPs, the cell viability and luciferase expression was analyzed using ONE-Glo + Tox assay kits. The luciferase activity and cell viability were normalized to those of untreated cells. All transfection assays were performed in triplicate and the average with standard deviation was reported.
2.4. Cellular uptake
A549-Luc cells were seeded into an 8-well glass bottom chamber (30,000 cells/well) and allowed to attach overnight in RPMI 1640 medium with 5% FBS (5% CO2, 37 °C). 60 μL NPs (containing 150 ng of Cy5.5-siRNA and 4.5 μg of polyester) were added to the cells with 600 μL fresh medium. After incubation for 0.5 to 8 h, the media was removed and the cells were fixed with 4% (vol%) paraformaldehyde (PFA) and stained with DAPI for confocal microscopy. For the co-localization of Cy5.5-siRNA and lysosomes, the cells were further incubated with 0.1% (vol%) LysoTracker Green for 30 min before fixation and DAPI staining. To identify the endocytic pathway(s) of siRNA polyplex nanoparticles to A549-Luc, chlorpromazine (28 μM, inhibitor of clathrin-mediated endocytosis), 5-(N-Ethyl-N-isopropyl)amiloride (EIPA) (75 μM, inhibitor of macropinocytosis), or genistein (200 μM, inhibitor of caveolar endocytosis) was added to the cell culture and incubated for 1 h prior to the addition of nanoparticles. Images were captured using a Zeiss LSM 700 confocal microscope with 20X objective lens and processed with consistent threshold using ImageJ.
2.5. Tumor models
All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of Texas Southwestern Medical Center and were consistent with local, state and federal regulations as applicable. Female athymic nude Foxnlnu/nu mice were purchased from Harlan Laboratories (Indianapolis, IN) and used for tumor models. A549-Luc cells were harvested, centrifuged, washed with PBS, and resuspended in Hanks’ balanced salt solution (HBSS) with 50% Matrigel (vol%). 5 million cells (100 μL A549-Luc suspension) were subcutaneously injected into the right hind leg of mice. ~6 mm tumor xenografts (110 mm3 in volume) formed in ~2 weeks. For the orthotopic A549-Luc lung tumor model, 3 million A549-Luc cells (25 μL, 50% Matrigel) were orthotopically implanted to the left lung of the nude mouse to develop single module orthopotic lung tumor. Strong luciferase signal was detected by bioluminescence imaging in 2-3 weeks after surgical implantation.
2.6. siRNA silencing in xenograft lung tumors
NPs containing 50 ng/μL siLuc were prepared as described above and intravenously injected into mice bearing A549-Luc xenograft tumors (1 mg/kg, 200 μL). After 24 or 48 h, 80 μL of 40 g/L luciferin PBS solution was injected subcutaneously 10 min prior to bioluminescence imaging on an IVIS Lumina imaging system (Caliper Life Science). The luciferase gene silencing was evaluated by the decrease of bioluminescence signal after treatment with siLuc NPs. To quantitate the luciferase knockdown, the tumor tissue was collected and homogenized in 2 mL of 25 mM HEPES (pH 7.5, 5 mM MgCl2, 1 mM EDTA). The luciferase in the supernatant was measured using a Luciferase Assay System kit (Promega) and normalized to the total protein as determined by the BCA assay (Piece). For biodistribution imaging, 200 μL Cy5.5-siRNA NPs were injected intravenously to mice bearing xenograft tumors. The mice were imaged at 4 and 24 h after i.v. administration of Cy5.5-siRNA NPs. The mice were sacrificed and the biodistribution of Cy5.5-siRNA in the tumor and organs was evaluated by Cy5.5 fluorescence measurement using an IVIS Lumina instrument. Statistical analysis was performed with two-tailed Student’s t-test; 95% confidence interval.
2.7. siRNA silencing in orthotopic lung tumors
The same NP solution was diluted 2-fold with PBS delivered to mice bearing orthotopic A549-Luc lung tumors (1 mg/kg, total NP volume of 400 μL) as an aerosol mist using a micropump nebulizer (Aeroneb Lab, Kent Scientific). The luciferase signal in the lungs of living mice was measured at 24 and 48 h after aerosol inhalation as described above. The biodistribution of NPs through aerosol inhalation was investigated by replacing the siLuc with Cy5.5-siRNA following the same procedure above. Statistical analysis was performed with two-tailed Student’s t-test; 95% confidence interval.
3. Results and discussion
3.1. Synthesis of a functional polyester library
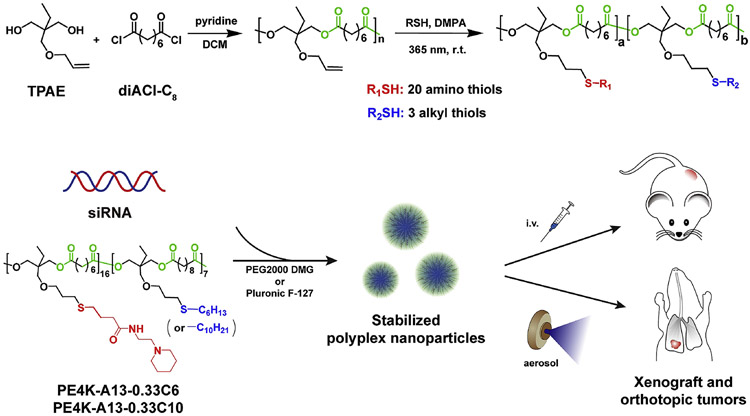
As a class, functional degradable polymers are particularly well suited for small RNA delivery [6, 28, 29, 32-36]. It has been demonstrated that NP delivery of siRNA requires a balance between cationic charge and hydrophobicity, where tertiary amines and hydrophobic alkyl chains provide pH-dependent small RNA binding and NP stabilization [6, 23, 29-31, 37-44]. Nevertheless, incorporation of these functional groups, particularly amines, into degradable polyesters remains challenging due to potential hydrolysis of the ester backbone. To overcome this issue, we recently developed a scalable (100+ gram) synthetic strategy based on the polymerization of trimethylolpropane allyl ether (TPAE) with diacid chlorides (poly(TPAE-co-AC)s) [28]. The side chain – ene groups could be easily modified via thiol-ene addition under UV irradiation in the presence of a free radical generator. These –ene groups could also be oxidized using 3-chloroperbenzoic acid (mCPBA) to epoxides, for further reaction with nucleophiles including amines to generate amino alcohols. Such chemistries are useful in preparing both functional linear polymers and cross-linked hydrogels [28]. Subsequently, we found that the thiol-ene addition route was particularly versatile, and could be expanded to generate combinatorial polymer libraries [23] (Figure 1) using commercially available and synthetic amino thiols via aminolysis of γ-thiobutyrolactone (Scheme S1).
