Abstract
Objective
Sepsis often prompts clinicians to start empirical antibiotics in suspected neonates while awaiting diagnosis. The next-generation testing with point-of-care (POC) techniques offers a lead-time advantage that could bridge the gap by providing a timely diagnosis.
Materials and Methods
We conducted a prospective diagnostic study in 82 neonates enrolled between May and October 2022 in a level III neonatal intensive care unit. All neonates with a new episode of clinically suspected sepsis were included. Diagnostic accuracy of POC testing of C-reactive protein (CRP), interleukin-6 (IL-6), and procalcitonin (PCT) with standard laboratory methods was performed.
Results
The mean gestation age and birth weight of the neonates were 33.17 ± 4.25 weeks and 1,695.4 ± 700.74 grams, respectively. Most neonates were preterm (75%) with nearly equal proportions of early (51.22%) and late-onset (48.78%) sepsis. The POC CRP correlated well with standard CRP (r = 0.8001, 95% CI: 0.706–0.867, p < 0.0001). Among the three biomarkers, CRP had the maximum diagnostic accuracy (area under the curve [AUC] – 0.73) followed by PCT (AUC – 0.65) and IL-6 (0.55). There was no significant difference in the diagnostic accuracy of CRP (p = 0.46), PCT (p = 0.29), and IL-6 (p = 0.60) in early- and late-onset sepsis. The mean time for POC estimation of IL-6, PCT, and CRP was 12 ± 3 min which was significantly less compared to 366 ± 61 min for standard techniques (p < 0.001).
Conclusion
POC CRP correlates well with standard techniques of estimation, and CRP alone and in combination with PCT has good diagnostic accuracy in neonatal sepsis.
Keywords: Bedside testing, Biological markers, Neonatal intensive care units, Diagnosis
Highlights of the Study
Point-of-care (POC) C-reactive protein (CRP) levels correlate well with standard laboratory estimation.
CRP alone and in combination with procalcitonin (PCT) has maximum diagnostic accuracy in neonatal sepsis.
POC testing can be used in both early- and late-onset sepsis with similar diagnostic accuracy.
The mean time required for POC estimation of interleukin-6, PCT, and CRP is nearly 12 min which is significantly less than standard techniques.
Introduction
Sepsis remains an important cause of neonatal morbidity and mortality, and due to the frequent overlap of symptoms with other neonatal morbidities and the lack of specific signs, investigations are often needed to confirm the diagnosis [1, 2]. The gold standard of diagnosis to date is blood culture. Despite innovations, the turnaround time is around 36–48 h using the BacT/Alert microbial detection system [3]. Traditional screening methods rely on hematological indices and acute phase reactants that require a few hours to obtain results. In neonatal intensive care units (NICUs), a “sepsis screen” comprising of total leukocyte count, absolute neutrophil count, immature to total leukocyte count ratio, C-reactive protein (CRP), and micro-erythrocyte sedimentation rate (ESR) is commonly used. However, blood counts tend to rise after 8–10 h to detectable levels [4]. Interleukin-6 (IL-6) and procalcitonin (PCT) have been found to rise around the time of onset of sepsis and can give early clues to diagnosis [5]. Acute phase reactants such as CRP and PCT are produced by the liver secondary to cytokines released following sepsis. CRP rises within 10–12 h after bacterial infection and peaks at 36–48 h. Serial measurements of CRP have been shown to have increased sensitivity and specificity for monitoring response in neonates undergoing treatment for sepsis [5]. PCT rises rapidly within 2–4 h and peaks within 6–8 h, and additionally, it may be useful in differentiating between bacterial and viral etiologies, as it does not rise significantly in viral infection.
Recent advancements in the early diagnosis of sepsis include the use of biomarkers such as cell adhesion molecules and interleukins [6]. Hence, in the current scenario, diagnostics that allow rapid confirmation in suspect cases are of vital importance. This highlights the need for an ideal technology that has high diagnostic accuracy, is not time-consuming, and is readily available bedside. This has opened new areas of research focused on finding the ideal biomarker and validation of point-of-care (POC) testing for neonatal sepsis. POC devices have been developed on the principles of sandwich enzyme-linked immunosorbent assay, which detects and quantifies the biomarkers by forming antibody-biomarker-antibody complexes and recently with microfluidic technology [7]. In addition to early diagnosis leading to timely intervention, they also aid in limiting the use of empiric antibiotics to prevent the emergence of multidrug-resistant organisms. However, there are few studies evaluating the diagnostic accuracy of POC biomarkers to facilitate rapid diagnosis of neonatal sepsis.
