Abstract
Background
Lymphopenia is common in respiratory viral infection. However, no studies elucidated the impact of prolonged lymphopenia on worse outcome in the way of quantitative risk.
Methods
Adult patients with laboratory-confirmed respiratory virus infection (influenza, SARS-CoV-2, and other viruses) between January 1st, 2016, and February 1st, 2023 were enrolled in this retrospective cohort study. Serial data of laboratory examination during hospitalization were acquired. The primary outcome was in-hospital all-cause death, and all information was obtained from the electronic medical records system. Legendre orthogonal polynomials (LOP), restricted cubic splines, and multivariable logistic regression were performed.
Results
Finally, 2388 inpatients were involved in this study, including 436 patients with influenza, 1397 with SARS-CoV-2, and 319 with other respiratory virus infections. After being adjusted for age, corticosteroids, chronic kidney disease, chronic respiratory disease, cardiovascular disease, lymphopenia on admission and length of hospital stay, prolonged lymphopenia was significantly associated with death in influenza (OR 7.20, 95 % CI 2.27–22.77, p = 0. 0008 for lasting for 3–7 days; OR 17.80, 95 % CI 5.21–60.82, p < 0.0001 for lasting for more than 7 days) and SARS-CoV-2 (OR 3.07, 95 % CI 1.89–5.01, p < 0.0001 for lasting for 3–7 days; OR 6.28, 95 % CI 3.53–11.18, p < 0.0001 for lasting for more than 7 days), compared with a transient lymphopenia of 1–2 days, while no significant association was found in other respiratory viruses. Prolonged lymphopenia was also associated with multi-organ damage in influenza and SARS-CoV-2 infections.
Conclusions
Prolonged lymphopenia was significantly associated with worse clinical prognoses in influenza and SARS-CoV-2 infections, but not in other respiratory virus infections.
Keywords: Prolonged lymphopenia, Clinical prognoses, Influenza, SARS-CoV-2
1. Introduction
Influenza virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses, including respiratory syncytial virus (RSV), parainfluenza virus (PIV), human rhinovirus, coronavirus, human metapneumovirus (HMPV) and adenovirus (AdV), are major pathogens in lower respiratory infection other than bacteria. According to previous studies, nearly 40% of inpatients hospitalized with respiratory virus infection would develop sepsis after viral infection [1,2]. During sepsis, the cytokine storm seriously impacts the clinical prognoses by inducing immune dysfunction and multiorgan damages [[3], [4], [5]]. Thus, earlier clearance of the virus is crucial, which relies on the key role of lymphocyte, especially human natural killer (NK) cells and T cells in the immune system [6].
However, under many circumstances, massive apoptosis and exhaustion of lymphocytes would be induced by virus and cytokine storm, particularly in severe cases [[7], [8], [9], [10]], resulting in lymphopenia. Lymphopenia has been identified strongly associated with disease severity and poor outcomes of virus infection, e.g., sepsis, multi-organ dysfunction syndrome, and death [[11], [12], [13], [14]]. A previous meta-analysis also reported a 3-4-fold high risk of developing severe Coronavirus Disease 2019 (COVID-19) or death after lymphopenia occurred [15].
Nevertheless, lymphocyte count keeps changing with the disease progression and the change would be more rapid in severe conditions. Using single observation of peripheral lymphocyte count for afterwards prognoses estimation, which have been applied by most previous studies, would increase the risk of bias, as more information was hidden in the whole hospitalization [16]. However, no studies elucidated the impact of prolonged lymphopenia on worse outcome in the way of quantitative risk among inpatients with SARS-CoV-2 and influenza virus infection. Data were even less reported in other respiratory virus infections.
Therefore, in this study, we aimed to detailly describe the dynamic change of lymphocytes and estimate the quantitative risk of prolonged lymphopenia for disease progression and clinical prognoses, by extracting the time-series data of patients with SARS-CoV-2, influenza virus and other respiratory virus infections under routine clinical practice during hospitalization, thus provide more precise and relevant information to clinical practice.
2. Methods
2.1. Study design and patients
This was a retrospective cohort study among adult inpatients admitted into China-Japan Friendship Hospital between January 1st, 2016, and February 1st, 2023. Patients with laboratory-confirmed respiratory virus infection within 48 h after admission were involved. Pregnant women were excluded. Patient's information was acquired from the electronic medical records system (EMR), including clinical features, all measures of laboratory findings, prescriptions, and medical operations, etc. As only sporadic COVID-19 cases were reported between April 2020 and December 2022 in China, only those who admitted to China-Japan Friendship Hospital during the pandemic of omicron strain in December 2022 to January 2023 were included in this study. Finally, a total of 2388 inpatients were involved in this study, including 1440 (60.3 %) males and 948 (39.7 %) females, and the median age was 66 years old.
2.2. Patient consent statement
Ethical approval was obtained from China-Japan Friendship Hospital Ethics Committee (approval number: 2023-KY-078-1). As an observational study based on retrospective data, written informed consent forms (ICF) were exempted and only routine clinical microbiology and laboratory tests and respiratory samples collection were permitted.
2.3. Virological detection
Respiratory specimens (including nasopharyngeal swab, sputum, bronchoalveolar lavage fluid, and endotracheal aspirate) were collected for the detection of influenza virus, SARS-CoV-2, and other respiratory viruses (RSV, PIV, human rhinovirus, coronavirus, HMPV and AdV). Viruses were detected by real-time PCR (TaqMan Array Microfluidic Cards; Applied Biosystems, Foster City, CA, USA).Quick tests by antigens were also applied for influenza virus (Clearview Exact Influenza A&B; Alere, Waltham, MA, USA).
2.4. Outcomes and definitions
The primary outcome was in-hospital all-cause death. The secondary outcomes included multi-organ damages, lymphopenia, suspected secondary bacterial infection, intensive care unit (ICU) admission, noninvasive mechanical ventilation (NIMV), invasive mechanical ventilation (IMV), extracorporeal membrane oxygenation (ECMO), vasoactive drug administration and length of hospital stay.
Acute kidney injury (AKI) was defined according to the “Kidney Disease: Improving Global Outcomes” (KDIGO) guidelines [17]. Renal insufficiency was defined as an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, assessed by CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula [18]. Liver injury was recognized when at least one of the following abnormal liver function tests were found: aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 3 times the upper reference limit (URL), bilirubin above the URL, albumin <35 g/L [19]. Myocardial injury was defined as hypersensitive troponin I (HsTNI) ≥ 0.0198 ng/ml or troponin T (TNT) ≥ 0.014 ng/ml [20]. Hyperckemia was defined as creatine kinase >1000 U/L. Elevated brain natriuretic peptide (BNP)/N-terminal pro-brain natriuretic peptide (NT-proBNP) was defined as BNP ≥100 pg/ml or NT-proBNP ≥125 pg/ml. Suspected secondary bacterial infection was defined as procalcitonin (PCT) ≥ 0.5 ng/ml during hospitalization. Lymphopenia was defined as the absolute lymphocyte count <1.1 × 109/L. Low platelet was defined as platelet count <50 × 109/L.
2.5. Statistical analysis
Baseline patients’ characteristics were expressed in terms of descriptive statistics. Categorical variables were summarized as frequency (percentage). Continuous variables were presented as median (interquartile range, IQR). P values were calculated by Mann-Whitney U test, χ2 test or Fisher exact test where appropriate. The data imputation was not performed in this study. All tests were two-sided and were considered statistically significant at a p-value of <0.05.
We divided respiratory virus infections into three categories according to previous experiences and study: influenza virus, SARS-CoV-2, and other respiratory viruses [2]. Considering the absolute values of lymphocytes to track changes over time does not adequately capture its impact on clinical outcomes and may lead to unexpecting bias, the duration of days below the lower threshold can offer insights into the progression of the immune dysregulation. Therefore, in this study, we introduced a statistical metric to describe the absolute counts change of peripheral lymphocyte: the duration below the lower bound, .
Where, represented the index for the patient at time . denoted the change at time . was typically one day but could be shorter or longer, depending on the frequency of laboratory test. The duration of lymphopenia was then divided into lasting for 1–2 days, 3–7 days and >7 days.
