Abstract
Study Objectives
Although short sleep could promote neurodegeneration, long sleep may be a marker of ongoing neurodegeneration, potentially as a result of neuroinflammation. The objective was to evaluate sleep patterns with age of expected Alzheimer’s disease (AD) onset and neuroinflammation.
Methods
We tested 203 dementia-free participants (68.5 ± 5.4 years old, 78M). The PREVENT-AD cohort includes older persons with a parental history of AD whose age was nearing their expected AD onset. We estimated expected years to AD onset by subtracting the participants’ age from their parent’s at AD dementia onset. We extracted actigraphy sleep variables of interest (times of sleep onset and morning awakening, time in bed, sleep efficiency, and sleep duration) and general profiles (sleep fragmentation, phase delay, and hypersomnia). Cerebrospinal fluid (CSF) inflammatory biomarkers were assessed with OLINK multiplex technology.
Results
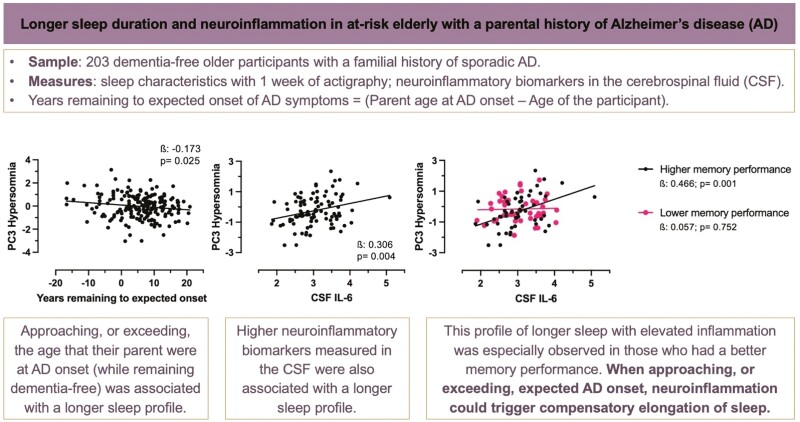
Proximity to, or exceeding, expected age of onset was associated with a sleep profile suggestive of hypersomnia (longer sleep and later morning awakening time). This hypersomnia sleep profile was associated with higher CSF neuroinflammatory biomarkers (IL-6, MCP-1, and global score). Interaction analyses revealed that some of these sleep-neuroinflammation associations were present mostly in those closer/exceeding the age of expected AD onset, APOE4 carriers, and those with better memory performance.
Conclusions
Proximity to, or exceeding, parental AD dementia onset was associated with a longer sleep pattern, which was related to elevated proinflammatory CSF biomarkers. We speculate that longer sleep may serve a compensatory purpose potentially triggered by neuroinflammation as individuals are approaching AD onset. Further studies should investigate whether neuroinflammatory-triggered long sleep duration could mitigate cognitive deficits.
Keywords: Dementia, apolipoprotein, MCI, mild cognitive impairment, inflammation, cytokines, cerebrospinal fluid, total sleep time, circadian
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Long sleep duration has been associated with increased risk of Alzheimer’s disease (AD). As it is unclear whether longer sleep duration is detrimental in itself, this long sleep pattern has been hypothesized to reflect neurodegeneration and/or neuroinflammation. We have found that as individuals are getting closer to or exceeding expected age of AD onset (i.e. the age that their parent developed AD), they presented longer sleep duration, which in turn, was associated with elevated biomarkers of neuroinflammation, especially in individuals with better cognitive performance. Neuroinflammatory processes in the AD trajectory could trigger compensatory increase in sleep duration. Future studies should evaluate whether long sleep duration could result in beneficial impact on the brain, such as delayed age of onset.
Introduction
Self-reports of both short and long sleep duration are associated with increased risk of Alzheimer’s disease (AD) [1–3]. Short sleep duration potentially represents chronic sleep loss and deprivation, which may promote AD pathology [4]. By contrast, associations between long sleep duration and AD risk are more intriguing, having been suggested to indicate either neurodegenerative damage to structures that promote wakefulness [5], or a compensatory increase potentially in response to neurodegenerative-related processes such as neuroinflammation [6]. The latter mechanism appears especially plausible, because AD progression is associated with neuroinflammatory processes [7, 8], and long sleep duration is known to be associated with elevated inflammatory levels [9]. Specifically, experimental studies have shown that higher interleukin-6 (IL-6) levels in the brain promote deeper sleep [6], potentially to help resolve inflammation and remove debris through increased enhanced glymphatic clearance [10–12].
Thus, a U-shaped association between sleep duration and AD risk is well substantiated. Questions remain, however, about the sleep profile observed years before AD onset. Only a few experimental designs allow the study of these phenomena in high-risk older persons who are nearing a likely onset of AD symptoms. The PRe-symptomatic EValuation of Novel or Experimental Treatments for Alzheimer’s Disease (PREVENT-AD) cohort, begun in 2011, offers an excellent opportunity for such study [13]. This longitudinal study has followed participants, most aged 65 years and older and all cognitively unimpaired at study entry. They were also required to have a parental or multiple-sibling history of “sporadic” AD, which could place them at a genetic risk—that is two- to three-fold higher—of developing AD [13–17]. We have previously reported that the proximity of their age to their parent’s age of AD onset (or exceeding it) is associated with higher amyloid costs, especially in APOE4 carriers [15]. Here, we describe efforts to characterize the sleep profile observed in participants with a limited brief number of expected years remaining before their own age of AD onset (EYO), defined by convention as the difference between their age and that of AD onset in their first-affected parent. In this study, we aimed to investigate sleep patterns measured with actigraphy in association with the EYO score, and in association with neuroinflammatory biomarkers. We tested the hypothesis that specific sleep patterns, such as longer sleep duration, appear as people are getting closer to expected AD onset; a sleep pattern that could be due in part to neuroinflammatory processes, which are known to play a role in sleep regulation. For this work, we selected and tested in the cerebrospinal fluid (CSF) two cytokines/chemokines that are well established as being involved in AD progression, namely IL-6 and monocyte chemotactic protein 1 (MCP-1, also known as CCL-2) [18–20]. IL-6 is central in regulating inflammatory cascades in feedback loops [21], and its levels in the brain have repeatedly been shown to regulate sleep [6]. In the central nervous system, MCP-1 is also a key neuroinflammatory species that promotes cellular migration and blood-brain barrier permeability changes that propagate inflammation [22]. Secondly, we investigated whether the associations between sleep patterns and neuroinflammatory biomarkers differed according to markers of AD progression and risk. We hypothesized that the association between sleep patterns and neuroinflammatory biomarkers would be especially apparent in those displaying markers of AD progression. These markers of AD progression were the EYO score; delayed recall memory performance given its predictive value for AD [23, 24]; mild cognitive impairment (MCI) progressor status; the APOE4 allele carrier status; and AD biomarkers measured in the CSF.
Materials and Methods
PREVENT-AD cohort and expected years remaining before clinical onset (EYO) scores
The PREVENT-AD Cohort is based in Montreal [13]. It evaluates older participants potentially at risk using a longitudinal multi-modal examination protocol. Participants have been tested annually since 2011 with cognitive assessments as well as with actigraphy and fluid biomarkers at selected years. Here, 203 participants who remained free of dementia underwent actigraphy testing. Of note, a few participants presented with MCI at the time of actigraphy (Table 1). They provided signed informed consent before their participation, and the protocol was approved by the ethics committee of McGill University. EYO scores were calculated as described above, that is, by subtracting the age of the participant from the age of their first-affected parent’s onset. EYO scores were calculated at the time of actigraphy. A positive value for EYO indicates a participant who was younger than their parent’s age of onset, while a negative number means that the participant’s age exceeded this value while remaining dementia-free. For example, a person aged 75 years old with a parent who developed AD at 80 has an EYO score of 5. A person aged 65 years old with a parent who developed AD at 75 years old has an EYO score of 10. A person aged 72 years old and dementia-free with a parent who developed AD at 70 years old has a score of −2. We analyzed EYO scores only for participants with a parental AD history (not with a multiple-sibling history). However, participants with a multiple-sibling history of AD were included in analyses that evaluated the association between sleep characteristics and inflammatory biomarkers. As a sensitivity analysis, we explored whether our findings were changed when using an alternative EYO score that considers those with a multi-sibling history.
