Abstract
Melioidosis is a tropical infectious disease that ranks as northeastern Thailand's third most common infectious cause of death. The manifestations of melioidosis vary depending on the organs involved and often resemble malignancy and tuberculosis.
We present a case of an atypical melioidosis presentation in a patient with low-grade fever and facial swelling without any risk factors. Chest CT revealed a 3.3-cm heterogeneous enhancing right lower paratracheal lymph nodes with thrombosis of the superior vena cava and azygos vein. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph node was performed, and Burkholderia pseudomallei was identified through lymph node culture. The patient underwent a three-week intravenous course of ceftazidime and a 12-week oral course of trimethoprim-sulfamethoxazole. Oral anticoagulation was also administered. Follow-up computed tomography of the thorax after completion of treatment revealed no residual lymphadenopathy and thrombosis.
Keywords: Mediastinal melioidosis, EBUS-TBNA, Atypical melioidosis presentation, SVC thrombosis, Mediastinal lymphadenopathy, Burkholderia pseudomallei
1. Introduction
Melioidosis is a tropical infectious disease characterized by a wide range of clinical manifestations and varying disease severity. The disease is most prevalent in Southeast Asia, particularly in northeastern Thailand, northern Australia, and India. It is caused by an infection with Burkholderia pseudomallei. Melioidosis primarily affects individuals with impaired neutrophil function, such as poorly controlled diabetes, chronic alcohol use, or chronic renal disease. However, it can also occur in patients without any significant risk factors.
The pulmonary system is the most commonly affected site of infection. The spectrum of human disease can vary from localized presentations with no systemic manifestations to severe septicemia and multiorgan dysfunction syndrome, depending on the different stages of melioidosis. Radiographic investigations commonly reveal nodular, alveolar, or mixed pulmonary consolidations with or without cavities, predominantly in the upper lobes. In rare cases, mediastinal involvement of melioidosis may be observed, particularly in conjunction with superior vena cava (SVC) thrombosis.
This report presents an extraordinary manifestation of melioidosis in a non-diabetic patient characterized by mediastinal lymphadenopathy, superior vena cava (SVC), and azygos vein thrombosis.
2. Case presentation and clinical course
A man in his 60s, without significant comorbidities, presented to the outpatient department with a 4-week history of low-grade fever and facial swelling, raising suspicion of superior vena cava (SVC) obstruction. He denied experiencing any symptoms of dyspnea, weight loss, hemoptysis, or other constitutional symptoms. However, he did have a 30-pack-year history of smoking. The patient's history and physical examination were unremarkable except for facial swelling.
A chest radiograph demonstrated the widening of the right paratracheal stripe. A thorax computed tomography (CT) revealed a 3.3-cm heterogenous enhancing lymph node at the right paratracheal region. An intraluminal filling defect was detected in the SVC and azygos vein, consistent with thrombosis. There was neither osteolytic nor osteoblastic lesion.
For diagnostic purposes, an endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed on the right paratracheal and subcarinal lymph nodes (Fig. 1). During the EBUS-TBNA procedure, ultrasound imaging displayed heterogeneous and well-demarcated lymph nodes, resembling malignant lymph nodes. However, the pathological examination of the lymph nodes revealed necrotic material without any signs of malignancy or granuloma formation. Bacterial culture of the lymph node aspiration yielded Burkholderia pseudomallei. Polymerase chain reaction testing for tuberculosis (TB) and mycobacterial culture from the lymph node aspiration yielded negative results. Other laboratory findings included a negative anti-HIV test, a normal hemoglobin A1C level, and normal liver function test results. Additionally, the blood culture showed negative results as well.
Fig. 1.
(A and B) The endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was conducted on the right paratracheal and subcarinal lymph nodes due to a high risk of malignancy.
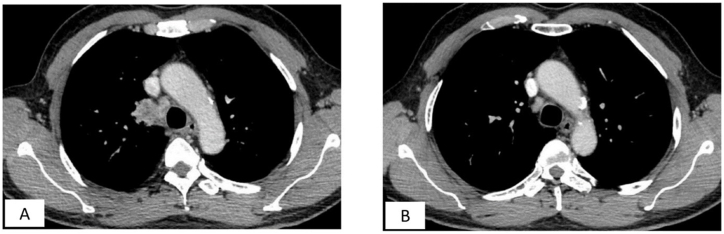
The patient underwent simultaneous treatment for mediastinal melioidosis and superior vena cava (SVC) thrombosis. Intravenous ceftazidime was administered for a duration of 3 weeks during the initial intensive phase, followed by a 12-week course of oral trimethoprim-sulfamethoxazole. After completing the full course of medication, a CT scan of the thorax showed significant improvement with near resolution of the lymph node in the right paratracheal region (Fig. 2) and complete resolution of the SVC and azygos vein thrombosis (Fig. 3).