2.5.1. Legendre orthogonal polynomials
In this study, we applied Legendre orthogonal polynomials (LOP) to capture the serial trends of different markers to minimize the information bias in data collection due to missing data. Legendre polynomials were a classical series of orthogonal polynomials widely used in mathematics and physics. They could be employed to approximate any function, particularly those defined on the interval [−1, 1]. The approximation of a function using Legendre polynomials was a numerical method that involves expressing the function as a linear combination of these orthogonal polynomials. The Legendre polynomial basis was defined as follows,
Where, represents degrees of Legendre polynomials.
Compared to a polynomial basis, Legendre polynomials had orthogonality over the interval [−1, 1], i.e.
Since the orthogonal property of this basis made it the most excellent basis, higher orders did not affect the contribution of lower orders, which could not be avoided for polynomial bases . To determine the appropriate order of fit for LOP, we utilized the Bayesian information criterion (BIC) method.
Where, represented the maximum likelihood estimate for LOP, denoted the number of samples, and signified the count of parameters.
2.5.2. Restricted cubic splines (RCS)
RCS were methods for analyzing the nonlinear relationships between features and outcomes. This approach employed polynomials to fit data across specified intervals, ensuring smoothness at the intersecting knots. Restricted cubic spline functions were typically expressed as follows,
Where, Spline basis functions were . meant that if , then took ; otherwise, took 0.
Moreover, to quantitively estimate the risk of prolonged lymphopenia during hospitalization, multivariable logistic regressions (adjusted for age, corticosteroids, chronic kidney disease, chronic respiratory disease, cardiovascular disease, length of hospital stay and lymphopenia on admission) were performed. Analyses were performed using SAS 9.4 software (Cary, NC, USA) or python 3.7 (package "sklearn" and "mlxtend"), where appropriate.
3. Results
3.1. Demographic and clinical characteristics on admission
As is shown in Table 1, patients died of COVID-19 or other respiratory virus infections were significantly older, comorbided with cardiovascular disease or CKD than those survived (P < 0.05). Most of the deaths were males or with CKD in influenza infection and COVID-19. The rate of diabetes mellitus was higher in death group of COVID-19, while for patients with influenza infection, the higher rate was in survival group. (Table 1).
Table 1.
Demographic and clinical characteristics of patients on admission.
|
Characteristics |
Influenza |
SARS-CoV-2 |
Other respiratory viruses |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Survival N = 385 |
Death N = 51 |
p | Survival N = 1286 |
Death N = 211 |
p | Survival N = 291 |
Death N = 28 |
p | |
| Demographic and clinical characteristics | |||||||||
| Age, years | 61.0 (49.0–72.0) | 63.0 (49.0–72.0) | 0.7647 | 67.0 (54.0–79.0) | 80.0 (68.0–88.0) | <0.0001 | 62.0 (48.0–72.0) | 69.0 (55.5–79.0) | 0.0512 |
| >65 | 136 (35.3) | 21 (41.2) | 0.4133 | 691 (53.7) | 163 (77.3) | <0.0001 | 118 (40.5) | 18 (64.3) | 0.0153 |
| Male | 217 (56.4) | 36 (70.6) | 0.0531 | 760 (59.1) | 144 (68.2) | 0.0118 | 169 (58.1) | 21 (75.0) | 0.0814 |
| Hypertension | 150 (39.0) | 20 (39.2) | 0.9720 | 577 (44.9) | 114 (54.0) | 0.0134 | 107 (36.8) | 11 (39.3) | 0.7923 |
| Chronic obstructive lung disease | 57 (14.8) | 5 (9.8) | 0.3366 | 96 (7.5) | 15 (7.1) | 0.8549 | 43 (14.8) | 5 (17.9) | 0.6700 |
| Malignancy | 34 (8.8) | 3 (5.9) | 0.4572 | 210 (16.3) | 19 (9.0) | 0.0061 | 33 (11.3) | 5 (17.9) | 0.3358 |
| Diabetes mellitus | 110 (28.6) | 8 (15.7) | 0.0516 | 405 (31.5) | 82 (38.9) | 0.0342 | 68 (23.4) | 6 (21.4) | 0.8164 |
| Chronic kidney disease | 41/384 (10.7) | 16/51 (31.4) | <0.0001 | 130/1250 (10.4) | 41/198 (20.7) | <0.0001 | 26/289 (9.0) | 10/28 (35.7) | 0.0003 |
| Chronic respiratory disease | 151/384 (39.3) | 15/51 (29.4) | 0.1710 | 301/1250 (24.1) | 58/198 (29.3) | 0.1145 | 137/289 (47.4) | 14/28 (50.0) | 0.7929 |
| Cardiovascular disease | 92/384 (24.0) | 17/51 (33.3) | 0.1466 | 339/1250 (27.1) | 124/198 (62.6) | <0.0001 | 87/289 (30.1) | 16/28 (57.1) | 0.0035 |
| Hepatopathy | 28/384 (7.3) | 6/51 (11.8) | 0.2906 | 49/1250 (3.9) | 7/198 (3.5) | 0.7942 | 11/289 (3.8) | 1/28 (3.6) | 0.9500 |
| Laboratory findings | |||||||||
| PT, s | 13.6 (13.0–14.4) | 14.6 (13.6–16.0) | <0.0001 | 13.6 (13.1–14.4) | 14.6 (13.8–16.1) | <0.0001 | 13.8 (13.2–14.7) | 16.0 (14.9–21.0) | <0.0001 |
| APTT, s | 40.7 (35.7–47.1) | 43.1 (38.3–51.4) | 0.0421 | 38.1 (35.3–41.2) | 50.0 (37.3–50.1) | 0.1426 | 39.0 (35.3–45.0) | 46.1 (41.0–56.5) | 0.0003 |
| Prolonged PT or APTT | 160/360 (44.4) | 31/50 (62.0) | 0.0197 | 69/1135 (6.1) | 55/204 (27.0) | <0.0001 | 97/274 (35.4) | 19/25 (76.0) | <0.0001 |
| BNP, pg/ml | 78.0 (39.9–216.0) | 142.8 (66.8–404.2) | 0.0068 | 87.0 (39.0–205.0) | 232.0 (111.5–569.0) | <0.0001 | 93.5 (36.6–281.6) | 235.0 (95.2–700.5) | 0.0390 |
| NT-proBNP, pg/ml | 300.0 (91.0–964.0) | 992.0 (313.0–5766.0) | 0.0004 | 368.5 (114.0–1031.5) | 1298.5 (497.5–4938.5) | <0.0001 | 232.0 (90.0–925.0) | 1786.0 (1071.0–8739.0) | <0.0001 |
| Elevated BNP/NT-proBNP | 180/306 (58.8) | 42/48 (87.5) | 0.0001 | 664/1047 (63.4) | 192/203 (94.6) | <0.0001 | 147/254 (57.9) | 21/24 (87.5) | 0.0046 |
| White blood cell count, × 109/L | 6.3 (4.4–9.0) | 9.0 (4.4–11.3) | 0.0064 | 5.9 (4.4–8.0) | 8.3 (5.4–11.3) | <0.0001 | 7.2 (5.4–9.7) | 10.2 (5.8–14.8) | 0.0244 |
| 4-10 | 246/383 (64.2) | 24/51 (47.1) | 0.0087 | 855/1227 (69.7) | 105/209 (50.2) | <0.0001 | 181/286 (63.3) | 10/27 (37.0) | 0.0090 |
| <4 | 65/383 (17.0) | 8/51 (15.7) | 222/1227 (18.1) | 25/209 (12.0) | 38/286 (13.3) | 3/27 (11.1) | |||
