Abstract
Background and Purpose
Posterior cerebral artery occlusion (PCAo) can cause long-term disability, yet randomized controlled trials to guide optimal reperfusion strategy are lacking. We compared the outcomes of PCAo patients treated with endovascular thrombectomy (EVT) with or without intravenous thrombolysis (IVT) to patients treated with IVT alone.
Methods
From the multicenter retrospective Posterior cerebraL ArTery Occlusion (PLATO) registry, we included patients with isolated PCAo treated with reperfusion therapy within 24 hours of onset between January 2015 and August 2022. The primary outcome was the distribution of the modified Rankin Scale (mRS) at 3 months. Other outcomes comprised 3-month excellent (mRS 0–1) and independent outcome (mRS 0–2), early neurological improvement (ENI), mortality, and symptomatic intracranial hemorrhage (sICH). The treatments were compared using inverse probability weighted regression adjustment.
Results
Among 724 patients, 400 received EVT+/-IVT and 324 IVT alone (median age 74 years, 57.7% men). The median National Institutes of Health Stroke Scale score on admission was 7, and the occluded segment was P1 (43.9%), P2 (48.3%), P3–P4 (6.1%), bilateral (1.0%), or fetal posterior cerebral artery (0.7%). Compared to IVT alone, EVT+/-IVT was not associated with improved functional outcome (adjusted common odds ratio [OR] 1.07, 95% confidence interval [CI] 0.79–1.43). EVT increased the odds for ENI (adjusted OR [aOR] 1.49, 95% CI 1.05–2.12), sICH (aOR 2.87, 95% CI 1.23–6.72), and mortality (aOR 1.77, 95% CI 1.07–2.95).
Conclusion
Despite higher odds for early improvement, EVT+/-IVT did not affect functional outcome compared to IVT alone after PCAo. This may be driven by the increased risk of sICH and mortality after EVT.
Keywords: Endovascular thrombectomy, Intravenous thrombolysis, Posterior cerebral artery, Posterior circulation stroke
Introduction
Endovascular thrombectomy (EVT) has become the standard of care for patients with large vessel occlusion (LVO) of the proximal anterior and posterior circulations in recent years [1-3]. However, there are no data from randomized controlled trials (RCTs) on EVT for patients with medium or distal vessel occlusion. Patients with posterior cerebral artery occlusion (PCAo) may present with severe neurological symptoms and harbor significant long-term disability, especially in more proximal occlusions [4,5]. Observational studies comparing EVT and best medical management (BMM) have not found a difference in 3-month functional outcome, yet their sample sizes have been mostly modest [6-10].
Our previous report from the international multicenter Posterior cerebraL ArTery Occlusion (PLATO) study comprising 1,023 patients with acute isolated PCAo revealed that patients treated with EVT experienced more often early neurological improvement (ENI) and excellent 3-month functional outcome compared to BMM despite an increased risk of symptomatic intracranial hemorrhage (sICH) and mortality [11]. However, BMM included intravenous thrombolysis (IVT) in only 45.5% of the patients, even though IVT is the guideline-based treatment in this indication [12,13]. A study from the Swiss Stroke Registry (n=136 in the propensity score matched cohort) compared EVT with or without bridging to IVT alone and observed no significant difference in functional outcome, sICH, or mortality but did not have data on ENI [14]. In recent meta-analyses, patients undergoing EVT for isolated PCAo had higher odds for ENI, but other clinical or safety outcomes did not differ significantly from patients treated with IVT, even though there was a trend for excellent functional outcome after EVT [15,16].
Due to the inconclusive results, our aim was to further investigate the clinical outcomes and safety of EVT versus IVT in acute PCAo. We compared the outcomes of patients treated with EVT +/- bridging IVT to those of patients treated with IVT alone from the updated multicenter PLATO cohort of isolated PCAo patients. We hypothesized that the functional recovery at 3 months would not differ based on reperfusion strategy.
Methods
Ethics
All centers obtained ethics committee or local institutional review board approval. Patient written informed consent was not required due to the study’s retrospective and anonymized design. The study was reported according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline. Anonymized data are available upon reasonable request to the corresponding author following national legislation.
Study population
The PLATO study (NCT05291637) is an international, multicenter, retrospective cohort study of consecutive patients aged ≥18 years with acute PCAo treated between January 2015 and August 2022. Since the primary report [11], there was an expansion to 31 centers and 9 countries in Europe, North America, and Asia. Only patients with an isolated verified occlusion of the P1, P2, P3, or P4 segment, fetal posterior cerebral artery (PCA), or bilateral PCAs who were treated with EVT and/or IVT within 24 hours of symptom onset were included. The choice of reperfusion therapy was made at the discretion of the treating physician according to local standards. Patients with no reperfusion therapy or pre-stroke modified Rankin Scale (mRS) >3 were excluded, as were patients with incomplete covariate data.
Baseline characteristics, clinical presentation, imaging results, and outcome variables were collected from each site to a data sheet that included definitions of all collected variables. The details of data collection have been described previously [11].
Outcomes
The primary outcome was the distribution of the mRS (ordinal shift) at 3 months. The 3-month mRS was prospectively collected at each site by investigators who were not systematically blinded to the treatment. The secondary outcomes comprised 3-month independent outcome (mRS 0–2), 3-month excellent outcome (mRS 0–1), ENI defined as a decrease of at least 2 points in National Institutes of Health Stroke Scale (NIHSS) at 24 hours or discharge, and visual field recovery. Visual field recovery was judged based on change either in confrontation perimetry or standard perimetry at 1 to 3 months after stroke and categorized as complete, partial, none, or worse [11].
The safety outcomes were 3-month all-cause mortality and sICH. sICH was defined as local or remote parenchymal hemorrhage type 2, subarachnoid hemorrhage, or intraventricular hemorrhage, causing neurological deterioration of ≥4 NIHSS points from baseline or death.
