Abstract
Ascorbic acid functions as an antioxidant and facilitates other biochemical processes such as collagen triple helix formation, and iron uptake by cells. Animals which endogenously produce ascorbic acid have a functional gulonolactone oxidase gene (GULO); however, humans have a GULO pseudogene (GULOP) and depend on dietary ascorbic acid. In this study, the conservation of GULOP sequences in the primate haplorhini suborder were investigated and compared to the GULO sequences belonging to the primates strepsirrhini suborder. Phylogenetic analysis suggested that the conserved GULOP exons in the haplorhini primates experienced a high rate of mutations following the haplorhini/strepsirrhini divergence. This high mutation rate has decreased during the evolution of the haplorhini primates. Additionally, indels of the haplorhini GULOP sequences were conserved across the suborder. A separate analysis for GULO sequences and well-conserved GULOP sequences focusing on placental mammals identified an in-frame GULO sequence in the Brazilian guinea pig, and a potential GULOP sequence in the pika. Similar to haplorhini primates, the guinea pig and lagomorph species have experienced a high substitution rate when compared to the mammals used in this study. A shared synteny to examine the conservation of local genes near GULO/GULOP identified a conserved inversion around the GULO/GULOP locus between the haplorhini and strepsirrhini primates. Fischer’s exact test did not support an association between GULOP and the chromosomal inversion. Mauve alignment showed that the inversion of the length of the syntenic block that the GULO/GULOP genes belonged to was variable. However, there were frequent rearrangements around ~ 2 million base pairs adjacent to GULOP involving the KIF13B and MSRA genes. These data may suggest that genes acquiring deleterious mutations in the coding sequence may respond to these deleterious mutations with rapid substitution rates.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00239-024-10165-0.
Keywords: Ascorbic acid, Vitamin C, GULO, GULOP, Neanderthal, Primates, Phylogeny
Introduction
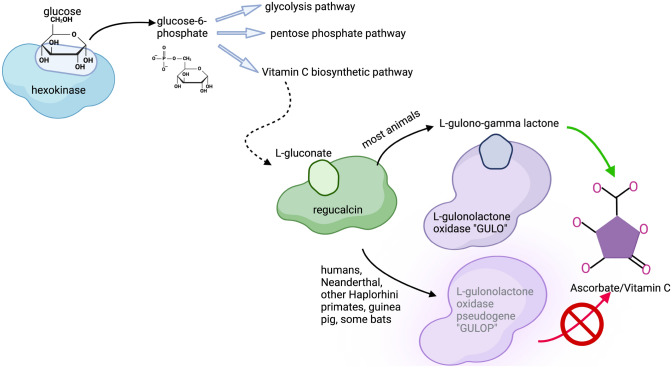
The gulonolactone oxidase gene (GULO) encodes L-gulonolactone oxidase (Gulo), a key enzyme in animals that provides an alternative metabolic pathway for D-glucuronate degradation (Chatterjee et al. 1960; Nishikimi and Yagi 1991). In this alternative pathway, the enzyme regucalcin commits the intermediate molecule L-gulonate to L-gulono-1,4-lactone which can be converted to L-ascorbate (vitamin C) by the Gulo enzyme (Fig. 1).
Fig. 1.
Ascorbic acid synthesis pathway in the presence of GULO or GULOP. Glucose is the starting carbohydrate for ascorbic acid synthesis. Phosphorylation of glucose by hexokinase forms glucose-6-phosphate which can be shuttled to the ascorbic acid synthesis pathway or used in glycolysis or the pentose phosphate pathway. In animals that have a functional GULO gene, the L-gulono-gamma lactone precursor is converted to ascorbate (vitamin C), but in animals with a GULOP gene, the pathway is non-functional. Those animals require dietary vitamin C and are prone to scurvy in its absence
Despite the importance of L-gulonolactone oxidase in ascorbic acid synthesis, some animals cannot endogenously synthesize ascorbic acid, and consequently these animals may develop scurvy if their diets lack significant sources of ascorbic acid (Chatterjee et al. 1960; Drouin et al. 2011; Linster and Van Schaftingen 2007; Nishikimi et al. 1992; Nishikimi and Yagi 1991). Dr. James Lind, in what was considered the first human clinical trial, demonstrated that sailors given a ration of oranges and lemons were cured of scurvy within about six days of eating them (Lind 1753). The lack of a Gulo enzyme was first documented by Grollman and Lehninger in 1957 (Grollman and Lehninger 1957) when they found that the liver in mammals and the kidney in birds and reptiles contains the vitamin C synthetic pathway. Specifically, they identified the three enzymes and vitamin C synthesis in rat, mouse, rabbit, dog, cat, pig, cow, chicken, pigeon, and turtle, but were unable to find Gulo in guinea pig, cynomolgus monkey, rhesus monkey and 5 different humans (Grollman and Lehninger 1957). Grollman and Lehninger noted that the four mammals which did not have the third enzyme were obligate consumers of vitamin C. Subsequent papers by Nishimiki and colleagues (Nishikimi et al. 1992, 1988; Nishikimi and Yagi 1991) identified an aberrant and non-expressing GULO gene in both humans and the domestic guinea pigs. This irregular GULO gene was identified as a unitary pseudogene of GULO (GULOP). Though some pseudogenes have functional roles (Petrov and Hartl 2000; Pink et al. 2011), GULOP does not have a known functional role. The bat genera Pteropodidae, Noctilionida, Mormoopidae, Phyloostomidae, Natalidae, Vespertilionidae, and Molossidase have lost Gulo enzymatic activity (Cui et al. 2011a) by a stepwise accumulation of mutations during GULO evolution in these species (Cui et al. 2011b). Interestingly in bats, the more recent the speciation event for the bat, the more accumulated mutations were identified, with Cui and colleagues hypothesizing that the GULO gene was becoming redundant in bats (Cui et al. 2011b). A diagram of the ascorbic acid synthesis pathway is shown in Fig. 1.
There are two primate suborders which are the haplorhini and strepsirrhini primates. These suborders are estimated to have diverged 70 million-years-ago (MYA) (Pozzi et al. 2014). GULOP is found in all haplorhini primates while the strepsirrhini primates have a functional GULO gene (Drouin et al. 2011). Within the haplorhini suborder are the hominid primates consisting of the genera Pongo, Gorilla, Pan, and Homo which are predicted to have diverged 12–16 MYA (Pozzi et al. 2014). Though pseudogenes like GULOP have equal synonymous and nonsynonymous mutation rates (Lachapelle and Drouin 2011; Nishikimi and Yagi 1991), this relatively short divergence time among the hominids suggests that there may be high GULOP sequence similarity between the genera.
