Abstract
Background:
High-volume pancreatic surgery centers require a significant investment in expertise, time, and resources to achieve optimal patient outcomes. A detailed understanding of the economics of major pancreatic surgery is limited among many clinicians and hospital administrators. A greater consideration of these financial aspects may in fact have implications for enhancing clinical care and for a broader sustainability of high-volume pancreatic surgery programs.
Methods:
In this retrospective observational study, patients who underwent pancreaticoduodenectomy (PD), total pancreatectomy, or distal pancreatectomy at one academic medical center during the fiscal year 2021 were evaluated. Detailed hospital charges and professional fees were obtained for patients using the Qlik perioperative database. Clinical data for the study cohort were gathered from a prospectively maintained, IRB-approved pancreatic surgery database. Charges for the 91-day perioperative period were included. A P < 0.05 was considered significant.
Results:
During the study period, 159 evaluable patients underwent 1 of 3 designated pancreatic resections included in the analysis. Ninety-seven patients (61%) were diagnosed with adenocarcinoma and 70% (n = 110) underwent PD. The total charges (combined professional and hospital charges) for the cohort encompassing the entire perioperative period were $20,661,759. The median charge per patient was $130,306 (interquartile range [IQR], $34,534). The median direct cost of care was $23,219 (IQR, $6321) and the median contribution margin per case was $10,092 (IQR, $22,949). The median surgeon professional fee charges were $7700 per patient (IQR, $1296) as compared to $3453 (IQR, $1,144) for professional fee receipts (45% of the surgeon charge). The differences between the professional fee charges and receipts per patient were also considerable for other health care professionals such as anesthesiologists ($4945 charges vs $1406 receipts [28%]) and pathologists ($3035 charges vs $680 receipts [22%]). The surgeon professional fees were only 6% of the total charges, while the professional fees for anesthesiology and pathology were 4% and 2% of the total charges, respectively. Supply charges were 3% of the total charges. Longer operative time was correlated with increased hospital and anesthesia charges, without a significant increase in surgeon charges (P < 0.001, P < 0.001, and P = 0.2, respectively). Male sex, diabetes, and low serum albumin correlated with greater total hospital charges (P = 0.01, P = 0.01, and P = 0.03, respectively).
Conclusions:
The role of the surgeon in the perioperative clinical care of major pancreatic resection patients is crucial and important and is by no means limited to the operative day. Nevertheless, in the context of the current US health care system, the reimbursement to the surgeon in the form of professional fees is a relatively small fraction of the total health care receipts for these patients. This imbalance necessitates a substantial financial partnership between hospitals and their pancreatic surgery units to ensure the long-term viability of these programs.
Keywords: pancreatic surgery, whipple, pancreatectomy, economic, financial, charges, cost, reciepts, financial model
An understanding of the economics of major pancreatic surgery is limited among many clinicians and hospital administrators. Evaluation of major pancreatic resection patients through the year of 2021 was performed, demonstrating an imbalance that necessitates a financial partnership between hospitals and their pancreatic surgery units to ensure the long-term viability of these programs.
INTRODUCTION
High-volume academic pancreatic surgery centers have a complex tripartite mission: to provide excellent clinical care for patients with complex benign and malignant hepato-pancreato-biliary disease, to engage in innovative research, and to train the next generation of clinicians and surgeons. In order to succeed in these domains, a tremendous investment in time and resources is required. An understanding of the financial foundations of pancreatic surgical practice will be important for the long-term growth and viability of pancreatic surgery centers and may have implications towards the design of future healthcare systems and reimbursement practices for complex clinical care.
Relative value units (RVUs) is a system designed to provide relative economic values for medical care in the United States, based on the cost of the service. This includes mainly physician work, practice expenses, and professional liability, and it is the foundation of the “fee-for-service” model currently in widespread use.1 Fee for service is a system of health insurance payment, by which the hospital submits charges to a third-party payer (insurance body) who is contracted out to pay a portion as reimbursement. A health care provider is paid a fee for each particular service rendered; hence, this system is essentially rewarding medical providers for volume and quantity of services provided, regardless of the outcome and independent of value. It has become clear that understanding the professional charges and receipts for surgical care is imperative, with implications toward the long-term viability of complex surgery programs such as pancreatic surgery.