Statistical analyses
Baseline categorical variables between the treatment groups were compared with the χ2 test, or Fisher exact test when appropriate, and continuous variables with the Mann-Whitney U test. The latter were presented as medians and interquartile ranges (IQR).
We obtained crude odds ratios (ORs) and 95% confidence intervals (CIs) with univariable logistic regression analyses using the generalized estimating equation (GEE) model. An independence covariance matrix was used to account for within-site clustering of patients. GEE is robust to the specification of the working correlation structure and allows for the proper estimation of standard errors through robust sandwich estimators. The model specifications included a cumulative logit link function and multinomial distribution for the ordinal outcome (1-point shift towards the lower value in mRS) and a logit link function and binomial distribution for the binary outcomes (other outcomes).
To acquire adjusted ORs, we combined propensity score-based weighting and outcome regression by using doubly robust inverse probability weighted regression adjustment (IPWRA) models [17]. In this approach, stabilized weights were applied for treatment weighting by inverse probability [18] and the doubly robust effect of treatment on the outcome was estimated by utilizing a logistic regression exposure model (EVT+/-IVT vs. IVT) and an outcome logistic regression model (3-month mRS). We used the multinomial logistic regression model for the ordinal primary outcome (mRS shift) and the binomial logistic regression model for the binary outcomes, using similar model specifications as in the unadjusted analyses. The exposure and outcome models were adjusted with a priori chosen variables of age, sex, hypertension, diabetes, atrial fibrillation, NIHSS score at baseline, the level of occlusion [10], the posterior circulation Acute Stroke Prognosis Early CT Score (pc-ASPECTS) [19] at baseline, and the year of treatment.
We tested the interaction of the treatment with onset-to-treatment time ≤4.5 hours, P1 occlusion, and baseline NIHSS as an ordinal or categorical (NIHSS<10) variable. In addition, we performed sensitivity analyses with onset-to-treatment time ≤4.5 hours as an additional covariate, excluding patients with missing data (n=59) on that variable. Finally, to further analyze the difference between the reperfusion strategies, we compared the outcomes of patients treated with direct EVT and EVT+/-IVT using similar IPWRA models described above. We performed the analyses with SPSS Statistics, Version 28 (IBM Corp., Armonk, NY, USA). Patients with missing covariates were excluded and missing data were not imputed.
Results
Baseline characteristics
The PLATO cohort included altogether 1,357 patients. After excluding patients treated with medical management only (n=557), other exclusion criteria (n=54), or missing covariate data (n=22), the final cohort comprised 724 patients (Figure 1): 400 patients treated with EVT with or without IVT and 324 patients treated with IVT alone.
Figure 1.
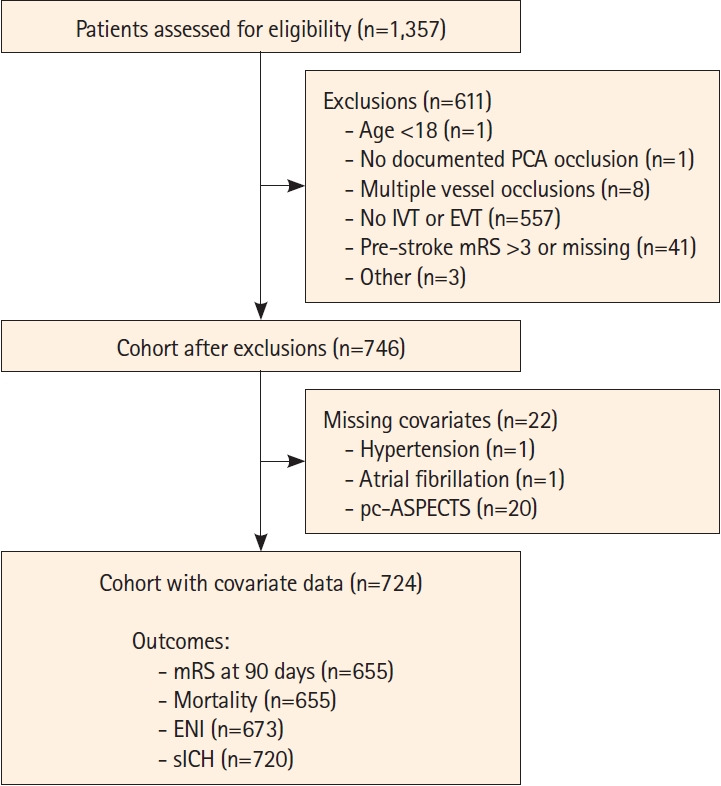
Flowchart of the study cohort. PCA, posterior cerebral artery; IVT, intravenous thrombolysis; EVT, endovascular thrombectomy; mRS, modified Rankin Scale; pc-ASPECTS, posterior circulation Acute Stroke Prognosis Early CT Score; ENI, early neurological improvement; sICH, symptomatic intracranial hemorrhage.
In the overall cohort, the median (IQR) age was 74 (64–82) years, and 57.7% were men (Table 1). The occlusion site was the P1 segment in 43.9%, P2 in 48.3%, P3 or P4 in 6.1%, posterior communicating artery in 0.7%, and bilateral PCA in 1.0%. The median baseline NIHSS score was 7 (4–11), and 449 of 641 patients (70.0%) had a visual field deficit on admission. The median onset-to-treatment time was 2.9 (1.8–4.7) hours. The patients treated with EVT were more often on oral anticoagulation before stroke (16.8% vs. 7.2%, P<0.01), had a higher baseline median (IQR) NIHSS score (8 [5–12] vs. 6 [3–10], P<0.01), were less frequently imaged with computed tomography, had more proximal occlusions (P1: 54.3% vs. 31.2%, P<0.01), lower baseline pc-ASPECTS, and different etiological distribution (more large artery atherosclerosis and less cardioembolic strokes) (Table 1). In addition, they had longer median (IQR) onset-to-treatment times (3.8 [2.4–7.2] h vs. 2.3 [1.5–3.3] h, P<0.01) and more often wake-up stroke.
Table 1.