Potential Neanderthal bones date from 350,000 years ago, but most fossils are from 130,000 years and earlier, with the transition to modern humans ~ 40,000 years ago (Villa and Roebroeks 2014). Thus, like the bat story, evolution of Hominids (which include extinct and modern Great Apes) occurs in the more recent evolutionary history. The Neanderthal genome was not available when the GULOP pseudogene was originally described. Thus, the question arises as to whether, like bats recent hominid evolution show a similar accumulation of mutations, compared to more ancient members of the Hominidae family. The Neanderthal draft genome was established from DNA found in the Vindija Cave located in Croatia, but as a draft genome, it did not have complete coverage of all regions, including those encompassing the chromosome 8 GULO/GULOP region (Green et al. 2010). In December 2013, the complete Neanderthal genome was obtained from the toe bone of a woman from Siberia (Prufer et al. 2014). This genome has 99.9% coverage of at least tenfold, and an average of 50-fold for all unique regions of the genome (https://bioinf.eva.mpg.de/) (2015).
In this study, the GULO or GULOP genes from haplorhini and strepsirrhini primates was studied with phylogenetic analysis to examine the evolution of the remaining homologous exons to test the hypothesis that more recent speciation events in Hominidae would accumulate more variants in the GULO gene. Particular attention in this analysis was given to the Neanderthal GULOP sequence because this species most recently diverged from humans between 680,000 and 350,000 years ago (Pozzi et al. 2014; Villa and Roebroeks 2014). The GULOP sequence of the haplorhini primates has significantly degenerated from the common 12 exon and 440 amino acid long sequence. Therefore, a separate phylogenetic analysis was conducted on mammals with well-conserved GULOP sequences for a robust comparison of mutations between GULO and GULOP sequences. Lastly, the GULO and GULOP sequences are positioned on opposite strands of the haplorhini and strepsirrhini primates. We hypothesized that the strand position of GULO may be associated with the occurrence of its pseudogene among mammals. Chromosomal inversions around GULO were scored and a Fischer’s exact test was conducted which did not support our hypothesis. Despite this finding, the orientation of the syntenic CLU gene is consistently anti-parallel to GULO/GULOP, and it may be a useful landmark to identify the GULO/GULOP gene in mammalian genomes where it is not annotated.
Material and Methods
Sequence Acquisition
GULO and the human GULOP sequences were acquired from Ensembl™ Release 107 (Zerbino et al. 2018) or from NCBI (Supplemental File 1). The Altai Neanderthal chromosome 8 sequence was acquired from the Max Plank Institute for Evolutionary Anthropology (Green et al. 2010; Prufer et al. 2014). These data are made freely available for individuals that are studying an individual gene or individual features of the genome. The Integrative Genomics Viewer (IGV; https://software.broadinstitute.org/software/igv/) was used to align the human chromosome 8 sequence with the Neanderthal chromosome 8 sequence (Robinson et al. 2017). SNVs in the Neanderthal GULOP were identified by a 90% deviance allele threshold from the reference sequence.
GULOP sequences were identified by BLAT analysis with a GULO gene or GULOP sequence from a taxonomically similar species. BLAT differs from BLAST in that BLAT allows for faster analysis by building an index of the database as opposed to indexing the query sequence, and BLAT can account for exon/intron splice sites in RNA and DNA alignments (Kent 2002). If nucleotides were missing on either the 5’ or 3’ ends of the BLAT hit when compared to GULO exon sequences, an additional set of nucleotides corresponding to the missing nucleotides were added after BLAT analysis. All BLAT hits (edited or unedited) were then reciprocally BLAT against the former query sequence (Supplemental File 1). Only the nucleotide sequences aligned to the reciprocal BLAT were accepted as GULOP sequences. For example, a BLAT using human GULOP as the query against the Gorilla genome was used. A preliminary Gorilla GULOP sequence was acquired. Then, the preliminary Gorilla GULOP sequence was BLAT back to the human genome. Only the reciprocating BLAT sequence from this search was used in all analyses.
Phylogenetic Analyses
The MEGA XI: Molecular Evolutionary Genetics Analysis across computing platforms (https://www.megasoftware.net/) (Tamura et al. 2021) program was used for the generation of the multiple sequence alignments (MSA) and phylogenetic trees. The Clustal Omega algorithm with default settings was used to generate the MSA. All sites where gaps occurred were removed from the analysis. Substitution models were simulated in MEGA XI with a maximum composite likelihood statistical method and using the Tamura-Nei model. The substitution model with the lowest corrected Akaike Information Criterion (AICc) value was used for rendering Bayesian inferred phylogenies using MrBayes. MrBayes was used for phylogenetic tree rendering, and the number of iterations used was dependent upon when the average standard deviation in an analysis was below 0.01. MrBayes uses Metropolis coupling which allows for parallel sampling of phylogenetic trees using heated and cold chains (Altekar et al. 2004). The parameters Swapfreq, Nswaps, Nchains, and Temp establish the number and behavior of the heated and cold chains. These parameters were left at the default values provided by MrBayes which specifies for 3 heated chains and 1 cold chain. Additionally, one chain was swapped upon every MCMC iteration. Consensus trees were generated with a 25% burn-in, and the consensus trees were subsequently rooted in FigTree version 1.4.4.
Pairwise Distance Analysis
The pairwise distances are calculated as nucleotide substitutions per site. The Tamura 3-parameter model with a gamma rate among sites of 1 was used to calculate the pairwise distances between species. All sites where gaps were present were deleted from the analysis. Both transition and transversion substitutions were included. This analysis was conducted in MEGA XI (Tamura and Nei 1993; Tamura et al. 2021).
Exon/Intron Analysis
The exon/intron analyses were conducted with NNSPLICE 0.9. Briefly, the coding strand of a GULO or GULOP sequence of interest was submitted to NNSPLICE 0.9 which was operated with default settings.