In this prospective observational study in neonates with clinically suspected sepsis, we evaluated the accuracy of the POC CRP and laboratory CRP for the diagnosis of sepsis and determined the correlation of values obtained by two techniques. Diagnostic accuracy of POC CRP, IL-6, and PCT was also carried out and evaluated as per the timing of onset of sepsis, namely, early- and late-onset of sepsis.
Materials and Methods
This prospective study was carried out from May to October 2022 in a level III NICU in a tertiary care hospital in Western India after obtaining permission from Institutional Ethics Committee. All neonates with a new episode of clinically suspected sepsis were included after obtained written informed consent from the parents. Neonates with life-threatening congenital malformations were excluded. The sample size was based on unit (unpublished) data of 40% of the neonates with a positive CRP in case of a new episode of clinically suspected sepsis. We calculated the sample size as 82 neonates with a new episode of clinically suspect sepsis to determine a sensitivity of 95% and specificity of 85% (with a 95% confidence interval of ±5%) for POC CRP, IL-6, and PCT.
Clinically Suspected Sepsis
A neonate was identified with a new episode of clinically suspected sepsis with the onset of clinical signs and symptoms suggestive of sepsis and mandated starting of empirical antibiotics or upgradation of ongoing antibiotics while the blood cultures were awaited. Depending upon the onset of sepsis, the neonates were classified as early-onset (within 72 h of life) and late-onset (beyond 72 h of life) sepsis.
Standard Laboratory Investigations
For all neonates with clinically suspected sepsis, a sepsis screen and blood culture were considered standard laboratory investigations. The sepsis screen consisted of total leukocyte count, absolute neutrophil count, immature to total neutrophil ratio, micro-ESR, and CRP. A total count of >25,000/mm3 or <5,000/mm3, absolute neutrophil count <1,750/mm3, and an immature to total neutrophil ratio greater than 20% were considered abnormal. The micro-ESR >15 mm at the end of 1 h and CRP of more than 10 mg/L were considered abnormal. A free-flowing venous sample was obtained under an aseptic technique for the determination of complete blood count and CRP. Complete blood count and differentials were determined by ERBA 5-part and Sysmex 200 5-part analyzers (Sysmex America, Inc., USA). Abnormal blood counts were confirmed by a microscopic smear evaluation by a pathologist. CRP was analyzed by immunoturbidimetric assay by Randox Daytona automated analyzer (Crumlin, Northern Ireland). Micro-ESR was done in the unit side laboratory, where blood was collected in a pre-heparinized microhematocrit tube with a length of 75 mm, an internal diameter of 1.1 mm, and an external diameter of 1.5 mm. One end of the capillary was sealed by soap wax, and the microhematocrit tube was fixed vertically and left undisturbed for an hour. The micro-ESR level was determined by the height of the plasma column of each tube. A “positive” screen was defined as two out of five parameters being abnormal as per the cutoffs. Blood culture was done by the BACTEC automation system (Becton Dickinson, Ireland). Clinical decisions were made based on standard laboratory investigations. Additional evaluation in the form of lumbar puncture, chest radiography, or urine culture was performed as clinically indicated.
POC Testing
A simultaneous POC quantitative estimation of CRP, IL-6, and PCT was done for all included neonates by collecting 0.1 mL of whole blood during the same venipuncture. The samples were analyzed by the Boditech Med NICU panel of AFIAS-6 (Boditech Med Inc., South Korea). The POC testing was based on fluorescence immunoassay, which used a sandwich immuno-detection method in which the detector antibodies in the buffer bind to antigens in the sample, forming antigen-antibody complexes. These complexes then migrate onto the nitrocellulose matrix to be captured by the other immobilized antibodies on the test strip. More antigens in the sample led to stronger fluorescence signals by detector antibodies. The cartridge part contains the membrane called a test strip which has streptavidin at the test line and chicken IgY at the control line. The detector part has two granules containing the anti-NT-proBNP-fluorescence conjugate, biotin-anti-NT-proBNP conjugate and anti-chicken IgY-fluorescence conjugate, and sodium azide as a preservative in Tris-Cl buffer. Three separate cartridges were used for estimating CRP, IL-6, and PCT. Blood from the sample was manually instilled into three cartridges which were then inserted into the machine and then run simultaneously. Hence, the samples were processed independently in the separate cartridges.
