Abstract
While the exact pathogenesis of IBD remains unclear, genetic, environmental and nutritional factors as well as the composition of the gut microbiome play crucial roles. Food additives, which are increasingly consumed in the Western diet, are being investigated for their potential effects on IBD. These additives can affect gut health by altering the composition of the microbiota, immune responses, and intestinal permeability, contributing to autoimmune diseases and inflammation. Despite the growing number of studies on food additives and IBD, the specific effects of carrageenan have not yet been sufficiently researched. This review addresses this gap by critically analyzing recent studies on the effects of carrageenan on the gut microbiota, intestinal permeability, and inflammatory processes. We searched the MEDLINE and SCOPUS databases using the following terms: carrageenan, carrageenan and inflammatory bowel disease, carrageenan and cancer, food additives and microbiome, food additives and intestinal permeability, and food additives and autoimmune diseases. In animal studies, degraded carrageenan has been shown to trigger intestinal ulceration and inflammation, highlighting its potential risk for exacerbating IBD. It can affect the gut microbiota, reduce bacterial diversity, and increase intestinal permeability, contributing to “leaky gut” syndrome. Some studies suggest that carrageenan may inhibit the growth of cancer cells by influencing the progression of the cell cycle, but the anti-cancer effect is still unclear. Carrageenan may also increase glucose intolerance and insulin resistance. Further research is needed to determine whether carrageenan should be excluded from the diet of individuals with IBD.
Keywords: inflammatory bowel disease, carrageenan, inflammation, health, food additives, microbiome
1. Introduction
The term inflammatory bowel disease (IBD) refers to diseases of the gastrointestinal tract of unknown etiology [1,2], characterized by chronic inflammation [3,4]. They progress with alternating periods of relapse and remission [5]. In IBDs, Crohn’s disease (CD) and ulcerative colitis (UC) are the most common [1]. In Crohn’s disease, the lesions are segmental and transmural and can be localized throughout the gastrointestinal tract, from the mouth to the anus, but most commonly form in the terminal ileum [6], and much less frequently in the upper gastrointestinal tract [3], while ulcerative colitis affects the mucosa of the large intestine, particularly the colon and rectum [3,7]. These diseases can occur at any age. Differences in the age of onset are observed depending on geographical region [4] and gender [8]. The most common symptoms are diarrhea [3], rectal bleeding, bowel movement urgency, and tenesmus [1]. Other common symptoms are abdominal pain, nausea, vomiting [1], decreased appetite, and weight loss [3,9]. Inflammatory markers, such as an increased erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), leucocytosis, and calprotectin, indicate active inflammation [9]. Extraintestinal manifestations occur in 25–40% of IBD cases, with joint manifestations such as arthritis and ankylosing spondylitis being the most common. Skin changes such as erythema nodosum or pyoderma gangrenosum, liver complications including primary sclerosing cholangitis, and ocular complications are less frequent [1,9]. In 2019, there were approximately 4.9 million IBD cases worldwide, with the highest number in China, followed by the USA [10] and 1.3 million cases in Europe in 2020 [8]. The pooled global prevalence of CD and UC per 100,000 person-years in 2017–2023 was 186.18 and 255.92, respectively [11].
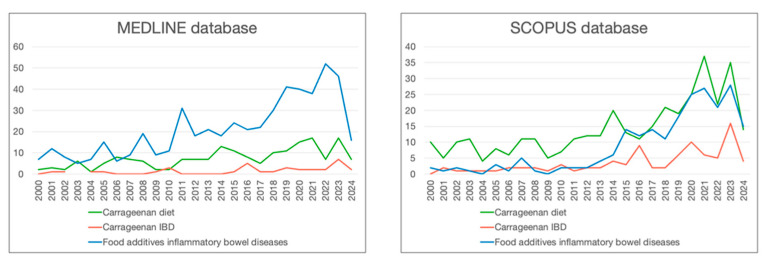
The etiology of IBD is multifactorial. It is not yet fully understood, but key factors include genetic factors [3,4,12], dietary factors [13,14,15], an inappropriate response to environmental factors [2], an abnormal mucosal immune response in predisposed individuals [14], and the composition of the gut microbiome [5,12,16]. A diet rich in vegetables, fruit, omega-3 fatty acids, and low in omega-6 fatty acids [17], with an adequate intake of dietary fiber, has a protective effect in IBDs [15,17]. It appears that a sufficient serum concentration of vitamin D, which is known for its extensive health-promoting effects [18,19], may also play a protective role [12,20]. The ‘Western diet’ is characterized by high fat consumption, increased intake of proteins and monosaccharides, and highly processed foods, and is linked to a higher risk of developing IBD. This effect is amplified by emulsifiers like carboxymethylcellulose, mentioned in the 2023 ESPEN recommendations, and other additives, including carrageenan, also included in this dietary pattern [14,15,17,21,22]. Due to the increasing consumption of food additives and the observed increase in the incidence of IBD [4,5], our study focuses on investigating the role of food additives with a particular reference to carrageenan. While the number of studies on food additives and IBD is growing, as shown in Figure 1, few focus specifically on the role of carrageenan. This review addresses this gap by critically analyzing recent studies on the effects of carrageenan on gut microbiota, intestinal permeability, and inflammatory processes. By integrating findings from different perspectives, the review provides a holistic view of the need for future research leading to better knowledge with practical benefits for IBD patients.
Figure 1.
Studies on carrageenan and food additives in MEDLINE and SCOPUS databases from 2000 to 2014.
2. Food Additives
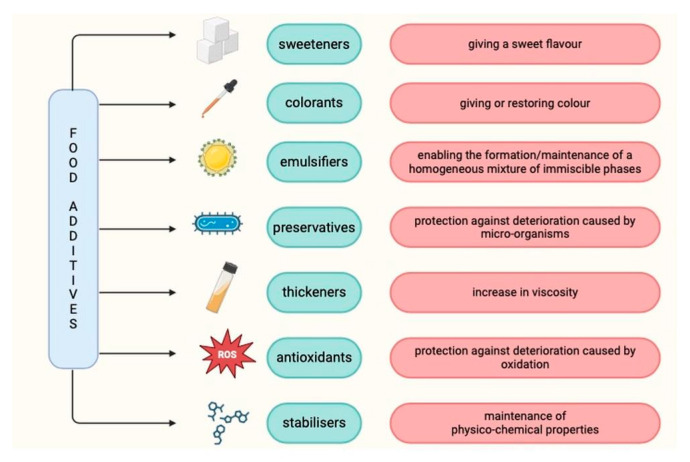
Food additives are defined as substances that are not a typical component of food and [23] are added to food to enhance technological capabilities [22,23,24] or to improve flavor or presentation [25]. These include colorings, sweeteners and thickeners, antimicrobials, and antioxidants. Food additives may be added if their use is technologically necessary, safe, not misleading, and beneficial to the consumer [23]. In the European Union, food additives have their own E-number, which is indicated on the product label [26]. The role of selected food additives is shown in Figure 2.
Figure 2.
The role of selected food additives based on [23].
2.1. Food Additives and Autoimmune Diseases
Diet affects the immune system and inflammation, particularly the Western diet with its highly processed foods [27]. The consumption of food additives appears to be associated with an increased incidence of autoimmune diseases [28,29] and inflammation in general. For example, chronic ingestion of polysorbate 80 leads to increased expression of TNF-α factor in muscle [30]. However, there are also reports of the anti-inflammatory effects of food additives. For example, sodium benzoate inhibits LPS-stimulated TNF-α and IL-1β expression in mouse microglia [31]. It was also shown that the administration of 80 mg of curcumin daily for 3 months to patients with osteoarthritis led to a decrease in CRP levels (compared to an increase in the placebo group). Moreover, it resulted in a reduction in CD4+ and CD8+ T-lymphocytes and Th17 cells, an increase in the proportion of Treg cells, an increase in FOXP3, and a decrease in the proportion of B-lymphocytes [32]. The effect also appears to cause a loosening of tight junctions and an increase in intestinal permeability (as described later in this article). This may result in the migration of toxins and antigens into the gut, leading to activation of the immune cascade [27,29], as well as increasing the risk of impaired nutrient absorption [27].