Figure 1.
Stabilized NPs were developed for aerosol delivery to orthotopic lung tumors. Functional polyesters were prepared by the polycondensation of trimethylolpropane allyl ether (TPAE) and suberoyl chloride (diACl-C8) in dichloromethane (DCM) using pyridine as promotor, followed by the thiol-ene reaction with different ratios of 20 amino thiols and 3 alkyl thiols functional thiols (Scheme S1). High throughput in vitro screening of the functional polyester library enabled the discovery of efficacious polymeric carriers for siLuc delivery to A549-Luc cancer cells. Stabilized PE4K-A13-0.33C6 and PE4K-A13-0.33C10 NPs were able to silence gene expression in A549-Luc xenograft and orthotopic tumors by administration of intravenous injection or aerosol inhalation, respectively.
In addition to functional group modification, polymeric carriers offer the ability to modulate the molecular weight, which plays an important role in the stability of siRNA-loaded NPs and delivery efficacy. These cumulative physiochemical properties allow NPs to overcome delivery barriers including RNA NP stability, cellular internalization, intracellular release, and cancer cell targeting [1, 5, 12]. Using rational design and high throughput screening approaches, we have discovered NPs that are efficacious in animal models of liver [6] and lung cancer [23], in some cases selectively killing tumor cells but normal cells. We have shown that carriers can exhibit cell type specificity without attachment of targeting ligands [23]. Moreover, i.v. delivery of these NPs largely resulted in liver accumulation. Coupled to cell selectivity, the specific delivery to non-liver tissues is still challenging for systemic administration of RNA nanoparticles.
In this paper, we focused on using an alternative delivery method: aerosol inhalation. We found that the biodegradable siRNA delivery carriers could accumulate exclusively in the lungs and silence luciferase expression in orthotopic lung tumors. We re-synthesized a library of 540 functional polyesters via combinatorial modification of polyesters (Mw of 4,200 and 8,300 prior to thiol-ene modification) with 20 amino thiols and 3 alkyl thiols (Scheme S1). The polymers are named by the starting MW, followed by the amino thiol, the alkyl thiol feed ratio, and alkyl thiol.
3.2. Functional polyester library screening for siLuc silencing in A549-Luc lung cancer cells
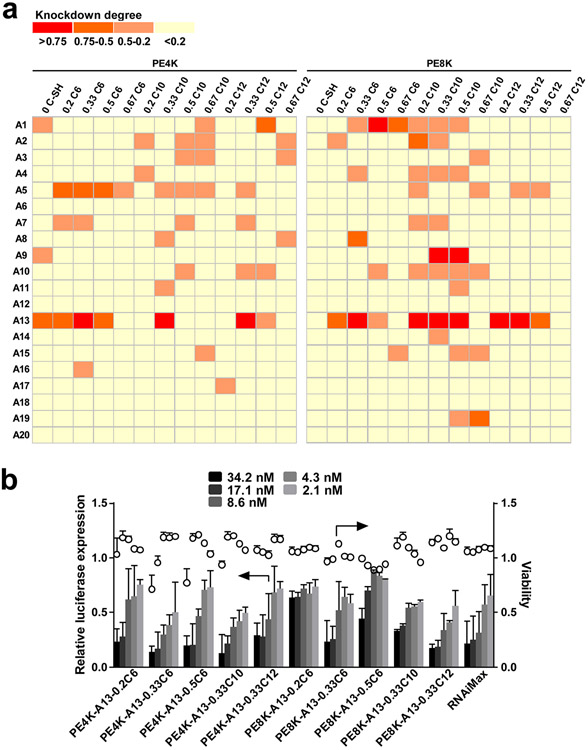
Our previous study demonstrated that functional poly(TPAE-co-AC)s with Mw between 4,000-8,000 g/mol exhibited siRNA delivery capability to HeLa cervical cancer cells. Using this library of polyesters, polyplex NPs were prepared and evaluated for siLuc delivery to A549-Luc lung cancer cells (Figure 2 and Figure S1). High throughput screening of 540 siLuc-polymer NPs shows that a large number of functional polyesters are capable of efficient luciferase gene silencing. In particular, the modification with 4-mercapto-N-(2-(piperidin-1-yl)ethyl)butanamide (A13) or 1-(4-(2-hydroxyethyl)piperazin-1-yl)-4-mercaptobutan-1-one (A5) endows the polyesters with the highest luciferase knockdown and lowest cytotoxicity. The delivery efficacy was validated by dose response experiments for the selected A13 modified polyesters (Figure 2b). The top performing polymers show comparable siRNA delivery capability to the commercial benchmark RNAiMax.