Table 1.
Descriptive Characteristics of the Sample
| Characteristics | Full sample (n = 203) |
CSF subsample (n = 100) |
|---|---|---|
| Age, years | 68.25 (5.41) | 67.45 (5.46)* |
| Sex, n men (%) | 78 (38.4) | 29 (29.0) |
| Education, years | 15.16 (3.37) | 15.13 (3.17) |
| Retirement, n (%) | 143 (70.4) | 61 (61.0)* |
| Body mass index, kg/m2 | 27.08 (4.83) | 27.50 (4.28) |
| Geriatric Depression Scale, score | 1.50 (2.14) | 1.21 (1.79) |
| Geriatric Anxiety Inventory, score | 2.17 (3.63) | 1.77 (3.02) |
| Hypertension or usage of antihypertensive, n (%) | 61 (30.1) | 30 (30.0) |
| Usage of statins, n (%) | 49 (24.1) | 26 (26.0) |
| History of atrial fibrillation, n (%) | 23 (11.3) | 8 (8.0) |
| Years remaining before estimated expected onset (EYO), years** | 5.64 (7.56) | 5.81 (7.41) |
| Up to 5 years before expected onset, n (%) | 79 (38.9) | 36 (36.0) |
| 5 to 10 years before expected onset, n (%) | 56 (27.6) | 31 (31.0) |
| More than 10 years before expected onset, n (%) | 54 (26.6) | 26 (26.0) |
| MCI progressors, n (%) | 34 (16.8) | 17 (17.0) |
| MCI at the time of actigraphy, n (%) | 10 (4.9) | 6 (6.0) |
| Delayed memory, RBANS score | 105.27 (10.54) | 104.96 (11.24) |
| APOE4 allele carrier status, n (%) | 78 (38.4) | 40 (40.0) |
| CSF IL-6 levels, npx | — | 3.04 (0.55) |
| CSF MCP-1 levels, npx | — | 13.85 (0.48) |
| CSF t-tau/Aβ42 | — | 0.24 (0.22) |
| CSF p-tau181/Aβ42 | — | 0.049 (0.031) |
| Sleep characteristics, weekly average | ||
| Time of sleep onset, hh:mm | 23:18 (00:58) | 23:22 (00:58) |
| Time of last morning awakening, hh:mm | 07:11 (01:06) | 07:01 (01:07)* |
| Time in bed, min | 504.22 (55.11) | 492.23 (50.98)* |
| Sleep efficiency, % | 86.97 (5.95) | 86.69 (6.75) |
| Sleep duration, min | 438.79 (52.98) | 426.34 (50.93)* |
CSF, cerebrospinal fluid; EYO, expected years remaining before clinical onset; MCI, mild cognitive impairment; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; APOE4, carriers with > 1 ε4 allele; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein 1; npx, normalized protein expression levels.
*Significant difference between the full and CSF subsample.
**EYO for those with a history of parental sporadic AD (n = 189), excluding those with a multiple-sibling history of AD. The group “up to 5 years before expected onset” include those with an EYO score between 0 and 5 that are proximal to age of parental onset, as well as those with a negative EYO score that exceeded age of parental onset while remaining dementia-free.
Actigraphy protocol and processing
Participants wore the Actiwatch (Philips Respironics, Murrysville, PA, USA) actigraphy device for six to seven consecutive days. The large majority of participants (96.1%) completed this protocol without interruption. The remainder had 4 or 5 days of continuous observations. Actigraphy data were collected in epochs of 15 seconds and processed using Actiware® (Phillips Respironics) analytic software with a medium threshold for wake-detection sensitivity (40 activity counts/per minute). This threshold reduces nighttime bias in sleep detection [25]. During the actigraphy period, participants also recorded a sleep diary. When participants provided sufficiently precise and accurate data, the time in bed used for sleep detection by the Actiware software was determined from sleep diaries, and confirmed with light and movement data. For the large majority of the participants (90.6%), sleep diaries were of good quality with sufficiently precise and accurate data. In 19 participants (9.4%), sleep diaries were fully or partly poorly filled (e.g. missing days, large difference between reported hours and actigraphy data, evidence of diary filled once at the end of the week rather than day-to-day). In those cases, time in bed was set using the moment of correspondence between lights off/lights on data with mobile/immobile movement data of the actigraph. We evaluated whether removing these participants affected our results.
Sleep characteristics
Selected sleep characteristics.
Sleep characteristics of interest were times of sleep onset and of last morning awakening, time in bed, sleep efficiency (sleep duration/time in bed), and sleep duration. These were selected because they grossly represent sleep timing, sleep habits, and sleep quality/quantity to dress a general portrait of sleep patterns. We averaged these data over all days of actigraphy. As many participants were not precise enough when filling sleep diaries regarding naps, daytime sleep was not considered. For purposes of correlations, sleep timing (time of sleep onset and time of last morning awakening) was expressed in 24-hour time. If the time of sleep onset exceeded 23h59, we added + 24 so that later time of sleep onset was represented by a higher value (e.g. sleep onset at 1:00 am was coded as 25).
Principal component analysis to derive sleep profiles.
Because the selected variables do not represent sleep structure as a whole, we also explored a wider array of sleep variables. Data reduction was accomplished using principal component analysis with varimax rotation to extract sleep profiles. Variables entered in the principal component analysis were each participant’s mean over days of actigraphy for sleep midpoint (midpoint between times of onset and last morning awakening), time of sleep onset, time of last morning awakening, time in bed, activity counts per minute, sleep onset latency, sleep efficiency (sleep duration/time in bed), wake after sleep onset, sleep duration, proportion of sleep over the sleep period (from sleep onset to last morning awakening), number of continuous sleep bouts, and the fragmentation index (percent of mobile + immobile < 1min bouts over the sleep period, indicating restlessness). The sleep principal components analysis is presented in Table 2. The first principal component (PC) included multiple variables that suggested disrupted and fragmented sleep. We therefore called this the “PC1-Sleep fragmentation” component. The second component included later sleep onset, midpoint, and latest morning awakening, thus being called “PC2-Phase delay.” “PC3-Hypersomnia” included longer time in bed, longer sleep duration, and a later time of morning awakening.
Table 2.
Principal Component Analyses of Sleep Characteristics
| PC1–sleep fragmentation 40.9% of the variance |
PC2–phase delay 21.9% of the variance |
PC3–hypersomnia 19.9% of the variance |
|
|---|---|---|---|
| Time of sleep midpoint | 0.039 | 0.978 | 0.171 |
| Time of sleep onset | 0.018 | 0.944 | −0.311 |
| Time of morning awakening | 0.051 | 0.82 | 0.558 |
| Time in bed | 0.289 | 0.068 | 0.905 |
| Activity count per min | 0.832 | 0.031 | −0.029 |
| Sleep onset latency | 0.511 | 0.277 | −0.075 |
| Sleep efficiency | −0.868 | −0.069 | 0.244 |
| Wake after sleep onset | 0.915 | −0.061 | 0.236 |
| Sleep duration | −0.244 | 0.015 | 0.963 |
| Sleep proportion | −0.956 | 0.07 | −0.007 |
| Number of sleep bouts | 0.734 | −0.025 | 0.293 |
| Fragmentation index | 0.877 | 0.081 | −0.009 |
PC, principal component; bold values represent those that loaded > 0.5 and contributed the most to the component.