Shared Synteny
Bat species not included in the phylogeny were included for the synteny to increase the representation of GULOP sequences in forward and reverse directions. Species represented in the gene synteny analysis are included in Supplemental File 1. All species were independently examined for the genetic rearrangement events which may have occurred around GULO/GULOP and between the genes STMN4 and NUGGC or STMN4 and BAG1. Species with identical orders, families, or genera and identical chromosome arrangements were excluded from this analysis to prevent redundant counting. Small gene fragments and non-coding genes which occurred between the synteny range were disregarded. For example, the haplorhini primates have identical genomic arrangements around GULOP with some non-coding genes interspersed between the synteny range. Therefore, only the human chromosome 8 region was included to represent all haplorhini primates. A Fischer’s exact test was conducted with R 4.0.3.
Mauve Alignments
The chromosome and scaffold assemblies where GULO or GULOP is located was downloaded as a full Genbank sequence from NCBI for human, grey mouse lemur, dog (reference genome), cat, mouse (C57Bl/6 J), domestic guinea pig, pig (reference genome), and sheep. A progressive Mauve alignment was used for whole chromosome alignment between species of the same order (Darling et al. 2010). The seed size is automatically determined by the algorithm based on genome size. This seed size is multiplied by a value of three to determine locally colinear blocks (LCBs). The LCBs are regions of similar nucleotide identity which encode orthologous genes despite genomic rearrangements along a chromosome. Using the LCB weight slider in the Mauve GUI, a new LCB weight value was determined for each alignment performed, and the analysis was repeated with the new LCB weight value to remove spuriously identified LCBs. These final alignments were used for analysis.
Results
Analysis of Primate GULO Sequences
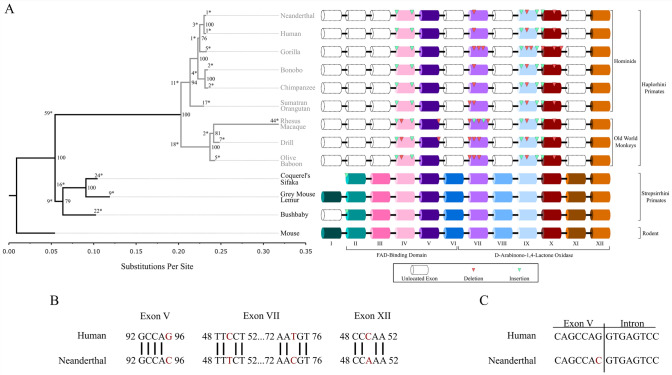
The mammalian GULO gene commonly has 12 exons which encode a 440 amino acid long protein. The first exon in mammalian GULO contains a 5’ untranslated region and the ATG start codon. The subsequent exons proceeding exon I code for the FAD-binding domain and the D-arabinono-1,4-lactone binding domain of L-gulonolactone oxidase. haplorhini GULOP sequences retain identity to exons IV, V, VII, IX, X, and XII suggesting that all other exons have degenerated beyond recognition (Fig. 2A). Phylogenetic analysis was conducted using the general time reversible (GTR) model with gamma parameter of the homologous GULO exons. The phylogeny rendered follows the current speciation events of the primate lineage. A discrepancy in the depiction of speciation events by phylogeny is the placement of the Gorilla GULOP adjacent to the genus Homo. The phylogeny depicts a greater number of substitutions per site between the haplorhini and strepsirhini primates. Indeed, there are a total of 59 conserved substitutions which occur at the node of the haplorhini suborder in the phylogeny. Within the haplorhini suborder, there is a reduction in the number of substitutions per site indicated by the phylogeny when compared to the initial haplorhini and strepsirrhini split (Fig. 2A, Supplemental File 2). Examination of the individual substitutions between the old-world monkeys and the hominids show 18 and 11 unique and conserved substitutions. These substitutions are further reduced apart from the rhesus macaque which had 44 unique substitutions when compared with all other primates. Like the substitutions, a separate examination of the indels between GULOP sequences showed conservation of some indels within the haplorhini GULOP suborder (Supplemental Fig. 1, Supplemental File 2). These findings suggest that the mutations accumulated in the GULOP exons occurred primarily at the haplorhini and strepsirrhini split.
Fig. 2.
GULO and GULOP gene structure among primates: A Phylogenetic tree showing GULO evolution among the primates. GULO/GULOP sequences were aligned in MEGA 11 with the Clustal Omega tool. Substitution matrices were calculated in MEGA11 with a complete deletion option for gaps and ambiguous sites. All sequences submitted for phylogenetic analysis had all gaps and ambiguous sites deleted. Bayesian-inferred phylogenies were rendered with 250,000 iterations in MrBayes with a GTR+G substitution model. The GTR+G model was the model with the lowest AICc value. A burn-in of 25% from the cold-chain generated phylogenies was used to generate a consensus tree. The tree was rooted to Mus musculus in FigTree version 1.4.4. A scale bar representing nucleotide substitution per site is provided. Numbers distinguished with an asterisk represent conserved substitutions at a node or unique substitutions of the species from the final alignment. Numbers without asterisks are the posterior probabilities of a node which were provided by MrBayes. An exon map showing the homologous exons of GULO and GULOP is provided next to each species. Exons in white with dashed lines are not detected. Indels are shown with light and dark triangles indicating insertions or deletions, respectively. B Alignment of GULOP exon sequences where SNVs occur between Homo sapiens and Homo neanderthalensis from untrimmed alignments. SNVs are labelled relative to their position in the exon sequence, and SNVs are indicated by the absence of a vertical line. C Exon/intron border of orthologous exon V and its 3’ intron. Exon/intron analysis was performed in NNSPLICE 0.9. Scores of 1.00 and 0.99 were computed for Homo sapiens and Homo neanderthalensis, respectively
Since the human and Neanderthal species diverged 400,000 years ago, we were curious with the extent of drift that occurred at the GULOP locus. Four SNVs were identified between humans and Neanderthals (Fig. 2B). The Neanderthal SNV G96C at exon V occurs at the last nucleotide position of this exon boundary. Nucleotide substitutions at exon/intron boundaries may affect how exons and introns are identified. We tested whether the G96C SNV in exon V would affect the exon/intron boundary with NNSPLICE version 0.9 (Fig. 2C). The G96C SNV does not significantly affect the recognition of the Neanderthal exon V boundary with its 3’ intron.