A small volume of literature currently exists regarding the complex relationship between clinical practice and hospital contribution margins for different operations,1–9 thus a detailed understanding of the economics of major pancreatic surgery is limited among clinicians. These complex operations are usually performed at large academic centers, necessitating multidisciplinary advanced teams and treatments and serving as an important component of hospital finance,5,6 including funds flow models.
During the past 2 decades, the field of pancreatic surgical care has burgeoned dramatically, with enhanced diagnostic capabilities and improvements in oncologic treatment regimens now widely adopted, increasing patient candidacy for resection and the complexity of procedures performed.10–12 Importantly, the multidisciplinary care team for pancreatic surgical patients has significantly evolved with patients often also treated by gastroenterologists, medical oncologists, radiation oncologists, anesthesiologists, and intensivists, to name the most common.
Despite these advancements, minimal attention has been paid to the financial implications as it pertains to the central role of the surgeon during the patient’s care “journey,” often translating to a primary caregiver/navigator role while fulfilling the academic tripartite mission. A greater consideration of these financial aspects may have implications for enhancing clinical care and for the broader sustainability of pancreatic surgery programs.
In this study, we aim to provide clarity concerning the financial implications of major pancreatic surgery for the hospital, the surgical team, and the other professional stakeholders, in one large academic medical center in the middle Atlantic region of the United States.
METHODS
Patient Cohort
This was a single-center, retrospective observational study designed to evaluate the financial implications of major pancreatic surgery for the surgeon, for other faculty (most notably anesthesiology and pathology), and for the hospital. Institutional review board approval was obtained. The study included all patients older than 18 years of age who underwent pancreaticoduodenectomy (PD), total pancreatectomy (TP), or distal pancreatectomy (DP) at Thomas Jefferson University Hospital (TJUH) during the fiscal year of 2021. The vast majority of operations were open resectional procedures. Clinical data of the study cohort was gathered from a prospectively maintained, IRB-approved pancreatic surgery database.
Hospital charges were accessed from the charge report section of our Qlik perioperative database. Professional fees were obtained from our Qlik PB charges, payments, and adjustments database. Facility payments, direct cost, and contribution margins were acquired from the financial decision support office at TJUH. Charges for the 91-day perioperative period (45 days pre- and postoperative and the operative day) are included. It is important to note that we chose not to include medical oncology or radiation oncology charges or receipts for either preoperative neoadjuvant treatment or postoperative adjuvant treatment, as both were deemed confounding.
Definitions
Hospital charge is the total (dollars) amount submitted to the payer by the hospital. Facility receipt represents the payment from the payer to the hospital. Facility direct cost entails the total hospital expenses for caring for the patient and facility contribution margin represents the hospital net profit. Professional charges are the amount submitted to the payer by the medical professional for a specific service, while professional receipts represent the payment from the payer to the professional. Total charge is the total dollar amount charged, for the hospital and professionals’ services.
Statistical Analyses
Continuous variables were summarized using medians and ranges, or means and standard deviations. Statistical analyses were performed using Mann–Whitney comparisons for nonparametric variables. Pearson and Spearman correlation analyses were carried to assess linear or directional correlations, respectively. P < 0.05 was considered significant. All analyses were conducted using SPSS (version 28.0.1.0, IBM).
RESULTS
Study Population
During the fiscal year of 2021, 172 patients were admitted for major pancreatic resection. Of those, 159 patients underwent the surgery as planned while 13 patients were excluded from the analysis after the planned surgery was aborted due to unresectability determined in the OR. The median age in the study cohort was 69 years (interquartile range [IQR], 13). Ninety-seven patients (61%) were diagnosed with adenocarcinoma, 23 (14%) with intraductal papillary mucinous neoplasm, and 10 (6%) with neuroendocrine tumor. Seventy-one patients (45%) had a smoking history and 18 patients (11%) ceased smoking only 3 to 8 weeks before surgery (smoking cessation is required to undergo pancreatic resection in our center). Comorbidities are further summarized in Table 1.
TABLE 1.
