Abstract
Performance fatigability is typically experienced as insufficient energy to complete daily physical tasks, particularly with advancing age, often progressing toward dependency. Thus, understanding the etiology of performance fatigability, especially cellular‐level biological mechanisms, may help to delay the onset of mobility disability. We hypothesized that skeletal muscle energetics may be important contributors to performance fatigability. Participants in the Study of Muscle, Mobility and Aging completed a usual‐paced 400‐m walk wearing a wrist‐worn ActiGraph GT9X to derive the Pittsburgh Performance Fatigability Index (PPFI, higher scores = more severe fatigability) that quantifies percent decline in individual cadence‐versus‐time trajectory from their maximal cadence. Complex I&II‐supported maximal oxidative phosphorylation (max OXPHOS) and complex I&II‐supported electron transfer system (max ETS) were quantified ex vivo using high‐resolution respirometry in permeabilized fiber bundles from vastus lateralis muscle biopsies. Maximal adenosine triphosphate production (ATPmax) was assessed in vivo by 31P magnetic resonance spectroscopy. We conducted tobit regressions to examine associations of max OXPHOS, max ETS, and ATPmax with PPFI, adjusting for technician/site, demographic characteristics, and total activity count over 7‐day free‐living among older adults (N = 795, 70–94 years, 58% women) with complete PPFI scores and ≥1 energetics measure. Median PPFI score was 1.4% [25th–75th percentile: 0%–2.9%]. After full adjustment, each 1 standard deviation lower max OXPHOS, max ETS, and ATPmax were associated with 0.55 (95% CI: 0.26–0.84), 0.39 (95% CI: 0.09–0.70), and 0.54 (95% CI: 0.27–0.81) higher PPFI score, respectively. Our findings suggested that therapeutics targeting muscle energetics may potentially mitigate fatigability and lessen susceptibility to disability among older adults.
Keywords: aging, fatigability, fatigue, gait, mitochondria
Among 795 older adults from the Study of Muscle, Mobility and Aging, we found that each 1 standard deviation lower max OXPHOS, max ETS, and ATPmax were associated with 0.55 (95% CI: 0.26–0.84), 0.39 (95% CI: 0.09–0.70), and 0.54 (95% CI: 0.27–0.81) higher PPFI score, respectively. Our findings suggested that therapeutics targeting muscle energetics may potentially mitigate fatigability and lessen susceptibility to disability among older adults.
Abbreviations
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- BMI
body mass index
- ETS
electronic transfer system
- OXPHOS
oxidative phosphorylation
- PCr
phosphocreatine
- PPFI
pittsburgh performance fatigability index
- ROS
reactive oxygen species
- SD
standard deviation
- SOMMA
Study of Muscle, Mobility and Aging
1. INTRODUCTION
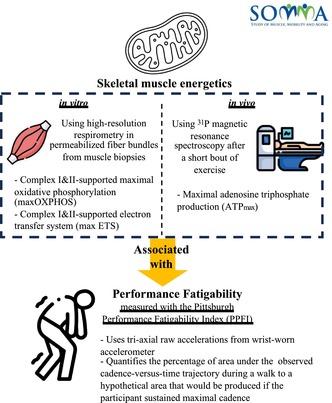
Skeletal muscle mitochondria produce the energy needed for muscle contraction and are the key energetic drivers of locomotion and mobility (Coen et al., 2013; Mau et al., 2022; Santanasto et al., 2016; Tian et al., 2023). With aging, skeletal muscle energetics decline, particularly for oxidative phosphorylation (OXPHOS) and adenosine triphosphate (ATP) generation capacity (Gonzalez‐Freire et al., 2015; Short et al., 2005). Meanwhile, performance fatigability, an energy disorder, worsens and manifests as insufficient energy to complete daily physical tasks (Alexander et al., 2010; Eldadah, 2010). In the aging literature, performance fatigability is defined as a decrement in performance during a standardized physical task or activity (Schrack et al., 2020; Van Geel et al., 2020) and has been associated with functional limitations/mobility decline (Murphy et al., 2017; Simonsick et al., 2014) and frailty (Schnelle et al., 2012) among older adults. Given the importance of skeletal muscle in physical performance and mobility (Gonzalez‐Freire et al., 2018; Lanza & Nair, 2010), better understanding of skeletal muscle energetics and performance fatigability may provide valuable insights to identify potential mitochondrial therapeutic targets that prevent fatigability and promote healthy aging.
To our knowledge, only two studies have directly examined the associations between muscle energetics and fatigability (Liu et al., 2021; Santanasto et al., 2015). However, they focused on perceived fatigability, a similar, but distinct, concept from performance fatigability (Loy et al., 2017; Simonsick et al., 2014). Studies by Santanasto et al. (2015) and Liu et al. (2021) both concluded that poorer skeletal muscle maximal ATP production (ATPmax), as measured with 31P magnetic resonance spectroscopy (MRS), was associated with more severe perceived fatigability. Extending this work by measuring performance fatigability objectively using accelerometry plus measuring mitochondrial respiration in addition to ATP production would capture skeletal muscle energetics more comprehensively. Specifically, high‐resolution respirometry has been recognized as the gold standard to measure OXPHOS in permeabilized muscle fibers from biopsies (Gnaiger, 2001), which provides mechanistic component of mitochondrial function that complements and extends assessments of ATP synthesis (i.e., ATPmax). Additionally, high‐resolution respirometry also assesses electron transfer system (ETS) capacity, which is quantified as the maximum oxygen flux stimulated by non‐coupled respiration with a short circuit of the proton cycle across the inner mitochondria membrane. By analyzing both respirometry and ATP production, we can further explore mechanical similarities and differences of maximal mitochondrial function capacity versus capacity under pathological conditions in relation to fatigability, which may further inform us on the optimal lifestyle intervention modality to mitigate fatigability severity.
Collectively, existing evidence provides a strong scientific premise for examining associations between skeletal muscle energetics and performance fatigability. The Study of Muscle, Mobility and Aging (SOMMA) included assessments of both skeletal muscle respirometry and ATP production in a large prospective cohort of older adults, as well as objectively measured performance fatigability called the Pittsburgh Performance Fatigability Index (PPFI). We hypothesized that lower Complex I&II‐supported maximal OXPHOS, Complex I&II‐supported ETS, and ATPmax would be significantly associated with higher PFFI score.