Evolution of the Mammalian GULO and GULOP Sequences
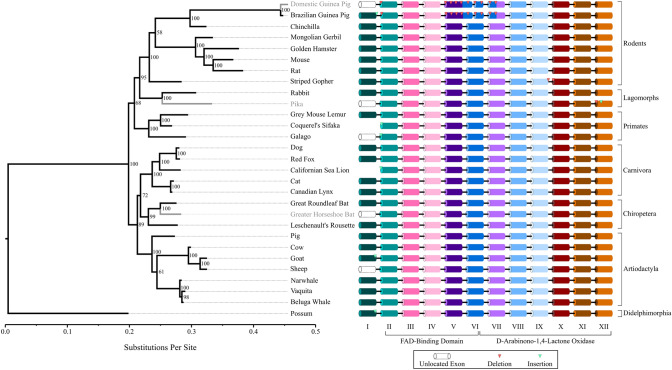
Next, we wanted to examine the evolution of other GULO and GULOP sequences with greater conservation of sequence information. A phylogeny of GULO was inferred with a GTR+G substitution matrix, which included placental mammals and the marsupial, possum, as an outgroup (Fig. 3). The haplorhini primates were not included in this analysis because a significant loss of nucleotide sequence would be incurred thus reducing the accuracy of the analysis. Phylogenetic analysis separates the species accordingly by order. In the placental mammal orders, the sequences have minor fluctuations in substitutions per site as indicated by the phylogeny, but the rodent order experiences a larger range in substitutions per site, especially in the families Muridae and Caviidae (Fig. 3, Supplemental File 2). Within the rodent order, the striped gopher GULO sequence is annotated by Ensembl™ as having two indels (Supplemental Fig. 2). NNSPLICE results suggest that there is a functional donor splice site and the 5-bp indel is an annotation error (Supplemental Fig. 3B). Additionally, the 1-bp deletion at the start of exon X in the striped gopher may be an annotation error as well, but NNSPLICE cannot identify the acceptor splice site for this exon in all species tested. Although the striped gopher is missing 6-bp from its GULO sequence, we used the Ensembl™ annotation for this analysis.
Fig. 3.
GULO and GULOP gene structure among mammals: DNA sequences of GULO and GULOP genes were acquired from Ensembl and NCBI databases. Sequences were aligned in MEGA11 with the Clustal Omega algorithm. A substitution matrix was calculated in MEGA11 with a complete deletion of gaps and ambiguous sites in the alignment. Trimmed sequences were used to generate Bayesian-inferred phylogenies with MrBayes. A GTR+G substitution model was used for modeling which had the lowest AICc value. 1,000,000 iterations were performed, and a consensus tree was drawn after discarding 25% of phylogenies from the cold chain. The phylogeny was rooted to the marsupial, possum, in FigTree 1.4.4. Exon/intron boundaries were tested with NNSPLICE 0.9. Taxa with phylogeny lines in grey contain either a pseudogene or do not express GULO. An exon schematic is provided for all species within the phylogeny where exons in white with dashed borders indicate exons which are not identified in the genome. Indels are shown by light and dark triangles indicating insertions and deletions, respectively
The domestic guinea pig has a well-described GULOP sequence, and we expected the Brazilian guinea pig to have a pseudogene as well. However, the Ensembl™ database has annotated a GULO-like gene in the genome of the Brazilian guinea pig. The main difference between the sequence of the domestic and Brazilian guinea pigs is an indel with either 2 or 1 nucleotides deleted, respectively (Supplemental Fig. 2). This 1 nucleotide deletion in the Brazilian guinea pig GULO maintains an open reading frame resulting in a putative 412 amino acid long sequence. Despite this gene being in-frame, there are significant rearrangements between exon V, exon VI, and exon VII along with numerous indels (Fig. 3, Supplemental Fig. 2). NNSPLICE version 0.9 was used to test if the resulting mRNA transcript could be properly spliced. The intron acceptor sites of these rearranged exons seem intact relative to the mouse GULO gene (Supplemental Fig. 3C). However, the exon donor splice sites in exons II, V, and VI were not identified (Supplemental Fig. 3D). Additionally, there is a high substitution rate identified in the Cavia genus which is similar to what was identified in the haplorhini primates.
Within the lagomorph order, the rabbit has an annotated GULO gene in Ensembl™, but the GULO gene in pika was not annotated by Ensembl™. Using the BLAT analysis, GULO-like exons were identified in the pika genome. We identified a 4-bp deletion and a 1-bp insertion in exon XII (Fig. 3, Supplemental Fig. 2). NNSPLICE version 0.9 was used to check if the pika exon XII acceptor site was functional (Supplemental Fig. 3A). NNSPLICE analysis suggests that the final 4-bp of the intron are duplicated and replace the 4-bp deletion identified by reciprocal BLAT. This splice site is moderately supported at the beginning of exon XII in pika. Although this 4-bp sequence was not included in the phylogenetic analysis, its inclusion would result in a frameshift mutation and the skipping of the termination codon during translation of this GULO transcript. Alternatively, the exon XII acceptor splice site of the pika GULO may not be recognized which would prevent the proper splicing of the mRNA transcript. Therefore, these results suggest that the GULO gene of pika is nonfunctional.
Similar to haplorhini primates, the chiroptera order was originally identified as having a pseudogene for GULO (Birney et al. 1976). However, previous work suggest that some bat species have spontaneously regained functionality of GULO (Drouin et al. 2011). The two bat species represented on the phylogeny have a similar substitution per site rate relative to other mammals (Fig. 3). This suggests that the GULO and GULOP sequences of the bats represented here may be a recent evolutionary event and has not diverged significantly from GULO despite transient reactivation or complete pseudogenization of the gene.
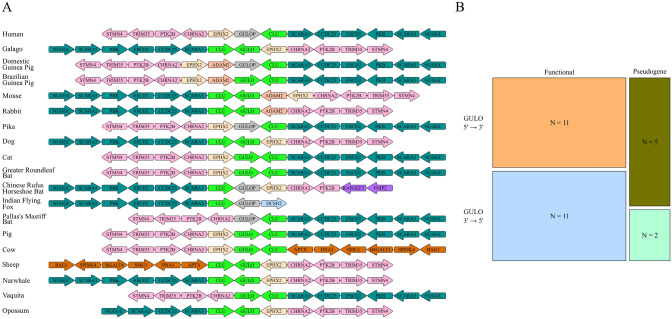
Gene Synteny Analysis of Chromosomal Inversion Around GULO
When searching for GULOP sequences in haplorhini primates, we used the syntenic CLU gene to identify where GULOP occurred in the primate genomes (Yang 2013). We noticed that the haplorhini primates examined had a chromosomal inversion of the surrounding genes relative to the strepsirrhini primates (Fig. 4A; Human and Galago). This chromosomal inversion suggests that the primate chromosome containing GULO (chromosome 8 in humans) was inverted at the haplorhini and strepsirrhini suborder split. Further examination of the chromosome region between STMN4 and NUGGC showed similar chromosomal rearrangement events occurring between the Caviidae and Muridae families and the rabbit and pika species. In the chiroptera order, there are few chromosomal assemblies for these animals, and the greater horseshoe bat previously analyzed does not have an assembly at this time. Therefore, we expanded the number of bat genomes for the synteny analysis and observed frequent chromosomal inversions around the GULO/GULOP locus (Fig. 4A).