Demographic Characteristics of the Entire Patients’ Cohort
| Characteristic (N = 159) | Number of Patients (%) |
|---|---|
| Gender | |
| Female | 75 (47) |
| Male | 84 (53) |
| Age at surgery (median, IQR) | 69 years (13) |
| BMI at surgery (median, IQR) | 25.4 (5.7) |
| Comorbidities | |
| Cardiac disease | 13 (8) |
| Diabetes (not newly diagnosed) | 36 (23) |
| HTN | 33 (21) |
| Pulmonary disease | 11 (7) |
| Pancreatitis | 18 (11) |
| Smoking history | 71 (45) |
| Resent smoking | 18 (11) |
BMI indicates body mass index; HTN, hypertension.
One hundred ten patients (70%) underwent PD, 32 patients (20%) underwent DP, 10 patients (6%) underwent TP, and 7 patients (4%) underwent other pancreatic resectional procedures.
Eighty patients (50%) had private medical insurance (the most common payers were Aetna and Blue Cross). The other half were covered by Medicare and a single patient was covered by Medicaid. All demographic characteristics are summarized in Table 1.
Fifty-one patients (32%) had jaundice at diagnosis. The median preoperative total bilirubin level was 0.6 mg/dL (IQR, 0.6) (bilirubin was recorded for all PD and TP patients). More preoperative assessments and interventions are detailed in Supplemental Table 1, see http://links.lww.com/AOSO/A270.
Complications
The median postoperative hospital length of stay for the entire cohort was 5 days (IQR, 1). Twenty-seven patients (17%) suffered severe complications (Clavien–Dindo ≥III)13: Nineteen patients (12%) experienced grade IIIa complications, 5 patients (3%) had grade IIIb, and 2 patients (1%) had grade IVa. There was a single mortality. Twenty-three patients (14%) developed an intra-abdominal abscess. Twenty-four (15%) patients required treatment by interventional radiology and a single patient had reoperation due to concerns for intra-abdominal hemorrhage. Four patients (2%) required postoperative endoscopic intervention by the gastroenterology team. Twenty-eight patients (18%) required readmission after discharge. Postoperative complications are summarized in Supplemental Table 1, see http://links.lww.com/AOSO/A270.
Hospital Charges
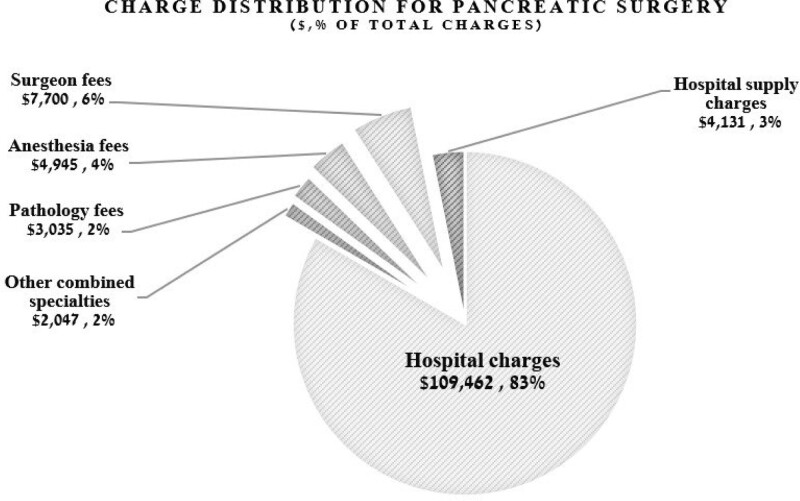
The total charges (combined professional and hospital charges) for the entire cohort encompassing the 91-day perioperative period were $20,661,759. The median total charge per patient was $130,306 (IQR, $34,534). Hospital charges included operating room (OR) and room/board charges (median charge per patient, $86,836; IQR, $17,878), anesthesia equipment charges (median per patient, $12,478; IQR, $2569), supply charges (median charge per patient, $4131; IQR, $3035), and postanesthesia care unit charges (median, $645; IQR, $3225) (Table 2, Fig. 1).
TABLE 2.
Total Median Charges per Pancreatic Resection Patient
| Charge | Median per Patient (IQR) | % of Total Charge |
|---|---|---|
| Hospital (including supply, OR, PACU, anesthesia) | $109,462 | 84 |
| OR | $86,836 ($17,878) | 67 |
| Anesthesia equipment | $12,478 ($2569) | 10 |
| Supply | $4131 ($3035) | 3 |
| PACU | $645 ($3228) | 0.5 |
| Surgeon (professional fee) | $7700 ($1295) | 6 |
| Anesthesia (professional fee) | $4954 ($1440) | 4 |
| Pathology (professional fee) | $3035 ($1790) | 2 |
PACU indicates postanesthesia care unit.
