Immune checkpoint inhibitors (ICIs) down-regulate inhibitory signals in T cells, thereby promoting antitumor immunity.1 ICIs, now approved for many advanced cancers, are especially effective in metastatic melanoma.1 Unsurprisingly, ICI for colitis (ICI-C) occurs in about 1% to 30% of patients depending on the agents, doses, and combinations used.2 Recent data suggest that ICI-C may be driven by expansion of cytotoxic CD8+ T cells, potentially derived from tissue-resident CD8+ T cells.3 These cytotoxic CD8+ T cells and inflammatory macrophages in ICI-C skew toward an interferon (IFN) gamma signature.3
Steroids are first-line therapy for ICI-C with infliximab (IFX) reserved refractory ICI-C.2 Case reports also suggest that vedolizumab and fecal microbiota transplantation may be effective.4,5 It was recently reported in a single case that tofacitinib is effective in refractory ICI-C.6 Tofacitinib selectively inhibits signaling downstream of janus kinase (JAK) 1 and 3. The JAKs are ubiquitously expressed and execute pleiotropic functions, including IFN gamma signaling (JAK1). Herein, we report that tofacitinib is effective for ICI-C.
Methods
The Institutional review board approved chart reviews from the University of Michigan and John’s Hopkins University. Extensive patient details are reported in the Supplementary Methods.
Results
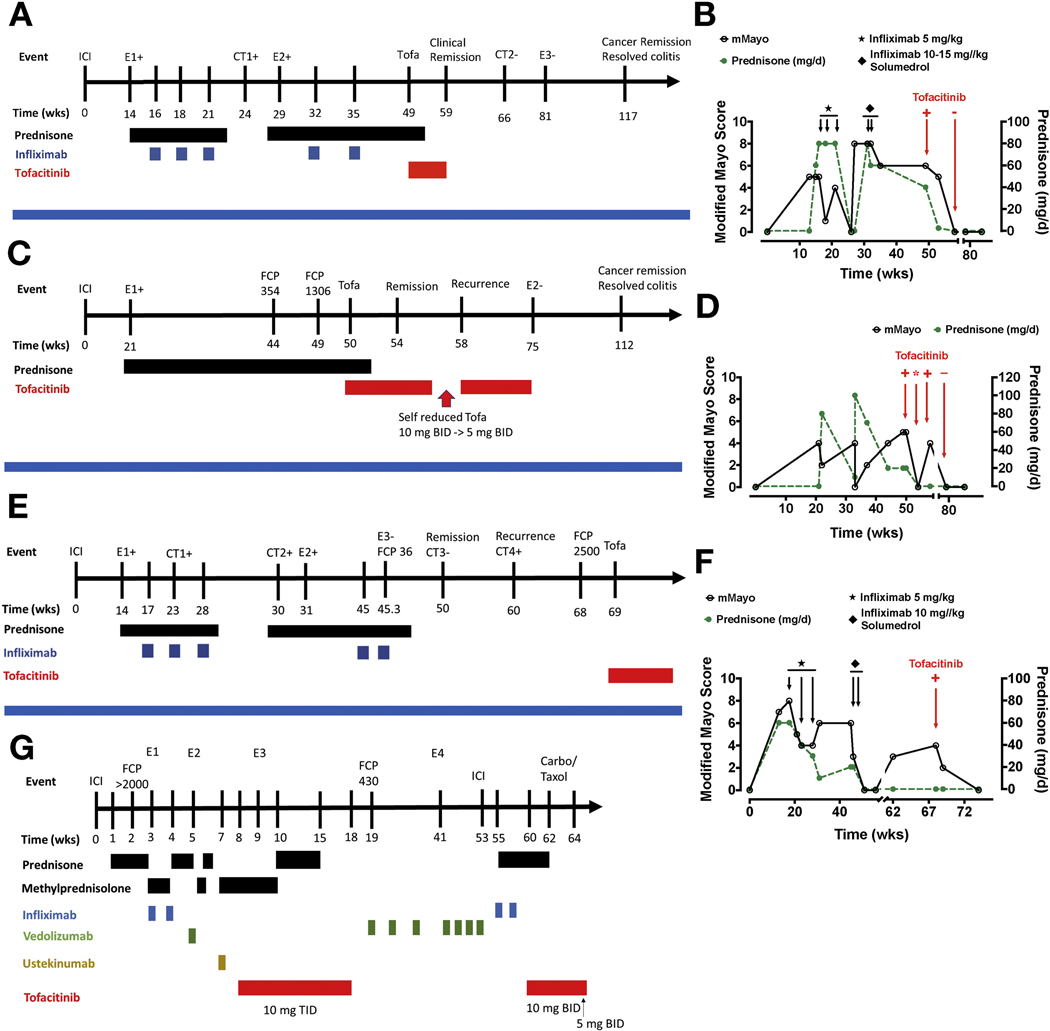
We identified 4 men treated with tofacitinib for ICI-C (Figure 1). Most patients received combination ipilimumab/nivolimumab for metastatic melanoma, whereas 1 patient (patient 2) received pembrolizumab and indoleamine-pyrrole 2,3-dioxygenase inhibitor as adjuvant therapy for lung adenocarcinoma. Most had failed biologics before tofacitinib (Figure 1). Prednisone was dosed at 0.7 to 1.3 mg/kg a day. Solumedrol and tofacitinib (5–10 mg, 2–3 times a day) doses varied.