2. METHOD
2.1. Study sample
A total of 879 community‐dwelling women and men (age ≥70 years) with gait speed of ≥0.6 m/s during a 4‐m walk and willing/eligible to have MRS and a skeletal muscle biopsy were enrolled in SOMMA (http://sommaonline.ucsf.edu) at two academic clinical centers (University of Pittsburgh and Wake Forest University School of Medicine) from April 2019 to December 2021 (Cummings et al., 2023). Participants were excluded if they reported an inability to walk one‐quarter of a mile or climb a flight of stairs; had body mass index (BMI) ≥40 kg/m2; had an active malignancy or dementia; or had any medical contraindication to biopsy or MR. Briefly, as a prospective, longitudinal ongoing cohort study, SOMMA aims to clarify the constellation of muscle properties that most strongly predict mobility disability, decline in fitness, walking speed, and muscle mass as people age, which might be targets for new interventions. The baseline visit generally included 3 days of assessments: Day 1—usual‐paced 400‐m walk and general clinic measures, Day 2—magnetic resonance imaging/spectroscopy, and Day 3—skeletal muscle biopsy. Full details of the study design and timing of clinical measurements have been published (Cummings et al., 2023). All participants provided written informed consent, and SOMMA was approved by the WIRB‐Copernicus Group Institutional Review Board (WCG IRB) as the single IRB for all participating sites.
2.2. Skeletal muscle energetics
2.2.1. Skeletal muscle biopsy collection and processing
The skeletal muscle biopsy was obtained from the right side medial vastus lateralis after an 12‐h fast and limited strenuous exercise for 48 h before the procedure. Participants also rested in a supine position for at least 15 min prior to the biopsy. Skeletal muscle biopsy specimens were obtained under local anesthesia (1% or 2% lidocaine), ~15 cm above the patella using a 5 or 6 mm Bergstrom‐style core biopsy needle with suction. Muscle tissue was immediately blotted dry and trimmed of visible adipose and connective tissue. A separate portion (~20 mg) of the biopsy specimen was placed in a relaxing and biopsy‐preserving solution for high‐resolution respirometry, as previously described (Coen et al., 2013). Myofiber bundles (~2–3 mg) were prepared by gently teasing apart the fibers using fine‐point tweezers. Then, myofiber bundles were chemically permeabilized for 30 min using saponin and were washed twice before determining their wet weight on an analytical balance (Mettler Toledo). Details on fiber bundle preparation can be found elsewhere (Coen et al., 2015).
2.2.2. Mitochondrial respiration
After weighing, the permeabilized fiber bundles were placed into the respirometer chambers of an Oxygraph‐2K (O2K, Oroboros Instruments). Assays were run at 37°C in a specific O2 concentration range (400–200 μM) in respiratory chambers. In the assay protocol, the following substrates were added in sequential steps to assess Complex I‐ and II‐supported maximal OXPHOS at state 3 respiration (max OXPHOS): pyruvate (5 mM) and malate (2 mM), ADP (4.2 mM) and Cytochrome c (10 μM), glutamate (10 mM), and succinate (10 mM). Then, a titration of the uncoupler FCCP was further added to determine Complex I and II‐supported ETS (max ETS). Any sample with the integrity of the outer mitochondrial membrane, as tested with Cytochrome c, greater than 15% was omitted from the analysis. Steady‐state oxygen flux was normalized to wet weight of the fiber bundle using Datlab 7.4 software. In SOMMA, the mean coefficient of variation for duplicates of max OXPHOS measurement was 11.5% across both clinical sites (Mau et al., 2022).
2.2.3. Maximal ATP production
31P MRS measures the rate of regeneration of phosphocreatine (PCr) after a short bout of exercise to quantify mitochondrial maximal ATP production (ATPmax). This is a functional measure of in vivo ATP production via oxidative phosphorylation (Coen et al., 2013). In SOMMA, participants were instructed to lie in a supine position with their lower leg strapped and right knee joint in 20°–30° of flexion. 31P spectra were collected in a 3 Tesla MR scanner (Siemens Medical System—Prisma at Pittsburgh site or Skyra at Wake Forest site) using a 12″ dual‐tuned, surface RF coil (PulseTeq, Limited) placed over the right distal vastus lateralis. Participants did two bouts of isometric knee extension against the resistance of an ankle strap. The first bout was as hard and fast as they could for 30 s, and the second was adjusted for the length of time of muscle contraction (18–36 s) based on the first bout to ensure 30%–50% PCr depletion which was needed to obtain a high signal‐to‐noise defining full PCr recovery without acidosis (PH < 6.8; Jubrias et al., 2003). PCr recovery rate after exercise until PCr returned to baseline levels was fit, and the time constant of the mono‐exponential fit (tau) was used to calculate ATPmax (Amara et al., 2008; Blei et al., 1993). Our previous study illustrated good reproducibility of ATPmax evidenced by a high correlation (r = 0.92) between same‐day repeat scans (Santanasto et al., 2015). In SOMMA, the mean coefficient of variation for duplicates of ATPmax measurement was 9.9% across both clinic sites (Mau et al., 2022).
2.3. Pittsburgh Performance Fatigability Index
Participants wore an ActiGraph GT9X (Link) accelerometer (ActiGraph LLC) on their nondominant wrist during the usual‐paced 400‐m walk. Triaxial raw accelerometer data were collected at a sampling frequency of 80 Hz. During the usual‐paced 400‐m walk, participants were instructed to complete the distance at their usual pace without overexerting themselves (20 m each, a total of 10 laps in an unobstructed long corridor with traffic cones on both ends; Simonsick et al., 2008).
Raw accelerometer data collected during the 400‐m walk were processed in R (Version 4.0) to calculate PPFI, shown as a ratio of comparing the area under an individual's observed cadence‐versus‐time trajectory during the walking task to a hypothetical area that would be observed in the absence of fatigue (i.e., if the participant sustained maximal cadence throughout the entire 400‐m walk; Figure S1). Individual‐level smoothed cadence trajectories were fitted using penalized regression splines. Specific details about the derivation of PPFI have been published (Qiao et al., 2022). Participants who completed the usual‐paced 400‐m walk within 6 min exhibited no performance fatigability during the walking task (i.e., negligible decline in cadence), thus were classified as PPFI = 0%. Higher PPFI score (range 0%–100%) indicates more severe performance fatigability. Sex‐ and task‐specific PPFI cut‐points that optimally discriminate usual gait speed were also used to categorize participants into three severity strata: (1) no performance fatigability (PPFI = 0%); (2) mild performance fatigability (0% < PPFI < 3.5% for women; 0% < PPFI < 5.4% for men); and (3) moderate‐to‐severe performance fatigability (PPFI ≥ 3.5% for women; PPFI ≥ 5.4% for men; Qiao et al., 2023).