FIGURE 1.
Charge distribution for pancreatic surgery. Hospital charge includes supply, OR, postanesthesia care unit, and anesthesia equipment.
The median facility payment per patient was $33,090 (IQR, $23,696). The median direct cost of care was $23,219 (IQR, $6321) and, therefore, the median facility contribution margin for major pancreatic resection was $10,092 per patient (IQR, $22,949).
As mentioned earlier, 110 patients underwent PD and 32 underwent DP. As the operative and perioperative management of those 2 procedures is different, one would expect significant differences in the cost of care. Assessment of the hospital charges in the different surgical procedures shows that for the PD group, the median hospital charge (not including professional fees) was $111,422 (IQR, $18,495), while the median facility payment, direct cost, and contribution margin were $33,903 (IQR, $24,775), $24,511 (IQR, $6128), and $9203 (IQR, $22,989), respectively. For the DP group, the median hospital charge was $84,127 (IQR, 37,498), and the median facility payment, direct cost, and contribution margin were $29,397 (IQR, $23,319), $17,613 (IQR, $6044), and $12,620 (IQR, $22,690), respectively. The difference between the hospital charges for the 2 operations was significant and so was the difference in the direct cost of care (P < 0.0001 each), without significant difference between hospital payments (P = 0.6) and contribution margin (P = 0.1).
Professional Charges and Receipts
The estimated median number of hours in the OR per week per surgeon was 12 hours. The median surgeon professional charges were $7700 per patient (IQR, $1296) as compared to $3453 (IQR, $1144) professional receipts (45% of the surgeon charge). The differences between the professional charges and receipts per patient were also considerable for other health care professionals such as anesthesiologists ($4945 charges vs $1406 receipts [28%]) and pathologists ($3035 charges vs $680 receipts [22%]) (Table 3).
TABLE 3.
Hospital and Professional Charges Versus Receipts
| Median Charges (IQR) | Median Receipts (IQR) | % of Charges | |
|---|---|---|---|
| Hospital | $109,462 ($26,393) | $33,090 ($23,696) | 30 |
| Surgeon | $7700 ($1295) | $3453 ($1143) | 45 |
| Anesthesia | $4954 ($1440) | $1406 ($1201) | 28 |
| Pathology | $3035 ($1790) | $680 ($418) | 22 |
Of the total charges, the surgeon professional charges were 6%, while professional fees for anesthesiology and pathology were 4% and 2% of the total charges, respectively (Fig. 1).
Comparison of professional charges in PD versus DP shows that surgeon charges and receipts were significantly greater (P < 0.0001) for PD, where median surgeon charges and receipts were $7700 (IQR, 0) and $3559 (IQR, $569, 45% of surgeon charges), respectively, as compared to $5225 (IQR, $1920) and $1812 (IQR, $965, 35% of surgeon charges), respectively, for DP.
The Effect on Total Charges Versus the Effect of Professional Fees
Notably, the payer’s identity did not significantly affect the hospital charges, professional charges, receipts, or contribution margin. In fact, receipts were almost identical between the various payer groups (this, of course, will vary across the United States, based upon contracts between payers and insurance companies).
Upon examining the entire cohort regardless of the specific procedure, male sex and existing diabetes correlated with greater hospital charges (P = 0.01 and P = 0.01, respectively) but not with greater professional charges. Higher levels of bilirubin and lower albumin levels correlated with greater hospital charges (P = 0.003 and P = 0.05, respectively). As concerns preoperative interventions, neoadjuvant chemotherapy correlated with greater hospital charges (P = 0.03) whereas neither preoperative endoscopic biliary stenting nor endoscopic ultrasound were associated with a significant impact on charges. We expanded our analysis to 45 days before surgery specifically to capture the abovementioned additional procedures and more, ERCP, biliary stenting, EUS, interventional radiology, etc., but unfortunately, only about one-third of the patients underwent those procedures in our hospital during that time frame, and in those cases the results did not have a significant impact on charges. This analysis did not include charge or receipt data on neoadjuvant or adjuvant therapy as this data was not available.