The cartridges were stored in the refrigerator at 4°C and brought to room temperature approximately 30 min before use. The cartridge was half inserted into the cartridge holder of the machine. A C-tip provided in the kit was placed in contact with the blood drop, and with capillary action, 0.03 mL of blood was drawn. The whole blood-filled C-tip was placed into the tip hole of the cartridge, and the rest of the cartridge was inserted into its holder. The test result was displayed in approximately 12 min.
For the estimation of IL-6, 0.03 mL whole blood sample was required, and a cutoff was 7 pg/mL with a working range of 2–2,500 pg/mL. CRP was detected by 0.01 mL whole blood sample, and a cutoff was 10 mg/L with a working range of 0.5–200 mg/L. For PCT, 0.05 mL whole blood sample was required, and a cutoff of 2 ng/mL with a working range of 0.1–100 ng/mL was used.
Data Collection and Analysis
Details of antenatal risk factors and birth details such as mode of delivery, gestational age, and birth weight were noted. Preterm rupture of membranes of any duration in preterm and of duration >18 h in term infants was taken as significant. The details of the day of onset of sepsis, clinical features, and results of standard laboratory testing were recorded.
Data were entered in MS Excel and analyzed using SPSS software version 23. An independent t test was used for continuous data and χ2 and Fisher exact tests for categorical data. A p value of <0.05 was considered statistically significant. The sensitivity, specificity, positive predictive value, and negative predictive value of POC CRP at 10 mg/L cutoff were determined against the standard laboratory estimation of CRP. The correlation of POC CRP with standard CRP was done using linear regression and the determination of the coefficient of correlation. The diagnostic accuracy of the POC testing of IL-6, PCT, and CRP was compared to standard laboratory testing (either a positive sepsis screen or a positive blood culture). The area under the curves (AUCs) was determined for individual biomarkers and for combinations of biomarkers.
Results