2.4. Covariates
Age, self‐reported sex, race (White, Black, Asian, Native American/Alaskan Native, Native Hawaiian/Pacific Islander, multiracial, or unknown), and smoking status (current/former/never) were obtained from each participant using self‐administered questionnaires. Race was further binarized for analyses as White and Black, Indigenous, and People of Color. Height (Harpenden stadiometers; Dyfed, UK) without shoes and weight (digital scales) with light clothing were assessed. The Short Physical Performance Battery (SPPB) consisted of three components: a balance battery with side‐by‐side, semi‐tandem, and tandem positions; a 4‐m usual pace walk; and five timed repeated chair stands. Each component was scored 0 (unable to complete) to 4 (best), and a summary score was calculated, ranging from 0 to 12, with higher scores indicating better physical function (Guralnik et al., 1994). Multimorbidity (yes/no) included self‐reported physician‐diagnosed hypertension, diabetes, heart diseases (including heart attack, coronary and myocardial infarction), stroke, lung diseases (including chronic obstructive lung disease, chronic bronchitis, asthma, emphysema, and chronic obstructive pulmonary disease), osteoporosis, and arthritis. Physical activity was measured by total activity count (Wolff‐Hughes et al., 2014) with the ActiGraph GT9X (Link) during a 7‐day free‐living period. We instructed participants to wear the device on their nondominant wrist at all times except underwater for more than 30 min. Valid days were defined as ≥17 h of wear time during a 24‐h period (00:00 to 23:59 for the same date) for ≥3 days (clinic visit day was excluded).
2.5. Statistical analyses
Among those enrolled in SOMMA (N = 879, 76.3 ± 5.0 years old, 59% women), 808 participants wore an accelerometer during the usual‐paced 400‐m walk, the source data used to derive PPFI, our key outcome of interest. Due to unidentifiable walking patterns based upon visual inspection of the accelerometer data, three participants were further excluded. Among participants with PPFI scores (n = 805), max OXPHOS data were available for 688 participants, max ETS data were available for 562 participants, and ATPmax data were available for 742 participants. The missingness for mitochondrial respiration was primarily due to assay data not meeting minimal quality control criteria or acidic conditions for ATPmax. Thus, the final analytical sample with PPFI score, at least one skeletal muscle energetics measure, and no missing values on covariates was 795. All analyses were performed using R (R Foundation for Statistical Computing, version 4.0). Alpha was set to 0.05, and two‐sided p values smaller than 0.05 were considered significant.
Descriptive characteristics of participants were reported as median [25th percentile, 75th percentile], mean ± standard deviation (SD), or frequencies (percentages) in the overall sample and by PPFI severity strata. Trends across PPFI severity strata were examined using Kruskal–Wallis analysis of variance (ANOVA) for non‐normally distributed continuous variables, ANOVA for normally distributed continuous variables, and chi‐square tests for categorical variables. To understand the representativeness of our analytical sample, we compared demographic characteristics and physical function between participants with a biopsy and our final sample.
First, we examined Spearman's correlations (rs) and used scatterplots to illustrate bivariate correlations between muscle energetics and PPFI. Then, we generated regression models to examine the associations between muscle energetics and PPFI with progressive covariate adjustments to account for established and potential confounders based on prior literature (Mau et al., 2022; Simonsick et al., 2014). All muscle energetics variables were standardized before analyses. Model 1 adjusted for technician/site, age, sex, and race; Model 2 further adjusted for height and weight; and Model 3 additionally adjusted for total activity count. Given that PPFI scores were bounded between 0 and 100 and right‐skewed, tobit regressions were applied with continuous PPFI score. Whereas for categorical PPFI severity strata, we used ordinal logistic regressions and examined the proportional odds ratio assumption using the Brant–Wald test. Given that previous studies found a higher prevalence of performance fatigability in women (Simonsick et al., 2014) and differential associations between skeletal muscle energetics and physical function by physical performance (Santanasto et al., 2016), we further examined interactions with continuous PPFI score and sex or SPPB score.
3. RESULTS
3.1. Participant characteristics
Participants (N = 795) were 76.4 ± 5.0 years old (range: 70–94), 58% women, and 86% White. Median PPFI score was 1.4% [0%, 2.9%], range of 0%–21.7%, with 283 (35.6%) participants having no performance fatigability, 400 (50.3%) participants having mild performance fatigability, and 112 (14.1%) participants having moderate‐to‐severe performance fatigability (Table 1). From lowest to highest PPFI severity strata, median max OXPHOS, median max ETS, and median ATPmax values were lower, all p trend < 0.001 (Table 1). Across PPFI severity strata, participants had slower usual gait speed, worse SPPB score, and lower total active count, all p trend ≤ 0.02 (Table 1). No differences were found across PPFI severity strata in terms of health conditions, except for arthritis (Table 1). Lastly, no differences were observed among participants with different skeletal muscle energetic measures (Table S1).
TABLE 1.
Baseline characteristics of the total sample (N = 795) and stratified by Pittsburgh Performance Fatigability Index (PPFI) severity strata in the Study of Muscle, Mobility and Aging (SOMMA).