Longer operative time correlated with increased hospital and anesthesia charges, without a significant impact on surgeon charges (P < 0.001, P < 0.001, and P = 0.2, respectively). Severe complications (Clavien–Dindo ≥III) correlated with significantly greater OR (P = 0.023), anesthesia (P = 0.004), gastroenterology (P = 0.022), and hospital charges (P = 0.017), without significant impact on the surgeon’s charge. In fact, there was no demographic, operative or perioperative characteristic that affected the surgeon’s charges or receipts significantly, likely due to the practice of global 90-day bundling of the surgeons’ professional fees.
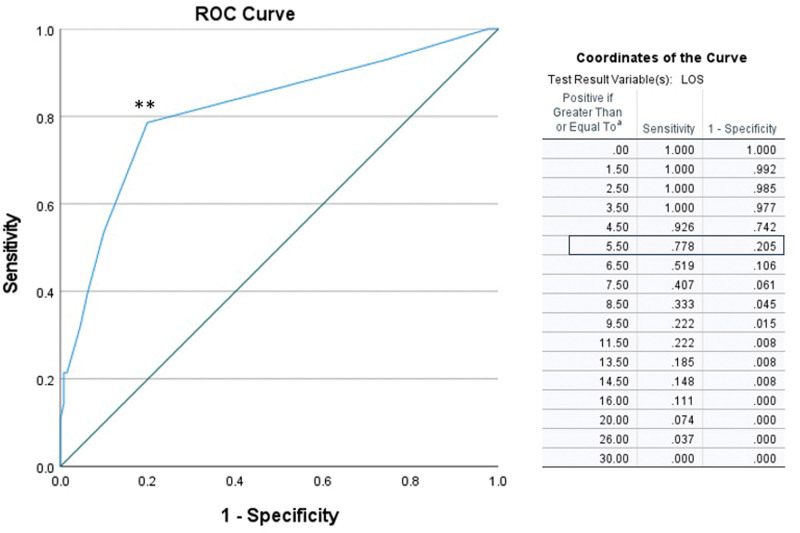
With regard to the length of stay (LOS), longer admissions (defined in this study as length of stay >6 days) significantly correlated with greater hospital charges. Median contribution margin for LOS <6 days was $11,383 (IQR, $20,810) while median contribution margin for LOS >6 days was $1097 (IQR, $23,690; RR, 8.4; P < 0.0001). In an effort to find the cutoff point above which a longer length of stay would not be profitable for the hospital, we performed a receiver–operator curve (ROC) analysis, which revealed that a length of stay above 5.5 days resulted, with a sensitivity and specificity of 0.8, in hospital net financial loss (Fig. 2). However, in our cohort, the majority of our patients were not admitted to the hospital for longer than 5 postoperative days and the number of patients who needed a longer admission was too small to allow a significant conclusion regarding the financial outcome.
FIGURE 2.
ROC analysis demonstrates the cutoff point above which greater admission would not be profitable for the hospital. In an effort to find this cutoff point, we performed an ROC analysis, which revealed that a length of stay above 5.5 days resulted, with a sensitivity and specificity of 0.8, in hospital net financial loss. Area under the curve is 0.8 (95% CI, 0.7–0.9). Sensitivity = 0.8 and specificity = 0.8 at the optimal cutoff point (marked **).
DISCUSSION
In this study, we provide a detailed analysis of the financial aspects of pancreatic surgery at a single academic medical center in the middle Atlantic region. We have also demonstrated an effect of the clinical sequela on the economic outcome and the implications on facility costs, charges, and contribution margin in comparison to the physician reimbursement.
We initiated this study because of the belief that there exists a lack of understanding and clarity between the advances in knowledge, techniques, and outcomes in the field of pancreatic diseases and surgery and the financial implications for both the hospitals and the health care providers rendering the care.
In reviewing the literature, there are few studies that have previously dealt with this subject. As we see it, it is of utmost importance for both health care delivery organizations and physicians alike to understand the current financial implications within this field and strive to make the system more equitable. This is particularly true in the domain of pancreatic cancer treatment, where the 5-year overall survival has increased from 2% to 11% over the last few decades and more patients are undergoing pancreatectomy.