A total of 82 neonates with a new episode of clinically suspected sepsis admitted to the NICU between May and October 2022 were enrolled. The mean maternal age was 27.18 ± 3.75 years, with 15.85% having multiple gestations. The mean gestation age and birth weight of the neonates were 33.17 ± 4.25 weeks and 1,695.4 ± 700.74 grams, respectively. The population consisted predominantly of preterm neonates (75%) with nearly equal proportions of early- and late-onset sepsis. Other key demographic characteristics are depicted in Table 1. The common clinical presentation of sepsis in neonates included the presence of respiratory distress (71.95%), feeding intolerance (35.37%), and hemodynamic instability (30.49%) (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000536678).
Table 1.
Baseline characteristics of the study population
| Characteristic (n = 82) | N (%) |
|---|---|
| Gestation age | |
| <37 completed weeks | 62 (75.61) |
| ≥37 weeks | 20 (24.39) |
| Birth weight, g | |
| <1,000 | 9 (10.98) |
| 1,000–1,499 | 32 (39.02) |
| 1,500–1,999 | 21 (25.61) |
| 2,000–2,499 | 7 (8.54) |
| >2,500 | 13 (15.85) |
| Male | 55 (67.07) |
| Small for gestation age | 35 (42.68) |
| Risk factors for sepsis | |
| Preterm premature rupture of membranes | 24 (29.27) |
| Spontaneous onset of preterm labor | 11 (13.41) |
| Mechanical ventilation | 29 (35.37) |
| Central line placement | 40 (48.78) |
| Mode of delivery | |
| Vaginal | 36 (43.90) |
| Cesarean section | 46 (56.1) |
| Resuscitation | 10 (12.19) |
| Suspect sepsis | |
| Early-onset (<72 h) | 42 (51.22) |
| Late-onset (>72 h) | 40 (48.78) |
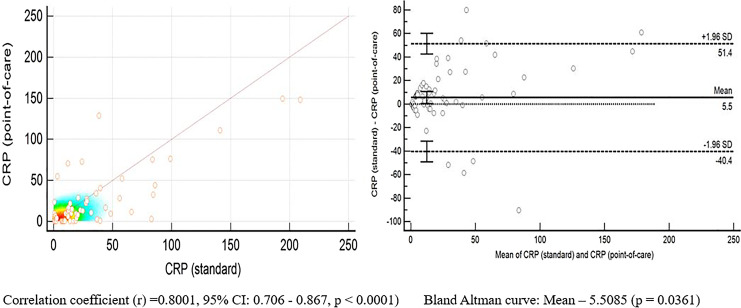
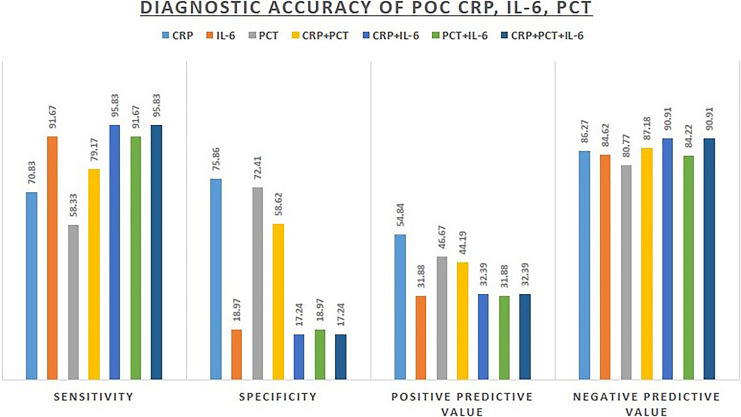
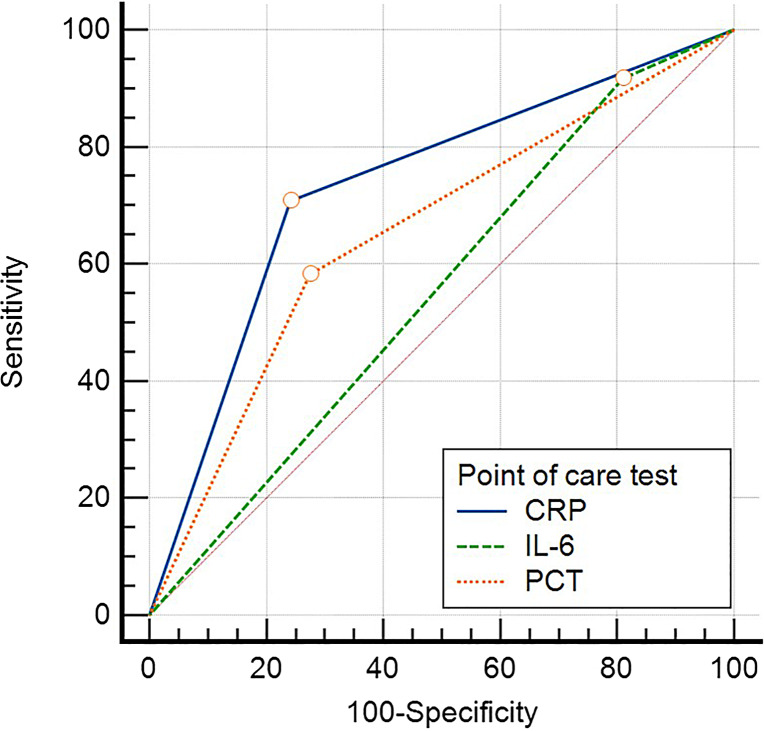
The POC CRP correlated well with standard CRP (r = 0.8001, 95% CI: 0.706–0.867, p < 0.0001) (Fig. 1). The diagnostic accuracy of POC testing of three biomarkers individually and in combinations is summarized in Figure 2. CRP had the highest diagnostic accuracy with the AUC of 0.733 (95% CI: 0.624–0.825). PCT had an AUC of 0.553 (95% CI: 0.439–0.663), and IL-6 had an AUC of 0.654 (95% CI: 0.541–0.755). There was a significant difference in the AUCs of CRP and IL-6 (p = 0.038). There was no significant difference in the AUCs of CRP and PCT (p = 0.33), and IL-6 and PCT (p = 0.21) (Fig. 3).
Fig. 1.
Correlation between POC CRP and standard laboratory CRP.
Fig. 2.
Diagnostic accuracy of POC testing of three biomarkers individually and in combinations.
Fig. 3.
Comparison of area under the curve (AUC) of three POC biomarkers (CRP, IL-6, and PCT) *Cutoffs used for CRP-10 mg/L, IL-6: 7 pg/mL, PCT: 2 ng/mL.