Fig. 2.
(A and B) Figure A showed a 3.3-cm heterogenous enhancing mediastinal lymph node at the right paratracheal region. Figure B demonstrated complete resolution of right paratracheal lymphadenopathy after treatment.
Fig. 3.
(A and B) Figure A revealed intraluminal filling defect in the SVC and azygos vein, resulting in thrombosis on a chest CT scan. Figure B demonstrated complete resolution of SVC thrombosis after complete treatment with standard antibacterial regimen along with anticoagulation.
3. Discussion
First recognized in 1911, melioidosis is a tropical infectious disease caused by Burkholderia pseudomallei, a gram-negative, non-spore-forming facultative anaerobic bacillus. The disease is highly endemic in Southeast Asia, particularly in northeastern Thailand, northern Australia, and India [1,2]. Although theoretically curable and preventable, melioidosis carries a high mortality rate of up to 40 % [3]. In Thailand, this organism is commonly found in soil and pooled surface water, such as rice paddies [4]. Burkholderia pseudomallei can thrive in harsh conditions, including distilled water, nutrient-depleted soil, and desert environments. The primary routes of human infection are through broken skin, inhalation, and ingestion. Certain environmental factors, such as rice farming, are known to increase the risk of exposure [1].
The clinical presentations, disease severity, morbidity, and mortality of melioidosis are influenced by factors such as the bacterial load at the time of exposure, route of infection, virulence strain, and individual risk factors [5]. In northeastern Thailand, melioidosis ranks as the third leading infectious cause of death, following HIV and tuberculosis [6]. Poorly controlled diabetes, chronic alcohol use, and chronic renal disease are known risk factors predisposing individuals to melioidosis, as they affect the function of innate immunity, particularly neutrophil function [1,[5], [6], [7], [8]]. Prolonged corticosteroid uses and immunosuppressive agents also increase the risk of melioidosis [4]. Diabetes mellitus, after adjusting for other factors, carries a 12-fold increased risk of infection. However, approximately 20 % of adult patients with melioidosis do not have any recognized risk factors [9,10]. Melioidosis can occur despite the absence of significant risk factors, likely attributable to a high bacterial load [1,6]. As observed in our case, the patient presented with melioidosis without any identifiable risk factors.
The spectrum of human disease in melioidosis varies, ranging from localized manifestations at bacterial entry sites with no systemic symptoms to severe septicemia and multiorgan dysfunction syndrome [1,3,9,11]. There are four distinct stages of melioidosis presentation. The acute form can rapidly manifest as acute septicemia or pneumonia. Secondly, the subacute type presents with prolonged low-grade fever, pleuritic chest pain, weight loss, and cough persisting for weeks to months. Thirdly, the chronic form may involve extrapulmonary manifestations or present as a chronic pulmonary condition. Lastly, the subclinical form may exhibit minimal or no symptoms and can be detected through abnormal chest radiography or serological testing [2,10,12]. Pulmonary involvement is the most common manifestation of melioidosis and can occur as the initial presentation [2,4,13]. Referred to as the “great mimicker,” the acute form of pulmonary melioidosis can resemble community-acquired bacterial pneumonia. Its chronic form can mimic malignancy or pulmonary tuberculosis [6,[13], [14], [15]]. Our patient was suspected of having a subacute to a chronic form of melioidosis based on the slow progression and mild symptoms, even though chronic disease is less commonly observed [13].
Radiologic findings in melioidosis commonly show nodular, alveolar, or mixed consolidation patterns, with or without cavities, particularly in the upper lobes. Pleural effusion, pericardial involvement, and multi-lobar lesions may also be observed [2,6]. However, mediastinal lymphadenopathy is an uncommon feature of melioidosis [2,6,[16], [17], [18]]. Several case reports have described melioidosis with mediastinal lymphadenopathy, which can present as necrotic nodes and may be mistaken for tuberculosis or malignancy, posing a diagnostic challenge [11,15,[19], [20], [21], [22], [23], [24]]. Initial blood cultures can yield negative results in cases of mediastinal melioidosis, similar to our case [[19], [20], [21]]. Most instances of mediastinal melioidosis occur in a chronic form [[20], [21], [22], [23]], as observed in our case, although some studies have reported cases with an acute onset [15].