| Characteristics | Total (N = 795) | No performance fatigability a (n = 283) | Mild performance fatigability a (n = 400) | Moderate‐to‐severe performance fatigability a (n = 112) | p Trend |
|---|---|---|---|---|---|
| PPFI, 0%–100% | 1.39 [0, 2.90] | 0 [0, 0] | 2.00 [1.27, 2.82] | 5.38 [4.21, 6.42] | |
| Max OXPHOS, pmol/(s × mg) | 56.9 [46.2, 69.5] | 62.4 [52.8, 77.8] | 54.1 [43.4, 65.1] | 53.7 [44.1, 60.5] | <0.001 |
| Missing | 107 (13.5) | 30 (10.6) | 53 (13.3) | 24 (21.4) | |
| Max ETS, pmol/(s × mg) | 77.0 [64.9, 92.2] | 81.5 [70.7, 97.7] | 74.1 [62.1, 90.0] | 70.4 [62.1, 85.6] | <0.001 |
| Missing | 233 (29.3) | 55 (19.4) | 135 (33.8) | 43 (38.4) | |
| ATPmax, mM/s | 0.51 [0.44, 0.61] | 0.55 [0.47, 0.67] | 0.50 [0.43, 0.60] | 0.47 [0.42, 0.54] | <0.001 |
| Missing | 53 (6.7) | 28 (9.9) | 21 (5.3) | 4 (3.6) | |
| Age, years | 76.4 ± 5.0 | 75.0 ± 3.8 | 77.1 ± 5.4 | 77.4 ± 5.5 | <0.001 |
| Sex, women | 462 (58.1) | 134 (47.3) | 240 (60.0) | 88 (78.6) | <0.001 |
| Race, white | 684 (86.0) | 255 (90.1) | 341 (85.3) | 88 (78.6) | 0.01 |
| Height, cm | 166.0 ± 9.8 | 168.2 ± 9.4 | 165.2 ± 10.0 | 163.0 ± 9.1 | <0.001 |
| Weight, kg | 76.3 ± 15.3 | 74.7 ± 15.1 | 77.5 ± 15.6 | 76.5 ± 14.6 | 0.14 |
| Body mass index, kg/m2 | 27.6 ± 4.6 | 26.2 ± 4.1 | 28.3 ± 4.7 | 28.7 ± 4.8 | <0.001 |
| 400‐m gait speed, m/s | 1.05 ± 0.18 | 1.23 ± 0.10 | 0.96 ± 0.11 | 0.89 ± 0.13 | <0.001 |
| Short Physical Performance Battery, 0–12 | 10.2 ± 1.8 | 11.1 ± 1.1 | 9.7 ± 1.8 | 9.3 ± 2.0 | <0.001 |
| Hypertension b | 411 (51.7) | 148 (52.3) | 200 (50.0) | 63 (56.3) | 0.70 |
| Diabetes c | 123 (15.5) | 32 (11.3) | 70 (17.5) | 21 (18.8) | 0.37 |
| Heart diseases c | 56 (7.0) | 18 (6.4) | 32 (8.0) | 6 (5.4) | 0.74 |
| Stroke c | 21 (2.6) | 9 (3.2) | 6 (1.5) | 6 (5.4) | 0.41 |
| Lung disease c | 105 (13.2) | 34 (12.0) | 51 (12.8) | 20 (17.8) | 0.80 |
| Osteoporosis c | 140 (17.6) | 47 (16.6) | 64 (16.0) | 29 (25.9) | 0.30 |
| Arthritis c | 443 (55.7) | 127 (44.9) | 245 (61.3) | 71 (63.4) | <0.001 |
| Fall history d | 222 (27.9) | 67 (23.7) | 124 (31.0) | 31 (27.7) | 0.54 |
| Total activity count, 10,000 counts/d | 198 ± 58 | 213 ± 59 | 191 ± 57 | 187 ± 55 | <0.001 |
Note: All reported in median [25th percentile, 75th percentile], mean ± SD or n (%).
Abbreviations: ATP, adenosine triphosphate; ETS, electronic transfer system; OXPHOS, oxidative phosphorylation; PPFI, Pittsburgh Performance Fatigability Index.
No performance fatigability: PPFI = 0; mild performance fatigability: 0 < PPFI < 3.5 for women, and 0 < PPFI < 5.4 for men; moderate‐to‐severe performance fatigability: PPFI ≥ 3.5 for women and PPFI ≥ 5.4 for men.
Hypertension was classified by systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥80 mmHg.
Diabetes and all following health conditions were self‐reported physician diagnoses. Heart diseases included heart attack or myocardial infarction, heart failure, or atrial fibrillation. Lung diseases included chronic obstructive lung disease, chronic bronchitis, asthma, emphysema, and COPD.
Fall history was self‐reported and asked as “During the past 12 months, have you fallen and landed on the floor or ground, or fallen and hit an object like a table or chair?”
3.2. Associations between skeletal muscle energetics and continuous Pittsburgh Performance Fatigability Index scores
Among all, lower max OXPHOS (r s = −0.25), max ETS (r s = −0.21), and ATPmax (r s = −0.22) were significantly correlated with higher PPFI score (Figure 1). In Tobit regression models, lower max OXPHOS, max ETS, and ATPmax were all significantly associated with higher PPFI score after adjustment for covariates (Table 2, Model 3). The partial R 2 for muscle energetics in Model 3 was the highest with max ETS (partial R 2 = 0.22), followed by ATPmax (partial R 2 = 0.06) and max OXPHOS (partial R 2 = 0.04; Table 2), indicating that max ETS explained considerably more variance in PPFI score than ATPmax and max OXPHOS. Note: Partial R 2 for max OXPHOS and ATPmax did not change even when the sample was restricted to only those with a max ETS measure (n = 562; data not shown).
FIGURE 1.
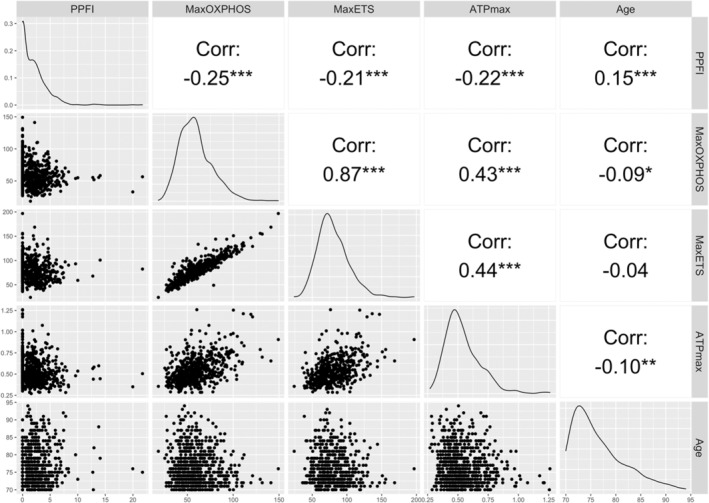
Spearman's correlations between skeletal muscle energetics, Pittsburgh Performance Fatigability Index (PPFI) score, and age in the Study of Muscle, Mobility and Aging (N = 795; there were n = 107 participants missing max OXPHOS, n = 233 missing max ETS, n = 53 missing ATPmax).***p < 0.001, **p < 0.01, *p < 0.05. ATP, adenosine triphosphate; ETS, electronic transfer system; OXPHOS, oxidative phosphorylation; PPFI, Pittsburgh Performance Fatigability Index.
TABLE 2.
Associations of skeletal muscle mitochondrial energetics and Pittsburgh Performance Fatigability Index (PPFI) in older adults in the Study of Muscle, Mobility and Aging a .
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | Partial R 2 | β (95% CI) | Partial R 2 | β (95% CI) | Partial R 2 | |
| Max OXPHOS (1 SD = 18.4 pmol/(s × mg); n = 688) | 0.65 (0.35, 0.94) | 0.04 | 0.60 (0.30, 0.89) | 0.04 | 0.55 (0.26, 0.84) | 0.04 |
| Max ETS (1 SD = 22.1 pmol/(s × mg); n = 562) | 0.48 (0.17, 0.79) | 0.23 | 0.43 (0.12, 0.74) | 0.22 | 0.39 (0.09, 0.70) | 0.22 |
| ATPmax (1 SD = 0.15 mM/s; n = 742) | 0.66 (0.39, 0.93) | 0.06 | 0.65 (0.38, 0.92) | 0.06 | 0.54 (0.27, 0.81) | 0.06 |
Abbreviations: ATP, adenosine triphosphate; ETS, electronic transfer system; OXPHOS, oxidative phosphorylation; PPFI, Pittsburgh Performance Fatigability Index.