| >10 | 72/383 (18.8) | 19/51 (37.3) | 150/1227 (12.2) | 79/209 (37.8) | 67/286 (23.4) | 14/27 (51.9) | |||
| Lymphocyte count, × 109/L | 1.1 (0.7–1.5) | 0.5 (0.3–0.7) | <0.0001 | 0.9 (0.6–1.4) | 0.5 (0.4–0.9) | <0.0001 | 1.2 (0.7–1.7) | 0.6 (0.4–1.0) | <0.0001 |
| Lymphocyte percentage | 17.3 (10.2–26.7) | 7.0 (3.6–10.3) | <0.0001 | 16.6 (9.7–26.4) | 7.5 (3.8–12.5) | <0.0001 | 16.9 (10.1–26.1) | 5.5 (4.2–10.7) | <0.0001 |
| Lymphopenia on admission | 201/383 (52.5) | 45/51 (88.2) | <0.0001 | 726/1225 (59.3) | 179/209 (85.6) | <0.0001 | 133/286 (46.5) | 21/27 (77.8) | 0.0019 |
| Hemoglobin, g/L | 126.0 (111.0–137.0) | 127.0 (103.0–141.0) | 0.6528 | 122.0 (109.0–136.0) | 116.0 (95.0–132.0) | 0.0006 | 122.0 (105.0–133.0) | 106.0 (91.0–121.0) | 0.0009 |
| Anemia | 116/383 (30.3) | 21/51 (41.2) | 0.1160 | 428/1227 (34.9) | 97/209 (46.4) | 0.0014 | 114/286 (39.9) | 20/27 (74.1) | 0.0006 |
| Platelet count, × 109/L | 189.5 (138.5–241.0) | 158.0 (118.0–199.0) | 0.0056 | 188.0 (143.0–246.0) | 157.0 (106.0–223.0) | <0.0001 | 214.0 (160.0–279.0) | 132.5 (106.0–181.0) | <0.0001 |
| 50 - 100 | 39/372 (10.5) | 6/49 (12.2) | 0.9097 | 66/1226 (5.4) | 35/209 (16.7) | <0.0001 | 12/273 (4.4) | 2/26 (7.7) | 0.0095 |
| <50 | 6/372 (1.6) | 1/49 (2.0) | 16/1226 (1.3) | 9/209 (4.3) | 5/273 (1.8) | 4/26 (15.4) | |||
| ≥100 | 327/372 (87.9) | 42/49 (85.7) | 1144/1226 (93.3) | 165/209 (78.9) | 256/273 (93.8) | 20/26 (76.9) | |||
| Alanine transaminase, U/L | 24.0 (16.0–44.0) | 34.0 (23.0–60.0) | 0.0073 | 23.0 (14.0–38.0) | 25.0 (16.0–38.0) | 0.1306 | 22.0 (14.0–36.0) | 28.0 (12.0–70.0) | 0.4078 |
| >40 | 108/373 (29.0) | 22/50 (44.0) | 0.0304 | 235/1048 (22.4) | 43/199 (21.6) | 0.7999 | 60/280 (21.4) | 9/27 (33.3) | 0.1570 |
| Creatinine, μmol/L | 67.5 (53.4–86.5) | 78.2 (54.1–108.5) | 0.0614 | 68.1 (53.7–89.7) | 88.3 (64.6–130.4) | <0.0001 | 69.4 (55.7–86.0) | 101.7 (53.9–152.1) | 0.0090 |
| >133 | 31/377 (8.2) | 8/50 (16.0) | 0.0967 | 136/1072 (12.7) | 49/200 (24.5) | <0.0001 | 25/279 (9.0) | 8/27 (29.6) | 0.0043 |
| Lactate dehydrogenase, U/L | 229.0 (190.0–339.0) | 472.0 (394.0–675.0) | <0.0001 | 229.5 (183.0–303.0) | 389.0 (280.0–547.0) | <0.0001 | 227.0 (177.0–333.0) | 358.0 (273.0–618.0) | <0.0001 |
| >245 | 129/309 (41.7) | 38/41 (92.7) | <0.0001 | 270/624 (43.3) | 140/161 (87.0) | <0.0001 | 100/241 (41.5) | 20/25 (80.0) | 0.0002 |
| Creatine kinase, U/L | 72.0 (44.0–156.0) | 93.0 (38.0–292.0) | 0.3178 | 55.0 (32.0–92.0) | 105.0 (49.0–243.0) | <0.0001 | 54.5 (33.5–98.0) | 205.0 (56.5–848.0) | 0.0013 |
| Creatine kinase >185, U/L | 63/306 (20.6) | 12/39 (30.8) | 0.1466 | 67/627 (10.7) | 51/157 (32.5) | <0.0001 | 35/240 (14.6) | 12/24 (50.0) | 0.0001 |
| D-dimer, μg/ml | 1.0 (0.4–2.3) | 3.2 (1.4–7.5) | <0.0001 | 0.8 (0.4–1.8) | 2.3 (1.1–4.9) | <0.0001 | 0.8 (0.4–2.2) | 3.4 (1.1–6.2) | 0.0004 |
| >0.5 | 79/347 (22.8) | 5/43 (11.6) | 0.0003 | 303/1097 (27.6) | 33/188 (17.6) | <0.0001 | 65/271 (24.0) | 4/21 (19.0) | 0.0023 |
| >1 | 170/347 (49.0) | 35/43 (81.4) | 463/1097 (42.2) | 148/188 (78.7) | 115/271 (42.4) | 16/21 (76.2) | |||
| ≤0.5 | 98/347 (28.2) | 3/43 (7.0) | 331/1097 (30.2) | 7/188 (3.7) | 91/271 (33.6) | 1/21 (4.8) | |||
| Serum Ferritin, μg/L | 344.3 (137.9–669.3) | 854.6 (551.6–2292.0) | 0.0028 | 366.6 (184.0–664.2) | 655.8 (334.9–1242.2) | <0.0001 | 225.6 (120.6–642.0) | 882.6 (685.7–1832.0) | 0.1308 |
| >300 | 40/69 (58.0) | 16/19 (84.2) | 0.0353 | 253/449 (56.3) | 79/97 (81.4) | <0.0001 | 34/76 (44.7) | 4/5 (80.0) | 0.1166 |
| Procalcitonin, ng/ml | 0.3 (0.2–0.8) | 0.9 (0.4–5.3) | <0.0001 | 0.2 (0.1–0.7) | 0.6 (0.3–2.1) | <0.0001 | 0.3 (0.2–0.6) | 0.8 (0.5–4.8) | <0.0001 |
| 0.1–0.24 | 78/266 (29.3) | 8/47 (17.0) | <0.0001 | 154/330 (46.7) | 26/123 (21.1) | <0.0001 | 79/176 (44.9) | 2/26 (7.7) | <0.0001 |
| 0.25–0.4 | 82/266 (30.8) | 4/47 (8.5) | 52/330 (15.8) | 27/123 (22.0) | 48/176 (27.3) | 6/26 (23.1) | |||
| <0.1 | 11/266 (4.1) | 1/47 (2.1) | 18/330 (5.5) | 3/123 (2.4) | 3/176 (1.7) | 0/26 (0.0) | |||
| ≥0.5 | 95/266 (35.7) | 34/47 (72.3) | 106/330 (32.1) | 67/123 (54.5) | 46/176 (26.1) | 18/26 (69.2) | |||
| Neutrophil count, × 109/L | 4.4 (2.9–7.1) | 8.0 (4.0–10.4) | <0.0001 | 4.2 (2.9–6.3) | 6.9 (4.5–10.4) | <0.0001 | 5.1 (3.4–7.4) | 8.9 (5.1–13.8) | 0.0007 |
| 1.8–6.3 | 235/383 (61.4) | 14/51 (27.5) | <0.0001 | 837/1225 (68.3) | 81/209 (38.8) | <0.0001 | 168/286 (58.7) | 8/27 (29.6) | 0.0120 |
| <1.8 | 31/383 (8.1) | 3/51 (5.9) | 87/1225 (7.1) | 12/209 (5.7) | 18/286 (6.3) | 2/27 (7.4) | |||
| >6.3 | 117/383 (30.5) | 34/51 (66.7) | 301/1225 (24.6) | 116/209 (55.5) | 100/286 (35.0) | 17/27 (63.0) | |||
| eGFR, ml/min/1.73 m2/L | 95.2 (74.9–107.7) | 84.4 (58.7–104.8) | 0.0494 | 88.2 (67.8–100.5) | 67.6 (44.0–89.4) | <0.0001 | 94.2 (78.1–106.2) | 60.4 (44.8–97.4) | 0.0004 |
| 60-90 | 102/368 (27.7) | 15/50 (30.0) | 0.1035 | 355/1031 (34.4) | 69/192 (35.9) | <0.0001 | 64/239 (26.8) | 5/23 (21.7) | 0.0004 |
| <60 | 56/368 (15.2) | 13/50 (26.0) | 205/1031 (19.9) | 77/192 (40.1) | 30/239 (12.6) | 11/23 (47.8) | |||
| ≥90 | 210/368 (57.1) | 22/50 (44.0) | 471/1031 (45.7) | 46/192 (24.0) | 145/239 (60.7) | 7/23 (30.4) | |||
| Corticosteroids | 102 (26.5) | 31 (60.8) | <0.0001 | 432 (33.6) | 93 (44.1) | 0.0031 | 104 (35.7) | 19 (67.9) | 0.0009 |
| Days from admission to corticosteroids treatment | 2.0 (1.0–4.0) | 2.0 (1.0–6.0) | 0.2808 | 1.0 (1.0–2.0) | 2.0 (1.0–4.0) | 0.0006 | 1.0 (1.0–4.0) | 2.0 (1.0–5.0) | 0.3934 |
Notes. Data were expressed as number (%) or median (interquartile range). P values were estimated by Chi-square test, Fisher's exact test or Kruskal-Wallis test, where appropriate. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus. Abbreviations: WBC, white blood cell; PT, thromboplastin time; APTT, activated partial thromboplastin time; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide; LDH, lactate dehydrogenase; eGFR, estimated glomerular filtration rate.