Fig. 4.
Synteny Analysis of Vertebrate GULO/GULOP Chromosome Regions: Species chromosomes were analyzed for independent chromosome rearrangements. Species order, family, and genera were considered with respect to GULO functionality and orientation when deciding on which species to include for analysis. A Synteny with representative species showing the genetic composition from the NUGGC gene to the STMN4 gene. The synteny is centered around GULO/GULOP. Coloring indicates syntenic blocks (GULOP is colored grey to distinguish it from GULO). B A Fischer’s Exact test was performed to test the hypothesis that the GULO functionality is dependent on the GULO orientation. This test was performed with R, and a p-value of 0.41 was calculated
The syntenic blocks frequently identified are STMN4 to CHRNA2, EPHX2, and SCARA3 to NUGGC which occur around the GULO/GULOP and CLU syntenic block (Fig. 4A). The ADAM2 gene is frequently rearranged to precede the EPHX2 gene in rodents. In other animal orders, ADAM2 is not found at the chromosomal locus between NUGGC and STMN4 which suggests that ADAM2 is rearranged independently of the other syntenic blocks near GULO/GULOP. Lastly, within the Artiodactyl order, the SCARA3 and NUGGC block is rearranged with a APTX and BAG1 block. These data suggest frequent rearrangements around the GULO/GULOP locus which may impact cis-regulatory elements that act on GULO.
We further hypothesized that the inversions of GULO could be associated with the formation of GULOP. To test this hypothesis, the chromosomal and gene rearrangements belonging to each species were categorized as independent events as described in the methods section (Supplemental Fig. 1). We found that although most of the GULOP pseudogenes are located on the 5’ to 3’ strand, there is equal distribution of GULO on the 5’ to 3’ and the 3’ to 5’ strand. A p-value of 0.41 was calculated by Fischer’s exact test suggesting that the strand orientation of GULO does not associate with its pseudogenization.
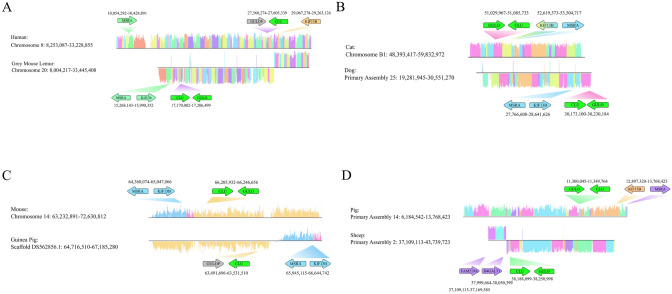
Next, we wanted to determine the breakpoints that resulted in the inversion. Whole chromosomes which contained the syntenic block of genes associated with GULO were analyzed by Mauve alignment. The domestic guinea pig genome is at a scaffold assembly level for this syntenic block and was the only scaffold assembly used. Using Mauve, the breakpoints between human and grey mouse lemur, cat and dog, mouse and guinea pig, and pig and sheep were identified (Supplemental Fig. 4). The breakpoints were found to be variable with the human containing the largest inverted segment of chromosomal DNA near the GULOP gene of ~ 16.5 million base pairs. The mouse and guinea pig were found to have the smallest inverted chromosome segment of ~ 7 million base pairs. From this analysis, it was found that the KIF13B and MSRA orthologues were syntenic and located about 1–2 million base pairs away from GULO and CLU (Fig. 5). In humans, the synteny between KIF13B and MSRA is disrupted with MSRA rearranged ~ 17 million base pairs away from GULOP (Fig. 5A). The gene orientation in cat and dog is reversed when compared between each other, but the overall synteny between KIF13B and MSRA is conserved (Fig. 5B). In the guinea pig scaffold, the KIF13B and MSRA block is rearranged to a slightly further position of ~ 2.5 million base pairs away from GULOP and on the opposite strand when compared to the mouse chromosome 14 segment (Fig. 5C). Lastly, the sheep KIF13B and MSRA gene segment is relocated ~ 37 million base pairs away (data not shown) from GULO, however an alternative set of genes is rearranged adjacent to the GULO-CLU syntenic block (Figs. 4 and 5D). This analysis may suggest that significant rearrangements of nearby gene blocks may occur more frequently in GULO-deficient animals.
Fig. 5.
Mauve Whole Genome Alignment of The Syntenic Blocks Surrounding GULO/GULOP: The reference species is the first species shown for each figure panel. LCB orientation is depicted by the placement of gene blocks over or under the black center line. Blocks with inverse alignment are shown below the center line. LCB blocks refer to regions of the genome between species which are similar and potentially free of genomic rearrangement. LCB height is proportional to the conservation of the specific block when compared to the reference genome. A Comparison of human and grey mouse lemur chromosomes 8 and 20, respectively. B Comparison of cat and dog chromosomes B1 and 25, respectively. C Comparison of mouse and guinea pig chromosome 14 and DS562856, respectively. D Comparison of pig and sheep chromosomes 14 and 2, respectively
Discussion
GULO, which encodes the protein necessary to catalyze the final step in ascorbic acid synthesis, has undergone independent pseudogenization events in mammals. The GULOP sequence of haplorhini primates has been rigorously investigated in previous works (Lachapelle and Drouin 2011; Nishikimi and Yagi 1991; Ohta and Nishikimi 1999; Yang 2013). The work presented shows that a significant quantity of substitutions were conserved in the GULOP sequence of the haplorhini primates. This finding is supported by the long branch length of the haplorhini primates indicating a greater substitution per site in the suborder. In a study examining the pseudogenization of UCP1 in placental mammals, a significantly greater substitution per site value was identified for the UCP1 gene of placental mammals when compared to its paralogs UCP2 and UCP3 (Gaudry and Campbell 2017). It is suggested that the higher rate of substitutions in the UCP1 gene was a result of relaxed purifying selection and enabled gain-of-function mutations for proton leakage. Though GULO is a unitary gene, diet can accommodate and replace endogenous ascorbic acid needs (Maeda et al. 2000). Therefore, the diet of haplorhini primates may have facilitated relaxed purifying selection or positive selection on GULO causing a significant accumulation of unique substitutions within the suborder. However, the substitution per site rate and total substitutions is reduced among species within the haplorhini suborder. Interestingly, miRNAs within processed pseudogenes of primates retain strong conservation and may conserve the underlying pseudogene the miRNA is derived from (Devor 2006). Yet, GULOP is an unprocessed pseudogene, which selection does not act on, so it is unclear how these former exons retain many conserved indels and substitutions. Taken together, these data suggest that the former GULO gene of haplorhini primates underwent a rapid substitution event at the beginning of the haplorhini/strepsirrhini split 70 MYA. The substitution per site rate has been reduced, and this may be a consequence of neutral evolution reducing a strong positive selection once experienced by the haplorhini GULOP. These data may provide insight towards identifying genes which may progress towards pseudogenization by having higher mutation rates when compared to orthologous genes in other species.