The evidence highlights the complex role of diet and food additives in modulating immune responses and inflammation. While some additives like polysorbate 80 exacerbate inflammatory processes, others like sodium benzoate and curcumin show anti-inflammatory potential. These findings suggest the dual nature of food additives, warranting further investigation on the mechanisms behind these effects and identifying which additives pose risks versus those that offer therapeutic benefits.
This appears particularly important considering the diet modifications in patients with autoimmune diseases.
2.2. Food Additives and Other Diseases
Food additives may cause allergic reactions and/or hypersensitivity [33,34,35]. Attention is also drawn to the potentially harmful effects of food additives on children’s health, particularly artificial colors (the ‘Southampton Six’ colors) and/or a sodium benzoate preservative, which may increase hyperactivity in children [36]. Additionally, artificial food colorings appear to exacerbate the symptoms of attention deficit hyperactivity disorder in young adults [37].
In mice, it was observed that the intake of emulsifiers (polysorbate 80, soy lecithin, gum arabic, carboxymethylcellulose) at a concentration of 1% led to a reduction in both the weight and length of the colon. However, the results were statistically only significant for polysorbate 80 (reduction in weight and length) and carboxymethylcellulose (length only). No changes were observed for cecal weight. In addition, the intake of emulsifiers resulted in an increase in weight (carboxymethylcellulose and polysorbate 80) and an elevated fasting glucose level (all emulsifiers mentioned above). The authors suggested that carboxymethylcellulose and polysorbate had the strongest effects on inflammatory and metabolic parameters, while soy lecithin and gum arabic had a lesser effect. One limitation of the study was that only the length of the colon was measured, which is considered to be an indirect marker of inflammation [38].
Sweeteners are often associated with impaired carbohydrate metabolism. The results come from both human and animal studies. In humans, saccharin and sucralose increase the glycemic response in a glucose tolerance test (OGTT) compared to glucose; this effect has not been reported for aspartame and stevia [39]. In 10-week-old C57Bl/6 mice, the intake of saccharin, sucralose, and aspartame in drinking water resulted in increased blood glucose levels, with the highest level observed for saccharin. In addition, saccharin caused higher blood glucose concentrations compared to glucose in mice fed a high-fat diet as well as in mice fed a normal diet [40]. Ingestion of 48 mg of sucralose by obese individuals, who did not consume sweeteners by default, resulted in statistically significantly higher glucose concentrations at 60 and 90 min, insulin concentrations at 60, 90, and 120 min, and C-peptide concentrations at 90, 120, and 150 min compared to ingestion of water [41]. Chronic ingestion of polysorbate 80 in mice resulted in elevated plasma insulin levels and increased insulin resistance [30]. In addition, it appears that sodium benzoate may have beneficial effects in the treatment of neurodegenerative diseases [31].
2.3. Food Additives and Microbiome
It appears that food additives commonly found in the “Western diet” may be associated with the pro-inflammatory potential of the microbiota [42]. Synthetic sweeteners (including sucralose, saccharin, aspartame, or acesulfame K), other emulsifiers including carboxymethylcellulose, polysorbate 80, and preservatives may have an impact on the gut microbiota [43,44]. In a randomized placebo-controlled study, Chassaing et al. showed that an intake of 15 g/day of carboxymethylcellulose reduced bacterial diversity in the gut (decrease in Faecalibacterium prausnitzii and Ruminococcus sp., increase in Roseburia sp. and Lachnospiraceae) and short-chain fatty acid production, and that highly processed foods may cause changes in the gut microbiota, that may lead to inflammation and the development or exacerbation of IBD [45]. In addition, carboxymethylcellulose and polysorbate 80 affect the bacterial taxa Proteus spp. and Veillonellaceae which have been associated with promoting the recurrence of CD. Moreover, polysorbate 80 and sodium sulphite have an inhibitory effect on the growth of Faecalibacterium prausnitzii, which appears to have a favorable effect on CD [46]. Glycerol monolaurate caused intestinal dysbiosis in mice, reduced the content of anti-inflammatory bacteria such as Akkermansia and increased levels of LPS [47]. The evidence on the pro-inflammatory potential of the gut microbiome should be interpreted with caution, since most of the studies were conducted on animals. However, reducing bacterial diversity and increasing pro-inflammatory taxa, as seen with carboxymethylcellulose and polysorbate 80, can exacerbate conditions like IBD and CD. Carboxymethylcellulose was listed in the ESPEN guidelines as potentially increasing the risk of IBD [17]. Future research should focus on long-term human studies to better understand the specific impacts of various food additives on gut health [17].
2.4. Food Additives and Intestinal Permeability
Increased intestinal permeability leads to bacterial translocation and lipopolysaccharide metabolites into the bloodstream, leading to metabolic endotoxemia and systemic inflammation [48], which is referred to as “leaky gut” [49]. Disruption of the intestinal barrier is seen in many diseases including autoimmune diseases, such as CD, irritable bowel syndrome (IBS), celiac disease, and diabetes [28,29]. Various additives can influence the permeability of the intestine. Decreased surface hydrophobicity of the proximal colon, middle colon, distal colon, and rectum was observed after oral administration of the nonionic detergent Brij 35 to rats and increased epithelial permeability to hydrophilic molecules and increased susceptibility of the mucosa to inflammation after administration of dextran sodium sulphate (DSS) [50]. It has been shown that chronic administration of P80 to mice resulted in an increased LPS in plasma, suggesting increased intestinal permeability [30].
A high-sugar diet in Drosophila melanogaster contributed to increased intestinal permeability, as demonstrated by the so-called “smurf assay”, in which the insect’s blue pigmentation suggests increased intestinal permeability. In addition, the high-sugar diet decreased the length and diameter of the intestine, the overall size of the animals with increased food intake, and decreased the activity of intestinal alkaline phosphatase, which is a protective factor against lipopolysaccharide, reduces inflammation and strengthens tight junctions both in vivo and in vitro [28].
In another study, the intake of emulsifiers and highly processed food among 657 people based on six 24 h dietary recalls was investigated. Antibodies against flagellin and lipopolysaccharide, as well as GlycA levels, were measured. Levels of those parameters were not associated with a higher intake of emulsifiers. In contrast, increased consumption of highly processed foods (%g/d) was associated with higher GlycA levels, and intake of highly processed foods, expressed as %kcal/d, with higher levels of LPS and antibody levels (sum of IgG and IgA for flagellin and LPS). In this case, the association with inflammation and intestinal permeability is due to the highly processed foods, not the emulsifiers themselves [51]. A study with human intestinal epithelial Caco-2 cells showed that sucrose monoester fatty acid loosens tight junctions and increases intestinal permeability, proven by changes in actin filament structure. This surfactant may also increase the paracellular uptake of food allergens [52]. “Leaky gut” plays a crucial role in the pathogenesis of many acute and chronic diseases, often exacerbating their severity. Mechanisms that damage the intestinal barrier can increase patient vulnerability and susceptibility to illness. While these findings are based on hypotheses and experimental studies, they highlight the need for personalized and in-depth dietary advice. Detailed knowledge on food additives included in personalized nutrition may consider individual susceptibility to intestinal permeability and could provide significant clinical benefits.
3. Carrageenan
3.1. Definition
One food additive is carrageenan, a linear, water-soluble polygalactan that belongs to the group of hydrocolloids [53,54]. It is obtained from the cell walls of red seaweeds, mainly from Chrondrus crispus, Kappaphycus alvarezii, and Eucheuma denticulatum [55]. It is an odorless powder with a white to yellow color [56]. This compound can be obtained in two ways. The first (traditional) method involves extracting the carrageenan from the seaweed, during which it is dissolved and removed. In the second method, carrageenan remains in the seaweed matrix and is not dissolved [55]—resulting in partially refined carrageenan [57]. Carrageenan is labelled with the identification number E407 (refined carrageenan) or E407a (partially refined carrageenan, otherwise processed Eucheuma seaweed) [58]. Processed Eucheuma seaweed contains more cellulose and fewer inorganic salts [59].