Figure 2.
Screening of a functional polyester library identified effective carriers for A549 lung cancer cells, notably amine A5 or A13 modified polyesters. a) siLuc (17.1 nM) was delivered in polymer-siRNA NPs in vitro to A549-Luc cells. b) Dose response of siLuc NPs with amine A13 modified polyesters validates the delivery capability of the selected NPs (mean ± s.d., n = 3).
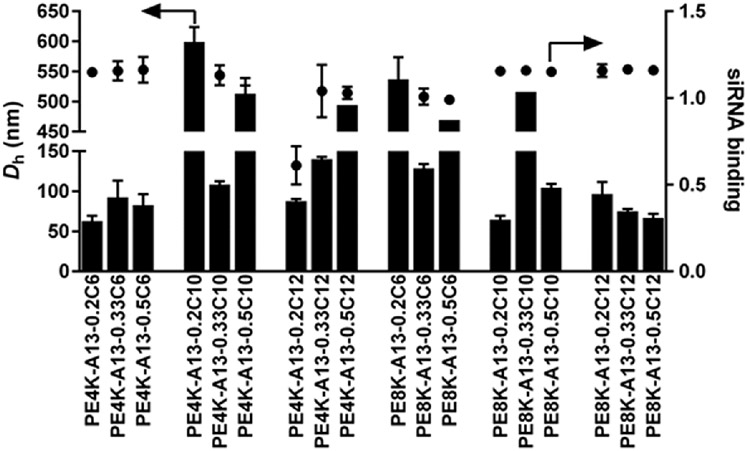
The NP size and siRNA binding with lead polyesters were measured (Figure 3). Most of the functional polyesters were able to completely bind the loaded siRNA under the complexation conditions (pH 4.2, 10 mM, and polymer/siRNA weight ratio of 30:1). It is known that the size of NPs plays a significant role in siRNA delivery. NPs larger than 200 nm are usually less effective [23]. It is worth noting that these polymers are not only capable of siLuc delivery to A549-Luc, but also show good delivery capability to HeLa-Luc and MDA-MB-231-Luc (Figures S2 and S3). Although selectivity can be advantageous, there is also a need for carriers that do not need customization. We therefore focused on these polyesters that can deliver to multiple cell types for further study.
Figure 3.
Size and siRNA binding for selected NPs (mean ± s.d., n = 3). The siRNA polyester NPs were prepared using the same protocol as in Figure 2.
3.3. Cellular uptake.
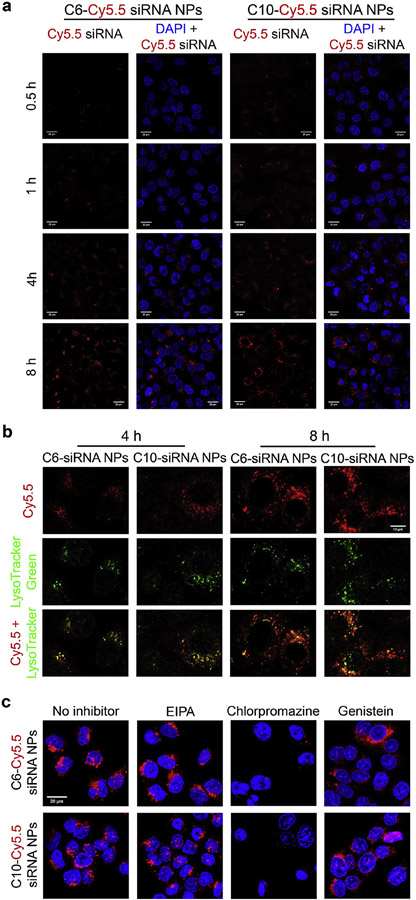
Successful modification of the polyesters with A13 and 1-hexanethiol (C6-SH)/1-decanethiol (C10-SH) was verified by the increase of MW in GPC curves (Figure S4) and complete disappearance of –ene groups after functionalization (NMR). Focusing on the most active polymers, we then studied the cellular uptake kinetics and in vivo efficacy of their siRNA NPs on different tumor models. Figure 4a shows that both Cy5.5-siRNA loaded PE4K-A13-0.33C6 (C6-Cy5.5-siRNA) NPs and Cy5.5-siRNA loaded PE4K-A13-0.33C10 (C10-Cy5.5-siRNA) NPs were endocytosed into A549 within 4 h. But in general, fluorescence signal in cells transfected by C10-Cy5.5-siRNA NPs was stronger than that for C6-Cy5.5-siRNA NPs in 0.5-4 h, indicating faster cellular uptake of C10-Cy5.5-siRNA NPs. The longer hydrophobic alkyl chains may facilitate the fusion of NPs with cell membrane and consequent internalization into A549 cells. To test the intracellular fate of Cy5.5-siRNA NPs after endocytosis, we examined the co-localization of Cy5.5-siRNA and lysosomes at 4 and 8 h after addition of NPs (Figure 4b). There is stronger co-localization between siRNA-Cy5.5 and LysoTracker green at 8 h than at 4 h. Moreover, some nanoparticles remain out of lysosomes, which gives the chance for siRNA loading to RNA-induced silencing complex (RISC) to silence targeted mRNAs. We further examined the endocytosis pathway(s) of these nanoparticles by analysing cellular uptake in the presence of various inhibitors of distinct endocytosis pathways (Figure 4c). Confocal images show that the internalization of both siRNA polyplex nanoparticles into A549-Luc cells is dominated by clathrin-mediated endocytosis.
Figure 4.