Baseline characteristics, metrics, and outcomes of patients with PCA occlusion treated with EVT with or without IVT versus IVT alone
| All (n=724) | EVT+/-IVT (n=400) | IVT (n=324) | P | Missing data | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age (yr) | 74 (64–82) | 74 (64–82) | 75 (65–82) | 0.23 | 0/0/0 |
| Male sex | 418 (57.7) | 228 (57.0) | 190 (58.6) | 0.71 | 0/0/0 |
| Pre-stroke mRS | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.39 | 0/0/0 |
| 0 | 458 (63.3) | 257 (64.3) | 201 (62.0) | 0.40 | 0/0/0 |
| 1 | 132 (18.2) | 76 (19.0) | 56 (17.3) | - | - |
| 2 | 72 (9.9) | 39 (9.8) | 33 (10.2) | - | - |
| 3 | 62 (8.6) | 28 (7.0) | 34 (10.5) | - | - |
| Hypertension | 539 (74.4) | 305 (76.3) | 234 (72.2) | 0.23 | 0/0/0 |
| Diabetes | 166 (22.9) | 92 (23.0) | 74 (22.8) | 1.00 | 0/0/0 |
| Hyperlipidemia | 330 (45.7) | 171 (42.9) | 159 (49.2) | 0.10 | 2/1/1 |
| Atrial fibrillation | 219 (30.2) | 131 (32.8) | 88 (27.2) | 0.11 | 0/0/0 |
| Current smoker | 104 (16.1) | 51 (15.0) | 53 (17.3) | 0.46 | 78/61/17 |
| Prior stroke | 106 (14.9) | 50 (12.9) | 56 (17.3) | 0.11 | 13-12-1 |
| Coronary heart disease | 122 (17.3) | 64 (16.7) | 58 (17.9) | 0.69 | 17/17/0 |
| Peripheral artery disease | 39 (6.2) | 18 (5.5) | 21 (6.9) | 0.51 | 90/72/18 |
| Dialysis | 27 (4.2) | 16 (4.8) | 11 (3.5) | 0.44 | 75/68/7 |
| Oral anticoagulation | 87 (12.4) | 64 (16.8) | 23 (7.2) | <0.01* | 23/18/5 |
| Statin | 232 (34.6) | 117 (33.0) | 115 (36.4) | 0.37 | 53/45/8 |
| NIHSS on admission | 7 (4–11) | 8 (5–12) | 6 (3–10) | <0.01* | 0/0/0 |
| Baseline visual field defect | 449 (70.0) | 228 (69.3) | 221 (70.8) | 0.73 | 83/71/12 |
| SBP (mm Hg) | 158 (140–176) | 159 (138–175) | 157 (141–176) | 0.80 | 92/73/19 |
| DBP (mm Hg) | 85 (75–97) | 84 (74–97) | 86 (75–98) | 0.31 | 97/77/20 |
| Baseline imaging | |||||
| Imaging modality | |||||
| CT | 656 (92.0) | 349 (89.7) | 307 (94.8) | 0.02* | 11/11/0 |
| MRI | 106 (14.9) | 53 (13.6) | 53 (16.4) | 0.34 | 11/11/0 |
| Perfusion | 437 (61.3) | 230 (59.1) | 207 (63.9) | 0.22 | 11/11/0 |
| pc-ASPECTS | 10 (9–10) | 10 (9–10) | 10 (9–10) | 0.04*† | 0/0/0 |
| Clot location | <0.01* | 0/0/0 | |||
| P1 | 318 (43.9) | 217 (54.3) | 101 (31.2) | - | - |
| P2 | 350 (48.3) | 159 (39.8) | 191 (59.0) | - | - |
| P3 or P4 | 44 (6.1) | 13 (3.3) | 31 (9.6) | - | - |
| PCom | 5 (0.7) | 4 (1.0) | 1 (0.3) | - | - |
| Bilateral | 7 (1.0) | 7 (1.8) | 0 | - | - |
| Perfusion mismatch >20% | 317 (91.6) | 161 (94.2) | 156 (89.1) | 0.12 | 378/229/149 |
| Treatment factors | |||||
| IVT | 488 (67.4) | 164 (41.0) | 324 (100) | <0.01* | 0/0/0 |
| Onset-to-treatment time (h) | 2.9 (1.8–4.7) | 3.8 (2.4–7.2) | 2.3 (1.5–3.3) | <0.01* | 59/54/5 |
| Time to treatment ≤4.5 h | 488 (73.4) | 206 (59.5) | 282 (88.4) | <0.01* | 59/54/5 |
| Wake-up stroke | 138 (19.9) | 106 (27.3) | 32 (10.4) | <0.01* | 29/12/17 |
| First-pass EVT technique | 54 | ||||
| SR | - | 44 (12.7) | - | - | - |
| Aspiration | - | 178 (51.4) | - | - | - |
| Combined SR and aspiration | - | 114 (32.9) | - | - | - |
| Intra-arterial thrombolysis | - | 5 (1.4) | - | - | - |
| Other | - | 5 (1.4) | - | - | - |
| No. of passes | - | 1 (1–2) | - | - | 18 |
| Recanalization | 3 | ||||
| TICI 0-2a | - | 81 (20.4) | - | - | - |
| TICI 2b-3 | - | 316 (79.6) | - | - | - |
| Etiology | 0.04* | 0/0/0 | |||
| Large artery atherosclerosis | 126 (17.4) | 83 (20.8) | 43 (13.3) | - | - |
| Cardioembolism | 285 (39.4) | 148 (37.0) | 137 (42.3) | - | - |
| Small vessel disease | 19 (2.6) | 7 (1.8) | 12 (3.7) | - | - |
| Other | 54 (7.5) | 28 (7.0) | 26 (8.0) | - | - |
| Undetermined | 240 (33.1) | 134 (33.5) | 106 (32.7) | - | - |
| Visual field recovery‡ | 0.62 | 287/156/131‡ | |||
| Complete | 96 (59.3) | 46 (63.9) | 50 (55.6) | - | - |
| Partial | 12 (7.4) | 5 (6.9) | 7 (7.8) | - | - |
| None | 35 (21.6) | 15 (20.8) | 20 (22.2) | - | - |
| Worse | 19 (11.7) | 6 (8.3) | 13 (14.4) | - | - |
Values are presented as median (interquartile range) or n (%).