Carrageenan comes in six different forms, the most common being: Kappa (κ), Iotta (ι), and Lambda (λ) [60]. They differ in their ability to gel and their solubility in water [61]. The κ form forms strong, stiff gels with potassium salts [57,62] and brittle gels with calcium salts [57], the ι form forms flexible gels with calcium salts, while carrageenan λ has no gelling ability [62] but can be used as a thickening agent [57,62]. In addition, the gel of ι carrageenan is transparent, in contrast to κ carrageenan [57]. All forms of carrageenan are soluble in warm water, but only λ is soluble in cold water [53,61]—this depends on the number of sulphate groups per galactose unit [53].
3.2. Carrageenan Market
The intake of carrageenan in a typical Western diet is estimated at 250 mg/person/day but can reach 2–4 g/person/day [63]. This compound is also the fourth most commonly consumed food additive in pediatric patients with CD [64]. The compound annual growth rate (CAGR) for the carrageenan market is estimated to reach 6.5% by 2028, primarily due to the increasing consumption of highly processed foods, but also due to the increasing demand for hydrocolloids. Europe is the fastest-growing market, and the Asia–Pacific region has the largest global market share, due to its significant production and consumption, and its favorable climatic conditions for cultivation and production. South America and Africa are characterized by their small market size [65]. It is estimated that the global market will reach USD 1.32 billion by 2030 [66].
3.3. Occurrence
As a food additive, carrageenan has a thickening, emulsifying, stabilizing, and gelling function [60]. It is used in dairy products where, when added in low concentrations, it prevents the separation of whey (κ form) and prevents the separation of protein and fat from milk and fat from cream. The addition of carrageenan (κ or λ) to cream improves the whipping process [57]. One study found that carrageenan is present in 66% of frozen desserts [64] and the largest amount is found in frosting mixes [61]. Another group involves meat products, where carrageenan binds free water and interacts with proteins to create a higher-quality product. The next group are carrageenan-based jellies, which are an alternative to gelatine-based products [57]. In addition to food, carrageenan is also used in cosmetics, animal feed, and in the pharmaceutical industry where it is used as a foaming, dissolving, or stabilizing agent [56,67]. It is used in the formulation of pharmaceutical capsules instead of gelatine and in biosensors. Carrageenan’s properties and advantages over synthetic polymers (including high availability and biodegradability) have also led to its use as an industrial material in the form of nanocomposites which eliminate pollution from the environment, functional textiles, as well as for controlled release technologies for fertilizers [67]. It can also be used in tissue engineering as a carrier matrix to transfer cells [68].
3.4. Safety of Carrageenan as a Food Additive
Carrageenan is widely regarded as safe. Its use as a food additive has been approved by the European Food Safety Authority (EFSA), the Joint FAO/WHO Expert Committee on Food Additives (JECFA) as well as the Food and Drug Administration (FDA) [58,69,70] (Table 1). However, the National Organic Standards Board (NOSB) in 2016 withdrew carrageenan from the list of substances permitted for use in organic foods. The report listed environmental contamination during the production of carrageenan, mentioning the fact that the function of carrageenan can be achieved using other additives such as xanthan gum, gellan gum, or guar gum. Another reason is its lack of compatibility with the definition of sustainable agriculture [71].
Table 1.
Reviews provided by selected organizations on the safety of carrageenan.
| Organisations | Conclusions | References |
|---|---|---|
| EFSA | No adverse effects were found in the context of carcinogenicity or genotoxicity. An ADI = 75 mg/kg b.w. was found to be temporary. However, degraded carrageenan cannot be used as a food additive. | [58] |
| JECFA | “The use of carrageenan in infant formula or formula for special medical purposes at concentrations up to 1000 mg/L is not of concern”. | [69] |
| FDA | Carrageenan can be used as a food additive and is considered safe under certain conditions, including that it is a hydrocolloid formed from red seaweed by extraction and that it is used as a stabilizing, emulsifying, thickening substance in the necessary amount. | [70] |
3.5. Impact on Inflammatory Bowel Diseases
Degraded carrageenan was used in the last century to induce intestinal ulceration in animals [72,73,74]. Their severity increased with the duration of supply of this compound [72]. The administration of a 5% solution of degraded carrageenan to guinea pigs in drinking water resulted in decreased body weight (loss of 15–25% compared to the beginning of the study), the appearance of blood in the feces (occult in all animals after 30 days and visible in one animal after 45 days of the study) and loose stools already at the end of the first week of the study [72]. Another study showed that administration of a 10% degraded carrageenan solution to mice resulted in bloody diarrhea and perianal inflammation, while weight changes were not significant. Histopathologically, deformations of the crypts and cellular inflammatory infiltrations in the lamina propria were observed [73]. Other studies have also been conducted with other animals including rabbits [74] and rats [75].
It was already noted in the previous century that the changes induced by degraded carrageenan in animals are in some ways similar to those found in UC in humans [76]. These reports were also confirmed in later years [77]. In 2016 Muynaka et al. described the changes induced by administrating of a 1% medium molecular weight carrageenan solution in drinking water to piglets. Swelling of the mucosa and submucosal layers of the intestines was observed, but without granulomatous inflammation, similar to the symptoms associated with UC, as the authors hypothesized [77].
Only a few human studies are looking at the effect of carrageenan on IBD. One randomized placebo-controlled study from 2017 investigated the effect of a carrageenan-free diet on UC activity. At the beginning, participants were instructed to follow a carrageenan-free diet. They were then randomized to take 100 mg carrageenan capsules (study group) or dextrose capsules (placebo group). The main outcome was the occurrence of relapse, i.e., an increase in at least two points on the Simple Clinical Colitis Activity Index (SCCAI) scale, along with an increase in the dose of maintenance medication or the introduction of a new medication for the patient if UC symptoms worsened. After the intervention, SCCAI scale scores increased by at least two points in three patients taking carrageenan capsules and in one patient in the placebo group (without increasing the dose of medication). Relapse was observed in three patients in the study group, two of whom were already taking medication. The study was completed by four patients in the placebo group and two patients in the carrageenan group without relapse. There was also a decrease in the SCCAI scale in four subjects in the group taking the dextrose capsules. In addition, an increase in IL-6 and fecal calprotectin levels was observed in subjects taking carrageenan capsules, while TNF-α levels decreased in two subjects in the control group and one subject in the study group. The results suggest that carrageenan may contribute to the development of UC relapses. It should also be pointed out that the doses of carrageenan used in this study were lower than those consumed in the “Western diet” [63].
In 2018, a study was published that investigated exposure to food additives among children and young adults with CD. The 24 h dietary recall of patients was used, from which a database of all food products was created and the intake of eight food additives, including carrageenan, was checked. The consumption of food additives was found to be widespread in this group, particularly xanthan gum, maltodextrin, soy lecithin, and carrageenan [64]. Moreover, after screening 116,087 adult participants aged 35–70 years, it was shown that a higher intake of ultra-processed foods (soft drinks, salty snacks, processed meat, refined sweetened foods) positively correlated with a higher risk of an IBD incident [78]. It has also been shown that a diet low in emulsifiers can have a positive impact on the symptoms associated with CD and is perceived as appetizing by most respondents [38].
In 2020, Sandall et al. published a study in which they investigated the feasibility and acceptability of a low-emulsifier diet, meaning that it excludes 65 food additives considered to be emulsifiers, in 20 patients with CD in remission. The subjects adhered to this diet for 14 days and evaluation was based on 7-day food diaries kept 7 days before the start of the study and during the last 7 days of the intervention. Prior to this, subjects received informational brochures containing products suitable and unsuitable for their diet, dietary counseling, and access to a dedicated app to help identify products containing emulsifiers. In this study, emulsifier intake was assessed by the frequency of consumption of products containing emulsifiers. Before the beginning of the study, 15/20 participants consumed emulsifiers daily, while the remaining 5 participants consumed emulsifiers on 6 of the 7 days in the week under evaluation. In total, 19 of 20 subjects followed the low-emulsifier diet well (reducing the frequency of emulsifier consumption by at least 75% between baseline and the end of the intervention). A total of 18 subjects declared following the diet for the entire study period, and two subjects for ¾ of the time, due to the need to have food away from home. Although 90% of the subjects rated the low-emulsifier diet as more difficult to follow than their usual diet, 95% found it appetizing. In addition, there was an improvement in symptoms of CD assessed by PRO-2 [38].