The AUC for CRP + IL-6 was 0.565 (0.451–0.675), CRP + PCT was 0.689 (0.577–0.787), PCT and IL-6 were 0.553 (0.439–0.663), and CRP + PCT + IL-6 were 0.565 (0.451–0.675). The differences in the AUCs of a combination of various biomarkers were not significant. The p values were 0.095 (CRP + IL-6 vs. CRP + PCT), 0.77 (CRP + IL-6 vs. PCT + IL-6), 1.00 (CRP + IL-6 vs. CRP + IL-6 + PCT), 0.09 (CRP + PCT vs. PCT + IL-6), 0.09 (CRP + PCT vs. CRP + PCT + IL-6) and 0.77 (PCT + IL-6 vs. CRP + PCT + IL-6).
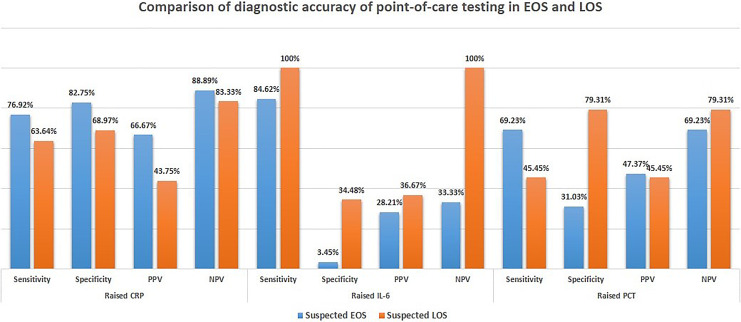
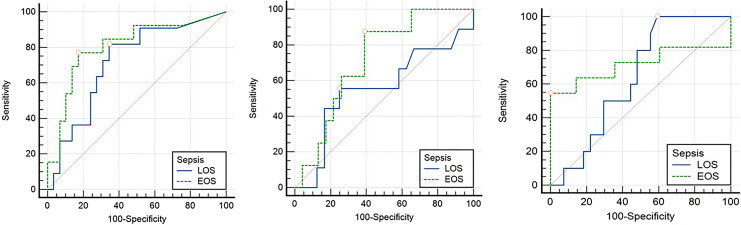
Figure 4 provides the comparison of the POC testing in early- and late-onset sepsis. There was no significant difference in the diagnostic accuracy of CRP (p = 0.46), PCT (p = 0.29), and IL-6 (p = 0.60) in early- and late-onset sepsis. The AUC for CRP, PCT, and IL-6 in early-onset sepsis was 0.718, 0.556, and 0.639, respectively. In late-onset sepsis, the AUC for CRP, PCT, and IL-6 were 0.805, 0.717, and 0.718, respectively (Fig. 5).
Fig. 4.
Diagnostic accuracy of POC testing of three biomarkers in early- and late-onset sepsis.
Fig. 5.
Comparison of area under the curve (AUC) of three POC biomarkers (CRP, IL-6, and PCT) in early- and late-onset sepsis. *Cutoffs used for CRP-10 mg/L, IL-6: 7 pg/mL, PCT: 2 ng/mL.
The mean time for estimation of the sepsis screen was 366 ± 61 min and 12 ± 3 min for POC estimation of IL-6, PCT, and CRP. The time for estimation by POC testing was significantly less than the time for estimation of the sepsis screen (p < 0.001).
Discussion
This study describes the diagnostic accuracy of POC testing of CRP, PCT, and IL-6 in 82 neonates with clinically suspected sepsis against the standard laboratory testing. There was a strong correlation of POC CRP with laboratory CRP with a correlation coefficient of 0.80. Among the three biomarkers tested for POC, CRP had the maximum diagnostic accuracy (AUC – 0.73) followed by PCT (AUC – 0.65) and IL-6 (0.55). A combination of CRP and PCT improved the diagnostic accuracy of PCT (AUC – 0.69). However, a combination of IL-6 with either of the other two biomarkers (CRP and PCT) did not increase the diagnostic accuracy of detecting sepsis in neonates.