Cellular uptake of C6-Cy5.5-siRNA NPs and C10-Cy5.5-siRNA NPs. a) Internalization kinetics of NPs within 0.5-8 h. The NPs were prepared following the procedure in in vitro delivery assays. After incubation with NPs for 0.5-8 h, A549 cells were washed with PBS, fixed by 4% PFA, and stained with DAPI for confocal microscopy. Scale bars = 20 μm. b) Co-localization (yellow) of siRNA-Cy5.5 (red) and lysozymes (green) at 4 and 8 h. The cells were incubated with LysoTracker green for 0.5 h before fixing and staining. Scale bars = 10 μm. c) Cellular uptake in the presence of various endocytosis inhibitors (8 h). Scale bars = 20 μm.
3.4. Effects of NP stabilizers on size, surface charge and in vitro delivery efficacy
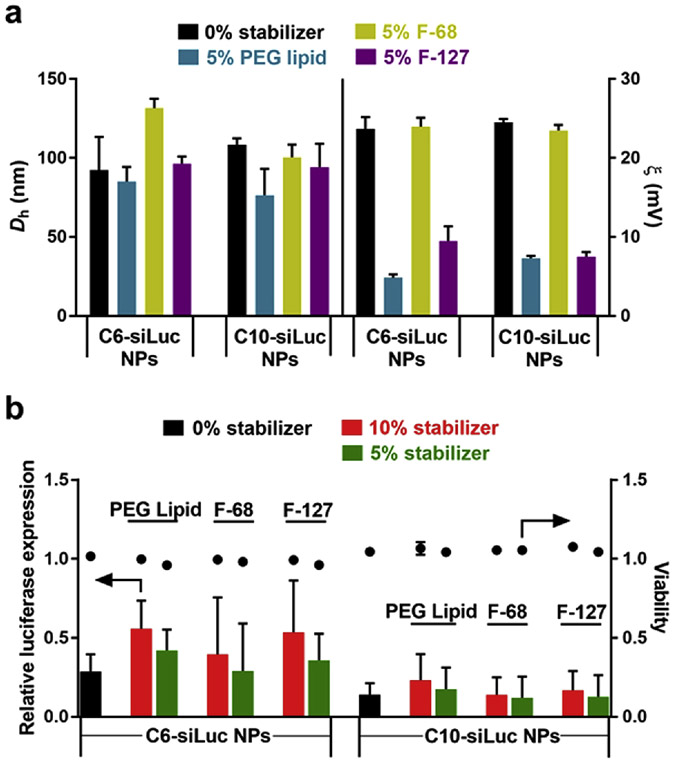
To reduce non-specific protein interactions and increase the stability of NPs in serum, we added amphiphilic Pluronic block copolymers (F-68 and F-127) or a PEG 2000 DMG lipid to polymer stock solutions prior to the preparation of siRNA polymer NPs. Coating nanoparticles with PEG and other hydrophilic non-fouling polymers is a well-established and effective approach to shield NPs from protein adsorption. Moreover, dense PEG brushes can improve transport through mucus [45-53]. Therefore, we examined F-68, F-127, and PEG 2000 DMG lipid as stabilizers. We found that these coatings did not significantly change the size of NPs, but that the surface charge decreased dramatically when formulated with PEG 2000 DMG lipid and F-127 (Figure 5a). Because the surface charge of NPs remained constant with addition of 5% F-68, this implied that there was no shielding effect by F-68. The hydrophobic PPO block of F-68 may be too short to make it well incorporated into NPs during the complexation of siRNA and polymers. In vitro transfection with the formulated NPs shows that the addition of 5% stabilizer does not compromise the luciferase gene knockdown or increase the cytotoxicity (Figure S5), in particular for C10-siLuc NPs (Figure 5b). However, the delivery efficacy decreases when 10% stabilizer was added to the NPs. More PEG on the surface may hinder the interaction between NPs and cell surface and decrease the cellular uptake. The addition of stabilizers shows similar effects on siRNA delivery to MDA-MB-231 breast cancer cells (Figure S6).
Figure 5.
Effects of stabilizers on physicochemical properties and delivery of efficacy of siLuc PE4K-A13-0.33C6 (C6-siLuc) NPs and siLuc PE4K-A13-0.33C10 (C10-siLuc) NPs. a) Size (Dh) and surface charge (ζ) of polyester NPs, and b) in vitro siRNA delivery efficacy of NPs (mean ± s.d., n = 3). 5% or 10% (weight ratio of stabilizer to the polymer) stabilizers (PEG lipid, F-68, or F-127) were added into the polymer stock solution before mixing with siLuc. NPs were prepared using the same protocol in screening assays and 17.1 nM siLuc was used.