Carrageenan may have pro-inflammatory effects. Oral administration of processed Eucheuma seaweed to rats led to deformation and destruction of intestinal villi, the presence of inflammatory infiltration of the small intestinal lamina propria and a decrease in the amount of goblet cells. In addition, eosinophils, macrophages, and lymphocytes were present in the intestinal lamina propria. This was consistent with an increase in inflammatory parameters, i.e., CRP and middle molecules [79]. It should be emphasized that in this study processed Eucheuma seaweed were used, which has been considered as safe and approved for use in food [58]. Carrageenan can also cause an increase in the activity of the transcription factor NF-κB in the intestinal epithelium, which results in an increased expression of the proinflammatory IL-8 gene [80] but another study showed that this only occurs in the presence of LPS [81]. In addition to this, there is an unbeneficial change in the distribution of tight junctions and a decrease in the overall density of ZO-1 protein [80]. It should be noted that most conclusions come from studies on cell lines. ZO-1 can be used as a marker of tight junction integrality in future studies on the effects of food additives on intestinal permeability.
Table 2 summarizes the results of animal studies. A summary of human studies focusing on the effects of carrageenan on IBD and the impact of the food additives is presented in Table 3.
Table 2.
Animal studies reporting effects of carrageenan on IBD.
| Carrageenan Solution | Type of Animals | Result | References |
|---|---|---|---|
| 1% medium molecular weight carrageenan solution |
Piglets | swelling of the mucosa and submucosal layers without granulomatous inflammation |
Munyaka et al. Front Microbiol, 2016 [77] |
| 5% solution of degraded carrageenan |
Guinea pigs | decreased body weight; appearance of blood in the feces and loose stools; ulcers in large intestine |
Watt et al. Gut, 1971 [72] |
| 10% degraded carrageenan solution |
Mice | bloody diarrhea; perianal inflammation | Fath et al. Digestion, 1984 [73] |
| 1% degraded carrageenan solution |
Rabbits | visible fecal blood; cecal ulceration |
Al-Suhail et al., Histochem J, 1984 [74] |
| 5% solution of degraded carrageenan |
Rats | cecal ulceration | Marcus et al. Lancet, 1971 [75] |
| processed Eucheuma seaweed |
Rats | destruction of intestinal villi, the presence of inflammatory infiltration of the small intestinal lamina propria and a decrease in the amount of goblet cells |
Pogozhykh et al. Int J Mol Sci, 2021 [79] |
Table 3.
A summary of human studies focusing on the effects of carrageenan on IBD and the impact of food additives.
| Study Group | Outcome/Conclusion | References |
|---|---|---|
| 12 adults with UC | Carrageenan supply may contribute to earlier relapse in patients with ulcerative colitis in remission | Bhattacharyya et al. Nutr Healthy Aging, 2017 [63] |
| 138 children with CD | Children with CD frequently consume food additives, particularly xanthan gum, maltodextrin, soy lecithin, and carrageenan | Lee et al. Dig Dis Sci, 2018 [64] |
| 116,087 adults | Higher intake of ultra-processed foods positively correlated with a higher risk of an IBD incident | Narula et al. BMJ, 2021 [78] |
| 20 patients with CD in remission |
90% of subjects rated the low-emulsifier diet as more difficult to follow than their usual diet, 95% found it appetizing; low-emulsifier diet led to improvement of symptoms | Sandall et al. Nutrients, 2020 [38] |
3.6. Impact on the Microbiota
The microbiota of IBD patients differs from that of healthy individuals and is characterized by a decrease in Firmicutes [46], Bacteroidetes, Lactobacillus, and Eubacterium [14], and an increase in Proteobacteria [46,82] and Enterobacteriaceae including Escherichia coli and Fusobacterium [13,14]. The intake of carrageenan can lead to changes in the gut microbiota and reduce bacterial diversity. Mi et al. demonstrated that administration of κ carrageenan in drinking water to C57BL/6J pathogen-free mice can exacerbate colitis while on a high-fat diet. A decrease in the anti-inflammatory Akkermansia muciniphila bacteria and an increase in pathogenic bacteria have been suggested as possible causes [83]. On the other hand, Wu et al. showed that the supply of κ carrageenan increased the total bacterial abundance and did not decrease microbiota diversity [84].
A reduction in Akkermansia muciniphila after administration of carrageenan was also observed in other studies [84,85]. Akkermansia muciniphila has been negatively correlated with serum TNF-α levels and plays an important role in maintaining intestinal homeostasis. It appears to have a protective effect on inflammatory diseases progressing with disruption of the intestinal barrier, including carrageenan-induced colitis [85].
Moreover, the intake of different types of carrageenan may affect other bacterial species. For example, ι carrageenan contributes to a reduction in Faecalibacterium [86] and κ carrageenan caused a decrease in Proteobacteria and Firmicutes [84,85]. It has been shown that low levels of Faecalibacterium spp. may be associated with higher risk of CD recurrence [46].
Given the variable effects of carrageenan on the intestinal microbiota observed across different studies, further research is essential to derive conclusive insights on this topic.
3.7. Carrageenan and Cancer
Cancers are one of the leading causes of premature death worldwide. They are estimated to be a cause of premature death in 57 countries in 2019, mainly in Canada, the United States, China, Australia, Peru, Argentina, Chile, Colombia, and Western Europe [87]. Globally, breast cancer is the most common, followed by prostate cancer, then lung cancer and colorectal cancer [88]. There are many types of anti-cancer therapies, including chemotherapy, radiotherapy, immunotherapy, and surgical treatment [89]. Many of these therapies result not only in the destruction of cancer cells, but also of healthy cells, which adversely affects the patient’s general condition and limits the effectiveness of the treatment [89,90]. Some studies have shown that carrageenan can inhibit cancer cell growth by arresting the cell cycle in specific phases and delaying cell cycle progression [91]. Others have shown decreased cell viability in tumor zones [92,93] and increased immune responses [94]. The cytotoxic effects of carrageenan may correlate with the number of sulphate groups which may be a result of its higher antioxidant activity [91,93] but the data are inconsistent because it has also been shown that the presence of these groups can inhibit cytotoxic effect [95]. The impact of carrageenan in the context of cancer is presented in Table 4.
Table 4.
Summary of the impact of carrageenan on cancer.
| Type of Carrageenan | Cell Type | Mechanism and/or Effect | Study |
|---|---|---|---|
| κ and λ | cervical carcinoma cell lines HeLa |
stopping the cell cycle in G1 (only λ carrageenan) and G2 phases (λ and κ carrageenan), delaying cycle progression and consequently inhibiting tumor cell growth | Prasedya et al. BMC Complement Altern Med, 2016 [91] |
| extracted from Gigartina pistillata |
colorectal cancer stem cells |
reduction in cell viability in tumor zones | Cotas et al. Mar Drugs, 2020 [93] |
| λ | breast cancer cells 4T1 and melanoma cells B16-F10 |
reduction in tumor weight and volume and increasing the tumor immune response by raising more activated CD4+ and CD8+ T-lymphocytes in the spleen and M1 macrophages that infiltrate the tumor (after intratumoral injection) | Luo et al. Sci Rep, 2015 [94] |
| κ | malignant breast cancer cell lines MCF-7 | decrease in cell viability, potential apoptotic effect |
Sayın et al. Aegean J Med Sci, 2022 [92] |
| λ extracted from Laurencia papillosa |
breast cancer cell line MDA-MB-231 |
decrease in cell viability, inhibition of cell proliferation, regulating genes involved in apoptosis, cell growth in the sub-G1 phase | Jazzara et al. Int J Cancer Manag, 2016 [96] |
| κ extracted from Hypnea musciformis |
breast cancer cell line MCF-7 and neuroblastoma cell line SH-SY5Y |
reduction in proliferative capacity, but lack of cytotoxic effects |
Souza et al. Int J Biol Macromol, 2018 [97] |
| κ- and λ-from Chondrus armatus and low-molecular weight degradation products | esophageal cancer cell lines KYSE30 and FLO1 | cytostatic activity (higher for low-molecular weight degradation products), monocytes induction to produce pro-inflammatory cytokines, induction of anti-inflammatory cytokine secretion (observed only for a λ carrageenan low-molecular weight degradation product) | Cicinskas et al. J Biomed Mater Res A, 2020 [98] |
Noteworthily, there are also reports that carrageenan has no cytotoxic effects on healthy cells, but only on cancer cells [91].
