Abstract
Messenger ribonucleic acid (mRNA) has emerged as a promising molecular preventive and therapeutic approach that opens new avenues for healthcare. Although the use of delivery systems, especially lipid nanoparticles (LNPs), greatly improves the efficiency and stability of mRNA, mRNA tends to accumulate in the liver and hardly penetrates physiological barriers to reach the target site after intravenous injection. Hence, the rational design of targeting strategies aimed at directing mRNA to specific tissues and cells remains an enormous challenge in mRNA therapy. High-throughput screening (HTS) is a cutting-edge targeted technique capable of synthesizing chemical compound libraries for the large-scale experiments to validate the efficiency of mRNA delivery system. In this review, we firstly provide an overview of conventional low-throughput targeting strategies. Then the latest advancements in HTS techniques for mRNA targeted delivery, encompassing optimizing structures of large-scale delivery vehicles and developing large-scale surface ligands, as well as the applications of HTS techniques in extrahepatic systemic diseases are comprehensively summarized. Moreover, we illustrate the selection of administration routes for targeted mRNA delivery. Finally, challenges in the field and potential solutions to tackle them are proposed, offering insights for future development toward mRNA targeted therapy.
Keywords: Messenger ribonucleic acid, High-throughput screening, Targeted delivery, Lipid nanoparticles
Graphical abstract
1. Introduction
Messenger ribonucleic acid (mRNA) is a single-stranded ribonucleic acid that carries genetic information from DNA and guides protein synthesis within cells. With the deepening development of molecular biology, therapies based on in vitro transcribed mRNA (IVT mRNA) have drawn growing interest in recent years [[1], [2], [3]]. Compared to other molecular therapeutic approaches, mRNA can directly initiate the protein translation process by binding to ribosomes in the cytoplasm without nuclear entry steps, thereby enhancing transfection efficiency and avoiding gene integration-induced mutations. Moreover, mRNA preparation and purification steps are simpler relative to traditional protein and peptide drugs. The short half-life, low toxicity, and low immunogenicity of mRNA also reduces the risk of foreign substances accumulation and immune rejection reactions in the course of treatment [4,5].
To exert its effects within cells, mRNA faces successive barriers from extracellular delivery to intracellular actions, including physiological barrier (e.g., skin mucosal barrier), transmembrane process upon reaching target cells, obstacle of intracellular membranes after entry into cells, and interference from various intracellular enzymes and molecules. The application of naked mRNA is limited by inherent defects such as instability, susceptibility to degradation by nucleases, and electrostatic repulsion by cell membranes because of negative charges, hampering the therapeutic potential [6]. Thus, addressing the effective delivery issues of mRNA has become a crucial theme in mRNA therapy. mRNA delivery refers to the use of specific methods or carriers to enhance the stability of mRNA, enabling the penetration of various barriers and expression of the target protein within target cells [7,8]. With the great success of SARS-CoV-2 mRNA vaccines, lipid nanoparticles (LNPs) have become the most common mRNA carriers. Despite the great efforts that have been made to improve the efficacy of LNPs through modifying structure and components [9], there are still technical difficulties in the design for overcoming specific barriers and enhancing targeting effect. In the current delivery mode, though the measures of using mRNA to target the liver and treat liver diseases have been extensively studied [10], mRNA targeting extrahepatic tissues and cell delivery efficiency remains low, leading to the occurrence of off-target effects. Therefore, to fully unleash the potential of mRNA technology and achieve extensive therapeutic development in various intracellular microenvironments, the prediction and rational design of targeting delivery strategies is crucial in mRNA therapy [11,12]. However, there is still a lack of systematic summarization of current methods and their applications for enhancing extrahepatic mRNA targeted delivery.
In recent years, emerging technologies to enhance mRNA targeted delivery have been developed. These include microscopic conventional low-throughput methods, and more universally applicable high-throughput screening (HTS) techniques, particularly applied to LNPs, as well as macroscopic choices in drug administration. Low-throughput methods primarily improve targeting effect of delivery systems by validating passive targeted delivery with structural modifications and active targeted delivery with surface ligands based on a few previous studies. These methods have the advantages of easy preparation and strong specificity but are limited in the rational design and large-scale evaluation of delivery systems. In contrast, HTS optimizes the inherent structure of the delivery system through the synthesis of chemical compound libraries and affinity experiments, which is available for the development of more effective drug delivery systems [13]. The large-scale validation of delivery systems enhances passive targeting and reduces off-target effects. Moreover, the principles of HTS can be applied in the improvement of structural improvements of delivery systems and surface ligand peptide segments, comprehensively enhancing the targeted delivery efficiency of mRNA.
In this review, we first summarized the use of low-throughput methods in mRNA targeted delivery. Second, we particularly discussed the technological improvements in delivery systems through HTS and elucidated the latest developments of HTS techniques in mRNA therapy for extrahepatic diseases. Third, we introduced the drug administration routes in mRNA therapy. Finally, we describe the challenges and opportunities for the application of HTS techniques in mRNA therapy in the future.
2. Low-throughput targeting strategies and applications
2.1. Optimizing structures of individual delivery vehicles
2.1.1. LNP
Despite the tremendous potential demonstrated by mRNA therapy based on various delivery systems, its efficacy is limited by biological barriers, including the distant targeting of tissues, excessive interference from the internal environment, and inefficient cellular delivery. For systemic intravenous administration, LNPs share physicochemical properties with very low-density lipoproteins. Due to its tendency to adsorb apolipoprotein E in the plasma and its natural inclination to accumulate in the liver, mRNA faces challenges in being delivered to target tissues outside the liver [7]. Low-throughput methods refer to the validation of individual or a few improvement strategies on a small scale to enhance mRNA targeted delivery. These methods are currently the mainstream approach for improving specificity due to their simplicity and strong targeting capabilities. Significant progress has been made in LNPs, especially in enhancing immune system targeting, offering viable improvement measures for mRNA vaccines and other immunotherapies.
LNPs are formed by the spontaneous interaction of lipid molecules and can encapsulate negatively charged mRNA, preventing its degradation by nucleases, and facilitating its delivery into target cells. When the carrier delivers mRNA to the target cells, most of it enters the cells through endocytosis, and the mRNA is then stored in endosomal vesicles. However, LNPs can mediate mRNA delivery by fusing the phospholipid bilayer of the liposome with the cell membrane, greatly reducing the time for mRNA release into the cytoplasm and significantly enhancing mRNA delivery efficiency [6]. LNP systems are primarily composed of four lipid components, including ionizable lipids, cholesterol or its variants, helper lipids, and polyethylene glycol (PEG)-lipids. They have high tunability compared to other carriers. By manipulating the four components of the chemically modified LNP systems, LNP carriers with high stability, large payload capacity, low toxicity, and other advantages can be developed [14].
The lipid composition, properties, and proportions of the LNP systems have a significant impact on intracellular mRNA delivery efficiency, stability, and targeting. The theoretical design and validation of individual LNP delivery systems to enhance the targeting and adjuvanticity of immune cells have been widely studied in mRNA vaccines [[15], [16], [17]] or adoptive NK cell therapy [18,19]. In vitro experiments have confirmed the aggregation of DC cells or NK cells, while in vivo, they tend to secondary lymphoid structures and enhance the activation effect of mRNA in immune responses. For example, replacing ionizable lipids with adjuvant-like lipid components imparts toll-like receptor 7/8 agonistic activity to LNPs, targeting the immune system and enhancing the efficacy of SARS-CoV-2 mRNA vaccine in mice [16]. Furthermore, since mRNA vaccines often exert their effects through intramuscular injection, it is essential to enhance skeletal muscle targeting through LNP structural modifications [20,21]. For example, researchers designed a skeletal muscle-specific modRNA translation system (skeletal muscle SMRTs) using the highly expressed miR-1 in skeletal muscle. The SMRTs system consists of two modRNA molecules: one carries the miR-1 recognition element and the L7Ae gene, while the other carries a K-turn sequence and the sequence of the target gene. In the presence of miR-1, the expression of L7Ae is suppressed, thereby allowing transgene expression and targeting skeletal muscle [21]. For the treatment of diseases such as tumors, the local lesions often exhibit a hypoxic state. Therefore, adding adenosine triphosphate to the LNP structure can passively enhance targeting in the microenvironment, improving the therapeutic effectiveness of disease [22].
Selective organ targeting (SORT) involves systematically designing the addition of a fifth chemically structured, tunable, charged molecule to the four-component LNP systems. The SORT molecule added to LNPs determines the composition of the protein corona (i.e., the interface layer of plasma proteins). It primarily achieves precise delivery of mRNA to target tissues in the body through an endogenous targeting mechanism, binding to specific proteins in the serum, facilitating LNP redirection, and cellular uptake in target organs [23]. The protein corona formed with the assistance of SORT molecules in LNPs is enriched with factors different from those associated with liver accumulation, including ApoE. Therefore, it can reduce the tendency to target the liver and promote interactions with receptors highly expressed in cells in the target organ [23]. Designing individual SORT molecules and adjusting their properties helps enhance targeting to specific cells or internal tissues such as the lungs, spleen, etc., thereby achieving the goal of targeting different cells or organs [[24], [25], [26]].
2.1.2. Other delivery system
Inorganic NPs are novel delivery materials consisting of inorganic particles and cations with a particle size ranging from 10 to 1000 nm. Inorganic NPs, characterized by high stability, good biocompatibility, and a high surface area-to-volume ratio, have become excellent carriers for mRNA delivery due to their high gene loading capacity compared to other carriers. However, most inorganic NPs lack biodegradability, leading to their accumulation in the body and potential cellular damage [27]. Mesoporous silica NPs (MSNPs) are mesoporous materials with a regular structure. Due to their high intrinsic stability and unique porous structure, they are widely used as delivery carriers in the field of anti-cancer therapy. MSNPs can mediate cancer targeting through specific endocytosis [28,29]. To achieve tissue specificity, researchers designed an organic-inorganic hybrid silica-based NP. mRNA was pre-mixed with cationic polymers, such as polyethyleneimine, and then electrostatically bound to the surface of MSNPs. This enhanced the stability of mRNA delivery, showing good targeting to the pancreas and mesentery after intraperitoneal injection [30]. Similarly structured silicon-coated polyelectrolyte complexes also demonstrated efficient macrophage-targeting activity [31].
Polymer carriers are typically composed of synthetic or natural polymers, forming polymeric nanomaterials through covalent structures to encapsulate mRNA. Dendritic polymers are large molecules with a complex three-dimensional structure, and their distinctive dendritic architecture sets them apart from other macromolecules effectively. Modifying polymers to regulate their charge and molecular weight can play a role in targeted mRNA delivery in vivo [32]. For instance, poly(β-amino ester) (PBAE) modified with PEG enhances the specific delivery efficiency to glioblastoma cancer cells [33]. PBAE terpolymers based on poly(ε-caprolactone) (PCL) can achieve mRNA-specific targeting to the spleen, showing promising applications in immunotherapy for tumors [34]. Polymer micelles are self-assembled stable aggregates formed by amphiphilic polymers, where hydrophilic groups are on the outer surface and hydrophobic groups are on the inner side. Due to their high safety, strong stability, and low toxicity, polymer micelles show great prospects in mRNA delivery [35]. Polymer micelles are widely used in mediating targeted mRNA delivery. For example, block copolymers synthesized from PEG, PCL, and trimethylene carbonate can self-assemble into polymer micelles. These micelles can specifically deliver drugs to the posterior segment of the eye through vitreous diffusion, providing prolonged and sustained effects [36]. Novel self-assembled polymer micelles based on poly(vinyl ethylene succinate) (PVES) modified with vitamin E succinate exhibit high cell viability when delivering mRNA. PVES/mRNA vaccines can induce strong immune responses [37]. Additionally, charge-altering releasable transporters (CART), composed of an initiator, one or more lipid segments, and a polycationic segment, can undergo acyl shift transformation to neutral at physiological pH, triggering the release of anionic cargo. Li et al. introduced a CART delivery system with a β-amino ester backbone (bAC), exhibiting high primary T cell-targeted transfection and enhancing the application of polymers in the immune system [38].
Biologically derived materials that balance targeting specificity and innate biocompatibility are garnering increasing attention. Nucleoside phospholipids, based on nucleoside-nucleoside interactions, are composed of covalent molecules with lipid moieties and nucleosides, facilitating additional hydrogen bonding between complementary bases, thus exhibiting low toxicity and immunogenicity. Li et al. developed a novel mRNA carrier, DNCA/CLD, containing cytosine cation, which reduces the risk of antibody-dependent enhancement (ADE) and overcomes the inherent instability and immune activation challenges during mRNA delivery, thereby enhancing mRNA absorption efficiency [39]. Due to its excellent biocompatibility and bioavailability, cell membrane derived from cells themselves can serve as an excellent targeted delivery vehicle. Cao et al. incorporated ionizable lipid YK-009 into the cell membrane of dendritic cells (DCs) to develop simulated NPs (DCMNPs) for targeted mRNA delivery, exhibiting strong targeting towards lymphoid organs and lymphocytes, offering significant prospects in the mRNA delivery immunotherapy field [40].
2.2. Developing individual surface ligand
Ligand-based active targeting design has always been one of the main research directions to enhance targeting. The current challenge is how to improve the targeting of ligands and allow the delivery vehicle to exert its delivery function stably and effectively in the body for an extended period. Ligands include antibodies, peptides, other types of proteins, aptamers, oligonucleotides, and small molecules, etc. They can undergo structural transformations and exert biological activity based on charge density, hydrophilicity, and structural conformation, thereby crossing biological barriers and achieving selective localization [41,42]. In addition to LNPs, the biological membrane delivery carrier system utilizes cellular membranes or vesicles mimicking cellular membranes, disguising themselves as NPs, and encapsulating mRNA. Through the process of membrane fusion, mRNA is delivered into the cytoplasm. Due to retaining crucial molecular structures and physicochemical properties of the live cell surface, this system not only evades clearance by the immune system but is also easily degradable, preventing accumulation in the body and minimizing cellular damage. Importantly, the excellent biocompatibility of the biological membrane delivery carrier is important for enhancing mRNA targeted delivery. In the application of extracellular vesicle delivery, the modification of targeting peptides on exosomes is one of the commonly used methods [43].
Applying monoclonal antibodies (mAb) to modify LNPs and flexibly switching between different targeting mAbs, this series of delivery carriers can effectively deliver mRNA molecules to various subsets of leukocytes in vivo [44,45]. This strategy has been preliminarily applied in T-cell engineering [46,47]. For example, research has confirmed that binding CD4 antibody to LNPs can achieve specific targeting and mRNA intervention for CD4+ cells, including T cells, with good targeted delivery effects [47]. Epidermal growth factor receptor (EGFR) is a common surface receptor in the body and is highly expressed in some solid tumors, becoming a tumor-associated antigen. Therefore, anti-EGFR antibodies can be effective targeting delivery peptides [48]. Research has shown that using a protein linker to covalently couple EGFR-targeting peptides with red blood cell-derived extracellular vesicles (RBC-EVs) can effectively promote the internalization of RBC-EVs by EGFR-positive cells through the formation of stable chemical bonds [49]. EVs are nanoscale vesicles with a double-membrane structure actively secreted by cells, containing complex molecules such as RNA, proteins, lipids, etc. EVs deliver the carried mRNA to target cells through paracrine or endocrine mechanisms [50]. Liposomes share a similar main structure with EVs, both having a double-layered phospholipid membrane, thus possessing excellent biocompatibility. By chemically modifying the surface phospholipids, cholesterol, and PEGylated lipid molecules, their targeting capabilities can be enhanced. The hybrid vesicles formed by combining liposomes with EVs simultaneously exhibit the advantages of both, becoming high-quality targeted delivery carriers [51,52]. Hybridizing EVs derived from breast cancer cells with liposomes and modifying the surface of hybrid vesicles with anti-EGFR antibodies significantly enhances the targeted delivery capability of extracellular vesicles [53].
In addition, peptides are common ligands in mRNA delivery, carrying various active functional groups on their side chains. They can chemically modify the carried mRNA to avoid degradation by nucleases. The polymer formed by the binding of peptides and nucleic acids is taken up into the cell membrane through endocytosis, thereby achieving mRNA delivery functionality [54]. For example, the glutathione-modified delivery system significantly enhances the drug's ability to specifically target the kidneys [55]. Among them, the arginine-glycine-aspartic acid (RGD) peptide, which specifically binds to integrin receptors overexpressed on the membrane of tumor cells, is a classic pathway. It can enhance affinity and increase the effectiveness of anti-tumor mRNA therapy [[56], [57], [58]]. Exosomes are nanoscale vesicles with a diameter of about 50–100 nm, containing complex RNA and proteins. Due to their small and uniform size, high stability, complex composition, and diverse functions, exosomes are increasingly being applied in various fields, such as cancer and inflammation treatment [59,60]. Exosomes, with their inherent characteristics, can overcome physiological barriers in the body, such as the blood-brain barrier, and are particularly useful in the treatment of certain central nervous system diseases [61]. Conjugating the functional ligand cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyK)] with exosomes derived from mesenchymal stem cells (MSCs) allows for more efficient penetration of the blood-brain barrier, specifically targeting the brain ischemic lesion area [58].
Other non-protein molecular ligands have also been widely applied. Gold NPs (AuNPs) are the most extensively studied inorganic nanocarriers. They can encapsulate mRNA by connecting it to the NP core through thiol groups. After surface modification through chemical means, AuNPs can deliver mRNA into cells [62]. AuNPs have the advantage of easy preparation, and their size and dimensions can be adjusted according to specific application needs, making it easier to screen for delivery systems with higher targeting efficiency [63]. AuNPs coupled with citrate can regulate miRNA in triple-negative breast cancer cells, effectively controlling tumorigenic inflammation. Glucose-coated AuNPs enhance the radiosensitivity of B16F0 melanoma cells, making it easier to induce apoptosis in breast cancer [64]. Furthermore, utilizing hyaluronic acid (HA) coating on AuNPs, the affinity of HA for the CD44 receptors overexpressed on cell membranes during eye diseases, along with the excellent biocompatibility of AuNPs, facilitates the delivery of large-molecule drugs to the retina and posterior segment of the eye. This suggests that HA-coated AuNPs can serve as a potential effective carrier for delivering large-molecule nucleic acids [65].
3. High-throughput screening technologies
3.1. Optimizing structures of large-scale delivery vehicles
3.1.1. LNP
HTS technology, as an unbiased large-scale optimization technique, provides a comprehensive tool for characterizing the targeting of delivery systems [66]. The attachment of additional substances has increased the complexity of developed delivery systems and reduced their in vitro and in vivo stability. This, in turn, diminishes the clinical translatability of these systems. Therefore, there is an urgent need for the development of self-optimizing methods to enhance affinity. First, constructing a library and then applies certain selection conditions for in vitro and in vivo experiments or reads out the interested intracellular information through barcoding and sequencing technologies, thereby identifying delivery methods and their mechanisms with higher affinity, effectiveness, stability, or immunoadjuvanticity to enhance passive targeting and reduce off-target effects.