CSF biomarkers of inflammation
For 100 out of 203 of the included participants, lumbar punctures had been performed. When multiple lumbar punctures were available, we selected the procedure closest in time to actigraphy. Thus, CSF collection was performed either in the few years before or the same annual evaluation of the actigraphy, with an average of 1.84 ± 1.46 years before the actigraphy. Lumbar punctures were performed following an overnight fast, and samples were centrifuged and frozen within four hours of collection. The McGill Genome Center then analyzed aliquots using the OLINK Target 96 Inflammatory Panel (Uppsala, Sweden). Standard quality control measures included assurance of sufficient sensitivity and aptness of comparison with internal controls. We were thereby able to use 60 individual inflammatory biomarkers for further analysis. First, we studied CSF IL-6 and MCP-1, which are included in the OLINK panel, as we hypothesized that IL-6 and MCP-1 specifically would be biomarkers of inflammation related to AD and sleep dysfunction.
Secondly, to condense inflammatory biomarkers into a single score, we performed a principal component analysis that forced a single solution, resulting in a unique score for each participant labeled as “PC-OLINK.” Creating an inflammatory score, the single solution CSF PC-OLINK, representing 52.4% of the variance, is described in Supplementary Table S1. IL-6 loaded at a coefficient of 0.421 whereas MCP-1 loaded at 0.738. Whereas loading coefficient shows that all 60 inflammatory biomarkers contributed to the PC-OLINK inflammatory score, there were twelve biomarkers that loaded higher than 0.85, indicating a strong contribution to the PC-OLINK single inflammatory score.
Effect modifiers: APOE4 genotyping, delayed memory performance, diagnoses of MCI, and CSF AD biomarkers
All participants underwent APOE genotyping. DNA extraction from buffy coat was automated and performed using the QiaSymphony DNA mini kit. Genotypes were then determined using the PyroMark Q96 pyrosequencer (Qiagen). Participants were categorized by presence of one or more APOE4 alleles (carriers vs. non-carriers). Adjudicated diagnosis of MCI was made by consensus committees including expert clinical and research staff, based on cognitive testing that included at a minimum the mini-mental state examination [26] and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [27]. Consideration of a diagnosis of MCI was performed at all annual follow-ups, including those before, concomitant to, and after the actigraphy testing. In progressors, MCI onset occurred on average 0.82 ± 2.08 years after actigraphy testing. Therefore, MCI progressors (n = 34) included both participants who already had MCI at the time of actigraphy (n = 10), as well as those who developed MCI after actigraphy (n = 24, up to 4 years after actigraphy). All participants except one had their CSF collection before MCI onset. We also extracted the delayed recall memory domain from the RBANS as a distinct variable measured at the latest time point, on average 1.19 ± 0.84 years after the actigraphy. CSF AD biomarkers were measured according to standard procedures [28] with Innotest ELISA kits (Fujirebio, Ghent, Belgium). AD biomarkers were CSF t-tau/Aβ42 and p-tau181/Aβ42 ratios, as they discriminate between AD stages better than single non-ratio biomarkers [29–31].
Statistical analyses
Statistical analyses were performed using SPSS26. Two-tailed tests for principal variables were considered significant at p < .05. Unless otherwise specified, beta coefficients in linear regression models were standardized to allow comparison of effect size estimates. All analyses were adjusted for age, sex, and when applicable, the time interval between CSF collection and actigraphy.
Primary analyses: EYO and sleep.
Continuous EYO scores were entered in linear regression models as predictors of selected sleep characteristics (times of sleep onset and last morning awakenings, time in bed, sleep efficiency, and sleep duration) as outcomes in separate models. As well, additional models considered continuous EYO scores and the sleep profile scores computed from the principal component analysis (PC1, PC2, and PC3) of actigraphy-derived sleep characteristics.
Primary analyses: inflammation and sleep.
CSF biomarkers of inflammation (individual measures of IL-6, MCP-1 as well as the PC-OLINK inflammatory score) were entered in separate linear regression models as predictors of individual sleep characteristics (times of sleep onset and last morning awakenings, time in bed, sleep efficiency, and sleep duration), and sleep principal components (PC1, PC2, and PC3).
Secondary and sensitivity analyses.
Additional covariates: For significant primary models only, separate models included the following variables as potential confounders: (1) body mass index (BMI), a strong correlate of obstructive sleep apnea (OSA) [32]; (2) retirement status as this could affect time available to sleep; (3) use of psychoactive medications (e.g. antidepressants, benzodiazepines, and hypnotics) that could influence sleep patterns; the (4) Geriatric Depression Scale score and the; (5) Geriatric Anxiety Inventory score as anxio-depressive symptoms that can be associated with sleep patterns; cardiometabolic factors that may increase dementia risk, including; (6) hypertension (>140 mmHg systolic blood pressure, >90 mmHg diastolic blood pressure, or usage of antihypertensive agents); (7) statin use as proxy for hypercholesterolemia; and (8) history of atrial fibrillation.
Descriptive and sensitivity analyses: For descriptive purposes, we also evaluated whether EYO scores were associated with delayed memory performance and MCI progressor status, adjusted for age and sex. We evaluated the association between CSF AD biomarkers with sleep patterns using linear regressions adjusted for age, sex, and time between CSF collection and actigraphy. We performed a series of sensitivity analyses to evaluate whether small modifications to our primary models changed our results: (1) removing the 9.4% of participants with poorer quality of sleep diaries, (2) changing the EYO scores to an alternative EYO score that also included participants with multi-sibling history into the calculation, and (3) removing statistical outliers (>3SD) in analyses with CSF inflammatory biomarkers.
Sleep duration categories: Because of the known U-shaped relationship between sleep duration and dementia (with both short and long duration being associated with adverse outcomes), we performed primary analyses again, replacing continuous sleep duration with a categorical classification of <7, 7–8, and >8 hours (one-way ANOVA).
Interaction models between EYO scores and inflammation on sleep: We evaluated whether EYO scores play a moderating role in the relationship between inflammation and sleep patterns. We performed further analyses for selected significant primary models. In linear regression models between inflammation and sleep, we included an interaction term by EYO score (dichotomized to facilitate further stratification analyses, split at 5 years before expected onset). When significant interactions were observed, we further stratified regressions between inflammation and sleep by EYO score split at 5 years.
Interaction analyses with other markers of AD progression: In order to evaluate whether the associations between inflammation and sleep differ according to markers of AD progression, we performed further analyses for selected significant primary models. We performed linear regression models between inflammation and sleep including interaction terms with markers of AD progression. Selected markers of AD progression were delayed recall memory performance (split at the median), MCI progressor status, APOE4 allele carriers, and CSF AD biomarkers (t-tau/Aβ42, p-tau181/Aβ42). When significant interactions were observed, we further stratified by those markers of AD progression.
Effect of time lag: For significant primary models, we investigated whether time lag between actigraphy and CSF collection (<2 years vs. 2 years and more) affected the association between CSF inflammatory biomarkers and sleep characteristics using linear regression models and interaction terms.