The investigation of SNVs between the human and neanderthal GULOP sequence revealed four SNVs. There is debate as to whether humans and Neanderthals are distinct species or subspecies. Despite these arguments, the human and Neanderthal populations are estimated to have split ~ 370,000 years ago (Noonan et al. 2006). Therefore, the neutral mutations that accumulated within GULOP occurred rapidly on an evolutionary time scale. The G96C SNV in the orthologous exon V of Neanderthals occurred at the last nucleotide position of the 3’ end which suggested that it could impact the exon donor recognition site. Exon/intron analysis of exon V reveals that this SNV does not impact the splice site. A caveat of this analysis is that the Neanderthal genome was sequenced from three bones acquired from Vindija samples (Green et al. 2010). Therefore, this sequencing represents a small sample when compared to the human genome sequencing project, and the SNVs in GULOP may not be representative of all Neanderthals.
When examining multiple GULO/GULOP sequences from placental mammals, the GULO/GULOP sequences are phylogenetically separated by order. This suggests overall conservation of the GULO gene. Indeed, previous findings have shown support for GULO conservation and for the evolution of GULO to conform to the current understanding of mammalian speciation (Yang 2013). Our findings extend upon previous work with the inclusion of the Brazilian guinea pig. The Brazilian guinea pig has an annotated GULO sequence in Ensembl™ version 107 which was used to identify the orthologous exon V region of the domestic guinea pig which was not originally identified (Nishikimi et al. 1992). Exon V of the Brazilian and domestic guinea pigs differ by a single indel which results in an in-frame or out-of-frame mutation, respectively. Both guinea pig species show a high rate of mutations within the rodent order, and without experimental testing, it is unknown if the Brazilian guinea pig can endogenously synthesize ascorbic acid. For these analyses, it was assumed that this was a functional gene transcript, despite the considerable genetic rearrangements which occurred within this GULO locus. Additionally, the pika was previously reported as having a functional GULO (Yang 2013). However, our findings suggest that the pika may not have a functional GULO gene. It would be interesting to examine guinea pig species and Lagomorphs for the ability to endogenously synthesize ascorbic acid as this may provide new information towards the evolution of GULO and the pseudogenes associated with it.
The GULOP of the chiroptera order which consists of bats has been studied in similar depth as the GULOP of haplorhini primates (Birney et al. 1976; Cui et al. 2011a, b; Drouin et al. 2011). However, two bat species, Rousettus leschenaultia and Hipposideros armiger, are thought to have independently regained a functional GULO (Drouin et al. 2011). In the present study, it is shown that there is significant gene conservation between the great roundleaf bat (Hipposideros armiger) and the greater horseshoe bat which contain GULO and GULOP, respectively. Interestingly, it has been shown that bats with GULOP can still express some Gulo protein, but only the Gulo protein from Hipposideros armiger was functional (Cui et al. 2011b). Cui and colleagues suggest that bat species may still produce L-gulonolactone oxidase, but the protein may be losing its function. The expression of a non-functional L-gulonolactone oxidase protein implies that the chiroptera promoter may be conserved. Taken together, the chiroptera order may have only recently lost GULO, and it is possible that drift has not altered much of the GULOP sequences or the promoter sequence. Functional promoters for human metallothionein pseudogenes have been identified despite these pseudogenes serving no known function similar to GULOP (Laukens et al. 2009). Therefore, regulatory elements of pseudogenes may remain intact, and this may facilitate pseudogenes regaining function as suggested by Drouin and colleagues (Drouin et al. 2011) or maintaining some degree of conservation as observed in this study.
While using BLAT analysis to find GULOP sequences in haplorhini primates, it was observed that the haplorhini GULOP was annotated in the 5’ to 3’ direction while the strepsirrhini GULO was annotated in the 3’ to 5’ direction. Additionally, a previously reported syntenic block for GULO suggested that CLU was syntenic with GULO (Yang 2013). Indeed, only the CLU gene is syntenic with GULO, and it was found to be inverted with GULO which suggested a larger chromosomal arrangement has occurred involving GULO and CLU. Chromosomal inversions play a significant role in evolution by preventing recombination during meiosis and facilitating speciation (Westram et al. 2022). A direct effect inversions may have on gene expression is by displacing enhancers or insulators from the respective gene target (Kleinjan and van Heyningen 1998). Therefore, a hypothesis was tested that this chromosomal inversion may be a causative factor for the pseudogenization of GULO. A chromosomal region containing GULO between the genes STMN4 and NUGGC was examined. Several species with GULOP were consistently found to have a chromosomal rearrangement where GULOP ran 5’ to 3’. However, the GULO gene occurred on either strand with equal frequency. A chi-square test to determine if GULO pseudogenization was dependent upon GULO orientation did not achieve statistical significance.
Though the hypothesis was not supported, the chromosomal location around GULO has undergone several genomic rearrangements throughout evolution. Large chromosomal rearrangements are present within human evolution such as the 17q21.31 MAPT polymorphic inversion. In this polymorphism, the H1 configuration indicates the original orientation and the H2 configuration indicates the inverted configuration among human populations. However, it is the H2 configuration that is suggested to be the ancestral orientation and is similar to the syntenic region in chimpanzees (Zody et al. 2008). This implies that the H1 configuration is inverted relative to the ancestral state of this locus, and this finding is further supported by a greater number of polymorphic SNPs among the H1 orientation differing from the position matched H2 and ancestral alleles. These findings suggest that chromosomal inversions may increase nucleotide variability within populations and across species. It may be possible that the chromosomal arrangement of GULOP may influence some of the genetic variability observed within the haplorhini suborder and other species tested.