PCA, posterior cerebral artery; EVT, endovascular thrombectomy; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; CT, computed tomography; MRI, magnetic resonance imaging; pc-ASPECTS, posterior circulation Acute Stroke Prognosis Early CT Score; PCom, posterior communicating artery; SR, stent retriever; TICI, Thrombolysis In Cerebral Infarction.
P<0.05;
The distribution was higher in the IVT group;
Of the 449 patients with visual field defect on the baseline.
During EVT, the most frequently used first-pass technique was contact aspiration (51.4%), followed by combined aspiration and stent retriever use (32.9%) and stent retriever alone (12.7%). The median number of passes was 1 (IQR 1–2), and 79.6% achieved TICI 2b-3 recanalization. Of patients treated with EVT, 41.0% received bridging IVT.
Outcomes
The primary outcome data were available for 655 patients (90.5%). Median 3-month mRS was 2 (1–4) in the EVT+/-IVT group and 2 (1–3) in the IVT-alone group (P=0.27) (Figure 2). Excellent outcome was observed in 31.8% of the EVT+/-IVT and 28.4% of the IVT-alone group (P=0.39) and independent outcome in 50.4% and 55.7% (P=0.18), respectively. ENI occurred more often after EVT+/-IVT than after IVT alone (64.7% vs. 53.2%, P<0.01) (Table 2). Data on visual fields at 1 to 3 months were available for 162 of the 449 patients (36.1%) with visual field defect at baseline; there was no difference in the vision field recovery between the groups (Table 1). Mortality at 3 months was higher in the EVT+/-IVT group than in the IVT group (16.2% vs. 10.1%, P=0.03), as was the sICH rate (6.1% vs. 2.5%, P=0.03).
Figure 2.
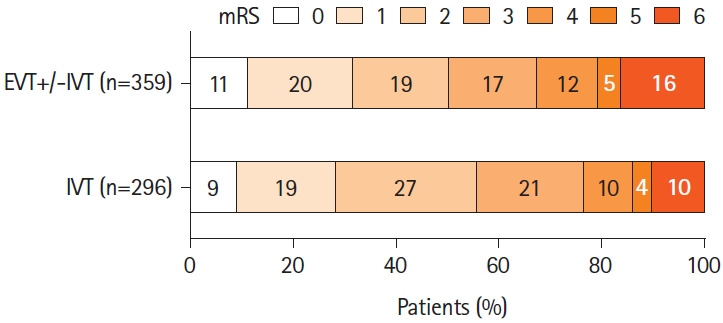
Distribution of the 3-month modified Rankin Scale (mRS) in patients with isolated posterior cerebral artery occlusion by endovascular therapy (EVT) with or without intravenous thrombolysis (IVT) versus IVT alone.
Table 2.
Univariable logistic regression and doubly robust regression analyses of outcomes according to treatment (EVT+/-IVT vs. IVT alone)
| EVT+/-IVT (n=400) | IVT (n=324) | P | OR (95% CI)† | aOR (95% CI)‡ | Missing data | |
|---|---|---|---|---|---|---|
| mRS at 3 months | 2 (1–4) | 2 (1–3) | 0.27 | 0.86 (0.66–1.12)§ | 1.07 (0.79–1.43)§ | 41/28 |
| mRS 0–1 at 3 months | 114 (31.8) | 84 (28.4) | 0.39 | 1.17 (0.84–1.64) | 1.39 (0.96–2.02)ǁ | 41/28 |
| mRS 0–2 at 3 months | 181 (50.4) | 165 (55.7) | 0.18 | 0.81 (0.59–1.10) | 1.07 (0.73–1.58) | 41/28 |
| ENI | 236 (64.7) | 164 (53.2) | <0.01* | 1.61 (1.18–2.19)* | 1.49 (1.05–2.12)* | 35/16 |
| Mortality | 58 (16.2) | 30 (10.1) | 0.03* | 1.71 (1.07–2.74)* | 1.77 (1.07–2.95)*ǁ | 41/28 |
| sICH | 24 (6.1) | 8 (2.5) | 0.03* | 2.55 (1.13–7.75)* | 2.87 (1.23–6.72)*ǁ | 4/0 |
Values are presented as median (interquartile range) or n (%) unless otherwise indicated.
EVT, endovascular thrombectomy; IVT, intravenous thrombolysis; OR, odds ratio; CI, confidence interval; aOR, adjusted OR; mRS, modified Rankin Scale; ENI, early neurological improvement; sICH, symptomatic intracerebral hemorrhage.
P<0.05;
Univariable generalized estimating equation analysis using multinomial distribution and cumulative logit link function (mRS shift) or binomial distribution and logit link function (other outcomes);
Doubly robust regression model (inverse probability weighted regression adjustment model) including treatment group (EVT+/-IVT vs. IVT alone), age, sex, hypertension, diabetes, atrial fibrillation, NIHSS score at baseline, posterior circulation Acute Stroke Prognosis Early CT Score (pc-ASPECTS) at baseline, level of occlusion, and year of treatment;
Common OR;
The model did not converge with the level of occlusion, so it was excluded.
According to the IPWRA analyses, there was no difference in the distribution of 3-month functional outcome (adjusted common OR 1.07 [95% CI 0.79–1.43]) between the treatment groups (Table 2). The patients treated with EVT+/-IVT were more likely to achieve ENI (adjusted OR 1.49 [95% CI 1.05–2.12]) compared to those receiving only IVT but had higher odds for mortality (adjusted OR 1.77 [95% CI 1.07–2.95]) and sICH (adjusted OR 2.87 [95% CI 1.23–6.72]). There was a trend for more frequent excellent 3-month functional outcome after EVT+/-IVT (adjusted OR 1.39 [95% CI 0.96–2.02]), but odds for independent outcome did not differ between the treatments (adjusted OR 1.07 [95% CI 0.73–1.58]).