Model 1 adjusted for technician/site, age, sex, race; Model 2 further adjusted for height and weight on top of Model 1; Model 3 additionally adjusted for total activity count from wrist‐worn ActiGraph GT9X.
After full adjustment, each 1 SD lower max OXPHOS (18.4 pmol/(s × mg)), max ETS (22.1 pmol/(s × mg)), and ATPmax (0.15 mM/s) were associated with 0.55 (95% CI: 0.26, 0.84), 0.39 (95% CI: 0.09, 0.70), and 0.54 (95% CI: 0.27, 0.81) higher PPFI score, respectively (Table 2). No sex interactions were found (all p interaction > 0.05). The association for max OXPHOS with PPFI significantly varied by physical function (p interaction = 0.046); interactions were not significant for max ETS (p interaction = 0.34) and ATPmax (p interaction = 0.10). Thus, when stratified by physical function (better: SPPB score ≥10 vs. worse: SPPB < 10), lower max OXPHOS was only associated with higher PPFI score for participants with better physical function (β = 0.52 [95% CI: 0.20, 0.85] for SPPB ≥ 10 and β = 1.56 [95% CI: −0.44, 0.76] for SPPB < 10).
3.3. Associations between skeletal muscle energetics and Pittsburgh Performance Fatigability Index severity strata
In ordinal logistic regression models, lower max OXPHOS, max ETS, and ATPmax yielded greater odds of being in a higher PPFI severity stratum before adjustment for total activity count (Figure 2, Model 2). However, max ETS was no longer associated with PPFI severity strata after adjusting for total activity count (odds ratio (OR) = 1.20 [95% CI: 1.00, 1.43]) (Figure 2, Model 3). Each 1 SD lower max OXPHOS and lower ATPmax were associated with 34% (95% CI: 1.12, 1.59) and 38% (95% CI: 1.17, 1.61) greater odds of being in a higher PPFI severity stratum, respectively (Figure 2, Model 3). Similar to the analyses with continuous PPFI score, only max OXPHOS showed differential associations with PPFI severity strata when stratified with physical function. Specifically, each 1 SD lower max OXPHOS was associated with 32% greater odds of being in a higher PPFI severity stratum among participants with better physical function (OR = 1.32 [95% CI: 1.07, 1.60] for SPPB ≥ 10 and OR = 1.22 [95% CI: 0.68, 1.67] for SPPB < 10).
FIGURE 2.
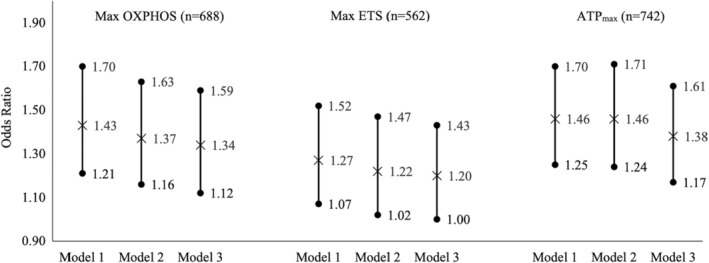
Associations of skeletal muscle energetics and Pittsburgh Performance Fatigability Index (PPFI) severity strata in older adults in the Study of Muscle, Mobility and Aging (SOMMA). ATP, adenosine triphosphate; ETS, electronic transfer system; OXPHOS, oxidative phosphorylation; PPFI, Pittsburgh Performance Fatigability Index. No performance fatigability: PPFI = 0; mild performance fatigability: 0 < PPFI < 3.5 for women, and 0 < PPFI < 5.4 for men; moderate‐to‐severe performance fatigability: PPFI ≥ 3.5 for women and PPFI ≥ 5.4 for men. Model 1 adjusted for technician/site, age, sex, race. Model 2 further adjusted for height and weight on top of Model 1. Model 3 additionally adjusted for total activity count from wrist‐worn ActiGraph GT9X.
4. DISCUSSION
Lower skeletal muscle energetics, particularly max OXPHOS, and ATPmax were significantly associated with higher PPFI score and greater odds of being in a higher PPFI severity stratum, whereas lower max ETS was only significantly associated with higher PPFI score, but not PPFI severity after adjustment for total activity count. To our knowledge, this is the first study to evaluate the associations between muscle energetics both in vivo and ex vivo with performance fatigability in a large cohort of older adults. In addition, the inverse associations between ATPmax with performance fatigability align with previous studies examining ATPmax and perceived fatigability (Liu et al., 2021; Santanasto et al., 2015). Furthermore, given that max ETS (22%) explained more of the variance in performance fatigability than max OXPHOS (4%) and ATPmax (6%), it implies that there might be a differential impact on performance fatigability across various aspects of mitochondrial function. Although developing lifestyle or pharmaceutical interventions that can differentially target specific muscle energetics may be challenging, our findings suggest that improving mitochondrial function or content in general would be beneficial to reduce performance fatigability.
Max ETS is an assessment of the maximal capacity to transport electrons when membrane potential has collapsed due to uncoupling and is not limited by the capacity of the phosphorylation system. It is important to note that max ETS measured ex vivo is not physiologically evident in human tissue per se because completely uncoupled respiration does not occur naturally in skeletal muscle (Gnaiger et al., 2019). However, max ETS is a valuable, focused assessment of electron transport system function, a fundamental constituent of mitochondrial energetics, under standardized experimental conditions. Studies have indicated that ETS dysfunction was associated with lower mitochondrial energy production and higher reactive oxygen species (ROS). It also corresponds to onset and development of obesity, aging, and pathology in many organ systems including cardiovascular diseases (Nolfi‐Donegan et al., 2020). Thus, lower max ETS not only indicates less oxygen flux to support membrane potential and OXPHOS, but also suggests potential decrements due to aging or chronic health conditions. Given that performance fatigability has been proposed as a manifestation of diminishing reserve between daily energy utilization and one's energy capacity (Alexander et al., 2010), it was expected that lower max ETS was associated with higher PPFI score. A caveat is that max ETS capacity is also considered a proxy measure for mitochondrial content (e.g., mtDNA count). Future studies are needed to examine markers of mitochondrial content to confirm the physiological effect of ETS capacity per se on performance fatigability, rather than the effect reflecting differences in mitochondrial content. An ancillary study in SOMMA is currently analyzing mtDNA copy number (a marker of mitochondrial content) from collected biopsies.