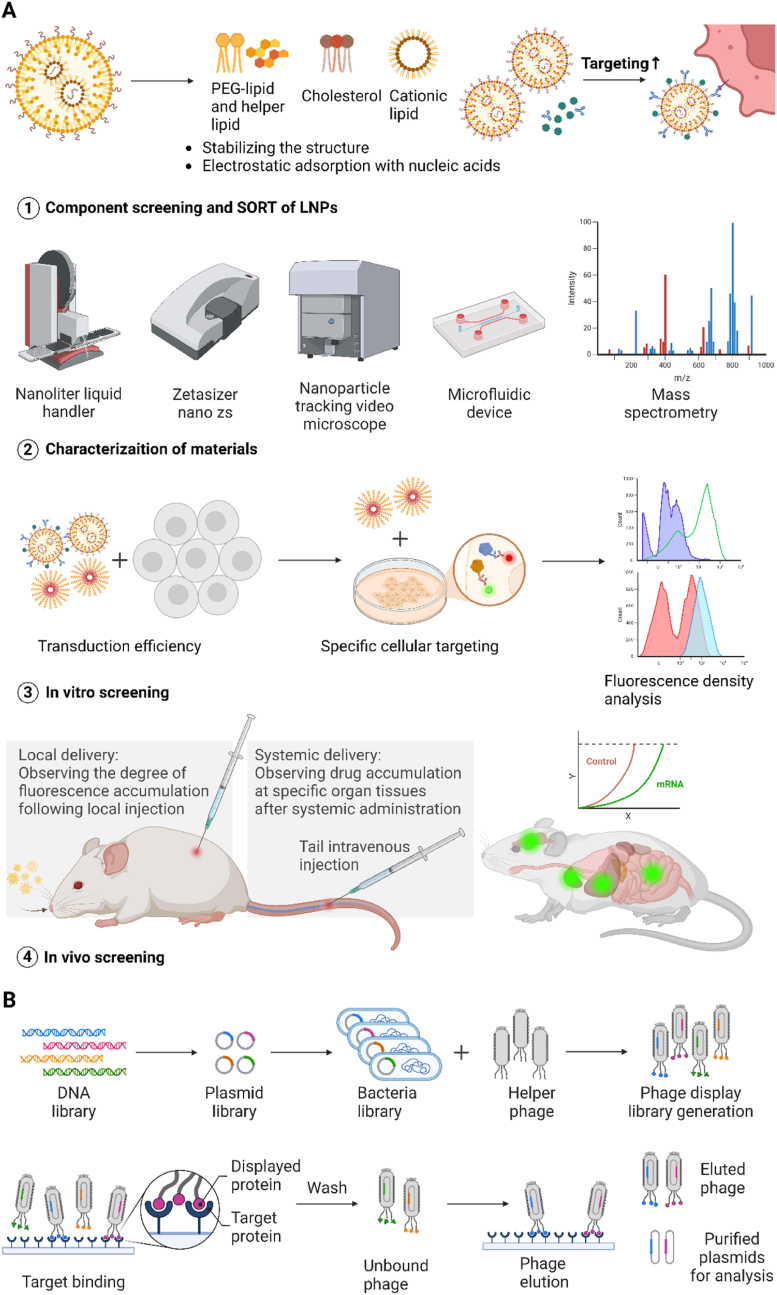
HTS techniques typically use combinatorial chemistry methods to alter lipid functionality and modulate the physicochemical properties of LNPs to improve mRNA delivery, typically involving library design, material characterization, in vitro screening, and in vivo screening or validation as four steps (Fig. 1A). The structural characteristics of LNPs and standards based on pKa have been proven to enhance the efficiency and biosafety of mRNA delivery, as well as predict the targeting specificity for delivery to specific organs in vivo. LNPs of different sizes and shapes can traverse different physiological barriers and induce biological accumulation. For instance, particles with diameters exceeding 200 nm may possess splenic targeting capabilities, while disc-shaped NPs are more likely to target vascular walls [12]. Additionally, the surface properties and carrier charges of LNPs are the most crucial physicochemical properties [12]. A possible mechanism involves LNP coming into contact with serum after intravenous injection, leading to protein adsorption on its surface and the formation of a unique protein corona. The formation of a protein corona at the interface is believed to affect the interaction between LNPs with different pKa values and serum proteins, which is closely associated with the in vivo biodistribution [23]. Through the design of orthogonal experiments, researchers can determine the optimal LNP formulation by preparing LNPs with different substance ratios, ensuring that changes in targeted delivery are not driven by specific ratios of the four components. This allows to produce large-scale LNPs with different chemical structures. These LNPs formed with different formulations are usually characterized and screened for physicochemical properties in vitro or ex vivo environments. This can be determined through methods such as dynamic light scattering (DLS), transmission electron microscopy (TEM), and chromatography to assess the impact of different components on physicochemical properties, including LNP size, zeta potential, pKa, mRNA encapsulation efficiency, etc.for initial screening, followed by subsequent targeted analysis and screening.
Fig. 1.
High-Throughput Screening Technologies. (A) Optimizing structures of large-scale delivery vehicles. HTS for improving material structures typically involves four steps. Firstly, design a large-scale library of structures and ratios for the four main components or SORT molecules of LNPs. Secondly, characterize the physicochemical properties of formed LNPs with different formulations using techniques such as DLS, TEM, and chromatography, including LNP size, zeta potential, pKa, mRNA encapsulation efficiency, etc. Thirdly, conduct in vitro screening, verifying expression efficiency in immortalized cell lines and targeting specificity for specific cell lines through fluorescence enzyme activity. Finally, perform in vivo screening in mice through systemic or local administration, observing the targeting of specific organs or tissues. (B) Phage display library screening. Phage display library screening can be used for developing large-scale surface ligands. Plasmids are constructed from a large DNA library, and the phage display library is built by binding with a helper phage. After multiple rounds of in vivo affinity selection, screening and enrichment are performed, unbound phages are washed away, phages with target-specific peptides are identified, and the corresponding DNA sequences are determined through sequencing.
Therefore, HTS techniques could be combined with various experimental methods to observe in vitro cellular protein expression, and the delivery of LNPs to target organs and cells through in vitro and in vivo screening experiments. This will ultimately determine the optimal novel LNP structure to enhance mRNA delivery targeting. In vitro screening, these LNPs are typically first used to deliver luciferase mRNA or Cre recombinase mRNA to immortalized cell lines (such as Hela cells, 293T cells, etc.) to reflect the cell transfection efficiency and intracellular mRNA translation ability of this delivery method, as well as the ability of LNPs to escape endosomal membranes within cells, promote mRNA release and translation, and assess the corresponding cell toxicity. Subsequently, for the validation of cell targeting, LNPs are used to transfect cell lines that simulate the corresponding tissue microenvironment in vitro, and the delivery efficiency is also assessed through in vitro luciferase assay. For instance, researchers used an LNP library to transfect B16F10, CT26, and Raw264.7 cells (representing epithelial cells, fibroblasts, and macrophages, respectively) and identified macrophage cell-type-specific lipids by evaluating mRNA delivery [67]. However, most of the time, in vitro screening cannot fully replicate the influence of the in vivo-specific biological barriers affecting LNPs, making the ability to predict in vivo biodistribution and efficacy less accurate. Therefore, in vivo screening to validate the tissue and cell affinity of LNPs is a crucial step. This involves monitoring fluorescence signals at different sites after systemic intravenous injection or local administration, and substituting mRNA cargoes to demonstrate the corresponding roles of the materials obtained from high-throughput screening in specific therapeutic functions.
The main challenge faced by HTS is the difficulty in biological tracking, especially in vivo [68]. Generally, mRNA transfection efficiency and biodistribution in vivo can be identified through fluorescence readings, but quantification may be limited due to the resolution constraints of microscopy. Enhancing fluorescence can be achieved by incorporating a fluorescent tricyclic cytosine analog, 1,3-diaza-2-oxophenoxazine (tCO) [69]. Additionally, imaging techniques such as transmission electron microscopy, super-resolution stochastic optical reconstruction microscopy [70], and others provide feasible approaches for observing mRNA delivery in vivo. However, HTS analysis still requires a more precise and efficient in vivo tracking imaging system [71,72]. Rui et al. used galectin-8 (Gal8) for image-based quantification of endosomal escape, establishing an efficient and robust high-throughput NP screening system, which holds significant importance for the detection and optimization of new materials [73]. In recent years, researchers have developed new methods to quantitatively evaluate variables in the in vivo microenvironment, including DNA barcoding, mRNA barcoding, and peptide barcoding signals [68,[74], [75], [76]]. The DNA barcoding system based on next-generation sequencing (NGS) allows each type of LNPs to carry a unique DNA barcode oligonucleotide. After isolating tissues or cells in vivo and recovering the oligonucleotides, PCR amplification with indexed primers is performed to amplify the signal of the oligonucleotides. Each tissue/animal is labeled, and deep sequencing analysis of the biological distribution of LNPs is conducted. This method reveals LNPs with good targeting in the liver and lungs. By combining DNA barcodes with ligands, this high-throughput nanoparticle barcoding system can also be used to rapidly identify effective targeting sequences through a process similar to phage display [75]. Additionally, researchers have developed single-cell nanoparticle targeting sequencing (SENT-seq), enabling each type of LNP to simultaneously carry DNA barcodes corresponding to the LNP formulation and mRNA encoding antibody heavy-chain single-domain (aVHH). Through single-cell sequencing analysis, the levels of DNA barcodes and anti-aVHH antibodies coupled with DNA tags in different cells are examined, revealing the cellular heterogeneity and downstream transcriptomic characteristics in response to different LNPs [74]. In the context of mRNA-LNP in vivo screening, encapsulating additional b-DNA may alter the LNP structure. Therefore, utilizing functional customized barcode mRNA (b-mRNA) incorporated into the 3′ untranslated region (UTR) allows for direct quantification of the in vivo delivery of each LNP formulation through deep sequencing [76]. However, LNPs containing nucleic acid barcodes have been shown to result in significantly different biodistributions, which may introduce additional biases [76]. Rhym et al. developed a peptide barcoding screening system based on liquid chromatography and tandem mass spectrometry (LC-MS/MS). They used mRNA encoding different peptide tags to barcode each NP, directly quantifying protein yield. Using enzyme-linked immunosorbent assay to quantify the expression of short epitope tags, they confirmed the sensitivity of the peptide barcoding system and used it to develop LNP RM133-3-21 with improved performance [68].
There are several ways to construct LNPs libraries. Cationic lipids are the core components of LNPs. Under physiological conditions, they are neutral. When the pH is lower than the dissociation constant pKa of the LNP itself, amine substances such as tertiary amines comprising the head of cationic lipids are pH-sensitive. They can bind protons to form cationic compounds that adsorb mRNA with negative charge. Their structure usually includes one or more amine heads that bind mRNA at low pH, multiple alkyl tails for hydrophobic self-assembly, and ester linkages connecting the two [77]. One of the important ways to establish LNPs libraries is to alter the cationic lipids in their own structure, including the head, linker, and tail. The hydrophilic head can promote the disassembly of LNP-mRNA complexes inside cells. The length of the hydrophobic tail is inversely proportional to the apparent pKa of LNPs. Its degree of unsaturation also affects characteristics related to membrane instability, thereby influencing the delivery efficiency of LNPs [12]. Therefore, altering the length, number, and degree of unsaturation of hydrophobic tails [78,79], as well as disulfide bond modifications [71], in conjunction with cholesterol, helper lipids, and PEG anchored lipids in set ratios, and using an improved microfluidic device to mix with mRNA, can be used to prepare large-scale libraries of novel LNPs [80]. Exploration of replacing cationic lipids with other components has also been explored in high-throughput screening. For instance, Zhang et al. synthesized 50 unique quaternary ammonium salts consisting of 8 amines, 3 acrylic acid esters, and 9 alkylated bromides through combinatorial chemistry. They screened for AMB-POC18 based on the transfection efficiency of EGFP mRNA (mEGFP), formulation size, and zeta potential in Hela cell lines. AMB-POC18 was incorporated into LNP formulations and the transfection efficiency of different formulations was screened again, followed by characterization of their physicochemical properties and stability. Subsequently, cellular internalization and endosomal escape validation in vitro, spleen targeting verification in vivo, and assessment of the immunogenicity and in vivo antitumor effects of AMB-POC18 LNPs were conducted. This led to the development of esterase-triggered deionized quaternary ammonium salt lipid LNPs, laying the foundation for the development of tumor vaccines [81]. Additionally, low-temperature transmission electron microscopy and molecular dynamics simulations have identified that helper lipids are the main components on the surface of LNPs. Improving helper lipids in a similar high-throughput manner [[66], [82], [83], [84]], replacing traditional cholesterol [[85], [86], [87]], and designing different LNP stereostructures [88] are also important measures for enhancing targeting efficiency. Researchers synthesized 8 different C12-200 lipids by altering the stereochemistry and hydrophobic tail length. They prepared a total of 128 different LNPs using 16 methods. Preliminary screening of particle size and DLS spectra identified 55 LNPs. These LNPs were loaded with Cre mRNA and injected into Ai14 mice for in vivo screening. Fluorescence-activated cell sorting (FACS) was used to isolate tdTomato + cells aggregated in the liver, lungs, spleen, kidneys, and bone marrow. DNA barcodes from each cell type were then separated and read using NGS. Subsequently, LNP-42S, which was screened out, underwent individual in vivo experimental validation, confirming its enhanced protein production capability and good in vivo tolerance [88]. Lastly, the systematic integration of high-throughput screening ideas with SORT could also be a feasible exploration direction to enhance targeting efficiency. Large-scale design of SORT molecular libraries for screening, such as Lee et al. who used modular reactions to screen 91 unsaturated thiol-synthesized amino lipid libraries, evaluated cell viability and Luc expression of LNPs in vitro, and found that the core molecule 4A3 with a citronellol moiety (4A3-cit) significantly improved mRNA transfection efficiency through the SORT method. Subsequently, in vivo validation was conducted by tail vein injection of LNP series carrying Luc mRNA based on 4A3, revealing its ability to promote lipid fusion and endosomal escape [79].
3.1.2. Other delivery system
The concept of HTS can also be applied to the improvement of other delivery systems. For instance, lipid-like NPs (LLNs) and polymer NPs are also representative materials widely used for in vivo mRNA delivery. By combining the core structure of N1,N3,N5-tri(2-aminoethyl)benzene-1,3,5-tricarboxamide (TT) with different types of biodegradable lipid chains, a series of functionalized TT (FTT) LLNs were designed. Their delivery efficiency was screened through in vitro and in vivo experiments, and the best-performing material, FTT5 LLNs, was identified [89]. In addition, polymer NPs have scalable production and high safety. Libraries can be easily generated through the Michael-type addition synthesis of different amines and divinyl monomers. Utilizing a similar screening approach, specific NP formulations with targeting properties for particular tissues and cells can be selected [90,91]. For instance, Rodrigues et al. conducted HTS of polymer formulations prepared by electrostatic complexation of polymers with mRNA encoding Cre recombinase. They used a fibroblast model sensitive to the enzymatic activity of translated proteins to assess the functional delivery of mRNA, resulting in the identification of polymers with fibroblast affinity associated with various diseases [90].
Specifically, amphiphilic carbon dots (ACDs) themselves possess bioimaging capabilities and excellent biocompatibility. A series of novel ACDs were synthesized through a combinatorial synthetic strategy, facilitating the convenient screening of delivery systems with high transfection efficiency and strong targeted delivery capabilities. Through the screening of ACDs, O12-Tta-CDs were identified as having the optimal mRNA transfection efficiency and spleen-targeted delivery ability. They effectively transduce bone marrow dendritic cells (BMDCs), facilitate efficient antigen presentation, activate immune responses, and exhibit promising therapeutic effects in tumor treatment [92].
3.2. Developing large-scale surface ligand
Cell-penetrating peptides (CPPs) can be internalized into cells by covalent or non-covalent attachment of small molecules to the cell membrane, and they are easily modified by specific amino acids to improve targeted delivery [93,94]. Research indicates that modifying the functional groups on CCPs and designing CCPs with different properties, such as cyclic, branched, and amphiphilic CCPs, can enhance the stability of CCP transport structures through the HTS approach, providing a foundation for better targeting capabilities [95].
Phage display peptide library screening technology utilizes HTS principles. A specific length of randomly synthesized gene fragment is directionally inserted into the coding gene of the phage capsid protein. This allows the gene-encoded exogenous peptide to be expressed and displayed on the surface of phage particles in a fusion protein manner. A large library of peptides or proteins is thus formed. Through multiple rounds of in vivo affinity selection, i.e., the "affinity adsorption-elution-recovery-amplification" process, screening and enrichment are performed. Unbound or non-specifically bound phages are washed away. This process allows the selection of phages that specifically bind to target tissues from the peptide library. The amplified phages are then directly sequenced to quickly and conveniently obtain the DNA sequence of the peptide/protein that specifically binds to the tissue of interest [96] (Fig. 1B). Barrera et al. used a heptapeptide library based on M13 phage to identify peptide sequences that bind to the neural retina in vivo. Three rounds of in vivo affinity selection, elution, and amplification were performed in mice to obtain an enriched library, which was then reinjected. The screened MH42 increased the binding affinity for 661w cone PR cells when administered to the retina, confirming the feasibility of peptide-conjugated LNPs [97]. The involvement of phage display peptide library technology in LNPs targeting optimization will be more widely applied in the future, driving larger-scale discovery of affinity peptides.