Figure 1.
The clinical course (left) and associated modified Mayo score and prednisone dose (right) of patients 1 to 4 are shown in A through G, respectively. A modified Mayo score could not be easily computed in patient 4 because of a complex disease course. Time is presented as weeks after starting ICI therapy. Serial endoscopies (E) and computed tomographies (CT) are denoted by number and positive (+) or negative (−), indicating presence or absence of colitis, respectively. FCP (μg/g) at select times is presented.
Efficacy of Tofacitinib
Patient 1 developed prednisone-dependent ICI-C after ipilimumab/nivolimumab. He had a partial response to 3 infusions of IFX (5 mg/kg) with relapse verified by computed tomography and endoscopy (Figure 1A and B). He then failed IFX (10–15 mg/kg × 2) despite prednisone. Tofacitinib was thus started for refractory ICI-C, resulting in steroid-free remission (SFR) within 4 weeks, with a normal follow-up endoscopy (Figure 1A, Supplementary Figure 1A). He remains ICI-C free 60 weeks after stopping tofacitinib and received a total of 9 weeks of tofacitinib (10 mg twice daily).
Patient 2 developed prednisone-dependent ICI-C after pembrolizumab and an indoleamine-pyrrole 2,3-dioxygenase inhibitor (Figure 1C and D). He started tofacitinib after 28 weeks of steroid dependence and promptly achieved SFR within 4 weeks. His symptoms recurred with dose reduction but abated with tofacitinib increase, suggesting a dose–response effect (Figure 1C and D). Follow-up endoscopy demonstrated remission (Supplementary Figure 1A). He remains ICI-C free 37 weeks after stopping tofacitinib and received a total of 25 weeks of tofacitinib (5–10 mg twice daily).
Patient 3 developed ICI-C after ipilimumab/nivolimumab. He received IFX (5 mg/kg × 3) for prednisone-dependent ICI-C with partial response and fluctuating fecal calprotectin (FCP; >1000 to 413 μg/g) (Figure 1E and F). Active ICI-C with prednisone taper was verified on computed tomography and endoscopy (Figure 1E, Supplementary Figure 1A). IFX level was 6.9 μg/mL, prompting IFX (10 mg/kg × 2) with good response, SFR, and normal follow-up FCP (36 μg/g). Unfortunately, he developed ICI-C recurrence with elevated FCP verified by computed tomography (Figure 1E). Tofacitinib was thus started, with clinical remission and normal FCP (<30 μg/g) within 6 weeks.
Patient 4 developed severe ICI enterocolitis (ICI-EC) after a single dose of ipilimumab/nivolimumab verified on endoscopy (Figure 1G, Supplementary Figure 1B). Importantly, he had pre-existing mild small intestinal Crohn’s disease, which had been managed off therapy for years without disease progression. His ICI-EC was refractory to steroids, IFX (5 mg/kg × 2), vedolizumab (300 mg × 1), and ustekinumab (390 mg intravenously), eventually requiring parenteral nutrition (Figure 1G, Supplementary Figure 1B). Tofacitinib 10 mg 3 times a day resulted in prompt improvement with normalization of C-reactive protein within 3 days (Figure 1G). Despite this response, he failed tofacitinib de-escalation and transition to oral steroids with endoscopically confirmed colitis. Tofacitinib was thus increased (10 mg 3 times a day) with successful SFR within 6 weeks (Figure 1G). Tofacitinib was stopped after 14 weeks because of clinically resolved ICI-EC but progressive malignancy. However, he rapidly developed symptoms with elevated FCP (430 mg/g) off tofacitinib. Thus, he was switched to vedolizumab and achieved clinical remission after intensification and a course of topical steroids (Supplementary Figure 1B).