In addition to the inversion of the syntenic blocks around GULO, the KIF13B and MSRA genes are rearranged in human, guinea pig, and sheep genomes where MSRA or both KIF13B and MSRA are displaced from GULO. This frequent rearrangement may be partially responsible for the strand inversion the GULO syntenic block has undergone in evolution. Alternatively, the displacement of MSRA was of interest since it is expressed in liver and alleviates oxidative damage similar to GULO expression and indirectly related to the function of GULO (Harrison et al. 2010; Tabula Muris et al. 2018; Weissbach et al. 2005). There may be an enhancer region associated with the MSRA gene, which facilitates GULO expression, and its rearrangement may influence the expression of GULO. Animals like the sheep and cow have a dramatically altered genetic architecture near the GULO gene which displaces MSRA, but this region may provide a comparable cis-regulatory element to maintain GULO expression.
There are some weaknesses with the approach taken to test the impact of the chromosomal inversion on GULO pseudogenization. First, species where independent chromosomal inversions were selected by tracing the inversion taxonomically. For example, haplorhini primates show this chromosomal inversion suggesting that the inversion occurred during the haplorhini and strepsirrhini split. Therefore, this inversion event was only counted once to avoid redundant counting. Another weakness is regarding the accuracy of the chromosome assemblies used in this study. Species such as the greater horseshoe bat and Chinese rufous horseshoe bat belong to the same genus, but the chromosomal arrangements are different. These species were separated and counted as independent chromosomal rearrangement events. These species may have inaccuracies in the current assemblies provided for them resulting in the differences observed. Additionally, only a few full chromosome maps of animals tested for the Mauve alignment could be used in this study. This low sample number limited examining additional rearrangements along with the breakpoints surrounding the syntenic block GULO belongs to. As chromosome assemblies are improved with the rapid advancements in whole genome sequencing, a robust analysis can be performed to accurately examine the frequency of the chromosomal rearrangements and the occurrence of GULOP.
Conclusion
GULO encodes the final protein for de novo vitamin C synthesis, and its loss is thought to occur as a neutral mutation in some organisms which can acquire sufficient dietary ascorbic acid. Our results to investigate nutritional evolutionary genetics has led to new findings, including previously undescribed SNVs in Neanderthals that have occurred in the GULOP sequences since the human and Neanderthal split 680,000 years ago (Pozzi et al. 2014). Neutral evolution, consisting of mutation and genetic drift, without selection has triggered rapid nucleotide changes between these sequences. In examining other extinct species such as Denisovans unique SNVs attributed to drift in GULOP may be identified. Additionally, there is a period of rapid nucleotide substitution between GULO sequences of strepsirrhini and haplorhini primates which is followed by a period of reduced substitution rates in the GULOP sequences. When examining the GULO evolution of other placental mammals, there are independent losses in guinea pigs, bats, and possibly pika. The Brazilian guinea pig has an open reading frame for GULO unlike the domestic guinea pig, but both sequences have undergone extensive rearrangements and substitutions. It is unknown if the Brazilian guinea pig retains a functional L-gulonolactone oxidase protein. Lastly, rearrangements of the GULOP locus attributed to chromosomal rearrangement may not be responsible for pseudogenization of GULO. Instead, the chromosomal rearrangement may have been conserved simultaneously with GULOP during haplorhini primate evolution.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- GULO
Gulonolactone oxidase
- GULOP
Gulonolactone oxidase pseudogene
- BLAT
Blast-like alignment tool
- SNV
Single-nucleotide variant
- MYA
Million years ago
- MSA
Multiple sequence alignment
- LCBs
Linear colinear blocks
Author Contributions
Both authors contributed to the study conception and design and have approved the final version of the manuscript.
Data Availability
A list of common, abbreviated, and full genus species names is given in Supplemental File 1.
Declarations
Conflict of interest
The authors have no competing interests to declare. No external funding supported this work. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Altekar G, Dwarkadas S, Huelsenbeck JP, Ronquist F. Parallel metropolis coupled markov chain monte carlo for bayesian phylogenetic inference. Bioinformatics. 2004;20:407. doi: 10.1093/bioinformatics/btg427. [DOI] [PubMed] [Google Scholar]
- (2015) A high-quality Neandertal genome sequence https://www.eva.mpg.de/genetics/genome-projects/neandertal/. Ancient Genome Browser. Ancient Genome Browser
- Birney EC, Jenness R, Ayaz KM. Inability of bats to synthesise L-ascorbic acid. Nature. 1976;260:626. doi: 10.1038/260626a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee IB, Chatterjee GC, Ghosh NC, Ghosh JJ, Guha BC. Biological synthesis of L-ascorbic acid in animal tissues: conversion of D-glucuronolactone and L-gulonolactone into L-ascorbic acid. Biochem J. 1960;76:279. doi: 10.1042/bj0760279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Pan YH, Zhang Y, Jones G, Zhang S. Progressive pseudogenization: vitamin C synthesis and its loss in bats. Mol Biol Evol. 2011;28:1025. doi: 10.1093/molbev/msq286. [DOI] [PubMed] [Google Scholar]
- Cui J, Yuan X, Wang L, Jones G, Zhang S. Recent loss of vitamin C biosynthesis ability in bats. PLoS One. 2011;6:e27114. doi: 10.1371/journal.pone.0027114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor EJ. Primate microRNAs miR-220 and miR-492 lie within processed pseudogenes. J Hered. 2006;97:186. doi: 10.1093/jhered/esj022. [DOI] [PubMed] [Google Scholar]