Max OXPHOS and ATPmax showed similar strong associations with continuous PPFI score and PPFI severity strata. There are a few potential explanations for their similar associations with performance fatigability. First, both max OXPHOS and ATPmax reflect ATP production via oxidative phosphorylation, and they were moderately correlated with each other in our study (r s = 0.43). Second, as mutations in mtDNA accumulate with aging and diseases, function and structure of OXPHOS complexes can be altered and lead to skeletal muscle energetics dysfunction which accelerates ROS generation (Bua et al., 2006; Wallace, 2010). Thus, lower max OXPHOS as measured ex vivo and lower ATPmax as measured in vivo may lead to a lower rate of ADP phosphorylation and increased ROS accumulation, which in turn limits skeletal muscle energy to maintain a faster walking speed potentially resulting in higher performance fatigability. Third, the in vivo ATPmax measure is dependent on more physiological factors beyond OXPHOS, such as oxygen delivery, muscle perfusion, and neuromuscular activation (Casas et al., 2014; Hochachka & McClelland, 1997). Also, our results concur with previous findings in terms of associations between ATPmax and perceived fatigability (Liu et al., 2021; Santanasto et al., 2015). Collectively, our results imply that therapeutics aimed at directly improving ATP production at the mitochondria level or peripheral physiological factors that influence oxygen delivery could both potentially mitigate fatigability and lessen susceptibility to disability among older adults.
In the current study, skeletal muscle energetics were standardized when modeling. To facilitate a better understanding of the magnitude of the associations, we provide context as to what 1 SD of muscle energetics means in regard to age and cardiorespiratory fitness. In general, comparing adults in their 30s to adults in their 70s, OXPHOS and ATP production were lower by 30%–50% (Boffoli et al., 1994; Cooper et al., 1992; Trounce et al., 1989). For example, younger adults (n = 9, aged 25–48 years) had ATPmax of 6.8 ± 0.6 mM, while older adults (n = 40, aged 65–80 years) had ATPmax of 5.9 ± 0.2 mM (Conley et al., 2000), similar to our data in SOMMA. Thus, 1 SD of ATPmax in SOMMA approximated the difference observed with two‐decade difference in age. While this may seem like a substantial change, a study of 24 weeks of either endurance training or resistance training among 40 older adults (mean age = 69.2 ± 0.6 years old) both showed improvement in ATPmax from 0.54 to 0.70 mM/s and 0.63 to 0.99 mM/s, respectively (Jubrias et al., 2001), which approximately equaled to 1 SD ATPmax in SOMMA. Prospective longitudinal data are needed to estimate annual changes in muscle energetics to better contextualize meaningful differences in skeletal muscle energetics.
Additionally, we found that only max OXPHOS was associated with performance fatigability among participants with better physical function but not among participants with worse physical function. Yet, no differential associations were observed for max ETS nor ATPmax. Although we need more studies to confirm the effect modification by physical function in relation to muscle energetics and performance fatigability, our findings could be better understood by a previous study that found ATPmax was only associated with time to walk 400 m among those with better physical function (Santanasto et al., 2016). Santanasto et al. (2016) postulated that those with higher levels of ATPmax and longer 400‐m walk completion times might have biomechanical inefficiency manifested as a lower mitochondrial efficiency (P/O) and higher energetic cost of walking. We will further investigate the plausibility of these postulations in SOMMA in future work, in order to understand the interrelationships between skeletal muscle energetics, energetic cost of walking, and performance fatigability among lower‐functioning older adults.
There are several strengths and some limitations of our study. First, SOMMA had both ex vivo assessment of skeletal muscle mitochondrial respiration (including max OXPHOS and max ETS) and in vivo assessment of ATPmax, in a large sample of older women and men ranging from 70 to 94 years old, providing comprehensive skeletal muscle energetics. Given our observed differences in PPFI score and muscle energetics values between white versus non‐white subgroups in SOMMA, the generalizability of our results might be limited for more diverse race/ethnicity populations, due to the largely white sample. Yet, our study participants had comparable muscle energetics values with the same age group demonstrated in other studies (Berg et al., 2020; Coen et al., 2013). Third, we excluded participants with missingness for certain muscle energetic measures in their relevant analyses. However, we found no significant differences in covariates among participants with different muscle energetic measures and no significant differences among our final analytical sample versus excluded participants. Lastly, given that a usual‐paced 400‐m walk is a less strenuous task, we may have observed lower PPFI scores and found more conservative associations between muscle energetics and fatigability. Nonetheless, PPFI is validated for use in conjunction with a usual‐paced 400‐m walk with good validity (Qiao et al., 2023).
In conclusion, we found that lower skeletal muscle energetics were associated with more severe performance fatigability. Our understanding of skeletal muscle energetics and performance fatigability may inform new directions for lifestyle interventions and potential mitochondrial medicine aimed at reducing fatigability, improving functional performance, and preventing mobility disability.
AUTHOR CONTRIBUTIONS
Drs. Qiao and Glynn had full access to all of the data for the study and take responsibility for the integrity of the data and accuracy of the data analyses. All authors: interpretation of data, critical revision of manuscript for important intellectual content. All authors read and approved the submitted manuscript.
ACKOWLEDGEMENTS
We acknowledge all the staff and investigators, and we appreciate all the SOMMA participants.
FUNDING INFORMATION
The Study of Muscle, Mobility and Aging is supported by funding from the National Institute on Aging (AG 059416). Study infrastructure support was funded in part by NIA Claude D. Pepper Older American Independence Centers at University of Pittsburgh (P30 AG024827) and Wake Forest University (P30 AG021332) and the Clinical and Translational Science Institutes, funded by the National Center for Advancing Translational Science at Wake Forest University (UL1 0TR001420). K.D.M. was supported by the Pittsburgh Epidemiology of Aging Training Program (NIA T32 AG000181).
CONFLICT OF INTEREST STATEMENT
None declared.
Supporting information
Appendix S1.
Qiao, Y. , Santanasto, A. J. , Coen, P. M. , Cawthon, P. M. , Cummings, S. R. , Forman, D. E. , Goodpaster, B. H. , Harezlak, J. , Hawkins, M. , Kritchevsky, S. B. , Nicklas, B. J. , Toledo, F. G. S. , Toto, P. E. , Newman, A. B. , & Glynn, N. W. (2024). Associations between skeletal muscle energetics and accelerometry‐based performance fatigability: Study of Muscle, Mobility and Aging. Aging Cell, 23, e14015. 10.1111/acel.14015
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available at http://sommaonline.ucsf.edu.