Results
Characteristics of the sample
Among the 203 participants with actigraphy, 189 had a parental history of sporadic AD (others had a multiple-siblings history of AD), which enabled analyses using EYO scores. In the subsample of 100 participants with CSF testing, 93 had a parental history of AD. Characteristics of the full sample and the CSF donor sample are presented in Table 1. Participants were aged between 58 and 89 years old at the time of actigraphy, and about 38% were men. Participants, all free of dementia, were on average 5.6 years younger than their parent’s age at symptom onset. The full sample was about equally distributed between those up to 5 years away, those between 5 and 10 years away, and those more than 10 years away from their parent’s age at symptom onset. The range of EYO scores was from −16.71 up to 20.92 years. Of note, 37 participants had a negative EYO score (19.58% of the full sample), meaning that, at the time of actigraphy, they were older than their parents were at AD onset, although these participants were still dementia-free. EYO scores were not associated with delayed memory performance of MCI progressor status. Participants in the CSF subsample were very slightly younger with a lower proportion of retired people. The CSF subsample also woke up slightly earlier, spent less time in bed, and had a shorter sleep duration than the full sample. Based on published cutoffs [33, 34], 12.8% of the CSF donor sample was positive for AD pathology. The association between CSF AD biomarkers and sleep characteristics are presented in Supplementary Table S2. Higher CSF t-tau/Aβ42 and p-tau/Aβ42 were associated with longer time in bed. Other regressions were trending with higher ratios in association with earlier time of sleep onset, longer sleep duration, and higher PC3-Hypersomnia score.
Associations between EYO scores and sleep characteristics
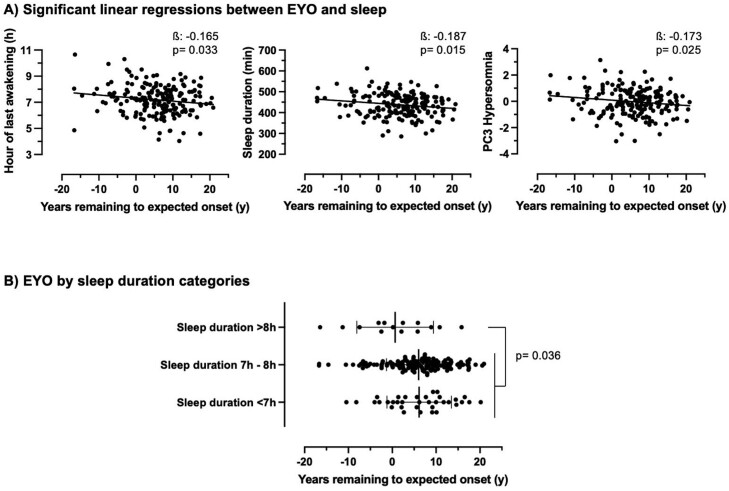
Proximity to, or exceeding, parental age of AD onset, as evidenced by lower EYO scores, was associated with a later time of morning awakening, longer sleep duration, and higher PC3-Hypersomnia (Figure 1A, Supplementary Table S3, small effect sizes). After additional adjustments (BMI, retirement, psychoactive medications, anxio-depressive symptoms, and cardiometabolic risk factors), all associations remained statistically significant except for the association between lower EYO and later time of morning awakening, which remained at trend level (p = .079) when adjusted for the BMI. Removing the participants with poorer sleep diary quality (9.4%) did not affect the results. Moreover, using an alternative EYO score calculated from age of parental onset or age of sibling onset resulted in mostly the same findings (Supplementary Table S4). No other sleep characteristics or components were associated with the EYO score.
Figure 1.
(A) Significant associations between lower EYO scores with later time of last morning awakening, longer sleep duration, and higher PC3-Hypersomnia score, adjusted for age and sex. (B) Lower EYO was observed in those with longer sleep duration (>8h) as compared to other sleep duration categories. Note that a lower EYO score includes negative values, representing dementia-free people older than their parent’s age at symptom onset. Betas are standardized. EYO, estimated years remaining to expected onset; PC3-Hypersomnia, third principal component of sleep characteristics representing a sleep pattern suggestive of hypersomnia.
When comparing sleep duration groups (<7, 7–8, and >8 hours) with a one-way ANOVA, participants with sleep duration >8 hours had markedly lower EYO (0.66 ± 8.75 years, p = .036) than those with short (<7 hours) and average (7–8 hours) sleep duration (6.15 ± 7.36 and 6.02 ± 7.36 years, respectively, Figure 1B), without any age difference between the sleep duration groups at the time of actigraphy.
Associations between CSF inflammatory biomarkers and sleep characteristics
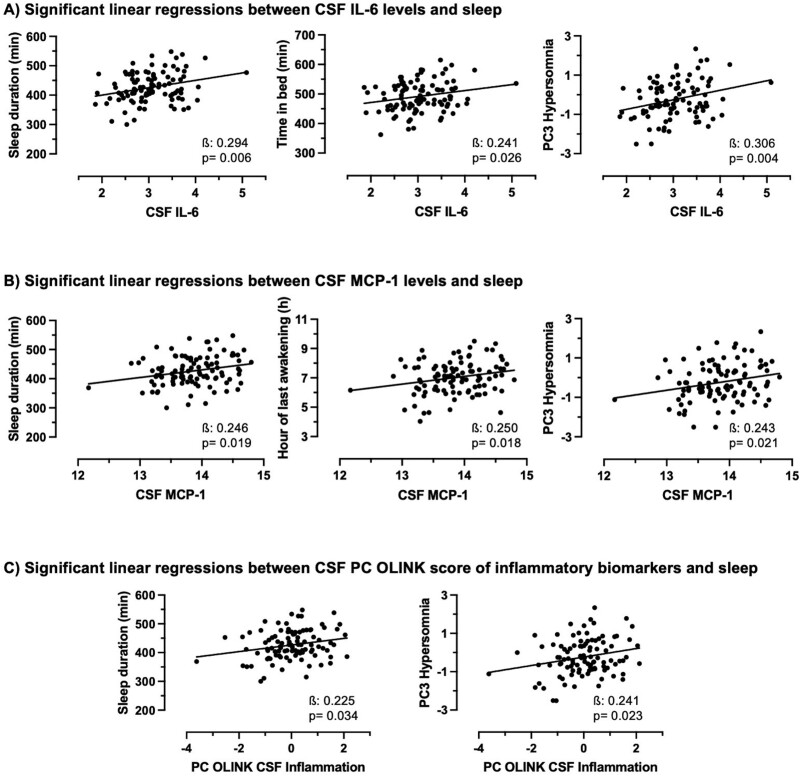
Higher CSF levels of IL-6, MCP-1, and the PC-OLINK inflammatory score were all associated with longer sleep duration and higher PC3-Hypersomnia (Figure 2, Supplementary Table S3, medium effect sizes). Higher CSF IL-6 was also associated with longer time in bed whereas higher CSF MCP-1 was associated with later time of morning awakening. After additional adjustments (BMI, retirement, psychoactive medications, anxio-depressive symptoms, and cardiometabolic risk factors), all associations remained statistically significant except for the associations between higher CSF MCP-1 with PC3-Hypersomnia, and higher CSF PC-OLINK inflammatory score with sleep duration and PC3-Hypersomnia, which remained at trend level (p = .057, p = .069, and p = .052, respectively) when adjusting for the Geriatric Depression Scale score. When removing statistical outliers (>3 SD, one outlier observed per CSF inflammatory marker), all associations remained significant. Time lag between CSF collection and actigraphy did not interact with CSF inflammatory biomarkers in their association with sleep characteristics. Removing the participants with poorer sleep diary quality (9.4%) did not affect the results. No other sleep characteristics or components were associated with CSF inflammatory individual biomarkers or the inflammatory score.
Figure 2.
(A) Significant associations between higher CSF IL-6 with longer sleep duration, longer time in bed, and higher PC3-Hypersomnia score. (B) Significant associations between higher CSF MCP-1 with longer sleep duration, later time of last morning awakening, and higher PC3-Hypersomnia score. (C) Significant associations between higher CSF PC-OLINK of inflammatory biomarkers with longer sleep duration and higher PC3-Hypersomnia score. All analyses are adjusted for age, sex and time interval between CSF collection and actigraphy. Betas are standardized. CSF, cerebrospinal fluid; PC3-Hypersomnia, third principal component of sleep characteristics representing a hypersomnia sleep pattern; PC-OLINK, single solution principal component of OLINK inflammatory biomarkers in the CSF.