4. High-throughput screening for improvement of targeted delivery
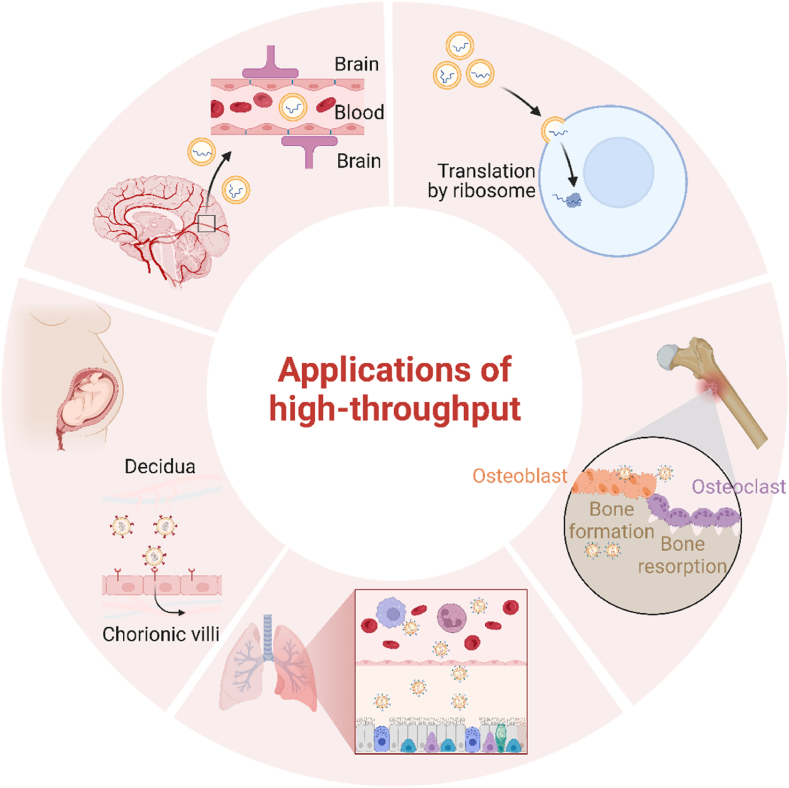
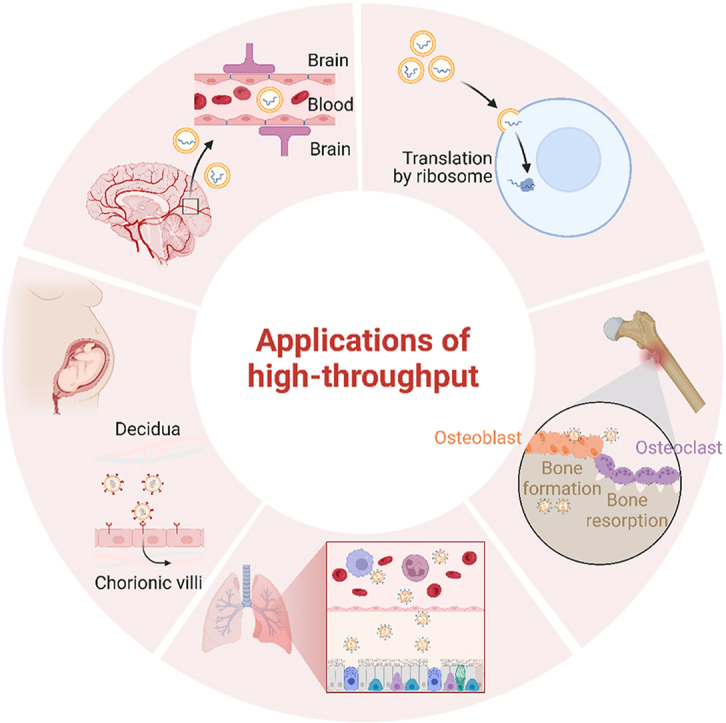
HTS technology, as an unbiased platform for optimizing delivery vehicles, provides a comprehensive characterization of the targeting specificity and delivery efficiency of delivery systems. It has shown outstanding insights in improving mRNA delivery. In previous studies, HTS technology has been applied to target mRNA carriers in various systems, especially in the optimization of LNPs, leading to significant breakthroughs (Fig. 2 &Table 1).
Fig. 2.
An Overview of High-throughput Screening Applications for mRNA Targeted Delivery. The applications of high-throughput screening primarily encompass immunotherapy, respiratory system diseases, central nervous system diseases, pregnancy disease, and other localized diseases such as skeletal disorders. Materials capable of traversing in vivo barriers and selectively targeting specific tissues can be screened.
Table 1.
Representative studies of high-throughput screening for targeted delivery improvement.
| Screening method | Encoded protein | Target tissue or cells | Delivery system | Discovered strategy | Discovered strategy type | Functions of discovered strategy | Study model | Ref. |
|---|---|---|---|---|---|---|---|---|
| Immunotherapy | ||||||||
| In vitro, ex vivo | CD19 CAR, FLuc | T cells | LNPs | C14-4 | Ionizable lipids | Increase potent mRNA delivery to T cells with lower cytotoxicity and induce cancer cell killing. | Jurkat cells, primary human T cells, Nalm-6 ALL cells | [98] |
| In vitro, ex vivo | CD19 CAR, FLuc | T cells | LNPs | C14–4: DOPE: Chol: PEG = 40:30:25: 2.5 | Excipient compositions | Increase potent mRNA delivery to T cells with lower cytotoxicity and induce cancer cell killing. | Jurkat cells, primary human T cells, Nalm-6 ALL cells | [99] |
| In vitro, ex vivo | FLuc | T cells | LNPs | 25 % and 50 % 7α-hydroxycholesterol | Hydroxycholesterols | Increase colocalization with the endosome and generation of late endosomes, and reduce endosomal recycling in T cells | Jurkat cells, primary human T cells | [87] |
|
In vitro, ex vivo in vivo |
FLuc, Cre-recombinase, OVA, Trp2 | Muscle tissue | LNPs | iso-A11B5C1 | Ionizable lipids | Increase potent mRNA delivery to muscle tissues, reduce off-targeting, and remain effective in triggering cellular immune responses. | HeLa cells, mTmG reporter mice, THP-1 cells, BMDCs, B16–F10Luc cells, C57BL/6 mice | [100] |
|
In vitro, ex vivo, in vivo |
FLuc, spike protein of the Delta SARS-CoV-2 variant |
DCs | LNPs | SAL12 | Non-nucleotide SALs | Increase delivering mRNAs encoding the Spike glycoprotein of SARS-CoV-2, activating the STING pathway in DCs, and eliciting neutralizing antibodies. | BMDCs, C57BL/6 mice, HEK293T-ACE2 cells | [101] |
| In vitro, ex vivo | GFP, OVA | Antigen-presenting cells, macrophage cells | LNPs | C12-200-cho-10%DOPE, C12-200sito-10%DOPE, cKK-E12-cho-10%DOPE, cKK-E12-sito-30%DOPE | β-sitosterol, helper lipid DOPE content | Increase potent mRNA delivery to immune cells and result in enhancing robust T cell proliferation and IFN-γ, TNF-α, IL-2 expression. | HeLa cells, Calu3 cells, DC2.4 cells, THP-1 cells, RAW264.7 cells, BMDCs, OT-I cells | [85] |
| In vitro, in vivo, | OVA,Fluc | Spleen | LNPs | AMB-POC18 LNPs | Esterase-triggered decationizable quaternium lipid-like molecules | Increase mRNA transfection efficiency and formulation stability, exhibit potent therapeutic and prophylactic efficacy against melanoma without side effects | C57BL/6 mice, B16F10, Balb/c mice | [81] |
|
In vitro, in vivo, ex vivo |
OVA, TRP2, GP100 | DCs | NPs | R18D NPs | PBAE architectures | Increase potent mRNA targeted delivery to the spleen and preferential transfection of dendritic cells without the need for surface functionalization of target ligands. | MC38-OVA, murine DC2.4 cell, BMDCs, C57BL/6J mice | [102] |
| In vitro, in vivo | OVA,Fluc | Lipids with branched hydrophobic tails | LNPs | arylates and varying amines | Ionizable lipids | Increased activation of CD8+ T cells in the tumor microenvironment resulted in the suppression of the growth of invasive B16-OVA tumors. | IGROV 1 cells, BALB/c mice, B16-OVA | [103] |
|
In vitro, in vivo, ex vivo |
OVA, FLuc | DCs | LPNs | DOPE: DOTMA = 70 %:30 % | Excipient compositions | Increase the uptake and mRNA transfection efficiency of lipid-polymer nanoparticles (LPN). | murine DC2.4 cell, BMDCs, C57BL/6J mice, primary mouse T cells | [104] |
|
in vitro, in vivo, ex vivo |
OVA, TRP2, Fluc | Lymph node | LNPs | 113-O12B | reduction-responsive lipids | Increased delivering mRNA to lymph nodes, impeding the formation of metastatic nodules in the lungs. | B16F10-OVA cells, Ai14 mice, PBMCs, C57BL/6J mice | [105] |
|
In vitro, in vivo, ex vivo |
Fluc | Splenocytes | LNPs | AA3-DLin LNPs | Ionizable lipid | Increasing mRNA delivery efficiency and long-term storage capability. | BALB/c mice, Hek 293 cell, ACE2-293T cells | [106] |
| In vitro, in vivo | Fluc, OVA, Cre | Lymph node | LNPs | A18 LNP | Ionizable lipid-like materials | Increasing targeted adjuvant stimulation through the STING pathway to enhance effective mRNA delivery. | B16-OVA, B16F10, TC-1 cells, HeLa cells, Ai14 mice | [107] |
|
In vitro, in vivo, ex vivo |
Gp100, TRP2, OVA, Fluc | Lymph node | LNPs | L17-F05 | Ionizable lipid | Increasing the efficiency of mRNA delivery into the lymph nodes and promoting the maturation of cDCs enhance the anti-tumor efficacy of the vaccine. | Myeloid Cells, B16F10, BMDMs BMDCs, C57BL/6 mice | [108] |
|
In vitro, in vivo, ex vivo |
Fluc,OVA, Cre, Trp2, Gp100 | DCs | LNPs | C10 LNPs | Helper lipid | Increase the delivery of tumour-antigen-encoding mRNA to dendritic cells and their immune-activation profile towards enhanced antitumour activity | BMDCs, Ai9 mice, C57BL/6 mice, B16-OVA cells | [83] |
| In vitro, in vivo | Fluc | Spleen, liver | LNPs | DOPE with AOPE | Helper lipid | Increase mRNA delivery to the spleen and liver, contributing to the future design of LNPs for nucleic acid therapeutics. | C57BL/6 mice | [109] |
| In vitro, in vivo | aVHH, Fluc | HNSCC | LNPs | LNPHNSCC | Ionizable lipid | Increasing the effective delivery of therapeutic mRNA to head and neck squamous cell carcinoma (HNSCC) tumor cells while minimizing side effects on the liver. | FaDu cells, Cal-27 cells, HEK-293 cells, NU/J mice | [110] |
| In vitro, in vivo, | Fluc, Omicron | Muscle tissue, Spleen, DCs | LNPs | YK009 | Ionizable lipid | Increase the inherent instability and immune activation challenges in modulating mRNA delivery process have been addressed, resulting in improved efficiency of mRNA uptake and demonstrating good efficacy and safety. | HEK-293 cells, DC2.4 cells, RD cells, BALB/c mice | [111] |
| Respiratory system diseases | ||||||||
| In vitro, in vivo | Fluc, Cre, aVHH | Lung cells | LNP | Cat-LNP | Cationic helper lipids | Increase the efficiency of mRNA specific targeting to the lungs. | Ai14 mice, BL/6mice | [66] |
| In vitro, in vivo, | Fluc, Cre | CD11b hi Macrophages, liver | LNPs | Lipid 16, Lipid 23 | Ionizable lipid | Increase the efficiency of mRNA targeting to CD11bhi macrophages and targeting the liver. | B16F10, CT26, Raw264.7, TdTomato animal model, C57BL/6 mice | [67] |
| In vitro, in vivo | Fluc, Cas9, Cre, | Lung | LNPs | RCB - 4 - 8LNP | Ionizable lipid | Increasing the efficiency of mRNA targeted delivery to pulmonary epithelial cells provides a pathway for gene therapy in congenital lung diseases. | A549 cells, Ai9 mice | [112] |
| In vitro, in vivo | Tsc2, Cas9, Fluc | Lung | LNPs | 306-N16B | Ionizable lipids | Increase mRNA targeting delivery to the lungs, achieving significant therapeutic effects in reducing tumor burden. | Ai14 mice, C57BL/6 mice, TSC2-null TTJ cells, Balb/c mice | [113] |
| In vitro, in vivo | Fluc | Lung | LNPs | Molar ratio of poly-(ethylene) glycol (PEG)-lipid | lipid compositions | Increase mRNA targeted delivery to the lungs, with the potential application in aerosol-mediated pulmonary mRNA delivery. | HEK-293, NuLi-1 cells, Balb/c mice | [114] |
| Central nervous system diseases | ||||||||
|
In vitro, in vivo, ex vivo |
Cre, Fluc | Brain | LNPs | C3LNP | Ionizable lipids | Increasing the stability and specificity of mRNA therapeutic drug delivery to the perinatal brain provides a translatable delivery platform for gene editing in the fetal and neonatal central nervous system. | BALB/c, Neuro-2a cells | [115] |
|
In vitro, in vivo, ex vivo |
Fluc | Brain | LNPs | C12-494 | Ionizable lipids | Increasing the delivery of mRNA to the brain in vivo, which can be utilized for the treatment of neurological disorders. | hCMEC/D3 cells, C57BL/6 mice | [116] |
| Pregnancy diseases | ||||||||
|
In vitro, in vivo, ex vivo |
Cre, Cas9, Fluc | Utero heart, diaphragm, muscle | LNPs | D-Lin-MC3-DMA: DOPE: cholesterol:DMG-PEG2000 = 36.8 %: 23.8 %: 38.2 %: 1.2 % | Excipient compositions | Increase delivering mRNA to organs outside of the liver. | Ai9 CRE reporter mice, E15.5 pregnant mice | [117] |
|
In vitro, in vivo |
Fluc, EPO | Utero | LNPs | Several | Ionizable lipids | Increase the fetal liver delivery and transfection efficiency of mRNA with good safety. | C57BL/6 mice, human dendritic cells | [118] |
|
In vitro, in vivo |
Fluc | Placenta | LNPss | LNP A4 | Ionizable lipid | Increase the efficiency of mRNA-specific delivery to the placenta and induce placental vascular dilation, thereby treating placental diseases. | JEG-3 cells, Nonpregnant and Pregnant Mice | [119] |
|
In vitro, in vivo, |
Fluc, PIGF | Placenta | LNPs | C12-200: DOPE: cholesterol: DMPE-PEG = 35:10:53.5:1.5 | Excipient compositions | Increase the delivery of placental growth factor mRNA and LNPs show no toxicity to pregnant mice and fetuses. | BeWos, CD1 mice | [120] |
|
In vitro, in vivo |
Fluc | Placenta | LNPs | LNP C5 | Ionizable lipid | Increasing the efficiency of mRNA delivery to the placenta in pregnant mice, mediating mRNA delivery to placental trophoblast cells, endothelial cells, and immune cells. | BeWo b30 trophoblast cells, pregnant mice | [121] |
|
In vitro, in vivo, ex vivo |
Fluc | Utero intraamniotic | LNPs | A-12 | Excipient compositions | Increase the efficiency of mRNA delivery within the amniotic sac, contributing to the optimization of non-viral therapeutic approaches for treating congenital diseases in utero. | HeLa, Balb/c mice, Primary Fetal Lung Cells | [122] |
| Other localized diseases | ||||||||
|
In vitro, in vivo, |
Fluc | Hepatic stellate cells | LNPs | CL15A6 | Excipient compositions | Increase the efficiency and stability of mRNA delivery to hepatic stellate cells, as well as enhancing ex vivo stability and in vivo biosafety. | LX-2, FL83B, BALB/c mice, C. KOR/StmSlc-Apoe/mice | [123] |
|
In vitro, in vivo, ex vivo |
BMP-2, Fluc | Bone microenvironment | LNPs | 490BP-C14 | Bisphosphonate Lipid-like Materials | Increasing mRNA expression in the bone microenvironment, moreover, enhancing the mRNA delivery and secretion of therapeutic bone morphogenetic protein-2. | C57BL/6 mice, HeLa | [124] |
CAR Chimeric antigen receptor; FLuc Firefly luciferase; SAL STING agonist-derived amino lipids; OVA Ovalbumin; BMDCs Bone marrow dendritic cells; GFP Green fluorescent protein; Cas9 CRISPR-associated protein 9; aVHH single-domain antibody; BeWos BeWo b30 trophoblast cells; PIGF Placental growth factor; Tsc2 tuberous sclerosis complex 2; Cre Cre recombinase; TRP2 tyrosinase-related protein 2; GP100 Melanoma-associated antigen gp100; p53 Tumor protein 53; ICG Indocyanine Green; EPO Erythropoietin; BMP-2 Bone morphogenetic protein-2.
4.1. Immunotherapy
Harnessing methods such as mRNA to artificially modify the function of the immune system for the treatment of diseases is a promising therapeutic approach, mainly used for the prevention or treatment of tumors and infectious diseases. Firstly, by transiently expressing specific immune antigen proteins through mRNA, mRNA vaccines can be created to trigger the host's active immune response, which is the most common application of mRNA therapy at present [125]. Compared to traditional vaccines, mRNA vaccines have a faster design and production speed. They do not need to be assembled into complete proteins and exhibit high adjustability and adaptability. They can be modified and optimized as needed to specifically prevent large-scale outbreaks of variable pathogens. Additionally, mRNA vaccines do not contain live pathogens or cells, eliminating the risk of infection and significantly improving safety while ensuring efficacy [126]. mRNA vaccines are primarily used to prevent infectious pathogens, including viruses [16,[101], [127], [128], [129], [130], [131], [132]], bacteria [[133], [134], [135]], parasites [[136], [137], [138], [139]], and therapeutic vaccines for specific tumor destruction, including melanoma [140,141], gastrointestinal tumors [[142], [143], [144]], head and neck tumors [145], etc. mRNA vaccines induce effective antigen-specific binding and innate immunity, followed by the activation of lymphocyte-specific immunity to prevent pathogens and treat tumors. Therefore, the clinical efficacy is often limited by insufficient protein expression in antigen-presenting cells (APCs) in immune organs and inadequate activation of immune functions.
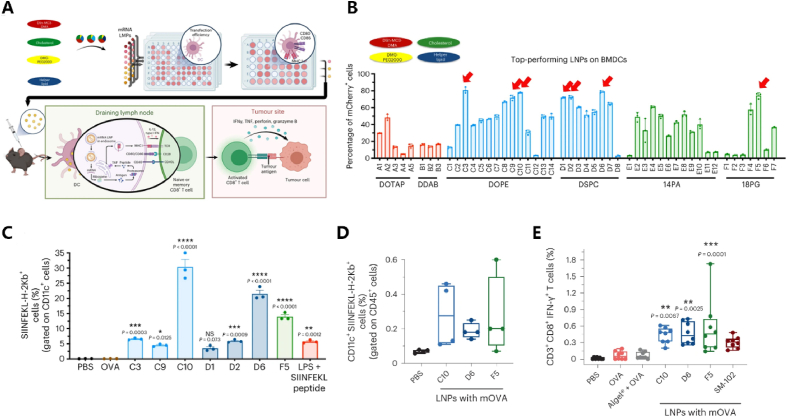
Utilizing mRNA in immunotherapy and enhancing immune treatment based on HTS is a feasible strategy. Commonly used mRNA vaccines are typically administered through intramuscular injection, and constructing a muscle-specific mRNA delivery ionizable lipid library through HTS can enhance mRNA delivery precision. Chen et al. identified through HTS that the lipid iso-A11B5C1 can centrally deliver mRNA to muscles and confirmed its potent therapeutic performance as a melanoma vaccine [100]. In most cases, the immune function in the body is primarily achieved by delivering mRNA to secondary peripheral lymphoid organs (such as draining lymph nodes or spleen) of APCs, and enhancing the innate immune stimulation in APCs through adjuvants, leading to better preventive and therapeutic effects [146]. Research has shown that the IFN response in innate immunity is crucial for the clonal expansion and activation of CD8+ T cells. Therefore, adjuvants that modulate the strength and quality of the IFN response are often co-administered with vaccines. Various pathways activating the secretion of type I IFN have been identified, primarily including stimulators of the interferon gene (STING) agonists, which have been demonstrated to enhance the therapeutic effects of cancer and anti-pathogen vaccines [147]. In recent years, numerous studies have induced different fluorescent mRNA-LNP complex libraries into 96-well plates of BMDCs through in vitro experiments to explore their immunotargeting effects. By embedding the gene encoding ovalbumin (OVA-mRNA) in the LNP library, activation of BMDCs in the spleen or lymph nodes of tumor model mice was achieved to enhance T cell proliferation and immune response. The therapeutic effects were evaluated to identify LNPs delivery systems that simultaneously possess high immunotargeting and immunostimulatory capabilities (adjuvanticity), leading to significant achievements [81,83,85,92,101,[102], [103], [104], [105], [106], [107], [108], [109], [148]]. For example, Miao et al. optimized auxiliary lipid types and lipid component ratios through in vitro screening to enhance the delivery of tumor antigen-encoding mRNA to DC cells and immune activation. The functionality was validated in vivo. The results indicate that through in vitro screening, it was discovered that LNPs composed of various lipid components efficiently expressed a fluorescent enzyme in the immortalized DC 2.4 cell line. The antigen presentation effect was superior, and the immune activation functionality was validated in vivo, including antigen presentation and T-cell activation [83] (Fig. 3). Furthermore, for the COVID-19 virus, research has shown that through the rational design and screening of various ionizable lipids, the YK009-LNP-Omicron mRNA vaccine can induce safe and effective humoral immune responses and Th1 cell immune responses, providing protection against the SARS-CoV-2 Omicron variant virus when administered to mice [111]. Furthermore, the direct targeted delivery of mRNA into cancer cells for cancer treatment has also been explored through HTS [149,110].