3.5. Intravenous siRNA NP delivery to xenograft tumors.
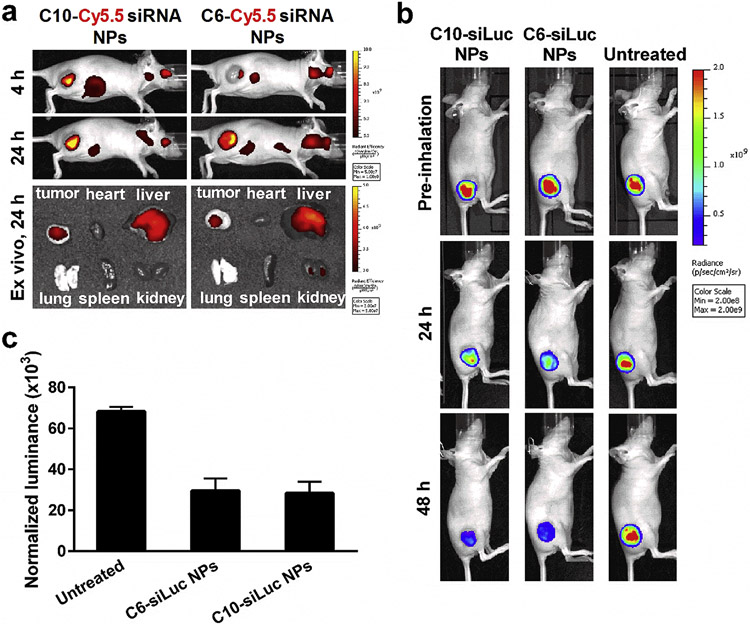
Before evaluating the luciferase knockdown in vivo, we measured the biodistribution of Cy5.5-siRNA NPs in mice upon i.v. tail vein injection. Live animal imaging indicates that NPs accumulated by tumors at 4 h after i.v. administration and the accumulation increased with time up to 24 h (Figure 6a). Ex vivo images clearly show that both C10-Cy5.5 NPs and C6-Cy5.5 NPs were mainly distributed in liver and tumor after 24 h, which corroborates with the luciferase gene silencing in the tumor by siLuc NPs. We also examined biodistribution of i.v. administered Cy5.5-siRNA NPs to mice bearing orthotopic lung tumors, which confirmed major accumulation in liver and minimal accumulation in the lungs after tail vein injection (Figure S7). These data suggested that i.v. administration may be suitable for xenograft tumors, but that aerosol administration may be required for orthotopic tumors.
Figure 6.
Lead NPs can deliver siRNA in vivo to xenograft tumors. a) Biodistribution of Cy5.5-siRNA NPs upon tail vein injection (1 mg/kg Cy5.5-siRNA, 100 μL). Live animal imaging shows efficient accumulation of Cy5.5-siRNA NPs in xenograft tumors at 4 and 24 h. ex vivo images verify that the NPs are mainly distributed in liver and tumor at 24 h after i.v. administration. b) Bioluminescence images of mice with i.v. injection of C10-siLuc NPs or C6-siLuc NPs (1 mg/kg siLuc, 200 μL). The decrease of bioluminescence intensity indicates the siLuc-mediated luciferase knockdown in xenograft tumors after administration of siLuc NPs. One of three mice was shown as a representative for each group. c) Efficient luciferase knockdown was confirmed by the decrease of protein normalized bioluminescence in the homogenized tumor tissues with injection of siLuc NPs (mean ± s.d., n = 3). ***P < 0.0005.
To evaluate in vivo siRNA delivery efficacy of siRNA polymer NPs, we first generated A549 xenograft tumors by subcutaneously injecting A549-Luc cells to the right hind leg of the nude mice. The effective delivery of siRNA against luciferase to the tumor induces the knockdown of luciferase gene and consequently suppresses the expression of luciferase protein. By i.v. injection of C10-siLuc or C6-siLuc NPs, the bioluminescence in the tumors significantly decreased while no remarkable change of bioluminescence was seen for the untreated mice, suggesting the siLuc-mediated luciferase silencing through i.v. administration of siLuc NPs (Figure 6b). To quantify the decrease of luciferase expression, the tumor tissue was homogenized and the luciferase in the suspension was measured and normalized to total protein. Results show that a single i.v. injection of siLuc NPs at the dose of 1 mg/kg enabled ~ 50% luciferase knockdown in A549 xenograft tumors in 48 h (Figure 6c). However, this administration route (i.v.) does not solve the non-specific liver uptake that hinders siRNA NPs for use in treatment of non-liver diseases. Therefore, we aerosolized the polyplex NPs and applied the siRNA NPs to the mice with surgical implanted orthotopic lung tumors.
3.6. Aerosol siRNA NP delivery to orthotopic A549 tumors.
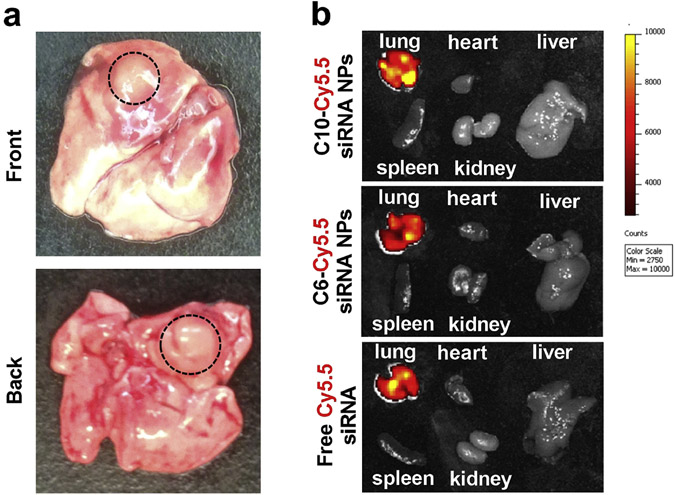
To further explore the potential of functional polyesters in lung cancer therapy, we developed an orthotopic lung tumor model by surgical implantation of A549-Luc cells to the lung of nude mouse. 3-4 mm (diameter) tumors were generated by 3 weeks after surgical implantation under these conditions (Figure 7a). Using this more clinically relevant tumor model and the pulmonary disease-specific aerosol administration method, the biodistribution of Cy5.5-siRNA NPs and in vivo efficacy of siLuc NPs were investigated. Ex vivo images show that the aerosol inhalation of Cy5.5-siRNA NPs or free Cy5.5-siRNA to mice led to the highly specific accumulation of siRNA in lung. No Cy5.5 fluorescence was observed in any other organs, which confirmed the advantage of the aerosol inhalation for pulmonary diseases (Figure 7b).