A one-way ANOVA revealed that those with longer sleep duration (>8 hours) had significantly higher IL-6 (p = .032) than those with short (<7 hours) and average (7–8 hours) sleep duration, but no such association was observed for MCP-1 and the PC-OLINK inflammatory score.
Interaction between EYO and inflammation on sleep patterns
Interaction by EYO scores were performed to further evaluate the association between inflammation (CSF IL-6 and PC-OLINK inflammatory score) with sleep patterns (sleep duration and PC3-Hypersomnia component). These specific variables were selected for further analyses because they represented most of the associations observed in primary models, and covered both hypothesis-driven (IL-6, sleep duration) and data-driven (principal components) variables. A significant interaction was observed between EYO and CSF PC-OLINK inflammatory score in its association with PC3-Hypersomnia (p = .017). When stratifying by EYO, we observed that higher CSF PC-OLINK was associated with higher PC3-Hypersomnia only in those with an EYO < 5 (includes those whose age exceeded their parental age at onset; β = 0.577, p < .001), whereas it was not observed in those with further EYO > 5 (β = 0.141, p = .345). No other interaction by EYO were observed between selected CSF inflammatory variables and sleep patterns.
Interactions by other markers of AD progression
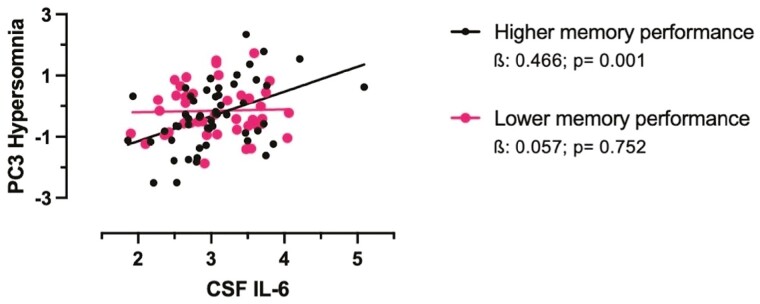
We evaluated the interaction of markers of AD progression in the association between inflammation (IL-6, PC-OLINK inflammatory score) with sleep patterns (sleep duration and PC3 Hypersomnia). Delayed recall memory performance interacted with CSF IL-6 levels and with PC-OLINK inflammatory score in their association with PC3-Hypersomnia (p = .033 and .042): The association between higher CSF IL-6 levels with higher PC3-Hypersomnia was only observed in those with better-delayed recall memory performance (β = 0.466, p = .001, Figure 3), whereas it was not in those with poorer delayed memory performance (β = 0.057, p = .752). In like fashion, the association between higher CSF PC-OLINK inflammatory score with higher PC3-Hypersomnia was only observed in those with better delayed memory performance (β = 0.358, p = .012), whereas it was not in those with poorer delayed memory performance (β = −0.005, p = .975).
Figure 3.
Significant associations between higher CSF IL-6 with higher PC3-Hypersomnia were observed only in those with better delayed recall memory performance (RBANS delayed recall memory, median split). All analyses are adjusted for age, sex and time interval between CSF collection and actigraphy. Betas are standardized. CSF, cerebrospinal fluid; PC3-Hypersomnia, the third principal component of sleep characteristics representing a sleep pattern suggestive of hypersomnia.
Similar interactions were observed between CSF IL-6 levels and MCI progressor status. The concurrent presence or future development of MCI interacted with CSF IL-6 levels in their association with sleep duration (p = .027) and PC3-Hypersomnia score (p = .009): the association between higher CSF IL-6 levels with longer sleep duration and PC3-Hypersomnia was only observed in MCI non-progressors (β = 0.384, p = .001; β = 0.396, p = .001), whereas it was not in those with concurrent or future MCI (β = −0.455, p = .250; β = −0.473, p = .215).
Finally, we observed an interaction at a trend level between APOE4 carrier status and CSF PC-OLINK inflammatory score (p = .056) in its association with sleep duration. Only carriers demonstrated an association between higher CSF PC-OLINK score and longer sleep duration (β = 0.454, p = .008), which was not observed in non-carriers (β = 0.082, p = .553).
CSF AD biomarker levels did not interact with CSF IL-6 levels or CSF PC-OLINK in their association with sleep duration or PC3-Hypersomnia.
Discussion
We observed that proximity to, or exceeding, parental age of onset for sporadic AD was associated in dementia-free individuals with a sleep pattern suggestive of hypersomnia characterized by longer sleep duration. The same sleep pattern was associated with elevated CSF inflammatory biomarkers, including IL-6 and MCP-1. By contrast, composite scores indicating disrupted sleep (PC1) or phase delay (PC2) were not associated with EYO or biomarkers of neuroinflammation. We further observed that some of the associations between sleep patterns suggestive of hypersomnia and elevated neuroinflammatory biomarkers were observed specifically in those within 5 years of (or exceeding) their parents’ age at AD onset or in APOE4 carriers. Perhaps surprisingly, some of those sleep/neuroinflammation associations were further limited to those with better memory performance and those who did not develop MCI. One interpretation of these findings is that neuroinflammatory processes might promote the elongation of sleep as a compensatory mechanism with higher pre-symptomatic risk, and that this sleep pattern might, in turn, protect cognitive function. Other interpretations are certainly possible, and the investigation of the causes of longer sleep duration in those potentially in the preclinical stages of AD is warranted.
To the best of our knowledge, this is the first study to evaluate sleep patterns in association with EYO. A few studies have used various measures to investigate sleep and/or inflammation in the different steps of the AD trajectory. One investigator group showed that self-reported sleep duration and time in bed increased progressively throughout the years before clinical AD onset [35]. Likewise, in dementia-free middle-aged individuals with a parental history of AD, CSF intercellular adhesion molecule 1 (ICAM-1) levels were associated with better sleep quality in African Americans [36]. One group computed the ratio of CSF MCP-1 on Aβ42 to investigate concomitant elevated neuroinflammation and amyloid deposition. They observed that higher CSF MCP-1/Aβ42 was associated with sleepiness in cognitively normal adults [37]. Similarly, higher CSF IL-6 and biomarkers of neurodegeneration were previously shown to be associated with higher sleepiness levels [38]. Among cognitively normal but amyloid-positive people, one group observed a U-shape association with sleep duration, where both self-reported short sleep and long sleep duration were associated with elevated CSF TREM2, another important neuroinflammatory biomarker [39]. For uncertain reasons, we did not observe an association between neuroinflammatory biomarkers and short sleep duration in our sample of potentially at-risk elderly.
Supporting the idea that longer sleep duration may mitigate adverse cognitive outcomes, we note that sleep is essential for memory functions [40], and that IL-6 is known to promote deeper sleep in both animal and human experiments studies [6]. In one series of experiments, injection of lipopolysaccharide (bacterial wall protein) was associated with a predictable cytokine response paralleled by enhanced fatigue and sleepiness [41]. In hospitalized older men, higher plasma inflammatory levels were associated with better cognitive performance only in those with longer sleep duration [42], suggesting a potential beneficial impact of inflammatory processes and long sleep duration on cognition. Similarly, we observed higher CSF inflammatory biomarkers with a longer sleep duration pattern only in those with better delayed recall memory performance and in MCI non-progressors. This sort of mechanism may no longer hold; however, in persons with dementia. One group observed that daytime napping was associated with both worse cognitive performance and higher plasma IL-6 levels in such persons [43]. An extended sleep pattern is associated with increased dementia-specific mortality over 13 years [44], and longer sleep duration is associated with increased risk of all-cause dementia in persons already experiences MCI [45]. In such persons at least, elongation of sleep may indicate more aggressive progression of AD, and not necessarily protect cognitive functions.