Fig. 3.
HTS application in mRNA vaccines.(A)The entire process of HTS involves assessing the efficiency of entering DC cells in vitro using porous plates and screening material candidates based on cell phenotypes, followed by exploring their functionality through injection into mice in vivo.(B)In BMDCs, through the assay of fluorescence enzyme expression activity, it was found that a series of auxiliary lipid components including C3, C10, D6, and F5 exhibited better effectiveness.(C)Testing the antigen-presenting ability of DC cells after selected auxiliary lipids carrying OVA enter, it was found that C10 exhibited the best effectiveness, while D6 and F5 showed similarly good effects.(D)In vivo validation in mice showed that LNPs with OVA using C10, D6, and F5 demonstrated antigen-presenting function, with C10 and F5 exhibiting better effectiveness.(E)In vivo validation in mice demonstrated that C10, D6, and F5 exhibited good activation function on CD8+ T cells [83]. Copyright © 2023, The Author(s), under exclusive licence to Springer Nature Limited.
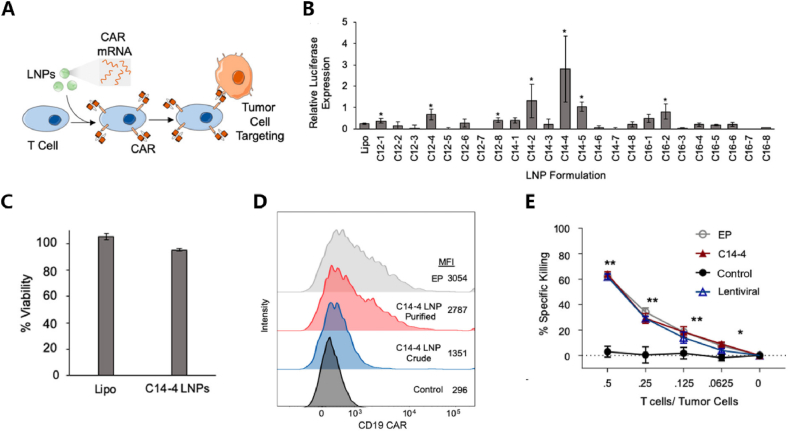
Secondly, mRNA-based adoptive cell therapy (ACT) has made significant progress in directly and specifically combating tumor cells or other pathogens [150]. ACT typically involves isolating autologous or allogeneic immune-active cells, amplifying them after in vitro modification, and reinfusing them into the patient's body, aiming to achieve direct targeted killing of diseased cells or modulation of the body's immune response. ACT based on T cells is a focal point in immunotherapy, especially with CAR-T cell therapy. Transiently expressed CAR-T cells can typically be generated by introducing mRNA encoding CAR sequences into autologous CD8+ T cells ex vivo through electroporation or LNP. After constructing CAR-T cells, they are then reinfused into the patient's body, significantly reducing genetic risks and manufacturing costs. The transient anti-tumor efficacy and safety of CAR-T cells have been validated in various in vitro and in vivo tumor models [[151], [152], [153], [154], [155]]. For instance, CD5-targeted LNP can deliver mRNA encoding fibroblast activation protein (FAP) CAR molecules into T cells in the human body, enabling the in vivo generation of CAR-T cells against cardiac fibrosis. Targeting activated fibroblasts improves myocardial remodeling and restores cardiac function [46]. Jurkat (an immortalized T cell line) cells were used for mRNA delivery using an LNP library with different compositional formulas. Various methods, including protein expression, cytotoxicity screening, and co-cultivation with tumor cells, were employed to assess improvement methods for delivery systems available in T cell engineering [87,99,98]. This lays the foundation for future work. For example, Billingsley et al. conducted HTS using in vitro methods in Jurkats cell line and ex vivo methods in human primary T cells. They found that C14-4 exhibited the highest fluorescent enzyme effect. Its feasibility in CAR-T engineering was validated through CD19 CAR gene expression and killing of acute lymphoblastic leukemia tumor cells [98] (Fig. 4).
Fig. 4.
HTS application in CAR-T cell therapy.(A)LNP libraries carrying mRNA encoding CAR sequences can enter T cells, enabling the selection of materials targeted for T cell delivery by observing extracellular CAR expression and tumor-killing capabilities.(B)During in vitro screening, it was found that LNP composed of C14-4 exhibited the strongest fluorescence enzyme activity in human primary T cells, indicating higher transfection efficiency.(C)Compared to Lipofectamine, C14-4 showed no significant decrease in cell activity, indicating its good safety profile.(D)The targeted delivery efficacy of C14-4 was validated through CAR expression, demonstrating that purified C14-4 LNP exhibited higher CD19-CAR expression efficiency compared to the control group and crude C14-4 LNP.(E)When co-cultured with tumor cells, the cytotoxic effect of CAR-T cells constructed with C14-4 LNP was significantly better than that of the control group. Similar to CAR-T cells obtained through lentivirus transfection, this demonstrates the potential of C14-4 in adoptive cell therapy [98]. Copyright © 2020, American Chemical Society.
4.2. Respiratory system diseases
The application of mRNA therapy in the lungs is a crucial aspect. For instance, cystic fibrosis results from defective gene mutations in cystic fibrosis transmembrane conductance regulator, leading to impaired water and salt flow in and out of cells in organs. This dysfunction results in mucus blocking the airways in the lungs, causing chronic pulmonary infections and progressive lung damage. This highlights the significance of mRNA respiratory delivery and targeted enhancement for lung applications [156]. HTS techniques have been widely employed for lung targeting [67,66,84,[112], [113], [114], [157], [158]]. This is one of the most common research directions for enhancing in vivo targeting specificity of mRNA delivery systems in extrahepatic systems.
Tracheal inhalation administration facilitates mRNA delivery to the airway epithelium or other lung cells for treating various congenital pulmonary diseases. However, the lung presents unique challenges for delivery due to its distinctive cell types, mucus barrier, and mucociliary clearance. Researchers synthesized and screened a combination library of mRNA inhalable delivery carriers for repetitive tracheal administration, achieving effective gene editing in pulmonary epithelium [112].
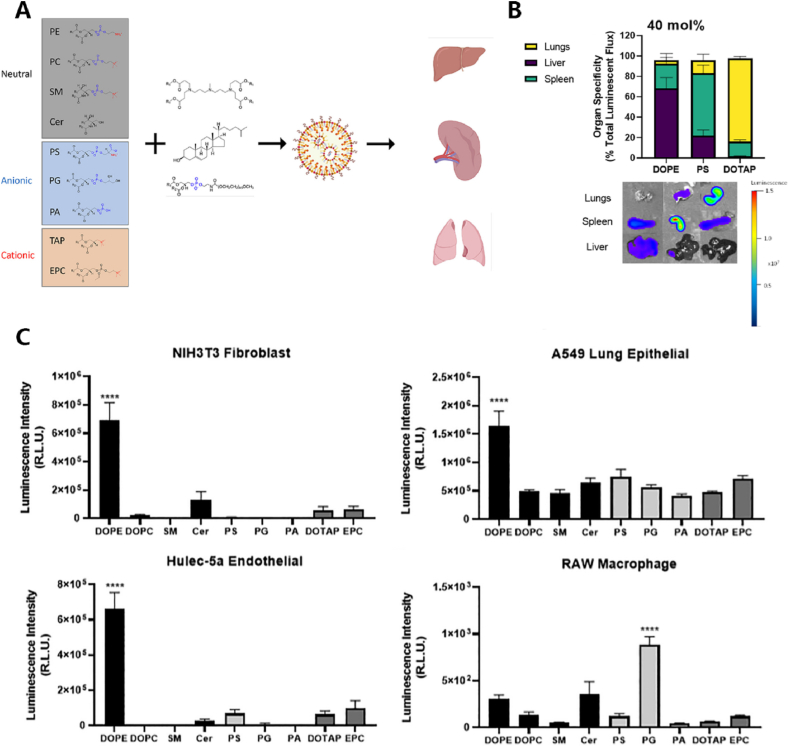
However, during systemic administration, HTS technology indicates that specific structures of ionizable lipids or auxiliary lipids also tend to aggregate mRNA in the lungs. For instance, study by Lopresti et al. involved in vivo screening of various attributes of auxiliary lipids, confirming that most neutral, anionic, and cationic auxiliary lipids tend to deliver mRNA to the liver, spleen, and lungs, correlating with the physical properties of the materials [84](Fig. 5). Targeting different cell subtypes in the lungs, such as alveolar epithelial cells, lung macrophages, and fibroblasts, for mRNA delivery is also feasible. Naidu et al. discovered through HTS that an ionizable lipid exhibits a tendency to deliver mRNA to CD11bhi macrophages. They speculated that mRNA expression in the lungs may originate from the organ's macrophages, promoting the development of cell-specific delivery to the lungs [67].
Fig. 5.
HTS application in respiratory system diseases.(A)Through in vivo screening of LNP composed of various auxiliary lipids with different properties (neutral, negative, positive), their accumulation in the liver, spleen, and lungs was observed.(B)Through in vivo fluorescence activity and imaging, it was discovered that cationic DOTAP tended to target the lungs, while neutral and anionic auxiliary lipids tended to accumulate in the liver and spleen, respectively.(C)Not only organ-specific, but LNPs composed of different auxiliary lipids also play a role in targeted delivery in specific cell lines. Unlike fibroblasts, lung epithelial cells, and endothelial cells, PQ exhibits strong specificity in lung macrophages [84].
4.3. Central nervous system diseases
mRNA therapy holds tremendous potential for applications in the central nervous system (CNS). For instance, the introduction of neurotrophic factors is a crucial aspect of regenerative therapy against neurodegenerative diseases, ischemic diseases, and traumatic diseases [159]. The mRNA expression of neurotrophic factor-3 (NT-3) in Schwann cells can promote the mechanism of axon regeneration in the body, mediating the repair of peripheral nervous system injuries [160,161]. Endogenous overexpression of brain-derived neurotrophic factor can enhance neuronal survival and function, providing protective effects on the CNS by reducing neuronal death [162,163]. Due to the presence of the BBB, HTS is promising for mRNA targeting in the CNS.
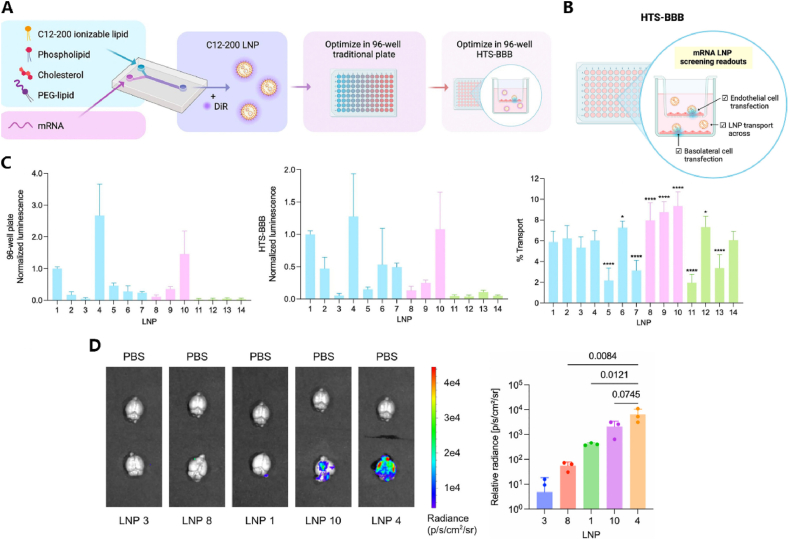
As mentioned earlier, mRNA therapy for the CNS is often administered through intraventricular or intrathecal injections. By screening an ionizable LNP library through intraventricular injections in the fetal and neonatal mouse brain, the optimal C3 LNP formulation has been identified, demonstrating greater mRNA delivery functionality in the perinatal brain compared to traditional LNPs [115]. However, local injections into the brain are susceptible to limitations such as high invasiveness, technical complexity, and limited diffusion. Therefore, systemic administration to deliver LNPs to the brain is a preferable approach. However, BBB is highly selective, blocks the transport of delivery systems, and has become a major obstacle for central delivery. In recent years, progress has been made in exploring new delivery platforms to penetrate BBB [164,165]. However, large-scale mRNA LNP library screening studies are limited, possibly because in vitro screening cannot establish a high-throughput compartmentalized system to simulate the BBB for simultaneous screening of endothelial transfection and transport [166]. Accordingly, Han et al. developed a BBB transwell model for HTS of mRNA LNPs. This model facilitates dual screening for mRNA LNP transfection and transport across the BBB. It has been employed in in vitro and in vivo experiments, revealing that LNP4 exhibits the highest fluorescent enzyme effect in brain endothelial cells in vitro and accumulates in mouse brain tissue in vivo [116]. This platform provides a foundation for subsequent LNP formulation screening and can be extended to other biological barriers such as the blood-retinal barrier and the blood-placental barrier (Fig. 6).
Fig. 6.
HTS application in central nervous system diseases. (A) Construct C12-200 LNPs with different lipid compositions, initially screening their transfection efficiency through fluorescence enzyme activity in standard 96-well plates, followed by optimizing LNP components using the HTS-BBB system.(B)The HTS-BBB system is a transwell model consisting of two layers (basal cells and endothelial cells), used for dual screening of mRNA LNP's cell transfection efficiency and transport efficiency across the blood-brain barrier.(C)During in vitro screening, LNP4 and LNP10 showed promising results in both standard 96-well plates and the HTS-BBB system, with LNP10 demonstrating superior transport efficiency.(D)During in vivo screening, LNP4 exhibited the best brain targeting specificity as evidenced by radiation brightness [116]. Copyright © 2024, American Chemical Society.
4.4. Pregnancy diseases
Due to the small size of the fetus, strong immune tolerance, and abundance of stem cells and progenitor cells, prenatal mRNA therapy can be conducted before irreversible pathological changes occur, resulting in higher efficacy and safety. Therefore, mRNA therapy during pregnancy has become one of the hot topics for early intervention in congenital diseases. Owing to the presence of the blood-placental barrier, targeting intrauterine structures is restricted, leading to widespread exploration of HTS techniques for improving delivery systems [167].
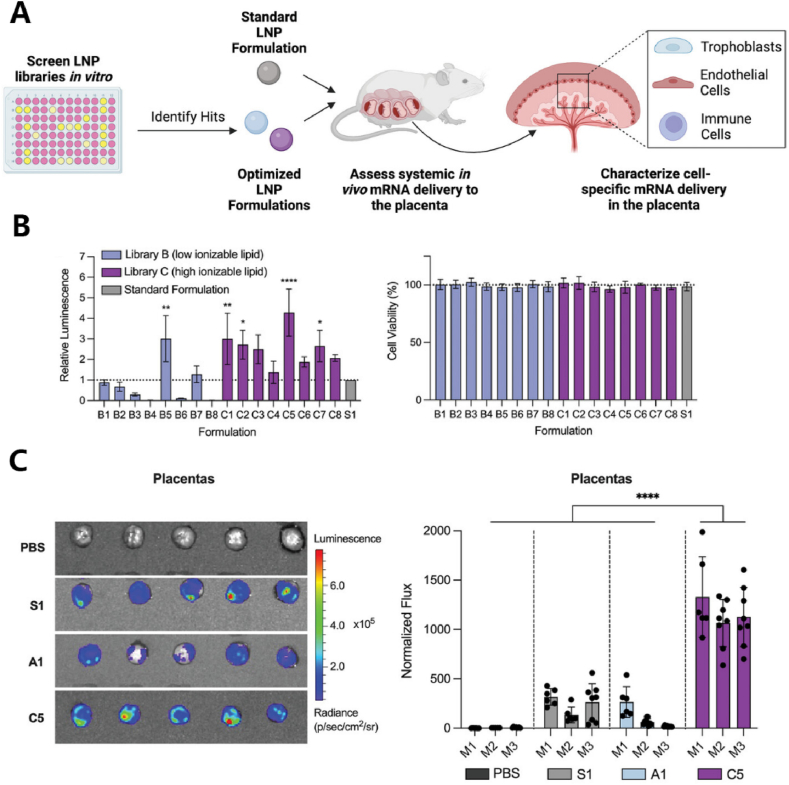
HTS can be used for pregnancy-related diseases, mainly involving intrauterine targeted treatment of the fetus or targeting fetal accessory structures such as the placenta and amniotic fluid. Researchers constructed an LNP library and injected it into pregnant mice via the yolk vein. mRNA expression was detected in widespread areas of the fetus, including the liver, lungs, heart, gastrointestinal tract, and brain. Through this, they identified ionizable lipid D-Lin [117] and polyamine structure [118] as having better prenatal treatment effects. Additionally, the placenta supports fetal growth during pregnancy, allowing the exchange of nutrients and oxygen between the mother and fetus. Serving as a biological barrier between maternal and fetal circulation, the placenta protects the fetus from harmful molecules. Placental diseases are significant causes of maternal and fetal mortality, leading to conditions such as preeclampsia and fetal growth restriction. Through in vitro culture cell lines, endothelial cell lines, and immune cell lines, along with in vivo experiments validating placental delivery, the enhancement of placental targeting for multiple LNPs with ionizable lipids may contribute to the treatment of placental insufficiency diseases during pregnancy [[119], [120], [121], [122]]. For instance, Safford et al. conducted in vitro screening of LNPA, B, C libraries, optimizing LNP formulations targeting the placenta. They identified C5 as having the highest fluorescent enzyme expression efficiency in cultured cells and confirmed its ability to target the placenta in vivo. This provides insights for research into the treatment of pregnancy-related and other reproductive system diseases [121](Fig. 7).