Patients died had more significant abnormalities than those survived in common laboratory markers for influenza, SARS-CoV-2 and other respiratory virus infections, manifesting as: higher prolonged thromboplastin time (PT) or activated partial thromboplastin time (APTT) rate, elevated BNP/NT-proBNP rate, white blood cell (WBC) count, lymphopenia rate, anemia, low platelet rate, lactate dehydrogenase (LDH), creatine kinase (CK), d-dimer, serum ferritin (SF), PCT, neutrophil count, low eGFR rate and high corticosteroids administration rate. Specially, patients died with influenza infection were observed higher rates of ALT >40 U/L, while for SARS-CoV-2 or other respiratory virus infections, more serum creatinine (Scr) > 133 μmol/L cases were found at admission. (Table 1).
3.2. Dynamic changes of laboratory results
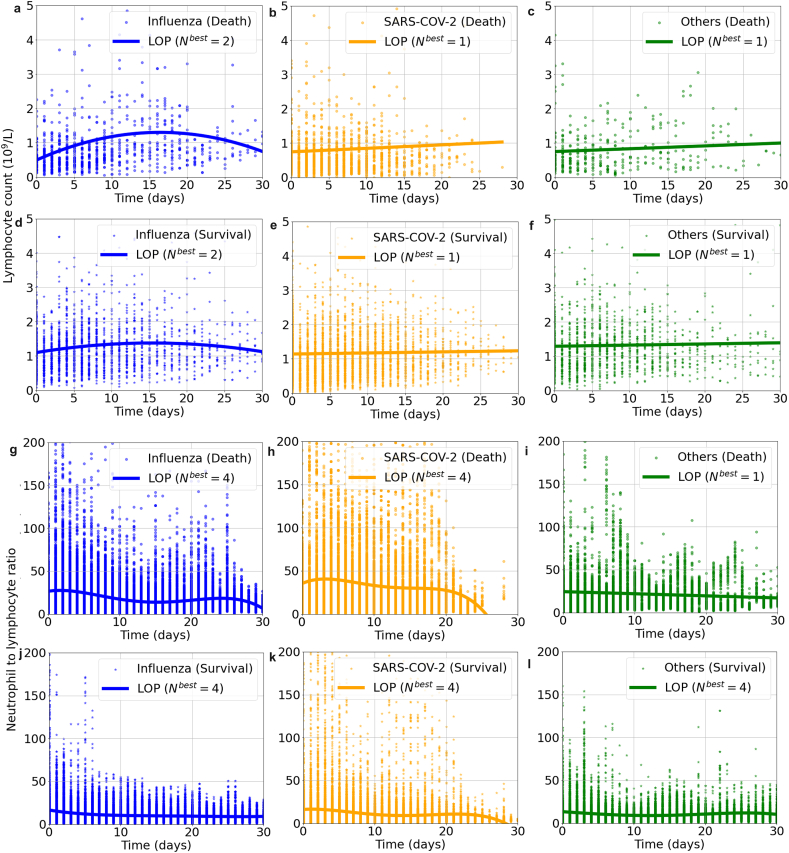
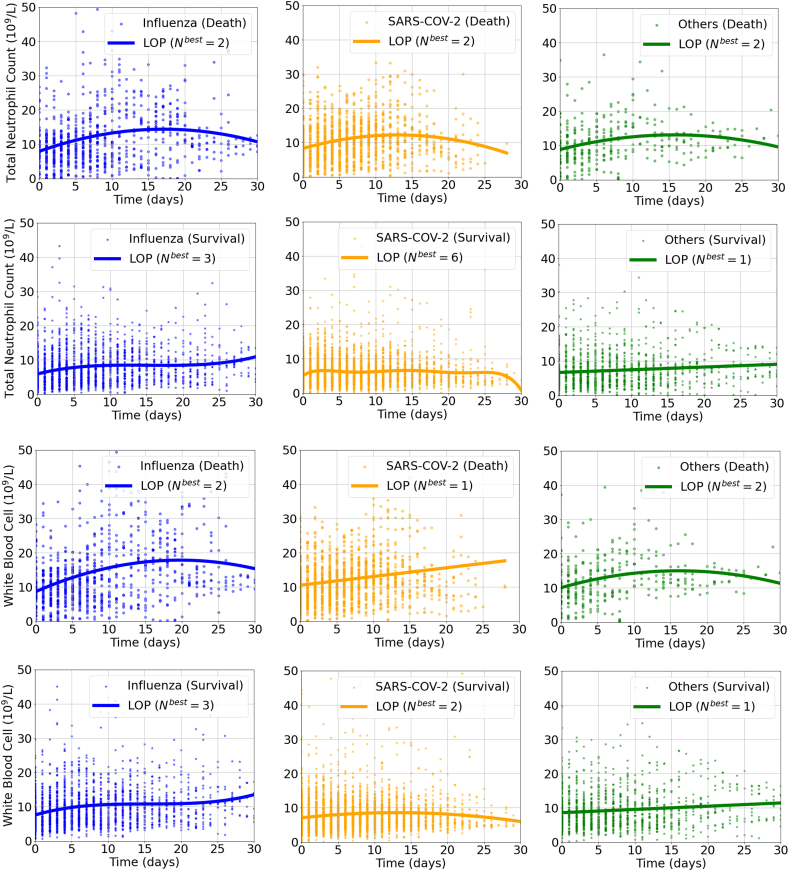
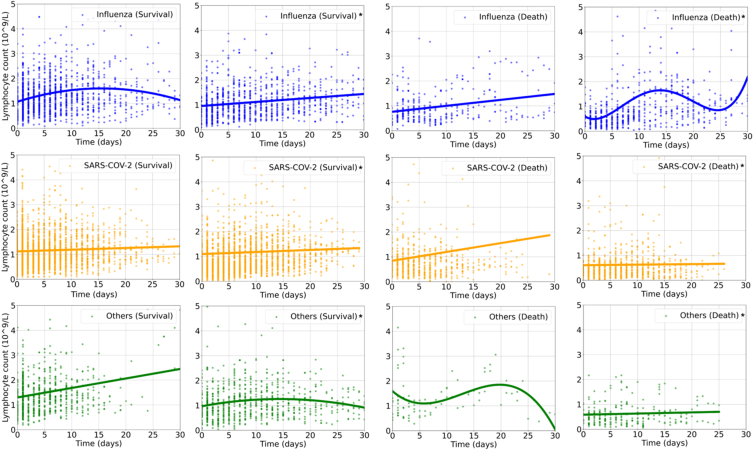
According to Fig. 1, Supplementary Fig. 1, Supplementary Fig. 2, and Supplementary Fig. 3, similar dynamic immune changes among different virus infection were noted during hospitalization: lymphocyte counts were generally lower in dead cases than the survivals and a trend of elevation was observed only in survival groups. In patients with influenza infection, the lymphocyte counts transiently increased from the baseline on admission, but then constantly declined as days of hospital stay prolonged, especially in those dead cases. Neutrophil to lymphocyte ratio (NLR) increased in death group of COVID-19, while maintained low and steady and gradually decreased in other subgroups. WBC counts also surged then slowly decreased during hospitalization in death groups, while changed relatively gently in survival groups (Fig. 1, Supplementary Fig. 1). While for those administered with corticosteroids, the lymphocyte counts were slightly lower during hospitalization. (Supplementary Fig. 2).