Neuroinflammatory processes appear to be in a delicate balance that promotes debris clearance, recovery, and repair to some extent, but can become detrimental and contribute to neuronal loss, enhanced pathology, and further neurodegeneration [46]. Our previous work has shown this sort of “delicate balance” where elevation of CSF inflammatory biomarkers is characteristic during tau pathogenesis, but is diminished in those who have developed only amyloid pathology [8]. Indeed, in some instances, inflammation may have beneficial effects to deter ongoing pathological events [20, 47–49]. Elevated brain IL-6 and MCP-1 levels have been associated with resiliency to AD pathology, suggesting that these may play an important role in clearance and inflammation resolution [20]. For example, higher CSF IL-6 levels have been associated with slower cognitive decline in AD [18], and with cognitive preservation in amyloid-positive (but tau-negative) APOE4 carriers [50]. It is unknown, but possible, that some of these protective effects could be mediated by elongation of sleep duration, which is well known to be important for cognitive function, brain health, and glymphatic clearance [10, 40].
The interplay between sleep quantity, inflammation, and AD is complex. Both short and long sleep duration as well as both lower and higher levels of inflammation were associated with higher AD risk and progression [1–3, 8, 47–49, 51–54], albeit at different points in the AD trajectory. What complexifies these relationships even more are the hypothesized bidirectional relationships between all these pathological concepts: disrupted sleep promotes low-grade inflammation and inflammation regulates sleep [6, 41, 55–58], whereas inflammation and disrupted sleep are thought of as both risk factors of AD and consequences of AD pathology [59, 60]. Therefore, the directionality of the associations observed here remains unknown. We have interpreted our findings according to the hypothesis that neuroinflammatory processes promote the elongation of sleep patterns in individuals at risk for AD. However, it remains a possibility that other factors could be related to both phenomena, such as neurodegenerative processes damaging sleep–wake structures and promoting inflammation, or comorbidities (e.g. depression and cardiovascular diseases) associated with elevated AD risk could extend sleep duration and elevate inflammation. Alternatively, longer sleep duration could promote elevated inflammatory processes. However, mechanisms explaining how longer sleep could promote neuroinflammation and enhanced AD risk in the literature are unclear, and potentially unlikely.
Strengths and limitations
The main strengths of our study include the invaluable PREVENT-AD cohort of older persons who were cognitively intact at enrollment but could bear a high genetic risk of developing AD. The present study of events during the potential preclinical stages of AD pathogenesis is an example of the rationale for the construction and longitudinal observation of the cohort. On the other hand, our study is limited by its lack of polysomnography to assess OSA, which is associated with both AD risk and inflammation [61, 62]. To offset this limitation somewhat, we analyze the confounding role of BMI, which is closely linked to the pathophysiology and intensity of OSA [32]. These analyses did not appear to show an important role for BMI in our models. OSA does result in sleep fragmentation, but it is intuitively unlikely that it would promote the sleep patterns suggestive of hypersomnia that proved to be important in our work. Moreover, there might have been a selection bias in participants accepting to undergo CSF collection.
Conclusions
Both proximity to parental age of sporadic AD onset and higher neuroinflammatory biomarker levels were associated with a pattern of longer sleep duration akin to hypersomnia. These novel findings shed a light on the potential underpinnings of longer sleep duration in the likely preclinical stage of AD. At preclinical AD stages represented in at least part of our sample, neuroinflammatory processes and prolonged sleep duration may mitigate the cognitive consequences of the ongoing AD process. Further study of the causes and consequences of sleep dysfunction in preclinical AD appears to be well warranted.
Supplementary Material
Acknowledgments
We wish to thank Jennifer Tremblay-Mercier, Doris Dea, Justin Miron, and Louise Théroux for their individual contribution at different stages of the project. Data used in preparation of this article were obtained from the program of PRe-symptomatic EValuation of Novel or Experimental Treatments for Alzheimer’s Disease (PREVENT-AD) at the Center for Studies on Prevention of Alzheimer’s Disease (StoP-AD). PREVENT-AD is the result of efforts of many other co-investigators from a range of academic institutions and private corporations, as well as an extraordinarily dedicated and talented clinical and technical assistants, students, and post-doctoral fellows. A complete listing of the PREVENT-AD Research Group can be found at: https://prevent-alzheimer.net/?page_id=94&lang=en. The authors acknowledge the generosity and commitment of all research participants who volunteered for this work and placed their trusts and hope in this research program. These findings were previously presented in an abstract form at the Alzheimer’s Association International Conference. The work was performed at the Douglas Mental Health University Institute.
Contributor Information
Andrée-Ann Baril, Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Coeur de Montréal, CIUSSS-NIM, Montréal, QC, Canada; Department of Medicine, Université de Montréal, Montréal, QC, Canada.
Cynthia Picard, Center for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute, McGill University, Montreal, QC, Canada.
Anne Labonté, Center for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute, McGill University, Montreal, QC, Canada.
Erlan Sanchez, Sunnybrook Research Institute, University of Toronto, Toronto, ON, Canada.
Catherine Duclos, Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Coeur de Montréal, CIUSSS-NIM, Montréal, QC, Canada; Department of Anesthesiology and Pain Medicine, Université de Montréal, Montréal, QC, Canada.
Béry Mohammediyan, Center for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute, McGill University, Montreal, QC, Canada.
John C S Breitner, Center for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute, McGill University, Montreal, QC, Canada.
Sylvia Villeneuve, Center for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute, McGill University, Montreal, QC, Canada.
Judes Poirier, Center for Studies on Prevention of Alzheimer's Disease, Douglas Mental Health University Institute, McGill University, Montreal, QC, Canada.
PREVENT-AD Research Group:
Sylvia Villeneuve, Mallar Chakravarty, Nathan Spreng, Véronique Bohbot, Louis Collins, Alan Evans, Rick Hoge, Jamie Near, Natasha Rajah, Jean-Paul Soucy, Sylvain Baillet, Judes Poirier, Daniel Auld, Gerhard Multhaup, Claudio Cuello, David G Morgan, Nathalie Arbour, John Breitner, Maiya Geddes, Simon Ducharme, Andrée-Ann Baril, Pedro Rosa-Neto, Samir Das, Cécile Madjar, Justin Kat, Jennifer Tremblay-Mercier, Stephanie Dyke, Yasser Iturria Medina, Jeannie-Marie Leoutsakos, Kaj Blennow, Henrik Zetterberg, Michelle M Mielke, Rik Ossenkoppele, Philippe Amouyel, Anne Labonté, Cynthia Picard, Christine Tardif, Lisa-Marie Münter, Pierre Orban, Vladimir Fonov, Holly Newbold, Masha Dadar, Pierre-François Meyer, Stéphanie Tullo, and Étienne Vachon-Presseau
Data Availability
Parts of the PREVENT-AD cohort data is open access and available online through data releases following requests (https://prevent-alzheimer.net/ and https://openpreventad.loris.ca/). Remaining data can be provided upon request.
Disclosure Statement
Financial disclosure: JP serves as a scientific advisor to the Alzheimer Society of France, outside of the submitted work. AB received speaker fees from Eisai, outside of the submitted work. All other authors have nothing to disclose.
Nonfinancial disclosure: none.