Fig. 7.
HTS application in pregnancy diseases.(A)After in vitro screening, selected Hits LNP formulations are locally injected into pregnant mice to observe the cell-specific transport effects on the placenta.(B)During in vitro screening, co-cultured with nurturing cells, C5 LNP exhibited the highest fluorescence enzyme activity compared to the standard S1, and the safety profiles of all materials were relatively good.(C)During in vivo validation, compared to standard S1, C5 exhibited significantly higher placental targeting specificity [121]. © 2023 Wiley‐VCH GmbH.
4.5. Other localized diseases
While liver is the primary organ targeted by systemic administration, secondary cell populations outside the liver increase the challenges of achieving mRNA selective delivery. Research has constructed an LNP lipid screening platform for delivering mRNA to activated hepatic stellate cells (aHSCs). The pKa of LNPs and pH-sensitive lipid hydrophobic scaffolds significantly influences the efficiency and selectivity of mRNA delivery in the liver. Ultimately, it was revealed that CL15A6 is superior to other studied lipids, enabling ligand-free, efficient, and potent mRNA delivery to aHSCs. It possesses high in vitro stability and in vivo biocompatibility, making it highly promising for applications in liver fibrosis treatment [123].
mRNA can be used for the treatment of bone and cartilage diseases. For example, WNT16 in chondrocytes can antagonize the typical β-catenin/WNT3a signal, leading to increased lubricin production and reduced chondrocyte apoptosis, thereby treating osteoarthritis [168]. However, due to certain biological barriers such as low bone blood flow, the blood-bone marrow barrier, and low affinity between drugs and bone minerals, therapeutic doses are hindered in the bone microenvironment, making bone therapy delivery still a significant challenge. Constructing a library of bisphosphonate (BP)-based lipid materials with high affinity for bone minerals has significant potential applications in targeting the bone microenvironment for therapies such as bone regeneration and hematopoietic stem cell treatment. Researchers identified a BP-LNP lead formulation, 490BP-C14, which, compared to 490-C14 LNPs without BP, showed enhanced expression and localization of mRNA in the bone microenvironment in mice. This can be further utilized for the treatment of bone diseases [124].
5. Selection of administration routes
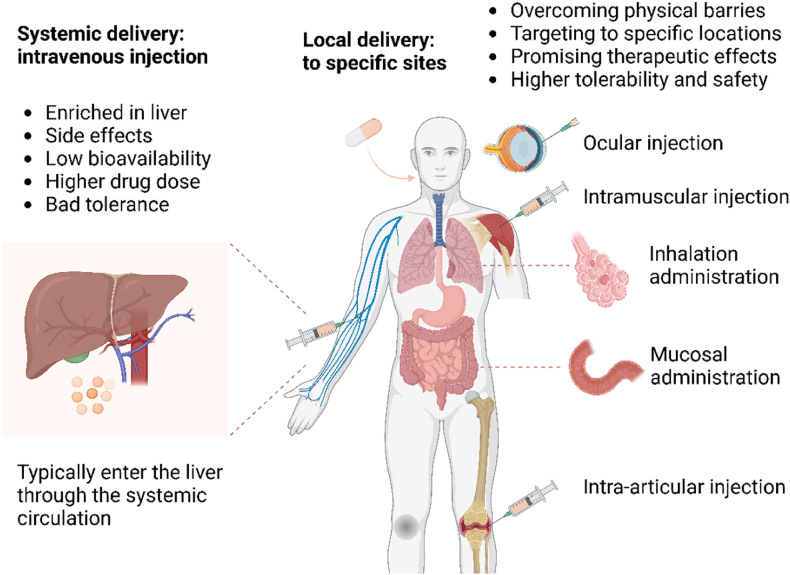
Adopting different administration methods to alter the destination of mRNA delivery is an intuitive macroscopic approach. Intravenous injection is the most used method, where drugs usually enter the liver through the circulatory system, undergoing filtration and absorption. Systemic administration leads to the majority of drugs accumulating in the liver, making it challenging for drug concentrations to reach therapeutic thresholds at the corresponding target cells. Therefore, precise control and enhanced targeting of delivery can be achieved by developing local or site-specific administration methods [9] (Fig. 8).
Fig. 8.
Selection of Administration Routes. The delivery of mRNA into the human body typically involves traditional systemic administration, intravenous injection, which accumulates in the liver through the circulatory system. This method triggers a series of side effects, resulting in lower bioavailability, higher drug concentrations, and poorer adaptability. Adopting local administration to reach specific locations is a feasible improvement method. This mainly includes intraventricular administration, intraocular injection, intramuscular injection, gastrointestinal administration, respiratory administration, and intra-articular injection. It can effectively overcome physical barriers to enhance targeting, achieve more promising therapeutic effects, and has higher availability and safety.
The local injection method for direct administration to specific sites allows drugs to bypass interference from other physiological structures, overcome inherent physiological barriers, and quickly reach the target site. Compared to systemic administration, local injection is relatively limited in range, making it more suitable for targeted treatment of diseases localized to specific positions. It can focus more on the effects of specific organs and is commonly applicable for delivering to local tissues and organs, including intraocular injection, intraventricular injection, intranasal injection, intramuscular injection, and others [9]. In recent years, more and more local drug delivery methods have been improved. For example, intramuscular injection, as a common injection method for most vaccines, allows drugs to spread from the injection site in the muscles throughout the body, deliver antigens, and activate the immune response. As mentioned above, the pKa of LNPs may affect the formation of specific protein coronas, thereby influencing the material's biodistribution. Unlike the optimal pKa value of 6.2–6.5 for liver targeting during intravenous injection [169], Tilstra et al. found that LNPs composed of an ethanolamine core with an apparent pKa value between 6.6 and 6.9, combined with ionizable lipids, maximally improve mRNA delivery in muscles. Notably, this formulation also exhibited the highest immunogenicity [170].This provides a foundation for the subsequent construction of intramuscular injection carriers and offers insights for the design of future vaccines [20]. Intraocular injection, through injecting mRNA into the eye, can effectively target photoreceptor cells and retinal pigment epithelial cells (RPEs), providing inherent advantages for the treatment of eye diseases. Intravitreal injection is mainly divided into intravitreal injection and subretinal injection. Intravitreal injection has higher safety, but the process of therapeutic drugs reaching target cells still faces many barriers [97,171]. Subretinal injection is closer to the target site, but potential damage to vision cannot be ignored. Therefore, improvements in carriers are crucial for enhancing the safety and efficacy of ocular injections. Barrera et al. screened a phage peptide library to identify surface ligands that bind to photoreceptor cells after intravitreal injection, further improving the targeting of photoreceptor cells [97]. In addition, for retinitis pigmentosa (RP) caused by mutations in the USH2A gene, the antisense oligonucleotide drug QR-421a, administered via intravitreal injection, targets photoreceptors and has entered clinical trials (NCT03780257), providing insights into mRNA-based therapy for ocular diseases [172]. Moreover, studies have found that adjusting the PEG content in LNPs to 0.5 % for subretinal injection results in improved RPE transduction, enhancing targeting [171]. Similarly, intraperitoneal injection involves injecting drugs into the peritoneum, stimulating pancreatic beta cells to increase protein levels. This discovery has made gene therapy for stubborn pancreatic diseases such as diabetes and cancer possible. The local injection triggering intraperitoneal effects can reduce systemic toxicity, allowing drugs to more selectively contact peritoneal organs, thereby enhancing bioavailability [173]. Additionally, it is difficult to achieve therapeutic effects on the central nervous system with systemic intravenous administration due to the presence of the blood-brain barrier. Intraventricular administration involves directly injecting mRNA-LNP complexes into the ventricles, bypassing the blood-brain barrier (BBB) to directly increase the levels of the target protein in the brain. This serves as a reference for the treatment of subsequent central nervous system diseases [174]. Similarly, intrathecal injection delivers the corresponding mRNA-LNP complex directly into the cerebrospinal fluid of patients via lumbar puncture, bypassing the blood-brain barrier to target specific areas of brain tissue [175]. For instance, Nabhan et al. utilized LNP-packaged frataxin (FXN) protein mRNA for intrathecal injection into the cerebrospinal fluid of mice and detected recombinant human FXN protein in the dorsal root ganglia, demonstrating that intrathecal injection can bypass the blood-brain barrier to reach the brain [176]. Nasal administration, taking advantage of the proximity between the nasal cavity and the brain, can deliver drugs to the brain through the nasal cavity, making it more accessible and causing less damage to the brain itself [177]. This provides a new feasible drug delivery option for neurological diseases; however, both local injection methods require attention to brain protection, and considerations for potential biological damage in the design of injection carriers.
Local administration not only has a direct effect on the organs at the administration site but also avoids the reduction in drug efficacy or drug loss caused by systemic blood circulation transport. For example, mucosal administration can be used to deliver mRNA for diseases in body cavities. For locally restricted diseases in the lungs such as cystic fibrosis, inhalation therapy is commonly administered through nebulization. Utilizing the direct connection of the respiratory tract with the external environment, inhalation therapy can deliver drugs more rapidly and directly to cells (e.g., tracheal epithelium) in the respiratory system with good efficacy. LNPs are commonly used as carriers for inhalation therapy [178,179]. Through nebulized inhalation, LNPs containing full-length mRNA of cystic fibrosis transmembrane conductance regulator can be directly transported to pulmonary epithelial cells, controlling the progression of the disease at its source. Additionally, polymers can be used as carriers; researchers have optimized a biodegradable polyamine-co-ester polymer inhalation formulation through end-group modification and polyethylene glycol, improving drug absorption efficiency in the lungs. Currently, the mRNA product MRT5005 for the treatment of cystic fibrosis has entered clinical trials (NCT03375047). MRT5005 shows good tolerance and safety, whereas effective improvement in lung function has not been observed yet [180]. Considering the relatively slow overall absorption, larger required doses, and susceptibility to ciliary clearance in inhalation therapy, with individual variations in treatment outcomes, clinical applications should focus more on improving absorption efficiency. Besides the respiratory tract, mucosal administration is also applicable to the gastrointestinal tract (oral and rectal administration), typically for diseases of the digestive system such as inflammatory bowel disease, and has achieved certain success [[181], [182], [183]]. Sung and colleagues designed self-assembled LNPs encapsulating IL-22-mRNA, which entered the mouse body non-invasively, increased the target protein content in the mouse mucosa, and showed good therapeutic effects on ulcerative colitis [181]. Researchers also utilized polyphenols to directly deliver mRNA to the intestines through rectal administration by complexing with nucleic acids, achieving significant therapeutic effects in a model of ulcerative colitis induced by sodium sulfate [183].
6. Conclusions and future perspective
The development of mRNA delivery has garnered increasing attention. In this review, we primarily discussed the progress of various technologies applied to overcome biological barriers encountered in mRNA delivery. This includes common low-throughput strategies in microscopic methods and macroscopic approaches such as route selection. This review also emphasized the validation and research of improved delivery systems, particularly focusing on HTS. The development of HTS technologies is an inevitable trend in molecular biology research as it gradually becomes more precise, requires specific binding, and delves into intricate details. With the rapid advancement of biotechnology, the application of HTS techniques in the field of mRNA therapy has similarities to the laboratory simulation of natural evolutionary processes, aiming at the targeted remodeling of mRNA delivery systems. The complementary advantages of these technologies provide a foundation for the improvement of targeted mRNA delivery.
The main differences between HTS and traditional low-throughput techniques lie in their efficiency (the number of compounds screened in a single run and the time taken to screen useable materials). Low-throughput screening to determine the optimal formulation is accomplished by testing individual formulations or modifying existing formulations, with the main issue being the time and efficiency constraints associated with low-throughput screening. Before the advent of HTS, drug screening was a slow and expensive process, which could hinder the identification of formulations with the highest potential and bias towards combinations tailored for specific applications. In contrast to low-throughput screening of individual or very few materials, libraries used by most large pharmaceutical companies engaged in HTS typically range from 0.5 to 4*106 compounds, greatly enhancing screening efficiency. Additionally, the effectiveness of screened materials in mRNA delivery (mRNA transfection rate, protein production, etc.), targeting effects (in vitro cell targeting, fluorescence intensity in various organs, barcode sequencing results, etc.), therapeutic effects (intensity of immune responses both in vitro and in vivo, anti-tumor or anti-infection effects, reduction of disease symptoms, etc.), and safety (acute inflammatory response, acute hemolysis, etc.) can also be compared between HTS and traditional low-throughput methods. For example, LNPs synthesized from the ionizable lipid iso-A11B5C1 identified through high-throughput screening for muscle injection of mRNA vaccines exhibited mRNA transfection efficiency comparable to commercially used SM-102 and MC3 LNPs. Analysis of fluorescence intensity revealed their higher muscle targeting efficiency, with no significant differences in acute inflammatory response, and induced a stronger immune response. Therefore, validation of high-throughput screening results can be achieved by comparing mRNA transfection efficiency, fluorescence intensity, and functional validation with low-throughput modified materials to confirm their effectiveness and safety. Moreover, the use of HTS allows researchers to understand the predictive value of parameters such as molecular weight, pKa, hydrophobicity of hydrocarbon chains, types of amine groups, types of terminal functional groups, degradation kinetics, number and saturation level of hydrocarbon chains, and other indicators for the targeting of delivery systems, making the results more applicable.
The validation of HTS through wet and dry experiments makes the targeted design of mRNA delivery more reliable, further enhancing delivery efficiency. Through large-scale screening of targeting sequences, researchers can discover ligand combinations that better meet expectations and provide improved therapeutic effects. Additionally, HTS aids in the development of efficient and safe regulatory elements, expanding the design space of mRNA delivery systems. Apart from the delivery system, screening of the mRNA structure itself can also enhance efficiency. For instance, high-throughput experiments can be employed to compare the sequences and structures of different mRNAs, along with assessing their stability and expression efficiency. This helps improve targeted design, and the advantages of large-scale samples can aid in identifying universal optimization patterns. Furthermore, the precision quantification and specific binding advantages provided by HTS enable mRNA targeted delivery to have higher controllability. This approach can comprehensively assess safety, toxicity, and stability in clinical applications. When combined with other therapeutic modalities, it is easier to achieve synergistic effects. The characteristics of being large-scale, efficient, and cost-effective also increase the likelihood of clinical translation.
In addition to constructing various material systems for mRNA targeted delivery using HTS, high-throughput technology can also be applied to other aspects and combined with mRNA interventions to bring more choices to gene therapy. For example, leveraging the characteristics of large-scale and diverse samples with HTS, extensive screening and in-depth analysis in model animals or patient samples can provide rich information on gene expression and cell states. This can lead to the discovery of new therapeutic targets or biomarkers, identification of suitable targeting sequences for mRNA delivery, expedite the development of related drugs, and offer new possibilities for treating various diseases. With its considerable scale and affinity screening capabilities, HTS offers the potential to overcome individualized response differences in the mRNA treatment process, possibly advancing the development of precision medicine. Combining HTS with multi-omics analysis may allow for the assessment of changes in gene expression levels before and after mRNA treatment, providing feedback for the design and improvement of mRNA targeted delivery.
However, how high-throughput technologies can play a more sensitive and efficient role in the research field of mRNA delivery systems remains one of the pressing technical bottlenecks. Due to their large scale, high specificity of results, and diverse screening objects and application scenarios, even minor differences in each sample can affect the analysis of results. High interference traits lead to reduced measurability and limited repeatability of results due to the appearance of false positive signals, posing some challenges that this technology must address.
In terms of quality control issues and result reproducibility in HTS, the specificity of the screening itself is also one of the factors limiting its development. The speed and volume of data generated by high-throughput technologies far exceed those of traditional experimental techniques. Therefore, standardization and repeatability of the technology are crucial for data reliability. While expanding screening throughput, how to maintain the purity between samples and reduce irrelevant factors' interference deserves technical discussion. The construction of in vitro models is a frequently discussed issue in this regard. Due to the high specificity of HTS and the limitations of existing models in simulating the in vivo microenvironment, results obtained from in vitro models may suffer from false positive issues and may not adapt well to in vivo application scenarios. New cell culture technologies such as 3D cell models and organoid construction, combined with high-throughput in vitro screening, may offer more effective in vitro screening results, thereby reducing the cost of in vivo experiments. The universality of in vivo-specific screening results may also be influenced by the characteristics of biology itself. Therefore, by combining single-cell technologies and artificial intelligence models such as machine learning to predict potential targets in advance, quantitative HTS can be conducted at the single-cell resolution. This approach may to some extent ensure the predictability and repeatability of experiments.
The analysis of HTS results often relies on background noise filtering of measurement platforms and integration analysis of positive signals. Therefore, maintaining signal stability, delaying quenching time in existing detection environments, improving response strategies, and ensuring the measurability of high-throughput technologies are among the challenges to be addressed. Experiments using high-throughput technologies may exhibit "all or nothing" results. Therefore, apart from false positive results, signal loss during the screening process is also a limiting factor in technology development. Due to the characteristics of automated analysis, the aforementioned challenges can also hinder our understanding of the mechanism of action of specific targeting molecules. Therefore, it may be beneficial to consider using fluorescent probes with higher affinity to specific cellular components or delivery system structures during the experimental process to assist in the screening process. With the assistance of artificial intelligence predictions, setting positive thresholds reasonably can be achieved. For complex mRNA drug delivery system design processes, the commercialization of technologies such as microfluidics and droplet sorting may enable analysis at a population level with optimized systems under more stringent standardized operating procedures and quality control measures. This approach can retain more data on interactions between cells or molecules, thus ensuring more comprehensive information analysis.
With continuous improvement and optimization, the application prospects of high-throughput technology in mRNA therapy and other biomedical fields will become broader. The interdisciplinary integration is also helpful to understand the complexity of biological systems more comprehensively, and it is expected to bring revolutionary changes to disease diagnosis, treatment, and personalized medicine.
Funding
This work was supported by National Natural Science Foundation of China (No. 82372374), Guangdong Basic and Applied Basic Research foundation (No. 2024B1515020015), Science and Technology Program of Guangzhou (No. 2024A04J4713).
Availability of data and materials
Data will be made available on request.