- Drouin G, Godin JR, Page B. The genetics of vitamin C loss in vertebrates. Curr Genomics. 2011;12:371. doi: 10.2174/138920211796429736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry MJ, Campbell KL. Evolution of UCP1 transcriptional regulatory elements across the mammalian phylogeny. Front Physiol. 2017;8:670. doi: 10.3389/fphys.2017.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, Hansen NF, Durand EY, Malaspinas AS, Jensen JD, Marques-Bonet T, Alkan C, Prufer K, Meyer M, Burbano HA, Good JM, Schultz R, Aximu-Petri A, Butthof A, Hober B, Hoffner B, Siegemund M, Weihmann A, Nusbaum C, Lander ES, Russ C, Novod N, Affourtit J, Egholm M, Verna C, Rudan P, Brajkovic D, Kucan Z, Gusic I, Doronichev VB, Golovanova LV, Lalueza-Fox C, de la Rasilla M, Fortea J, Rosas A, Schmitz RW, Johnson PLF, Eichler EE, Falush D, Birney E, Mullikin JC, Slatkin M, Nielsen R, Kelso J, Lachmann M, Reich D, Paabo S. A draft sequence of the Neandertal genome. Science. 2010;328:710. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman AP, Lehninger AL. Enzymic synthesis of L-ascorbic acid in different animal species. Arch Biochem Biophys. 1957;69:458. doi: 10.1016/0003-9861(57)90510-6. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Meredith ME, Dawes SM, Saskowski JL, May JM. Low ascorbic acid and increased oxidative stress in gulo(-/-) mice during development. Brain Res. 2010;1349:143. doi: 10.1016/j.brainres.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ. BLAT–the BLAST-like alignment tool. Genome Res. 2002;12:656. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan DJ, van Heyningen V. Position effect in human genetic disease. Hum Mol Genet. 1998;7:1611. doi: 10.1093/hmg/7.10.1611. [DOI] [PubMed] [Google Scholar]
- Lachapelle MY, Drouin G. Inactivation dates of the human and guinea pig vitamin C genes. Genetica. 2011;139:199. doi: 10.1007/s10709-010-9537-x. [DOI] [PubMed] [Google Scholar]
- Laukens D, Waeytens A, De Bleser P, Cuvelier C, De Vos M. Human metallothionein expression under normal and pathological conditions: mechanisms of gene regulation based on in silico promoter analysis. Crit Rev Eukaryot Gene Expr. 2009;19:301. doi: 10.1615/CritRevEukarGeneExpr.v19.i4.40. [DOI] [PubMed] [Google Scholar]
- Lind J (1753) Treatise of the Scurvy in Three Parts
- Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS. 2007;274:1. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA. 2000;97:841. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi M, Yagi K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am J Clin Nutr. 1991;54:1203S. doi: 10.1093/ajcn/54.6.1203s. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Koshizaka T, Ozawa T, Yagi K. Occurrence in humans and guinea pigs of the gene related to their missing enzyme L-gulono-gamma-lactone oxidase. Arch Biochem Biophys. 1988;267:842. doi: 10.1016/0003-9861(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Kawai T, Yagi K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J Biol Chem. 1992;267:21967. doi: 10.1016/S0021-9258(19)36707-9. [DOI] [PubMed] [Google Scholar]
- Noonan JP, Coop G, Kudaravalli S, Smith D, Krause J, Alessi J, Chen F, Platt D, Paabo S, Pritchard JK, Rubin EM. Sequencing and analysis of Neanderthal genomic DNA. Science. 2006;314:1113. doi: 10.1126/science.1131412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Nishikimi M. Random nucleotide substitutions in primate nonfunctional gene for L-gulono-gamma-lactone oxidase, the missing enzyme in L-ascorbic acid biosynthesis. Biochim Biophys Acta. 1999;1472:408. doi: 10.1016/S0304-4165(99)00123-3. [DOI] [PubMed] [Google Scholar]
- Petrov DA, Hartl DL. Pseudogene evolution and natural selection for a compact genome. J Hered. 2000;91:221. doi: 10.1093/jhered/91.3.221. [DOI] [PubMed] [Google Scholar]
- Pink RC, Wicks K, Caley DP, Punch EK, Jacobs L, Carter DR. Pseudogenes: pseudo-functional or key regulators in health and disease? RNA. 2011;17:792. doi: 10.1261/rna.2658311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L, Hodgson JA, Burrell AS, Sterner KN, Raaum RL, Disotell TR. Primate phylogenetic relationships and divergence dates inferred from complete mitochondrial genomes. Mol Phylogenet Evol. 2014;75:165. doi: 10.1016/j.ympev.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prufer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, Heinze A, Renaud G, Sudmant PH, de Filippo C, Li H, Mallick S, Dannemann M, Fu Q, Kircher M, Kuhlwilm M, Lachmann M, Meyer M, Ongyerth M, Siebauer M, Theunert C, Tandon A, Moorjani P, Pickrell J, Mullikin JC, Vohr SH, Green RE, Hellmann I, Johnson PL, Blanche H, Cann H, Kitzman JO, Shendure J, Eichler EE, Lein ES, Bakken TE, Golovanova LV, Doronichev VB, Shunkov MV, Derevianko AP, Viola B, Slatkin M, Reich D, Kelso J, Paabo S. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43. doi: 10.1038/nature12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Wenger AM, Zehir A, Mesirov JP. Variant review with the integrative genomics viewer. Cancer Res. 2017;77:e31. doi: 10.1158/0008-5472.CAN-17-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabula Muris C, Overall c, Logistical c, Organ c, processing, Library p, sequencing, Computational data a, Cell type a, Writing g, Supplemental text writing g, Principal i Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562:367. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, Roebroeks W. Neandertal demise: an archaeological analysis of the modern human superiority complex. PLoS One. 2014;9:e96424. doi: 10.1371/journal.pone.0096424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach H, Resnick L, Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Westram AM, Faria R, Johannesson K, Butlin R, Barton N. Inversions and parallel evolution. Philos Trans R Soc Lond B Biol Sci. 2022;377:20210203. doi: 10.1098/rstb.2021.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Conserved or lost: molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis. Biochem Genet. 2013;51:413. doi: 10.1007/s10528-013-9574-0. [DOI] [PubMed] [Google Scholar]
- Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Giron CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hut SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P. Ensembl 2018. Nucleic Acids Res. 2018;46:D754. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zody MC, Jiang Z, Fung HC, Antonacci F, Hillier LW, Cardone MF, Graves TA, Kidd JM, Cheng Z, Abouelleil A, Chen L, Wallis J, Glasscock J, Wilson RK, Reily AD, Duckworth J, Ventura M, Hardy J, Warren WC, Eichler EE. Evolutionary toggling of the MAPT 17q21.31 inversion region. Nat Genet. 2008;40:1076. doi: 10.1038/ng.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A list of common, abbreviated, and full genus species names is given in Supplemental File 1.