A possible reason for the lack of literature and widespread knowledge of this subject may be the fact that it is not always feasible to acquire the financial data regarding pancreatic resection-specific charges, payments, and contribution margin. The fact that we were able to parse actual charges and receipts collected per patient, per surgeon, and per payer represents one of the strengths of this study. Of note, this analysis includes actual dollar amounts from a specific hospital facility, recognizing that in the United States, payments and receipts may vary based upon geography and payer mix.
As noted in this study, not surprisingly, longer postoperative hospital admissions were less profitable for the hospital with lower facility contribution margins or even net losses. The median facility contribution margin for major pancreatic surgery was $10,092 per patient (IQR, $22,949), but this number was dependent on a very good outcome and a short hospital postoperative length of stay. Indeed, the contribution margin was significantly greater with a shorter length of stay. We also demonstrate using ROC analysis that in cases where patients were discharged from the hospital on postoperative day 5 or earlier, the chances for hospital profit were greater, while cases with longer admissions were more likely to result in hospital loss. One should bear in mind that this is an experience based on the financial results from a single hospital (TJUH) and that length of hospitalization after major pancreatic resection may vary and can be much longer with different practices or in different countries.
These findings should not be interpreted as a need to discharge patients prematurely, rather to promote pathways for accelerated recovery such as the Whipple accelerated recovery pathway (WARP) our department reported in 2019.14 WARP was developed in our department for patients undergoing PD at low-to-moderate risk for perioperative complications, under the hypothesis that a specialized, accelerated postoperative care pathway could facilitate the completion of in-hospital recovery after PD within 5 days. Importantly, the WARP includes establishment of early discharge goals with patients and families, shortened intensive care unit stay, a modified postoperative dietary and drain management algorithm, rigorous physical therapy with an in-hospital gym visit, standardized rectal suppository administration, and close telehealth follow-up after hospital discharge. After this randomized controlled trial, the WARP effectively supported a facilitated recovery from pancreatic surgery. Moreover, hospital LOS, postoperative weight loss, the time to commencement of adjuvant therapy, and hospital charges and cost were all reduced after implementing the WARP. Consequently, according to the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) 2022,15 TJUH had the lowest LOS after PD of all hospitals in NSQIP (surgery dates July 1, 2020 to June 30, 2021)—5 days on average, as compared to the average LOS after PD in other high-volume centers in the country, which was 8 days. Indeed, we consider the WARP a critical component that led to the shorter LOS after pancreatic resection in our department.
In this study, treatment of complex patients with more comorbidities, advanced disease, longer operation times, and more severe complications may have had an effect of raising hospital charges, yet it did not influence the surgeon’s fee, which remained the same. Such is a result of the bundled global surgeon’s professional fee practice. With such findings, this work joins several others in the literature criticizing the existing reimbursement system,1–4 stating that the current system is overly simplistic and lacks recognition of several very important qualities in any surgeon’s (or physician in general) clinical effort.
Specifically, the current work relative value unit (wRVU)-based system disincentives clinicians from focusing on those behaviors that are essential to deliver better outcomes. Within our current system, the wRVU is supposed to represent the physician’s work as a whole. It formally takes into account the total time spent on care together with the clinical skills, and for surgeons, it bundles the perioperative charges (preoperative office visits, day of surgery, and postoperative visits) into a global surgeon charge. The reasons our current wRVU system is flawed is fourfold.
First, as we previously posited in the introduction to this article, the role of the surgeon in the multidisciplinary care of pancreatic surgical patients has become central. The pancreatic surgeon is the team leader and decision maker, and furthermore, as the operation itself is the crucial step to achieve cure, we can argue that the talented, well-trained surgeon is a critical component for patient’s survival. Unfortunately, this role is not well reflected in the current system of reimbursement.
Second, there remains a significant disadvantage within the wRVU system when wRVU payments apply only to billable procedures. Notably, mentoring younger physicians and handling other tasks outside of basic perioperative care are not billable and not financially rewarding in this current format. Furthermore, wRVUs reward the volume of care, not the quality of care. With mounting pressure for increasingly more wRVUs, it can be tempting for some physicians to focus on quantity over quality. For instance, a surgeon who cares for complex patients, operates meticulously and educates trainees in perioperative and intraoperative care, conducts research to advance the field, and assumes responsibility for postoperative complications, such a surgeon, may lose his or her incentive to persevere when receiving lower financial compensation compared to a surgeon who is not as collaborative and chooses to spend considerably less time and effort on the other parts of this “mission” or who is involved primarily in lower complexity care.