CRediT authorship contribution statement
Yuchen Zhang: Writing – original draft, Visualization, Methodology, Data curation. Zhifei Gao: Writing – original draft, Validation, Data curation. Xiao Yang: Writing – original draft, Data curation. Qinglong Xu: Writing – original draft, Data curation. Yao Lu: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Rohner E., Yang R., Foo K.S., Goedel A., Chien K.R. Unlocking the promise of mRNA therapeutics. Nat Biotechnol. 2022;40:1586–1600. doi: 10.1038/s41587-022-01491-z. [DOI] [PubMed] [Google Scholar]
- 2.Kang D.D., Li H., Dong Y. Advancements of in vitro transcribed mRNA (IVT mRNA) to enable translation into the clinics. Adv Drug Deliv Rev. 2023;199 doi: 10.1016/j.addr.2023.114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin L., Pei Y., Li Z., Luo D. Progress and challenges of mRNA vaccines. Interdisciplinary Medicine. 2023;1 doi: 10.1002/INMD.20220008. [DOI] [PubMed] [Google Scholar]
- 4.Wei H.-H., Zheng L., Wang Z. mRNA therapeutics: new vaccination and beyond. Fundamental Research. 2023;3:749–759. doi: 10.1016/j.fmre.2023.02.022. [DOI] [Google Scholar]
- 5.Liang D., Wang Y., Qian K. Nanozymes: applications in clinical biomarker detection. Interdisciplinary Medicine. 2023;1 doi: 10.1002/INMD.20230020. [DOI] [Google Scholar]
- 6.Zhang W., Jiang Y., He Y., Boucetta H., Wu J., Chen Z., He W. Lipid carriers for mRNA delivery. Acta Pharmaceutica Sinica. B. 2023;13:4105–4126. doi: 10.1016/j.apsb.2022.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Y., Tang Z., Huang X., Chen W., Zhou J., Liu H., Liu C., Kong N., Tao W. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem. Soc. Rev. 2022;51:3828–3845. doi: 10.1039/D1CS00617G. [DOI] [PubMed] [Google Scholar]
- 8.G.-A. I, R.-C. J, V.-P. M, R.-G. A. Má S., A D.P.-R. Nanomedicines to deliver mRNA: state of the art and future Perspectives. Nanomaterials (Basel, Switzerland) 2020;10 doi: 10.3390/nano10020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung S., Lee C.M., Zhang M. Advances in nanoparticle-based mRNA delivery for liver cancer and liver-associated infectious diseases. Nanoscale Horiz. 2022;8:10–28. doi: 10.1039/d2nh00289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan M., Han Z., Liang Y., Sun Y., He B., Chen W., Li F. mRNA nanodelivery systems: targeting strategies and administration routes. Biomater Res. 2023;27:90. doi: 10.1186/s40824-023-00425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L., Su K., Cheng Q., Liu S. Targeting materials and strategies for RNA delivery. Theranostics. 2023;13:4667–4693. doi: 10.7150/thno.87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.I T., V F., H F., L F. High-throughput screening of nanoparticles in drug delivery. APL Bioengineering. 2021;5 doi: 10.1063/5.0057204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong M., Lee Y., Park J., Jung H., Lee H. Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv Drug Deliv Rev. 2023 doi: 10.1016/j.addr.2023.114990. [DOI] [PubMed] [Google Scholar]
- 15.Haabeth O.A.W., Lohmeyer J.J.K., Sallets A., Blake T.R., Sagiv-Barfi I., Czerwinski D.K., McCarthy B., Powell A.E., Wender P.A., Waymouth R.M., Levy R. An mRNA SARS-CoV-2 vaccine employing charge-altering releasable transporters with a TLR-9 agonist induces neutralizing antibodies and T cell memory. ACS Cent Sci. 2021;7:1191–1204. doi: 10.1021/acscentsci.1c00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han X., Alameh M.-G., Butowska K., Knox J.J., Lundgreen K., Ghattas M., Gong N., Xue L., Xu Y., Lavertu M., Bates P., Xu J., Nie G., Zhong Y., Weissman D., Mitchell M.J. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat Nanotechnol. 2023;18:1105–1114. doi: 10.1038/s41565-023-01404-4. [DOI] [PubMed] [Google Scholar]
- 17.Islam M.A., Rice J., Reesor E., Zope H., Tao W., Lim M., Ding J., Chen Y., Aduluso D., Zetter B.R., Farokhzad O.C., Shi J. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. 2021;266 doi: 10.1016/j.biomaterials.2020.120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douka S., Brandenburg L.E., Casadidio C., Walther J., Garcia B.B.M., Spanholtz J., Raimo M., Hennink W.E., Mastrobattista E., Caiazzo M. Lipid nanoparticle-mediated messenger RNA delivery for ex vivo engineering of natural killer cells. J Control Release. 2023;361:455–469. doi: 10.1016/j.jconrel.2023.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T., Nakade T., Sato Y., Harashima H. Delivering mRNA to a human NK cell line, NK-92 cells, by lipid nanoparticles. Int J Pharm. 2023;636 doi: 10.1016/j.ijpharm.2023.122810. [DOI] [PubMed] [Google Scholar]
- 20.Tilstra G., Couture-Senécal J., Lau Y.M.A., Manning A.M., Wong D.S.M., Janaeska W.W., Wuraola T.A., Pang J., Khan O.F. Iterative design of ionizable lipids for intramuscular mRNA delivery. J Am Chem Soc. 2023;145:2294–2304. doi: 10.1021/jacs.2c10670. [DOI] [PubMed] [Google Scholar]
- 21.Żak M.M., Kaur K., Yoo J., Kurian A.A., Adjmi M., Mainkar G., Yoon S., Zangi L. Modified mRNA formulation and stability for cardiac and skeletal muscle delivery. Pharmaceutics. 2023;15:2176. doi: 10.3390/pharmaceutics15092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y., Fenton O.S. An efficacy and mechanism driven study on the impact of Hypoxia on lipid nanoparticle mediated mRNA delivery. J Am Chem Soc. 2023;145:11375–11386. doi: 10.1021/jacs.3c02584. [DOI] [PubMed] [Google Scholar]
- 23.Dilliard S.A., Cheng Q., Siegwart D.J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2109256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J., Atochina-Vasserman E.N., Maurya D.S., Shalihin M.I., Zhang D., Chenna S.S., Adamson J., Liu M., Shah H.U.R., Shah H., Xiao Q., Queeley B., Ona N.A., Reagan E.K., Ni H., Sahoo D., Peterca M., Weissman D., Percec V. Screening libraries to discover molecular design principles for the targeted delivery of mRNA with one-component ionizable amphiphilic Janus Dendrimers derived from plant Phenolic acids. Pharmaceutics. 2023;15:1572. doi: 10.3390/pharmaceutics15061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dilliard S.A., Sun Y., Brown M.O., Sung Y.-C., Chatterjee S., Farbiak L., Vaidya A., Lian X., Wang X., Lemoff A., Siegwart D.J. The interplay of quaternary ammonium lipid structure and protein corona on lung-specific mRNA delivery by selective organ targeting (SORT) nanoparticles. J Control Release. 2023;361:361–372. doi: 10.1016/j.jconrel.2023.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heuer-Jungemann A., Feliu N., Bakaimi I., Hamaly M., Alkilany A., Chakraborty I., Masood A., Casula M.F., Kostopoulou A., Oh E., Susumu K., Stewart M.H., Medintz I.L., Stratakis E., Parak W.J., Kanaras A.G. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem Rev. 2019;119:4819–4880. doi: 10.1021/acs.chemrev.8b00733. [DOI] [PubMed] [Google Scholar]
- 28.Chang J., Mo L., Song J., Wang X., Liu H., Meng C., Wu Y. A pH-responsive mesoporous silica nanoparticle-based drug delivery system for targeted breast cancer therapy. Journal of Materials Chemistry. B. 2022;10:3375–3385. doi: 10.1039/d1tb02828f. [DOI] [PubMed] [Google Scholar]
- 29.Amin M.U., Ali S., Ali M.Y., Tariq I., Nasrullah U., Pinnapreddy S.R., Wölk C., Bakowsky U., Brüßler J. Enhanced efficacy and drug delivery with lipid coated mesoporous silica nanoparticles in cancer therapy. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V. 2021;165:31–40. doi: 10.1016/j.ejpb.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Dong S., Feng Z., Ma R., Zhang T., Jiang J., Li Y., Zhang Y., Li S., Liu X., Liu X., Meng H. Engineered design of a mesoporous silica nanoparticle-based Nanocarrier for efficient mRNA delivery in vivo. Nano Lett. 2023;23:2137–2147. doi: 10.1021/acs.nanolett.2c04486. [DOI] [PubMed] [Google Scholar]
- 31.Kamegawa R., Naito M., Uchida S., Kim H.J., Kim B.S., Miyata K. Bioinspired Silicification of mRNA-loaded polyion complexes for macrophage-targeted mRNA delivery. ACS Appl Bio Mater. 2021;4:7790–7799. doi: 10.1021/acsabm.1c00704. [DOI] [PubMed] [Google Scholar]
- 32.Tehrani S.F., Bharadwaj P., Leblond Chain J., Roullin V.G. Purification processes of polymeric nanoparticles: how to improve their clinical translation? J Control Release. 2023;360:591–612. doi: 10.1016/j.jconrel.2023.06.038. [DOI] [PubMed] [Google Scholar]
- 33.Kim J., Mondal S.K., Tzeng S.Y., Rui Y., Al-Kharboosh R., Kozielski K.K., Bhargav A.G., Garcia C.A., Quiñones-Hinojosa A., Green J.J. Poly(ethylene glycol)-Poly(beta-amino ester)-based nanoparticles for Suicide gene therapy enhance brain penetration and Extend survival in a Preclinical human glioblastoma Orthotopic Xenograft model. ACS Biomaterials Science & Engineering. 2020;6:2943–2955. doi: 10.1021/acsbiomaterials.0c00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capasso Palmiero U., Kaczmarek J.C., Fenton O.S., Anderson D.G. Poly(β-amino ester)-co-poly(caprolactone) terpolymers as Nonviral Vectors for mRNA delivery In vitro and In vivo. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800249. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh B., Biswas S. Polymeric micelles in cancer therapy: state of the art. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2021;332:127–147. doi: 10.1016/j.jconrel.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Junnuthula V., Sadeghi Boroujeni A., Cao S., Tavakoli S., Ridolfo R., Toropainen E., Ruponen M., van Hest J.C.M., Urtti A. Intravitreal polymeric nanocarriers with Long ocular Retention and targeted delivery to the retina and optic nerve head region. Pharmaceutics. 2021;13:445. doi: 10.3390/pharmaceutics13040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J., Cao Y., Li L., Wang X., Lu H., Yang J., Wang S. Self-assembled polymeric micelle as a novel mRNA delivery carrier. J Control Release. 2021;338:537–547. doi: 10.1016/j.jconrel.2021.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z., Amaya L., Pi R., Wang S.K., Ranjan A., Waymouth R.M., Blish C.A., Chang H.Y., Wender P.A. Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism. Nat Commun. 2023;14:6983. doi: 10.1038/s41467-023-42672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L., Long J., Sang Y., Wang X., Zhou X., Pan Y., Cao Y., Huang H., Yang Z., Yang J., Wang S. Rational preparation and application of a mRNA delivery system with cytidinyl/cationic lipid. J Control Release. 2021;340:114–124. doi: 10.1016/j.jconrel.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y., Long J., Sun H., Miao Y., Sang Y., Lu H., Yu C., Zhang Z., Wang L., Yang J., Wang S. Dendritic cell-mimicking nanoparticles promote mRNA delivery to lymphoid organs. Adv Sci (Weinh) 2023 doi: 10.1002/advs.202302423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y., Fourniols T., Labrak Y., Préat V., Beloqui A., des Rieux A. Surface modification of lipid-based nanoparticles. ACS Nano. 2022;16:7168–7196. doi: 10.1021/acsnano.2c02347. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Zhan J., Huang J., Wang X., Chen Z., Yang Z., Li J. Dynamic responsiveness of self-assembling peptide-based nano-drug systems. Interdisciplinary Medicine. 2023;1 doi: 10.1002/INMD.20220005. [DOI] [Google Scholar]
- 43.Zhao Y., Li X., Zhang W., Yu L., Wang Y., Deng Z., Liu M., Mo S., Wang R., Zhao J., Liu S., Hao Y., Wang X., Ji T., Zhang L., Wang C. Trends in the biological functions and medical applications of extracellular vesicles and analogues. Acta Pharmaceutica Sinica. B. 2021;11:2114–2135. doi: 10.1016/j.apsb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veiga N., Goldsmith M., Granot Y., Rosenblum D., Dammes N., Kedmi R., Ramishetti S., Peer D. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat Commun. 2018;9:1–9. doi: 10.1038/s41467-018-06936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain A.K., Bataille C.J.R., Milhas S., Miller A., Zhang J., Rabbitts T.H. Immunopolymer lipid nanoparticles for delivery of macromolecules to antigen-expressing cells. ACS Appl Bio Mater. 2020;3:8481–8495. doi: 10.1021/acsabm.0c00857. [DOI] [PubMed] [Google Scholar]
- 46.Rurik J.G., Tombácz I., Yadegari A., Méndez Fernández P.O., Shewale S.V., Li L., Kimura T., Soliman O.Y., Papp T.E., Tam Y.K., Mui B.L., Albelda S.M., Puré E., June C.H., Aghajanian H., Weissman D., Parhiz H., Epstein J.A. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–96. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tombácz I., Laczkó D., Shahnawaz H., Muramatsu H., Natesan A., Yadegari A., Papp T.E., Alameh M.-G., Shuvaev V., Mui B.L., Tam Y.K., Muzykantov V., Pardi N., Weissman D., Parhiz H. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol Ther. 2021;29:3293–3304. doi: 10.1016/j.ymthe.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roskoski R. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res. 2019;139:395–411. doi: 10.1016/j.phrs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Pham T.C., Jayasinghe M.K., Pham T.T., Yang Y., Wei L., Usman W.M., Chen H., Pirisinu M., Gong J., Kim S., Peng B., Wang W., Chan C., Ma V., Nguyen N.T.H., Kappei D., Nguyen X.-H., Cho W.C., Shi J., Le M.T.N. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. Journal of Extracellular Vesicles. 2021;10 doi: 10.1002/jev2.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mori T., Giovannelli L., Bilia A.R., Margheri F. Exosomes: potential next-generation nanocarriers for the therapy of inflammatory diseases. Pharmaceutics. 2023;15:2276. doi: 10.3390/pharmaceutics15092276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du R., Wang C., Zhu L., Yang Y. Extracellular vesicles as delivery vehicles for therapeutic nucleic acids in cancer gene therapy: progress and challenges. Pharmaceutics. 2022;14:2236. doi: 10.3390/pharmaceutics14102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evers M.J.W., van de Wakker S.I., de Groot E.M., de Jong O.G., Gitz-François J.J.J., Seinen C.S., Sluijter J.P.G., Schiffelers R.M., Vader P. Functional siRNA delivery by extracellular vesicle-liposome hybrid nanoparticles. Adv Healthc Mater. 2022;11 doi: 10.1002/adhm.202101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W., Ngo L., Tsao S.C.-H., Liu D., Wang Y. Engineered cancer-derived small extracellular vesicle-liposome hybrid delivery system for targeted treatment of breast cancer. ACS Appl Mater Interfaces. 2023;15:16420–16433. doi: 10.1021/acsami.2c22749. [DOI] [PubMed] [Google Scholar]
- 54.Hadianamrei R., Zhao X. Current state of the art in peptide-based gene delivery. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2022;343:600–619. doi: 10.1016/j.jconrel.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Lai X., Geng X., Tan L., Hu J., Wang S. A pH-responsive system based on fluorescence enhanced gold nanoparticles for Renal targeting drug delivery and fibrosis therapy. Int. J. Nanomed. 2020;15:5613–5627. doi: 10.2147/IJN.S260069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu M., Feng C., Yuan Q., Liu C., Ge B., Sun F., Zhu X. Lantern-shaped flexible RNA origami for Smad4 mRNA delivery and growth suppression of colorectal cancer. Nat Commun. 2023;14:1307. doi: 10.1038/s41467-023-37020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grant-Serroukh D., Hunter M.R., Maeshima R., Tagalakis A.D., Aldossary A.M., Allahham N., Williams G.R., Edbrooke M., Desai A., Hart S.L. Lipid-peptide nanocomplexes for mRNA delivery in vitro and in vivo. J Control Release. 2022;348:786–797. doi: 10.1016/j.jconrel.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 58.Tian T., Zhang H.-X., He C.-P., Fan S., Zhu Y.-L., Qi C., Huang N.-P., Xiao Z.-D., Lu Z.-H., Tannous B.A., Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X., Liu M., Sun L., Cao Y., Tan S., Luo G., Liu T., Yao Y., Xiao W., Wan Z., Tang J. Circulating small extracellular vesicles microRNAs plus CA-125 for treatment stratification in advanced ovarian cancer. J. Transl. Med. 2023;21:927. doi: 10.1186/s12967-023-04774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L., Yu X., Zhou J., Su C. Extracellular vesicles for drug delivery in cancer treatment. Biol. Proced. Online. 2023;25:28. doi: 10.1186/s12575-023-00220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ray R., Chowdhury S.G., Karmakar P. A vivid outline demonstrating the benefits of exosome-mediated drug delivery in CNS-associated disease environments. Arch. Biochem. Biophys. 2024;753 doi: 10.1016/j.abb.2024.109906. [DOI] [PubMed] [Google Scholar]
- 62.Fan J., Cheng Y., Sun M. Functionalized gold nanoparticles: synthesis, properties and biomedical applications. Chemical Record (New York, N.Y.) 2020;20:1474–1504. doi: 10.1002/tcr.202000087. [DOI] [PubMed] [Google Scholar]
- 63.Entezari M., Yousef Abad G.G., Sedghi B., Ettehadi R., Asadi S., Beiranvand R., Haratian N., Karimian S.S., Jebali A., Khorrami R., Zandieh M.A., Saebfar H., Hushmandi K., Salimimoghadam S., Rashidi M., Taheriazam A., Hashemi M., Ertas Y.N. Gold nanostructure-mediated delivery of anticancer agents: biomedical applications, reversing drug resistance, and stimuli-responsive nanocarriers. Environ. Res. 2023;225 doi: 10.1016/j.envres.2023.115673. [DOI] [PubMed] [Google Scholar]
- 64.Bemidinezhad A., Mirzavi F., Gholamhosseinian H., Gheybi F., Soukhtanloo M. Gold-containing liposomes and glucose-coated gold nanoparticles enhances the radiosensitivity of B16F0 melanoma cells via increasing apoptosis and ROS production. Life Sci. 2023;318 doi: 10.1016/j.lfs.2023.121495. [DOI] [PubMed] [Google Scholar]