REFERENCES
- Alexander, N. B. , Taffet, G. E. , Horne, F. M. , Eldadah, B. A. , Ferrucci, L. , Nayfield, S. , & Studenski, S. (2010). Bedside‐to‐Bench conference: Research agenda for idiopathic fatigue and aging. Journal of the American Geriatrics Society, 58(5), 967–975. 10.1111/j.1532-5415.2010.02811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara, C. E. , Marcinek, D. J. , Shankland, E. G. , Schenkman, K. A. , Arakaki, L. S. L. , & Conley, K. E. (2008). Mitochondrial function in vivo: Spectroscopy provides window on cellular energetics. Methods, 46(4), 312–318. 10.1016/j.ymeth.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, O. K. , Kwon, O. S. , Hureau, T. J. , Clifton, H. L. , Thurston, T. S. , Le Fur, Y. , Jeong, E. K. , Trinity, J. D. , Richardson, R. S. , Wang, E. , & Layec, G. (2020). Skeletal muscle mitochondrial adaptations to maximal strength training in older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(12), 2269–2277. 10.1093/gerona/glaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blei, M. L. , Conley, K. E. , & Kushmerick, M. J. (1993). Separate measures of ATP utilization and recovery in human skeletal muscle. The Journal of Physiology, 465, 203–222. 10.1113/jphysiol.1993.sp019673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffoli, D. , Scacco, S. C. , Vergari, R. , Solarino, G. , Santacroce, G. , & Papa, S. (1994). Decline with age of the respiratory chain activity in human skeletal muscle. Biochimica et Biophysica Acta, 1226(1), 73–82. 10.1016/0925-4439(94)90061-2 [DOI] [PubMed] [Google Scholar]
- Bua, E. , Johnson, J. , Herbst, A. , Delong, B. , McKenzie, D. , Salamat, S. , & Aiken, J. M. (2006). Mitochondrial DNA‐deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. American Journal of Human Genetics, 79(3), 469–480. 10.1086/507132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas, M. , Buvinic, S. , & Jaimovich, E. (2014). ATP signaling in skeletal muscle: From fiber plasticity to regulation of metabolism. Exercise and Sport Sciences Reviews, 42(3), 110–116. 10.1249/JES.0000000000000017 [DOI] [PubMed] [Google Scholar]
- Coen, P. M. , Jubrias, S. A. , Distefano, G. , Amati, F. , Mackey, D. C. , Glynn, N. W. , Manini, T. M. , Wohlgemuth, S. E. , Leeuwenburgh, C. , Cummings, S. R. , Newman, A. B. , Ferrucci, L. , Toledo, F. G. , Shankland, E. , Conley, K. E. , & Goodpaster, B. H. (2013). Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68(4), 447–455. 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen, P. M. , Menshikova, E. V. , Distefano, G. , Zheng, D. , Tanner, C. J. , Standley, R. A. , Helbling, N. L. , Dubis, G. S. , Ritov, V. B. , Xie, H. , Desimone, M. E. , Smith, S. R. , Stefanovic‐Racic, M. , Toledo, F. G. , Houmard, J. A. , & Goodpaster, B. H. (2015). Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes, 64(11), 3737–3750. 10.2337/db15-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley, K. E. , Jubrias, S. A. , & Esselman, P. C. (2000). Oxidative capacity and ageing in human muscle. The Journal of Physiology, 526 Pt 1(Pt 1), 203–210. 10.1111/j.1469-7793.2000.t01-1-00203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J. M. , Mann, V. M. , & Schapira, A. H. (1992). Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: Effect of ageing. Journal of the Neurological Sciences, 113(1), 91–98. 10.1016/0022-510x(92)90270-u [DOI] [PubMed] [Google Scholar]
- Cummings, S. R. , Newman, A. B. , Coen, P. M. , Hepple, R. T. , Collins, R. , Kennedy, K. , Danielson, M. , Peters, K. , Blackwell, T. , Johnson, E. , Mau, T. , Shankland, E. G. , Lui, L. Y. , Patel, S. , Young, D. , Glynn, N. W. , Strotmeyer, E. S. , Esser, K. A. , Marcinek, D. J. , … Cawthon, P. M. (2023). The Study of Muscle, Mobility and Aging (SOMMA). A Unique Cohort Study about the Cellular Biology of Aging and Age‐related Loss of Mobility. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, glad052. 10.1093/gerona/glad052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldadah, B. A. (2010). Fatigue and fatigability in older adults. PM & R: The Journal of Injury, Function, and Rehabilitation, 2(5), 406–413. 10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- Gnaiger, E. (2001). Bioenergetics at low oxygen: Dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respiration Physiology, 128(3), 277–297. 10.1016/s0034-5687(01)00307-3 [DOI] [PubMed] [Google Scholar]
- Gnaiger, E. , Arnould, T. , Detraux, D. , & Storder, J. (2019). Mitochondrial respiratory states and rates. MitoFit Preprint Archives. 10.26124/mitofit:190001.v5 [DOI] [Google Scholar]
- Gonzalez‐Freire, M. , de Cabo, R. , Bernier, M. , Sollott, S. J. , Fabbri, E. , Navas, P. , & Ferrucci, L. (2015). Reconsidering the role of mitochondria in aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(11), 1334–1342. 10.1093/gerona/glv070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Freire, M. , Scalzo, P. , D'Agostino, J. , Moore, Z. A. , Diaz‐Ruiz, A. , Fabbri, E. , Zane, A. , Chen, B. , Becker, K. G. , Lehrmann, E. , Zukley, L. , Chia, C. W. , Tanaka, T. , Coen, P. M. , Bernier, M. , de Cabo, R. , & Ferrucci, L. (2018). Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell, 17(2), e12725. 10.1111/acel.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik, J. M. , Simonsick, E. M. , Ferrucci, L. , Glynn, R. J. , Berkman, L. F. , Blazer, D. G. , Scherr, P. A. , & Wallace, R. B. (1994). A short physical performance battery assessing lower extremity function: Association with self‐reported disability and prediction of mortality and nursing home admission. Journal of Gerontology, 49(2), M85–M94. 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W. , & McClelland, G. B. (1997). Cellular metabolic homeostasis during large‐scale change in ATP turnover rates in muscles. Journal of Experimental Biology, 200(Pt 2), 381–386. 10.1242/jeb.200.2.381 [DOI] [PubMed] [Google Scholar]
- Jubrias, S. A. , Esselman, P. C. , Price, L. B. , Cress, M. E. , & Conley, K. E. (2001). Large energetic adaptations of elderly muscle to resistance and endurance training. Journal of Applied Physiology, 90(5), 1663–1670. 10.1152/jappl.2001.90.5.1663 [DOI] [PubMed] [Google Scholar]