Third, another flaw of the system for the surgeons is the need to cover other expenses. The contribution margin from professional fee receipts is very much in the negative when taking into account surgeon salary and benefits, professional liability insurance, and practice-based costs. Add to that the additional “unfunded missions” of research and education and this type of clinical practice would not be sustainable without a financial partnership with the hospital.
Finally, in addition to all of the above, without a deeper understanding of the nuances of each operation and the intensity of work required for management of pancreatic surgical patients, wRVU simply do not accurately represent the actual surgical complexity. Several studies have evaluated whether wRVU measurement, as a surrogate for surgical complexity, is actually associated with perioperative outcomes that can be correlated with surgical complexity and have demonstrated that the current wRVU-based system poorly correlates with certain metrics of surgeon work such as length of stay and complications.16,17 In addition, similar studies likewise have shown that there are other less easily quantifiable factors that comprise surgeon work, such as those listed above, not to mention administrative work, which has increased substantially, comprising 10% to 15% of surgeon work.18 These factors strengthen the claim that the wRVU system is flawed.
The above factors are often mitigated with a “funds flow” model whereby the hospital supports clinical departments individually or as a combined “service line” where care for certain diseases stretch across multiple departments. With that said, how this model is constructed at each AMC varies from institution to institution and depends on countless variables, from human resources, through research and academic missions, to geography, yet the principle that a transparent financial partnership between the hospital and the clinical program forms the basis for the long-time viability and development of the individual clinical program. Given this, we are in the process of performing this type of analysis for other fields that deliver complex care at our institution, such as cardiac and transplant surgery.
In summary, after reviewing the financial implications of pancreatic surgery for both the hospital and the physicians, it is apparent that the current wRVU model is far from optimal as a reimbursement system in general and for care delivered to the pancreatectomy patient in particular. The reimbursement mechanism should be revisited and adjusted, with different models, based upon an equitable partnership between hospitals and their pancreatic surgery units.
LIMITATIONS
This study has several limitations—this is a retrospective study and potentially subject to selection biases and errors of omission and classification. However, the study is based on a prospectively maintained database that includes a well-characterized cohort of pancreatectomy patients and is, therefore, less likely to contain selection bias or errors of omission. We also strived to verify the accuracy of the data by multiple data cross-checking to minimize any errors. Other limitations include the single institution nature of this analysis and the high-volume nature of our pancreatic resection practice. The latter 2 elements may limit the generalizability of the results. Because this study was conducted in a high-volume specialized center, the nature of the patient cohort may bias certain patient characteristics unique to high-volume tertiary referral centers. As a multidisciplinary referral center for pancreatic-related diseases, we should bear in mind that the majority of patients had health insurance and were able to assume the personal and financial burdens of treatment in a referral center. Furthermore, it remains well established that a variable percentage of patients with pancreatic-associated diseases in the United States still receive their care at nonuniversity-based, nontertiary referral centers within their respective communities and thus do not undertake their care at specialized centers such as ours. Therefore, this study perhaps could be viewed as more specific for designated referral centers and as a proof of principle contribution with clinical and administrative imperatives more suitable for a high-volume center. Furthermore, these data likely apply best to an academic medical center in the urban northeast. Insurance contracts, payer mix, and average expected outcomes vary by institution and by region. In addition, the lack of information on adjuvant chemotherapy or radiotherapy are significant limitations when considering the overall financial impact of care for pancreatic surgery patients. We also note that given the complexity of the economics of healthcare, it is challenging to demonstrate the conclusions of this study via a retrospective study of a limited group of patients, without considering the full account of total cost of care, fixed costs, billing efficiency, billing cycle, and profit and loss statements for hospital and physicians.
CONCLUSIONS
Pancreatic surgery centers have a tripartite mission: to provide excellent clinical care, to focus on education, and to maintain innovative research. To sustain each of those goals at the highest level while achieving optimal patient outcomes, a change in the financial paradigm is necessary. The medical system should acknowledge the surgeon’s role in the global perioperative clinical care of pancreatic surgery patients, which is by no means limited to the operative day. However, the reimbursement to the surgeon in the form of professional fees is currently a relatively small fraction of the total health care receipts for these patients. This imbalance necessitates a substantial financial partnership between hospitals and their pancreatic surgery units to ensure the long-term viability of these programs.