- 65.Laradji A., Karakocak B.B., Kolesnikov A.V., Kefalov V.J., Ravi N. Hyaluronic acid-based gold nanoparticles for the topical delivery of therapeutics to the retina and the retinal pigment epithelium. Polymers. 2021;13:3324. doi: 10.3390/polym13193324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radmand A., Lokugamage M.P., Kim H., Dobrowolski C., Zenhausern R., Loughrey D., Huayamares S.G., Hatit M.Z.C., Ni H., Del Cid A., Da Silva Sanchez A.J., Paunovska K., Schrader Echeverri E., Shajii A., Peck H., Santangelo P.J., Dahlman J.E. The Transcriptional response to lung-targeting lipid nanoparticles in vivo. Nano Lett. 2023;23:993–1002. doi: 10.1021/acs.nanolett.2c04479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naidu G.S., Yong S.-B., Ramishetti S., Rampado R., Sharma P., Ezra A., Goldsmith M., Hazan-Halevy I., Chatterjee S., Aitha A., Peer D. A combinatorial library of lipid nanoparticles for cell type-specific mRNA delivery. Adv Sci (Weinh) 2023;10 doi: 10.1002/advs.202301929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rhym L.H., Manan R.S., Koller A., Stephanie G., Anderson D.G. Peptide-encoding mRNA barcodes for the high-throughput in vivo screening of libraries of lipid nanoparticles for mRNA delivery. Nat Biomed Eng. 2023;7:901–910. doi: 10.1038/s41551-023-01030-4. [DOI] [PubMed] [Google Scholar]
- 69.Baladi T., Nilsson J.R., Gallud A., Celauro E., Gasse C., Levi-Acobas F., Sarac I., Hollenstein M.R., Dahlén A., Esbjörner E.K., Wilhelmsson L.M. Stealth fluorescence labeling for live microscopy imaging of mRNA delivery. J Am Chem Soc. 2021;143:5413–5424. doi: 10.1021/jacs.1c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wojnilowicz M., Glab A., Bertucci A., Caruso F., Cavalieri F. Super-resolution imaging of proton Sponge-triggered Rupture of Endosomes and Cytosolic release of small interfering RNA. ACS Nano. 2019;13:187–202. doi: 10.1021/acsnano.8b05151. [DOI] [PubMed] [Google Scholar]
- 71.Chen Z., Tian Y., Yang J., Wu F., Liu S., Cao W., Xu W., Hu T., Siegwart D.J., Xiong H. Modular design of biodegradable ionizable lipids for improved mRNA delivery and precise cancer Metastasis Delineation In vivo. J Am Chem Soc. 2023;145:24302–24314. doi: 10.1021/jacs.3c09143. [DOI] [PubMed] [Google Scholar]
- 72.Xiong H., Liu S., Wei T., Cheng Q., Siegwart D.J. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J Control Release. 2020;325:198–205. doi: 10.1016/j.jconrel.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rui Y., Wilson D.R., Tzeng S.Y., Yamagata H.M., Sudhakar D., Conge M., Berlinicke C.A., Zack D.J., Tuesca A., Green J.J. High-throughput and high-content bioassay enables tuning of polyester nanoparticles for cellular uptake, endosomal escape, and systemic in vivo delivery of mRNA. Sci Adv. 2022;8 doi: 10.1126/sciadv.abk2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dobrowolski C., Paunovska K., Schrader Echeverri E., Loughrey D., Da Silva Sanchez A.J., Ni H., Hatit M.Z.C., Lokugamage M.P., Kuzminich Y., Peck H.E., Santangelo P.J., Dahlman J.E. Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery. Nat Nanotechnol. 2022;17:871–879. doi: 10.1038/s41565-022-01146-9. [DOI] [PubMed] [Google Scholar]
- 75.Dahlman J.E., Kauffman K.J., Xing Y., Shaw T.E., Mir F.F., Dlott C.C., Langer R., Anderson D.G., Wang E.T. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc Natl Acad Sci U S A. 2017;114:2060–2065. doi: 10.1073/pnas.1620874114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guimaraes P.P.G., Zhang R., Spektor R., Tan M., Chung A., Billingsley M.M., El-Mayta R., Riley R.S., Wang L., Wilson J.M., Mitchell M.J. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J Control Release. 2019;316:404–417. doi: 10.1016/j.jconrel.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eygeris Y., Gupta M., Kim J., Sahay G. Chemistry of lipid nanoparticles for RNA delivery. Acc Chem Res. 2022;55:2–12. doi: 10.1021/acs.accounts.1c00544. [DOI] [PubMed] [Google Scholar]
- 78.Wei Y., He T., Bi Q., Yang H., Hu X., Jin R., Liang H., Zhu Y., Tong R., Nie Y. A cationic lipid with advanced membrane fusion performance for pDNA and mRNA delivery. J Mater Chem B. 2023;11:2095–2107. doi: 10.1039/d2tb02783f. [DOI] [PubMed] [Google Scholar]
- 79.Lee S.M., Cheng Q., Yu X., Liu S., Johnson L.T., Siegwart D.J. A systematic study of unsaturation in lipid nanoparticles leads to improved mRNA transfection In vivo. Angew Chem Int Ed Engl. 2021;60:5848–5853. doi: 10.1002/anie.202013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui L., Pereira S., Sonzini S., van Pelt S., Romanelli S.M., Liang L., Ulkoski D., Krishnamurthy V.R., Brannigan E., Brankin C., Desai A.S. Development of a high-throughput platform for screening lipid nanoparticles for mRNA delivery. Nanoscale. 2022;14:1480–1491. doi: 10.1039/d1nr06858j. [DOI] [PubMed] [Google Scholar]
- 81.Zhang R., Shao S., Piao Y., Xiang J., Wei X., Zhang Z., Zhou Z., Tang J., Qiu N., Xu X., Liu Y., Shen Y. Esterase-labile Quaternium lipidoid enabling improved mRNA-LNP stability and spleen-selective mRNA transfection. Adv Mater. 2023 doi: 10.1002/adma.202303614. [DOI] [PubMed] [Google Scholar]
- 82.Liu S., Cheng Q., Wei T., Yu X., Johnson L.T., Farbiak L., Siegwart D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat Mater. 2021;20:701–710. doi: 10.1038/s41563-020-00886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu Y., Ma J., Shen R., Lin J., Li S., Lu X., Stelzel J.L., Kong J., Cheng L., Vuong I., Yao Z.-C., Wei C., Korinetz N.M., Toh W.H., Choy J., Reynolds R.A., Shears M.J., Cho W.J., Livingston N.K., Howard G.P., Hu Y., Tzeng S.Y., Zack D.J., Green J.J., Zheng L., Doloff J.C., Schneck J.P., Reddy S.K., Murphy S.C., Mao H.-Q. Screening for lipid nanoparticles that modulate the immune activity of helper T cells towards enhanced antitumour activity. Nat Biomed Eng. 2023 doi: 10.1038/s41551-023-01131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LoPresti S.T., Arral M.L., Chaudhary N., Whitehead K.A. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J Control Release. 2022;345:819–831. doi: 10.1016/j.jconrel.2022.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng Y., Escalona-Rayo O., Knol R., Kros A., Slütter B. Lipid nanoparticle-based mRNA candidates elicit potent T cell responses. Biomater Sci. 2023;11:964–974. doi: 10.1039/d2bm01581a. [DOI] [PubMed] [Google Scholar]
- 86.Li Y., Jarvis R., Zhu K., Glass Z., Ogurlu R., Gao P., Li P., Chen J., Yu Y., Yang Y., Xu Q. Protein and mRNA delivery enabled by Cholesteryl-based biodegradable lipidoid nanoparticles. Angew Chem Int Ed Engl. 2020;59:14957–14964. doi: 10.1002/anie.202004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel S.K., Billingsley M.M., Frazee C., Han X., Swingle K.L., Qin J., Alameh M.-G., Wang K., Weissman D., Mitchell M.J. Hydroxycholesterol substitution in ionizable lipid nanoparticles for mRNA delivery to T cells. J Control Release. 2022;347:521–532. doi: 10.1016/j.jconrel.2022.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Da Silva Sanchez A.J., Zhao K., Huayamares S.G., Hatit M.Z.C., Lokugamage M.P., Loughrey D., Dobrowolski C., Wang S., Kim H., Paunovska K., Kuzminich Y., Dahlman J.E. Substituting racemic ionizable lipids with stereopure ionizable lipids can increase mRNA delivery. J Control Release. 2023;353:270–277. doi: 10.1016/j.jconrel.2022.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X., Zhao W., Nguyen G.N., Zhang C., Zeng C., Yan J., Du S., Hou X., Li W., Jiang J., Deng B., McComb D.W., Dorkin R., Shah A., Barrera L., Gregoire F., Singh M., Chen D., Sabatino D.E., Dong Y. Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Sci Adv. 2020;6:eabc2315. doi: 10.1126/sciadv.abc2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodrigues A.F., Rebelo C., Simões S., Paulo C., Pinho S., Francisco V., Ferreira L. A polymeric nanoparticle formulation for targeted mRNA delivery to fibroblasts. Adv Sci (Weinh) 2023;10 doi: 10.1002/advs.202205475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ulkoski D., Munson M.J., Jacobson M.E., Palmer C.R., Carson C.S., Sabirsh A., Wilson J.T., Krishnamurthy V.R. High-throughput automation of Endosomolytic polymers for mRNA delivery. ACS Appl Bio Mater. 2021;4:1640–1654. doi: 10.1021/acsabm.0c01463. [DOI] [PubMed] [Google Scholar]
- 92.Chen P., He X., Hu Y., Tian X.-L., Yu X.-Q., Zhang J. Spleen-targeted mRNA delivery by amphiphilic carbon dots for tumor immunotherapy. ACS Appl Mater Interfaces. 2023;15:19937–19950. doi: 10.1021/acsami.3c00494. [DOI] [PubMed] [Google Scholar]
- 93.Porosk L., Härk H.H., Arukuusk P., Haljasorg U., Peterson P., Kurrikoff K. The development of cell-penetrating peptides for efficient and selective In vivo expression of mRNA in spleen tissue. Pharmaceutics. 2023;15:952. doi: 10.3390/pharmaceutics15030952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Periyasamy K., Maloverjan M., Biswas A., Remm A., Pook M., Rebane A., Pooga M. PepFect14 mediates the delivery of mRNA into human primary keratinocytes and in vivo. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1219761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu B.R., Chen C.-W., Huang Y.-W., Lee H.-J. Cell-penetrating peptides for Use in development of transgenic Plants. Molecules (Basel, Switzerland) 2023;28:3367. doi: 10.3390/molecules28083367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jaroszewicz W., Morcinek-Orłowska J., Pierzynowska K., Gaffke L., Węgrzyn G. Phage display and other peptide display technologies. FEMS Microbiology Reviews. 2022;46 doi: 10.1093/femsre/fuab052. fuab052. [DOI] [PubMed] [Google Scholar]
- 97.Herrera-Barrera M., Ryals R.C., Gautam M., Jozic A., Landry M., Korzun T., Gupta M., Acosta C., Stoddard J., Reynaga R., Tschetter W., Jacomino N., Taratula O., Sun C., Lauer A.K., Neuringer M., Sahay G. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci Adv. 2023;9:eadd4623. doi: 10.1126/sciadv.add4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Billingsley M.M., Singh N., Ravikumar P., Zhang R., June C.H., Mitchell M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–1589. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Billingsley M.M., Hamilton A.G., Mai D., Patel S.K., Swingle K.L., Sheppard N.C., June C.H., Mitchell M.J. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 2022;22:533–542. doi: 10.1021/acs.nanolett.1c02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen J., Xu Y., Zhou M., Xu S., Varley A.J., Golubovic A., Lu R.X.Z., Wang K.C., Yeganeh M., Vosoughi D., Li B. Combinatorial design of ionizable lipid nanoparticles for muscle-selective mRNA delivery with minimized off-target effects. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2309472120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y., Yan J., Hou X., Wang C., Kang D.D., Xue Y., Du S., Deng B., McComb D.W., Liu S.-L., Zhong Y., Dong Y. STING agonist-derived LNP-mRNA vaccine enhances protective immunity against SARS-CoV-2. Nano Lett. 2023;23:2593–2600. doi: 10.1021/acs.nanolett.2c04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ben-Akiva E., Karlsson J., Hemmati S., Yu H., Tzeng S.Y., Pardoll D.M., Green J.J. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2301606120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan Y., Liu X., Wang L., Wu C., Shuai Q., Zhang Y., Liu S. Branched hydrophobic tails in lipid nanoparticles enhance mRNA delivery for cancer immunotherapy. Biomaterials. 2023;301 doi: 10.1016/j.biomaterials.2023.122279. [DOI] [PubMed] [Google Scholar]
- 104.Kliesch L., Delandre S., Gabelmann A., Koch M., Schulze K., Guzmán C.A., Loretz B., Lehr C.-M. Lipid-polymer hybrid nanoparticles for mRNA delivery to dendritic cells: impact of lipid composition on performance in different Media. Pharmaceutics. 2022;14:2675. doi: 10.3390/pharmaceutics14122675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen J., Ye Z., Huang C., Qiu M., Song D., Li Y., Xu Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Z., Zhang X.-Q., Ho W., Li F., Gao M., Bai X., Xu X. Enzyme-catalyzed one-Step synthesis of ionizable cationic lipids for lipid nanoparticle-based mRNA COVID-19 vaccines. ACS Nano. 2022;16:18936–18950. doi: 10.1021/acsnano.2c07822. [DOI] [PubMed] [Google Scholar]
- 107.Miao L., Li L., Huang Y., Delcassian D., Chahal J., Han J., Shi Y., Sadtler K., Gao W., Lin J., Doloff J.C., Langer R., Anderson D.G. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat Biotechnol. 2019;37:1174–1185. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 108.Kubara K., Yamazaki K., Miyazaki T., Kondo K., Kurotaki D., Tamura T., Suzuki Y. Lymph node macrophages drive innate immune responses to enhance the anti-tumor efficacy of mRNA vaccines. Mol Ther. 2024 doi: 10.1016/j.ymthe.2024.01.020. S1525-0016(24)00020–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang R., El-Mayta R., Murdoch T.J., Warzecha C.C., Billingsley M.M., Shepherd S.J., Gong N., Wang L., Wilson J.M., Lee D., Mitchell M.J. Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomater Sci. 2021;9:1449–1463. doi: 10.1039/d0bm01609h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huayamares S.G., Lokugamage M.P., Rab R., Da Silva Sanchez A.J., Kim H., Radmand A., Loughrey D., Lian L., Hou Y., Achyut B.R., Ehrhardt A., Hong J.S., Sago C.D., Paunovska K., Echeverri E.S., Vanover D., Santangelo P.J., Sorscher E.J., Dahlman J.E. High-throughput screens identify a lipid nanoparticle that preferentially delivers mRNA to human tumors in vivo. J Control Release. 2023;357:394–403. doi: 10.1016/j.jconrel.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Long J., Yu C., Zhang H., Cao Y., Sang Y., Lu H., Zhang Z., Wang X., Wang H., Song G., Yang J., Wang S. Novel ionizable lipid nanoparticles for SARS-CoV-2 Omicron mRNA delivery. Adv Healthc Mater. 2023;12 doi: 10.1002/adhm.202202590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li B., Manan R.S., Liang S.-Q., Gordon A., Jiang A., Varley A., Gao G., Langer R., Xue W., Anderson D. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat Biotechnol. 2023 doi: 10.1038/s41587-023-01679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qiu M., Tang Y., Chen J., Muriph R., Ye Z., Huang C., Evans J., Henske E.P., Xu Q. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang H., Leal J., Soto M.R., Smyth H.D.C., Ghosh D. Aerosolizable lipid nanoparticles for pulmonary delivery of mRNA through design of experiments. Pharmaceutics. 2020;12:1042. doi: 10.3390/pharmaceutics12111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palanki R., Bose S.K., Dave A., White B.M., Berkowitz C., Luks V., Yaqoob F., Han E., Swingle K.L., Menon P., Hodgson E., Biswas A., Billingsley M.M., Li L., Yiping F., Carpenter M., Trokhan A., Yeo J., Johana N., Wan T.Y., Alameh M.-G., Bennett F.C., Storm P.B., Jain R., Chan J., Weissman D., Mitchell M.J., Peranteau W.H. Ionizable lipid nanoparticles for therapeutic base editing of congenital brain disease. ACS Nano. 2023;17:13594–13610. doi: 10.1021/acsnano.3c02268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han E.L., Padilla M.S., Palanki R., Kim D., Mrksich K., Li J.J., Tang S., Yoon I.-C., Mitchell M.J. Predictive high-throughput platform for dual screening of mRNA lipid nanoparticle blood-brain barrier transfection and crossing. Nano Lett. 2024 doi: 10.1021/acs.nanolett.3c03509. [DOI] [PubMed] [Google Scholar]
- 117.Gao K., Li J., Song H., Han H., Wang Y., Yin B., Farmer D.L., Murthy N., Wang A. In utero delivery of mRNA to the heart, diaphragm and muscle with lipid nanoparticles. Bioact Mater. 2023;25:387–398. doi: 10.1016/j.bioactmat.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Riley R.S., Kashyap M.V., Billingsley M.M., White B., Alameh M.-G., Bose S.K., Zoltick P.W., Li H., Zhang R., Cheng A.Y., Weissman D., Peranteau W.H., Mitchell M.J. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci Adv. 2021;7 doi: 10.1126/sciadv.aba1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Swingle K.L., Safford H.C., Geisler H.C., Hamilton A.G., Thatte A.S., Billingsley M.M., Joseph R.A., Mrksich K., Padilla M.S., Ghalsasi A.A., Alameh M.-G., Weissman D., Mitchell M.J. Ionizable lipid nanoparticles for In vivo mRNA delivery to the placenta during pregnancy. J Am Chem Soc. 2023;145:4691–4706. doi: 10.1021/jacs.2c12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Young R.E., Nelson K.M., Hofbauer S.I., Vijayakumar T., Alameh M.-G., Weissman D., Papachristou C., Gleghorn J.P., Riley R.S. Lipid nanoparticle composition Drives mRNA delivery to the placenta. bioRxiv. 2022 doi: 10.1101/2022.12.22.521490. 2022.12.22.521490. [DOI] [Google Scholar]
- 121.Safford H.C., Swingle K.L., Geisler H.C., Hamilton A.G., Thatte A.S., Ghalsasi A.A., Billingsley M.M., Alameh M.-G., Weissman D., Mitchell M.J. Orthogonal design of experiments for engineering of lipid nanoparticles for mRNA delivery to the placenta. Small. 2023 doi: 10.1002/smll.202303568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Swingle K.L., Billingsley M.M., Bose S.K., White B., Palanki R., Dave A., Patel S.K., Gong N., Hamilton A.G., Alameh M.-G., Weissman D., Peranteau W.H., Mitchell M.J. Amniotic fluid stabilized lipid nanoparticles for in utero intra-amniotic mRNA delivery. J Control Release. 2022;341:616–633. doi: 10.1016/j.jconrel.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Younis M.A., Sato Y., Elewa Y.H.A., Kon Y., Harashima H. Self-homing nanocarriers for mRNA delivery to the activated hepatic stellate cells in liver fibrosis. J Control Release. 2023;353:685–698. doi: 10.1016/j.jconrel.2022.12.020. [DOI] [PubMed] [Google Scholar]