On the other hand, administration of 15% undegraded carrageenan (Viscarin 402) to female F344 rats was shown to increase the incidence of colon tumors induced by subcutaneously injected azoxymethane or rectally administered methylnitrosourea [99]. Additionally degraded κ carrageenan increased the incidence of high-grade dysplasia and tumor incidence in a mouse model of azoxymethane-induced colorectal cancer [100]. Future studies should focus on well-designed animal models and human trials to evaluate the safety and efficacy of carrageenan in cancer therapy. It will be critical to understand the exact mechanisms by which carrageenan interacts with cancer cells and its long-term effects on healthy and cancerous tissue. This research will help determine whether carrageenan can be safely integrated into cancer therapy or whether its use should be restricted to avoid potential adverse effects.
3.8. Impact on Other Diseases
In addition to its effects on inflammation, cancer and IBD, carrageenan has also been shown to have effects on other diseases. The administration of carrageenan to mice in drinking water can lead to increased glucose intolerance compared to a control group of mice that did not receive this additive, additionally leading to higher insulin levels and greater insulin resistance. The authors suggested that this compound may increase the risk of diabetes [101]. There have also been reported cases of IgE-mediated allergy after carrageenan supply [102,103]. There are also reports that ι carrageenan can inhibit the spread of murine cytomegalovirus [104], carrageenans λ, κ, and ι may have a protective effect against herpes simplex virus type 2 (HSV-2) infection in mice [105]. Carrageenan-based gels (Carraguard) have also been tested for efficacy, safety, and acceptability in the context of human immunodeficiency virus (HIV). It appears to be safe and acceptable, but there is evidence that it is not effective in preventing HIV infection [106,107,108,109]. It was shown that κ carrageenan extracted from Hypnea musciformis exhibited antibacterial activity against Staphylococcus aureus and antifungal activity against Candida albicans. Furthermore, in vitro, the same compound showed neuroprotective effects in 6-hydroxydopamine-induced neurotoxicity in human neuroblastoma cells [97].
4. Carrageenan, Degraded Carrageenan, and Poligeenan—Differences
It is important to note the difference between carrageenan, degraded carrageenan, and poligeenan. Poligeenan is formed from carrageenan during acid hydrolysis at pH 0.9–1.3 and temperature > 80 degrees Celsius under laboratory conditions. The term ‘degraded carrageenan’ currently refers to the products of the test material used in nutritional research. Both compounds are formed under laboratory conditions and are not part of the molecular weight profile of carrageenan. All of the above compounds have different molecular weights. Poligeenan has a low molecular weight, i.e., 10,000–20,000 Da, degraded carrageenan an average of 20,000–40,000 Da, while the carrageenan used in food products has a high molecular weight (200,000–800,000 Da). As suggested by the authors of the above review, confusing nomenclature causes problems in the precise understanding of studies [62] which was also noted during the writing of this paper. It is necessary to pay particular attention to the nomenclature and to distinguish between carrageenan found in food products and degraded carrageenan and poligeenan.
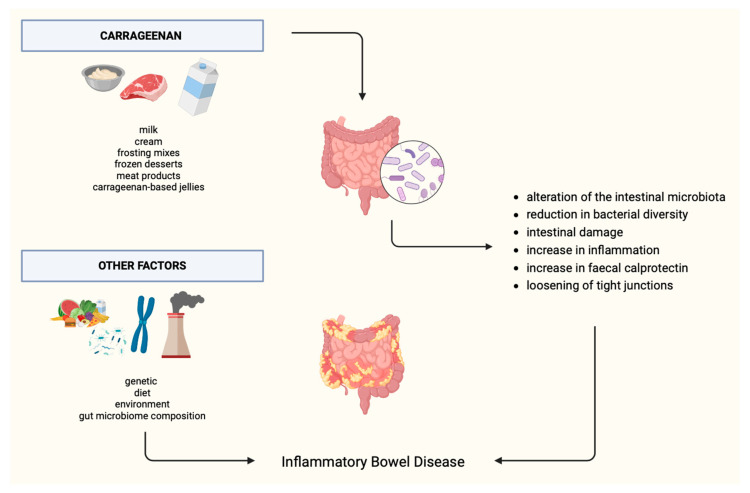
5. Conclusions
Carrageenan is a commonly used food additive that has been generally regarded as safe. However, recently there have been new studies assessing the effects of carrageenan on health, including IBD. Potentially pro-inflammatory properties, increasing intestinal permeability, impairing glucose tolerance, and the composition of the intestinal microbiome were observed. The effect of carrageenan on IBD is presented in Figure 3. On the other hand, there are studies suggesting that carrageenan may inhibit the growth of cancer cells; however, more studies are needed to determine the role of this additive in oncogenesis. Based on the available literature, it can be concluded that additional, particularly randomized clinical trials are needed to answer whether carrageenan (and other food additives) should be excluded from the diet of individuals exposed to and/or suffering from IBD. In addition, special attention should be paid to the exact nomenclature of carrageenan when writing scientific papers or constructing studies. Making a distinction between carrageenan presented in food, degraded carrageenan, and polygeenan is essential. The research should focus on examining both the effects of degraded carrageenan and carrageenan that is approved for human consumption.
Figure 3.
Effect of carrageenan on IBD.
Abbreviations
| ADI | adequate daily intake |
| CAGR | compound annual growth rate |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| DSS | dextran sodium sulphate |
| EFSA | European Food Safety Authority |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| ESR | erythrocyte sedimentation rate |
| FDA | Food and Drug Administration |
| HIV | human immunodeficiency virus |
| HSV-2 | herpes simplex virus type 2 |
| IBD | inflammatory bowel disease |
| IBS | irritable bowel syndrome |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| LPS | lipopolysaccharide |
| NF-κB | nuclear factor-κB |
| NOSB | National Organic Standards |
| OGTT | oral glucose tolerance test |
| SCCAI | Simple Clinical Colitis Activity Index |
| TNF-α | tumor necrosis factor α |
| UC | ulcerative colitis |
| ZO-1 | zonula occludens-1 |
Author Contributions
Conceptualization, and methodology: N.K., K.G.-C. and M.F.; writing—original draft preparation, N.K.; writing—review and editing, N.K., M.F., K.G.-C., M.P. and D.M.-W.; visualization, N.K.; supervision, M.F. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest in this topic.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bernstein C., Eliakim A., Fedail S., Fried M., Gearry R., Goh K.L., Hamid S., Khan A.G., Khalif I., Ng S.C., et al. World Gastroenterology Organisation Global Guidelines. J. Clin. Gastroenterol. 2016;50:803–818. doi: 10.1097/MCG.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 2.Jairath V., Feagan B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2020;5:2–3. doi: 10.1016/S2468-1253(19)30358-9. [DOI] [PubMed] [Google Scholar]
- 3.Mrowicki J., Mrowicka M., Majsterek I. Czynniki środowiskowe zwiększające ryzyko aktywacji i rozwoju chorób zapalnych jelit. Postep. Biochem. 2020;66:167–175. doi: 10.18388/pb.2020_318. [DOI] [PubMed] [Google Scholar]
- 4.Mak W.Y., Zhao M., Ng S.C., Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020;35:380–389. doi: 10.1111/jgh.14872. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 6.Fabián O., Kamaradová K. Morphology of inflammatory bowel diseases (IBD) Ceskoslovenska Patol. 2022;58:27–37. [PubMed] [Google Scholar]
- 7.Raine T., Bonovas S., Burisch J., Kucharzik T., Adamina M., Annese V., Bachmann O., Bettenworth D., Chaparro M., Czuber-Dochan W., et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis. 2022;16:2–17. doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M., Gönczi L., Lakatos P.L., Burisch J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis. 2021;15:1573–1587. doi: 10.1093/ecco-jcc/jjab029. [DOI] [PubMed] [Google Scholar]
- 9.Wojtuń S., Gil J., Szwed Ł., Dyrla P. Basic symptoms and differentiation of inflammatory bowel diseases. Pediatr. I Med. Rodz. 2014;10:61–66. doi: 10.15557/PiMR.2014.0009. [DOI] [Google Scholar]
- 10.Wang R., Li Z., Liu S., Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023;13:e065186. doi: 10.1136/bmjopen-2022-065186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorospe J., Windsor J., Hracs L., Coward S., Buie M., Quan J., Caplan L., Markovinovic A., Cummings M., Goddard Q., et al. Trends in Inflammatory Bowel Disease Incidence and Prevalence across Epidemiologic Stages: A Global Systematic Review with Meta-Analysis. Inflamm. Bowel. Dis. 2024;30((Suppl. S1)):S00. doi: 10.1093/IBD/IZAE020.085. [DOI] [Google Scholar]
- 12.Zhang Y.Z., Li Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba M., Nakane K., Komatsu M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm. J. 2019;23:18–107. doi: 10.7812/TPP/18-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mentella M.C., Scaldaferri F., Pizzoferrato M., Gasbarrini A., Miggiano G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients. 2020;12:944. doi: 10.3390/nu12040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruemmele F.M. Role of Diet in Inflammatory Bowel Disease. Ann. Nutr. Metab. 2016;68:33–41. doi: 10.1159/000445392. [DOI] [PubMed] [Google Scholar]
- 16.Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019;2019:7247238. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bischoff S.C., Bager P., Escher J., Forbes A., Hébuterne X., Hvas C.L., Joly F., Klek S., Krznaric Z., Ockenga O., et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023;42:352–379. doi: 10.1016/j.clnu.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Bouillon R., Manousaki D., Rosen C., Trajanoska K., Rivadeneira F., Richards J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022;18:96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aranow C. Correspondence: Cynthia Aranow 350 Community Drive Manhasset, NY 11030. J. Investig. Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharifi A., Hosseinzadeh-Attar M.J., Vahedi H., Nedjat S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi J. Gastroenterol. 2016;22:316–323. doi: 10.4103/1319-3767.187606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adolph T.E., Meyer M., Schwärzler J., Mayr L., Grabherr F., Tilg H. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2022;19:753–767. doi: 10.1038/s41575-022-00658-y. [DOI] [PubMed] [Google Scholar]
- 22.Martino J.V., Van Limbergen J., Cahill L.E. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front. Pediatr. 2017;5:96. doi: 10.3389/fped.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. [(accessed on 5 May 2024)]. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj.