Figure 7.
Aerosol inhalation is an effective method to increase NP and siRNA localization to the lungs. a) Representative ex vivo pictures of the lung with orthotopic tumor. b) ex vivo images show that Cy5.5-siRNA NPs or free Cy5.5-siRNA could be specifically accumulated in lung at 24 h after administration through aerosol inhalation (1 mg/kg Cy5.5-siRNA, 400 μL).
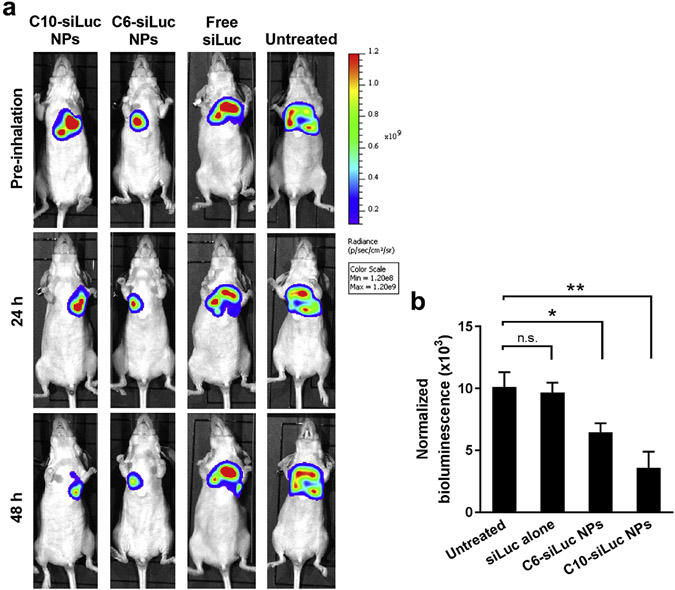
Using a similar protocol, siLuc NPs were aerosolized and inhaled to mice bearing A549-Luc tumors in their lungs. The luciferase signal decreased dramatically at 48 h after aerosol inhalation of C10-siLuc or C6-siLuc NPs comparing to that before inhalation (Figure 8a), indicating the efficient luciferase gene knockdown by the delivered siLuc. There was no significant change of the bioluminescence intensity for the untreated mice and the mice inhaled with free siLuc. The naked siLuc could be locally accumulated in lung through aerosol inhalation, but it cannot be efficiently internalized into tumor cells and mediate gene silencing without a delivery vehicle. The quantitative luciferase in homogenized lungs further verified the luciferase knockdown by this delivery approach (Figure 8b).
Figure 8.
Aerosolized NPs can deliver siRNA in vivo to surgically implanted orthotopic lung tumors. a) The decrease of luminance intensity in the lung indicates the siLuc-mediated luciferase knockdown in orthotopic A549 tumors after inhalation of siLuc NPs (1 mg/kg siLuc, 400 μL). One of three mice was shown as a representative for each group. b) Efficient luciferase knockdown was confirmed by the decrease of protein normalized luminance in the homogenized lung with aerosol inhalation of siLuc NPs (mean ± s.d., n = 3). n.s. P > 0.05; *P < 0.05; **P < 0.005.
4. Conclusions
In this paper, we utilized a combinatorial functional polyester library that satisfies biodegradability and multifunctionality requirements of polymeric carriers for siRNA delivery. High throughput screening of the 540-member polyester library allowed discovery of the synthetic amine A13 modified polyester series as being potent for siRNA delivery to A549-Luc lung cancer cells. Using the best performing polyesters, siRNA polyplex NPs were prepared for detailed investigation of cellular uptake and in vivo gene knockdown in different tumor models. siRNA-polymer NPs were efficiently internalized into A549-Luc cells within 4 hours and started to co-localize with lysosomes by 8 hours. The addition of PEG 2000 DMG lipid and Pluronic F-127 onto the surface of the polyplexes reduced the surface charge of NPs, therefore decreasing the nonspecific binding and increasing the stability in serum. This surface modification did not significantly change the size of NPs or their siRNA delivery efficacy in vitro. By i.v. injection to mice bearing xenograft tumors, the siRNA NPs effectively accumulated in tumor tissues, which resulted in ~50% siLuc-mediated luciferase gene silencing at 48 hours. Furthermore, the in vivo delivery efficacy of NPs was evaluated by aerosol inhalation to mice bearing orthotopic lung tumors. Bio-distribution studies showed that aerosol inhalation enabled specific accumulation of NPs in the lung. Using this non-invasive delivery approach, NPs successfully delivered siLuc to orthotopic lung tumors and enabled ~65% knockdown of luciferase expression in the tumors. Because of the biodegradability, diverse functionality, and the ability to be aerosolized, these polyesters may provide great potential for the treatment of lung cancers.
Supplementary Material
Acknowledgements
D.J.S. acknowledges financial support from the Cancer Prevention and Research Institute of Texas (CPRIT) (R1212 and RP140110), the Welch Foundation (I-1855), the American Cancer Society (ACS-IRG-02-196), the Friends of the Comprehensive Cancer Center, and the UTSW Translational Pilot Program via the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (UL1TR001105). D.A.B. acknowledges the NIH (NCI R01 CA201489-01). L.L. acknowledges CPRIT (RP160617). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the above mentioned funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biomaterials.2016.00.000.