There was no interaction between the treatment group and onset-to-treatment time ≤4.5 hours (P=0.48), occlusion of the P1 segment (P=0.13), or baseline NIHSS as a binary (NIHSS<10) (P=0.52) or ordinal variable (P=0.34) in the primary outcome model. In the sensitivity analysis adjusted for onset-to-treatment time, the results on the primary outcome remained unchanged (Supplementary Table 1). However, unlike in the main analysis including the whole cohort, the EVT+/-IVT group had significantly higher odds for excellent outcome (adjusted OR 1.66 [95% CI 1.10–2.50]), and mortality no longer differed between the groups (adjusted OR 1.33 [95% CI 0.75–2.35]).
The distribution of 3-month mRS of the patients treated with EVT only, IVT+EVT, and IVT alone are presented in Supplementary Figure 1. The patients treated with direct EVT had lower odds for a 1-point shift towards more favorable mRS (adjusted OR 0.65 [95% CI 0.44–0.96], for mRS 0–1 (adjusted OR 0.60 [95% CI 0.37–0.98]), and mRS 0–2 (adjusted OR 0.55 [95% CI 0.34–0.89]) compared to the patients receiving EVT+/-IVT, whereas the safety outcomes and ENI did not differ between these groups (Supplementary Table 2). Of note, when we compared the safety outcomes of EVT and IVT alone, EVT alone had higher mortality (adjusted OR 2.06 [95% CI 1.18–3.61]) and a trend for an increased sICH rate (adjusted OR 2.43 [95% CI 0.97–6.09]) compared to IVT alone.
Discussion
In this multicenter cohort of 724 patients with isolated PCAo, there was no difference in the 3-month functional outcome according to treatment either with EVT with or without bridging IVT or with IVT alone. EVT increased the odds for early improvement in the NIHSS score to the detriment of an increased risk of sICH and mortality. There was also a trend for more frequent excellent 3-month functional outcome after EVT.
Several prior observational studies have implied that EVT may promote ENI [7,11], vision recovery [8,11], and recanalization [8] in patients with isolated PCAo in comparison to BMM. Yet they have not shown a robust effect on functional recovery [6-8,10,14], the only exception being the more frequent excellent functional outcome (mRS 0–1) after EVT in the previous analysis from our registry [11]. Some studies have also observed an increased risk of sICH [6,11], mortality [11], or early neurological deterioration [6] after EVT. However, BMM in the previous studies has included either IVT or no reperfusion therapy. As IVT is the guideline-recommended treatment for acute ischemic stroke within 4.5 hours irrespective of the occlusion location [12], we focused on the question of whether EVT improves the outcome in addition to or in lieu of this standard of care.
Two previous observational studies compared EVT+/-IVT to IVT alone and found no difference in functional outcome, ENI, mortality, or sICH—potentially due to small sample sizes [9,14]. A subgroup analysis of the earlier PLATO cohort (n=613) did not detect a significant difference in ENI or mortality between patients receiving EVT+/-IVT versus IVT only, even though there were higher odds for excellent functional outcome and sICH in the EVT group [11]. On the other hand, a meta-analysis combining the previous observational data reported more frequent ENI after EVT, whereas other outcomes did not differ between the treatments [15]. However, the analysis was heavily driven by the previous results from the PLATO cohort and used unadjusted data with significant baseline imbalances, including higher baseline NIHSS in the EVT group. Thus, the results are susceptible to confounding.
Taken together, our results are largely in line with prior reports, strengthening the notion that EVT could increase the odds for early and excellent recovery at the potential cost of an increased risk of bleeding complications and mortality, neutralizing the effect on the distribution of mRS at 3 months. Our findings are also compatible with outcomes reported for PCAo patients who did not receive reperfusion therapy. As 43% of conservatively treated patients (IVT rate 3.8%) with isolated PCA stroke achieve mRS 0–1 and 69% mRS 0–3 at 1 month [4], there is less potential for a significant mRS shift with EVT compared to patients with anterior circulation LVO [1] or basilar artery occlusion [20,21]. Moreover, early recanalization after IVT is more likely with smaller vessel size, narrowing the gap of possible benefit of EVT in addition to IVT [22]. The same applies to the low mortality (0% to 8%) of PCA stroke patients at 1 month [4,23,24]. Therefore, it is important to weigh the bleeding risk against the potential benefit and consider EVT primarily for patients with reasonable probability for significant recovery, e.g., patients presenting with high severity of NIHSS.
The current data also suggest that if EVT is opted for PCAo, optimizing procedural safety is of uttermost importance. The sICH rate of 6.1% in the EVT+/-IVT group of our study was slightly higher than in the anterior circulation LVO [1] and approximately equal to reports in basilar artery occlusion [20,21,25,26]; however, different sICH definitions across studies hinder direct comparisons. Smaller vessel diameter, thinner vessel wall, and more distal and tortuous access to medium- or distal-vessel occlusions might increase the risk of complications, including perforation, dissection, and vasospasm [27,28]. However, a study on distal PCAo (P2 and P3 segments) observed an overall low frequency of procedural complications after EVT and no difference in the frequency of sICH or mortality compared to BMM [10]. Regardless, targeting increasingly distal and small arteries challenges interventionalists and device manufacturers to realize the potential benefit of EVT.
Interestingly, when we examined the patients treated with EVT according to whether they received bridging IVT or not, the poorest outcome was discovered after direct EVT. This contrasts the previous analysis from the Swiss stroke registry, where the outcomes did not differ depending on the bridging IVT [14], but is in line with the observational data on reperfusion treatment of basilar artery occlusion [29]. Although our data do not reveal the reasons for withholding IVT, some of which may be associated with poorer outcomes, this observation underlines the benefits of IVT as the first-line treatment for eligible patients undergoing EVT with isolated PCAo–similar to the current guideline recommendations for EVT in anterior circulation LVO [30]. Furthermore, there was no significant difference in the sICH rate between patients who received bridging IVT and those who received EVT alone.