- Jubrias, S. A. , Crowther, G. J. , Shankland, E. G. , Gronka, R. K. , & Conley, K. E. (2003). Acidosis inhibits oxidative phosphorylation in contracting human skeletal muscle in vivo. The Journal of Physiology, 553(Pt 2), 589–599. 10.1113/jphysiol.2003.045872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, I. R. , & Nair, K. S. (2010). Mitochondrial function as a determinant of life span. Pflugers Archiv: European Journal of Physiology, 459(2), 277–289. 10.1007/s00424-009-0724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, F. , Wanigatunga, A. A. , Zampino, M. , Knuth, N. D. , Simonsick, E. M. , Schrack, J. A. , & Ferrucci, L. (2021). Association of mitochondrial function, substrate utilization, and anaerobic metabolism with age‐related perceived fatigability. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 76(3), 426–433. 10.1093/gerona/glaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy, B. D. , Taylor, R. L. , Fling, B. W. , & Horak, F. B. (2017). Relationship between perceived fatigue and performance fatigability in people with multiple sclerosis: A systematic review and meta‐analysis. Journal of Psychosomatic Research, 100, 1–7. 10.1016/j.jpsychores.2017.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau, T. , Lui, L.‐Y. , Distefano, G. , Kramer, P. A. , Ramos, S. V. , Toledo, F. G. S. , Santanasto, A. J. , Shankland, E. G. , Marcinek, D. J. , Jurczak, M. J. , Sipula, I. , Bello, F. M. , Duchowny, K. A. , Molina, A. J. A. , Sparks, L. M. , Goodpaster, B. H. , Hepple, R. T. , Kritchevsky, S. B. , Newman, A. B. , … Coen, P. M. (2022). Mitochondrial energetics in skeletal muscle are associated with leg power and cardiorespiratory fitness in the Study of Muscle, Mobility, and Aging (SOMMA). The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 78, 1367–1375. 10.1093/gerona/glac238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S. L. , Kratz, A. L. , & Schepens Niemiec, S. L. (2017). Assessing fatigability in the lab and in daily life in older adults with osteoarthritis using perceived, performance, and ecological measures. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(1), 115–120. 10.1093/gerona/glw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolfi‐Donegan, D. , Braganza, A. , & Shiva, S. (2020). Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biology, 37, 101674. 10.1016/j.redox.2020.101674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Y. S. , Harezlak, J. , Cawthon, P. M. , Cummings, S. R. , Forman, D. E. , Goodpaster, B. H. , Hawkins, M. , Moored, K. D. , Nicklas, B. J. , Toledo, F. G. S. , Toto, P. E. , Santanasto, A. J. , Strotmeyer, E. S. , Newman, A. B. , & Glynn, N. W. (2023). Validation of the Pittsburgh performance fatigability index in the study of muscle, mobility and aging (SOMMA). The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, glad197. 10.1093/gerona/glad197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Y. S. , Harezlak, J. , Moored, K. D. , Urbanek, J. K. , Boudreau, R. M. , Toto, P. E. , Hawkins, M. , Santanasto, A. J. , Schrack, J. A. , Simonsick, E. M. , & Glynn, N. W. (2022). Development of a novel accelerometry‐based performance fatigability measure for older adults. Medicine and Science in Sports and Exercise, 54(10), 1782–1793. 10.1249/MSS.0000000000002966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanasto, A. J. , Coen, P. M. , Glynn, N. W. , Conley, K. E. , Jubrias, S. A. , Amati, F. , Strotmeyer, E. S. , Boudreau, R. M. , Goodpaster, B. H. , & Newman, A. B. (2016). The relationship between mitochondrial function and walking performance in older adults with a wide range of physical function. Experimental Gerontology, 81, 1–7. 10.1016/j.exger.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santanasto, A. J. , Glynn, N. W. , Jubrias, S. A. , Conley, K. E. , Boudreau, R. M. , Amati, F. , Mackey, D. C. , Simonsick, E. M. , Strotmeyer, E. S. , Coen, P. M. , Goodpaster, B. H. , & Newman, A. B. (2015). Skeletal muscle mitochondrial function and fatigability in older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(11), 1379–1385. 10.1093/gerona/glu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnelle, J. F. , Buchowski, M. S. , Ikizler, T. A. , Durkin, D. W. , Beuscher, L. , & Simmons, S. F. (2012). Evaluation of two fatigability severity measures in elderly adults. Journal of the American Geriatrics Society, 60(8), 1527–1533. 10.1111/j.1532-5415.2012.04062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrack, J. A. , Simonsick, E. M. , & Glynn, N. W. (2020). Fatigability: A prognostic indicator of phenotypic aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(9), e63–e66. 10.1093/gerona/glaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short, K. R. , Bigelow, M. L. , Kahl, J. , Singh, R. , Coenen‐Schimke, J. , Raghavakaimal, S. , & Nair, K. S. (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proceedings of the National Academy of Sciences of the United States of America, 102(15), 5618–5623. 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick, E. M. , Newman, A. B. , Visser, M. , Goodpaster, B. , Kritchevsky, S. B. , Rubin, S. , Nevitt, M. C. , Harris, T. B. , & Health, Aging and Body Composition Study . (2008). Mobility limitation in self‐described well‐functioning older adults: Importance of endurance walk testing. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 63(8), 841–847. 10.1093/gerona/63.8.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick, E. M. , Schrack, J. A. , Glynn, N. W. , & Ferrucci, L. (2014). Assessing fatigability in mobility‐intact older adults. Journal of the American Geriatrics Society, 62(2), 347–351. 10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Q. , Lee, P. R. , Walker, K. A. , & Ferrucci, L. (2023). Energizing mitochondria to prevent mobility loss in aging: Rationale and hypotheses. Exercise and Sport Sciences Reviews, 51(3), 96–102. 10.1249/JES.0000000000000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounce, I. , Byrne, E. , & Marzuki, S. (1989). Decline in skeletal muscle mitochondrial respiratory chain function: Possible factor in ageing. The Lancet, 1(8639), 637–639. 10.1016/s0140-6736(89)92143-0 [DOI] [PubMed] [Google Scholar]
- Van Geel, F. , Moumdjian, L. , Lamers, I. , Bielen, H. , & Feys, P. (2020). Measuring walking‐related performance fatigability in clinical practice: A systematic review. European Journal of Physical and Rehabilitation Medicine, 56(1), 88–103. 10.23736/S1973-9087.19.05878-7 [DOI] [PubMed] [Google Scholar]
- Wallace, D. C. (2010). Mitochondrial DNA mutations in disease and aging. Environmental and Molecular Mutagenesis, 51(5), 440–450. 10.1002/em.20586 [DOI] [PubMed] [Google Scholar]
- Wolff‐Hughes, D. L. , Bassett, D. R. , & Fitzhugh, E. C. (2014). Population‐referenced percentiles for waist‐worn accelerometer‐derived total activity counts in U.S. youth: 2003–2006 NHANES. PLoS One, 9(12), e115915. 10.1371/journal.pone.0115915 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available at http://sommaonline.ucsf.edu.