References
- [1].Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–77. [DOI] [PubMed] [Google Scholar]
- [2].Lorenzer C, Dirin M, Winkler AM, Baumann V, Winkler J. Going beyond the liver: Progress and challenges of targeted delivery of siRNA therapeutics. J Controlled Release. 2015;203:1–15. [DOI] [PubMed] [Google Scholar]
- [3].Wu SY, Lopez-Berestein G, Calin GA, Sood AK. RNAi therapies: Drugging the undruggable. Sci Transl Med. 2014;6:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Bio. 2006;2:689–700. [DOI] [PubMed] [Google Scholar]
- [5].Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discovery. 2009;8:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou K, Nguyen LH, Miller JB, Yan Y, Kos P, Xiong H, Li L, Hao J, Minnig JT, Zhu H, Siegwart DJ. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc Natl Acad Sci USA. 2016;113:520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zimmermann TS, Lee ACH, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–4. [DOI] [PubMed] [Google Scholar]
- [8].Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Jayaprakash KN, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DWY, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Love K, Mahon K, Levins C, Whitehead K, Querbes W, Dorkin J, Qin J, Cantley W, Qin L, Racie T, Frank-Kamenetsky M, Yip K, Alvarez R, Sah D, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson D. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107:1864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D, Bogorad RL, Yin H, Chen Y, Vegas AJ, Alabi CA, Sahay G, Olejnik KT, Wang W, Schroeder A, Lytton-Jean AKR, Siegwart DJ, Akinc A, Barnes C, Barros SA, Carioto M, Fitzgerald K, Hettinger J, Kumar V, Novobrantseva TI, Qin J, Querbes W, Koteliansky V, Langer R, Anderson DG. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci USA. 2014;111:3955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Semple SC, Akinc A, Chen JX, Sandhu AP, Mui BL, Cho CK, Sah DWY, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen QM, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–6. [DOI] [PubMed] [Google Scholar]
- [12].Wittrup A, Lieberman J. Knocking down disease: A progress report on siRNA therapeutics. Nat Rev Genet. 2015;16:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discovery. 2015;14:843–56. [DOI] [PubMed] [Google Scholar]
- [14].Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen QM, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DWY, Gollob JA, Suhr OB. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. New Engl J Med. 2013;369:819–29. [DOI] [PubMed] [Google Scholar]
- [15].Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18:1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi B, Keough E, Matter A, Leander K, Young S, Carlini E, Sachs AB, Tao W, Abrams M, Howell B, Sepp-Lorenzino L. Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery. J Histochem Cytochem. 2011;59:727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Asai T, Oku N. Systemic delivery of small RNA using lipid nanoparticles. Biol Pharm Bull. 2014;37:201–5. [DOI] [PubMed] [Google Scholar]
- [18].Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, Xing YP, Sager HB, Sahay G, Speciner L, Bader A, Bogorad RL, Yin H, Racie T, Dong YZ, Jiang S, Seedorf D, Dave A, Sandhu KS, Webber MJ, Novobrantseva T, Ruda VM, Lytton-Jean AKR, Levins CG, Kalish B, Mudge DK, Perez M, Abezgauz L, Dutta P, Smith L, Charisse K, Kieran MW, Fitzgerald K, Nahrendorf M, Danino D, Tuder RM, von Andrian UH, Akinc A, Panigrahy D, Schroeder A, Koteliansky V, Langer R, Anderson DG. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fehring V, Schaeper U, Ahrens K, Santel A, Keil O, Eisermann M, Giese K, Kaufmann J. Delivery of therapeutic siRNA to the lung endothelium via novel lipoplex formulation DACC. Mol Ther. 2014;22:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xue W, Dahlman JE, Tammela T, Khan OF, Sood S, Dave A, Cai W, Chirino LM, Yang GR, Bronson R, Crowley DG, Sahay G, Schroeder A, Langer R, Anderson DG, Jacks T. Small RNA combination therapy for lung cancer. Proc Natl Acad Sci USA. 2014;111:E3553–E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khan OF, Zaia EW, Yin H, Bogorad RL, Pelet JM, Webber MJ, Zhuang I, Dahlman JE, Langer R, Anderson DG. Ionizable amphiphilic dendrimer-based nanomaterials with alkyl-chain-substituted amines for tunable siRNA delivery to the liver endothelium in vivo. Angew Chem Int Ed. 2014;53:14397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khan OF, Zaia EW, Jhunjhunwala S, Xue W, Cai WX, Yun DS, Barnes CM, Dahlman JE, Dong YZ, Pelet JM, Webber MJ, Tsosie JK, Jacks TE, Langer R, Anderson DG. Dendrimer-inspired nanomaterials for the in vivo delivery of siRNA to lung vasculature. Nano Lett. 2015;15:3008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yan Y, Liu L, Xiong H, Miller JB, Zhou K, Kos P, Huffman KE, Elkassih S, Norman JW, Carstens R, Kim J, Minna JD, Siegwart DJ. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc Natl Acad Sci USA. 2016;113:E5702–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perez-Medina C, Abdel-Atti D, Tang J, Zhao YM, Fayad ZA, Lewis JS, Mulder WJM, Reiner T. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat Commun. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiang HL, Xu CX, Kim YK, Arote R, Jere D, Lim HT, Cho MH, Cho CS. The suppression of lung tumorigenesis by aerosol-delivered folate-chitosan-graft-polyethylenimine/Akt1 shRNA complexes through the Akt signaling pathway. Biomaterials. 2009;30:5844–52. [DOI] [PubMed] [Google Scholar]
- [26].Dolovich MB, Dhand R. Aerosol drug delivery: Developments in device design and clinical use. Lancet. 2011;377:1032–45. [DOI] [PubMed] [Google Scholar]