Our large multicenter cohort builds upon the previous observational data on reperfusion therapies in isolated PCAo, as there are yet no available RCTs on the topic. The cohort is composed of patients treated after 2015, so it represents the era of EVT as the routine stroke treatment with modern catheter technology. The baseline pc-ASPECTS of both treatment groups was high, reflecting little established ischemic changes within the posterior circulation that could have affected the outcome despite successful recanalization.
However, there were some limitations. As the study design was non-randomized, the treatment decisions were based on the treating physician’s discretion, which resulted in patients with more severe symptoms and proximal occlusions being treated with EVT more frequently. We used the robust IPWRA method to account for the baseline differences but acknowledge the risk of remaining confounding. Due to missing time metric data, we did not adjust the analyses for time in the overall cohort. However, there was no interaction of treatment strategy and onsetto-treatment time within 4.5 hours. Our primary outcome measure, mRS, is not ideal to assess non-motor disability that often dominates after PCA stroke [31]. Indeed, a previous study found better cognitive outcome after EVT in PCAo, even though there was no difference in mRS [8]. The assessment of radiological data was not centralized but performed in each participating center following instructions in the data collection sheet. Furthermore, visual field recovery was available only for a minority of patients and was assessed with variable methods, including contrast perimetry that may miss smaller defects [32]. Finally, there were 19 (2.6%) patients whose stroke etiology was recorded as small vessel disease, potentially reflecting concomitant stroke mechanisms in the same patient. However, we acknowledge this classification may be a limitation.
Conclusions
In our large, observational multicenter cohort, there was no difference in functional outcome at 3 months as measured by the distribution of mRS scores in patients treated with either EVT+/- IVT or IVT alone for isolated PCAo. EVT increased the likelihood of ENI but at the cost of an increased risk of sICH and mortality. Ongoing RCTs on medium- and distal-vessel occlusion may shed light on the best treatment strategy for these patients in the future [33-35].
Appendix 1. Disclosures of conflicts of interest
Dr Dabus reported consultancy for Cerenovus, Penumbra, Route 92, Medtronic, MicroVention, and Stryker and stock holdings in RIST and InNeuroCo.
Dr Fifi reported consultancy for Cerenovus, MicroVention, and Stryker; Data Safety Monitoring Board (DSMB) for MIVI; and stock holdings in Imperative Care and Sim&Cure.
Dr Fischer reported research support from the Swiss National Science Foundation (SNF), Medtronic, Stryker, Rapid Medical, Penumbra, and Phenox; consultancies for Stryker and CSL Behring; and is on the advisory board for Alexion/Portola, Boehringer Ingelheim, Biogen, and Acthera.
Dr Haussen reported consultancy for Vesalio, Cerenovus, Stryker, Brainomix, Poseydon Medical, and Chiesi USA; DSMB from Jacobs Institute; and stock options in Viz AI.
Dr Herweh reported consultancy for Brainomix and Speaker with Stryker.
Dr Jadhav reports consulting with Basking Biosciences; stock options in Gravity Medical Technology; a patent for a novel stent retriever device licensed to Basking Biosciences, and Editor-in-Chief for the S:VIN journal. Dr Kaesmacher reported grants from the Swiss Academy of Medical Sciences/Bangerter Foundation, Swiss Stroke Society, and Clinical Trials Unit Bern.
Dr Kaiser reported grants from the Joachim Herz Foundation.
Dr Kuramatsu reports grants from Alexion Pharmaceuticals, Bayer Healthcare, Sanofi Pasteur, and Biogen Idec.
Dr Marto reported consulting from Amicus Therapeutics and Boehringer Ingelheim and Speaker with Boehringer Ingelheim.
Dr Marto reported consulting and speaker fees from Amicus Therapeutics and Boehringer Ingelheim.
Dr Michel reported grants from the University of Lausanne and Swiss National Science Foundation.
Dr Möhlenbruch reported grants from Medtronic, Stryker, and MicroVention.
Dr Mokin reported stock holdings in BrainQ, Serenity Medical, Synchron, and Bendit Technology and consulting from MicroVention, Medtronic, and Johnson & Johnson.
Dr Nagel reported consultancy for Brainomix and is a speaker with Boehringer Ingelheim and Pfizer.
Dr Nguyen reported Associate Editor of Stroke, advisory board with Idorsia, Brainomix.
Dr Nogueira reported consultancy for Biogen, Brainomix, Corindus, Cerenovus, Stryker, Medtronic, Ceretrieve, Anaconda Biomed, Vesalio, Imperative Care, NeuroVasc Technologies, Viz AI, Genentech, Prolong Pharmaceuticals, Perfuze, Phenox, and RapidPulse; stock options in Viz AI, Vesalio, Perfuze, Corindus, Brainomix, and Ceretrieve; grants from Cerenovus and Stryker.
Dr Nolte reported research report from compensation from Novartis, AstraZeneca, Deutsches Zentrum für Herz-Kreislaufforschung, and Deutsches Zentrum für Neurodegenerative Erkrankungen and consultancy for Alexion, Daiichi Sankyo, Novartis, AstraZeneca, Bayer Healthcare, Pfizer, Alexion, and Bristol Myers Squibb.
Dr Psychogios reported grants from Penumbra, Rapid Medical, Medtronic, Phenox, Bangerter-Rhyner Stiftung, SNF, Siemens Healthineers, and Stryker Neurovascular; travel support from Medtronic, Siemens Healthineers, Phenox, Penumbra, and Stryker; and consultancy for Siemens Healthineers.
Dr Puetz reported being a lecturer for Daiichi Sankyo.
Dr Ribo reported consultancy for Medtronic MiniMed, Cerenovus, AptaTargets, Stryker, and Philips and stock holdings in Methinks, Nora, and Anaconda Biomed.