Fig. 1.
The dynamic changes of lymphocyte count [panel a–f] and neutrophil to lymphocyte ratio [panel g–l] in patients hospitalized with respiratory virus infections by Legendre orthogonal polynomials.
Note: Nbest refers to the appropriate order of fit for LOP by the Bayesian information criterion method. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LOP, Legendre orthogonal polynomials.
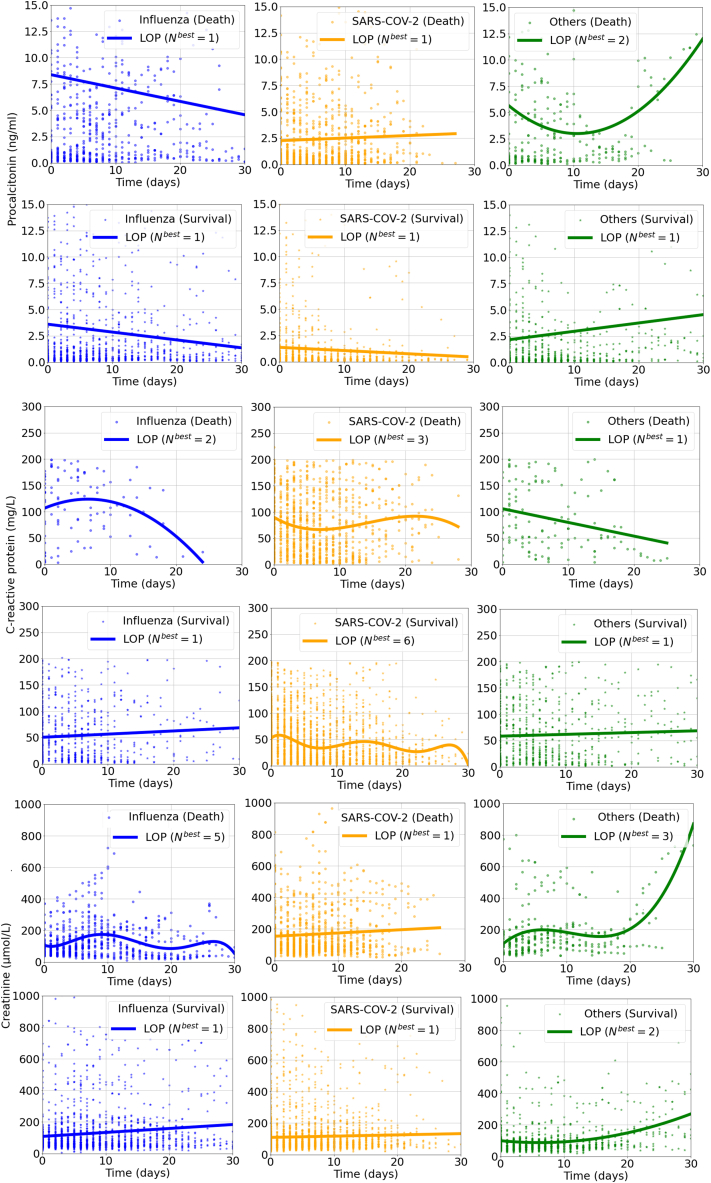
As for other biomarkers, the PCT, C-reactive protein (CRP), Scr and SF levels were higher in death groups than in the survival groups for all respiratory virus infections. Of note, patients infected with SARS-CoV-2 had generally numerically lower PCT level during hospitalization in both death and survival groups than those infected with other viruses. (Supplementary Fig. 3).
3.3. Clinical prognoses
As shown in Table 2, the death rate in SARS-CoV-2 was 14.0 % (211/1497), followed by 11.7 % (51/436) in influenza and 8.8 % (28/319) in other respiratory virus infections. During hospitalization, longest duration of lymphopenia, lymphopenia, the rates of longest duration of lymphopenia, AKI, liver injury, myocardial injury, elevated BNP/NT-proBNP, low platelet, suspected secondary bacterial infection, machinal ventilation, ECMO, vasoactive drug and ICU admission were all higher, and the length of hospital stay was all longer in death groups for all viruses. Of note, the longest duration of lymphopenia was 8 days (IQR 5.0–12.0) in death group of influenza, numerically longer than that of SARS-CoV-2 and other respiratory virus infections. Hyperckemia was found significantly higher in only death groups of SARS-CoV-2 and other respiratory virus infections. For influenza virus, 335 (87.0 %) patients were with at least one secondary outcome and 50 (13.0 %) were without any secondary outcome among survivors; for SARS-CoV-2, the cases (proportions) were 1060 (82.4 %) and 226 (17.6 %), respectively; for other respiratory viruses, they were 231 (79.4 %) and 60 (20.6 %). (Table 2).
Table 2.
Clinical prognoses of patients hospitalized with respiratory virus infection.
|
Characteristics |
Influenza |
SARS-CoV-2 |
Other respiratory viruses |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Survival N = 385 |
Death N = 51 |
p | Survival N = 1286 |
Death N = 211 |
p | Survival N = 291 |
Death N = 28 |
p | |
| Lymphopenia during hospitalization | 240/383 (62.7) | 51/51 (100.0) | <0.0001 | 867/1227 (70.7) | 198/209 (94.7) | <0.0001 | 158/286 (55.2) | 25/27 (92.6) | 0.0002 |
| Lasting for 1–2 days | 111/383 (29.0) | 4/51 (7.8) | <0.0001 | 338/1226 (27.6) | 39/207 (18.8) | <0.0001 | 70/286 (24.5) | 7/27 (25.9) | 0.0002 |
| Lasting for 3–7 days | 77/383 (20.1) | 20/51 (39.2) | 234/1226 (19.1) | 68/207 (32.9) | 45/286 (15.7) | 9/27 (33.3) | |||
| Lasting for more than 7 days | 52/383 (13.6) | 27/51 (52.9) | 294/1226 (24.0) | 89/207 (43.0) | 43/286 (15.0) | 9/27 (33.3) | |||
| Longest duration of lymphopenia | 1.0 (0.0, 4.0) | 8.0 (5.0, 12.0) | <0.0001 | 1.0 (0.0, 7.0) | 7.0 (3.0, 10.0) | <0.0001 | 1.0 (0.0, 5.0) | 6.0 (2.0, 9.0) | <0.0001 |
| Acute kidney injury | 68/377 (18.0) | 32/50 (64.0) | <0.0001 | 112/1074 (10.4) | 95/201 (47.3) | <0.0001 | 47/279 (16.8) | 16/27 (59.3) | <0.0001 |
| Liver injury | 80/373 (21.4) | 38/50 (76.0) | <0.0001 | 154/1049 (14.7) | 89/199 (44.7) | <0.0001 | 58/280 (20.7) | 20/27 (74.1) | <0.0001 |
| Hyperckemia | 17/306 (5.6) | 3/39 (7.7) | 0.6053 | 9/627 (1.4) | 14/157 (8.9) | <0.0001 | 9/240 (3.8) | 7/24 (29.2) | 0.0001 |
| Myocardial injury | 142/238 (59.7) | 28/30 (93.3) | 0.0003 | 524/1058 (49.5) | 200/205 (97.6) | <0.0001 | 102/227 (44.9) | 23/23 (100.0) | <0.0001 |
| Elavated BNP/NTproBNP | 186/306 (60.8) | 46/48 (95.8) | <0.0001 | 732/1049 (69.8) | 204/206 (99.0) | <0.0001 | 161/255 (63.1) | 24/24 (100.0) | 0.0003 |
| Low platelet | 16/373 (4.3) | 24/50 (48.0) | <0.0001 | 35/1226 (2.9) | 44/209 (21.1) | <0.0001 | 12/273 (4.4) | 11/26 (42.3) | <0.0001 |
| Suspected secondary bacterial infection | 111/269 (41.3) | 47/49 (95.9) | <0.0001 | 160/354 (45.2) | 121/145 (83.4) | <0.0001 | 63/183 (34.4) | 26/27 (96.3) | <0.0001 |
| Oxygen support | 20 (5.2) | 11 (21.6) | 0.0003 | 122 (9.5) | 15 (7.1) | 0.2669 | 26 (8.9) | 2 (7.1) | 0.7422 |
| NIMV | 69 (17.9) | 20 (39.2) | 0.0004 | 26 (2.0) | 21 (10.0) | <0.0001 | 22 (7.6) | 3 (10.7) | 0.5706 |
| IMV | 57 (14.8) | 45 (88.2) | <0.0001 | 22 (1.7) | 67 (31.8) | <0.0001 | 20 (6.9) | 20 (71.4) | <0.0001 |
| ECMO | 10 (2.6) | 17 (33.3) | <0.0001 | 2 (0.2) | 8 (3.8) | <0.0001 | 3 (1.0) | 5 (17.9) | 0.0001 |