- [27].Lam JKW, Liang WL, Chan HK. Pulmonary delivery of therapeutic siRNA. Adv Drug Deliver Rev. 2012;64:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yan Y, Siegwart DJ. Scalable synthesis and derivation of functional polyesters bearing ene and epoxide side chains. Polym Chem. 2014;5:1362–71. [Google Scholar]
- [29].Hao J, Kos P, Zhou K, Miller JB, Xue L, Yan Y, Xiong H, Elkassih S, Siegwart DJ. Rapid synthesis of a lipocationic polyester library via ring-opening polymerization of functional valerolactones for efficacious siRNA delivery. J Am Chem Soc. 2015;137:9206–9. [DOI] [PubMed] [Google Scholar]
- [30].Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, Ma ML, Lytton-Jean A, Vegas A, Fenton P, Levins CG, Love KT, Lee H, Cortez C, Collins SP, Li YF, Jang J, Querbes W, Zurenko C, Novobrantseva T, Langer R, Anderson DG. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc Natl Acad Sci USA. 2011;108:12996–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yan YF, Xue L, Miller JB, Zhou KJ, Kos P, Elkassih S, Liu L, Nagai A, Xiong H, Siegwart DJ. One-pot synthesis of functional poly(amino ester sulfide)s and utility in delivering pDNA and siRNA. Polymer. 2015;72:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo ST, Huang YY, Wei T, Zhang WD, Wang WW, Lin D, Zhang X, Kumar A, Du QA, Xing JF, Deng LD, Liang ZC, Wang PC, Dong AJ, Liang XJ. Amphiphilic and biodegradable methoxy polyethylene glycol-block-(polycaprolactone-graft-poly(2-(dimethylamino)ethyl methacrylate)) as an effective gene carrier. Biomaterials. 2011;32:879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhou J, Patel TR, Fu M, Bertram JP, Saltzman WM. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33:583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hong BJ, Chipre AJ, Nguyen ST. Acid-degradable polymer-caged lipoplex (PCL) platform for siRNA delivery: Facile cellular triggered release of siRNA. J Am Chem Soc. 2013;135:17655–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Islam MA, Park TE, Singh B, Maharjan S, Firdous J, Cho MH, Kang SK, Yun CH, Choi YJ, Cho CS. Major degradable polycations as carriers for DNA and siRNA. J Controlled Release. 2014;193:74–89. [DOI] [PubMed] [Google Scholar]
- [36].Zhou K, Kos P, Yan Y, Xiong H, Min YL, Kinghorn KA, Minnig JT, Miller JB, Siegwart DJ. Intercalation-mediated nucleic acid nanoparticles for siRNA delivery. Chem Commun. 2016;52:12155–8. [DOI] [PubMed] [Google Scholar]
- [37].Nelson CE, Kintzing JR, Hanna A, Shannon JM, Gupta MK, Duvall CL. Balancing cationic and hydrophobic content of PEGylated siRNA polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo. ACS Nano. 2013;7:8870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].deRonde BM, Posey ND, Otter R, Caffrey LM, Minter LM, Tew GN. Optimal hydrophobicity in ring-opening metathesis polymerization-based protein mimics required for siRNA internalization. Biomacromolecules. 2016;17:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wong S, Sood N, Putnam D. Combinatorial evaluation of cations, pH-sensitive and hydrophobic moieties for polymeric vector design. Mol Ther. 2009;17:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhi DF, Zhang SB, Wang B, Zhao YN, Yang BL, Yu SJ. Transfection efficiency of cationic lipids with different hydrophobic domains in gene delivery. Bioconjugate Chem. 2010;21:563–77. [DOI] [PubMed] [Google Scholar]
- [41].Philipp A, Zhao XB, Tarcha P, Wagner E, Zintchenko A. Hydrophobically modified oligoethylenimines as highly efficient transfection agents for siRNA delivery. Bioconjugate Chem. 2009;20:2055–61. [DOI] [PubMed] [Google Scholar]
- [42].Liu Z, Zhang Z, Zhou C, Jiao Y. Hydrophobic modifications of cationic polymers for gene delivery. Prog Polym Sci. 2010;35:1144–62. [Google Scholar]
- [43].Kurisawa M, Yokoyama M, Okano T. Transfection efficiency increases by incorporating hydrophobic monomer units into polymeric gene carriers. J Controlled Release. 2000;68:1–8. [DOI] [PubMed] [Google Scholar]
- [44].Hao J, Elkassih S, Siegwart DJ. Progress towards the synthesis of amino polyesters via ring-opening polymerization (ROP) of functional lactones. Synlett. 2016;27:2285–92. [Google Scholar]
- [45].Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliver Rev. 2009;61:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu QG, Ensign LM, Boylan NJ, Schon A, Gong XQ, Yang JC, Lamb NW, Cai ST, Yu T, Freire E, Hanes J. Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano. 2015;9:9217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernandez S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano. 2015;9:6996–7008. [DOI] [PubMed] [Google Scholar]
- [48].Hak S, Helgesen E, Hektoen HH, Huuse EM, Jarzyna PA, Mulder WJM, Haraldseth O, Davies CD. The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targeting studied in vivo by dual modality imaging. ACS Nano. 2012;6:5648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ensign LM, Schneider C, Suk JS, Cone R, Hanes J. Mucus penetrating nanoparticles: Biophysical tool and method of drug and gene delivery. Adv Mater. 2012;24:3887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cone RA. Barrier properties of mucus. Adv Drug Deliver Rev. 2009;61:75–85. [DOI] [PubMed] [Google Scholar]
- [51].Cu Y, Saltzman WM. Controlled surface modification with poly(ethylene)glycol enhances diffusion of PLGA nanoparticles in human cervical mucus. Mol Pharmaceut. 2009;6:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, Dawson KA. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133:2525–34. [DOI] [PubMed] [Google Scholar]
- [53].Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci USA. 2009;106:19268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.