Nivolimumab was restarted, but he suffered recurrent ICI-EC after the second infusion. He then developed in infusion reaction with IFX indicative of antibodies to IFX. Tofacitinib was therefore restarted (10 mg twice daily), with prompt improvement and SFR over 30 days (Figure 1G). Thus, he received a total of 10 weeks of tofacitinib, followed by a second course of 6 weeks, and achieved SFR after each course. At the last follow-up, he was tapered to tofacitinib 5 mg twice daily with plans to stop.
Cancer Outcomes With Tofacitinib
Three patients achieved cancer remission before starting tofacitinib, all of whom remain cancer free 12 to 71 weeks after tofacitinib (Supplementary Figure 2A–C). Patient 4 had not achieved cancer remission when he received tofacitinib, and his cancer progressed through ICI-C therapy.
Discussion
Treatment for ICI-C is based on expert guidelines and diverges from inflammatory bowel disease despite their similarities.2 We found tofacitinib is effective for ICI-C and even led to SFR in 1 patient who had ICI-C refractory to multiple biologics (Figure 1G). Our patients responded within days and achieved SFR within 4 to 10 weeks. We dosed tofacitinib based on the dose for inflammatory bowel disease (10 mg twice daily), except in patient 4, who received a higher dose because of severe ICI-EC. Although this patient’s cancer progressed on tofacitinib, he had only received 1 dose of ICI and received multiple biologics before tofacitinib, thus complicating the picture. It is possible his ICI-C was severe because of underlying inflammatory bowel disease.7
Loss-of-function mutations in JAK1 are associated with resistance to PD-1 blockade in melanoma.8 This is believed to be due to loss of IFN-driven tumor cell growth arrest.8 Additionally, IFN gamma signaling in effector cells may be involved in antitumor immunity.3,9 Thus, given that IFN-driven JAK1-dependent responses may be necessary for tumor clearance, we advise caution when considering tofacitinib for ICI-C. Indeed, most of our patients had resolved their malignancies when they start tofacitinib. Overall, our data suggest that further investigations of tofacitinib for ICI-C are warranted.
Supplementary Material
Funding
This study was funded by a Crohn’s and Colitis Foundation Career Development Award (no. 598997) and K08DK123403 (to S.B.), K08 DK114478 (to J.M.), and R01 DK11815403 (to P.D.R.H.).
Abbreviations used in this paper:
- FCP
fecal calprotectin
- ICI
immune checkpoint inhibitor
- ICI-C
immune checkpoint inhibitor colitis
- ICI-EC
immune checkpoint inhibitor enterocolitis
- IFN
interferon
- IFX
infliximab
- JAK
janus kinase
- SFR
steroid-free remission
Footnotes
CRediT Authorship Contributions
Shrinivas Bishu, MD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead; Writing – review & editing: Lead).
Joanna Melia, MD (Conceptualization: Equal; Investigation: Equal;
Methodology: Equal; Writing – original draft: Equal; Writing – review & editing: Equal).
William Sharfman, MD (Investigation: Supporting; Writing – review & editing: Supporting).
Christopher D. Lao, MD (Investigation: Supporting; Writing – review & editing: Supporting).
Leslie A. Fecher, MD (Investigation: Supporting; Writing – review & editing: Supporting).
Peter D. R. Higgins, MD, MSc (Investigation: Supporting; Writing – review & editing: Supporting).
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/j.gastro.2020.10.029.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Postow MA, et al. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos-Casals M, et al. Nat Rev Dis Primers 2020;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luoma AM, et al. Molecular Cell 2020;182:655–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Sbeih H, et al. J Immunother Cancer 2018;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, et al. Nat Med 2018;24:1804–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esfahani K, et al. N Engl J Med 2020;382:2374–2375. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Sbeih H, et al. J Clin Oncol 2020;38:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaretsky JM, et al. N Engl J Med 2016;375:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riaz N, et al. Cell 2017;171:934–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.