| vasoactive drug | 106 (27.5) | 36 (70.6) | <0.0001 | 76 (5.9) | 108 (51.2) | <0.0001 | 81 (27.8) | 25 (89.3) | <0.0001 |
| ICU admission | 95 (24.7) | 47 (92.2) | <0.0001 | 64 (5.0) | 72 (34.1) | <0.0001 | 45 (15.5) | 21 (75.0) | <0.0001 |
| Length of stay | 11.0 (8.0–16.0) | 12.0 (8.0–22.0) | 0.2948 | 12.0 (8.0–16.0) | 10.0 (6.0–14.0) | 0.0002 | 11.0 (8.0–15.0) | 8.5 (5.5–17.5) | 0.1350 |
| At least one secondary outcome | 335 (87.0) | 51 (100.0) | 0.0062 | 1060 (82.4) | 209 (99.1) | <0.0001 | 231 (79.4) | 27 (96.4) | 0.0285 |
| Without any secondary outcome | 50 (13.0) | 0 (0.0) | 0.0062 | 226 (17.6) | 2 (0.9) | <0.0001 | 60 (20.6) | 1 (3.6) | 0.0285 |
Notes. Data were expressed as number (%) or median (interquartile range). P values were estimated by Chi-square test, Fisher's exact test or Kruskal-Wallis test, where appropriate. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus. Secondary outcomes referred to lymphopenia during hospitalization, acute kidney injury, liver injury, hyperckemia, myocardial injury, elevated BNP/NTproBNP, low platelet, suspected secondary bacterial infection, NIMV, IMV, ECMO, vasoactive drug and ICU admission.
Abbreviations: BNP, brain natriuretic peptide; NT-pro BNP, N-terminal pro-brain natriuretic peptide; NIMV, noninvasive mechanical ventilation; IMV, invasive mechanical ventilation; ECMO, extracorporeal membrane oxygenation.
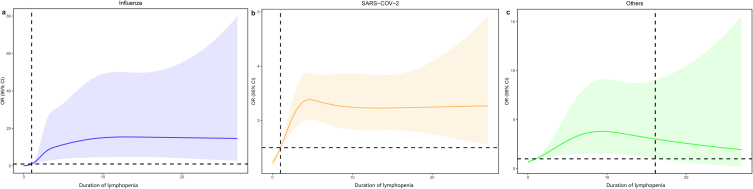
The RCS and inflection points of duration of lymphopenia were shown in Fig. 2. Prolonged lymphopenia rapidly increased death risk in the first several days for all respiratory infection and gradually turned into high but stable level. (Fig. 2).
Fig. 2.
Associations of prolonged lymphopenia with death in patients hospitalized with influenza [panel a], SARS-CoV-2 [panel b] and other respiratory virus infections [panel c].
Notes: The OR curves were portrayed by restricted cubic splines. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; OR, odds ratio; 95 % CI, 95 % confidence interval.
The logistic regressions in Table 3 and Supplementary Table 1 showed that prolonged lymphopenia was significantly associated with death in influenza and SARS-CoV-2, but not in other respiratory viruses. The associations were stronger in influenza as the lymphopenia prolonged in influenza virus infection than that in SARS-CoV-2 infection. After the adjustment for age, corticosteroids, chronic kidney disease, chronic respiratory disease, cardiovascular disease, lymphopenia on admission and length of hospital stay, the duration of lymphopenia was significantly associated with death in influenza (OR 7.20, 95 % CI 2.27–22.77, p = 0. 0008 for lasting for 3–7 days; OR 17.80, 95 % CI 5.21–60.82, p < 0.0001 for lasting for more than 7 days) and SARS-CoV-2 (OR 3.07, 95 % CI 1.89–5.01, p < 0.0001 for lasting for 3–7 days; OR 6.28, 95 % CI 3.53–11.18, p < 0.0001 for lasting for more than 7 days), compared with a transient lymphopenia of 1–2 days, while no association was found in other respiratory virus infections (Table 3).
Table 3.
Multivariable analysis of the association between prolonged lymphopenia and death outcome.
| Risk factors | Influenza |
SARS-CoV-2 |
Other respiratory viruses |
|||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p | OR (95 % CI) | p | OR (95 % CI) | p | |
| Duration of lymphopenia | ||||||
| 1–2 days | Reference | Reference | Reference | |||
| 3–7 days | 7.20 (2.27–22.77) | 0.0008 | 3.07 (1.89–5.01) | <0.0001 | 1.61 (0.46–5.66) | 0.4538 |
| More than 7 days | 17.80 (5.21–60.82) | <0.0001 | 6.28 (3.53–11.18) | <0.0001 | 3.23 (0.79–13.21) | 0.1031 |
| Length of stay | 0.96 (0.93–1.01) | 0.0862 | 0.85 (0.81–0.89) | <0.0001 | 0.92 (0.85–1.00) | 0.0417 |
| Age >65 years | 1.05 (0.50–2.22) | 0.8988 | 2.06 (1.34–3.17) | 0.0009 | 2.52 (0.92–6.94) | 0.0737 |
| Corticosteroids | 2.08 (1.04–4.15) | 0.0371 | 1.33 (0.91–1.93) | 0.1360 | 3.07 (1.05–8.99) | 0.0406 |
| Chronic kidney disease | 2.37 (1.04–5.38) | 0.0395 | 1.52 (0.96–2.43) | 0.0768 | 6.04 (1.92–19.01) | 0.0021 |
| Chronic respiratory disease | 0.83 (0.37–1.85) | 0.6526 | 1.33 (0.90–1.98) | 0.1512 | 1.01 (0.38–2.71) | 0.9782 |
| Cardiovascular disease | 0.84 (0.40–1.77) | 0.6397 | 3.19 (2.21–4.60) | <0.0001 | 1.45 (0.52–3.99) | 0.4765 |
| Lymphopenia on admission | 0.78 (0.27–2.25) | 0.6392 | 0.88 (0.46–1.67) | 0.6909 | 0.54 (0.13–2.20) | 0.3926 |
Notes. P values, odds ratios and 95 % confidence intervals were estimated by Logistic regression. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus.
Abbreviations: OR, odds ratio; CI, confidence interval.