- 24.Carocho M., Morales P., Ferreira I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015;45:284–295. doi: 10.1016/j.tifs.2015.06.007. [DOI] [Google Scholar]
- 25.Wu L., Zhang C., Long Y., Chen Q., Zhang W., Liu G. Food additives: From functions to analytical methods. Crit. Rev. Food Sci. Nutr. 2022;62:8497–8517. doi: 10.1080/10408398.2021.1929823. [DOI] [PubMed] [Google Scholar]
- 26.Valluzzi R.L., Fierro V., Arasi S., Mennini M., Pecora V., Fiocchi A. Allergy to food additives. Curr. Opin. Allergy Clin. Immunol. 2019;19:256–262. doi: 10.1097/ACI.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 27.Mazzucca C.B., Raineri D., Cappellano G., Chiocchetti A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients. 2021;13:3956. doi: 10.3390/nu13113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira M.T., Malik M., Nostro J.A., Mahler G.J., Musselman L.P. Effect of dietary additives on intestinal permeability in both Drosophila and a human cell co-culture. DMM Dis. Models Mech. 2018;11:dmm034520. doi: 10.1242/dmm.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerner A., Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015;14:479–489. doi: 10.1016/j.autrev.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura S., Aoi W., Kodani H., Kobayashi Y., Wada S., Kuwahata M., Higashi A. Polysorbate 80-induced leaky gut impairs skeletal muscle metabolism in mice. Physiol. Rep. 2020;8:e14629. doi: 10.14814/phy2.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmachari S., Jana A., Pahan K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Reduces Microglial and Astroglial Inflammatory Responses. J. Immunol. 2009;183:5917–5927. doi: 10.4049/jimmunol.0803336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atabaki M., Shariati-Sarabi Z., Tavakkol-Afshari J., Mohammadi M. Significant immunomodulatory properties of curcumin in patients with osteoarthritis; a successful clinical trial in Iran. Int. Immunopharmacol. 2020;85:106607. doi: 10.1016/j.intimp.2020.106607. [DOI] [PubMed] [Google Scholar]
- 33.Bush R.K., Zoratih E., Taylor S.L. Diagnosis of sulfite and aspirin sensitivity. Clin. Rev. Allergy. 1990;8:159. doi: 10.1007/BF02914443. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson D., Simon R., Lumry W., Mathison D. Adverse reactions to tartrazine. J. Allergy Clin. Immunol. 1986;78:182–191. doi: 10.1016/0091-6749(86)90011-4. [DOI] [PubMed] [Google Scholar]
- 35.Balatsinou L., Di Gioacchino G., Sabatino G., Cavallucci E., Caruso R., Gabriele E., Ramondo S., Di Giampaolo L., Verna N., Di Gioacchino M. Asthma Worsened by Benzoate Contained in Some Antiasthmatic Drugs. Int. J. Immunopathol. Pharmacol. 2004;17:225–226. doi: 10.1177/039463200401700215. [DOI] [PubMed] [Google Scholar]
- 36.Mccann D., Barrett A., Cooper A., Crumpler D., Dalen L., Grimshaw K., Kitchin E., Lok K., Porteous L., Prince E., et al. Articles Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet. 2007;370:1560–1567. doi: 10.1016/S0140-6736(07)61306-3. [DOI] [PubMed] [Google Scholar]
- 37.Kirkland A.E., Langan M.T., Holton K.F. Artificial food coloring affects EEG power and ADHD symptoms in college students with ADHD: A pilot study. Nutr. Neurosci. 2022;25:159–168. doi: 10.1080/1028415X.2020.1730614. [DOI] [PubMed] [Google Scholar]
- 38.Sandall A.M., Cox S.R., Lindsay J.O., Gewirtz A.T., Chassaing B., Rossi M., Whelan K. Emulsifiers impact colonic length in mice and emulsifier restriction is feasible in people with Crohn’s disease. Nutrients. 2020;12:2827. doi: 10.3390/nu12092827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suez J., Cohen Y., Valdés-Mas R., Mor U., Dori-Bachash M., Federici S., Zmora N., Leshem A., Heinemann M., Linevsky R., et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. 2022;185:3307–3328.e19. doi: 10.1016/j.cell.2022.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 41.Pepino M.Y., Tiemann C.D., Patterson B.W., Wice B.M., Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 2013;36:2530–2535. doi: 10.2337/dc12-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al Bander Z., Nitert M.D., Mousa A., Naderpoor N. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public Health. 2020;17:7618. doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao Y., Liu H., Qin N., Ren X., Zhu B., Xia X. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci. Technol. 2020;99:295–310. doi: 10.1016/j.tifs.2020.03.006. [DOI] [Google Scholar]
- 44.Gültekin F. Food Additives And Microbiota. North. Clin. Istanb. 2019;7:192–200. doi: 10.14744/nci.2019.92499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chassaing B., Compher C., Bonhomme B., Liu Q., Tian Y., Walters W., Nessel L., Delaroque C., Hao F., Gershuni V., et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology. 2022;162:743–756. doi: 10.1053/j.gastro.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loayza J.J.J., Kang S., Schooth L., The J.J., de Klerk A., Noon E.K., Zhang J., Hu J., Hamilton A.L., Wilson-O’Brien A., et al. Effect of food additives on key bacterial taxa and the mucosa-associated microbiota in Crohn’s disease. The ENIGMA study. Gut Microbes. 2023;15:2172670. doi: 10.1080/19490976.2023.2172670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bancil A.S., Sandall A.M., Rossi M., Chassaing B., Lindsay J.O., Whelan K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohn’s Colitis. 2021;15:1068–1079. doi: 10.1093/ecco-jcc/jjaa254. [DOI] [PubMed] [Google Scholar]
- 48.De Siena M., Raoul P., Costantini L., Scarpellini E., Cintoni M., Gasbarrini A., Rinninella E., Mele M.C. Food Emulsifiers and Metabolic Syndrome: The Role of the Gut Microbiota. Foods. 2022;11:2205. doi: 10.3390/foods11152205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu Q., Kirby J., Reilly C.M., Luo X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugea A., Salas A., Casalot J., Guarner F., Malagelada J.-R. Surface Hydrophobicity of the Rat Colonic Mucosa is a Defensive Barrier against Macromolecules and Toxins. [Online] 2000. [(accessed on 8 May 2024)]. Available online: http://gut.bmj.com/ [DOI] [PMC free article] [PubMed]
- 51.Um C., Hodge R., Gewirtz A., Stevens V., Jacobs E., Mccullough M. Emulsifier and Highly Processed Food Intake and Biomarkers of Intestinal Permeability and inflammation in the Cancer Prevention Study-3 Diet Assessment Sub-Study. Curr. Dev. Nutr. 2020;4:1498. doi: 10.1093/cdn/nzaa061_126. [DOI] [PubMed] [Google Scholar]
- 52.Mine Y., Zhang J.W. Surfactants Enhance the Tight-Junction Permeability of Food Allergens in Human Intestinal Epithelial Caco-2 Cells. Int. Arch. Allergy Immunol. 2003;130:135–142. doi: 10.1159/000069009. [DOI] [PubMed] [Google Scholar]
- 53.Błaszak B., Gozdecka G., Shyichuk A. Carrageenan as a functional additive in the production of cheese and cheese-like products. Acta Sci. Pol. Technol. Aliment. 2018;17:107–116. doi: 10.17306/J.AFS.0550. [DOI] [PubMed] [Google Scholar]
- 54.Necas J., Bartosikova L. Carrageenan: A review. Veterinární Med. 2013;58:187–205. [Google Scholar]
- 55.Thevenet F. Food Stabilisers, Thickeners and Gelling Agents. Blackwell Publishing Ltd.; Hoboken, NJ, USA: 2009. [Google Scholar]
- 56.Borsani B., De Santis R., Perico V., Penagini F., Pendezza E., Dilillo D., Bosetti A., Zuccotti G.V., D’Auria E. The role of carrageenan in inflammatory bowel diseases and allergic reactions: Where do we stand? Nutrients. 2021;13:3402. doi: 10.3390/nu13103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. [(accessed on 8 May 2024)]. Available online: https://www.fao.org/3/y4765e/y4765e0a.htm.