In addition to mediastinal involvement, superior vena cava (SVC) thrombosis in melioidosis is uncommon. Our patient presented with facial swelling, raising suspicion of SVC obstruction. The initial chest CT scan revealed a mediastinal mass in the right paratracheal region, accompanied by SVC and azygos vein thrombosis, likely resulting from perilesional inflammation. Some studies have described melioidosis as a rare benign cause of SVC obstruction due to mediastinal fibrosis or compression from nearby lymph nodes [25]. SVC thrombosis associated with mediastinal melioidosis has shown a good response to anticoagulant and antimicrobial treatment. Only one reported mediastinal melioidosis with SVC thrombosis [25]. Thus, our case demonstrates an extraordinary manifestation of melioidosis with mediastinal involvement and SVC thrombosis. Notably, the recent advances in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) played a crucial role in achieving a less invasive diagnosis. In an endemic area for melioidosis, sending specimens obtained from transbronchial needle aspirations for bacterial culture is essential to identify Burkholderia pseudomallei.
Our patient received the standard antibiotic therapy for melioidosis, starting with a 3-week course of ceftazidime as part of the intensive phase. Previous studies have demonstrated favorable responses to ceftazidime in cases of mediastinal melioidosis [15,21]. Following ceftazidime, a 12-week course of trimethoprim-sulfamethoxazole was administered during the eradication phase, which is strongly supported by research [7]. Additionally, anticoagulant treatment was initiated in the initial phase to address SVC thrombosis and continued for 3 months. The follow-up chest CT scan after completion of treatment revealed complete resolution of the mediastinal lymphadenopathy and no evidence of SVC thrombosis.
4. Conclusion
-
•
We reported a rare presentation of melioidosis as mediastinal and SVC thrombosis in a patient with no known risk factor.
-
•
Given its variable presentations, melioidosis always needs to be in the differential diagnosis along with tuberculosis and malignancy in the endemic area especially in patients with mediastinal lymphadenopathy.
-
•
The hemocultures can be nonbeneficial in this presentation.
-
•
EBUS-TBNA with lymph node culture can make an accurate diagnosis and assure immediate treatment.
Declaration of competing interest
No conflict.
Handling Editor: DR AC Amit Chopra
References
- 1.Wiersinga W.J., Virk H.S., Torres A.G., Currie B.J., Peacock S.J., Dance D.A.B., et al. Melioidosis. Nat. Rev. Dis. Prim. 2018 Jun 7;4(1) doi: 10.1038/nrdp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burivong W., Wu X., Saenkote W., Stern E.J. Thoracic radiologic manifestations of melioidosis. Curr. Probl. Diagn. Radiol. 2012 Nov;41(6):199–209. doi: 10.1067/j.cpradiol.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Currie B.J., Peacock S.J. Melioidosis. N. Engl. J. Med. 2012 Sep 13;367(11):1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A.C., Currie B.J. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005 Apr;18(2):383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currie B.J. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. 2015 Feb;36(1):111–125. doi: 10.1055/s-0034-1398389. [DOI] [PubMed] [Google Scholar]
- 6.Reechaipichitkul W. Clinical manifestation of pulmonary melioidosis in adults. Southeast Asian J. Trop. Med. Public Health. 2004;35(3):6. [PubMed] [Google Scholar]
- 7.Anunnatsiri S., Chaowagul W., Teparrukkul P., Chetchotisakd P., Tanwisaid K., Khemla S., et al. A comparison between 12 versus 20 Weeks of trimethoprim-sulfamethoxazole as oral eradication treatment for melioidosis: an open-label, pragmatic, multicenter, non-inferiority, randomized controlled trial. Clin. Infect. Dis. 2021 Dec 6;73(11):e3627–e3633. doi: 10.1093/cid/ciaa1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetchotisakd P., Porramatikul S., Mootsikapun P., Anunnatsiri S., Thinkhamrop B. Randomized, double‐blind, controlled study of cefoperazone‐sulbactam plus cotrimoxazole versus ceftazidime plus cotrimoxazole for the treatment of severe melioidosis. Clin. Infect. Dis. 2001 Jul;33(1):29–34. doi: 10.1086/320878. [DOI] [PubMed] [Google Scholar]