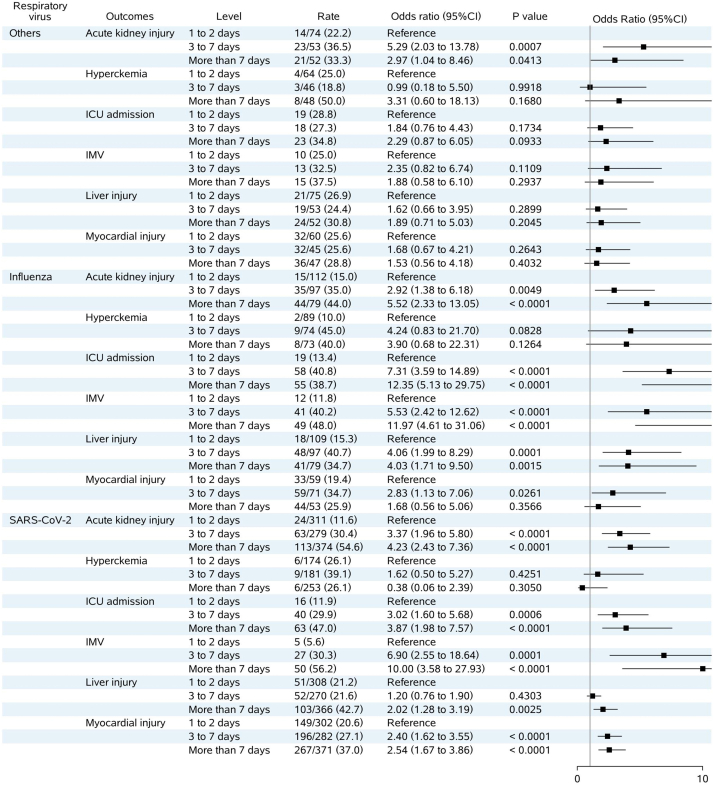
For other clinical prognoses, analyses in Supplementary Fig. 4 showed that prolonged lymphopenia also associated with similar worse prognoses, e.g. AKI, ICU admission, invasive mechanical ventilation, liver injury and myocardial injury. While for other respiratory virus infections, prolonged lymphopenia was only associated with AKI. (Supplementary Fig. 4).
4. Discussion
In this retrospective cohort study, we described the dynamic change of lymphocyte count and its associations with clinical prognoses among patients hospitalized with influenza, SARS-CoV-2, and other respiratory virus infections. We innovatively quantified the increased risk of prolonged lymphopenia on prognoses in these patients, especially for those with influenza or SARS-CoV-2 infection. Compared with the previous study on the comparison of clinical features and outcomes among different respiratory viruses [21], our study provided the most comprehensive description on lab markers and multi-organ damages for patients hospitalized with common respiratory virus infections.
Lymphopenia had been found associated with disease severity and poor outcomes in patients infected by respiratory viruses [14,22]. It was demonstrated that lymphopenia was a prominent feature of COVID-19 and influenza infections. Lymphocyte counts in peripheral blood maybe a useful and readily available biomarker for differential diagnosis [23], predicting severity and clinical outcome.However, sample sizes in previous studies were small and the mechanisms were different between SARS-CoV-2 and influenza [13,15,24,25]. For SARS-CoV-2, it had a broad tropism of major immune cells, including neutrophils, B cells, T cells and NK cells [26], and may directly infect CD4+ T cell and induce apoptosis in the hypoxia-inducible factor-1a (HIF-1a)-dependent pathway [8]. Afterwards, strong inflammation would also be triggered by dying CD4+ T cell and furtherly impact the adaptive immune system [8]. While during influenza infection, the virus directly infects NK cells and induces cell apoptosis [27]. As observed in mice during influenza infection, a transient increase of NK cytotoxicity is followed by a marked decrease in NK cell activity, with a virus dose-dependent effect [27]. However, during infections with very high load of influenza virus, increased NK cells facilitated its act in reducing T cell immunity, resulting in viral persistence and T cell exhaustion [28].
Prolonged lymphopenia and its quantitative effect on clinical prognoses have never been analyzed. Our innovative finding that prolonged lymphopenia had increased risk for death in influenza infection and relatively lower risk in SARS-CoV-2 infection was of higher clinical significance. Clinically, lymphopenia caused by corticosteroids was transient after a single administration of corticosteroids but could be sustained with the chronic elevation of plasma corticosteroids [10]. The effect of corticosteroids treatment on lymphocytes count was also considered in our study and did not show strong impact on the duration of lymphopenia, which might need more studies to verify. Nevertheless, our study indicated the impact of infection on lymphopenia may be stronger in influenza infection than that in COVID-19, as we found longer median duration of lymphopenia and higher magnitude in OR values. However, the underlying mechanisms remain to be revealed. Moreover, we found that for other respiratory virus infections, the occurrence of lymphopenia was important for worse prognoses prediction, rather than how long the lymphopenia would be lasting.
A high NLR implies an aberrant immune response and has been studied as a marker of poor prognosis for respiratory infections [[29], [30], [31]]. It was reported that NLR was lower at admission and has more prognostic value in COVID-19 patients compared with influenza and RSV, and had the potential for distinguishing COVID-19 from influenza infection [29,30]. In our study, the NLR of dead cases of COVID-19 kept highest compared with other virus infections during hospitalization, which may be resulted from the excessive neutrophil generation and activation [4] and the massive decline of lymphocyte in severe or critical COVID-19. The predictive value of NLR was verified in our study with the pandemic omicron strain, consistent with previous strains in 2020 [[32], [33], [34]]. However, like lymphopenia, no studies discussed the impact of higher NLR on prognoses from a dynamic perspective during hospitalization, which implies that more further studies are needed.
Besides all-cause death during hospitalization, the association between prolonged lymphopenia and major organ damage were demonstrated in our study as well. Prolonged lymphopenia, especially lasting for longer days, had stronger association with kidney, liver and cardiac injury, and higher level of respiratory support. This indicated that lymphopenia might be involved in more important roles during the progression after influenza or SARS-CoV-2 infection, and more research are warranted to verify these findings.
Complete blood count is one of the commonest tests in clinical practice, always with several repetition during hospitalization thus it would be instant and economic if more information could be obtained from it [31]. The investigation of clinical big data increases rapidly in recent years, and was one of the aims in our study. In this paper, we employed the LOP method which can be utilized to interpret complex, dispersed medical data. Our study exhibited a successful attempt in the application of mathematical methods on temporal and large amounts of clinical data. Another advantage of this study was that we compared the dynamic changes and impact of lymphopenia among the commonest respiratory viruses. It has been reported that the severity and outcomes of influenza and COVID-19 patients, but lymphopenia was not able to provide distinguishable information for those two kinds of virus [21]. We found the risk magnitude of lymphopenia differed between different virus infections. That might be used as a distinguished way to identify different viruses.
Some limitations must be claimed in this study. Firstly, in this single-center, retrospective study, selection bias was inevitable. Most patients involved in our study were severe to critical, with high rates of organ damage and death, especially in those hospitalized with influenza infection. Therefore, the comparisons between different virus infections were inappropriate. Secondly, the laboratory tests were performed following clinical necessity, and the missing values were difficult to traceback, so our analyses could only depend on the current available data. For other respiratory virus infections, more laboratory findings were absent than influenza and SARS-CoV-2, which led to potential imbalance in model fitting. However, the application of LOP method helped to describe temporal trends during hospitalization and were accurately portrayed as a curve with optimal fit of goodness, despite the nature of medical series data was discrete in distribution and the random missing. Thirdly, the subtypes of influenza were not recorded accurately in the retrospective database, which limited further investigation on the difference on different subtypes. Fourthly, among the subtypes of SARS-CoV-2, only the data of omicron variant were available in our study, so the difference between different SARS-CoV-2 variants were unavailable, and we could only compare our findings with previous literatures, regardless the kind of variant. Fifthly, duration of lymphopenia was valuable, but could only predict events occurring within or after the specified duration range category. For patients died before specific days of persistent lymphopenia, they would be excluded from specific analysis and the sample size would be smaller. Finally, although bacterial infection might be indicated by PCT, it was hard to be confirmed without the result of culture. Thus, we only described the condition as “suspected bacterial infection”. According to previous study, bacteria co-infections were common in both influenza and SARS-CoV-2 infections [35,36]. In our study, we observed that almost all dead cases in influenza were comorbid suspected secondary bacterial infection, while those with SARS-CoV-2 were not. The reason may lie in the acute alter in patients’ systemic Toll-like receptor (TLR) responses during the infection of influenza virus [36]. Nevertheless, more studies are necessary to investigate the bacterial or even viral cross infection after hospitalization with respiratory virus infection.