ACKNOWLEDGMENTS
We wish to thank Jean M. McNeil and Patricia Daniel in the TJUH Financial Decision Support Office for providing the financial information critical to this analysis. We also wish to thank the Jefferson surgical residents, nurses, and other stuff who contributed to the care of these complex patients.
Supplementary Material
Footnotes
Published online 21 December 2023
Disclosure: The authors declare that they have nothing to disclose.
Presented at the Pancreas Club Annual Meeting, May 2023, Chicago, IL. This work was also presented at The Halsted Society 97th annual meeting, Charlottesville, VA, USA, 2023 and The American College of Surgeons clinical congress, Boston, MA, USA, 2023
Prepared for Annals of Surgery Open October 2023.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Belay ES, Charalambous LT, Saltzman EB, et al. Relative value units underestimate reimbursement for revision shoulder arthroplasty. J Am Acad Orthop Surg. 2022;30:416–420. [DOI] [PubMed] [Google Scholar]
- 2.Stecker EC, Schroeder SA. Adding value to relative-value units. N Engl J Med. 2013;369:2176–2179. [DOI] [PubMed] [Google Scholar]
- 3.Nurok M, Gewertz B. Relative value units and the measurement of physician performance. JAMA. 2019;322:1139–1140. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder SA, Frist W; National Commission on Physician Payment Reform. Phasing out fee-for-service payment. N Engl J Med. 2013;368:2029–2032. [DOI] [PubMed] [Google Scholar]
- 5.Resnick AS, Corrigan D, Mullen JL, et al. Surgeon contribution to hospital bottom line: not all are created equal. Ann Surg. 2005;242:530–7; discussion 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doria C, De Deyne P, Dolan S, et al. Municipality and adjusted gross income influence outcome of patients diagnosed with pancreatic cancer in a newly developed cancer center in Mercer County New Jersey, USA, a single center study. Cancers (Basel). 2021;13:1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber TS, Carlton LM, O’Hern DG, et al. Financial impact of tertiary care in an academic medical center. Ann Surg. 2000;231:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taheri PA, Butz DA, Greenfield LJ. Paying a premium: how patient complexity affects costs and profit margins. Ann Surg. 1999;229:807–11; discussion 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perri JL, Zwolak RM, Goodney PP, et al. Reimbursement in hospital-based vascular surgery: physician and practice perspective. J Vasc Surg. 2017;66:317–322. [DOI] [PubMed] [Google Scholar]
- 10.Hu Q, Wang D, Chen Y, et al. Network meta-analysis comparing neoadjuvant chemoradiation, neoadjuvant chemotherapy and upfront surgery in patients with resectable, borderline resectable, and locally advanced pancreatic ductal adenocarcinoma. Radiat Oncol. 2019;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouypoudat C, Buscail E, Cossin S, et al. FOLFIRINOX-based neoadjuvant chemoradiotherapy for borderline and locally advanced pancreatic cancer: a pilot study from a tertiary centre. Dig Liver Dis. 2019;51:1043–1049. [DOI] [PubMed] [Google Scholar]
- 12.Nanda RH, El-Rayes B, Maithel SK, et al. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. J Surg Oncol. 2015;111:1028–1034. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavu H, McCall NS, Winter JM, et al. Enhancing patient outcomes while containing costs after complex abdominal operation: a randomized controlled trial of the Whipple accelerated recovery pathway (WARP). J Am Coll Surg. 2019;228:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2020 ACS NSQIP Participant Use Data File (PUF). Available at: https://www.facs.org/media/yaol5yoj/nsqip_puf_userguide_2020.pdf. October 2023.
- 16.Shah DR, Bold RJ, Yang AD, et al. Relative value units poorly correlate with measures of surgical effort and complexity. J Surg Res. 2014;190:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen KT, Gart MS, Smetona JT, et al. The relationship between relative value units and outcomes: a multivariate analysis of plastic surgery procedures. Eplasty. 2012;12:e60. [PMC free article] [PubMed] [Google Scholar]
- 18.Satiani B. Use, misuse, and underuse of work relative value units in a vascular surgery practice. J Vasc Surg. 2012;56:267–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.