- 58.Younes M., Aggett P., Aguilar F., Crebelli R., Filipič M., Frutos M.J., Galtier P., Gott D., Gundert-Remy U., Kuhnle G.G., et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018;16:e05238. doi: 10.2903/j.efsa.2018.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen S.M., Ito N. A Critical Review of the Toxicological Effects of Carrageenan and Processed Eucheuma Seaweed on the Gastrointestinal Tract. Crit. Rev. Toxicol. 2002;32:413–444. doi: 10.1080/20024091064282. [DOI] [PubMed] [Google Scholar]
- 60.David S., Shani Levi C., Fahoum L., Ungar Y., Meyron-Holtz E.G., Shpigelman A., Lesmes U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018;9:1344–1352. doi: 10.1039/c7fo01721a. [DOI] [PubMed] [Google Scholar]
- 61.Tobacman J.K. Review of Harmful Gastrointestinal Effects of Carrageenan in Animal Experiments. [Online] [(accessed on 8 May 2024)];2001 doi: 10.1289/ehp.01109983. Available online: http://ehpnet1.niehs.nih.gov/docs/2001/109p983-994tobacman/abstract.html. [DOI] [PMC free article] [PubMed]
- 62.McKim J.M., Willoughby J.A., Blakemore W.R., Weiner M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Crit. Rev. Food Sci. Nutr. 2019;59:3054–3073. doi: 10.1080/10408398.2018.1481822. [DOI] [PubMed] [Google Scholar]
- 63.Bhattacharyya S., Shumard T., Xie H., Dodda A., Varady K.A., Feferman L., Halline A.G., Goldstein J.L., Hanauer S.B., Tobacman J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging. 2017;4:181–192. doi: 10.3233/NHA-170023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee D., Swan C.K., Suskind D., Wahbeh G., Vanamala J., Baldassano R.N., Leonard M.B., Lampe J.W. Children with Crohn’s Disease Frequently Consume Select Food Additives. Dig. Dis. Sci. 2018;63:2722–2728. doi: 10.1007/s10620-018-5145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. [(accessed on 8 May 2024)]. Available online: https://www.mordorintelligence.com/industry-reports/global-carrageenan-market-industry.
- 66. [(accessed on 8 May 2024)]. Available online: https://www.researchandmarkets.com/report/carrageenan?gclid=CjwKCAjw38SoBhB6EiwA8EQVLg2cRVvixQPdRQhCpReJE64yyMJdLM42ZTDRXdgqopAB3RJcTNLtmxoCuwsQAvD_BwE.
- 67.Liu F., Duan G., Yang H. Recent advances in exploiting carrageenans as a versatile functional material for promising biomedical applications. Int. J. Biol. Macromol. 2023;235:123787. doi: 10.1016/j.ijbiomac.2023.123787. [DOI] [PubMed] [Google Scholar]
- 68.Popa E.G., Gomes M.E., Reis R.L. Cell delivery systems using alginate-carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules. 2011;12:3952–3961. doi: 10.1021/bm200965x. [DOI] [PubMed] [Google Scholar]
- 69.Jecfa/and/Sc. Summary Report of the Seventy-Ninth Meeting of JECFA. [Online] [(accessed on 8 May 2024)]. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/en/andhttp://www.who.int/foodsafety/chem/jecfa/en/index.html.
- 70. [(accessed on 8 May 2024)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.620.
- 71. [(accessed on 8 May 2024)]; Available online: https://www.ams.usda.gov/rules-regulations/organic/petitioned-substances/carrageenan.
- 72.Marcus R. Carrageenan-induced ulceration of the large intestine in the guinea pig. Gut. 1971;12:164–171. doi: 10.1136/gut.12.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fath R.B., Deschner E.E., Winawer S.J., Dworkin B.M. Degraded Carrageenan-Induced Colitis in CF! Mice A Clinical, Histopathological and Kinetic Analysis. Digestion. 1984;29:197–203. doi: 10.1159/000199033. [DOI] [PubMed] [Google Scholar]
- 74.Al-Suhail A.A., Reid P.E., Culling C.F.A., Dunn W.L., Clay M.G. Studies of the degraded carrageenan-induced colitis of rabbits. I. Changes in the epithelial glycoproteinO-acylated sialic acids associated with ulceration. Histochem. J. 1984;16:543–553. doi: 10.1007/BF01041354. [DOI] [PubMed] [Google Scholar]
- 75.Marcus R., Watt J. Colonic Ulceration in Young Rats Fed Degraded Carrageenan. Lancet. 1971;298:765–766. doi: 10.1016/S0140-6736(71)92130-1. [DOI] [PubMed] [Google Scholar]
- 76.Sharratt M., Grasso P., Carpanini F., Gangolli S.D. Carrageenan ulceration as a model for human ulcerative colitis. Lancet. 1971;297:192–193. doi: 10.1016/S0140-6736(71)91971-4. [DOI] [PubMed] [Google Scholar]
- 77.Munyaka P.M., Sepehri S., Ghia J.E., Khafipour E. Carrageenan gum and adherent invasive Escherichia coli in a piglet model of inflammatory bowel disease: Impact on intestinal mucosa-associated microbiota. Front. Microbiol. 2016;7:462. doi: 10.3389/fmicb.2016.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Narula N., Wong E.C.L., Dehghan M., Mente A., Rangarajan S., Lanas F., Lopez-Jaramillo P., Rohatgi P., Lakshmi P.V.M., Varma R.P., et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ. :2021. doi: 10.1136/bmj.n1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pogozhykh D., Posokhov Y., Myasoedov V., Gubina-Vakulyck G., Chumachenko T., Knigavko O., Polikarpova H., Kalashnyk-Vakulenko Y., Sharashydze K., Nakonechna O., et al. Experimental Evaluation of Food-Grade Semi-Refined Carrageenan Toxicity. Int. J. Mol. Sci. 2021;22:11178. doi: 10.3390/ijms222011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi H.J., Kim J., Park S.-H., Do K.H., Yang H., Moon Y. Pro-inflammatory NF-κB and early growth response gene 1 regulate epithelial barrier disruption by food additive carrageenan in human intestinal epithelial cells. Toxicol. Lett. 2012;211:289–295. doi: 10.1016/j.toxlet.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 81.Wu W., Zhen Z., Niu T., Zhu X., Gao Y., Yan J., Chen Y., Yan X., Chen H. K-Carrageenan Enhances Lipopolysaccharide-Induced Interleukin-8 Secretion by Stimulating the Bcl10-NF-kB Pathway in HT-29 Cells and Aggravates C. freundii-Induced Inflammation in Mice. Mediat. Inflamm. 2017;2017:8634865. doi: 10.1155/2017/8634865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mi Y., Chin Y.X., Cao W.X., Chang Y.G., Lim P.E., Xue C.H., Tang Q.J. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020;147:284–294. doi: 10.1016/j.ijbiomac.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 84.Wu W., Zhou J., Xuan R., Chen J., Han H., Liu J., Niu T., Chen H., Wang F. Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022;277:118830. doi: 10.1016/j.carbpol.2021.118830. [DOI] [PubMed] [Google Scholar]
- 85.Shang Q., Sun W., Shan X., Jiang H., Cai C., Hao J., Li G., Yu G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017;279:87–95. doi: 10.1016/j.toxlet.2017.07.904. [DOI] [PubMed] [Google Scholar]
- 86.Naimi S., Viennois E., Gewirtz A.T., Chassaing B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome. 2021;9:66. doi: 10.1186/s40168-020-00996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 88. [(accessed on 8 May 2024)]. Available online: https://gco.iarc.fr.