For further clinical perspective, studies are required to mechanically prove the clinical manifestations revealed by our study. Beyond those, the method conducted in our study is also promising for adjuvant clinical decision, such as optimizing the opportunity of corticosteroids and other immune regulatory medications. For example, a clinical trial by Cheng et al. reported that recombinant human granulocyte colony-stimulating factor (rhG-CSF) may have the potential to reduce deterioration or death COVID-19 patients with lymphopenia [37]. Our study may provide a clue for the selections of treatment timing and specific patients to acquire better effect.
In summary, we found prolonged lymphopenia was significantly associated with worse clinical prognoses in influenza and SARS-CoV-2 infections, compared with a transient lymphopenia of 1-2 days, but not in other respiratory virus infections. The dynamic immune status changes and organ damages after infection were different between viruses.
Data availability statement
Data will be made available on request: readers can access the data by contacting the corresponding authors to acquire permission.
Financial support
This work has been supported by Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-001, 2021-I2M-1–044, 2021-I2M-1–048); CAMS Institute of Respiratory Medicine Grant for Young Scholars (2023-ZF-8); National High Level Hospital Clinical Research Funding; Elite Medical Professionals. Project of China-Japan Friendship Hospital (NO. ZRJY2023-QM20).
Role of the funder/sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CRediT authorship contribution statement
Guohui fan: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Wuyue Yang: Writing – original draft, Software, Methodology, Formal analysis, Data curation. Dingyi Wang: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Feiya Xu: Writing – review & editing, Writing – original draft. Yeming Wang: Investigation, Conceptualization. Chaozeng Si: Software, Data curation. Zhenguo Zhai: Writing – review & editing, Supervision, Project administration, Conceptualization. Zhongjie Li: Writing – review & editing, Supervision, Methodology. Rongling Wu: Writing – review & editing, Supervision. Bin Cao: Writing – review & editing, Supervision, Funding acquisition. Weizhong Yang: Writing – review & editing, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge all healthcare personnels who were devoted to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31733.
Contributor Information
Chaozeng Si, Email: supersi@aliyun.co.
Rongling Wu, Email: ronglingwu@bimsa.cn.
Bin Cao, Email: caobin_ben@163.com.
Weizhong Yang, Email: yangweizhong@cams.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
The dynamic changes of total neutrophil count and white blood cell count in patients hospitalized with respiratory virus infections by Legendre orthogonal polynomials. Note: Nbest refers to the appropriate order of fit for LOP by the Bayesian information criterion method. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LOP, Legendre orthogonal polynomials
Supplementary Fig. 2.
The dynamic changes of lymphocyte count according to clinical outcomes and corticosteroids treatment in patients hospitalized with respiratory virus infections by Legendre orthogonal polynomials. Note: *, administered with corticosteroids during hospitalization. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LOP, Legendre orthogonal polynomials.
Supplementary Fig. 3.
The dynamic changes of C-reactive protein, creatinine and serum ferritin in patients hospitalized with respiratory virus infections by Legendre orthogonal polynomials. Note: Nbest refers to the appropriate order of fit for LOP by the Bayesian information criterion method. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LOP, Legendre orthogonal polynomials.
Supplementary Fig. 4.
Forest plot of the association between duration of lymphopenia and clinical prognoses in patients hospitalized with respiratory virus infections. Note: OR and 95 % CI were estimated by logistic model, adjusted for age, corticosteroids treatment, chronic kidney disease, chronic respiratory disease cardiovascular disease, lymphopenia on admission and length of hospital stay. Other respiratory viruses included respiratory syncytial virus, parainfluenza virus, human rhinovirus, coronavirus, human metapneumovirus and adenovirus.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IMV, invasive mechanical ventilation; ICU, intensive care unit; OR, odds ratio; 95 % CI, 95 % confidence interval.
References
- 1.van der Poll T., et al. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017;17(7):407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F., et al. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China Network. Eur. Respir. J. 2019;54(2) doi: 10.1183/13993003.02406-2018. [DOI] [PubMed] [Google Scholar]
- 3.Liontos A., et al. Inflammation and venous thromboembolism in hospitalized patients with COVID-19. Diagnostics. 2023;13(22) doi: 10.3390/diagnostics13223477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y.M., et al. Blood molecular markers associated with COVID-19 immunopathology and multi-organ damage. EMBO J. 2020;39(24) doi: 10.15252/embj.2020105896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar B.V., Connors T.J., Farber D.L. Human T cell development, localization, and function throughout life. Immunity. 2018;48(2):202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boomer J.S., et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen X.R., et al. ACE2-independent infection of T lymphocytes by SARS-CoV-2. Signal Transduct. Targeted Ther. 2022;7(1):83. doi: 10.1038/s41392-022-00919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y., et al. Dynamic changes of lymphocyte counts in adult patients with severe pandemic H1N1 influenza A. J. Infect. Public Health. 2019;12(6):878–883. doi: 10.1016/j.jiph.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan S., Wu G. Is lymphopenia different between SARS and COVID-19 patients? Faseb. J. 2021;35(2) doi: 10.1096/fj.202002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth C.M., et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao B., et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N. Engl. J. Med. 2009;361(26):2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- 14.Lalueza A., et al. Severe lymphopenia in hospitalized patients with influenza virus infection as a marker of a poor outcome. Inf. Disp. 2019;51(7):543–546. doi: 10.1080/23744235.2019.1598572. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q., et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int. J. Infect. Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahneman D., Lovallo D., Sibony O. Before you make that big decision. Harv. Bus. Rev. 2011;89(6):50–60. 137. [PubMed] [Google Scholar]
- 17.Outcomes K.D.I.G. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2(1):138. [Google Scholar]
- 18.Levey A.S., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biegus J., et al. Liver function tests in patients with acute heart failure. Pol. Arch. Med. Wewn. 2012;122(10):471–479. doi: 10.20452/pamw.1413. [DOI] [PubMed] [Google Scholar]
- 20.Thygesen K., et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 21.Cobb N.L., et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann Am Thorac Soc. 2021;18(4):632–640. doi: 10.1513/AnnalsATS.202007-805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 23.Shimoni Z., Glick J., Froom P. Clinical utility of the full blood count in identifying patients with pandemic influenza A (H1N1) J. Infect. 2013;66(6):545–547. doi: 10.1016/j.jinf.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Tan L., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Targeted Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharon N., et al. Transient lymphopenia and neutropenia: pediatric influenza A/H1N1 infection in a primary hospital in Israel. Isr. Med. Assoc. J. 2011;13(7):408–412. [PubMed] [Google Scholar]
- 26.Delorey T.M., et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595(7865):107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao H., et al. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J. Virol. 2009;83(18):9215–9222. doi: 10.1128/JVI.00805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waggoner S.N., et al. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481(7381):394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prozan L., et al. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 compared with Influenza and respiratory syncytial virus infection. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-00927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H.A., et al. Clinical impact of monocyte distribution width and neutrophil-to-lymphocyte ratio for distinguishing COVID-19 and influenza from other upper respiratory tract infections: a pilot study. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terpos E., et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan X., et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study. J. Med. Virol. 2020;92(11):2573–2581. doi: 10.1002/jmv.26061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit. Care. 2020;24(1):647. doi: 10.1186/s13054-020-03374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasizadeh R., et al. Beyond the virus: exploring coinfections in the COVID-19 pandemic. Open Microbiol. J. 2023;17(1) [Google Scholar]
- 36.Heltzer M.L., et al. Immune dysregulation in severe influenza. J. Leukoc. Biol. 2009;85(6):1036–1043. doi: 10.1189/jlb.1108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng L.L., et al. Effect of recombinant human granulocyte colony-stimulating factor for patients with coronavirus disease 2019 (COVID-19) and lymphopenia: a randomized clinical trial. JAMA Intern. Med. 2021;181(1):71–78. doi: 10.1001/jamainternmed.2020.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request: readers can access the data by contacting the corresponding authors to acquire permission.