Dr Ringleb reported travel support from Bayer and Bristol Myers Squibb, consultancy for Daiichi Sankyo Company and Boehringer Ingelheim.
Dr Sheth reported consultancy for Imperative Care, Viz AI, and Penumbra; compensation from Motif Neurosciences (other services); and grants from the National Institutes of Health.
Dr Siddiqui reported an ownership stake in Integra Lifesciences and Medtronic; consultancy for Cordis, Rapid Medical, MicroVention, Medtronic Vascular, Vassol, IRRAS USA, Boston Scientific, Amnis Therapeutics, Minnetronix Neuro, Canon Medical Systems USA, Cardinal Health 200, Johnson & Johnson–Latin America, Corindus, Penumbra, Apellis Pharmaceuticals, W.L. Gore & Associates, Stryker Corporation, and Viz AI; stock holdings in E8, Spinnaker Medical, Endostream Medical, Cerebrotech Medical Systems, Adona Medical, Bend IT Technologies, Whisper Medical, Neurotechnology Investors, Collavidence, Instylla, Q’Appel Medical, Serenity Medical, Borvo Medical, NeuroRadial Technologies, Sense Diagnostics, Tulavi Therapeutics, Synchron, Neurolutions, Viseon, BlinkTBI, Radical Catheter Technologies, and Truvic Medical; stock options in Viz AI, StimMed, Three Rivers Medical, Silk Road Medical, Imperative Care, CVAID, Cerevatech Medical, InspireMD, and PerFlow Medical; and security holdings in Vastrax, Launch NY, QAS.ai, VICIS, Neurovascular Diagnostics, Cognition Medical, and SongBird Therapy.
Dr. Strbian reported Assistant Editor of Stroke, Editorial board of European Stroke Journal, advisory board with Boehringer Ingelheim, Alexion/Astra Zeneca, BMS/Janssen, research support from the Boehringer Ingelheim; consultancies for Orion, Herantis Pharma, CSL Behring.
The other authors report no conflicts.
Footnotes
Funding statement
None
Conflicts of interest
The disclosures of conflict of interest of all authors are provided in Appendix 1.
Author contribution
Conceptualization: TNN, SN, DS. Study design: SR, TNN, SN, DS. Methodology: SR, TNN, MMQ, DS. Data collection: all authors. Investigation: all authors. Statistical analysis: SR, MMQ, DS. Writing—original draft: SR. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2024.00458.
Doubly robust regression analyses of outcomes according to treatment (EVT+/-IVT vs. IVT alone) in a sensitivity cohort excluding patients with missing data on onset-to-treatment time
Doubly robust regression analyses of outcomes according to treatment (EVT only vs. EVT+IVT)
Distribution of the 3-month modified Rankin Scale (mRS) in patients with isolated posterior cerebral artery occlusion by endovascular therapy (EVT) alone versus EVT with intravenous thrombolysis (IVT) versus IVT alone.
References
- 1.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 2.Räty S, Nguyen TN, Nagel S, Puetz V, Alemseged F, Abdalkader M, et al. What is the evidence for endovascular thrombectomy in posterior circulation stroke? Semin Neurol. 2023;43:345–355. doi: 10.1055/s-0043-1771210. [DOI] [PubMed] [Google Scholar]
- 3.Starikova N, Räty S, Strbian D, Kaiser DPO, Gerber JC, Huo X, et al. Endovascular thrombectomy for anterior circulation large vessel occlusion stroke: an evolution of trials. Semin Neurol. 2023;43:397–407. doi: 10.1055/s-0043-1771454. [DOI] [PubMed] [Google Scholar]
- 4.Ntaios G, Spengos K, Vemmou AM, Savvari P, Koroboki E, Stranjalis G, et al. Long-term outcome in posterior cerebral artery stroke. Eur J Neurol. 2011;18:1074–1080. doi: 10.1111/j.1468-1331.2011.03384.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40:3834–3840. doi: 10.1161/STROKEAHA.109.561787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabben C, Charbonneau F, Delvoye F, Strambo D, Heldner MR, Ong E, et al. Endovascular therapy or medical management alone for isolated posterior cerebral artery occlusion: a multicenter study. Stroke. 2023;54:928–937. doi: 10.1161/STROKEAHA.122.042283. [DOI] [PubMed] [Google Scholar]
- 7.Herweh C, Abdalkader M, Nguyen TN, Puetz V, Schöne D, Kaiser D, et al. Mechanical thrombectomy in isolated occlusion of the proximal posterior cerebral artery. Front Neurol. 2021;12:697348. doi: 10.3389/fneur.2021.697348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strambo D, Bartolini B, Beaud V, Marto JP, Sirimarco G, Dunet V, et al. Thrombectomy and thrombolysis of isolated posterior cerebral artery occlusion: cognitive, visual, and disability outcomes. Stroke. 2020;51:254–261. doi: 10.1161/STROKEAHA.119.026907. [DOI] [PubMed] [Google Scholar]
- 9.Cunha B, Baptista M, Pamplona J, Carvalho R, da Câmara CP, Alves M, et al. Acute treatment of isolated posterior cerebral artery occlusion: single center experience. J Stroke Cerebrovasc Dis. 2022;31:106239. doi: 10.1016/j.jstrokecerebrovasdis.2021.106239. [DOI] [PubMed] [Google Scholar]
- 10.Meyer L, Stracke CP, Jungi N, Wallocha M, Broocks G, Sporns PB, et al. Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol. 2021;78:434–444. doi: 10.1001/jamaneurol.2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TN, Qureshi MM, Strambo D, Strbian D, Räty S, Herweh C, et al. Endovascular versus medical management of posterior cerebral artery occlusion stroke: the PLATO study. Stroke. 2023;54:1708–1717. doi: 10.1161/STROKEAHA.123.042674. [DOI] [PubMed] [Google Scholar]