- 89.Liu Z., Gao T., Yang Y., Meng F., Zhan F., Jiang Q., Sun X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules. 2019;24:4286. doi: 10.3390/molecules24234286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El-Deeb N.M., Ibrahim O.M., Mohamed M.A., Farag M.M.S., Farrag A.A., El-Aassar M.R. Alginate/κ-carrageenan oral microcapsules loaded with Agaricus bisporus polysaccharides MH751906 for natural killer cells mediated colon cancer immunotherapy. Int. J. Biol. Macromol. 2022;205:385–395. doi: 10.1016/j.ijbiomac.2022.02.058. [DOI] [PubMed] [Google Scholar]
- 91.Prasedya E.S., Miyake M., Kobayashi D., Hazama A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement. Altern. Med. 2016;16:270. doi: 10.1186/s12906-016-1199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sayın M., Akan H.S., Atalay Ö., Kubat E., Gürpınar A. Cytotoxic Activity of Carrageenan on Malignant MCF-7 Breast Cancer and The Non-Malignant SVCT Breast Epithelial Cell Lines. Ege Tıp Bilim. Derg. 2022;5:35–39. doi: 10.33713/egetbd.1110113. [DOI] [Google Scholar]
- 93.Cotas J., Marques V., Afonso M.B., Rodrigues C.M.P., Pereira L. Antitumour potential of gigartina pistillata carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs. 2020;18:50. doi: 10.3390/md18010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo M., Shao B., Nie W., Wei X.W., Li Y.L., Wang B.L., He Z.Y., Liang X., Ye T.H., Wei Y.Q. Antitumor and Adjuvant Activity of λ-carrageenan by Stimulating Immune Response in Cancer Immunotherapy. Sci. Rep. 2015;5:srep11062. doi: 10.1038/srep11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calvo G.H., Cosenza V.A., Sáenz D.A., Navarro D.A., Stortz C.A., Céspedes M.A., Mamone L.A., Casas A.G., Di Venosa G.M. Disaccharides obtained from carrageenans as potential antitumor agents. Sci. Rep. 2019;9:6654. doi: 10.1038/s41598-019-43238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jazzara M., Ghannam A., Soukkarieh C., Murad H. Anti-proliferative activity of λ-carrageenan through the induction of apoptosis in human breast cancer cells. Int. J. Cancer Manag. 2016;9:e3836. doi: 10.17795/ijcp-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Souza R.B., Frota A.F., Silva J., Alves C., Neugebauer A.Z., Pinteus S., Rodrigues J.A.G., Cordeiro E.M.S., de Almeida R.R., Pedrosa R., et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018;112:1248–1256. doi: 10.1016/j.ijbiomac.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 98.Cicinskas E., Begun M.A., Tiasto V.A., Belousov A.S., Vikhareva V.V., Mikhailova V.A., Kalitnik A.A. In vitro antitumor and immunotropic activity of carrageenans from red algae Chondrus armatus and their low-molecular weight degradation products. J. Biomed. Mater. Res. A. 2020;108:254–266. doi: 10.1002/jbm.a.36812. [DOI] [PubMed] [Google Scholar]
- 99.Watanabe K., Reddy B.S., Wong C.Q., Weisburger J.H. Effect of dietary undegraded carrageenan on colon carcinogenesis in F344 rats treated with azoxymethane or methylnitrosourea. Cancer Res. 1978;38:4427–4430. [PubMed] [Google Scholar]
- 100.Wang X., Fang Y., Liang W., Liang M., Xu L., Yu J. IDDF2022-ABS-0253 Dietary additive carrageenan metabolized by human GUT microbiota and promoting colorectal cancer. Gut. 2022;71:A66. doi: 10.1136/gutjnl-2022-IDDF.79. [DOI] [Google Scholar]
- 101.Bhattacharyya S., O-Sullivan I., Katyal S., Unterman T., Tobacman J.K. Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signalling in HepG2 cells and C57BL/6J mice. Diabetologia. 2012;55:194–203. doi: 10.1007/s00125-011-2333-z. [DOI] [PubMed] [Google Scholar]
- 102.Tarlo S., Dolovich J., Listgarten C. Anaphylaxis to carrageenan: A pseudo–latex allergy. J. Allergy Clin. Immunol. 1995;95:933–936. doi: 10.1016/S0091-6749(95)70091-9. [DOI] [PubMed] [Google Scholar]
- 103.Kular H., Dean J., Cook V. A Case of Carrageenan Allergy in a Pediatric Patient. Ann. Allergy Asthma Immunol. 2018;121:S119. doi: 10.1016/j.anai.2018.09.395. [DOI] [Google Scholar]
- 104.Hamasuna R., Eizuru Y., Minamishima Y. Inhibition by iota-carrageenan of the spread of murine cytomegalovirus from the peritoneal cavity to the blood plasma. J. Gen. Virol. 1994;75:111–116. doi: 10.1099/0022-1317-75-1-111. [DOI] [PubMed] [Google Scholar]
- 105.Zacharopoulos V.R., Phillips D.M. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin. Diagn. Lab. Immunol. 1997;4:465–468. doi: 10.1128/cdli.4.4.465-468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van de Wijgert J.H.H.M., Braunstein S.L., Morar N.S., Jones H.E., Madurai L., Strickfaden T.T., Moodley M., Aboobaker J., Ndlovu G., Ferguson T.M., et al. Carraguard Vaginal Gel Safety in HIV-Positive Women and Men in South Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2007;46:538–546. doi: 10.1097/QAI.0b013e318159d5a8. [DOI] [PubMed] [Google Scholar]
- 107.Ramjee G., Morar N.S., Braunstein S., Friedland B., Jones H., van de Wijgert J. Acceptability of Carraguard, a candidate microbicide and methyl cellulose placebo vaginal gels among HIV-positive women and men in Durban, South Africa. AIDS Res. Ther. 2007;4:20. doi: 10.1186/1742-6405-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kilmarx P.H., Blanchard K., Chaikummao S., Friedland B.A., Srivirojana N., Connolly C., Witwatwongwana P., Supawitkul S., Mock P.A., Chaowanachan T., et al. A Randomized, Placebo-Controlled Trial to Assess the Safety and Acceptability of Use of Carraguard Vaginal Gel by Heterosexual Couples in Thailand. Sex. Transm. Dis. 2008;35:226–232. doi: 10.1097/OLQ.0b013e31815d6e0d. [DOI] [PubMed] [Google Scholar]
- 109.Skoler-Karpoff S., Ramjee G., Ahmed K., Altini L., Plagianos M.G., Friedland B., Govender S., De Kock A., Cassim N., Palanee T., et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]