- 9.Limmathurotsakul D., Wongratanacheewin S., Teerawattanasook N., Wongsuvan G., Chaisuksant S., Chetchotisakd P., et al. Increasing incidence of human melioidosis in northeast Thailand. Am. J. Trop. Med. Hyg. 2010 Jun;82(6):1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie B.J., Jacups S.P., Cheng A.C., Fisher D.A., Anstey N.M., Huffam S.E., et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop. Med. Int. Health. 2004;9(11):1167–1174. doi: 10.1111/j.1365-3156.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 11.Saravu K., Vishwanath S., Kumar R.S., Barkur A.S., Varghese G.K., Mukhyopadhyay C., et al. Melioidosis — a case series from south India. Trans. Roy. Soc. Trop. Med. Hyg. 2008 Dec 1;102(Supplement_1):S18–S20. doi: 10.1016/S0035-9203(08)70006-3. [DOI] [PubMed] [Google Scholar]
- 12.Currie B.J., Ward L., Cheng A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 Year Darwin prospective study. PLoS Neglected Trop. Dis. 2010 Nov 30;4(11):e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meumann E.M., Cheng A.C., Ward L., Currie B.J. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin. Infect. Dis. 2012 Feb 1;54(3):362–369. doi: 10.1093/cid/cir808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidyalakshmi K., Chakrapani M., Shrikala B., Damodar S., Lipika S., Vishal S. Tuberculosis mimicked by melioidosis. Int. J. Tubercul. Lung Dis. 2008 Oct 1;12(10):1209–1215. [PubMed] [Google Scholar]
- 15.Saravu K., Mukhopadhyay C., Eshwara V.K., Shastry B.A., Ramamoorthy K., Krishna S., et al. Melioidosis presenting with mediastinal lymphadenopathy masquerading as malignancy: a case report. J. Med. Case Rep. 2012 Dec;6(1):28. doi: 10.1186/1752-1947-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maneechotesuwan K. An exotic pulmonary infection in Thailand: melioidosis. Respirology. 1999;4(4):419–422. doi: 10.1046/j.1440-1843.1999.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Tshibwabwa E.T., Richenberg J.L., Aziz Z.-A. Lung radiology in the tropics. Clin. Chest Med. 2002 Jun 1;23(2):309–328. doi: 10.1016/s0272-5231(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 18.Muttarak M., Peh W.C., Euathrongchit J., Lin S.E., Tan A.G., Lerttumnongtum P., Sivasomboon C. Spectrum of imaging findings in melioidosis. Br. J. Radiol. 2009 Jun;82(978):514–521. doi: 10.1259/bjr/15785231. [DOI] [PubMed] [Google Scholar]
- 19.Ashraf O., Iyer A., Krishnan R., Yadav S. Thoracic melioidosis: a diagnostic dilemma. Asian Cardiovasc. Thorac. Ann. 2015 Feb 1;23(2):219–220. doi: 10.1177/0218492314521823. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J., Yap A., Wu E., Yap J. A mimic of bronchogenic carcinoma-pulmonary melioidosis. Respiratory Med. Case Rep. 2020 Jan 1;29 doi: 10.1016/j.rmcr.2020.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan H.P., Yip H.S. Mediastinal lymphadenopathy: melioidosis mimicking tuberculosis. Trop. Med. Health. 2015;43(2):93–94. doi: 10.2149/tmh.2014-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong T., Naidoo P., Kumar B., Stefanski D., Woolley I., Farmer M. InC54. CASE REPORTS: INTERVENTIONAL PULMONOLOGY. American Thoracic Society; 2012 May. Acute mediastinal melioidosis: diagnosis by endobronchial ultrasound-guided transbronchial needle aspiration. (pp. A4568-A4568) [Google Scholar]
- 23.Kho S.S., Ho Y.F., Chan S.K., Tie S.T. Mediastinal melioidosis masquerading as malignancy of the lung. Lancet. 2021 Mar 13;397(10278) doi: 10.1016/S0140-6736(21)00200-2. [DOI] [PubMed] [Google Scholar]
- 24.Chlebicki M.P., Tan B.H. Six cases of suppurative lymphadenitis caused by Burkholderia pseudomallei infection. Trans. Roy. Soc. Trop. Med. Hyg. 2006 Aug 1;100(8):798–801. doi: 10.1016/j.trstmh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Reechaipichitkul W., Thongpaen S. Etiology and outcome of superior vena cava (SVC) obstruction in adults. Southeast Asian J. Trop. Med. Public Health. 2004;35(2):5. [PubMed] [Google Scholar]