- 12.Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I–LXII. doi: 10.1177/2396987321989865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 14.Maulucci F, Disanto G, Bianco G, Pileggi M, Fischer U, Padlina G, et al. Endovascular therapy outcome in isolated posterior cerebral artery occlusion strokes: a multicenter analysis of the Swiss Stroke Registry. Eur Stroke J. 2023;8:575–580. doi: 10.1177/23969873221150125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkhiri A, Alamri AF, Alharbi AR, Almaghrabi AA, Alansari N, Niaz AA, et al. Endovascular therapy versus best medical management for isolated posterior cerebral artery occlusion: a systematic review and meta-analysis. Eur Stroke J. 2024;9:69–77. doi: 10.1177/23969873231201715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berberich A, Finitsis S, Strambo D, Michel P, Herweh C, Meyer L, et al. Endovascular therapy versus no endovascular therapy in patients receiving best medical management for acute isolated occlusion of the posterior cerebral artery: a systematic review and meta-analysis. Eur J Neurol. 2022;29:2664–2673. doi: 10.1111/ene.15410. [DOI] [PubMed] [Google Scholar]
- 17.Zetterqvist J, Sjölander A. Doubly robust estimation with the R package drgee. Epidemiol Methods. 2015;4:69–86. [Google Scholar]
- 18.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273–277. doi: 10.1111/j.1524-4733.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. doi: 10.1161/STROKEAHA.107.511162. [DOI] [PubMed] [Google Scholar]
- 20.Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C. Trial of Thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387:1373–1384. doi: 10.1056/NEJMoa2207576. [DOI] [PubMed] [Google Scholar]
- 21.Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387:1361–1372. doi: 10.1056/NEJMoa2206317. [DOI] [PubMed] [Google Scholar]
- 22.Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. 2016;47:2409–2412. doi: 10.1161/STROKEAHA.116.014181. [DOI] [PubMed] [Google Scholar]
- 23.Cals N, Devuyst G, Afsar N, Karapanayiotides T, Bogousslavsky J. Pure superficial posterior cerebral artery territory infarction in the Lausanne Stroke Registry. J Neurol. 2002;249:855–861. doi: 10.1007/s00415-002-0742-0. [DOI] [PubMed] [Google Scholar]
- 24.Arboix A, Arbe G, García-Eroles L, Oliveres M, Parra O, Massons J. Infarctions in the vascular territory of the posterior cerebral artery: clinical features in 232 patients. BMC Res Notes. 2011;4:329. doi: 10.1186/1756-0500-4-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langezaal LCM, van der Hoeven EJRJ, Mont’Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. 2021;384:1910–1920. doi: 10.1056/NEJMoa2030297. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19:115–122. doi: 10.1016/S1474-4422(19)30395-3. [DOI] [PubMed] [Google Scholar]
- 27.Saver JL, Chapot R, Agid R, Hassan A, Jadhav AP, Liebeskind DS, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke. 2020;51:2872–2884. doi: 10.1161/STROKEAHA.120.028956. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TN, Abdalkader M, Qureshi MM, Michel P, Strambo D, Strbian D, et al. First-line stent retriever versus contact aspiration or combined technique for endovascular therapy of posterior cerebral artery occlusion stroke: the PLATO study. Stroke Vasc Interv Neurol. 2024;4:e001004. [Google Scholar]
- 29.Nie X, Wang D, Pu Y, Wei Y, Lu Q, Yan H, et al. Endovascular treatment with or without intravenous alteplase for acute ischaemic stroke due to basilar artery occlusion. Stroke Vasc Neurol. 2022;7:190–199. doi: 10.1136/svn-2021-001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turc G, Tsivgoulis G, Audebert HJ, Boogaarts H, Bhogal P, De Marchis GM, et al. European Stroke Organisation - European Society for Minimally Invasive Neurological Therapy expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischaemic stroke and anterior circulation large vessel occlusion. Eur Stroke J. 2022;7:I–XXVI. doi: 10.1177/23969873221076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Haan R, Limburg M, Bossuyt P, van der Meulen J, Aaronson N. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke. 1995;26:2027–2030. doi: 10.1161/01.str.26.11.2027. [DOI] [PubMed] [Google Scholar]
- 32.Pandit RJ, Gales K, Griffiths PG. Effectiveness of testing visual fields by confrontation. Lancet. 2001;358:1339–1340. doi: 10.1016/S0140-6736(01)06448-0. [DOI] [PubMed] [Google Scholar]
- 33. Psychogios M, Brehm A. EnDovascular therapy plus best medical treatment (BMT) versus BMT alone for medIum vessel occlusion stroke - a pragmatic, international, multicentre, randomized triaL (DISTAL) [Internet]. Bethesda: National Library of Medicine; December 9, 2021 [accessed January 25, 2024]. Available from: www.clinicaltrials.gov/study/NCT05029414.
- 34. Hill MD. A multicentre, prospective, randomized, parallel group, open-label design to determine the efficacy and safety of endovascular thrombectomy for ischemic stroke patients with symptomatic acute medium vessel intracranial occlusions [Internet]. Bethesda: National Library of Medicine; April 15, 2022 [accessed January 25, 2024]. Available from: www.clinicaltrials.gov/study/NCT05151172.
- 35. Clarençon F. Evaluation of mechanical thrombectomy in acute ischemic stroke related to a distal arterial occlusion: a randomized controlled trial [Internet]. Bethesda: National Library of Medicine; November 14, 2021 [accessed January 25, 2024]. Available from: www.clinicaltrials.gov/study/NCT05030142.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Doubly robust regression analyses of outcomes according to treatment (EVT+/-IVT vs. IVT alone) in a sensitivity cohort excluding patients with missing data on onset-to-treatment time
Doubly robust regression analyses of outcomes according to treatment (EVT only vs. EVT+IVT)
Distribution of the 3-month modified Rankin Scale (mRS) in patients with isolated posterior cerebral artery occlusion by endovascular therapy (EVT) alone versus EVT with intravenous thrombolysis (IVT) versus IVT alone.