Funding
JP is supported by the Fonds de recherche en santé du Québec (FRSQ), the Canadian Institutes of Health Research (CIHR) (# PJT 153287, 178210), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the J.L. Levesque Foundation. SV is supported by the FRQS, the Alzheimer’s Association, the CIHR (PJT: 178385) and Brain Canada. J.C.S. Breitner is supported by the CIHR (PJT: 451830). AAB is supported by the Banting Fellowship Program (#454104), the Alzheimer Society of Canada and the CIHR. ES is supported by a fellowship from the CIHR. CD is supported by the FRQS, the NSERC (RGPIN-2022-04220), the Brain Canada, a Healthy Brain for Healthy Lives Neuro-Commercialization Grant, the International Anesthesia Research Society, and the CIHR (480995). As foundations and government granting agencies, the funding sources played no role in the conduct of the research nor the decision to publish.
COLLABORATORS IN THE PREVENT-AD RESEARCH GROUP
A complete listing of the PREVENT-AD Research Group can be found at: https://prevent-alzheimer.net/?page_id=94&lang=en. The current list of the PREVENT-AD Research Group on May 27th, 2023 is listed below:
Sylvia Villeneuve, Mallar Chakravarty, Nathan Spreng, Véronique Bohbot, Louis Collins, Alan Evans, Rick Hoge, Jamie Near, Natasha Rajah, Jean-Paul Soucy, Sylvain Baillet, Judes Poirier, Daniel Auld, Gerhard Multhaup, Claudio Cuello, David G. Morgan, Nathalie Arbour, John Breitner, Maiya Geddes, Simon Ducharme, Andrée-Ann Baril, Pedro Rosa-Neto, Samir Das, Cécile Madjar, Justin Kat, Jennifer Tremblay-Mercier, Stephanie Dyke, Yasser Iturria Medina, Jeannie-Marie Leoutsakos, Kaj Blennow, Henrik Zetterberg, Michelle M. Mielke, Rik Ossenkoppele, Philippe Amouyel, Anne Labonté, Cynthia Picard, Christine Tardif, Lisa-Marie Münter, Pierre Orban, Vladimir Fonov, Holly Newbold-Fox, Masha Dadar, Pierre-François Meyer, Stéphanie Tullo, Étienne Vachon-Presseau,
References
- 1. Chen JC, Espeland MA, Brunner RL, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimers Dement. 2016;12(1):21–33. doi: 10.1016/j.jalz.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liang Y, Qu LB, Liu H.. Non-linear associations between sleep duration and the risks of mild cognitive impairment/dementia and cognitive decline: a dose-response meta-analysis of observational studies. Aging Clin Exp Res. 2019;31(3):309–320. doi: 10.1007/s40520-018-1005-y [DOI] [PubMed] [Google Scholar]
- 3. Ohara T, Honda T, Hata J, et al. Association between daily sleep duration and risk of dementia and mortality in a Japanese community. J Am Geriatr Soc. 2018;66(10):1911–1918. doi: 10.1111/jgs.15446 [DOI] [PubMed] [Google Scholar]
- 4. Ahnaou A, Drinkenburg W.. Sleep, neuronal hyperexcitability, inflammation and neurodegeneration: does early chronic short sleep trigger and is it the key to overcoming Alzheimer’s disease? Neurosci Biobehav Rev. 2021;129:157–179. doi: 10.1016/j.neubiorev.2021.06.039 [DOI] [PubMed] [Google Scholar]
- 5. Oh JY, Walsh CM, Ranasinghe K, et al. Subcortical neuronal correlates of sleep in neurodegenerative diseases. JAMA Neurol. 2022;79(5):498–508. doi: 10.1001/jamaneurol.2022.0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Besedovsky L, Lange T, Haack M.. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradburn S, Murgatroyd C, Ray N.. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res Rev. 2019;50:1–8. doi: 10.1016/j.arr.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 8. Meyer PF, Savard M, Poirier J, et al.; Alzheimer’s Disease Neuroimaging Initiative. Bi-directional association of cerebrospinal fluid immune markers with stage of Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2018;63(2):577–590. doi: 10.3233/JAD-170887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He L, Yang N, Ping F, et al. Long sleep duration is associated with increased high-sensitivity c-reactive protein: a nationwide study on chinese population. Diabetes Metab Syndr Obes. 2020;13:4423–4434. doi: 10.2147/DMSO.S265465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moreno-Frias C, Figueroa-Vega N, Malacara JM.. Sleep extension increases the effect of caloric restriction over body weight and improves the chronic low-grade inflammation in adolescents with obesity. J Adolesc Health. 2020;66(5):575–581. doi: 10.1016/j.jadohealth.2019.11.301 [DOI] [PubMed] [Google Scholar]
- 12. Livingston WS, Rusch HL, Nersesian PV, Baxter T, Mysliwiec V, Gill JM.. Improved sleep in military personnel is associated with changes in the expression of inflammatory genes and improvement in depression symptoms. Front Psychiatry. 2015;6:59. doi: 10.3389/fpsyt.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breitner JCS, Poirier J, Etienne PE, Leoutsakos JM.. Rationale and structure for a new center for Studies on Prevention of Alzheimer’s Disease (StoP-AD). J Prev Alzheimers Dis. 2016;3(4):236–242. doi: 10.14283/jpad.2016.121 [DOI] [PubMed] [Google Scholar]
- 14. Green RC, Cupples LA, Go R, et al.; MIRAGE Study Group. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–336. doi: 10.1001/jama.287.3.329 [DOI] [PubMed] [Google Scholar]
- 15. Villeneuve S, Vogel JW, Gonneaud J, et al.; Presymptomatic Evaluation of Novel or Experimental Treatments for Alzheimer Disease (PREVENT-AD) Research Group. Proximity to parental symptom onset and amyloid-beta burden in sporadic Alzheimer disease. JAMA Neurol. 2018;75(5):608–619. doi: 10.1001/jamaneurol.2017.5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wingo TS, Lah JJ, Levey AI, Cutler DJ.. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol. 2012;69(1):59–64. doi: 10.1001/archneurol.2011.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168 [DOI] [PubMed] [Google Scholar]
- 18. Taipa R, das Neves SP, Sousa AL, et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol Aging. 2019;76:125–132. doi: 10.1016/j.neurobiolaging.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 19. Lee WJ, Liao YC, Wang YF, Lin IF, Wang SJ, Fuh JL.. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Sci Rep. 2018;8(1):1280. doi: 10.1038/s41598-018-19807-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barroeta-Espar I, Weinstock LD, Perez-Nievas BG, et al. Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol Dis. 2019;121:327–337. doi: 10.1016/j.nbd.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Del Giudice M, Gangestad SW.. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 22. Sawyer AJ, Tian W, Saucier-Sawyer JK, et al. The effect of inflammatory cell-derived MCP-1 loss on neuronal survival during chronic neuroinflammation. Biomaterials. 2014;35(25):6698–6706. doi: 10.1016/j.biomaterials.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowe DA, Balsis S, Benge JF, Doody RS.. Adding delayed recall to the ADAS-cog improves measurement precision in mild Alzheimer’s disease: implications for predicting instrumental activities of daily living. Psychol Assess. 2015;27(4):1234–1240. doi: 10.1037/pas0000133 [DOI] [PubMed] [Google Scholar]
- 24. Weissberger GH, Strong JV, Stefanidis KB, Summers MJ, Bondi MW, Stricker NH.. Diagnostic accuracy of memory measures in alzheimer’s dementia and mild cognitive impairment: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(4):354–388. doi: 10.1007/s11065-017-9360-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao C, Li P, Morris CJ, et al. Actigraphy-based sleep detection: validation with polysomnography and comparison of performance for nighttime and daytime sleep during simulated shift work. Nat Sci Sleep. 2022;14:1801–1816. doi: 10.2147/NSS.S373107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Folstein MF, Robins LN, Helzer JE.. The mini-mental state examination. Arch Gen Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016 [DOI] [PubMed] [Google Scholar]