These POC devices have been developed on the principles of sandwich enzyme-linked immunosorbent assay, which detects and quantifies the biomarkers by forming antibody-biomarker-antibody complexes. In the current study, we used the AFIAS-6 system (Boditech Inc., South Korea), in which fluorescence immunoassay was used for the detection of these biomarkers. We compared 82 paired CRP samples and found the POC CRP values to correlate well with laboratory CRP with a correlation coefficient of 0.80 (0.7056–0.8666) and a sensitivity of 70.8% and specificity of 75.9%. Similarly, Zecca et al. [8] tested the reliability of two different POC methods in 72 paired samples and compared them to standard laboratory testing. The methods were immunoturbidometric assay and sandwich-format immunometric assays with an excellent correlation with standard laboratory testing. The sensitivity was 97.2% and 94.4%, respectively, and the specificities were 80.5% and 83.3%, respectively. However, the accuracy of both of them was less when CRP concentrations were more than 100 mg/L [8]. In a study by Prince et al. [9] in 2019, POC conducted with an analyzer based on a solid phase immunochemical assay compared 139 paired CRP samples with the laboratory analyzer based on immunoturbidimetry. The population was mainly preterm (82.7%) with 44% neonates with early-onset sepsis and 56% with late-onset sepsis. The cutoff for CRP was 10 mg/L, and the sensitivity was 97.4% and specificity was 99% of POC CRP with laboratory CRP (AUC of 0.99) [9]. This highlights that the POC CRP by various methods correlates well with standard lab testing. However, the sensitivity and specificity of POC CRP in our study were lower as compared to studies by Zecca et al. and Prince et al. [9, 10] as we compared it to standard diagnostic methods for detecting sepsis and were not restricted only to raised CRP as taken in these studies.
Despite the availability of POC testing, its diagnostic accuracy needs to be established in comparison with standard diagnostic methods. In the present study, we found the POC CRP at cutoff >10 mg/L to have a good performance as a diagnostic biomarker in clinically suspected sepsis with an AUC of 0.73 in comparison to standard laboratory testing. PCT is being increasingly used for diagnosing and managing neonatal sepsis, and a meta-analysis was done in 2017 which analyzed data from 1,086 neonates and 322 children with microbiologically proven sepsis or those who had sepsis based upon clinical, radiological, and laboratory results, found sensitivity of 84% and specificity of 51% for PCT at a cutoff of 2 ng/mL [10]. In the present study, at a similar cutoff for PCT, we found a lower sensitivity of 58.33% but a higher specificity of 72.41%. With an AUC of 0.65, PCT had a moderate accuracy for detecting sepsis.
We also detected IL-6 levels in these neonates, and at a cutoff of 7 pg/mL, it had a high sensitivity of 91.67%, however, with an unacceptably low specificity (18.97%) with an AUC of 0.55, which questions its utility alone as a diagnostic biomarker in clinically suspected sepsis. A recent systematic review by Eichberger and Resch [11] also concluded that IL-6 has a high sensitivity but was poorly specific in early-onset sepsis. This concludes that it could serve as a useful tool to screen for sepsis in clinically suspected cases, however, needs to be combined with other tests with higher specificity to establish the diagnosis. We found IL-6 to have a low specificity in the detection of sepsis alone or in combination, albeit with a high sensitivity. In the present study, a result of IL-6 >7 pg/mL was used as a cutoff based on the manufacturer’s recommendations. However, this cutoff is probably low and hence decreased the specificity of the test considerably. A recent study analyzed IL-6 in 1,695 neonates, including 752 very preterm infants and 701 very low-birth-weight infants. They found cutoff values of 80 pg/mL on day of life 1, 40 pg/mL on day of life 2–7, and 30 values pg/mL after day of life 7 to achieve a sensitivity of 75% and a specificity of 81% for culture-confirmed sepsis [12].
On subgroup analysis, the diagnostic accuracy of CRP, PCT, and IL-6 was not statistically significantly higher in early-onset sepsis as compared to late-onset sepsis. However, Eschborn and Weitkamp who evaluated 29 studies found PCT and CRP to have higher sensitivity but less specificity in late-onset sepsis [13]. This variation is possibly due to the lack of power for subgroup analysis of early- and late-onset sepsis in our study.
The mean time for POC estimation of IL-6, PCT, and CRP was 12 ± 3 min, which was significantly less than the time for estimation of the sepsis screen (p < 0.001). Other studies found POC testing to yield results in <5 min [8, 9] which highlights the lead time advantage with these methods and ability to limit the exposure and duration of antibiotics and thereby promote antibiotic stewardship. These benefits are already being explored, and in a large multicentric randomized control trial conducted by Stocker et al. [14] in 1,710 neonates, with suspected early-onset sepsis, antibiotic duration in one of the arms was determined by the PCT as compared to standard therapy, and there was a significant reduction in the duration of the antibiotics in the former.