- 124.Xue L., Gong N., Shepherd S.J., Xiong X., Liao X., Han X., Zhao G., Song C., Huang X., Zhang H., Padilla M.S., Qin J., Shi Y., Alameh M.-G., Pochan D.J., Wang K., Long F., Weissman D., Mitchell M.J. Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J Am Chem Soc. 2022;144:9926–9937. doi: 10.1021/jacs.2c02706. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y., Zhang Z., Luo J., Han X., Wei Y., Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20:33. doi: 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malla R., Srilatha M., Farran B., Nagaraju G.P. mRNA vaccines and their delivery strategies: a journey from infectious diseases to cancer. Mol Ther. 2023 doi: 10.1016/j.ymthe.2023.10.024. S1525-0016(23)00601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li B., Jiang A.Y., Raji I., Atyeo C., Raimondo T.M., Gordon A.G.R., Rhym L.H., Samad T., MacIsaac C., Witten J., Mughal H., Chicz T.M., Xu Y., McNamara R.P., Bhatia S., Alter G., Langer R., Anderson D.G. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nat Biomed Eng. 2023 doi: 10.1038/s41551-023-01082-6. [DOI] [PubMed] [Google Scholar]
- 128.Zhang L., More K.R., Ojha A., Jackson C.B., Quinlan B.D., Li H., He W., Farzan M., Pardi N., Choe H. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. NPJ Vaccines. 2023;8:156. doi: 10.1038/s41541-023-00751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McMahon M., O'Dell G., Tan J., Sárközy A., Vadovics M., Carreño J.M., Puente-Massaguer E., Muramatsu H., Bajusz C., Rijnink W., Beattie M., Tam Y.K., Kirkpatrick Roubidoux E., Francisco I., Strohmeier S., Kanekiyo M., Graham B.S., Krammer F., Pardi N. Assessment of a quadrivalent nucleoside-modified mRNA vaccine that protects against group 2 influenza viruses. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2206333119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van de Ven K., Lanfermeijer J., van Dijken H., Muramatsu H., Vilas Boas de Melo C., Lenz S., Peters F., Beattie M.B., Lin P.J.C., Ferreira J.A., van den Brand J., van Baarle D., Pardi N., de Jonge J. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci Adv. 2022;8 doi: 10.1126/sciadv.adc9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang P., Narayanan E., Liu Q., Tsybovsky Y., Boswell K., Ding S., Hu Z., Follmann D., Lin Y., Miao H., Schmeisser H., Rogers D., Falcone S., Elbashir S.M., Presnyak V., Bahl K., Prabhakaran M., Chen X., Sarfo E.K., Ambrozak D.R., Gautam R., Martin M.A., Swerczek J., Herbert R., Weiss D., Misamore J., Ciaramella G., Himansu S., Stewart-Jones G., McDermott A., Koup R.A., Mascola J.R., Finzi A., Carfi A., Fauci A.S., Lusso P. A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat Med. 2021;27:2234–2245. doi: 10.1038/s41591-021-01574-5. [DOI] [PubMed] [Google Scholar]
- 132.Awasthi S., Hook L.M., Pardi N., Wang F., Myles A., Cancro M.P., Cohen G.H., Weissman D., Friedman H.M. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aaw7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Touray B.J.B., Hanafy M., Phanse Y., Hildebrand R., Talaat A.M. Protective RNA nanovaccines against Mycobacterium avium subspecies hominissuis. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1188754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X., Liu C., Rcheulishvili N., Papukashvili D., Xie F., Zhao J., Hu X., Yu K., Yang N., Pan X., Liu X., Wang P.G., He Y. Strong immune responses and protection of PcrV and OprF-I mRNA vaccine candidates against Pseudomonas aeruginosa. NPJ Vaccines. 2023;8:76. doi: 10.1038/s41541-023-00672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naveed M., Mughal M.S., Jabeen K., Aziz T., Naz S., Nazir N., Shahzad M., Alharbi M., Alshammari A., Sadhu S.S. Evaluation of the whole proteome to design a novel mRNA-based vaccine against multidrug-resistant Serratia marcescens. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.960285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ganley M., Holz L.E., Minnell J.J., de Menezes M.N., Burn O.K., Poa K.C.Y., Draper S.L., English K., Chan S.T.S., Anderson R.J., Compton B.J., Marshall A.J., Cozijnsen A., Chua Y.C., Ge Z., Farrand K.J., Mamum J.C., Xu C., Cockburn I.A., Yui K., Bertolino P., Gras S., Le Nours J., Rossjohn J., Fernandez-Ruiz D., McFadden G.I., Ackerley D.F., Painter G.F., Hermans I.F., Heath W.R. mRNA vaccine against malaria tailored for liver-resident memory T cells. Nat Immunol. 2023 doi: 10.1038/s41590-023-01562-6. [DOI] [PubMed] [Google Scholar]
- 137.O'Leary K. An mRNA vaccine against tick bites. Nat Med. 2021 doi: 10.1038/d41591-021-00071-z. [DOI] [PubMed] [Google Scholar]
- 138.Chuang Y.-M., Alameh M.-G., Abouneameh S., Raduwan H., Ledizet M., Weissman D., Fikrig E. A mosquito AgTRIO mRNA vaccine contributes to immunity against malaria. NPJ Vaccines. 2023;8:88. doi: 10.1038/s41541-023-00679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y., Li D., Shen Y., Li S., Lu S., Zheng B. Immunization with a novel mRNA vaccine, TGGT1_216200 mRNA-LNP, prolongs survival time in BALB/c mice against acute toxoplasmosis. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1161507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sittplangkoon C., Alameh M.-G., Weissman D., Lin P.J.C., Tam Y.K., Prompetchara E., Palaga T. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.983000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waghela I.N., Mallory K.L., Taylor J.A., Schneider C.G., Savransky T., Janse C.J., Lin P.J.C., Tam Y.K., Weissman D., Angov E. Exploring in vitro expression and immune potency in mice using mRNA encoding the Plasmodium falciparum malaria antigen, CelTOS. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1026052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bird L. mRNA vaccine for treating pancreatic cancer. Nat Rev Immunol. 2023;23:413. doi: 10.1038/s41577-023-00899-1. [DOI] [PubMed] [Google Scholar]
- 143.Tockary T.A., Abbasi S., Matsui-Masai M., Hayashi A., Yoshinaga N., Boonstra E., Wang Z., Fukushima S., Kataoka K., Uchida S. Comb-structured mRNA vaccine tethered with short double-stranded RNA adjuvants maximizes cellular immunity for cancer treatment. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2214320120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cafri G., Gartner J.J., Zaks T., Hopson K., Levin N., Paria B.C., Parkhurst M.R., Yossef R., Lowery F.J., Jafferji M.S., Prickett T.D., Goff S.L., McGowan C.T., Seitter S., Shindorf M.L., Parikh A., Chatani P.D., Robbins P.F., Rosenberg S.A. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest. 2020;130:5976–5988. doi: 10.1172/JCI134915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo M., Duan X., Peng X., Jin Z., Huang H., Xiao W., Zheng Q., Deng Y., Fan N., Chen K., Song X. A lipid-based LMP2-mRNA vaccine to treat nasopharyngeal carcinoma. Nano Res. 2023;16:5357–5367. doi: 10.1007/s12274-022-5254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao T., Cai Y., Jiang Y., He X., Wei Y., Yu Y., Tian X. Vaccine adjuvants: mechanisms and platforms. Sig Transduct Target Ther. 2023;8:1–24. doi: 10.1038/s41392-023-01557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Van Herck S., Feng B., Tang L. Delivery of STING agonists for adjuvanting subunit vaccines. Advanced Drug Delivery Reviews. 2021;179 doi: 10.1016/j.addr.2021.114020. [DOI] [PubMed] [Google Scholar]
- 148.Gomi M., Sakurai Y., Sato M., Tanaka H., Miyatake Y., Fujiwara K., Watanabe M., Shuto S., Nakai Y., Tange K., Hatakeyama H., Akita H. Delivering mRNA to secondary lymphoid tissues by Phosphatidylserine-loaded lipid nanoparticles. Adv Healthc Mater. 2023;12 doi: 10.1002/adhm.202202528. [DOI] [PubMed] [Google Scholar]
- 149.Zhou H., Liao Y., Han X., Chen D.S., Hong X., Zhou K., Jiang X., Xiao Y., Shi J. ROS-responsive nanoparticle delivery of mRNA and Photosensitizer for combinatorial cancer therapy. Nano Lett. 2023;23:3661–3668. doi: 10.1021/acs.nanolett.2c03784. [DOI] [PubMed] [Google Scholar]
- 150.Raimondo T.M., Reed K., Shi D., Langer R., Anderson D.G. Delivering the next generation of cancer immunotherapies with RNA. Cell. 2023;186:1535–1540. doi: 10.1016/j.cell.2023.02.031. [DOI] [PubMed] [Google Scholar]
- 151.Foster J.B., Griffin C., Rokita J.L., Stern A., Brimley C., Rathi K., Lane M.V., Buongervino S.N., Smith T., Madsen P.J., Martinez D., Delaidelli A., Sorensen P.H., Wechsler-Reya R.J., Karikó K., Storm P.B., Barrett D.M., Resnick A.C., Maris J.M., Bosse K.R. Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin L., Cho S.-F., Xing L., Wen K., Li Y., Yu T., Hsieh P.A., Chen H., Kurtoglu M., Zhang Y., Stewart C.A., Munshi N., Anderson K.C., Tai Y.-T. Preclinical evaluation of CD8+ anti-BCMA mRNA CAR T-cells for treatment of multiple myeloma. Leukemia. 2021;35:752–763. doi: 10.1038/s41375-020-0951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parayath N.N., Stephan S.B., Koehne A.L., Nelson P.S., Stephan M.T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11:6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Si K., Dai Z., Li Z., Ye Z., Ding B., Feng S., Sun B., Shen Y., Xiao Z. Engineered exosome-mediated messenger RNA and single-chain variable fragment delivery for human chimeric antigen receptor T-cell engineering. Cytotherapy. 2023;25:615–624. doi: 10.1016/j.jcyt.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 155.Ye Z., Chen J., Zhao X., Li Y., Harmon J., Huang C., Chen J., Xu Q. In vitro engineering chimeric antigen receptor macrophages and T cells by lipid nanoparticle-mediated mRNA delivery. ACS Biomater Sci Eng. 2022;8:722–733. doi: 10.1021/acsbiomaterials.1c01532. [DOI] [PubMed] [Google Scholar]
- 156.Ong T., Ramsey B.W. Cystic fibrosis: a review. JAMA. 2023;329:1859–1871. doi: 10.1001/jama.2023.8120. [DOI] [PubMed] [Google Scholar]
- 157.Massaro M., Wu S., Baudo G., Liu H., Collum S., Lee H., Stigliano C., Segura-Ibarra V., Karmouty-Quintana H., Blanco E. Lipid nanoparticle-mediated mRNA delivery in lung fibrosis. Eur J Pharm Sci. 2023;183 doi: 10.1016/j.ejps.2023.106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abd Elwakil M.M., Gao T., Isono T., Sato Y., Elewa Y.H.A., Satoh T., Harashima H. Engineered ε-decalactone lipomers bypass the liver to selectively in vivo deliver mRNA to the lungs without targeting ligands. Mater Horiz. 2021;8:2251–2259. doi: 10.1039/d1mh00185j. [DOI] [PubMed] [Google Scholar]
- 159.Burns T.C., Quinones-Hinojosa A. Regenerative medicine for neurological diseases-will regenerative neurosurgery deliver? BMJ. 2021;373:n955. doi: 10.1136/bmj.n955. [DOI] [PubMed] [Google Scholar]
- 160.Puhl D.L., Funnell J.L., Fink T.D., Swaminathan A., Oudega M., Zha R.H., Gilbert R.J. Electrospun fiber-mediated delivery of neurotrophin-3 mRNA for neural tissue engineering applications. Acta Biomater. 2023;155:370–385. doi: 10.1016/j.actbio.2022.11.025. [DOI] [PubMed] [Google Scholar]
- 161.Yang Z., Yang Y., Xu Y., Jiang W., Shao Y., Xing J., Chen Y., Han Y. Biomimetic nerve guidance conduit containing engineered exosomes of adipose-derived stem cells promotes peripheral nerve regeneration. Stem Cell Res Ther. 2021;12:442. doi: 10.1186/s13287-021-02528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fukushima Y., Uchida S., Imai H., Nakatomi H., Kataoka K., Saito N., Itaka K. Treatment of ischemic neuronal death by introducing brain-derived neurotrophic factor mRNA using polyplex nanomicelle. Biomaterials. 2021;270 doi: 10.1016/j.biomaterials.2021.120681. [DOI] [PubMed] [Google Scholar]
- 163.Crowley S.T., Fukushima Y., Uchida S., Kataoka K., Itaka K. Enhancement of motor function recovery after Spinal Cord injury in mice by delivery of brain-derived neurotrophic factor mRNA. Mol Ther Nucleic Acids. 2019;17:465–476. doi: 10.1016/j.omtn.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ogawa K., Kato N., Yoshida M., Hiu T., Matsuo T., Mizukami S., Omata D., Suzuki R., Maruyama K., Mukai H., Kawakami S. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J Control Release. 2022;348:34–41. doi: 10.1016/j.jconrel.2022.05.042. [DOI] [PubMed] [Google Scholar]
- 165.Sakurai Y., Watanabe H., Nishio K., Hashimoto K., Harada A., Gomi M., Suzuki M., Oyama R., Handa T., Sato R., Takeuchi H., Taira R., Tezuka K., Tange K., Nakai Y., Akita H., Uchida Y. pH-Responsive lipid nanoparticles achieve efficient mRNA transfection in brain Capillary endothelial cells. Pharmaceutics. 2022;14:1560. doi: 10.3390/pharmaceutics14081560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.D'Souza A., Dave K.M., Stetler R.A., Manickam D.S. Targeting the blood-brain barrier for the delivery of stroke therapies. Advanced Drug Delivery Reviews. 2021;171:332–351. doi: 10.1016/j.addr.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 167.Stock S.J., Aiken C.E. Barriers to progress in pregnancy research: how can we break through? Science. 2023;380:150–153. doi: 10.1126/science.adf9347. [DOI] [PubMed] [Google Scholar]
- 168.Yan H., Hu Y., Akk A., Rai M.F., Pan H., Wickline S.A., Pham C.T.N. Induction of WNT16 via peptide-mRNA nanoparticle-based delivery maintains cartilage Homeostasis. Pharmaceutics. 2020;12:73. doi: 10.3390/pharmaceutics12010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jayaraman M., Ansell S.M., Mui B.L., Tam Y.K., Chen J., Du X., Butler D., Eltepu L., Matsuda S., Narayanannair J.K., Rajeev K.G., Hafez I.M., Akinc A., Maier M.A., Tracy M.A., Cullis P.R., Madden T.D., Manoharan M., Hope M.J. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene Silencing In vivo. Angew Chem Int Ed Engl. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T., Mihai C., Lynn A., McFadyen I., Moore M.J., Senn J.J., Stanton M.G., Almarsson Ö., Ciaramella G., Brito L.A. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ryals R.C., Patel S., Acosta C., McKinney M., Pennesi M.E., Sahay G. The effects of PEGylation on LNP based mRNA delivery to the eye. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dulla K., Slijkerman R., van Diepen H.C., Albert S., Dona M., Beumer W., Turunen J.J., Chan H.L., Schulkens I.A., Vorthoren L., den Besten C., Buil L., Schmidt I., Miao J., Venselaar H., Zang J., Neuhauss S.C.F., Peters T., Broekman S., Pennings R., Kremer H., Platenburg G., Adamson P., de Vrieze E., van Wijk E. Antisense oligonucleotide-based treatment of retinitis pigmentosa caused by USH2A exon 13 mutations. Mol Ther. 2021;29:2441–2455. doi: 10.1016/j.ymthe.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Melamed J.R., Yerneni S.S., Arral M.L., LoPresti S.T., Chaudhary N., Sehrawat A., Muramatsu H., Alameh M.-G., Pardi N., Weissman D., Gittes G.K., Whitehead K.A. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci Adv. 2023;9 doi: 10.1126/sciadv.ade1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tanaka H., Nakatani T., Furihata T., Tange K., Nakai Y., Yoshioka H., Harashima H., Akita H. In vivo introduction of mRNA encapsulated in lipid nanoparticles to brain neuronal cells and Astrocytes via Intracerebroventricular administration. Mol Pharm. 2018;15:2060–2067. doi: 10.1021/acs.molpharmaceut.7b01084. [DOI] [PubMed] [Google Scholar]
- 175.Monine M., Norris D., Wang Y., Nestorov I. A physiologically-based pharmacokinetic model to describe antisense oligonucleotide distribution after intrathecal administration. J Pharmacokinet Pharmacodyn. 2021;48:639–654. doi: 10.1007/s10928-021-09761-0. [DOI] [PubMed] [Google Scholar]
- 176.Nabhan J.F., Wood K.M., Rao V.P., Morin J., Bhamidipaty S., LaBranche T.P., Gooch R.L., Bozal F., Bulawa C.E., Guild B.C. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich's ataxia. Sci Rep. 2016;6 doi: 10.1038/srep20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dhaliwal H.K., Fan Y., Kim J., Amiji M.M. Intranasal delivery and transfection of mRNA therapeutics in the brain using cationic liposomes. Mol Pharm. 2020;17:1996–2005. doi: 10.1021/acs.molpharmaceut.0c00170. [DOI] [PubMed] [Google Scholar]
- 178.Suberi A., Grun M.K., Mao T., Israelow B., Reschke M., Grundler J., Akhtar L., Lee T., Shin K., Piotrowski-Daspit A.S., Homer R.J., Iwasaki A., Suh H.-W., Saltzman W.M. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci Transl Med. 2023;15 doi: 10.1126/scitranslmed.abq0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cao Y., He Z., Chen Q., He X., Su L., Yu W., Zhang M., Yang H., Huang X., Li J. Helper-polymer based Five-element nanoparticles (FNPs) for lung-specific mRNA delivery with Long-Term stability after Lyophilization. Nano Lett. 2022;22:6580–6589. doi: 10.1021/acs.nanolett.2c01784. [DOI] [PubMed] [Google Scholar]
- 180.Rowe S.M., Zuckerman J.B., Dorgan D., Lascano J., McCoy K., Jain M., Schechter M.S., Lommatzsch S., Indihar V., Lechtzin N., McBennett K., Callison C., Brown C., Liou T.G., MacDonald K.D., Nasr S.Z., Bodie S., Vaughn M., Meltzer E.B., Barbier A.J. Inhaled mRNA therapy for treatment of cystic fibrosis: Interim results of a randomized, double-blind, placebo-controlled phase 1/2 clinical study. J Cyst Fibros. 2023 doi: 10.1016/j.jcf.2023.04.008. S1569-1993(23)00112–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sung J., Alghoul Z., Long D., Yang C., Merlin D. Oral delivery of IL-22 mRNA-loaded lipid nanoparticles targeting the injured intestinal mucosa: a novel therapeutic solution to treat ulcerative colitis. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121707. [DOI] [PubMed] [Google Scholar]
- 182.Abramson A., Kirtane A., Shi Y., Zhong G., Collins J., Tamang S., Ishida K., Hayward A., Wainer J., Rajesh N., Lu X., Gao Y., Karandikar P., Tang C., Lopes A., Wahane A., Reker D., Frederiksen M., Jensen B., Traverso G. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter. 2022;5 doi: 10.1016/j.matt.2021.12.022. [DOI] [Google Scholar]
- 183.Chen Z., Hao W., Gao C., Zhou Y., Zhang C., Zhang J., Wang R., Wang Y., Wang S. A polyphenol-assisted IL-10 mRNA delivery system for ulcerative colitis. Acta Pharm Sin B. 2022;12:3367–3382. doi: 10.1016/j.apsb.2022.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
Data will be made available on request.