- 27. Randolph C, Tierney MC, Mohr E, Chase TN.. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 28. Lleo A, Alcolea D, Martinez-Lage P, et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimers Dement. 2019;15(6):742–753. doi: 10.1016/j.jalz.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 29. Hansson O, Seibyl J, Stomrud E, et al.; Swedish BioFINDER study group. CSF biomarkers of Alzheimer’s disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470–1481. doi: 10.1016/j.jalz.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blennow K, Shaw LM, Stomrud E, et al. Predicting clinical decline and conversion to Alzheimer’s disease or dementia using novel Elecsys Abeta(1-42), pTau and tTau CSF immunoassays. Sci Rep. 2019;9(1):19024. doi: 10.1038/s41598-019-54204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park JE, Choi KY, Kim BC, et al. Cerebrospinal fluid biomarkers for the diagnosis of prodromal alzheimer’s disease in amnestic mild cognitive impairment. Dement Geriatr Cogn Dis Extra. 2019;9(1):100–113. doi: 10.1159/000496920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ciavarella D, Tepedino M, Chimenti C, et al. Correlation between body mass index and obstructive sleep apnea severity indexes - a retrospective study. Am J Otolaryngol. 2018;39(4):388–391. doi: 10.1016/j.amjoto.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 33. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al.; Alzheimer's Disease Neuroimaging Initiative. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim JH, Lim H, Lee JU, et al.; Alzheimer's Disease Neuroimaging Initiative. Cerebrospinal biomarker cut-off methods defined only by Alzheimer’s disease predict more precisely conversions of mild cognitive impairment. Dement Neurocogn Disord. 2017;16(4):114–120. doi: 10.12779/dnd.2017.16.4.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cavailles C, Carriere I, Wagner M, et al. Trajectories of sleep duration and timing before dementia: a 14-year follow-up study. Age Ageing. 2022;51(8):afac186. doi: 10.1093/ageing/afac186 [DOI] [PubMed] [Google Scholar]
- 36. Pak VM, Paul S, Swieboda D, Balthazar MS, Wharton W.. Sleep duration and biomarkers of inflammation in African American and white participants with a parental history of Alzheimer’s disease. Alzheimers Dement (N Y). 2022;8(1):e12332. doi: 10.1002/trc2.12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sprecher KE, Koscik RL, Carlsson CM, et al. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89(5):445–453. doi: 10.1212/WNL.0000000000004171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carvalho DZ, St Louis EK, Przybelski SA, et al. Sleepiness in cognitively unimpaired older adults is associated with csf biomarkers of inflammation and axonal integrity. Front Aging Neurosci. 2022;14:930315. doi: 10.3389/fnagi.2022.930315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu HY, Ma LZ, Hu H, et al. Associations of sleep characteristics with cerebrospinal fluid strem2 in cognitively normal older adults: the CABLE Study. Neurotox Res. 2021;39(4):1372–1380. doi: 10.1007/s12640-021-00383-5 [DOI] [PubMed] [Google Scholar]
- 40. Rasch B, Born J.. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lasselin J, Karshikoff B, Axelsson J, et al. Fatigue and sleepiness responses to experimental inflammation and exploratory analysis of the effect of baseline inflammation in healthy humans. Brain Behav Immun. 2020;83:309–314. doi: 10.1016/j.bbi.2019.10.020 [DOI] [PubMed] [Google Scholar]
- 42. Dzierzewski JM, Song Y, Fung CH, et al. Self-reported sleep duration mitigates the association between inflammation and cognitive functioning in hospitalized older men. Front Psychol. 2015;6:1004. doi: 10.3389/fpsyg.2015.01004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Basta M, Koutentaki E, Vgontzas A, et al. Objective daytime napping is associated with disease severity and inflammation in patients with mild to moderate dementia1. J Alzheimers Dis. 2020;74(3):803–815. doi: 10.3233/JAD-190483 [DOI] [PubMed] [Google Scholar]
- 44. Benito-Leon J, Louis ED, Villarejo-Galende A, Romero JP, Bermejo-Pareja F.. Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES). Neurology. 2014;83(17):1530–1537. doi: 10.1212/WNL.0000000000000915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Westwood AJ, Beiser A, Jain N, et al. Prolonged sleep duration as a marker of early neurodegeneration predicting incident dementia. Neurology. 2017;88(12):1172–1179. doi: 10.1212/WNL.0000000000003732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Novoa C, Salazar P, Cisternas P, et al. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol Res. 2022;55(1):39. doi: 10.1186/s40659-022-00404-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gabin JM, Saltvedt I, Tambs K, Holmen J.. The association of high sensitivity C-reactive protein and incident Alzheimer disease in patients 60 years and older: The HUNT study, Norway. Immun Ageing. 2018;15:4. doi: 10.1186/s12979-017-0106-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lima TA, Adler AL, Minett T, Matthews FE, Brayne C, Marioni RE; Medical Research Council Cognitive Function and Ageing Study. C-reactive protein, APOE genotype and longitudinal cognitive change in an older population. Age Ageing. 2014;43(2):289–292. doi: 10.1093/ageing/aft193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ritchie K, Carriere I, Berr C, et al. The clinical picture of Alzheimer’s disease in the decade before diagnosis: clinical and biomarker trajectories. J Clin Psychiatry. 2016;77(3):e305–e311. doi: 10.4088/JCP.15m09989 [DOI] [PubMed] [Google Scholar]
- 50. Motta C, Finardi A, Toniolo S, et al. Protective role of cerebrospinal fluid inflammatory cytokines in patients with amnestic mild cognitive impairment and early alzheimer’s disease carrying apolipoprotein E4 genotype. J Alzheimers Dis. 2020;76(2):681–689. doi: 10.3233/JAD-191250 [DOI] [PubMed] [Google Scholar]
- 51. Fan L, Xu W, Cai Y, Hu Y, Wu C.. Sleep duration and the risk of dementia: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc. 2019;20(12):1480–1487.e5. doi: 10.1016/j.jamda.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 52. Bubu OM, Brannick M, Mortimer J, et al. Sleep, Cognitive impairment, and Alzheimer’s disease: a Systematic Review and Meta-Analysis. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw032 [DOI] [PubMed] [Google Scholar]
- 53. Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, Hofman A.. Inflammatory markers and the risk of dementia and Alzheimer’s disease: a meta-analysis. Alzheimers Dement. 2018;14(11):1450–1459. doi: 10.1016/j.jalz.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 54. Zheng F, Xie W.. High-sensitivity C-reactive protein and cognitive decline: the English Longitudinal Study of Ageing. Psychol Med. 2018;48(8):1381–1389. doi: 10.1017/S0033291717003130 [DOI] [PubMed] [Google Scholar]
- 55. Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348–355. doi: 10.1016/j.nbd.2012.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wisor JP, Schmidt MA, Clegern WC.. Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. Sleep. 2011;34(3):261–272. doi: 10.1093/sleep/34.3.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weinberger JF, Raison CL, Rye DB, et al. Inhibition of tumor necrosis factor improves sleep continuity in patients with treatment resistant depression and high inflammation. Brain Behav Immun. 2015;47:193–200. doi: 10.1016/j.bbi.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Irwin MR, Olmstead R, Carroll JE.. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang C, Holtzman DM.. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45(1):104–120. doi: 10.1038/s41386-019-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT.. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leng Y, McEvoy CT, Allen IE, Yaffe K.. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 2017;74(10):1237–1245. doi: 10.1001/jamaneurol.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rocchi C, Valentina C, Totaro V, et al. Inflammation markers in moderate and severe obstructive sleep apnea: the influence of sex. Sleep Breath. 2022;26(4):1703–1709. doi: 10.1007/s11325-021-02537-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Parts of the PREVENT-AD cohort data is open access and available online through data releases following requests (https://prevent-alzheimer.net/ and https://openpreventad.loris.ca/). Remaining data can be provided upon request.