The strengths of the study include determining the diagnostic accuracy of three key biomarkers of neonatal sepsis using the latest POC technology individually as well as in combination. We analyzed the diagnostic accuracy in both early- and late-onset sepsis; however, the results were limited by the sample size of the study. Future studies focusing on the use of POC technology to direct antibiotic usage and determine the duration of therapy will be of utmost importance.
Conclusions
The POC diagnostic determination of CRP correlates well with standard techniques of estimation. CRP alone and in combination with PCT has good diagnostic accuracy in neonatal sepsis. The role of IL-6 needs to be further refined by the determination of appropriate cutoffs.
Statement of Ethics
The study was conducted after receiving ethical permission from the Institutional Ethics Committee at Seth G.S. Medical College and Kind Edward Memorial Hospital, Mumbai, India (EC/OA – 42/2022).
Conflict of Interest Statement
The authors have no conflicts of interest relevant to this article.
Funding Sources
No external funding was obtained for this manuscript.
Author Contributions
Medha Goyal, Dwayne Mascarenhas, and Prashanth RR conceptualized and designed the study, conducted the literature search, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. Anitha Haribalakrishna conceptualized and designed the study, coordinated and supervised data collection, critically reviewed and revised the manuscript for important intellectual content, and finalized the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding Statement
No external funding was obtained for this manuscript.
Data Availability Statement
All data generated during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Amare D, Mela M, Dessie G. Unfinished agenda of the neonates in developing countries: magnitude of neonatal sepsis: systematic review and meta-analysis. Heliyon. 2019;5(9):e02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Investigators of the Delhi Neonatal Infection Study DeNIS Collaboration, SankarHealth JN, Centre K. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health. 2016;4(10):e752–60. [DOI] [PubMed] [Google Scholar]
- 3. Kumar Y, Qunibi M, Neal TJ, Yoxall CW. Time to positivity of neonatal blood cultures. Arch Dis Child Fetal Neonatal Ed. 2000;85(3):F182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25–36. [DOI] [PubMed] [Google Scholar]
- 5. Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: a literature review. J Matern Fetal Neonatal Med. 2018;31(12):1646–59. [DOI] [PubMed] [Google Scholar]
- 6. Jyoti A, Kumar S, Kumar Srivastava V, Kaushik S, Govind Singh S. Neonatal sepsis at point of care. Clin Chim Acta. 2021;521:45–58. [DOI] [PubMed] [Google Scholar]
- 7. Bradley Z, Bhalla N. Point-of-care diagnostics for sepsis using clinical biomarkers and microfluidic technology. Biosens Bioelectron. 2023;227:115181. [DOI] [PubMed] [Google Scholar]
- 8. Zecca E, Barone G, Corsello M, Romagnoli C, Tiberi E, Tirone C, et al. Reliability of two different bedside assays for C-reactive protein in newborn infants. Clin Chem Lab Med. 2009;47(9):1081–4. [DOI] [PubMed] [Google Scholar]
- 9. Prince K, Omar F, Joolay Y. A comparison of point of care C-reactive protein test to standard C-reactive protein laboratory measurement in a neonatal intensive care unit setting. J Trop Pediatr. 2019;65(5):498–504. [DOI] [PubMed] [Google Scholar]
- 10. Pontrelli G, De Crescenzo F, Buzzetti R, Jenkner A, Balduzzi S, Calò Carducci F, et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: a meta-analysis. BMC Infect Dis. 2017;17:302–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eichberger J, Resch B. Reliability of interleukin-6 alone and in combination for diagnosis of early onset neonatal sepsis: systematic review. Front Pediatr. 2022;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Küng E, Unterasinger L, Waldhör T, Berger A, Wisgrill L. Cut-off values of serum interleukin-6 for culture-confirmed sepsis in neonates. Pediatr Res. 2022;93(7):1969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eschborn S, Weitkamp JH. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol. 2019;39(7):893–903. [DOI] [PubMed] [Google Scholar]
- 14. Stocker M, Van Herk W, El Helou S, Dutta S, Fontana MS, Schuerman FA, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet. 2017;390(10097):871–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.